Abstract
Background
The aim of this study was to determine whether the local application of tendon stem cells (TSCs) with chitosan/b-glycerophosphate/collagen(C/GP/Co) hydrogel promotes healing after an acute Achilles tendon injury in a rat model.
Material/Methods
Ninety-six Sprague-Dawley (SD) rats were used to make an Achilles tendon defect model, then the animals were randomly divided into 4 groups consisting of 8 rats each: control group, hydrogel group, TSCs group, and TSCs with hydrogel group. At 2, 4, and 6 weeks after treatment, tendon samples were harvested, and the quality of tendon repair was evaluated based on histology, immunohistochemistry, and biomechanical properties.
Results
Combining TSCs with C/GP/Co hydrogel significantly enhances tendon healing compared with the control, hydrogel, and TSCs groups. The improved healing was indicated by the improvement in histological and immunohistochemistry outcomes and the increase in the biomechanical properties of the regenerated tissue at both 4 and 6 weeks post-injury.
Conclusions
This study demonstrates that the transplantation of TSCs combined with C/GP/Co hydrogel significantly improved the histological, immunohistochemistry, and biomechanical outcomes of the regenerated tissue at 4 and 6 weeks after implantation. TSCs with C/GP/Co hydrogel is a potentially effective treatment for tendon injury.
MeSH Keywords: Hydrogel, Stem Cell Transplantation, Tendon Injuries, Tissue Engineering
Background
Tendons are an important component of the musculoskeletal system and transmit force between muscles and bones to enable joint movement [1].
Tendons are frequently injured during sports and other rigorous physical activities and often fail to heal optimally, which can affect personal and professional activities. Due to the limited regenerative capacity, natural tendon healing is often accompanied by fibrotic scar tissue formation, resulting in poor mechanical properties, and the healed tendon is susceptible to re-injury [2,3].
The repair of tendon injuries is a major clinical challenge for orthopedic medicine [4]. Tendon injuries are currently managed by conservative or surgical treatments, but these treatment methods have limitations; they cannot completely restore the tendon to its native composition, structure, and mechanical properties. The ideal treatment remains controversial, and there is a critical need for more effective treatments [5].
Stem cell therapy has the potential to regenerate damaged tendon tissue after tendon injury. Tendon stem cells (TSCs) are a type of mesenchymal stem cells derived from the tendon [6]. TSCs were identified in tendon tissues in humans, mice [7], rabbits [8], and rats [9]. TSCs have multi-differentiation potential and play a major role in the maintenance of tendon homeostasis and recovery after injury [1]. Tendon tissue engineering using TSCs is a promising strategy for tendon regeneration. TSCs require appropriate scaffolds to keep their stemness and orient tenogenic differentiation, and scaffolds play a critical role during the regeneration process [10,11].
Extracellular matrix (ECM) and its composition are critical to the development and maintenance of healthy tendons, and the ECM in tendons is mainly composed of type I collagen [12]. The ideal scaffold must closely mimic the structure of native tendon ECM. An ideal scaffold for tendon healing should be biodegradable and biocompatible [13]. Collagen has optimum biocompatibility, biodegradability, and bioactivity. Collagen is one of the most suitable biomaterials for tendon tissue engineering and for producing scaffolds [14].
A hydrogel system is a cost-effective way to produce tissue engineered scaffolds [13]. Hydrogels provide a carrier and living environment mimicking the ECM for the transplanted cells, and cells are easily seeded into hydrogels; small components such as nutrients and oxygen easily move inside hydrogels [15]. Hydrogels have promising applications for healing in a variety of tissues [16].
Our group previously developed and characterized a chitosan/β-glycerophosphate/collagen(C/GP/Co) hydrogel, which had a volume ratio of 6: 1: 8. We showed that the C/GP/Co hydrogel is highly permeable to nutrients and water-soluble metabolites, which promotes TSCs survival and growth. Additionally, the C/GP/Co hydrogel could be injected as a fluid to fill an injury space and form a gel at 37°C as a minimally invasive procedure.
The objective of this study was to evaluate the effect of TSCs with C/GP/Co hydrogel on tendon healing through histological and biomechanical assays in a rat Achilles tendon injury model.
Material and Methods
Animals
All experimental procedures were approved by the Animal Research Ethics Committee of the Third Military Medical University, China. All animals were treated according to institutional guidelines for laboratory animal treatment and care. Four Sprague-Dawley (SD) rats (3–4 weeks old; male) weighing 80–100 g were used for the isolation of TSCs. We used 114 6-week-old male SD rats weighing 200–220 g for the animal experiments; 18 SD rats that did not undergo an operation provided the healthy Achilles tendons as the intact controls for the biomechanical test. The remaining 96 SD rats were used for Achilles tendon healing experiments.
Isolation and culture of rat TSCs
The isolation and culture of rat TSCs were performed as previously described [17]. The intact Achilles tendons were excised from both limbs of each rat following euthanasia. Only the midsubstance tissue was collected; the tendon sheath and the surrounding paratenon were removed carefully. The tissues were minced into small pieces in sterile phosphate-buffered saline (PBS), digested for 2.5 h at 37°C with type I collagenase (3 mg/ml Sigma-Aldrich, St. Louis, MO, USA) and dispase (4 mg/ml STEMCELL Technologies Inc., Vancouver, BC, Canada). Then, the obtained suspensions were passed through a 70-μm cell strainer (Becton Dickinson, Franklin Lakes, NJ, USA) to yield a single-cell suspension.
