Abstract
Background
Calprotectin is a promising biomarker for granulocyte activation. It is mainly measured in faeces as a marker for inflammatory bowel disease. A limitation is that there is no widely accepted calibrator.
Aim
To establish a method for purification of calprotectin from human granulocytes that is easily reproducible, reliable, and could contribute to a better agreement between different calprotectin methods.
Methods and results
Calprotectin was purified from granulocyte extracts using ion‐exchange chromatography. The granulocytes were separated from blood bags. The purity was analysed by analysing pixel density of a picture of the sodium dodecyl sulfate polyacrylamide gel electrophoresis and by size exclusion chromatography. The calprotectin concentration of the pure antigen solution was determined using Biuret method. The purity was >95% for 3 preparations, and their concentrations were 1079, 1080, and 1813 mg/L.
Conclusion
It is possible to reproducibly prepare highly purified calprotectin antigen from human granulocytes. The preparations can be used for preparing calibrators, controls for immunological calprotectin assays, and immunisation for raising antibodies against human calprotectin in hens.
Keywords: antigen purification, calprotectin, chromatography, granulocyte, MRP8/MRP14, S100A8/S100A9
Short abstract
Background: Calprotectin is a promising biomarker for granulocyte activation. It is mainly measured in faeces as a marker for inflammatory bowel disease. A limitation is that there is no widely accepted calibrator.
Aim: To establish a method for purification of calprotectin from human granulocytes that is easily reproducible, reliable, and could contribute to a better agreement between different calprotectin methods.
Methods and results: Calprotectin was purified from granulocyte extracts using ion‐exchange chromatography. The granulocytes were separated from blood bags. The purity was analysed by analysing pixel density of a picture of the sodium dodecyl sulfate polyacrylamide gel electrophoresis and by size exclusion chromatography. The calprotectin concentration of the pure antigen solution was determined using Biuret method. The purity was >95% for 3 preparations, and their concentrations were 1079, 1080, and 1813 mg/L.
Conclusion: It is possible to reproducibly prepare highly purified calprotectin antigen from human granulocytes. The preparations can be used for preparing calibrators, controls for immunological calprotectin assays, and immunisation for raising antibodies against human calprotectin in hens.
1. INTRODUCTION
Calprotectin is mainly found in neutrophils, where it accounts for 30% to 40% of the cytosolic protein content.1 Several inflammatory conditions cause an increase in circulating calprotectin.2, 3 Calprotectin is, therefore, considered to be an important inflammation marker.4, 5 The calprotectin molecule is composed of 2 members of the S100 family, S100A8 (MRP8) and S100A9 (MRP14). However, the exact structure of calprotectin is debated. While Johne et al6 suggest it is a hetero trimer, Pepper et al7 suggest it is a heterodimer, and Steinbakk et al8 suggest it is a heterotetramer when calcium is present.
In faeces, elevated calprotectin concentration is used as a marker for inflammatory bowel disease (IBD), including Crohn disease.9 It has been suggested that serum or plasma calprotectin levels could be used as an important marker for several inflammatory diseases, such as sepsis,10 acute appendicitis, and rheumatoid arthritis.7, 11, 12 To be able to develop immunoassays measuring calprotectin reliably, it is important to use a highly purified calprotectin antigen at an early stage, ie, in the immunisation process. The purified antigen must be as similar as possible to the native protein that is to be measured, to avoid assay problems due to differences in antibody reactivity between calibrators, controls, and samples. Presently, calprotectin is mainly used as a faecal biomarker for distinguishing between IBD and irritable bowel syndrome.13, 14, 15 There are no generally accepted calibrators for calprotectin, for either blood or faeces. Method comparisons show that there are clear calibration differences between F‐calprotectin assays from different manufacturers.16 These differences make it difficult to interpret test results, as the physician must be aware of the calibration of the method used, to correctly evaluate the test results. If this is not considered, there is a risk that the results are misinterpreted and the patients get the wrong treatment. So far, there is a limited clinical use of serum or plasma calprotectin and no available standardised calibrator.
