Abstract
Studies in humans have shown a direct association between maternal plasma cholesterol concentrations and infant birthweight. Similarly, previous studies in our laboratory have shown that chow-fed mice lacking apolipoprotein (apo) A-I, the major protein in HDL, have low HDL-cholesterol (HDL-C) concentrations and smaller fetuses in midgestation. In the current study, we measured fetal weights in mice with varying levels of apoA-I gene dose (knockout, wild-type, and transgenic) and examined metabolic pathways known to affect fetal growth. As expected, we found the differences in apoA-I expression led to changes in HDL particle size and protein cargo as well as plasma cholesterol concentrations. Fetal masses correlated directly with maternal plasma cholesterol and apoA-I concentrations, but placental masses and histology did not differ between groups of mice. There was no significant difference in glucose or amino acid transport to the fetus or in expression levels of the glucose (glucose transporter 1 and 2) or amino acid (sodium-coupled neutral amino acid transporter 1 and 2) transporters in whole placentas, although there was a trend for greater uptake of both nutrients in the whole fetal unit (fetus + placenta) of mice with greater apoA-I levels; significant differences in transport rates occurred when mice without apoA-I (knockout) vs. mice with apoA-I (wild-type and transgenic) were compared. Glucose tolerance tests were improved in the mice with the highest level of apoA-I, suggesting increased insulin-induced uptake of glucose by tissues of apoA-I transgenic mice. Thus, maternal HDL is associated with fetal growth, an effect that is likely mediated by plasma cholesterol or other HDL-cargo, including apolipoproteins or complement system proteins. A direct role of enhanced glucose and/or amino acid transport cannot be excluded.—Rebholz, S. L., Melchior, J. T., Davidson, W. S., Jones, H. N., Welge, J. A., Prentice, A. M., Moore, S. E., Woollett, L. A. Studies in genetically modified mice implicate maternal HDL as a mediator of fetal growth.
Keywords: apolipoprotein A-I, cholesterol, placenta, pregnancy, fetus
Infants with low birthweight (LBW) (i.e., <2.5 kg) are at an increased risk for adverse acute and chronic conditions. The acute effects of LBW can range from poor cognitive development and neonatal health to high infant mortality (1,–5). The chronic effects can include metabolic diseases and cognitive impairments that last across the life course [reviewed by Barker (6) and Prentice and Moore (7)]. It is estimated that 15–20% of all births worldwide are LBW (8), averaging 20 million births each year. The incidence of LBW is especially high in resource-poor settings in Africa and Asia. This is a global problem, however, because a number of resource-rich countries also have high rates of LBW in specific regions or subgroups (9). Although comparisons are often made between infants of LBW and normal birthweight (≥2.5 kg), there is a continuous impact of birthweight on acute and chronic diseases even within the normal range of birthweights (10,–13). This has led to the understanding that even incremental changes in birthweight can affect health.
Birthweight is a product of fetal growth rates and length of gestation (14, 15). An infant can have a normal rate of fetal growth but be LBW if born prematurely. Likewise, reduced fetal growth rates can result in infants who are small for gestational age (SGA); this latter factor is the primary driver of LBW in low-income settings. One of the primary regulators of fetal growth is nutrient supply, including the macronutrients glucose, amino acids, and fatty acids (16, 17). Because the placenta forms the sole conduit for transfer of essential nutrients between the maternal and fetal circulations, nutrients must be taken up by and transported across the syncytiotrophoblast layer of the placenta to enter the fetal circulation. These processes are regulated by placental mass and function, which are regulated by factors in the maternal circulation, with the end point being optimal fetal growth based on the nutrient and health status of the mother and her capacity to host her growing fetus. Gestational length is governed by a number of factors, including ethnicity, genetics, stress, infection, reduced local progesterone action due to less local progesterone or altered membrane function, vascular disorders, and more recently placental insufficiency (18, 19).
An often neglected, but essential, metabolite in fetal growth is cholesterol. Previous studies in humans have shown a direct association between maternal plasma cholesterol levels and birthweight (20,–26), although results are inconsistent and may be confounded by associations with maternal body mass index, the inflammatory status of the mother, and gestational age at the time of sample collection (27,–32). Studies in mice with different plasma cholesterol concentrations, and specifically HDL-cholesterol (HDL-C) levels due to genetic disruption of apolipoprotein (apo) A-I, also have shown reduced fetal growth in dams with lower maternal cholesterol levels (33). The reduced growth is not mediated by a change in gene expression in the fetus itself (33), suggesting that a factor in the maternal circulation is responsible for the change in fetal growth.
Cholesterol is carried in plasma by lipoproteins. Lipoprotein particles, including HDL, are taken up readily by lipoprotein receptors on syncytiotrophoblasts of the placenta. Whole HDL particles can be taken up by receptors, including cubulin and apoE-receptors, and HDL-cholesteryl ester can be selectively taken by the scavenger receptor class B type I. It has been suggested by Kramer et al. (34) that the ability of the placenta to take up HDL is important for fetal growth and low uptake rates (or low HDL levels) could yield adverse pregnancy outcomes. These researchers found an abundance of small HDL particles in the plasma of women with infants with lower birthweights, which they assume suggests a lack of HDL uptake by the placenta. It is unknown if HDL-C itself is mediating an effect or if another component of HDL is affecting metabolism, such HDL protein cargo.
The goal of the current studies was to determine if there was a direct correlation between fetal masses and plasma HDL/HDL-C concentrations in a mouse model of LBW and if the effect was mediated by a change in placental structure or function. These initial studies focused on nutrient transport as one of the key mediators of placenta-directed fetal growth. Understanding the relationship between maternal plasma HDL, apoA-I, or cholesterol concentrations and fetal growth could aid in devising simple strategies to improve fetal growth rates and/or in identifying a novel biomarker for infants with reduced growth in utero.
MATERIALS AND METHODS
Mice
Mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) at 12 wk of age. Mice lacked apoA-I (apoA-I−/−), had normal levels of apoA-I (wild-type mice; apoA-I+/+), or had overexpressed human apoA-I with mouse apoA-I present (apoA-Itg/tg). Upon arrival, mice were fed standard rodent chow (7012; Teklad, Madison, WI, USA) and placed in an environmentally controlled room with 12-h light/dark cycle. After acclimation (2 wk), 1 male and 3 female mice with the same genotype were placed together, and female mice were checked daily for postcopulatory plugs. Female mice were placed in a separate cage and were considered 0.5 d postconception (dpc) the morning a plug was detected. Female mice were studied at 14.5 dpc in the midlight cycle and after being unfed for 5–6 h unless stated otherwise. Samples were collected after anesthesia and exsanguination except where noted. Blood was collected from the vena cava. Plasma was separated and either assayed within 48 h or portioned in aliquots and frozen. All studies were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati.