The released cells were washed in PBS, centrifuged at 1100 rpm for 5 min, and resuspended in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Carlsbad, CA, USA) containing 20% fetal bovine serum (FBS), 100 U/ml penicillin,100 mg/ml streptomycin, and 2 mML-glutamine (all from HyClone, Logan City, Utah, USA). Following cell dilution to different densities, they were cultured at 37°C and 5% CO2 in a humidified incubator to form colonies.
On day 3, after the initial plating, the cells were washed twice with PBS to remove non-adherent cells. At day 10, the cells were trypsinized and mixed together as passage 0 (P0) cells. Cells from passage 3 were used for the remainder of the experiments. The culture medium was changed 2–3 times per week throughout the experiments.
Multi-differentiation potential
To differentiate the TSCs into osteogenic, adipogenic, and chondrogenic lineages, the multi-differentiation potential was examined.
Osteogenesis was induced by seeding the cells in 12-well plates at a density of 2×104 cells/cm2. Cells were allowed to adhere for 24 h and were cultured in osteogenic induction medium consisting of basic growth medium (DMEM,10% FBS) to which we added 0.1 μM dexamethasone,10 mM β-glycerol phosphate, and 0.05 mM ascorbic acid. The medium was refreshed every 3 days. After 28 days, the cells were fixed with 4% paraformaldehyde for 30 min and stained with 0.1% Alizarin red solution (all from Cyagen Biosciences Inc., Santa Clara, CA, USA) for 5 min.
Adipogenesis was induced by seeding the cells in 12-well plates at a density of 2×104 cells/cm2. Cells at confluence were treated with basic growth medium supplemented with 0.5 μM dexamethasone, 10 μg/ml insulin, 50 μM indomethacin, and 50 μM isobutylmethylxanthine (IBMX). The medium was refreshed every 3 days. After 21 days of culture, the cells were fixed with 4% paraformaldehyde for 30 min and stained with 0.5% fresh Oil-red O solution (all from Cyagen Biosciences Inc., Santa Clara, CA, USA) for 30 min.
For inducing chondrogenesis, a pellet culture system was used. Approximately 5×105 cells were pelleted into a micromass by centrifugation at 1000 rpm for 10 min in a 15-mL conical polypropylene tube and cultured in chondrogenic medium, which consisted of basic growth medium, 10−7 M dexamethasone, 10 ng/ml TGF-β 3,40 μg/ml proline, 50 μg/ml ascorbate-2-phosphate, 100 μg/ml sodium pyruvate, and 1% insulin-transferrin-selenious acid mix (ITS)+Premix. The tubes were incubated at 37°C in a 5% CO2 incubator. The medium was refreshed every 3 days. Chondrogenic pellets were harvested after 28 days in culture. The pellets were fixed with 4% paraformaldehyde, embedded in paraffin blocks, and sectioned into 5-μm sections. Samples were stained with Alcian blue (all from Cyagen Biosciences Inc., Santa Clara, CA, USA).
Preparation of C/GP/Co hydrogel
A 2.0 wt% totally dissolved chitosan (C; degree of deacetylation >95%, molecular weights = 700,000 Da, Meilun Technology, Inc., Dalian, China) solution was prepared by stirring powdered chitosan in 0.1 M acetic acid for 4 h at room temperature, followed by sterilization by autoclaving at 121°C for 10 min. Then, the solution’s pH was measured, and it was stored at 4°C for later use.
A 50 wt% β-glycerophosphate (GP, Ruiyong Technology, Inc., Shanghai, China) solution was obtained by dissolving 1 g of GP in 1 mL of deionized water and sterilized by passing through a 22-μm filter membrane prior to storage. Precooled sterile GP solution was added dropwise into the C solution at a volume ratio C/GP was 6: 1 while stirring in the ice bath; the resulting C/GP solution without collagen was then prepared.
A type I collagen (Co, Luwen Technology, Inc., Shanghai, China) solution with a concentration of 2 mg/ml was prepared by adding 400 μl collagen into 480 μl precooled deionized water. Then, the mixed solution was added into
24 μl of 0.1 mol/l NaOH solution. Finally, 100 μl PBS solution was added into the previous mixed solution. All steps were performed inside the ice bath. The C/GP/Co hydrogel was prepared by mixing the C/GP hydrogel solution with the collagen solution at the volume ratio of C/GP/Co of 6: 1: 8.
Determination of the pH and gelation time of the C/GP/Co hydrogel
The pH values of the ice-cold C/GP/Co solution were measured using an electronic pH meter (Fisherbrand Hydrus 300, Orin Research Incorporation, USA). After determination of the pH, the obtained C/GP/Co liquid solutions were sub-packaged into test tubes and transferred to a 37°C incubator. The gelation time was recorded and determined by evaluating the fluidity and color change.
The time to gelation was measured using a stopwatch. One milliliter of each hydrogel solution was dropped into a small tube, placed in an incubator at 37°C, and examined every 1 min by turning over the tube until the liquid failed to flow, and the gelation time was recorded.
TSCs encapsulation in C/GP/Co hydrogel
To encapsulate TSCs into hydrogel, the cells were trypsinized from the plate and resuspended in PBS. The cell suspension was then mixed with the C/GP/Co hydrogel solution. The final cell density was 106 cells in 300 μl.
Animal model and surgical procedures
Ninety-six SD rats were used for the Achilles tendon injury model. In each animal, the left hindlimb was selected to make a tendon defect, and the right one was left intact. After anesthesia with 2.5% pentobarbital (4.5 mg/kg body weight), the left hind limb of each rat was shaved and disinfected.