The aim of the present investigation was to develop a method to prepare purified calprotectin, such that it can be used as calibrator and control in immunological calprotectin assays, and for immunisation of hens to raise antibodies. Because of the complex structure of calprotectin, it is difficult to produce recombinant calprotectin in a reproducible way, and there is a risk that the tertiary structure of the recombinant calibrator may be different from the calprotectin present in the patients' samples.17 We thus chose to purify the calprotectin directly from granulocytes.
2. METHODS
2.1. Samples
Informed consent (approval to use excess material in research) was obtained from all blood bank donors. The use of anonymised excess material from the Ullevål Hospital blood bank was approved by the Ethics Committee at Uppsala University (Ups 01‐367).
2.2. Extraction
The buffy coats were collected from blood bags (Ullevål Hospital blood bank) after removal of plasma and erythrocytes. The buffy coats were mixed with 250 mL of erythrocyte lysis buffer (8.3 g/L ammonium chloride and 0.85 g/L sodium hydrogen carbonate, no pH adjustments) and incubated for 5 to 6 minutes, to lyse the red blood cells. The solution was then centrifuged at 65 g for 12 minutes at room temperature (Hettich Rotixa 50s), allowing the granulocytes to precipitate but not disintegrate. The supernatant was decanted carefully, and the granulocyte washing buffer (9 g/L NaCL, 1 g/L dipotassium EDTA dihydrate, and adjusted to pH 7.0) was then added. Granulocytes were then gently mixed into solution before being centrifuged at 65 g for 12 minutes. The supernatant was once more decanted, and the granulocytes were resuspended in the binding buffer (18.75 mM 5,5‐Diethylbarbituric acid; 0.623 mM dipotassium EDTA dihydrate; and adjusted to pH 7.4.), followed by incubation at −50°C. After at least 24 hours in the freezer, the solution was thawed and ultrasonicated (sonotrode s7 on UP200s, Hielcher Ultrasonics GmbH, Teltow, Germany) to release the cytosolic proteins. The solution was then centrifuged at 4000 g for 20 minutes (Heraeus Christ, Minifuge), and the supernatant was transferred to a new container and diluted with purified water (resistivity >1 MΩ/cm, Elix(R) Gulfstream C35, cat. No. ZWGSC5035, Millipore, Merck KGaA, Darmstadt, Germany), until conductivity reached a similar level as the binding buffer (<1000 mS/cm).
2.3. Purifying calprotectin antigen
The newly prepared granulocyte extracts were applied to an anion exchange column for purification. The volume of the extract from 4 donors was typically 13 mL. When diluted with purified water to required conductivity, the volume was typically 40 mL. For this volume of extract, a column with 30‐mL gel was applied. The gel (Sepharose DEAE, [GE Healthcare, Uppsala, Sweden, cat. No. 17‐0709‐01]) was equilibrated against the diemal buffer (18.75 mM 5,5‐Diethylbarbituric acid; 0.623 Dipotassium EDTA; and adjusted to pH 7.4) and then filled into the column (Econo‐Column Chromatography Columns, 1.5 × 30 cm, BioRad, Hercules, California, cat. No. 7371532). The extract was applied, and the column was rinsed with a gel‐washing buffer (85 mM diemal buffer and adjusted to pH = 8.6).