Plasma lipid and sterol concentrations
Plasma cholesterol and triglyceride concentrations were determined enzymatically in individual mice. Fresh plasma was pooled from some of the female mice studied within 48 h of one another and with the same genetic background. Lipoproteins were separated by size using fast performance liquid chromatography (FPLC) with 2 Sephadex columns in tandem as described (33). Cholesterol was measured enzymatically in each fraction. Plasma progesterone levels were measured by ELISA after extraction with methylene chloride (Cayman Chemicals, Ann Arbor, MI, USA).
HDL proteomics
HDL isolation
Frozen plasma was thawed, and non–apoB lipid-carrying particles consisting primarily of HDL were isolated by precipitation of apoB-containing particles with heparin-MnCl2 (35). Lipid-bound proteins were separated from lipid-free proteins with calcium silicate hydrate (lipid removal agent) (36). Lipid-bound proteins (i.e., those present in 25 µl of treated plasma) were digested directly off the lipid removal agent with trypsin. Peptides were reduced using DTT (200 mM; 30 min at 37°C) and carbamidomethylated using iodoacetamide (800 mM, 30 min at room temperature). Peptides were lyophilized to dryness and stored at −20°C until analyzed by mass spectrometry.
MS analysis
Dried peptides were reconstituted in 35 µl of 0.1% formic acid in water, and 2 µl of sample was applied to an Acquity ultraperformance liquid chromatography C18 reverse-phase column (Waters, Milford, MA, USA) maintained at 60°C using an Infinity 1290 autosampler and HPLC (Agilent Technologies, Santa Clara, CA, USA). Peptides were eluted at 0.1 ml/min using a varying mobile phase gradient from 95% phase A (FA/H2O 0.1/99.9, v/v) to 32% phase B (FA/ACN 0.1/99.9 v/v) for 60 min followed by 32 to 50% B for 2 min. Column cleaning was performed by varying the mobile phase gradient to 90% B for 10 min, and the column was re-equilibrated at 95% A for 10 min. Peptides were introduced to the mass spectrometer using a Jet Stream source (Agilent Technologies) as previously described (37). Spectra were acquired using an iFunnel Q-TOF (Agilent Technologies) operating in positive ion mode. Precursors were limited to acquisition of 2+ and 3+ ions with a minimum threshold of 1500 cts. Each cycle acquired the 20 most intense precursors, which were fragmented with a variable collision energy dependent on the precursor mass-to-charge (m/z) ratio: collision energy = k* (m/z) + b, with a slope (k) of 3 and an offset (b) of −2 or 2 for 2+ and 3+ ions, respectively. MS/MS spectra were acquired until 45,000 total counts were collected or a maximum accumulation time of 0.33 s.
Proteomic data analysis
Peptide spectral data were entered into the Swiss-prot Protein Knowledgebase for Mus musculus (release 2011, 533,657 sequences) using the Mascot (2.2.07) search engine. An additional search was entered into the UniProtKB/Swiss-Prot Protein Knowledgebase (release February 2016; 550,552 sequences) for Homo sapiens (20,273 sequences) for spectral data obtained from apoA-I human transgenic mice to determine spectral counts of mouse and human apoA-I. Data were constrained to enzymatic digestion with trypsin with a maximum of 3 missed cleavage sites. Carbamidomethylation was set as a fixed modification and Met oxidation as a variable modification. Peptide and MS/MS mass tolerance were set to 35 PPM and ±0.6 Da, respectively. Scaffold (v.4.3.4) was used for MS/MS-based peptide validation using X! Tandem (2010.12.01.1). Proteins and peptides were constrained to have >99.9 and 95% identification probability, respectively. Additionally, proteins were only accepted if they contained a minimum of 3 unique peptides. Raw spectral counts were normalized to the maximum spectral count found within respective sample sets to adjust for differences in protein mass injected onto the MS.
Placental histology
Placentas were collected and placed in 4% paraformaldehyde. Fixed placentas were embedded in paraffin and cut into 5-µm sections. Sections were deparaffinized, rehydrated, and stained with hematoxalin and eosin to identify nuclei and cytosol, respectively. Slides were imaged at ×10, 20, and 40 magnification by a blinded reviewer.
Glucose tolerance
Animals were injected with glucose (2 mg/g i.p.) after 6 h unfed; female mice in this study ranged from 14.5 to 15.5 dpc. Blood was collected from the tail at 0, 15, 30, 60, 120, and 180 min after injection, and glucose was measured in triplicate with a glucometer using glucose strips (Abbott Laboratories, Chicago, IL, USA).
Western blots
Animals were unfed for 5–6 h. Tissues were removed rapidly and frozen in liquid nitrogen; some mice were injected with insulin (0.75 mU/g) 15 min before tissue collection, which would not affect whole tissue expression of proteins. Tissues were homogenized, and samples were tested with primary antibodies for glucose transporter (GLUT)1, GLUT3, sodium-coupled neutral amino acid transporter (SNAT)1, and SNAT2. Relative expression levels were assessed using β-actin as a loading control and using a phosphoimager. All antibodies used were the same as those previously described (38).
Glucose and amino acid transport
Fed pregnant female mice were anesthetized with continuous isoflurane in the early afternoon, and the jugular vein was isolated. A bolus containing both [3H]methylglucose and [14C]methylaminoisobutyric acid was injected into the jugular vein of each female as described (38); an average of ≈16 µCi of [3H] and ≈3 µCi of [14C] was injected in each mouse as a single bolus. After 5 min, blood was collected from the vena cava of the still-anesthetized female mouse. Fetal units (3–7/dam) were removed rapidly, and fetuses and placentas were isolated. Fetal units were collected and separated into the fetus and the placenta. Tissues were solubilized separately in BioSolve (Lexington, MA, USA). Radiolabel was measured in maternal plasma, and solubilized tissues were measured by β scintillation. The dpm from each fetus and each placenta were averaged with other fetuses or placentas of each dam. Data are presented as dpm per fetus, per placenta, and per fetal unit (fetus + placenta) as a percentage of the radiolabel injected.