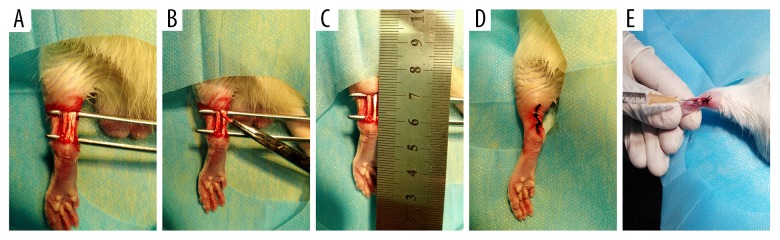
A 1.5-cm longitudinal skin incision was made over the left hind leg in SD rats to expose the Achilles tendon. Then, the central one-third of the Achilles tendon (10 mm×1 mm) was removed from the myotendinous junction to the calcaneus to create a tendon defect (Figure 1A–1C). After surgery, the skin incision was closed in layers (Figure 1D). The same method was applied to all animals by the same surgeon.
Figure 1.
Experimental protocol. A complete longitudinal incision was made 10 mm from the myotendinous junction to the calcaneus of the Achilles tendon (A–C). A clinical suture was used to suture the skin (D). Using a syringe, 300 μL C/GP/Co hydrogel solution with 106 TSCs or without cells or 106 TSCs only were injected around the injury site (E).
The animals were randomly divided into 4 groups consisting of 8 rats each: (1) control group, identical surgical procedure without any treatment; (2) hydrogel group, implantation with hydrogel solution (300 μL); (3) TSCs group, implantation with TSCs (106 cells); and (4) TSCs with hydrogel group, implantation with TSCs and hydrogel solution (106 cells in 300 μL). After the skin incision was closed, 300 μL C/GP/Co hydrogel solution containing 106 TSCs and 300 μL C/GP/Co hydrogel solution without cells or 106 TSCs alone were injected around the injury site by a 1-mL syringe (Figure 1E).
The animals were allowed to have free-cage activity. At weeks 2, 4, and 6 after injury, rats were euthanized via CO2 intoxication and both Achilles tendons were harvested (n=8 for each group at each time point). Six specimens at each time point were used for biomechanical testing, and the other 2 samples were used for histology and immunohistochemical staining. The fate of TSCs in the tendon injury site was followed by immunohistochemical staining of tenogenic-specific Scleraxis (SCX) and tendon ECM (type I and III collagen) markers.
Histological analysis
Tendon tissue was harvested at 2, 4, and 6 weeks after treatment. The tendon specimens were placed in 10% formalin solution for 24 h, embedded in paraffin, cut into 6-μm coronal sections, and stained with hematoxylin and eosin (HE) to assess the tendon morphology.
To quantify the differences among the groups, the HE-stained sections were evaluated using a semi-quantitative histopathological scale according to a previously reported grading system [1,18]. Six parameters – fiber structure, fiber arrangement, angiogenesis (increased vascularity), rounding of the nuclei, inflammation, and cell density – were quantified using a 0–3 grading scale: 0 (normal),1 (slightly abnormal), 2 (moderately abnormal), and 3 (maximally abnormal) (Table 1).
Table 1.
Grading system for histological evaluation.
| Tendon repair assessment score | ||||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Fiber structure | Continuous, long fiber | Slightly fragmented fiber | Moderately fragmented fiber | Severely fragmented fiber |
| Fiber arrangement | Compacted and parallel | Slightly loose and wavy | Moderately loose, wavy and crossing each other | No identifiable pattern |
| Rounding of the nuclei | Long spindle-shape cells | Slightly rounded | Moderately rounded | Severely rounded |
| Inflammation (area infiltrated by inflamed cells) | <10% | 10–20% | 20–30% | >30% |
| Increased vascularity (neovascular area) | <10% | 10–20% | 20–30% | >30% |
| Cell density | Normal | Slightly increased | Moderately increased | Severely increased |
The 6 parameters together yield a total score of 0 for a normal tendon and a score of 18 for a maximally abnormal tendon. Three sections were randomly selected from each sample at each time point and were evaluated by a single co-investigator in a blinded fashion. The average score was used for comparison. The total score was calculated by summing the scores of the 6 parameters.
Immunohistochemistry staining
The distributions of collagen type I, collagen type III, and SCX were assessed by immunohistochemical (IHC) staining. Briefly, after deparaffinization, the sections were rehydrated, quenched of endogenous peroxidase activity, and subjected to antigen retrieval. After blocking with 5% goat serum at 37°C for 30 min, the sections were incubated in diluted anti-collagen type I (1: 500), anti-collagen type III (1: 200), and anti-SCX (1: 500) antibody (all from Abcam, USA) at 4°C overnight. The spatial and temporal localization of the proteins were visualized by incubating with secondary antibodies for 1 h, incubating with a Streptavidin-Biotin Complex working solution (SABC, BOSTER, Wuhan, China) at 37°C for 30 min, developing in 3,3-diaminobenzidine (DAB, Sigma-Aldrich), and then counterstaining with hematoxylin. The primary antibody was replaced with blocking solution in the controls. All incubation times and conditions were strictly controlled. The sections were examined under light microscopy (Olympus BX51, Tokyo, Japan). After the IHC staining, sections stained with each antibody were quantitatively analyzed in random order with the Image-Pro Plus software (Media Cybernetics, Bethesda, MD, USA).
The procedure has been well-established [19]. Briefly, the integrated optical density (IOD; in arbitrary unit) of the immunopositive signal was measured from the sampled views inside the wound of each slide, and the mean IOD/μm2 was calculated. The assessor was blinded to the time points during image analysis.