For elution of bound calprotectin, a diemal buffer with calcium (18.75 mM diemal, 0.623 mM dipotassium EDTA, 10 mM CaCl2, and adjusted to pH 8.6) was applied. This procedure is in accordance with the method described in the patent from 1989, held by Fagerhol.18
The eluates were collected in 2‐mL fractions. The fractions were measured with the turbidimetric prototype Gentian Calprotectin Immunoassay (GCAL Gentian AS, Moss, Norway, cat. No. 1201 and 1251), as described in Nilsen et al,19 on the automatic clinical chemistry analyser, Mindray BS‐380 (Mindray, Shenzhen, Kina). The fractions with high calprotectin levels were pooled. The number of fractions collected varied between the preparations. The antigen solution was then concentrated, and the elution buffer was replaced with 0.9% saline (prepared in house) without any preservatives, applying Amicon Ultra centrifuge filters Ultracel 3 K from Merck Millipore (Tullagreen, Ireland, cat. No. Z740205). The solution was then centrifuged at 4000 g for 10 to 15 minutes (Heraeus Christ, Minifuge). The filtrates were measured with Gentian prototype calprotectin immunoassay to confirm no antigen passed through the filter.
2.4. Electrophoresis
Two‐dimensional polyacrylamide gel electrophoresis (PAGE) of the antigen solution was performed on an Amersham ECL Gel Box system from GE Healthcare, Uppsala Sweden, using Amersham ECL Gel Running Buffer 10× (cat. No. 28‐9902‐52) and Amersham ECL Gel 4% to 20% (cat. No. 28‐9901‐54). The samples were heated under reducing conditions for 5 minutes at 95°C using Lane Marker Reducing Sample Buffer 5× from Thermo Scientific (cat. No. 39000), before applying to the gel. The samples were separated in the gel at 160 V and 160 mA for approximately 60 minutes. The gel was then rinsed in purified water and stained overnight with PAGE blue protein Staining Solution from Thermo Scientific (Rockford, Illinois, cat. No. 24620). As a molecular weight reference (ladder), Protein Marker II (6.5‐200 kDa), prestained (AppliChem, Darmstadt, Germany, cat. No. A5418,0250) was used. The gel was analysed by the gel densitometry software, UN‐Scan‐it from Silk Scientific, Inc, Utah 84059, to establish a digital profile of each lane based on pixel density.20
2.5. Size exclusion chromatography
The antigen solution was analysed by size exclusion chromatography on Äkta FPLC system connected to a fraction collector Frac‐950. A total of 0.1 mL of the antigen solution at a concentration of 1.57 mg/mL was injected onto a GE HC Superdex 75 10/300 GL column, cat. No. 17‐5174‐01. The elution was performed with 0.1 M PBS pH 7.2, 150 mM NaCl at 0.4 mL/min. The fraction volumes were 0.5 mL, and a total of 20 mL of elution was collected. The fractions were then analysed by turbidimetry, and the profiles were saved for comparison in MS Excel 2003.
2.6. Biuret method
Biuret method was used to measure total protein concentration in the antigen solution.21, 22 A total of 0.33 mL of the purified antigen was mixed with 0.66 mL of BIOQUANT (Merck KGaA, Darmstadt, Germany, cat. No. 1.10307.0500). After an incubation of 30 minutes, the absorbance was measured at 546 nm on a Shimadzu Spectrophotometer. The absorbance was compared to an 8‐point calibration curve to calculate the concentration. The calibration curve was established from 7 bovine serum albumin standards (Pre‐diluted protein standards, Kit, Thermo Scientific, Rockford, Illinois, cat. No. 23208) and purified water as the zero standard. As blank sample, 0.9% saline with no preservatives was used. The standards were prepared and measured the same way as described for the sample.
2.7. Yield
The percentage yield was calculated from the concentration of the purified antigen, assigned by Biuret method, and the concentration of the extract measured on BS‐380 prior to the purification.
2.8. Stability of the antigen
One lot of the purified antigen solution was aliquoted into cryo tubes and stored at −50°C. A week later, 1 tube was thawed at room temperature for at least 1 hour and measured with Gentian prototype calprotectin immunoassay on Mindray BS‐380 in a 1:100 dilution in saline. The same tube was then placed back in −50°C for at least 24 hours. This procedure was repeated 3 times, in total 4 times over 7 days, to see if the purified antigen was stable through multiple freeze and thaw cycles.