Fatty acid composition
Whole fetuses and maternal plasma samples (100 µl) were analyzed. Samples were assayed as described previously (39). Briefly, samples were saponified, and fatty acids were converted to methyl esters prior to analysis by gas chromatography. Data are presented as a weight percentage of the total fatty acids for each sample. Fatty acids were summed together based on their double bonds: polyunsaturated fatty acid (PUFA) consisted of 18:2, 18:3, 20:3, 20:4, 22:4, 22:5, and 22:6 fatty acids; saturated fatty acid (SFA) consisted of 14:0, 16:0, and 18:0 fatty acids; and monounsaturated fatty acid (MUFA) consisted of 16:1 and 18:1 fatty acids. Because some samples had more unknown fatty acids than others, resulting in 85–100% of total fatty acids from the sum of 16:0, 16:1, 18:1, 18:2, 20:4, and 22:6, each total of PUFA, SFA, and MUFA was set to 100%, and the percentages of PUFA, SFA, and MUFA were calculated.
Statistics
Data are presented as means ± sem. When comparing mice of different genotypes, data were compared by 1-way ANOVA, followed by pairwise comparison with Holm-Sidak adjustments; if equivalence failed, Kruskal–Wallis 1-way ANOVA on ranks was used. In the case of fetal weights, which included several different studies, we estimated the overall effects of group (apoA-I−/− vs. apoA-I+/+ vs. apoA-Itg/tg) from all available studies by 2-factor (group and experiment) ANOVA, and, after confirming that group effects were homogeneous across experiments by the test of group-by-experiment interaction, we estimated the main effects of the group. Linear regression was used to determine the relationship of fetal weight (dependent) vs. plasma cholesterol level (independent), HDL proteins (dependent) vs. apoA-I levels (independent), and nutrient transport (dependent) vs. plasma cholesterol levels (independent). We did not account for multiple comparisons for the proteome because the likeliness of type I errors was less than that for type II errors and due to the rigor with which we verified the presence of specific proteins. Significance was determined as P < 0.05, and trends were determined as P = 0.05–0.159.
RESULTS
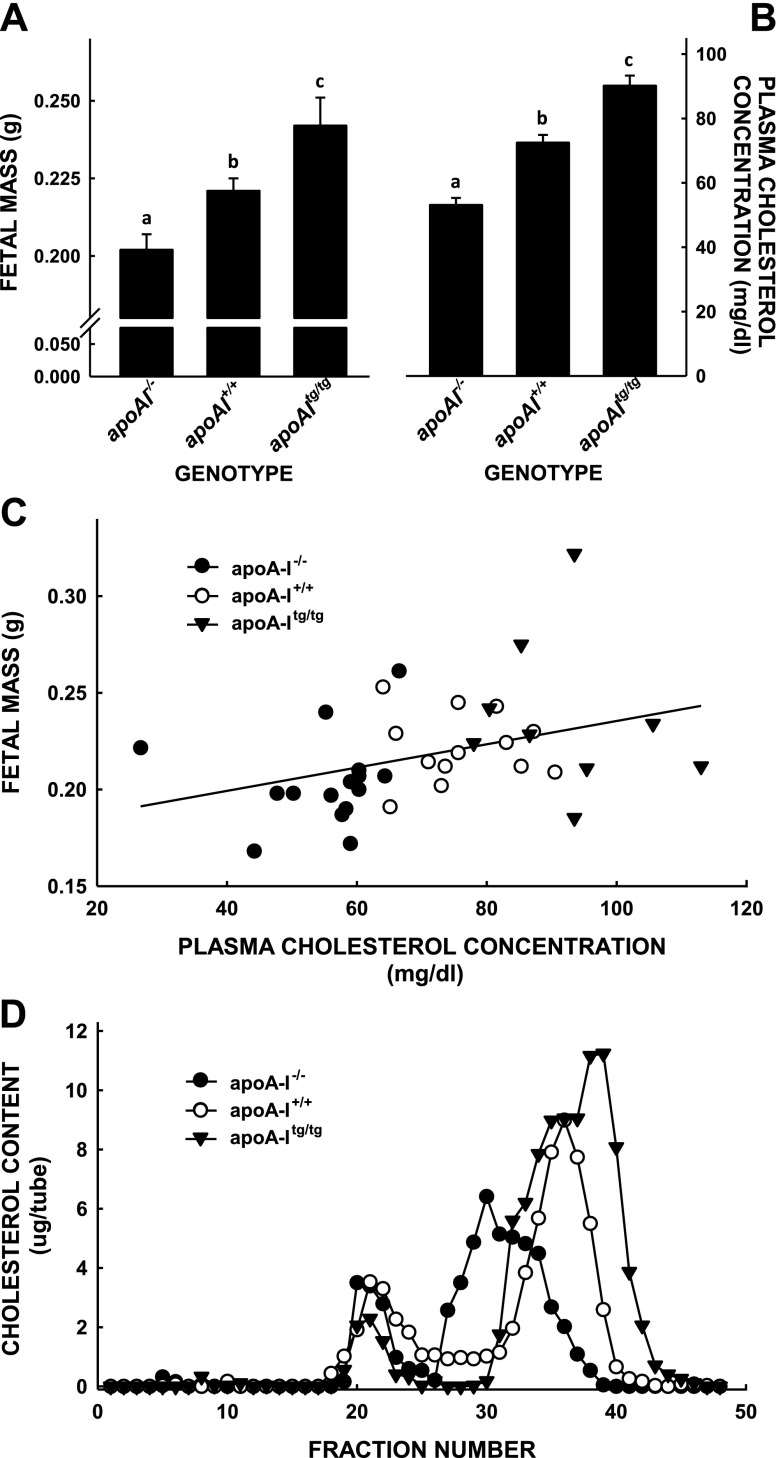
Similar to our previous study (33), there were significant differences in fetal masses when female mice had HDL-C concentrations varying from low in apoA-I−/− mice to high in apoA-Itg/tg (Fig. 1). Female mice with the lowest HDL-C concentrations that were lacking apoA-I had the smallest fetuses (0.202 ± 0.005 g), wild-type female mice with normal levels of HDL-C had the next largest fetuses (0.221 ± 0.004 g), and apoA-Itg/tg female mice with the greatest HDL-C levels had the largest fetuses (0.242 ± 0.009 g) (Fig. 1A). There was no difference in the number of pups per litter (6.0 ± 0.6, 6.0 ± 1.0, and 6.0 ± 2.5 fetuses per litter for apoA-I−/−, apoA-I+/+, and apoA-Itg/tg mice, respectively). The differences in fetal masses paralleled the differences in maternal cholesterol concentrations (53.1 ± 2.2 vs. 72.5 ± 2.9 vs. 90.2 ± 3.1 mg/dl, respectively) as an indication of HDL levels (Fig. 1B). Indeed, regression analysis revealed a direct, significant correlation between fetal mass and maternal plasma cholesterol concentration (Fig. 1C). As expected, differences in plasma cholesterol concentrations were due primarily to changes in HDL (Fig. 1D). We summed the amount of cholesterol in FPLC fractions containing HDL presented in Fig. 1C and measured 43.2, 50.3, and 86.6 mg/dl in the plasma of knockout, wild-type, and transgenic mice, respectively. There were bigger differences in HDL-C concentrations between pregnant mice without apoA-I or wild-type mice at 13 dpc (33). Compared with pregnant wild-type mice, the size of the HDL particles of apoA-I−/− pregnant mice appeared to be larger, and HDL particles of apoA-Itg/tg pregnant mice appeared to be smaller.
