Abstract
Aim
This study aimed to determine the 28‐day, 1‐year, and 5‐year survival probabilities in first‐ever stroke patients in a relatively understudied setting: a suburban hospital that serves a predominantly rural population in the east coast of Peninsular Malaysia.
Methods and results
A retrospective record review was conducted among 432 first‐ever stroke patients admitted to the Hospital Universiti Sains Malaysia, Kelantan, Malaysia. Data from between January 1, 2005 and December 31, 2011, were extracted from the medical records. The Kaplan‐Meier product limit estimator was applied to determine the 28‐day, 1‐year, and 5‐year survival probabilities. Log‐rank test was used to test the equality of survival time between different groups. A total of 101 patients died during the study period. The 28‐day, 1‐year, and 5‐year survival probabilities were 78.0% (95% confidence interval [CI]: 73.5–81.9), 74.2% (95% CI: 69.4–78.4), and 70.9% (95% CI: 65.1–75.9), respectively. There were significant differences in the survival time based on the types of stroke, Glasgow Coma Scale, hyperlipidaemia, atrial fibrillation, fasting blood glucose, and diastolic blood pressure.
Conclusion
This study, though retrospective, highlights several clinical parameters that influenced the survival probabilities among first‐ever stroke patients managed in a suburban setting in Malaysia, and compared them to those reported in more urban regions. Our data emphasise the need for wider establishment of specialized stroke units and teams, as well as for prospective multi‐centre studies on first‐ever stroke patients to better inform the development of stroke care provision in Malaysia.
Keywords: first‐ever stroke, ischaemic strokeintracerebral haemorrhage, Malaysia, subarachnoid haemorrhage, survival probabilities
Short abstract
This retrospective study determined the 28‐day, 1‐year, and 5‐year survival probabilities in 432 first‐ever stroke patients in a relatively understudied setting at a single hospital (Hospital Universiti Sains Malaysia, Kelantan) that served a predominantly rural population in the east coast of Peninsular Malaysia. The 28‐day, 1‐year, and 5‐year survival probabilities were 78.0% (95% confidence interval [CI]: 73.5‐81.9), 74.2% (95% CI: 69.4‐78.4), and 70.9% (95% CI: 65.1‐75.9), respectively. We highlighted several clinical parameters that influenced the survival probabilities in this suburban setting compared to the more urban regions.
1. INTRODUCTION
Non‐communicable diseases accounted for 38 million of the total 56 million deaths worldwide in 2012. Most deaths were due to cardiovascular diseases, including strokes (17.5 million), followed by cancer (8.2 million), respiratory diseases (4 million), and diabetes (1.5 million).1 It is estimated that in 2030, a total of 23.6 million deaths will be due to cardiovascular disease, chiefly from heart disease and stroke.2 The South East Asian region has seen one of the most dramatic increases in cases of non‐communicable diseases worldwide, from 6.7 million in year 2000, to 8.5 million in 2012. In Malaysia, stroke is the third largest cause of death after heart diseases and cancer.3 However, by 2020, stroke is projected to be the second leading cause of death and disability worldwide.4 Consequently, the impact of stroke is likely to impose continuing burden and strain on health care resources, particularly for countries with limited resources, such as that of middle‐income like Malaysia.
In Malaysia, variations are known to exist between the different states or districts, in particular in hospital bed utilisation. Our study setting in the north eastern state of Kelantan, a region with one of the lowest urbanization levels in the country (42.4%)5 located in the east coast of Peninsular Malaysia, has the second highest bed occupancy rate for publicly funded hospitals (70.6%), the highest one being in the state of Perlis (75.1%, Hospital Tuanku Fauziah, Kangar, Perlis).6 In relation to stroke, no contemporary data on survival probabilities of first‐ever stroke patients in Kelantan exists, despite such a high utilisation of hospital beds.
National data from relatively more urban catchment areas have indicated an overall stroke mortality of 11.1%, with a 3‐fold increase for haemorrhagic stroke (24.9%) compared with ischaemic stroke (8.1%).7 Another study, although with a 10‐year gap, at a tertiary centre in the nation's capital, Kuala Lumpur, also reported an overall stroke mortality of 11.7% and 27.3% for ischaemic and haemorrhagic stroke, respectively.8 Nevertheless, none of these local studies addressed short‐term and long‐term survival probabilities, or the relevant prognostic factors. Further, suburban/less urbanized areas remain largely uncharacterized in Malaysia, with regards to stroke mortality. Thus, this study aimed to determine the 28‐day, 1‐year, and 5‐year survival probabilities of first‐ever stroke patients in a largely suburban setting in Malaysia.
2. METHODS
2.1. Study design and participants
A retrospective record review was conducted among patients with first‐ever stroke admitted to the Hospital Universiti Sains Malaysia (HUSM), Kelantan, Malaysia. Data from between January 1, 2005 to December 31, 2011, were extracted from medical records, using standard data collection proforma. Patients who were clinically diagnosed as first‐ever stroke and aged 18 years and above were included in the study. Those with recurrent stroke or having any neurological deficits secondary to an infection, epilepsy, tumour, or traumatic causes, were excluded. Patients were informed, on admission, that their medical data could be used for medical and scientific research. Patients were given the option to opt out of this. The study received approval from Human Ethics Committee of Universiti Sains Malaysia (USMKK/PPP/JEPeM [256.4(2.9)]).
Stroke was defined as “rapidly developing clinical signs of focal (or global) disturbance of cerebral function, lasting more than 24 hours or leading to death with no apparent cause other than that of vascular origin.”1 A first‐ever stroke patient was classified as a patient with no evidence of previous stroke, as confirmed by computed tomography scan or magnetic resonance imaging, and neurological examination during admission. During the study period, acute stroke patients were either managed in general medical or neurointensive care wards, with no stroke unit or specific stroke team setting.
Sample size was determined by using Power and Sample Size Calculation software9 on various types of stroke, with requirements for level of significance (α) of 0.05 and with pre‐determined power (1 − β) of 0.80. The detectable hazard ratio of those with subarachnoid haemorrhage (SAH) to those with cerebral infarct of 2.3 was chosen based on clinical expert opinion. The median survival time of stroke patients with cerebral infarct (m1 = 84) and ratio of stroke patients with cerebral infarct to those with SAH patients (m = 5.54) were obtained from the literature.10 The accrual time (A) during which the patients were recruited was 84 months, with an additional follow‐up time (F) of 12 months. An estimated 10% was added to the final sample size estimation, in anticipation of missing data. The predetermined sample size was 417 patients. Systematic random sampling was applied from the list of stroke patients admitted to HUSM that was recorded in medical records for the period of 84 months (Figure 1).
Figure 1.