To investigate the stability of the antigen in a protein solution, a sample with a calprotectin concentration of approximately 10 mg/L was prepared by spiking the antigen into a HEPES (4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid) buffer (11 mM, pH = 7.4) containing casein (1.5%). The sample was measured the day after preparation (day 0). A total of 14 vials were stored at 44°C, 8 vials were stored at 33°C, and 6 vials were stored at 30°C and measured repeatedly in different intervals through the next 106 days, to observe if the values differed more than 10% from those on day 0. The temperatures chosen were in the range above room temperature and below temperatures, which potentially cause denaturation of proteins.
3. RESULTS
3.1. Purity of the calprotectin preparations
Buffy coats from 8 (lot 1), 3 (lot 2), and 4 (lot 3) donors were pooled to prepare 3 granulocyte extracts. The 3 extracts were purified with anion‐exchange chromatography, where 40 to 50 fractions of the elute were collected from each preparation. The fractions were pooled based on the concentrations measured with the prototype calprotectin immunoassay. The number of fractions pooled varied between each preparation and was determined by the measured concentrations. The pools were concentrated and washed into saline without preservatives applying the ultracentrifuge filters. The 3 calprotectin preparations were checked for purity by size exclusion chromatography and sodium dodecyl sulphate (SDS) PAGE.
3.2. Size exclusion chromatography
The ultraviolet absorption of the fractions from the size exclusion were compared with the measured calprotectin concentrations determined with the prototype calprotectin immunoassay (Figure 1 ). The calprotectin concentration profile correlated well with the absorbance values. The molecular weight corresponded to oligomers, for the peak at approximately 9.5 mL, and to heterodimers, for the peak at 10.5 mL. The smaller peak at approximately 15 mL corresponded to the size of monomers.
Figure 1.
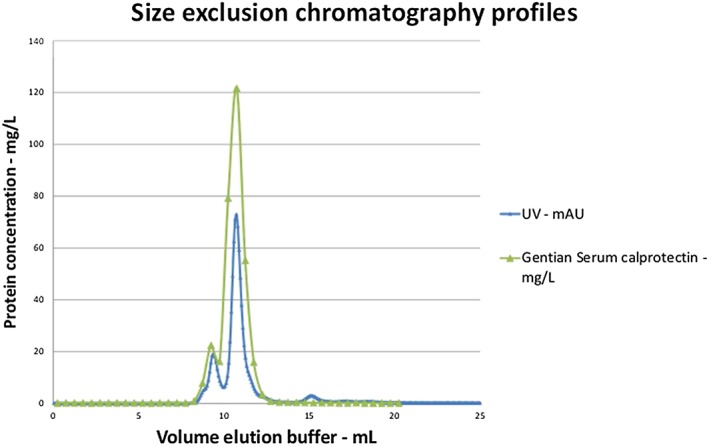
Size exclusion chromatography. All fractions from the size exclusion chromatography were measured with the Gentian prototype calprotectin immunoassay. The calprotectin concentrations were compared to the UV absorptions measured by the Äkta instrument
3.3. Sodium dodecyl sulfate polyacrylamide gel electrophoresis
The electrophoresis gel from the analysis of the 3 antigen batches is shown in Figure 2. Lot 1 in lanes 2 and 3 shows the 2 expected bands between 10 and 16 kDa. These bands correspond well with the size of the monomers MRP8 (10.8 kDa) and MRP14 (13.2 kDa). No other bands could be observed for this lot. Two dilutions of lot 2 were run, in lanes 4 and 5. The 2 expected bands between 10 and 16 kDa were observed. There was also a weak band between 16 and 23 kDa, which was unexpected. This unexpected band may be a salt from the buffers and was not considered as a contaminating protein. Lot 3 was run in lanes 6 and 7. The SDS electrophoresis profiles from the 3 antigen batches were similar.
Figure 2.
