Abstract
Background
This study aimed to investigate the efficacy and safety of treatment with transcutaneous vagus nerve stimulation (tVNS) for patients with refractory epilepsy by evaluation of the frequency of seizures, electroencephalogram (EEG) changes, and quality of life on follow-up at three months and six months.
Material/Methods
EEG evaluation followed baseline evaluation with EEG at three months and six months following tVNS treatment. The frequency of seizures was recorded during the six-month study period. Before and after tVNS treatment, patients completed the Self-Rating Anxiety Scale (SAS), the Self-Rating Depression Scale (SDS), the Liverpool Seizure Severity Scale (LSSS), the Quality of Life in Epilepsy Inventory (QOLIE-31), and the Pittsburg Sleep Quality Index (PSQI).
Results
Seventeen patients completed six months of tVNS treatment. Following three months of tVNS therapy, the frequency of epileptic seizures decreased in 13/17 subjects, with an average reduced seizure rate of 31.3%. Following six months of tVNS treatment, the frequency of epileptic seizures decreased in 16/17 subjects, with an average reduced seizure rate of 64.4%. There were 14/17 cases with abnormal EEG at baseline; 2/17 patients had improved EEGs by three months, and 10/17 patients had improved EEGs by six months. During the study period, there were no adverse events associated with tVNS treatment, but the effects on sleep were inconclusive.
Conclusions
This preliminary study showed that tVNS was an effective and safe adjuvant treatment for refractory epilepsy that reduced seizure frequency and reduced abnormal EEG changes following clinical improvement.
MeSH Keywords: Electroencephalography; Epilepsy, Absence; Patients
Background
Epilepsy is a common chronic central nervous system disease, which is caused by the abnormal discharge of neurons in the brain. Worldwide, in 2011, there were estimated to be approximately 70 million people living with epilepsy [1]. The prevalence of epilepsy in developing countries has been reported to be significantly higher than that in developed countries [2]. In China, in 2003, there were reported to be about 9 million people with epilepsy, with an estimated 40,000 new cases of epilepsy each year [3,4]. Worldwide, epilepsy and its management remain a significant public health problem.
The treatment of epilepsy can be challenging for the neurologist. The current treatment for epilepsy includes anti-epileptic drugs, craniotomy, neural regulation, and the use of ketogenic diets [5–7]. Clinical treatment with anti-epileptic drugs is the first-line treatment for epilepsy, which can be effective in 59% of patients who have new onset of epilepsy, resulting in control of seizures, but approximately 25% of patients are refractory to anti-epileptic drug treatment and suffer from further seizures [8]. Refractory epilepsy is a unique type of epilepsy, which characterized by the lack of response to standard anti-epilepsy drug treatment [9,10]. Worldwide, between 20–30% of people with epilepsy have been estimated to have refractory epilepsy [11]. Patients with epilepsy that is refractory to drug treatment may choose treatment with craniotomy, ketogenic diet, transcranial magnetic stimulation, or neural regulation using vagus nerve stimulation, or other treatment methods.
Some patients may be unsuitable for surgical treatment or choose not to undergo surgery, and the use of a ketogenic diet is more suitable for children with refractory epilepsy than for adults [12,13]. Recently, neural stimulation has been recognized as a safe and potentially effective method to treat refractory epilepsy [14]. Current neural stimulation treatments include vagus nerve stimulation (VNS), trigeminal nerve stimulation (TNS), repetitive transcranial magnetic stimulation (rTMS), anterior nucleus of the thalamus-deep brain stimulation (ANT-DBS) [15], and responsive neurostimulation (RNS) [16]. In 1997, the US Food and Drug Administration (FDA) approved the use of vagus nerve stimulation (VNS) for the treatment of refractory epilepsy, and clinical studies have shown that VNS is a safe and effective means of adjuvant therapy for refractory epilepsy [9,16]. However, direct VNS is an invasive procedure that involves the implant of a battery-powered device into the body, with an invasive procedure required to replace the battery [9,16]. Therefore the use of direct VNS for patients with refractory epilepsy can be costly and associated with some risk.
In 2000, Ventureyra first reported the use of transcutaneous vagus nerve stimulation (tVNS) for the treatment of partial epilepsy, which resulted in further studies on the application of this non-invasive therapy [17]. Compared to the implanted VNS, tVNS has several advantages, including the effectiveness of VNS but with lower cost, lack of the need for an invasive procedure, fewer side effects, no secondary tissue damage, and the ease of performing the tVNS procedure [18]. A previously published preliminary clinical study on the treatment of refractory epilepsy with tVNS showed clinical effectiveness that was comparable with VNS in the control of seizures [18]. Also, for patients with refractory epilepsy, treatment with tVNS has been shown to result in a reduction of seizure frequency by up to 48% after nine months of treatment, and improvement of the electroencephalogram (EEG) findings [19].