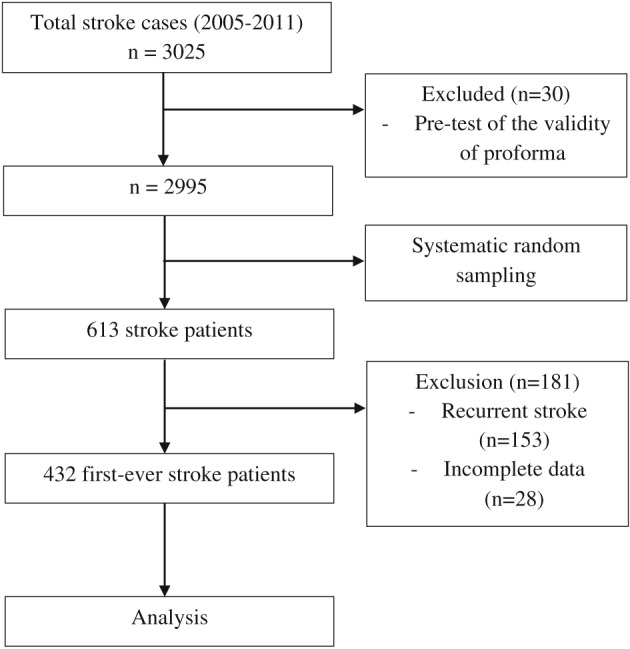
Flow chart of the patients analyzed in this study
2.2. Study factors and outcome
All required information, such as demographic characteristics, neurological status, vascular risk factors, and paraclinical parameters, was extracted from the medical records by a single researcher using standard data collection proforma and verified by another researcher. Demographic characteristics consisted of age at the time of diagnosis and sex. Age at the time of diagnosis was calculated based on difference of date of diagnosis and date of birth of the patient. This was then categorised as age less than 40 and equal, and more than 40 years old.
For neurological status, the variables included types of stroke and Glasgow Coma Scale (GCS).11 Types of stroke were broadly divided into 3 categories, namely ischaemic stroke, intracerebral haemorrhage (ICH), and SAH. Aetiologies for the ischaemic stroke sub‐types, such as large artery or small vessel diseases, were not documented. GCS status, which was used as an indicator of conscious level, was categorised into severe (GCS of 8 or less), moderate (9 to 12), and mild or no impairment (13 to 15).
The variables for vascular risk factors included high blood pressure, hyperlipidaemia, ischaemic heart disease, atrial fibrillation, and smoking status. Smoking status was categorised into non‐smoker, ever‐smoked, and current smoker. Paraclinical variables included fasting blood glucose, systolic blood pressure, and diastolic blood pressure. Fasting blood glucose was divided into less than 7.0 mmol/L and more and equal than 7.0 mmol/L.12 For systolic blood pressure, it was divided into Grade 1 (140–159 mmHg), Grade 2 (160–179 mmHg), and Grade 3 (≥180 mmHg), and for diastolic blood pressure, into Grade 1 (90–99 mmHg), Grade 2 (100–109 mmHg), and Grade 3 (≥110 mmHg).13
The event in this study was the survival time of first‐ever stroke patients, measured in days. The survival time was defined as the time interval between time of diagnosis and time of death, where death was the event of interest. The censored observations were patients who did not experience the event of interest, such as those who were still alive at the end of the study period and those who were lost to follow‐ups.
2.3. Statistical analysis
Data were entered and analyzed using Stata/SE version 11 software. The Kaplan‐Meier product limit estimator was used to measure the survival probabilities of first‐ever stroke patients at 28 days, 1 year, and 5 years, by incorporating the information from event and censored data. The result was expressed as survival probability and its 95% confidence interval (CI). The Kaplan‐Meier survival graph was obtained by plotting the estimated survival probability against survival time plotted to display an estimate of a survival probability for the categorical independent variables.14, 15, 16
In order to test the equality of survival time between different groups, log‐rank test was used, by testing the null hypothesis that no statistical difference in the survival time among the groups existed. For a categorical variable with 2 levels, the level of significance was set at 0.05. On the other hand, for a categorical variable with more than 2 levels, the P‐value obtained from the pair‐wise comparison was compared with the level of significance after Bonferroni correction. The level of significance (α = 0.05) was divided by the number of pairs in this correction. The P‐value obtained was then compared with this corrected level of significance. A decision was then reached in order to determine whether the difference of survival time was significant or not.14, 15, 16
3. RESULTS
A total of 432 (70.5%) out of 613 stroke patients who met the criteria were further analyzed. Those excluded had recurrent stoke (25.0%) and incomplete data from the medical records (4.5%). At the study endpoint, 101 (23.4%) deaths were identified, and 331 patients (76.6%) were still alive and were considered as censored. The median duration of survival time in this study was 119 days, with an interquartile range of 1073.5. The minimum and maximum survival times were 1 and 5545 days, respectively. The 28‐day, 1‐year, and 5‐year survival probabilities were 78.0% (95% CI: 73.5–81.9), 74.2% (95% CI: 69.4–78.4), and 70.9% (95% CI: 65.1–75.9), respectively (Figure 2).
Figure 2.
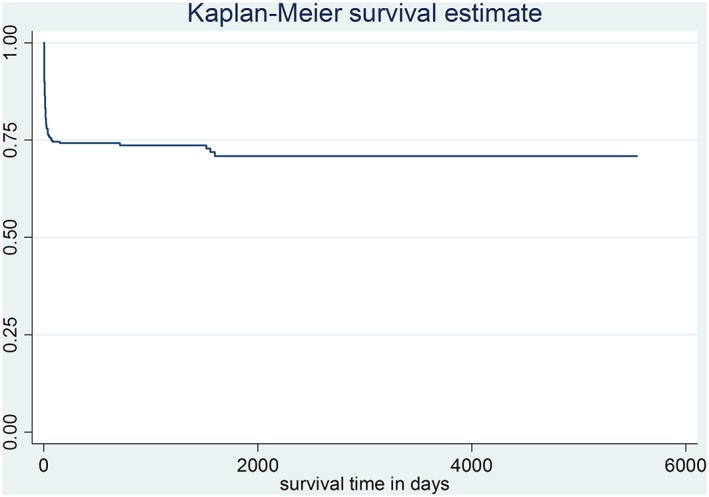
Overall survival probabilities of first‐ever stroke patients
Descriptive statistics of first‐ever stroke patients are shown in Table 1. Most patients (92.1%) were 40 years old or older at the time of diagnosis (mean: 59 years old; standard deviation: 13.5), with a slight male predominance (53.9%). More than half of the patients were diagnosed with ischaemic stroke (54.4%); ICH (37.5%) and SAH (8.1%) were the other most frequent diagnoses. Most patients (94.9%) had no atrial fibrillation. More than half were classified as non‐smokers (56.7%). Similarly, over half of the patients (57.4%) were found to have a fasting blood glucose of more than 7.0 mmol/L.
Table 1.