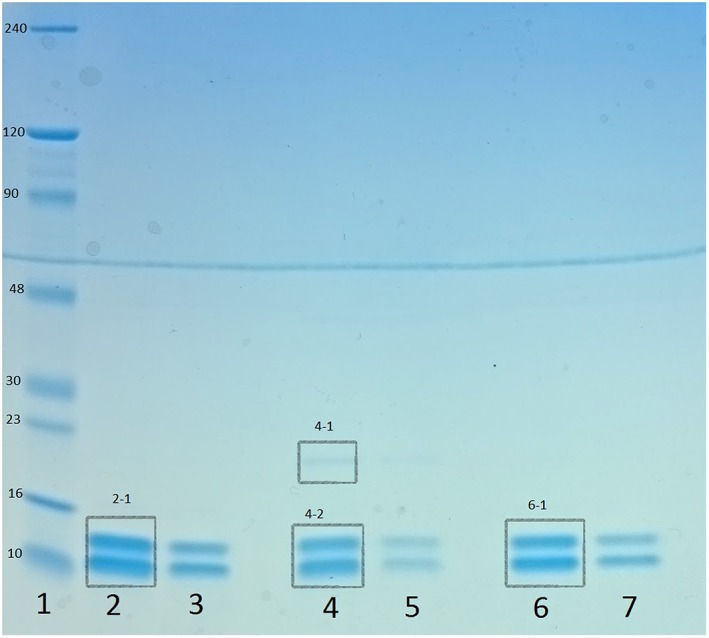
Sodium dodecyl sulphate polyacrylamide gel electrophoresis. The 3 preparations of antigen were analysed by electrophoresis under reducing conditions. Lane 1, ladder (Protein Marker II [6.5‐200 kDa], prestained, AppliChem). Lanes 2 and 3, preparation 1 diluted 1:4 and 1:10. Lanes 4 and 5, preparation 2 diluted 1:4 and 1:10. Lanes 6 and 7, preparation 3 diluted 1:4 and 1:10. Segments 2‐1, 4‐1, 4‐2, and 6‐1 were analysed with UN‐SCAN‐IT
3.4. Analysis of the gel by UN‐SCAN‐IT
The PAGE gel was then analysed by UN‐SCAN‐IT, transforming the segments 2‐1, 4‐1, 4‐2, and 6‐1 in Figure 2 into curves, based on the pixel density.
The pixel analysis by the software UN‐SCAN‐IT showed that segments 2‐1 contained 100% of all pixels in lane 2, indicating that it is only the 2 bands within the segment contributing to colouring the gel in this lane. This means there is no contamination of the protein solution. Segment 4‐1 contained 3.25% and 4‐2 96.8% of lane 4, which means that 3.25% of the content can be something other than calprotectin. In lane 6, segment 6‐1 contained 100% of the pixels. These results suggest that the purified calprotectin antigen solution mostly contains the subunits of the calprotectin molecule, S100A8 and S100A9, and are, therefore, pure. The contamination of undesired proteins was minimal.
3.5. Yield
We then determined the calprotectin concentration and the yield from the extracts.
The total protein concentrations determined by Biuret method spanned from 1079 to 1819 mg/L. On the basis of the results from the electrophoresis analysis and the size exclusion chromatography analysis, we conclude that these are the calprotectin concentrations of the solutions. The percentage yields from the granulocyte extracts varied from 46% to 76.87% in the 3 preparations and the average yield per donor from the 3 purifications spanned from 1.8 to 2.6 mg; see Table 1.
Table 1.
Yielda
| Lot | Concentration Extract, mg/L | Amount in Extract, mg | Concentration Purified Antigen, mg/L | Amount Antigen in Purified Solution, mg | Yield from Extract, % | Yield pr. Donor, mg |
|---|---|---|---|---|---|---|
| 1 | 1080 | 25.9 | 99.5 | 19.9 | 76.8 | 2.5 |
| 2 | 1079 | 12.9 | 119.2 | 6.0 | 46.0 | 1.8 |
| 3 | 1813 | 21.8 | 131.51 | 10.5 | 48.4 | 2.6 |
The percentage yield from the extracts varied from 46% to 76.8% for these preparations. The yield per donor spanned from 1.8 to 2.6 mg.