Biomechanical test
At weeks 2, 4, and 6 after injury, tendons for the biomechanical test (6 tendons in each group at each time point; 6 normal left Achilles tendons at each time point; 90 tendons in total) were harvested, which included part of the calf muscle (0.5 cm above the myotendinous junction), Achilles tendon, and calcaneus bone. All the harvested specimens were stored at −80°C to prevent tissue damage. Before mechanical testing, samples were thawed at room temperature in glass jars containing deionized water for more than 6 h.
Tendon thickness and width were measured with a vernier caliper at 4 locations at equal spacing from the top to the bottom of the tendons for the calculation of the cross-sectional area.
The tendon samples were mounted in a biomaterial testing machine (Exceed E44, Materials testing machine max, American) with 100 N load cell capacity. A preload of 0.1 N was applied to a tendon sample for straightening and adjustment. The length between the calcaneal bone and the myotendinous junction was measured as the initial length. Each tendon was then axially pulled at a constant speed of 10 mm/min until the maximum load to failure, and the maximum breaking forces of each tendon were noted. Ultimate load (N) and stiffness (N/mm2) were measured from each tendon’s stress-strain curve.
Statistical analysis
Data are shown in boxplots. As the data were not normally distributed, the comparison of more than 2 independent groups was done by the Kruskal-Wallis test followed by post hoc pairwise comparison using the Mann-Whitney U test. All analyses were performed using SPSS version 20.0 for Windows (SPSS, Inc., an IBM Company, Chicago, USA). P<0.05 was considered statistically significant.
Results
Cell morphology and multi-differentiation potential
The cells isolated from rat Achilles tendons formed colonies after 8–10 days. Rat TSCs exhibited slender cobblestone shapes with an irregular distribution (Figure 2A). The isolated rat TSCs were evaluated for chondrogenic, adipogenic, and osteogenic differentiation abilities.
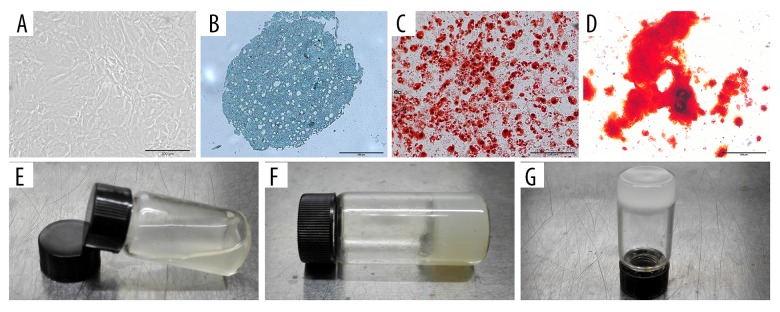
Figure 2.
Identity of rat tendon stem cells (TSCs). Photographs of thermo-sensitive C/GP/Co hydrogel. Rat TSCs were cobblestone-shaped (A). TSCs showed chondrogenic (B), adipogenic (C), and osteogenic (D) differentiation potential in vitro. Scale bar: 200 μm. The solution of C/GP/Co is liquid (E) at room temperature and gel at 37°C (F, G).
For chondrogenic differentiation, the pellets were cultured for 28 days; staining with Alcian blue revealed that TSCs increased in chondrogenic differentiation (Figure 2B). After induction of adipogenesis in adipogenic induction medium for 21 days, Oil-red O solution staining revealed multiple lipid droplets in the cells (Figure 2C). In a similar manner, inducing osteogenic differentiation resulted in alizarin-red-positive calcium deposits in the TSCs after 28 days (Figure 2D).
The pH and gelation time of C/GP/Co hydrogel
At room temperature, the C/GP/Co solution remained in the liquid state (Figure 2E). When the temperature was increased to 37°C, the C/GP/Co solution changed to a solid gel, as shown in Figure 2F, 2G. The pH of the C/GP/Co solution was 7.20 and the gelation time was approximately 8 min. The color of the solution changed from transparent to opaque upon gelation. Approximately 8 min of gelation time was sufficient for handling the cell mixture and the subsequent in vivo injection.
Histological analysis
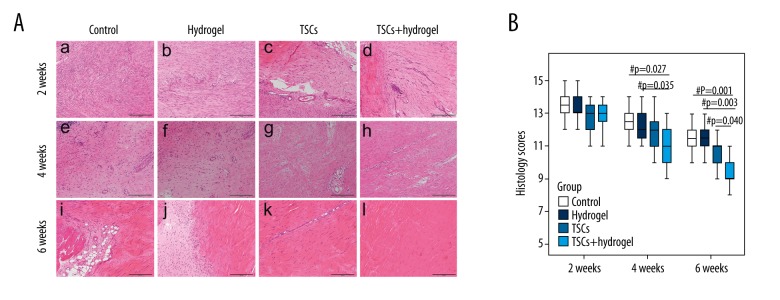
At 2 weeks post-surgery, a macroscopic examination showed more spindle-shaped cells aligned along the longitudinal axis of the tendon in the TSCs and TSCs with hydrogel groups than in the control and hydrogel groups. In addition, the TSCs with hydrogel group exhibited more ECM deposition and obvious longitudinal fibrous tissue than that of other groups [Figure 3A(a–d)]. At week 2, there were no differences in the average histology scores among all treated groups (including the control) (Figure 3B).
Figure 3.
Histology of healing tissues and histology scores of tendon repair. Photomicrographs showing the histology of the healing tissues at weeks 2 [A(a–d)], 4 [A(e–h)], and 6 [A(i–l)] post-injury, and boxplots showing the histology scores of repaired tendon (B). Magnification: ×100, Scale bar=200 μm. ‘#’ Represents p<0.05.