Descriptive statistics of first‐ever stroke patients admitted to HUSM (n = 432)
| Variable |
Total n (%) |
Died n (%) |
Censored n (%) |
|---|---|---|---|
| Demographic characteristics | |||
| Age at the time of diagnosis | |||
| <40 years | 34 (7.9) | 5 (14.7) | 29 (85.3) |
| ≥40 years | 398 (92.1) | 96 (24.1) | 302 (75.9) |
| Sex | |||
| Female | 199 (46.1) | 48 (24.1) | 151 (75.9) |
| Male | 233 (53.9) | 53 (22.8) | 180 (77.2) |
| Neurological status | |||
| Types of stroke | |||
| Ischaemic stroke | 235 (54.4) | 39 (16.6) | 196 (83.4) |
| Intracerebral haemorrhage | 162 (37.5) | 46 (28.4) | 116 (71.6) |
| Subarachnoid haemorrhage | 35 (8.1) | 16 (45.7) | 19 (54.3) |
| Glasgow Coma Scale | |||
| Mild (13–15) | 232 (53.7) | 19 (8.2) | 213 (91.8) |
| Moderate (9–12) | 120 (27.8) | 41 (34.2) | 79 (65.8) |
| Severe (less than 8) | 80 (18.5) | 41 (51.2) | 39 (48.8) |
| Vascular risk factor | |||
| Hyperlipidaemia | |||
| No | 317 (73.4) | 84 (26.5) | 233 (73.5) |
| Yes | 115 (26.6) | 17 (14.8) | 98 (85.2) |
| Atrial fibrillation | |||
| No | 410 (94.9) | 92 (22.4) | 318 (77.6) |
| Yes | 22 (5.1) | 9 (40.9) | 13 (59.1) |
| Smoking status | |||
| Never smoke | 245 (56.7) | 50 (20.4) | 195 (79.6) |
| Ever‐smoker | 66 (15.3) | 16 (24.2) | 50 (75.8) |
| Current smoker | 121 (18.0) | 35 (28.9) | 86 (71.1) |
| Selected paraclinical parameter | |||
| Fasting blood glucose | |||
| <7.0 mmol/L | 184 (42.6) | 34 (18.5) | 150 (81.5) |
| ≥7.0 mmol/L | 248 (57.4) | 67 (27.0) | 181 (73.0) |
| Systolic blood pressure | |||
| Grade 1 (140–159 mmHg) | 192 (44.4) | 41 (21.3) | 151 (78.7) |
| Grade 2 (160–179 mmHg) | 80 (18.5) | 19 (23.8) | 61 (76.2) |
| Grade 3 (≥180 mmHg) | 160 (37.0) | 41 (25.6) | 119 (74.4) |
| Diastolic blood pressure | |||
| Grade 1 (90–99 mmHg) | 259 (60.0) | 53 (20.7) | 206 (79.5) |
| Grade 2 (100–109 mmHg) | 67 (15.5) | 12 (17.9) | 55 (82.1) |
| Grade 3 (≥110 mmHg) | 106 (24.5) | 36 (34.0) | 70 (66.0) |
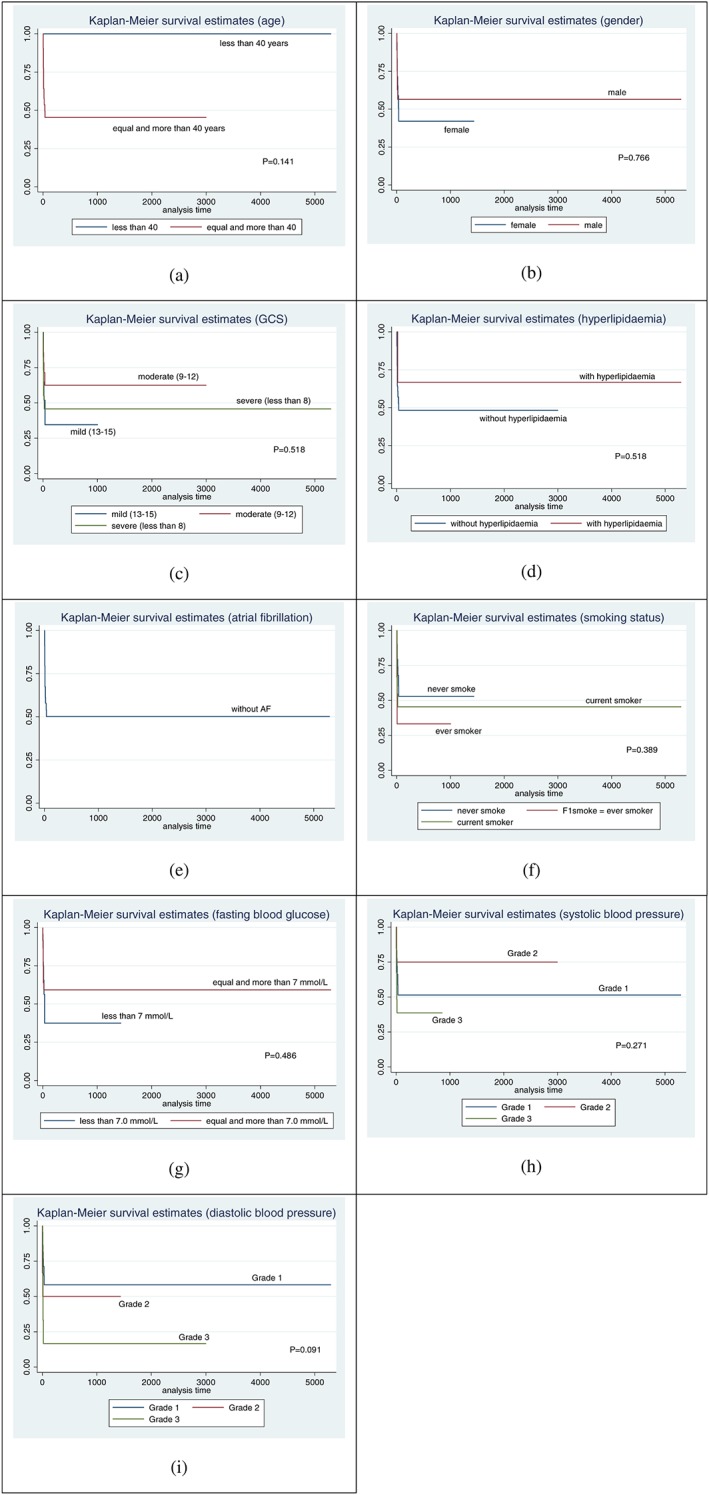
Comparisons of survival probabilities for the different time points on the basis of demographic characteristic, neurological status, vascular risk factors, and paraclinical parameters are summarised in Table 2. The Kaplan‐Meier survival probability curve for the different stroke types is shown in Figure 3 (ischaemic stroke), Figure 4 (ICH), and Figure 5 (SAH), respectively. Based on the log‐rank test results, there were significant differences of the overall survival probabilities in GCS, hyperlipidaemia, atrial fibrillation, fasting blood glucose, and diastolic blood pressure. Patients diagnosed with SAH had the lowest survival probability, followed by ICH and ischaemic stroke.
Table 2.