3.6. Stability of the purified antigen solution
The freeze thaw stability of the antigen was then tested, by measuring the concentration of calprotectin after repeated freeze and thaw cycles. The antigen concentration of the purified antigen solution dropped 14%, from 1566 to 1386 mg/L, after the first freeze and thaw cycle, but seemed to be stabilised at this level through the next 2 cycles. It then dropped, however, to 1251 mg/L after the fourth cycle. In this 1‐test sample, the concentration reduction was altogether of 20% through the 4 freeze and thaw cycles.
To further study the stability of the protein solution, we then tested it by measuring the calprotectin concentration in a HEPES casein buffer over time. The spiked sample measured within 10% from the value at day 0 at all measuring points for 70 days when stored at 44°C, 90 days when stored at 33°C, and for 106 days at 30°C; see Figure 3 .
Figure 3.
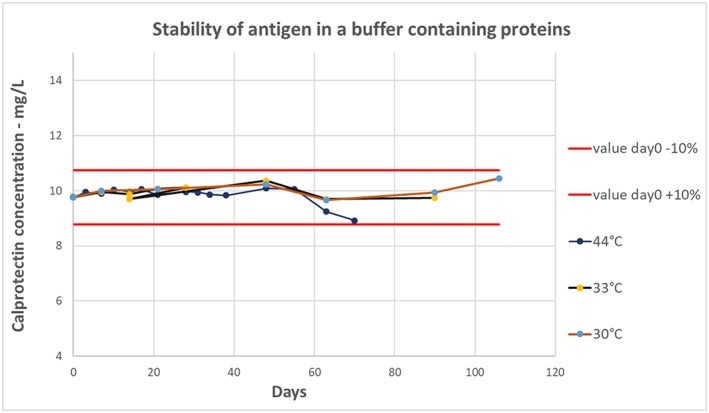
Stability of antigen in HEPES buffer. The antigen was spiked into a HEPES buffer containing casein and placed at 30°C, 33°C, and 45°C. The solutions were measured with the Gentian prototype calprotectin immunoassay multiple times within a period of 106 days. The measured concentrations were plotted against the days measured
4. DISCUSSION
From a commercial point of view, it is important to establish a purification method with high yields and high grade of purity, to ensure supplies of high demands of antigens for production of calibrators and controls, and for antibodies. For instance, determination of faecal calprotectin levels is a widely used assay to rule out IBD. It is estimated that approximately 11% of the population globally experience symptoms related to irritable bowel syndrome.23 Potentially, this requires substantial volumes of reagents, including calibrators and controls.
We collected buffy coats from 450‐mL blood bags to prepare the antigens. The number of neutrophil granulocytes in these bags may vary by donor because of several factors. In the literature, the reference range in 1 L of blood24 is given as 1.6−8.3 × 109. Using these values, estimates range from 0.72 × 109 to 3.74 × 109 neutrophil granulocytes in each blood bag. Hence, the average amount per neutrophil granulocyte is in the range 0.6 to 3.1 pg. The amount of calprotectin in each neutrophil granulocyte is not known, and the publications on purification of calprotectin from human granulocytes in this scale are few and not easily comparable. However, the amount of extracted and purified calprotectin in the 3 preparations herein evaluated was in the same range as that reported by Dale et al.25 The total yield in the purified calprotectin antigen solution varied between 40% and 80% compared with the measured amount of calprotectin in the extract before the purification.
In addition to the amount, the purity of the antigen solution is important when using the preparations for polyclonal antibody production. Any contamination may induce an immune response that will reduce the specificity of the final assay and should, therefore, be reduced as much as possible. The purity of the prepared antigens was >95%.