Although there have been several clinical studies on the use of tVNS in patients with epilepsy that is refractory to drug treatment, there have been no previous prospective clinical studies that have included the effects of tVNS on the prevention of seizures, EEG changes, and the impact on the quality of life of individuals with epilepsy. Therefore, this study aimed to investigate the efficacy and safety of treatment tVNS for patients with refractory epilepsy by evaluation of the frequency of seizures, the electrophysiological changes seen on EEG, and quality of life on follow-up at three months and six months.
Material and Methods
Ethical approval
This study was approved by the Xuanwu Hospital Ethics Committee. The patients were screened in the Department of Neurology of Xuanwu Hospital, from January 2013 to June 2014. All the subjects included in the study were recruited, clinically evaluated, and managed by epilepsy experts. All subjects voluntarily participated and signed informed consent to participate in the study.
Study design
The study was conducted at a single neurological center in China and included the screening of patients with epilepsy who did not respond to drug treatment. Clinical evaluation was performed by professional epilepsy experts. The baseline information recorded for all participants in the study included age, gender, the patient’s history of epilepsy, including the onset, course, seizure type, and baseline seizure frequency, and the past medical history, including any history of previous treatments, including previous surgery, and previous illnesses, including encephalitis or episodes of fever. Previous imaging data were recorded, including brain magnetic resonance imaging (MRI). The study participant’s family history and medication history (current medication type and the dose of anti-epileptic drugs) were recorded.
Baseline scalp electroencephalogram (EEG) data were collected for two hours in Xuanwu Hospital, and neuropsychological assessment was performed. Transcutaneous vagus nerve stimulation (tVNS) then commenced for a period of six months. Patient follow-up was performed monthly with telephone calls, with follow-up records of the frequency of epileptic seizures, any tVNS parameter adjustments required, and any adverse reactions. After three months of treatment, the scalp EEG was reviewed, and after six months of treatment, the scalp EEG was reviewed and evaluated again.
Study inclusion and exclusion criteria
Inclusion criteria for this study included: patient age between 12–65 years; a history of epilepsy refractory to anti-epileptic drug treatment: the previous use of two or more anti-epileptic drugs for at least two years; an average monthly epileptic seizure rate of four or more episodes; no adjustment of the type and dosage of the anti-epileptic drug treatment in the three months before the starting date of the study; a patient who was not suitable for surgical treatment or refused surgery; compliance with and participation in the study, including follow-up.
Exclusion criteria for this study included: a history of progressive central nervous system disease; women who were pregnant or lactating; patients with serious diseases of the heart, respiratory system, blood system, immune system; a history of rheumatism; a clinical history of mental illness, alcohol or drug abuse; patients with implantable medical devices, including a cardiac pacemaker; inability to understand or complete the follow-up questions and questionnaire surveys.
Recording of baseline clinical information
After obtaining the written informed consent of the subjects or their families, the patients were examined by qualified neurologists. Screening and baseline clinical procedures included: a general demographic survey (including name, gender, age, ethnicity, education, occupation) and clinical history (including the present illness, past medical history, family history); preliminary screening according to the inclusion and exclusion criteria; scalp EEG recordings and neuropsychological scale tests, as part of the initial screening. All subjects took a fixed dose of anti-epileptic drug throughout the course of the study.
Performance of scalp electroencephalograms (EEGs)
All electroencephalograms (EEGs) were performed in the Department of Neurology of Xuanwu Hospital by a professional neuro-electrophysiology laboratory technician or physician. A 64-channel video EEG acquisition system was used and EEG recording and analysis was performed using Medicare Systems software. The scalp electrodes used were composed of disk-shaped electrode wire (Compumedics Ltd., Melbourne, Australia) and electrocardiogram (ECG) electrodes (CONMED Corp., Utica, NY, USA). The patients were asked to wash their hair before the electrodes were sited according to the International 10–20 system for siting and recording from scalp electrodes, with conventional provocation testing including an eye test and response to hyperventilation. The total recording time was 2 hours, and EEG recordings were made during waking and sleep (1 hour for each).
Data analysis and processing from the EEG was performed by two senior EEG technicians or by a physician separately, with the average of two recordings and the operator blinded to the identity of the patient. Frequency measurements included a spike, slow wave, and sharp wave components in the unipolar lead in units per hour, with waves characteristic of epilepsy in two cases. The EEG instrument technical parameters included: a sampling frequency of 1024 Hz; a high-frequency filter of 70 Hz; a low-frequency filter of 0.5 Hz; and a wave amplitude of 100 V/mm.