Survival probabilities in 28‐day, 1‐year, and 5‐year of first‐ever stroke patients admitted to HUSM (n = 432)
| Variable | Survival Probability (95% CI) | P‐Value b | ||
|---|---|---|---|---|
| 28 days | 1 year | 5 years | ||
| Demographic characteristic | ||||
| Age at the time of diagnosis | ||||
| <40 years a | 84.9 (67.5, 93.4) | 84.9 (67.5, 93.4) | 84.9 (67.5, 93.4) | |
| ≥40 years | 77.4 (72.6, 81.5) | 73.2 (68.0, 77.6) | 69.5 (63.3, 74.9) | 0.150 |
| Sex | ||||
| Female a | 76.3 (69.1, 82.1) | 71.4 (63.6, 77.9) | 68.7 (59.1, 76.4) | |
| Male | 79.5 (73.4, 84.3) | 76.3 (69.9, 81.5) | 72.6 (65.0, 78.8) | 0.323 |
| Neurological status | ||||
| Types of stroke | ||||
| Ischaemic a | 83.4 (77.5, 87.9) | 80.3 (74.0, 85.3) | 80.3 (74.0, 85.3) | |
| Intracerebral haemorrhage | 75.3 (67.5, 81.5) | 71.1 (62.9, 77.9) | 62.2 (50.5, 71.9) | 0.009 c |
| Subarachnoid haemorrhage | 57.9 (39.3, 72.6) | 50.2 (31.6, 66.1) | 50.2 (31.6, 66.1) | <0.001 c |
| Glasgow Coma Scale | ||||
| Mild a | 93.2 (88.8, 95.9) | 90.5 (85.4, 93.8) | 90.5 (85.4, 93.8) | |
| Moderate | 68.9 (58.9, 77.0) | 62.4 (51.6, 71.4) | 46.5 (30.1, 61.3) | <0.001 c |
| Severe | 45.5 (33.3, 56.9) | 40.8 (28.5, 52.8) | 40.8 (28.5, 52.8) | <0.001 c |
| Vascular risk factor | ||||
| Hyperlipidaemia | ||||
| No a | 74.0 (68.4, 78.7) | 70.8 (64.9, 75.8) | 67.4 (60.4, 73.4) | 0.009 |
| Yes | 87.1 (78.8, 92.4) | 83.7 (74.7, 89.8) | 80.6 (69.3, 88.2) | |
| Atrial fibrillation | ||||
| No a | 79.5 (74.9, 83.3) | 75.4 (70.6, 79.6) | 72.0 (66.0, 77.1) | |
| Yes | 46.5 (20.8, 69.9) | 46.5 (20.8, 69.9) | 46.5 (20.8, 69.9) | 0.005 |
| Smoking status | ||||
| None smoker a | 80.7 (74.7, 85.4) | 77.0 (70.6, 82.3) | 73.6 (65.6, 80.1) | |
| Ever‐smoker | 78.5 (65.7, 87.0) | 74.5 (61.0, 83.9) | 71.8 (57.7, 81.9) | 0.535 c |
| Current smoker | 72.0 (62.6, 79.4) | 68.9 (59.3, 76.7) | 65.8 (54.5, 75.0) | 0.153 c |
| Selected paraclinical parameter | ||||
| Fasting blood glucose | ||||
| <7.0 mmol/L a | 81.6 (74.7, 86.8) | 79.4 (72.2, 85.0) | 77.2 (68.7, 83.7) | |
| ≥7.0 mmol/L | 74.9 (68.6, 80.1) | 70.2 (63.4, 76.0) | 66.1 (58.0, 73.0) | 0.033 |
| Systolic blood pressure | ||||
| Grade 1 a | 80.0 (73.1, 85.3) | 75.1 (67.7, 81.1) | 75.1 (67.7, 81.1) | |
| Grade 2 | 78.4 (67.0, 86.2) | 74.5 (62.2, 83.3) | 70.1 (55.1, 80.9) | 0.613 c |
| Grade 3 | 75.5 (67.5, 81.8) | 71.8 (63.3, 78.6) | 66.6 (55.6, 75.5) | 0.297 c |
| Diastolic blood pressure | ||||
| Grade 1 a | 79.9 (74.1, 84.6) | 76.2 (69.9, 81.3) | 76.2 (69.9, 81.3) | |
| Grade 2 | 85.9 (74.6, 92.5) | 85.9 (74.6, 92.5) | 75.9 (59.1, 86.5) | 0.424 c |
| Grade 3 | 66.7 (56.0, 75.4) | 61.0 (49.8, 70.5) | 56.6 (43.0, 68.2) | 0.006 c |
Reference group.
Level of significance alpha was set at 0.05.
Bonferroni correction was applied for prognostic factors ≥3 levels by correcting the level of significance alpha (α/number of pairs = 0.05/3 = 0.017).
Figure 3.
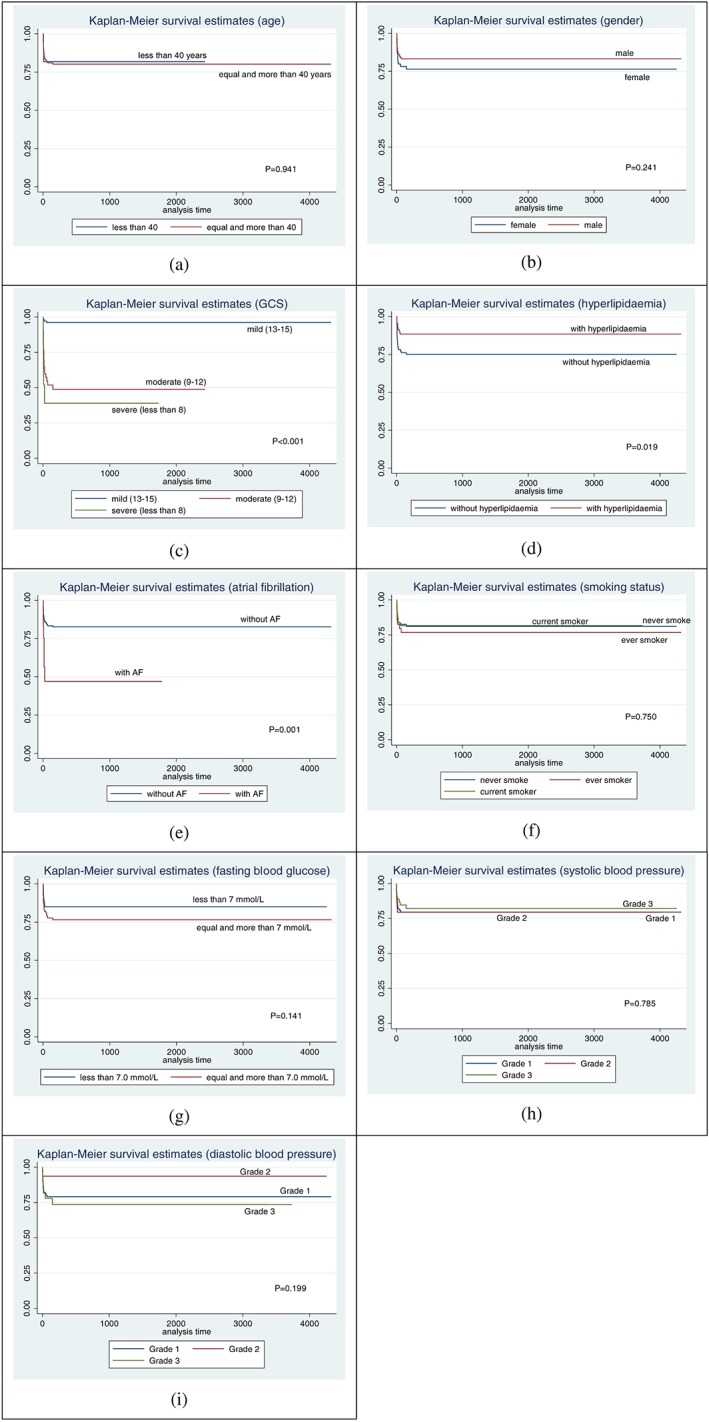
Kaplan Meier survival probability curves for ischaemic stroke, among first‐ever stroke patients, based on different clinical parameters
Figure 4.