Figure 1.
Fetal masses and total and lipoprotein cholesterol concentrations in apoA-I−/−, apoA-I+/+, and apoA-Itg/tg pregnant mice. Mice with different expression levels of apoA-I were mated with genetically similar male mice and studied 14.5 dpc, assuming the presence of a postcopulatory plug denoted 0.5 dpc. Masses of fetal mice (A) and maternal plasma cholesterol concentrations (B) were combined from several different studies and are presented as means ± sem [n = 14–20 (A); n = 12–20 (B)]. Different letters denote significant differences between groups. C) Averaged fetal masses from each litter plotted as a function of maternal plasma cholesterol concentration. There was a significant positive association between fetal mass and plasma cholesterol (P = 0.025; R2 = 0.136). D) Plasma from a subset of these genetically similar pregnant mice was pooled and separated into lipoproteins by FPLC within 1 d of collection, and cholesterol was measured in each fraction.
Next, we characterized the HDL proteomic fingerprint to determine if there were compositional differences between the experimental mouse groups (Table 1). When comparing samples of pregnant female mice with different levels of apoA-I, besides apoA-I, which was genetically altered, there were 5 complement or complement factor proteins (complements C3, C4-B, and C5 and complement factors B and H), 2 apolipoproteins (E and A-IV), and 3 proteins with metabolic functions (carboxypeptidase N subunit, vitronectin, and antithrombin-III) that had a significant group effect. Most of these proteins, as well as 5 additional proteins (Table 1), were correlated to apoA-I.
TABLE 1.
Representative proteins associated with HDL in pregnant mice with different levels of apoA-I
| Protein | Peptide counta | ||
|---|---|---|---|
| apoA-I−/− | apoA-I+/+ | apoA-Itg/tg | |
| Complement C3 | 105.6 ± 5.7 | 82.5 ± 5.4 | 69.2 ± 2.9bc |
| Inter-α-trypsin inhibitor | 65.4 ± 5.5 | 61.5 ± 1.5 | 49.7 ± 3.6c |
| Serotransferin | 41.9 ± 3.1 | 45.3 ± 5.8 | 24.2 ± 4.1c |
| α-2-HS-glycoprotein | 27.6 ± 1.4 | 24.3 ± 1.7 | 26.5 ± 1.6 |
| Kininogen-1 | 25.5 ± 1.1 | 25.5 ± 2.0 | 19.3 ± 1.3 |
| Apolipoprotein A-I | 0.0 ± 0.0 | 45.2 ± 2.6 | 296.0 ± 24.0b |
| Pregnancy zone protein | 15.3 ± 1.2 | 9.1 ± 1.2 | 10.6 ± 1.9 |
| Hemopexin | 28.4 ± 1.1 | 25.1 ± 2.5 | 21.4 ± 2.6c |
| Murinoglobulin | 15.3 ± 2.3 | 14.4 ± 0.4 | 11.9 ± 0.9 |
| Vitamin D–binding protein | 24.8 ± 2.6 | 23.9 ± 2.0 | 19.4 ± 2.0 |
| Fibrinogen α chain | 18.2 ± 1.1 | 15.2 ± 4.4 | 12.6 ± 1.7 |
| Apolipoprotein A-IV | 13.7 ± 1.6 | 15.2 ± 1.4 | 21.0 ± 1.1c |
| Gelsolin | 14.1 ± 5.4 | 12.9 ± 1.9 | 16.9 ± 2.3 |
| Plasminogen | 14.9 ± 3.1 | 14.1 ± 2.2 | 10.5 ± 0.8 |
| α-1-B glycoprotein | 16.8 ± 2.6 | 19.0 ± 1.0 | 13.6 ± 1.7 |
| Prothrombin | 20.1 ± 1.6 | 17.9 ± 0.4 | 14.3 ± 2.3c |
| Afamin | 7.9 ± 1.0 | 12.5 ± 2.3 | 10.9 ± 1.0 |
| Complement factor B | 17.3 ± 0.6 | 14.1 ± 0.7 | 10.9 ± 1.2bc |
| Fibronectin | 10.5 ± 3.0 | 3.0 ± 0.4 | 6.5 ± 3.1 |
| Leukemia inhibitory factor receptor | 21.9 ± 4.8 | 24.3 ± 1.0 | 14.3 ± 0.7 |
| Clusterin | 13.7 ± 1.7 | 8.8 ± 1.7 | 8.1 ± 1.1 |
| Complement factor H | 10.7 ± 1.3 | 7.2 ± 1.0 | 4.8 ± 0.4bc |
| Complement C4-B | 11.4 ± 0.7 | 8.0 ± 1.1 | 6.1 ± 1.1bc |
| Fibrinogen β chain | 11.7 ± 1.8 | 9.1 ± 0.1 | 8.8 ± 0.3 |
| Paroxonase | 8.5 ± 0.8 | 11.8 ± 0.4 | 7.1 ± 2.0 |
| Ceruloplasmin | 13.0 ± 1.9 | 15.6 ± 2.4 | 11.6 ± 0.3 |
| Inter-α-trypsin inhibitor heavy chain H2 | 8.3 ± 1.3 | 6.5 ± 2.1 | 7.4 ± 1.4 |
| Apolipoprotein E | 18.2 ± 0.9 | 10.6 ± 1.7 | 1.7 ± 0.7bc |
| Corticosteroid-binging globulin | 8.8 ± 0.9 | 10.6 ± 2.1 | 11.9 ± 1.2 |
| Fibrinogen γ chain | 9.3 ± 0.7 | 5.7 ± 1.8 | 5.2 ± 0.6 |
| Complement factor I | 5.4 ± 0.4 | 5.3 ± 1.4 | 6.1 ± 0.7 |
| Hemoglobin subunit β-1 | 5.0 ± 2.1 | 4.6 ± 1.3 | 10.7 ± 0.7c |
| α-1 antitrypsin 1 | 6.8 ± 1.1 | 8.0 ± 0.7 | 5.8 ± 0.7 |
| Carboxypeptidase N subunit | 9.5 ± 1.3 | 8.7 ± 1.0 | 4.4 ± 0.3bc |
| Hemoglobin subunit α | 6.4 ± 0.6 | 6.1 ± 2.7 | 3.4 ± 0.9 |
| Inter-α-trypsin inhibitor heavy chain H1 | 7.1 ± 1.1 | 5.7 ± 0.7 | 4.4 ± 0.8 |
| Fetuin-B | 5.8 ± 1.2 | 3.8 ± 0.8 | 3.7 ± 0.6 |
| Inhibitor of carbonic anhydrase | 4.1 ± 0.9 | 3.0 ± 1.0 | 5.8 ± 0.6 |
| Carboxyesterase | 3.7 ± 0.5 | 1.9 ± 0.4 | 5.4 ± 0.3 |
| β-2-glcoprotein 1 | 3.7 ± 0.5 | 3.4 ± 0.7 | 2.0 ± 0.6 |
| Inter-α-trypsin inhibitor heavy chain H3 | 5.1 ± 1.7 | 3.4 ± 0.7 | 2.7 ± 0.4 |
| Vitronectin | 5.9 ± 0.7 | 3.8 ± 1.0 | 1.0 ± 0.1bc |
| Complement C5 | 4.5 ± 0.8 | 4.2 ± 1.3 | 1.4 ± 0.3bc |
| Antithrombin-III | 3.4 ± 0.3 | 2.3 ± 0.7 | 0.7 ± 0.7bc |
Data are presented as means ± sem (n = 3). aNumber of peptides identified by mass spec fragmentation per protein. bUnadjusted significant difference between apoA-I−/−/apoA-I+/+/apoA-Itg/tg. cUnadjusted significant correlation with apoA-I (P < 0.05).