Neuropsychological testing
In this study, the neuropsychological scale evaluation was completed under the guidance of professional neuropsychologist to assess the quality of life, impact of epilepsy, mental state, and sleep quality. Self-completed questionnaires were used, including the Self-Rating Anxiety Scale (SAS), the Self-Rating Depression Scale (SDS), the Liverpool Seizure Severity Scale (LSSS), the Quality of Life in Epilepsy Inventory (QOLIE-31), and the Pittsburg Sleep Quality Index (PSQI).
Transcutaneous vagus nerve stimulation (tVNS)
The location of the vagal nerve stimulus was both ears and localized to the cavity of the auricular concha and the outside of the external ear canal, with the area of stimulation measuring approximately 3 cm2. The nerve stimulation apparatus used was the transcutaneous electrical nerve stimulation (TENS)-sm device (Suzhou Medical Audio Supplies Company Ltd., China), with the electrode clip through the wire and TENS connection. The pulsed mode was used for the biphasic waveform, with a pulse width of 200 sec, a stimulation frequency of 10 Hz, and the stimulation intensity of 4 mA. The intensity of tVNS was increased by 2 mA per week, until the patient was unable to tolerate the intensity or until a state of complete control of epileptic seizures was achieved. Following professional training, patients were able to perform the tVNS themselves as a daily routine treatment, and used the machine for 20 minutes a day, three times a day. The total treatment time was six months. The use of tVNS in this study is shown in Figure 1.
Figure 1.
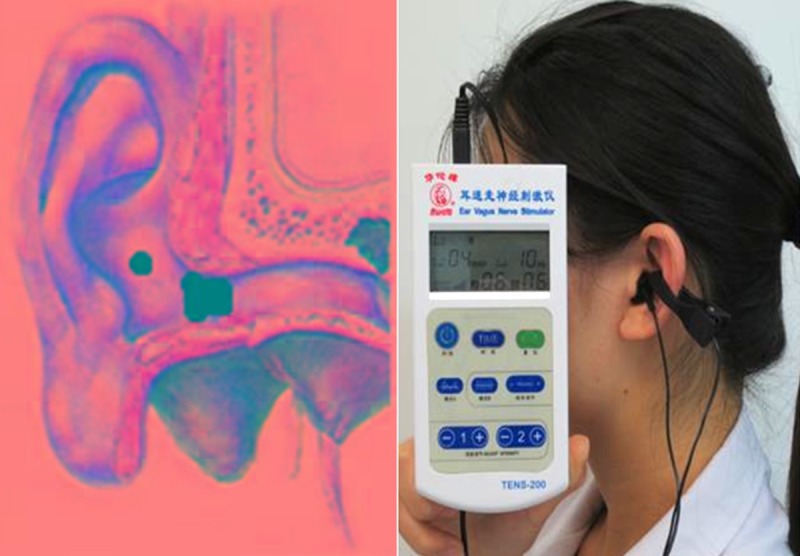
Location of transcutaneous vagus nerve stimulation (tVNS) points.
Data analysis
The study indicators that were analyzed included seizure frequency, effective rate (reduced frequency of epileptic seizures), and the frequency of seizures during tVNS treatment for three months and six months. Changes were compared with the baseline findings before tVNS treatment. The average number of seizures per month were recorded during the six-month study period. The classification of the curative effect on epileptic seizures used the improved Engel curative effect classification method [20], as follows: I, no seizures; II, >90% seizure reduction; III, seizure rate between 50–89%; IV, seizure rate reduced to <50%. The data from the EEG results included the number of abnormal waves, the duration of abnormal discharge, and the number of seizures. The data from the neuropsychological scales, side effects, and outcome of the tVNS treatment were recorded.
Results
Baseline information
A total of 24 patients with refractory epilepsy were initially enrolled in this study, with 17 patients who completed the six-month study of transcutaneous vagus nerve stimulation (tVNS) treatment (Table 1). Seven patients were lost to the study for reasons: poor treatment compliance (three cases); dizziness (one case); inability to self-treat (one case), a history of hepatocellular carcinoma (one case); and loss to follow-up (one case).
Table 1.
Baseline data of 17 patients.
| General situation | T-VNS subjects |
|---|---|
| Average age (years) | 27±9.4 |
| Sex (Male/Female) | 10/7 |
| Time of illness (year) | 14.2±12.3 |
| Medication species (species) | 2–3 |
| Febrile convulsions (with/without) | 3/14 |
| Type of attack (number) | GTCS (5) |
| CPS (9) | |
| GTCS and CPS (2) | |
| GTCS and SPS (1) |
GTCS – generalized tonic clonic seizures; CPS – complex partial seizures; SPS – simple partial seizures.