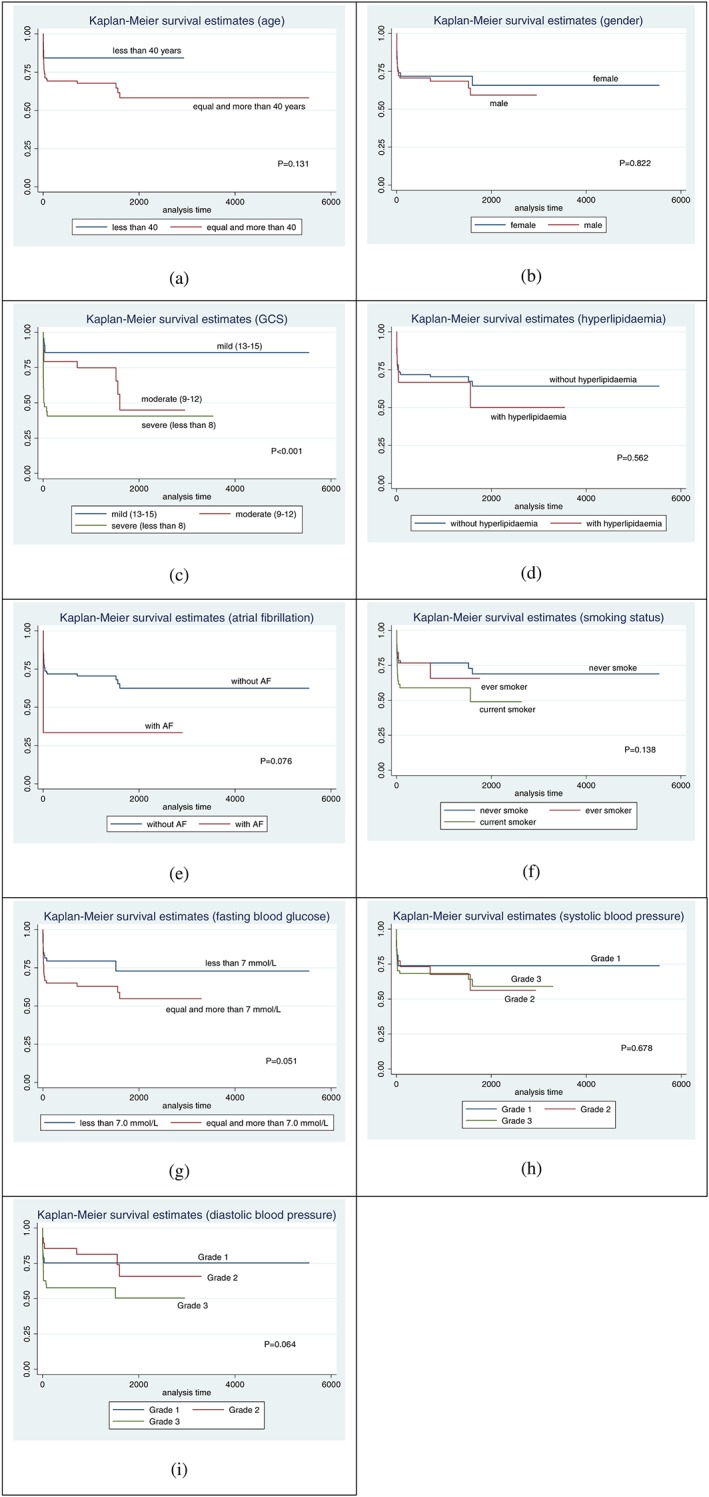
Kaplan Meier survival probability curves for intracerebral haemorrhage stroke, among first‐ever stroke patients, based on different clinical parameters
Figure 5.
Kaplan Meier survival probability curves for subarachnoid haemorrhage stroke, among first‐ever stroke patients, based on different clinical parameters
Patients with severe GCS at admission had the lowest survival probability, followed by those with moderate and mild GCS. The survival probability was higher in stroke patients with hyperlipidaemia. Even though there was only a small number of patients with atrial fibrillation, the survival probability still showed significant difference. Patients with atrial fibrillation had lower survival probability compared with patients with no atrial fibrillation (P‐value = 0.005). Patients with a fasting blood glucose of more than 7.0 mmol/L had lower survival probability compared with those with less than 7.0 mmol/L (P‐value = 0.033). Patients with grade 3 diastolic blood pressure had lower survival probability compared with those with grade 1 (P‐value = 0.006).
4. DISCUSSION
Although a few studies have evaluated stroke mortality in Malaysia, none of these have addressed short‐term and long‐term survival probabilities or the relevant prognostic factors. In addition, these reports have largely focused on urban regions, and suburban/less urbanized areas in this country have remained largely uncharacterized with regards to stroke mortality. In this study, we thus aimed to determine the 28‐day, 1‐year, and 5‐year survival probabilities of first‐ever stroke patients in a largely suburban setting in Malaysia.
Compared with previous studies done in developed countries,17, 18 in this study, the median age of first‐stroke patients was considerably lower, and there was a higher incidence of ICH. We found that the mortality following an acute stroke is highest in the first 28 days. Atrial fibrillation was much less commonly associated, but diabetes was much more commonly associated with acute ischemic stroke than in developed countries.
Short‐term or long‐term survival probabilities after first‐ever stroke event have been noted to differ between different populations, which highlights the need to evaluate these variables in different settings. The comparison of the results obtained in this study and other studies is summarised in Table 3. In this study, the 28‐day survival probability was 78.0% (95% CI: 73.5–81.9), comparable to the result obtained in a study conducted in the state of Penang, Malaysia (an urban setting), which was 79.7%.31 However, our 1‐month survival probability figure (78%) is lower than that reported in previous studies, which ranged from 80.2% to 95.2%.17, 24, 32
Table 3.