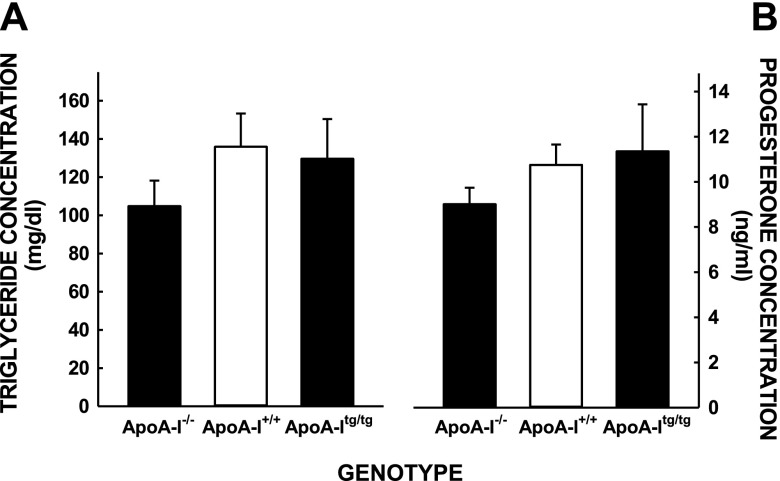
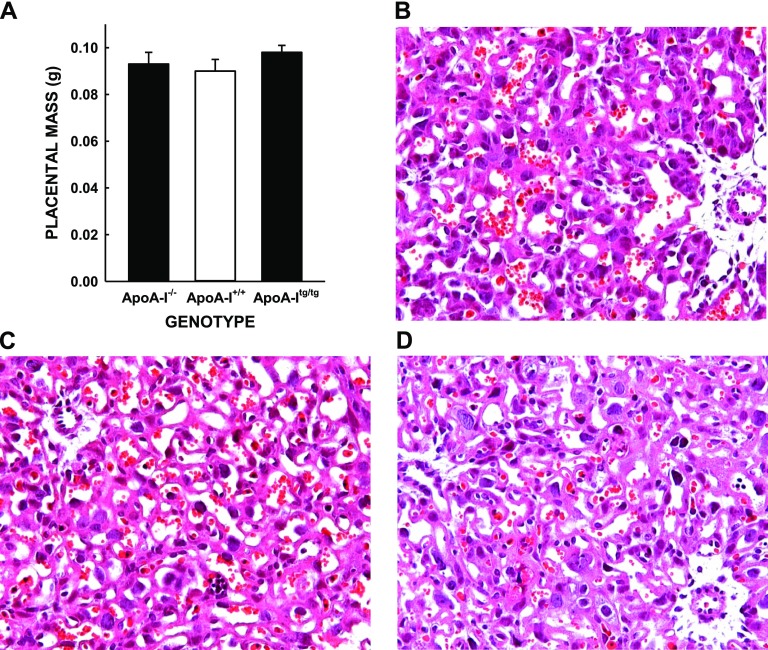
Various maternal plasma factors previously shown to be associated with fetal growth rates were also measured in the mice, including plasma triglyceride and progesterone levels (20, 40, 41). No differences were noted between plasma triglyceride or progesterone levels between the different groups of female mice (Fig. 2). Although we know that the differences in fetal masses were not due to a change in apoA-I expression levels in the fetus itself (33), placental structure and function were examined because the placenta is the gateway by which maternal nutrients are made available to the fetus. The differences in fetal masses were not due to changes in placental masses because those were similar in all female mice (Fig. 3A). The differences were also not due to a change in placental structure because these were also similar in female mice of all groups, as noted by hematoxylin and eosin staining of placentas (magnification, ×40) (Fig. 3B–D).
Figure 2.
Maternal plasma triglyceride (A) and progesterone (B) levels in mice with different levels of apoA-I. Data are presented from some of the studies used to obtain fetal masses as means ± sem [n = 3–5 (A); n = 5–15 (B)].
Figure 3.
Placental masses, histology, and cholesterol concentrations of apoA-I−/−, apoA-I+/+, and apoA-Itg/tg pregnant mice. A) Placentas were collected from a subset of mice and weighed (n = 9–10). Data are presented as means ± sem. Histology (hematoxylin and eosin) of representative placentas from a total of 11 placentas apoA-I−/−(B), apoA-I+/+(C), and apoA-Itg/tg (D) pregnant mice are shown.