Efficacy of transcutaneous vagus nerve stimulation (tVNS) on follow-up
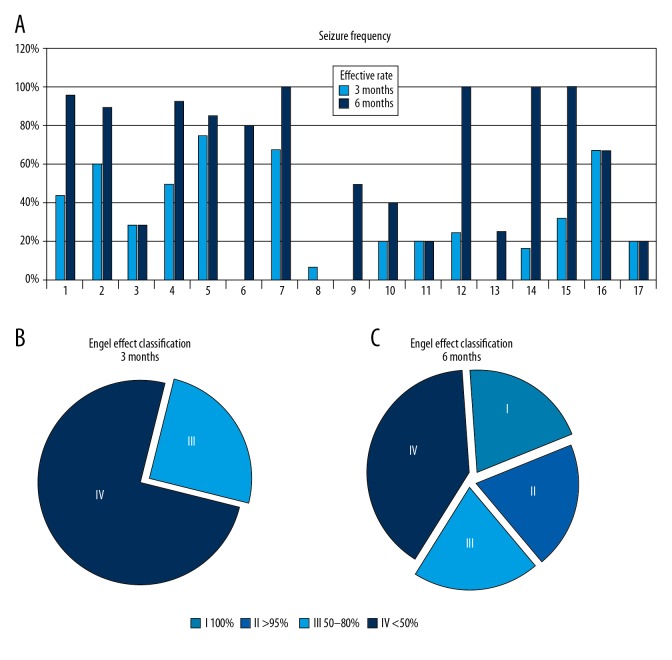
Figure 2 shows the results of the reduction in seizures at three months and six months in 17 patients treated with tVNS, according to the patient seizure diaries. A comparison of seizure frequency after three months and six months showed a reduction of seizures in 13 out of 17 and 16 out of 17 patients, respectively. According to the modified Engel evaluation system, five patients had Engel Class III outcomes and 12 patients had Engel Class IV outcomes after three months of treatment, 11 patients had less than Engel Class III outcome, and only four patients had Engel Class IV outcome after six months of treatment.
Figure 2.
Efficacy of control of seizure frequency after treatment with transcutaneous vagus nerve stimulation (tVNS). (A) Percentage of seizure reduction over time. (B) Curative effect classification (treatment for three months): Grade I, and Grade II: 0 cases; Grade III: 5 cases; Grade IV: 12 cases. (C) Curative efficacy classification (treatment for six months): Grade I: 4 cases; Grade II: 3 cases; Grade III: 4 cases; Grade IV: 6 cases.
Effects of tVNS on the electroencephalogram (EEG) changes
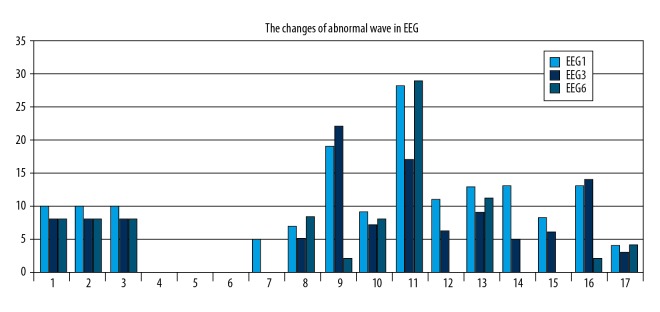
In this study, 14 patients showed abnormal epileptic discharge in the baseline period. Patient 2, Patient 4, Patient 5, and Patient 6 had baseline EEGs that did not show abnormal epileptiform discharge. Overall, 10/17 patients showed improvement in the EEG at three months and six months of tVNS treatment. However, the prevalence of abnormal waves in the EEG of Patient 8, Patient 10, Patient 11, and Patient 17 at six months was greater than at three months (Figure 3).
Figure 3.
The electroencephalogram (EEG) changes after three months and six months of treatment with transcutaneous vagus nerve stimulation (tVNS). Baseline electroencephalogram (EEG1). EEG after three months of transcutaneous vagus nerve stimulation (tVNS) treatment (EEG3). EEG after six months of tVNS treatment (EEG6).