At 4 weeks post-surgery, more relatively normal tendon tissues had formed in the TSCs with hydrogel group than in the other groups. Most cells in the TSCs and TSCs with hydrogel groups showed a spindle-shaped morphology and distribution along the longitudinal fibrous tissue of the tendon. By contrast, loose and thin longitudinal fibrous tissue had begun to appear in the control and hydrogel groups [Figure 3 A(e–h)]. The average histology scores were lower for the TSCs and TSCs with hydrogel groups than in the control and hydrogel groups at 4 weeks, but only the TSCs with hydrogel group had a significant difference compared to the control and hydrogel groups (P=0.027 and 0.035, respectively) (Figure 3B).
At 6 weeks, HE staining showed that the tendon fibers in the control, hydrogel, and TSCs groups were wavy and kinking and displayed a disordered collagen arrangement. In contrast, the fibers of the tendons in the TSCs with hydrogel group were well aligned; collagen fiber bundles were aligned parallelly along the axis of the tendon, and they closely approximated fibers in normal tendons [Figure 3A(i–l)]. At week 6, the average histology scores of the TSCs with hydrogel group also were significantly lower compared with the other groups (P=0.001, 0.003, and 0.040, respectively) (Figure 3B).
Immunohistochemistry
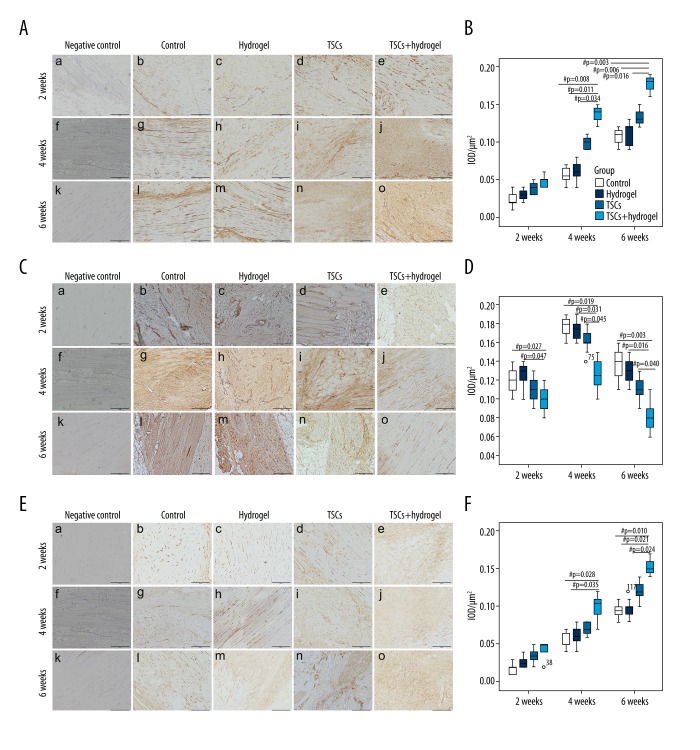
The expression of collagen type I increased from week 2 to week 6 in all 4 groups. There was weak expression of collagen type I in the tendon matrix and cells in all 4 groups (Figure 4A), and there was no significant change in the mean IOD at 2 weeks (Figure 4B). The expression of collagen type I increased in the tendon matrix and cells inside the wound in the TSCs and TSCs with hydrogel groups more than in the control and hydrogel groups, but only the TSCs with hydrogel group had a higher IOD of general collagen type I than the control, hydrogel, and TSCs groups at 4 weeks (P=0.008, 0.011, and 0.034, respectively) and 6 weeks (P=0.003, 0.006, and 0.016, respectively).
Figure 4.
Photomicrographs showing immunohistochemical staining of collagen type I (A) and collagen type III (C) and SCX (E) in the tendon injury sites at weeks 2, 4, and 6. Magnification: ×100, scale bar=200 μm. Boxplot showing the changes of mean IOD/μm2 of collagen type I (B), collagen type III (C), and SCX (F) at different times after tendon injury. ‘o’ Represents an outlier value of the dataset. ‘#’ Represents P<0.05.
There was mild expression of collagen type III in tendon fibroblasts and matrixes inside the wound at week 2 after injury (Figure 4C). The mean IOD of the control and hydrogel groups was higher than that of the TSCs and TSCs with hydrogel groups. However, only the IOD of the TSCs with hydrogel group was significantly different compared to the control and hydrogel groups (P=0.027 and 0.047, respectively) (Figure 4D). There was increased, intense expression of collagen type III in tendon fibroblasts and matrixes inside the wound at week 4 after injury (Figure 4C). The mean IOD of the control, hydrogel, and TSCs groups were higher than that in the TSCs with hydrogel group (P=0.019, 0.031, and 0.045, respectively) (Figure 4D). The expression of collagen type III inside the wound was significantly reduced at week 6 (Figure 4C), but the mean IOD of control, hydrogel, and TSCs groups remained significantly higher than in the TSCs with hydrogel group (P=0.003, 0.016, and 0.040, respectively) (Figure 4D).
There was weak expression of SCX in the tendon matrix and cells in all 4 groups at 2 weeks (Figure 4E), and there was no significant change in the mean IOD at 2 weeks (Figure 4F). The expression of SCX increased in the tendon matrix and cells inside the wound in the TSCs and TSCs with hydrogel groups more than that of the control and hydrogel groups at 4 and 6 weeks, but only the TSCs with hydrogel group had higher IOD of general SCX than the control and hydrogel groups at 4 weeks (P=0.028 and 0.035, respectively).The mean IOD of the TSCs with hydrogel group was higher than in the control, hydrogel, and TSCs groups at 6 weeks (P=0.010, 0.021, and 0.024, respectively) (Figure 4F).