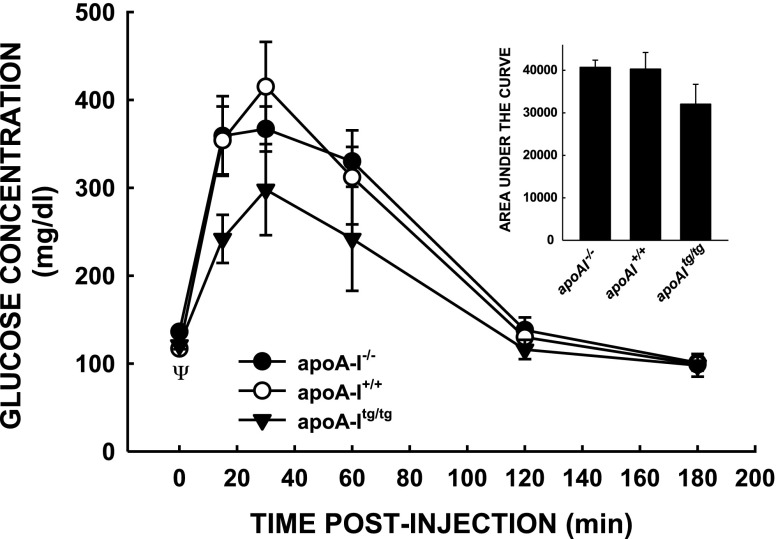
We next examined known promoters of fetal growth. One of the major promoters of fetal growth is glucose. As an estimation of insulin-induced glucose uptake by maternal and the placenta plus glucose production by the maternal liver, we performed a glucose tolerance test on the mice. Although there was no significant difference in glucose disposal after intraperitoneal glucose, apoA-Itg/tg female mice appeared to have enhanced glucose tolerance to an exogenous glucose load compared with female mice with lower HDL-C levels (apoA-I−/−, apoA-I+/+) (Fig. 4), although the effects were not significant (Fig. 4, inset). Interestingly, there was a between-group effect on fasting glucose concentrations (P = 0.049) with knockout mice having the greatest glucose concentration (136 ± 4 mg/dl), which was not statically different from glucose concentrations in wild-type (117 ± 1 mg/dl) or transgenic (121 ± 5 mg/dl) mice after multiple comparison procedures were performed.
Figure 4.
Glucose tolerance tests of apoA-I−/−, apoA-I+/+, and apoA-Itg/tg pregnant mice. Mice at 14.5 and 15.5 dpc were injected intraperitoneally with glucose after being unfed for 5–6 h, and blood was collected just before and for 3 h after the glucose injection. Data are presented as means ± sem (n = 3–5). Areas under the curves are presented for each genotype in the inset.
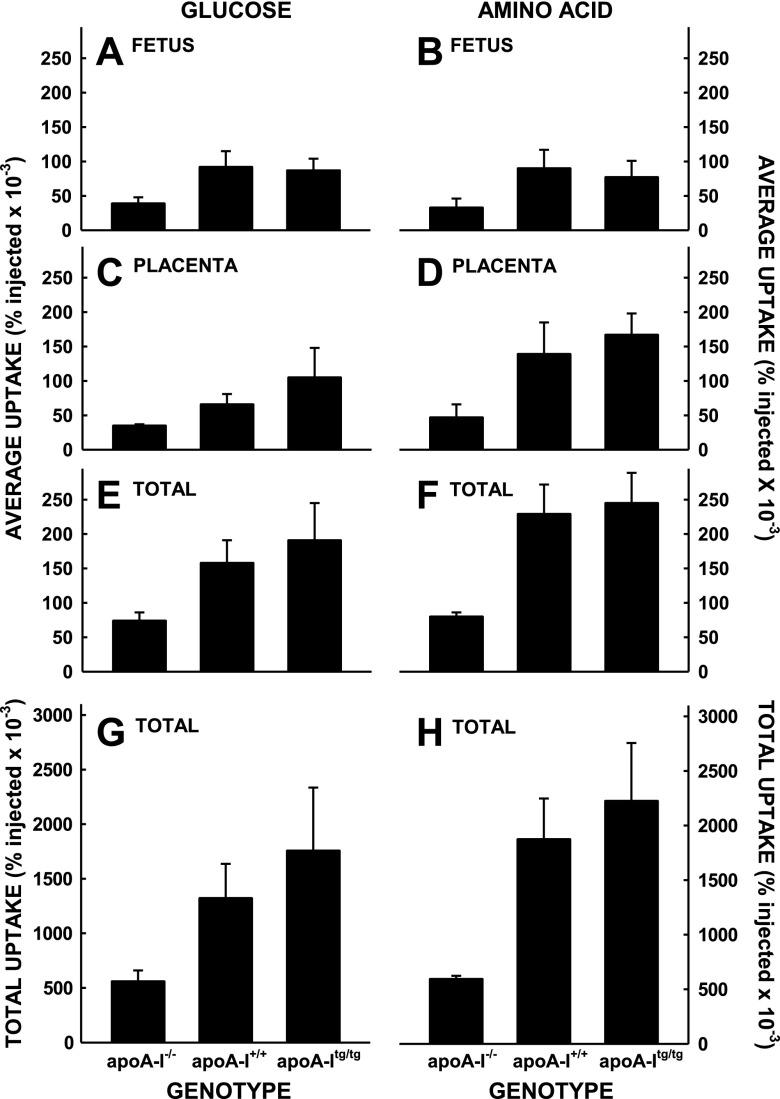
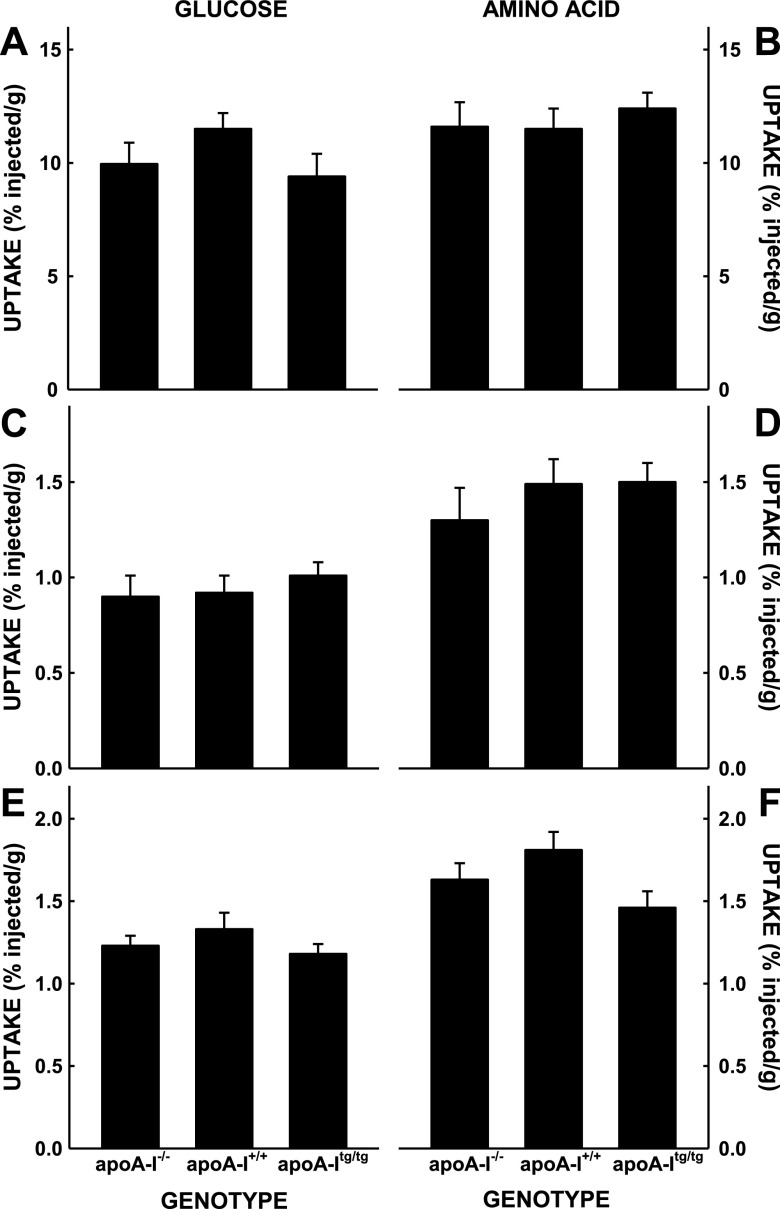
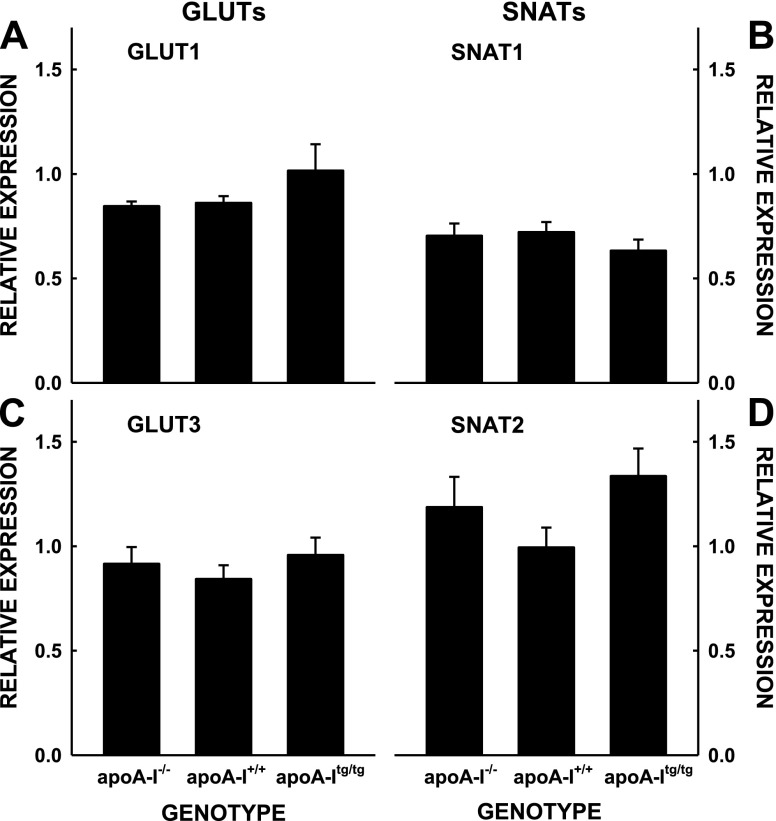
To directly test the transport of nutrients by the fetus, we measured the transport of maternal glucose and amino acids to the fetus and placenta. Although it appeared that there was a trend toward greater uptake and transport of maternal glucose (Fig. 5A) and amino acids (Fig. 5B) in the mice with the most vs. the least levels of plasma cholesterol, uptake rates were not significantly different. Additionally, there was no correlation between nutrient uptake and plasma cholesterol. The lack of differences was not due to changes in glucose or amino acid uptake in the whole maternal body because the percentage of radiolabel remaining in the plasma 5 min after injection was similar in all mice for each isotope (Table 2) and because the uptake of radiolabels in the maternal liver, adipose tissue, and skeletal muscle were similar (Fig. 6). When the fetus and placenta for each fetal unit were summed, there were trends for a group effect for glucose (P = 0.099) and amino