Studies on survival probabilities in stroke patients
| Authors | Study Population | Study Setting | Study Period | Survival Probability (%) | |||
|---|---|---|---|---|---|---|---|
| 28 days | 1 year | 5 years | |||||
| Hankey et al, 200019 | n = 362 first‐ever stroke | Perth Community Stroke Study | Feb 1989 – Aug 1990 | Overall | ‐ | ‐ | 39.9 |
| Bronnum‐Hansen et al, 200110 | n = 4162 first stroke | Community based in Copenhagen country | 1982–1991 | Overall | 72.0 | 59.0 | 40.0 |
| Collins et al, 200320 | n = 40 308 first stroke | Department of Veterans Affairs, US | 1994–1998 |
Ischaemic stroke Haemorrhagic stroke |
92.6 81.2 |
80.9 68.2 |
‐ ‐ |
| Kimura et al, 200521 | n = 10 981 acute ischemic stroke | Japan Multicenter Stroke Investigators' Collaboration study | May 1999 – April 2000 |
Overall Age ≤ 59 Age 60–69 Age 70–79 Age ≥ 80 Male Female HPT—Yes HPT—No Diabetes—Yes Diabetes—No HPL—Yes HPL—No Smoking—Yes Smoking—No AF—Yes AF—No Own home Institution |
‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ |
93.2 98.5 96.6 93.6 84.0 93.4 92.9 93.7 92.5 92.6 93.5 95.8 92.7 95.6 92.7 87.4 94.5 96.7 84.9 |
‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ |
| Lavados et al, 200522 | n = 380 stroke | Community‐based prospective study (PISCIS project) | July 2000 – June 2006 |
Overall Ischaemic stroke ICH SAH Undetermined |
76.7 82.2 71.1 60.0 60.9 |
‐ ‐ ‐ ‐ ‐ |
‐ ‐ ‐ ‐ ‐ |
| Kammersgaard and Olsen, 200623 | n = 899 ischemic stroke | Community‐based Copenhagen Stroke study |
AF—Yes AF—No IHD—Yes IHD—No Diabetes—Yes Diabetes—No Smoking—Yes Smoking—No Alcohol—Yes Alcohol—No |
‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ |
‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ |
20.6 45.4 32.2 45.7 32.8 44.1 45.7 40.8 49.1 40.6 |
|
| Jeng et al, 200824 | n = 850 acute stroke | Stroke ICU, Taiwan | Nov 2002 – Dec 2006 |
Ischaemic stroke Haemorrhagic stroke |
87.2 82.2 |
‐ ‐ |
‐ ‐ |
| Kong et al, 200925 | n = 2774 ischaemic stroke | Chengdu Stroke Registry | March 2002 – July 2007 | Overall | ‐ | 88.2 | ‐ |
| Chang et al, 201026 | n = 360 first‐ever ischemic stroke | Chang Gung Memorial Hospital, Taiwan | Sept 1998 – Oct 1999 | Overall | 93.1 | 87.8 | ‐ |
| Goulart et al, 201327 | n = 665 first‐ever stroke | The EMMA study, Sao Paulo, Brazil | April 2006 – Dec 2010 |
Ischaemic stroke Haemorrhagic stroke Age 35–59 Age 60–79 Age ≥ 80 Married Single Divorced Widowed Diabetes—Yes Diabetes—No |
‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ |
66.0 59.0 80.0 68.0 38.0 69.0 68.0 70.0 53.0 59.0 67.0 |
‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ |
| Hoffmeister et al, 201328 | n = 51 130 ischemic stroke | Retrospective Study Chilean Hospital, Chile | Jan 2003 – Dec 2007 |
Overall Male Female Low‐income Middle‐high income High income Age ≤ 60 Age 61–70 Age 71–80 Age > 80 |
81.9 83.4 80.3 80.0 86.3 91.4 88.9 85.5 81.1 73.3 |
69.9 71.8 67.9 67.0 76.1 85.4 84.2 77.1 67.6 53.1 |
‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ |
| Leyden et al, 201329 | n = 238 first‐ever stroke | Adelaide Stroke Incidence Study | July 2009 – July 2010 |
Overall Ischaemic stroke Haemorrhagic stroke |
82.0 84.0 65.0 |
‐ ‐ ‐ |
‐ ‐ ‐ |
| Medic et al, 201330 | n = 300 first‐ever ischaemic stroke | Neurological institutions, Belgrade | March 2008 | Overall | 81.0 | 78.3 | ‐ |
| Current study | n = 432 first‐ever stroke | Hospital Universiti Sains Malaysia, Kelantan, Malaysia | Jan 1, 2005 – Dec 31, 2011 | Overall | 78.0 | 74.2 | 70.9 |
The overall 1‐month survival probability for the different stroke types in this study was higher in ischaemic stroke patients (83.4%), followed by ICH (75.3%) and SAH (54.0%). These figures are comparable to the findings reported at Penang Hospital, with ischaemic stroke at 88.6% and haemorrhagic stroke at 53.2%.31 By contrast, the Danish Indicator Project reported 1‐month survival probabilities of 95.2% and 80.2% in ischaemic stroke and ICH, respectively.17 Several reasons may account for this marked difference. In developed countries, such as Denmark in this case, the general public is likely to be more informed about symptoms of stroke, and patients are more likely to seek timely hospital treatment. In addition, patients are likely to receive care in a stroke unit setting upon admission to the hospital, and/or to be cared for by a multi‐disciplinary stroke team. Another study on those managed in stroke intensive care unit also reported a higher 1‐month survival probability in ischaemic stroke (87.2%) and ICH (82.2%).24 Given that specialist stroke unit care is an evidence‐based model for stroke care, the marked difference in 1‐month survival probability is reflected by our study population of first‐ever stroke patients, which received care within a generic general medical ward setting without coordinated access to a multi‐disciplinary stroke team.
Management details for those with SAH were not collected within the study data collection proforma. However, all SAH patients were managed by the neurosurgical team at a dedicated neurosurgical intensive care unit in our setting. A prospective study to assess the management and outcome of SAH is warranted in our population.
The 1‐year survival probability in our study was 74.2% (95% CI: 69.4–78.4), which is comparable to that found in previous studies, which ranged from 59.0% to 93.2%.10, 21, 26, 30 While a lower figure (59%) was reported in a community‐based study in Copenhagen,10 the mean age of patients in that study was 61.4 years and 62.8 years for ischaemic stroke and ICH, respectively, which contrasts with our relatively younger stroke population (mean: 59 years old). Other studies have reported higher survival probability, which may derive from the fact that they solely included ischaemic stroke survivors.21, 26, 30
In this study, even though there was no statistically significant difference in the log‐rank test, the survival probability of 1‐year was higher in males (76.3%) compared with female patients (71.4%). However, this is in contrast to 1 Japanese study that reported a significant difference of survival probability between sex in patients more than 80 years old (P‐value = 0.033), where the survival probability appeared lower in males compared with females (82.0% and 85.6% respectively).21 Although the prospective nature of this Japanese data offers a more reliable conclusion than that of our retrospective, single‐centre data limitation, our patients were considerably younger, and, therefore, the results are not directly comparable.