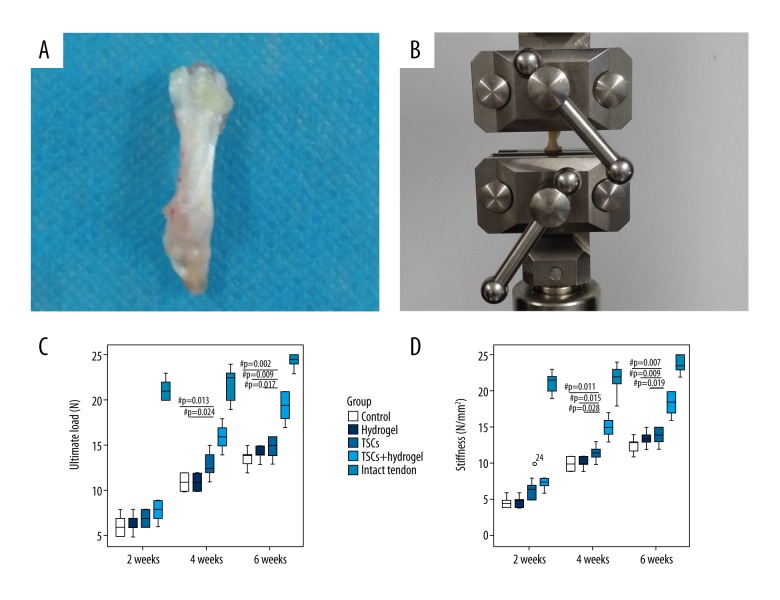
Biomechanical testing
At weeks 2, 4, and 6, the ultimate load and stiffness were lower than in the normal tendons (intact tendon group) for all treated groups (including the control). There were no significant differences in the ultimate load or stiffness for all treated groups at the early 2-week time point.
At week 4, the ultimate load of the TSCs with hydrogel group was significantly higher than in the control and hydrogel groups (P=0.013 and 0.024, respectively), but there was no significant difference between the TSCs with hydrogel group and the TSCs group. At week 4, the stiffness of the TSCs with hydrogel group was significantly higher than in the control, hydrogel, and TSCs groups (P=0.011, 0.015, and 0.028, respectively).
At week 6, the ultimate load of the TSCs with hydrogel group became significantly different from the other 3 groups (P=0.002, 0.009, and 0.017, respectively). At week 6, the stiffness of the TSCs with hydrogel group was significantly higher than in the control, hydrogel, and TSCs groups (P=0.007, 0.009, and 0.019, respectively).
Discussion
Tendon injuries are common and present a clinical challenge because they often respond poorly to treatment and can lead to long-term dysfunction. The blood supply to tendons is inferior to that of most other connective tissues. Tendons regenerate and repair slowly and inefficiently and do not regain their normal biological and biomechanical status after injury.
Following injury, tendons typically go through a healing and scar formation response with 3 mutually dependent and overlapping phases of healing: inflammation, collagen proliferation, and remodeling. In the initial, inflammatory phase, erythrocytes and inflammatory cells, particularly neutrophils, enter the site of injury. TSCs gradually migrate to the wound, and collagen type III synthesis is initiated. After a few days, the proliferative phase begins, and the synthesis of collagen type III peaks during this stage. After a few weeks, the remodeling phase commences, and a higher proportion of collagen type I is synthesized during this stage [20,21].
Stem cell-based therapy is a promising intervention for tendon repair. Previous studies have shown positive effects of bone-marrow-derived mesenchymal stem cells (BMSCs) [22], TSCs [23], and human adipose-derived mesenchymal stem cells (hASCs) [24] for tendon repair. TSCs are immune-privileged cells that have the potential for allogeneic transplantation [25]. Tendon regeneration using TSCs is a promising strategy for tendon injury.
Cell leakage after injection may be a problem with local injection of stem cells, and it is difficult for cells to stay in the injured area for a long time. Thus, a more appropriate method of cell delivery is required. The combination of cells with a supporting biomaterial scaffold has been considered as a potential approach to treat tendon injuries [26]. The ideal scaffold is biocompatible and does not incite a host inflammatory response [27]. Hydrogels are bioactive, biodegradable, and biocompatible, and our previous study developed and characterized a C/GP/Co hydrogel that closely mimics the structure of native tendon ECM.
Previous studies have shown positive effects of BMSCs [28], nucleus pulposus cells [29], and adipose tissue-derived stem cells (ADSCs) [30] on chitosan and β-glycerophosphate-based injectable hydrogels in vivo and in vitro studies. In this study, we showed that the TSCs with C/GP/Co hydrogel promoted the expression of collagen type I and SCX, which were reported to be tendon-specific markers at the tissue level [25]. TSCs with C/GP/Co hydrogel had reduced expression of collagen type III in the tendon matrix inside wounds in our injury model. Type III collagen is increased after injury and is abundant in wound bed granulation tissue. In the natural healing of tendon injuries, successful remodeling is typified by the replacement of type III collagen with type I collagen [31].
SCX is a key regulator of TSCs differentiation as a specific tendon marker [32]. Type I collagen is the predominant collagen in tendons, making up approximately 90% of the total collagen content [20,33]. The high expression of type I collagen and SCX indicated that repaired tendons had better collagen formation and organization. Type I collagen is considered to be responsible for the mechanical strength of tendon tissues. An optimized collagen type I/III ratio might account for the quality of the matrix organization in tendon healing [34].