Clinical outcome measurements of tVNS treatment
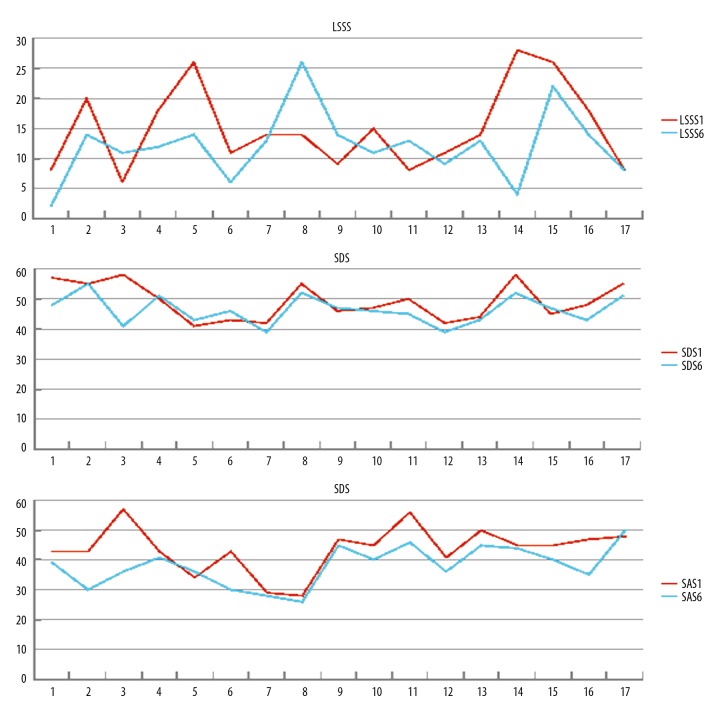
The clinical outcome measurements after six months of tVNS treatment are shown in Figure 4. There were 12/17 patients who showed reduced Liverpool Seizure Severity Scale (LSSS) scores compared with baseline following tVNS treatment, except Patients 3, 8, 9 and 11, who had increased LSSS scores when compared with baseline. The Self-Rating Anxiety Scale (SAS) scores following tVNS treatment were reduced in 15/17 patients. The Self-Rating Depression Scale (SDS) scores following tVNS treatment were reduced in 11/17 patients.
Figure 4.
Clinical outcome measurements after three months and six months of treatment with transcutaneous vagus nerve stimulation (tVNS). Results of the Self-Rating Anxiety Scale (SAS), the Self-Rating Depression Scale (SDS), and the Liverpool Seizure Severity Scale (LSSS), before (LSSS-1, SDS-1, SAS-1) and six months after treatment (LSSS-6, SDS-6, SAS-6) with transcutaneous vagus nerve stimulation (tVNS).
Quality of life (QOL) analysis oo tVNS treatment
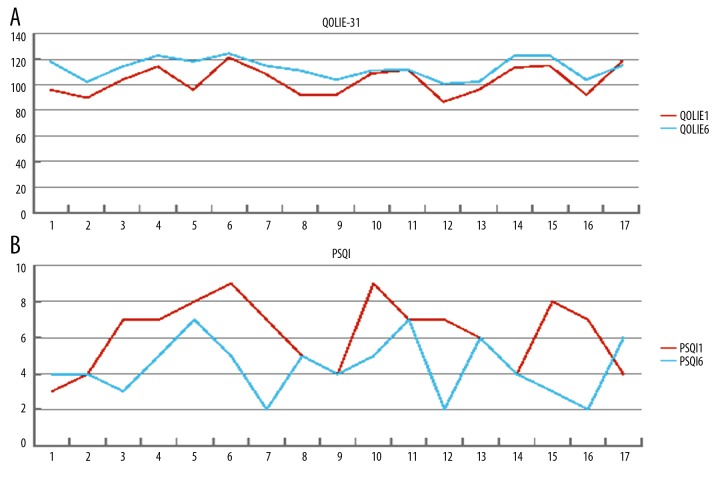
The overall quality of life (QOL) of participants in this study, as measured by the Quality of Life in Epilepsy Inventory (QOLIE-31) changed slightly from baseline to treatment at six months (Figure 5). In 15/17 patients, the QOLIE-31 scores were reduced at six months of treatment, while the QOLIE-31 score of Patient 17 increased compared to baseline. Using the Pittsburg Sleep Quality Index (PSQI) scoring system, the percentage of ‘good sleepers’ (with a score <5) was 53% after six months of treatment, compared with 29% at baseline. However, the PSQI scores of Patient 1 and Patient 17 were increased after tVNS treatment, compared with the scores before treatment.
Figure 5.
Quality of life and sleep analysis before and six months following transcutaneous vagus nerve stimulation (tVNS) treatment. (A) The changes in the scores of the Quality of Life in Epilepsy Inventory (QOLIE) before treatment (QOLIE-1), and six months after treatment (QOLIE-6) with transcutaneous vagus nerve stimulation (tVNS). (B) Changes in the scores of the Pittsburg Sleep Quality Index (PSQI) before treatment (PSQI-1), and six months after treatment (PSQI-6) with transcutaneous vagus nerve stimulation (tVNS).