acids (P = 0.159). When the number of fetuses per dam were taken into account, there was still a trend (P = 0.103) for a group effect of glucose. There also was no difference in expression of nutrient transporters among the groups with different levels of apoA-I (Fig. 7); the levels of GLUTs and SNAT for unfed mice injected with insulin or not injected with insulin were averaged together because insulin will not change protein expression in 15 min.
Figure 5.
Uptake of glucose and amino acids by fetuses in apoA-I−/−, apoA-I+/+, and apoA-Itg/tg pregnant mice. Mice at 14.5 dpc were injected with radiolabeled markers for glucose (A, C, E, G) and amino acids (B, D, F, H), and the amounts of radiolabel present in the fetuses 5 min later were measured. Data are presented as percent present in the whole fetus compared with that injected and as means ± sem (n = 4–8).
TABLE 2.
Percent dpm remaining in the plasma in mice with injected with radiolabeled glucose and amino acids and with different levels of apoA-I
| Dpm in plasmaa | Glucose (%) | Amino acid (%) |
|---|---|---|
| apoA-I−/− | 12.1 ± 1.6 | 7.9 ± 1.2 |
| poA-I+/+ | 16.3 ± 1.1 | 11.9 ± 1.0 |
| apoA-Itg/tg | 15.5 ± 1.0 | 10.2 ± 1.3 |
Data are presented as mean ± sem (n = 4–8). aPercentage dpm in plasma 5 min after injection.
Figure 6.
Uptake of glucose (A, C, E) and amino acids (B, D, F) by maternal tissues in apoA-I−/−, apoA-I+/+, and apoA-Itg/tg pregnant mice; the tissues are from the same mice described in Fig. 5. Data are presented as percent present per grams tissue and as means ± sem (n = 4–8).
Figure 7.
Relative expression levels of GLUT1 and GLUT3 (A, C) and SNAT1 and SNAT2 (B, D) of placentas of apoA-I−/−, apoA-I+/+, and apoA-Itg/tg pregnant mice. Densities are presented relative to β-actin and as means ± sem (n = 7–8).
Fatty acid transport was assessed by measuring the relative percentages of essential PUFAs (linoleic acid/18:2 + linolenic acid/18:3 + eicosatrienoic acid/20:3 + arachidonic acid/20:4 + docosapentaenoic acid/22:4 + docosapentaenoic acid/22:5 + docosahexaenoic acid/22:6), SFAs (myristic acid/14:0 + palmitic acid/16:0 + stearic acid/18:0), and MUFAs (palmitoleic acid/16:1 + oleic acid/18:1) measured in the fetuses and maternal circulations (42). Although there were greater percentages of PUFA in the fetuses of the transgenic mice, suggesting greater transport, there were greater percentages of PUFA in the maternal circulation as well, which was the source of the fatty acids (Table 3).