While the 5‐year survival probability of first‐ever stroke patients in our study was 70.9% (95% CI: 65.1–75.9), other studies have reported a 5‐year survival probability of approximately 40.0%.10, 19 In these studies, older age group was thought to increase the mortality among stroke patients.10, 19 By contrast, in our study, those aged 70 and above represented only 22.0% of the patients (mean age of all patients: 59 years old). Moreover, the mortality among the long‐term stroke survivors has been attributed to causes other than stroke and related complications, including accidents, cancer, suicide, and other morbidities.10
In relation to stroke types, our result for the long‐term survival probability was comparable with the short‐term survival probability. The survival probability was generally lower in haemorrhagic stroke (ICH and SAH) when compared with ischaemic stroke. However, in a study that was part of the Monitoring Trends and Determinants in Cardiovascular Disease (MONICA) Project, the long‐term survival probability for patients aged 65 and above was reported to be better for SAH than for other types of stroke.10
We found a significant difference (P‐value = 0.005) in 5‐year survival probability between stroke survivors with atrial fibrillation (46.5%) compared with those without atrial fibrillation (72.0%). While a small number of cases and sample bias may have influenced this finding, as over 90% of patients were 40 years old or older at the time of the diagnosis (mean: 59 years old; standard deviation: 13.5), similar finding were reported in the Copenhagen Stroke study, whereby the 5‐year mortality risk was significantly higher in stroke patients with atrial fibrillation (79.4%) than in those without atrial fibrillation (54.6%) (P‐value < 0.001).23 The small number of those found to have atrial fibrillation in this study was determined solely from the medical records: from the discharge summary entry of the diagnosis, as well as from any documented ECG and/ or prolonged cardiac monitoring where indicated.
There was no significant difference of short‐term and long‐term survival probabilities according to smoking status in this study. However, the survival probability was lower in current smoker patients compared with ever‐smoked and non‐smoker groups. A statistically significant association was found between 5‐year mortality risk and smoking status in the Copenhagen Stroke study (P‐value = 0.010). The cumulative risk was lower in smokers (54.3%) compared with non‐smokers (59.2%).23
In our study, even though there was no significant difference of overall survival probability between age groups (P‐value = 0.150), the survival probability was lower for patients aged more than 40 years compared with those aged less than 40 years. This is consistent with a previous study which reported the cumulative survival probabilities for patients aged 35 to 59 years, 60 to 79 years, and more than 80 years of 70.0%, 55.0%, and 15.0%, respectively.27
It is recognised that both the short and long‐term survival probabilities tend to reduce drastically with the increasing level of stroke severity. Glasgow Coma Scale (GCS) was used to guide stroke severity in our data, as this was routinely documented in our patients' medical records. Those with lower GCS appeared to have lowest survival probability. Studies using stroke‐specific scales to assess stroke severity, such as National Institutes of Health Stroke Scale (NIHSS) or World Federation of Neurological Surgeons Scale, had reported an increased risk of dying with stroke severity on admission. Indeed, a study in a Taiwanese setting reported increases in mortality rates from 7.8% to 58.6% as a result of an increase in NIHSS score,26 while another study revealed that high NIHSS score among stroke patients on admission was associated with early mortality.33
We recognise several limitations to this study and emphasise that the study findings be used and interpreted within its context. Firstly, this study was confined to 1 hospital, which limits the generalisability of the finding to the Malaysian population. Secondly, data were retrospectively extracted from secondary data sources. Because it was a retrospective record review study, information on variables under study were not as comprehensive and might have been subjected to sample bias. Additional information, such as assessment using a stroke‐specific scale, could not be obtained from this retrospective data. And thirdly, patients with incomplete observations were excluded from the analysis and this may have underestimated the overall results.
Despite these limitations, this study has several strengths to support the validity and reliability of the results obtained. A deductive sample size determination was performed prior to the study. The sampling method applied was appropriate to address the research questions of this study which was systematic random sampling.34 This sampling method was probability sampling, where everyone in the sampling frame had an equal probability of being chosen. This approach made the sample more representative of the population from which it was drawn. Lastly, there was no contemporary data on survival probabilities of first‐ever stroke in Malaysia; therefore, this study established to date, contemporary information on first‐ever stroke patients in Malaysia, specifically from an understudied suburban region.
5. CONCLUSION
In conclusion, this study though retrospective, provides some useful information about both short and long‐term survival probabilities among first‐ever stroke patients managed in a suburban hospital setting in Malaysia. Our data emphasise the need for the urgent development of specialist stroke units and also for prospective multi‐centre studies on first‐ever stroke patients to better inform the development of stroke care provision in Malaysia.
FUNDING
No external funding for the research.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
Conceptualization: Nadiah Wan‐Arfah, Nyi Nyi Naing, Mustapha Muzaimi
Formal Analysis: Nadiah Wan‐Arfah
Investigation: Nadiah Wan‐Arfah
Writing—review and editing: Nadiah Wan‐Arfah, Hanafi Muhammad Hafiz, Nyi Nyi Naing, Mustapha Muzaimi, Hamsaraj G M Shetty
Writing—original draft preparation: Nadiah Wan‐Arfah
ACKNOWLEDGMENTS
The authors would like to thank the Director of Hospital Universiti Sains Malaysia, to all the staff of Medical Records Unit for their cooperation to complete data collection in this study.
Wan‐Arfah N, Hafiz HM, Naing NN, Muzaimi M, Shetty HGM. Short‐term and long‐term survival probabilities among first‐ever ischaemic and haemorrhagic stroke patients at a hospital in the suburban east coast of Peninsular Malaysia. Health Sci Rep. 2018;1:e27 10.1002/hsr2.27
REFERENCES
- 1. World Health Organization . Deaths from NCDs. 2012. Retrieved from:http://www.who.int/gho/ncd/mortality_morbidity/ncd_total_text/en/index.html [Accessed April 16, 2012].
- 2. World Health Organization . Cardiovascular diseases. 2011. Retrieved from:http://www.wpro.who.int/mediacentre/factsheets/cardiovascular_disease/en/ [Accessed April 16, 2012].
- 3. Ministry of Health Malaysia . Health Facts 2015. 2016. Retrieved from:http://www.moh.gov.my/ [Accessed June 7, 2016].
- 4. Murray CJL, Lopez AD. Alternative projections of mortality and disability by cause 1990‐2020: global burden of disease study. Lancet. 1997;349:1498‐1504. [DOI] [PubMed] [Google Scholar]
- 5. Department of Statistics Malaysia . Population and Housing Census of Malaysia. 2010. Retrieved from:http://www.statistics.gov.my/portal/download_Population/files/census2010/Taburan_Penduduk_dan_Ciri-ciri_Asas_Demografi.pdf [Accessed September 21, 2014].