Adverse events
One patient had mild dizziness and left the study. There were no other complications or adverse events reported due to tVNS in the other patients in the study.
Discussion
The aims of this prospective clinical study were to investigate the efficacy and safety of treatment with transcutaneous vagus nerve stimulation (tVNS) for patients with epilepsy that was refractory to anti-epileptic drug treatment, epilepsy by evaluation of the frequency of seizures, electroencephalogram (EEG) changes, and quality of life on follow-up at three months and six months. This study included 17 patients. Although the study was preliminary in nature, the findings showed that tVNS was a non-invasive, safe and effective clinical treatment for refractory epilepsy. The clinical and neural electrophysiological study findings support that treatment using tVNS could achieve effective control of seizures and improve the quality of life for patients with epilepsy. The findings of this study are supported by those of previous studies that have shown improved EEG changes after tVNS treatment in patients with refractory epilepsy [21]. However, the present study not only evaluated the effects of tVNS treatment on reducing frequency of seizures and improving EEG findings, patients also completed the Self-Rating Anxiety Scale (SAS), the Self-Rating Depression Scale (SDS), the Liverpool Seizure Severity Scale (LSSS), the Quality of Life in Epilepsy Inventory (QOLIE-31), and the Pittsburg Sleep Quality Index (PSQI) during the six-month study period.
The findings of the present study showed that tVNS treatment for three months was effective in 76.5% of the subjects studied; at six months, tVNS treatment was effective in 94.1% of subjects. The EEG findings indicated that the frequency of the majority of generalized tonic-clonic seizures, simple partial seizures, complex partial seizures, in refractory epilepsy patients could be reduced by treatment with tVNS. Compared with tVNS treatment at three months and six months, the seizure frequency decreased from 31.3% to 64.4%, respectively, indicating that the effects of tVNS treatment improve with time, which is consistent with previously published studies [18,22].
It has previously been reported that in between 40–50% of patients, direct vagus nerve stimulation (VNS) treatment reduced the average frequency of epileptic seizures by up to 50% [23]. Direct VNS stimulates the vagus nerve trunk and would be expected to be more effective than tVNS in the control of seizures. However, the results of the present study showed that tVNS showed efficacy in seizure control and improved EEG changes. The question remains as to whether tVNS is effective in patients with specific types of epilepsy, such as generalized tonic-clonic seizures (GTCS) or ‘grand mal’ seizures, complex partial seizures (CPS), and simple partial seizures (SPS).
Previous studies have shown that tVNS treatment can be associated with side effects that include dizziness, drowsiness, pharyngitis, and nausea [24,25]. In the present study, during the initial stages of patient recruitment, one patient was treated with tVNS for one week, and when nerve stimulation intensity was increased to 4 mA, the patient complained of dizziness, which was reversed when tVNS treatment ceased. In the present study, no other adverse events or side effects were reported during tVNS treatment.
In this study, the evaluation of the EEG changes before and during tVNS treatment provided important information on treatment response. However, brain electrical activity is a dynamic process that can be affected by many factors, including genetic factors, age, external stimulation, temperature fluctuations, drugs, and the level of arousal. While it is difficult to control so many factors that might affect the EEG, the study design attempted to eliminate the influence of arousal level, by EEG monitoring during periods of wakefulness and sleep. In future studies, although the many external factors that might affect the EEG findings might be controlled, a previously published study has shown that factors such as seasonal and diurnal variations, and the stage in the menstrual cycle in women, had no significant effects on the EEG, which may be considered to be a stable and reliable form of patient evaluation [26].
In 2012, Stefan et al. undertook a nine-month of proof of concept (POC) study of tVNS in 10 patients with drug-resistant epilepsy, including follow-up, and confirmed that tVNS was a safe and effective adjuvant therapy for refractory epilepsy [19]. In this previous study, the EEG was used to evaluate the interval between seizures and found out that two patients had a significant improvement in the EEG that included a reduced number of sharp waves, and a reduction in the duration of the epileptic waves [19].
In the present study, following tVNS treatment for three months, two patients showed improvement in the EEG, and 10 patients showed improvement in the EEG following tVNS treatment for six months. The onset and number of abnormal waves on the EEG of Patient 1 were significantly reduced after six months of tVNS treatment, and complex partial seizures (CPS) decreased (Supplementary Figure 1). The improvements in the EEG in Patient 1 following tVNS treatment was consistent with the effect in most subjects, but Patient 3, Patient 11 and Patient 17 had no significant changes in the EEG. The possible reasons for these findings in three of the patients in the present study were that EEG improvement may have a lag time that was longer than the six-month duration of this study, which supports the need for future long-term studies. Also, the frequency of epileptic seizures was subjectively recorded by the patients in the study, and future studies should combine subjective records of seizures and objective EEG confirmation of seizure frequency, duration, and type.