The freeze‐thaw stability of the purified antigen stored in saline, however, was not what is expected from a calibrator. The pure antigen solution was prepared for immunisation purposes and was not meant to be used as a calibrator as it is. For immunisation purposes, freeze‐thaw stability is not a major issue, as it is stored at −50°C until use. However, when the antigen is used as calibrators or controls, stability of several months is required when stored at 2°C to 8°C. The sample prepared by spiking HEPES buffer containing casein is a potential solution for a calibrator. When the antigen is spiked into a solution with proteins, the concentration shows little variation within 106 days when stored at 30°C. The stability study was stopped at 106 days because all vials of the samples had been opened; it is expected, however, that the sample is stable for longer than 106 days. From this study design, it is hard to estimate the stability of the antigen in a protein solution when stored at 2°C to 8°C, but it is expected to be substantially longer than the 106 days evaluated for the vials stored at 30°C. To prepare a reference material, the stability of the antigen solution must be investigated further. A next step could be to lyophilisate the solution as an attempt to stabilise the antigen.
For further investigation, determination of the dry mass of the purified protein solution could be of interest. The observed dry mass from the dry mass determination can be applied for a value transfer, via radial immunodiffusion. The procedure is described in https://paperpile.com/c/9F3w2c/NLEHe.26
AUTHOR CONTRIBUTIONS
Investigation: Tom Nilsen
Methodology: Tom Nilsen
Supervision: Anders Larsson
Validation: Siri Helen Haugen
Writing – original draft: Tom Nilsen
Writing – review and editing: Siri Helen Haugen, Anders Larsson
CONFLICT OF INTEREST
Tom Nilsen and Siri Helen Haugen work at Gentian AS, which has developed and currently manufactures a turbidimetric faecal calprotectin immunoassay and a turbidimetric plasma calprotectin immunoassay.
ACKNOWLEDGEMENT
Thanks to Dr Arnaud David for running the size exclusion chromatography on the Äkta instrument and contributing in the writing by describing the size‐exclusion method. Tom Nilsen was funded by The Norwegian Research Council under the Industrial PhD programme.
Nilsen T, Haugen SH, Larsson A. Extraction, isolation, and concentration of calprotectin antigen (S100A8/S100A9) from granulocytes. Health Sci Rep. 2018;1:e35 10.1002/hsr2.35
REFERENCES
- 1. Berntzen HB, Fagerhol MK. L1, a major granulocyte protein; isolation of high quantities of its subunits. Scand J Clin Lab Invest. 1990a;50(7):769‐774. [DOI] [PubMed] [Google Scholar]
- 2. Golden BE, Clohessy PA, Russell G, Fagerhol MK. Calprotectin as a marker of inflammation in cystic fibrosis. Arch Dis Child. 1996;74(2):136‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hammer HB, Ødegård S, Syversen SW, et al. Calprotectin (a major S100 leucocyte protein) predicts 10‐year radiographic progression in patients with rheumatoid arthritis. Ann Rheum Dis. 2010;69(1):150‐154. [DOI] [PubMed] [Google Scholar]
- 4. Berntzen HB, Fagerhol MK. L1, a major granulocyte protein; isolation of high quantities of its subunits. Scand J Clin Lab Invest. 1990b;50(7):769‐774. [DOI] [PubMed] [Google Scholar]
- 5. Yui S, Nakatani Y, Mikami M. Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis‐inducing activity. Biol Pharm Bull. 2003;26(6):753‐760. [DOI] [PubMed] [Google Scholar]
- 6. Johne B, Fagerhol MK, Lyberg T, et al. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol Pathol. 1997;50(3):113‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pepper RJ, Hamour S, Chavele K‐M, et al. Leukocyte and serum S100A8/S100A9 expression reflects disease activity in ANCA‐associated vasculitis and glomerulonephritis. Kidney Int. 2013;83(6):1150‐1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steinbakk M, Naess‐Andresen CF, Lingaas E, Dale I, Brandtzaeg P, Fagerhol MK. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990;336(8718):763‐765. [DOI] [PubMed] [Google Scholar]