Our results show that combining TSCs with C/GP/Co hydrogel significantly enhances tendon healing compared with the other 3 groups in a model of rat Achilles tendon injury. Improved healing was indicated by the improvement of histological and immunohistochemistry outcomes and increase in biomechanical properties of the regenerated tissue. We confirmed that the relatively high proportion of type I collagen fibers to type III collagen in the TSCs with hydrogel group is a major factor in enhancing the mechanical properties of regenerated tissues.
TSCs alone had no significant therapeutic effect, which was consistent with the results of Chen et al. [18], who demonstrated that platelet-rich plasma (PRP) had a positive effect on tendon healing, which was significantly enhanced by TSCs, but TSCs injection alone did not improve tendon healing.
Other experiments that use stem cells to treat tendon injuries also take advantage of a wide variety of scaffolds, including tendon hydrogel [35], fibrin glue [23], and silk-collagen sponge scaffold [25].
There are limitations of this study. First, we did not track the TSCs by injecting labeled cells. Furthermore, a longer follow-up period after TSCs with C/GP/Co hydrogel injection would be useful to observe the long-term effects. Further study is necessary to determine the specific mechanism by which C/GP/Co hydrogel influences the exogenous TSCs to promote tendon tissue regeneration and repair in vivo. Additionally, further research is needed to decide on the optimal strategy for tendon regeneration and repair.
Conclusions
In summary, our study provides evidence to suggest that the transplantation of TSCs combined with C/GP/Co hydrogel promotes tendon tissue regeneration through the improvement of histological and immunohistochemistry outcomes and increases the biomechanical properties of the regenerated tendon tissues.
Figure 5.
Tendon sample that included part of the calf muscle (0.5 cm above the myotendinous junction), Achilles tendon, and calcaneus bone (A). The tendon samples were mounted in the biomaterial testing machine (B). Boxplots showing the ultimate load (C) and the mean stiffness (D) of tendon tissues. ‘o’ Represents an outlier value in the dataset. ‘#’ Represents P<0.05.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (grant no. 81230040)
Conflicts of interest
None.
References
- 1.Komatsu I, Wang JH, Iwasaki K, et al. The effect of tendon stem/progenitor cell (TSC) sheet on the early tendon healing in a rat Achilles tendon injury model. Acta Biomater. 2016;42:136–46. doi: 10.1016/j.actbio.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 2.Shen W, Chen X, Chen J, et al. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials. 2010;31:7239–49. doi: 10.1016/j.biomaterials.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 3.Yin Z, Guo J, Wu TY, et al. Stepwise differentiation of mesenchymal stem cells augments tendon-like tissue formation and defect repair in vivo. Stem Cells Transl Med. 2016;5:1106–16. doi: 10.5966/sctm.2015-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerquin MJ, Charvet B, Nourissat G, et al. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J Clin Invest. 2013;123:3564–76. doi: 10.1172/JCI67521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu K, Al-Ani MK, Sun Y, et al. Platelet-rich plasma activates tendon-derived stem cells to promote regeneration of Achilles tendon rupture in rats. J Tissue Eng Regen Med. 2017;11:1173–84. doi: 10.1002/term.2020. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Han W, Chen L, Tang K. Mechanism of osteogenic and adipogenic differentiation of tendon stem cells induced by sirtuin 1. Mol Med Rep. 2016;14:1643–48. doi: 10.3892/mmr.2016.5417. [DOI] [PubMed] [Google Scholar]
- 7.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–27. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Wang JH. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord. 2010;11:10. doi: 10.1186/1471-2474-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rui YF, Lui PP, Li G, et al. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A. 2010;16:1549–58. doi: 10.1089/ten.TEA.2009.0529. [DOI] [PubMed] [Google Scholar]
- 10.Yin Z, Chen X, Zhu T, et al. The effect of decellularized matrices on human tendon stem/progenitor cell differentiation and tendon repair. Acta Biomater. 2013;9:9317–29. doi: 10.1016/j.actbio.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Jiang D, Xu B, Yang M, et al. Efficacy of tendon stem cells in fibroblast-derived matrix for tendon tissue engineering. Cytotherapy. 2014;16:662–73. doi: 10.1016/j.jcyt.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Yokoya S, Mochizuki Y, Natsu K, et al. Rotator cuff regeneration using a bioabsorbable material with bone marrow-derived mesenchymal stem cells in a rabbit model. Am J Sports Med. 2012;40:1259–68. doi: 10.1177/0363546512442343. [DOI] [PubMed] [Google Scholar]
- 13.Moshiri A, Oryan A, Meimandiparizi A, Maffulli N. Collagen implants in experimental tendon injury in rabbits: A clinical, ultra-structural and biomechanical investigation. J Biol Regul Homeost Agents. 2014;28:381–97. [PubMed] [Google Scholar]