The present study included the evaluation of neuropsychological changes before and after tVNS treatment. The quality of life of 15 patients with epilepsy, as assessed by the SDS scores, was improved following tVNS treatment. However, in two patients, the LSSS score decreased, which may have been associated with a recent seizure. Patients with epilepsy commonly suffer from the comorbidities of anxiety and depression, although the subjects in the present study had the diagnostic criteria of mild anxiety and mild depression. The neuropsychological and quality of life aspects of epilepsy require further and long-term study.
Sleep disturbance is reported to be an important comorbidity by patients with epilepsy, which is why the PSQI was included in the present study. After tVNS treatment, the PSQI scores of nine patients were reduced, six patients had no change, and two patients had increased PSQI scores. Four patients who reported a history of insomnia had reduced PSQI scores following tVNS treatment. However, further studies are required to evaluate the effects of tVNS treatment on sleep.
This study had several limitations. This was a retrospective and uncontrolled study performed in a single center that included 17 patients with refractory epilepsy. The retrospective nature of the study depends on the quality of the recorded clinical data and can include reporter bias and selection bias. Because the study included a small cohort of 17 study participants, sampling bias might have occurred. Because seizure frequency was self-reported and assessed in a non-blinded manner, there is a possibility that positive responses regarding the outcome of tVNS treatment were over-reported or under-reported. Three patients did not report any improvement during the study and were treated with new anti-epileptic drugs. Therefore, large, multicenter, long-term controlled studies are required to support the efficacy of tVNS.
Conclusions
This retrospective clinical study included 17 patients with refractory epilepsy treated with transcutaneous vagus nerve stimulation (tVNS) who were treated and followed-up for six months. Treatment with tVNS was effective in reducing the number of epileptic seizures and reduced abnormal wave changes shown on electroencephalogram (EEG) monitoring. The EEG changes followed the reduction in the frequency of seizures. Treatment with tVNS also reduced levels of anxiety and depression, and improved patient quality of life. The effects of tVNS on sleep were inconclusive and require further large-scale controlled studies.
Supplementary Figure
Patient 16. The electroencephalogram (EEG) of a 24-year-old man with refractory epilepsy treated with transcutaneous vagus nerve stimulation (tVNS). A 24-year-old man (Patient 16) with refractory epilepsy treated with transcutaneous vagus nerve stimulation (tVNS). The electroencephalogram (EEG) showed a wide range (2–2.5Hz) of sharp slow waves with short-range and long-range bursts. The duration of epilepsy after tVNS treatment was significantly shorter compared with the duration of epilepsy before tVNS treatment.
Footnotes
Source of support: This study was supported by Beijing Key Laboratory of Neuromodulation (BZ0098), and The Capital Medical Development Foundation of China (TCM-SF-2009-II-15)
References
- 1.Ngugi AK, Kariuki SM, Bottomley C, et al. Incidence of epilepsy: A systematic review and meta-analysis. Neurology. 2011;77(10):1005–12. doi: 10.1212/WNL.0b013e31822cfc90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakano H, Inoue Y. [Epidemiology and cause of epilepsy]. Nihon Rinsho. 2014;72(5):785–89. [in Japanese] [PubMed] [Google Scholar]
- 3.Fang J, Rong P, Hong Y, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. 2016;79(4):266–73. doi: 10.1016/j.biopsych.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang WZ, Wu JZ, Wang DS, et al. The prevalence and treatment gap in epilepsy in China: an ILAE/IBE/WHO study. Neurology. 2003;60(9):1544–45. doi: 10.1212/01.wnl.0000059867.35547.de. [DOI] [PubMed] [Google Scholar]
- 5.Wu D, Chen L, Ji F, et al. The effects of oxcarbazepine, levetiracetam, and lamotrigine on semen quality, sexual function, and sex hormones in male adults with epilepsy. Epilepsia. 2018;59(7):1344–50. doi: 10.1111/epi.14450. [DOI] [PubMed] [Google Scholar]