- 6. Clinical Research Centre . National Healthcare Establishments and Workforce Statistics (Hospital) 2008–2009. 2011. Retrieved from:http://www.crc.gov.my/wp-content/uploads/documents/report/Hospitals_Report.pdf [Accessed September 21, 2014].
- 7. Nazifah SN, Azmi IK, Hamidon BB, Looi I, Zariah AA, Hanip MR. National Stroke Registry (NSR): Terengganu and Seberang Jaya experience. Med J Malaysia. 2012;67(3):302‐304. [PubMed] [Google Scholar]
- 8. Hamidon B, Raymond AA. Predictors of in‐hospital mortality after an acute ischaemic stroke. Neurol J Southeast Asia. 2003;8:5‐8. [Google Scholar]
- 9. Dupont WD, Plummer WD. Power and sample size calculation: a review and computed program. Control Clin Trials. 1990;11:116‐128. [DOI] [PubMed] [Google Scholar]
- 10. Bronnum‐Hansen H, Davidsen M, Thorvaldsen P. Long‐term survival and causes of death after stroke. Stroke. 2001;32:2131‐2136. [DOI] [PubMed] [Google Scholar]
- 11. Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The Glasgow coma scale at 40 years: standing the test of time. The Lancet Neurology. 2014;13:844‐854. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization . Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/ IDF consultation. 2006. Retrieved from:http://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf [Accessed January 1, 2014].
- 13. Kaplan N, Mendis S, Poulter N, Whitworth J. 2003 World Health Organization (WHO)/ International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21(11):1983‐1992. [DOI] [PubMed] [Google Scholar]
- 14. Goel MK, Khanna P, Kishore J. Understanding survival analysis: Kaplan‐Meier estimate. Int J Ayurveda Res. 2010;1(4):274‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hosmer DW, Lemeshow S, May S. Applied Survival Analysis: Regression Modeling of Time‐to‐Event Data. 2nd ed. Hoboken, New Jersey: John Wiley & Sons, Inc.; 2008. [Google Scholar]
- 16. Rich JT, Neely JG, Paniello RC, Voelker CCJ, Nussenbaum B, Wang EW. A practical guide to understanding Kaplan‐Meier curves. Otolaryngol Head Neck Surg. 2010;143(3):331‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andersen KK, Olsen TS, Dehlendorff C, Kammersgaard LP. Hemorrhagic and ischemic strokes compared: stroke severity, mortality, and risk factors. Stroke. 2009;40:2068‐2072. [DOI] [PubMed] [Google Scholar]
- 18. Bejot Y, Jacquin A, Rouaud O, et al. One‐year survival of demented stroke patients: data from the Dijon Stroke Registry, France (1985‐2008). Eur J Neurol. 2012;19(5):712‐717. 10.1111/j.1468-1331.2011.03613.x [DOI] [PubMed] [Google Scholar]
- 19. Hankey GJ, Jamrozik K, Broadhurst RJ, et al. Five‐year survival after first‐ever stroke and related prognostic factors in the Perth community stroke study. Stroke. 2000;31:2080‐2086. [DOI] [PubMed] [Google Scholar]
- 20. Collins TC, Petersen NJ, Menke TJ, Souchek J, Foster W, Ashton CM. Short‐term, intermediate‐term, and long‐term mortality in patients hospitalized for stroke. J Clin Epidemiol. 2003;56(1):81‐87. [DOI] [PubMed] [Google Scholar]
- 21. Kimura K, Minematsu K, Kazui S, Yamaguchi T. Mortality and cause of death after hospital discharge in 10,981 patients with ischemic stroke and transient ischemic attack. Cerebrovasc Dis. 2005;19:171‐178. [DOI] [PubMed] [Google Scholar]
- 22. Lavados PM, Sacks C, Prina L, et al. Incidence, 30‐day case‐fatality rate, and prognosis of stroke in Iquique, Chile: a 2‐year community‐based prospective study (PISCIS project). Lancet. 2005;365:2206‐2215. [DOI] [PubMed] [Google Scholar]
- 23. Kammersgaard LP, Olsen TS. Cardiovascular risk factors and 5‐year mortality in the Copenhagen Stroke Study. Cerebrovasc Dis. 2006;21(3):187‐193. 10.1159/000090531 [DOI] [PubMed] [Google Scholar]
- 24. Jeng JS, Huang SJ, Tang SC, Yip PK. Predictors of survival and functional outcome in acute stroke patients admitted to the stroke intensive care unit. J Neurol Sci. 2008;270:60‐66. [DOI] [PubMed] [Google Scholar]
- 25. Kong FY, Tao WD, Hao ZL, Liu M. Predictors of one‐year disability and death in Chinese hospitalized women after ischemic stroke. Cerebrovasc Dis. 2009;29:255‐262. [DOI] [PubMed] [Google Scholar]
- 26. Chang KC, Lee HC, Tseng MC, Huang YC. Three‐year survival after first‐ever ischemic stroke is predicted by initial stroke severity: a hospital‐based study. Clin Neurol Neurosurg. 2010;112:296‐301. [DOI] [PubMed] [Google Scholar]
- 27. Goulart AC, Fernandes TG, Santos IS, Alencar AP, Bensenor IM, Lotufo PA. Predictors of long‐term survival among first‐ever ischemic and hemorrhagic stroke in a Brazilian stroke cohort. BMC Neurol. 2013;13(51). 10.1186/1471-2377-13-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoffmeister L, Lavados PM, Murta‐Nascimento C, Araujo M, Olavarria VV, Castells X. Short‐ and long‐term survival after stroke in hospitalized patients in Chile: a nationwide 5‐year study. J Stroke Cerebrovasc Dis. 2013;22(8):E463‐E469. 10.1016/j.jstrokecerebrovasdis.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 29. Leyden JM, Kleinig TJ, Newbury J, et al. Adelaide stroke incidence study: declining stroke rates but many preventable cardioembolic strokes. Stroke. 2013;44(5):1226‐1231. 10.1161/STROKEAHA.113.675140 [DOI] [PubMed] [Google Scholar]
- 30. Medic S, Beslac‐Bumbasirevic L, Kisic‐Tepavcevic D, Pekmezovic T. Short‐term and long‐term stroke survival: the Belgrade Prognostic Study. J Clin Neurol. 2013;9:14‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ong TZ, Raymond AA. Risk factors for stroke and predictors of one‐month mortality. Singapore Med J. 2002;43(10):517‐521. [PubMed] [Google Scholar]
- 32. Andersen KK, Olsen TS. One‐month to 10‐year survival in the Copenhagen Stroke Study: interactions between stroke severity and other prognostic indicators. J Stroke Cerebrovasc Dis. 2011;20(2):117‐123. [DOI] [PubMed] [Google Scholar]
- 33. Nedeltchev K, Renz N, Karameshev A, et al. Predictors of early mortality after acute ischaemic stroke. Swiss Med Wkly. 2010;140(17–18):254‐259. [DOI] [PubMed] [Google Scholar]
- 34. Bellhouse DR. Systematic sampling methods. Wiley Stats Ref: Statistics Reference Online. 2014.