- 14.Moshiri A, Oryan A, Meimandi-Parizi A, Koohi-Hosseinabadi O. Effectiveness of xenogenous-based bovine-derived platelet gel embedded within a three-dimensional collagen implant on the healing and regeneration of the Achilles tendon defect in rabbits. Expert Opin Biol Ther. 2014;14:1065–89. doi: 10.1517/14712598.2014.915305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Wang S, Wei C, et al. In vivo differentiation of adipose-derived stem cells in an injectable poloxamer-octapeptide hybrid hydrogel. Tissue Cell. 2011;43:344–49. doi: 10.1016/j.tice.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Crowe CS, Chattopadhyay A, McGoldrick R, et al. Characteristics of reconstituted lyophilized tendon hydrogel: an injectable scaffold for tendon regeneration. Plast Reconstr Surg. 2016;137:843–51. doi: 10.1097/01.prs.0000480012.41411.7c. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Chen L, Tao X, Tang K. Phosphoinositide 3-kinase/Akt signaling is essential for prostaglandin E2-induced osteogenic differentiation of rat tendon stem cells. Biochem Biophys Res Commun. 2013;435:514–19. doi: 10.1016/j.bbrc.2012.11.083. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Liu JP, Tang KL, et al. Tendon derived stem cells promote platelet-rich plasma healing in collagenase-induced rat achilles tendinopathy. Cell Physiol Biochem. 2014;34:2153–68. doi: 10.1159/000369659. [DOI] [PubMed] [Google Scholar]
- 19.Yee LPP, Wong YM, Rui YF, et al. Expression of chondro-osteogenic BMPs in ossified failed tendon healing model of tendinopathy. J Orthop Res. 2011;29:816–21. doi: 10.1002/jor.21313. [DOI] [PubMed] [Google Scholar]
- 20.MacLean S, Khan WS, Malik AA, et al. Tendon regeneration and repair with stem cells. Stem Cells Int. 2012;2012 doi: 10.1155/2012/316281. 316281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 22.Hankemeier S, Hurschler C, Zeichen J, et al. Bone marrow stromal cells in a liquid fibrin matrix improve the healing process of patellar tendon window defects. Tissue Eng Part A. 2009;15:1019–30. doi: 10.1089/ten.tea.2008.0046. [DOI] [PubMed] [Google Scholar]
- 23.Ni M, Lui PP, Rui YF, et al. Tendon-derived stem cells (TDSCs) promote tendon repair in a rat patellar tendon window defect model. J Orthop Res. 2012;30:613–19. doi: 10.1002/jor.21559. [DOI] [PubMed] [Google Scholar]
- 24.Lee SY, Kwon B, Lee K, et al. Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. Am J Sports Med. 2017;45(6):1429–39. doi: 10.1177/0363546517689874. [DOI] [PubMed] [Google Scholar]
- 25.Shen W, Chen J, Yin Z, et al. Allogenous tendon stem/progenitor cells in silk scaffold for functional shoulder repair. Cell Transplant. 2012;21:943–58. doi: 10.3727/096368911X627453. [DOI] [PubMed] [Google Scholar]
- 26.Vuornos K, Björninen M, Talvitie E, et al. Human adipose stem cells differentiated on braided polylactide scaffolds is a potential approach for tendon tissue engineering. Tissue Eng Part A. 2016;22:513–23. doi: 10.1089/ten.tea.2015.0276. [DOI] [PubMed] [Google Scholar]
- 27.Hogan MV, Bagayoko N, James R, et al. Tissue engineering solutions for tendon repair. J Am Acad Orthop Surg. 2011;19:134–42. doi: 10.5435/00124635-201103000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Sun B, Ma W, Su F, et al. The osteogenic differentiation of dog bone marrow mesenchymal stem cells in a thermo-sensitive injectable chitosan/collagen/β-glycerophosphate hydrogel: In vitro and in vivo. J Mater Sci Mater Med. 2011;22:2111–18. doi: 10.1007/s10856-011-4386-4. [DOI] [PubMed] [Google Scholar]
- 29.Cheng YH, Yang SH, Su WY, et al. Thermosensitive chitosan-gelatin-glycerol phosphate hydrogels as a cell carrier for nucleus pulposus regeneration: an in vitro study. Tissue Eng Part A. 2010;16:695–703. doi: 10.1089/ten.TEA.2009.0229. [DOI] [PubMed] [Google Scholar]
- 30.Song K, Qiao M, Liu T, et al. Preparation, fabrication and biocompatibility of novel injectable temperature-sensitive chitosan/glycerophosphate/collagen hydrogels. J Mater Sci Mater Med. 2010;21:2835–42. doi: 10.1007/s10856-010-4131-4. [DOI] [PubMed] [Google Scholar]
- 31.Cross JA, Cole BJ, Spatny KP, et al. Leukocyte-reduced platelet-rich plasma normalizes matrix metabolism in torn human rotator cuff tendons. Am J Sports Med. 2015;43:2898–906. doi: 10.1177/0363546515608157. [DOI] [PubMed] [Google Scholar]
- 32.Scott A, Danielson P, Abraham T, et al. Mechanical force modulates scleraxis expression in bioartificial tendons. J Musculoskelet Neuronal Interact. 2011;11:124–32. [PubMed] [Google Scholar]
- 33.MKh A, Xu K, Sun Y, et al. Study of bone marrow mesenchymal and tendon-derived stem cells transplantation on the regenerating effect of achilles tendon ruptures in rats. Stem Cells Int. 2015;2015 doi: 10.1155/2015/984146. 984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eriksen HA, Pajala A, Leppilahti J, Risteli J. Increased content of type III collagen at the rupture site of human Achilles tendon. J Orthop Res. 2002;20:1352–57. doi: 10.1016/S0736-0266(02)00064-5. [DOI] [PubMed] [Google Scholar]
- 35.Crowe CS, Chiou G, McGoldrick R, et al. Tendon regeneration with a novel tendon hydrogel: In vitro effects of platelet-rich plasma on rat adipose-derived stem cells. Plast Reconstr Surg. 2015;135:981e–89e. doi: 10.1097/PRS.0000000000001268. [DOI] [PubMed] [Google Scholar]