- 6.Phung J, Mathern GW, Krogstad P. Timing and predictors of fever and infection after craniotomy for epilepsy in children. Pediatr Infect Dis J. 2013;32(5):450–59. doi: 10.1097/INF.0b013e318287b408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee BH, Smith T, Paciorkowski AR. Autism spectrum disorder and epilepsy: Disorders with a shared biology. Epilepsy Behav. 2015;47:191–201. doi: 10.1016/j.yebeh.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karceski S. Vagus nerve stimulation and Lennox-Gastaut syndrome: A review of the literature and data from the VNS patient registry. CNS Spectr. 2001;6(9):766–70. doi: 10.1017/s1092852900001516. [DOI] [PubMed] [Google Scholar]
- 9.Hong J, Desai A, Thadani VM, Roberts DW. Efficacy and safety of corpus callosotomy after vagal nerve stimulation in patients with drug-resistant epilepsy. J Neurosurg. 2018;128(1):277–86. doi: 10.3171/2016.10.JNS161841. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi K, Yoshinaga H, Ohtsuka Y. Drug-resistant epilepsy. N Engl J Med. 2011;365(23):2238–39. doi: 10.1056/NEJMc1111683. (author reply 2239–40) [DOI] [PubMed] [Google Scholar]
- 11.Brodie MJ, Barry SJ, Bamagous GA, et al. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78(20):1548–54. doi: 10.1212/WNL.0b013e3182563b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314–19. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 13.Nam SH, Lee BL, Lee CG, et al. The role of ketogenic diet in the treatment of refractory status epilepticus. Epilepsia. 2011;52(11):e181–84. doi: 10.1111/j.1528-1167.2011.03289.x. [DOI] [PubMed] [Google Scholar]
- 14.Asconape JJ. Epilepsy: New drug targets and neurostimulation. Neurol Clin. 2013;31(3):785–98. doi: 10.1016/j.ncl.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 16.DeGiorgio CM, Krahl SE. Neurostimulation for drug-resistant epilepsy. Continuum (Minneap Minn) 2013;19(3 Epilepsy):743–55. doi: 10.1212/01.CON.0000431397.61970.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ventureyra EC. Transcutaneous vagus nerve stimulation for partial onset seizure therapy. A new concept. Childs Nerv Syst. 2000;16(2):101–2. doi: 10.1007/s003810050021. [DOI] [PubMed] [Google Scholar]
- 18.Aihua L, Lu S, Liping L, et al. A controlled trial of transcutaneous vagus nerve stimulation for the treatment of pharmacoresistant epilepsy. Epilepsy Behav. 2014;39:105–10. doi: 10.1016/j.yebeh.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Stefan H, Kreiselmeyer G, Kerling F, et al. Transcutaneous vagus nerve stimulation (tVNS) in pharmacoresistant epilepsies: A proof of concept trial. Epilepsia. 2012;53(7):e115–18. doi: 10.1111/j.1528-1167.2012.03492.x. [DOI] [PubMed] [Google Scholar]
- 20.Elliott RE, Morsi A, Tanweer O, et al. Efficacy of vagus nerve stimulation over time: review of 65 consecutive patients with treatment-resistant epilepsy treated with VNS >10 years. Epilepsy Behav. 2011;20(3):478–83. doi: 10.1016/j.yebeh.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 21.Rong P, Liu A, Zhang J, et al. Transcutaneous vagus nerve stimulation for refractory epilepsy: A randomized controlled trial. Clin Sci (Lond) 2014 doi: 10.1042/CS20130518. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Rong P, Liu A, Zhang J, et al. An alternative therapy for drug-resistant epilepsy: Transcutaneous auricular vagus nerve stimulation. Chin Med J (Engl) 2014;127(2):300–4. [PubMed] [Google Scholar]
- 23.Meneses MS, Rocha SF, Simao C, et al. Vagus nerve stimulation may be a sound therapeutic option in the treatment of refractory epilepsy. Arq Neuropsiquiatr. 2013;71(1):25–30. doi: 10.1590/s0004-282x2013000100006. [DOI] [PubMed] [Google Scholar]
- 24.Bauer S, Baier H, Baumgartner C, et al. Transcutaneous vagus nerve stimulation (tVNS) for treatment of drug-resistant epilepsy: A randomized, double-blind clinical trial (cMPsE02) Brain Stimul. 2016;9(3):356–63. doi: 10.1016/j.brs.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Salanova V, Witt T, Worth R, et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84(10):1017–25. doi: 10.1212/WNL.0000000000001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagai Y, Trimble MR. Long-term effects of electrodermal biofeedback training on seizure control in patients with drug-resistant epilepsy: Two case reports. Epilepsy Res. 2014;108(1):149–52. doi: 10.1016/j.eplepsyres.2013.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient 16. The electroencephalogram (EEG) of a 24-year-old man with refractory epilepsy treated with transcutaneous vagus nerve stimulation (tVNS). A 24-year-old man (Patient 16) with refractory epilepsy treated with transcutaneous vagus nerve stimulation (tVNS). The electroencephalogram (EEG) showed a wide range (2–2.5Hz) of sharp slow waves with short-range and long-range bursts. The duration of epilepsy after tVNS treatment was significantly shorter compared with the duration of epilepsy before tVNS treatment.