- 9. Røseth AG, Fagerhol MK, Aadland E, Schjønsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992;27(9):793‐798. [DOI] [PubMed] [Google Scholar]
- 10. Simm M, Söderberg E, Larsson A, et al. Performance of plasma calprotectin as a biomarker of early sepsis: a pilot study. Biomark Med. 2016;10(8):811‐818. [DOI] [PubMed] [Google Scholar]
- 11. Schellekens DHSM, Hulsewé KWE, van Acker BAC, et al. Evaluation of the diagnostic accuracy of plasma markers for early diagnosis in patients suspected for acute appendicitis. Acad Emerg Med. 2013;20(7):703‐710. [DOI] [PubMed] [Google Scholar]
- 12. Terrin G, Passariello A, Manguso F, et al. Serum calprotectin: an antimicrobial peptide as a new marker for the diagnosis of sepsis in very low birth weight newborns. Clin Dev Immunol. 2011;2011(May):291085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bjarnason I. The use of fecal calprotectin in inflammatory bowel disease. Gastroenterol Hepatol. 2017;13(1). [PMC free article] [PubMed] [Google Scholar]
- 14. Heida A, Park KT, van Rheenen PF. Clinical utility of fecal calprotectin monitoring in asymptomatic patients with inflammatory bowel disease: a systematic review and practical guide. Inflamm Bowel Dis. 2017;23(6):894‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jang HW, Kim HS, Park SJ, et al. Accuracy of three different fecal z tests in the diagnosis of inflammatory bowel disease. Intestinal Research. 2016;14(4):305‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Sloovere MMW, De Smet D, Baert FJ, Debrabandere J, Vanpoucke HJM. Analytical and diagnostic performance of two automated fecal calprotectin immunoassays for detection of inflammatory bowel disease. Clin Chem Lab Med. 2017;55(9):1435‐1446. [DOI] [PubMed] [Google Scholar]
- 17. Flodin M, Hansson L‐O, Larsson A. Variations in assay protocol for the Dako Cystatin C method may change patient results by 50% without changing the results for controls. Clin Chem Lab Med. 2006;44(12):1481‐1485. [DOI] [PubMed] [Google Scholar]
- 18. Fagerhol, Magne K. , Dale Inge, and Naesgaard Inger. 1989. “Purified human granylocyte L1 proteins methods for their preparation, monospecific antibodies and test kits.” United States Patent and Trademark Office (USPTO), May. http://www.google.com/patents/US4833074.
- 19. Nilsen T, Sunde K, Larsson A. A new turbidimetric immunoassay for serum calprotectin for fully automatized clinical analysers. J Inflamm. 2015;12(July):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.“UN‐SCAN‐ITTM Version 5.” no date available Accessed November 29, 2017. https://www.silkscientific.com/manual/Manual70.pdf.
- 21. Karl H, von Norman MB. The biuret reaction and the cold nitric acid test in the recognition of protein. Biochem J. 1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sapan, C V , Lundblad R L, and Price N C 1999. “Colorimetric protein assay techniques” Biotechnol Appl Biochem 29 ( Pt 2) (April):99–108. [PubMed] [Google Scholar]
- 23. Canavan, Caroline , West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6(February):71‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.“Nasjonal Brukerhåndbok I Medisinsk Biokjemi.” last updated: 09.09.2014. Accessed September 9, 2016. http://brukerhandboken.no/index.php?action=showtopic&topic=d86fbb6f2c2b6e03a6f9&book_request=biokjemi&highlight=true
- 25. Dale I, Fagerhol MK, Naesgaard I. Purification and partial characterization of a highly immunogenic human leukocyte protein, the L1 antigen. Eur J Biochem. 1983;134(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 26. Blirup‐Jensen S. Protein standardization II: dry mass determination procedure for the determination of the dry mass of a pure protein preparation. Clin Chem Lab Med. 2001;39(11):1090‐1097. [DOI] [PubMed] [Google Scholar]