TABLE 3.
Fatty acid composition in fetuses and maternal plasma
| Tissue | PUFA (%) | SFA (%) | MUFA (%) |
|---|---|---|---|
| Fetus | |||
| apoA-I−/− | 24.2 ± 0.5a | 39.2 ± 0.5a | 21.1 ± 0.2a |
| apoA-I+/+ | 27.7 ± 0.1b | 40.0 ± 0.3a | 18.8 ± 0.1b |
| apoA-Itg/tg | 29.8 ± 0.5c | 40.3 ± 0.3a | 17.1 ± 0.5c |
| Maternal plasma | |||
| apoA-I−/− | 40.9 ± 1.6a | 46.2 ± 2.7a | 8.2 ± 0.6a |
| apoA-I+/+ | 52.1 ± 1.0b | 35.9 ± 1.5b | 10.0 ± 0.5a |
| apoA-Itg/tg | 52.7 ± 2.1b | 37.8 ± 2.1b | 9.2 ± 0.2a |
Letters (a, b) signify percentage differences for PUFA, SFA, or MUFA of fetuses or maternal plasma. Data are presented as means ± sem (n = 3–4 for fetuses; n = 3–5 for maternal plasma). P < 0.05.
DISCUSSION
Birthweight can be predictive of the short- and long-term health of an infant, with infants of lower birthweights at greater risk for adverse health outcomes. There is much interest in finding biomarkers or pathways to target in women at risk of having small infants so that physicians will know which female patients to target for observation or intervention. It seems plausible that maternal cholesterol could be indicative of fetal growth and thereby birthweight because of the integral role cholesterol has in development: cholesterol is a precursor for steroid hormones, is integral for every cell membrane, and can mediate metabolic functions. Indeed, some previous studies have shown a direct relationship between maternal cholesterol and infant birthweight (20,–26).
The series of studies presented here were designed to expand upon previous work in which mice with low HDL-C, due to genetic deletion of apoA-I, had smaller fetuses. Because the reduction in fetal mass was not due to a change in apoA-I levels in the fetus itself (33), the effect had to be due to a factor in the maternal circulation that changed placental function or was transported across the placenta and affected the fetus directly. Because there is a plethora of data demonstrating the key role that the placenta plays in regulating fetal growth rate, either through a change in size, structure, or function, the current studies focused on the placenta.
HDL has diverse functions, including lipid transport, oxidation, inflammation, hemostasis, immunity, and energy balance, which vary from individual to individual (43,–45). Thus, although HDL function is often thought to be inversely associated with heart disease, it also can play a role in insulin-resistance states, inflammatory states, the acute phase reaction, and infectious diseases (46). All of these functions can impinge on pregnancy outcomes. HDL function is related to the cargo carried by HDL, specifically the bioactive proteins or lipids. HDL particles can be composed from a palate of over 95 distinct proteins (44). The primary protein carried by HDL is apoA-I. Indeed, a direct relationship between plasma apoA-I levels and birthweight has been demonstrated, even in the absence of an association between maternal cholesterol and birthweight (47). HDL also carries paroxonase. As with apoA-I, lower paroxonase activity has been associated with adverse pregnancy outcomes (48). HDL also transports cholesterol between tissues and can result in removal of cholesterol from membranes or in deposition of cholesterol into cells leading to changes in membrane-based signaling [reviewed by Fielding and Fielding (49)]. Thus, HDL could have an impact on fetal growth by affecting placental function through altered HDL-protein cargo or changes in cellular sterol content.
Not surprisingly, HDL particles of pregnant knockout, wild-type, and transgenic mice in the current study had different protein cargo and varied in size. Our data are consistent with a recent study in which HDL was separated into different subpopulations of those with only apoA-I or those with apoA-I and apoA-II (50). We found that the proteins present in the 2 subpopulations of HDL varied significantly. The authors hypothesize that apoA-I, the primary protein on HDL, forms a scaffold that promotes the interaction of other proteins with various functions. Thus, a change in the amount of apoA-I would affect the proteins carried by HDL and thereby the function of HDL. Indeed, in our pregnant mice, the HDL particles lacking apoA-I were larger and contained greater amounts of other apolipoproteins (apoE and apoA-IV) and complement proteins. Because there was less plasma cholesterol, there may have been fewer particles as well. Thus, assuming there is a relationship between fetal growth and HDL composition and concentration, the lower fetal growth rates could have been the result of a change in protein cargo on HDL because complement proteins are associated with adverse pregnancy outcomes (51) or a reduction in HDL uptake by the placenta due to fewer circulating particles.
Because the placenta is the gatekeeper of nutrients required for fetal growth, the structure and function of the placenta was examined next. There was no difference in placental masses or structures in the mice with different apoA-I genotypes. In addition, there was no significant difference in the uptake of glucose or amino acid by the fetus or the placenta of mice with increasing levels of apoA-I. However, when the uptake in the whole fetal unit was calculated, there was a trend for a group interaction, with rates greater in mice with apoA-I and greater plasma cholesterol levels. In addition, when results of mice with apoA-I (wild-type and transgenic) were grouped and compared with mice without apoA-I (knockout), there was a significant difference in transport for glucose (P = 0.04) and amino acids (P = 0.006) in the whole fetal unit.
The results were somewhat surprising because a change in fetal growth is often related to nutrient transport, and therefore a more substantial difference was expected. Importantly, nutrient uptake rates in maternal tissues also were similar in all mice; these results contrast those of the glucose tolerance tests. The difference could be due to the fact that the transport study was done at a time of lower insulin levels, whereas the tolerance tests were performed after a glucose injection and more elevated insulin levels; even though mice were not unfed, mice eat mostly during the dark phase, suggesting low insulin levels at the time of the transport study. Thus, uptake rates might have been more different after insulin injection. Indeed, although some the glucose receptors are insulin independent, such as GLUT1, more recent studies show that glucose transport in the placenta can also be insulin dependent due to detection of more insulin-dependent transporters (52). Amino acid transporters can also be regulated by insulin (53). One could argue that the lack of differences in expression levels of the glucose and amino acid transporter levels in the whole placenta supports a lack of difference in transport rates. We might have seen a difference had just the labyrinth been used, where meaningful transport occurred, or if membranes had been isolated from the cytosol because transporter activity takes place in the membranes. However, whole placentas were used due to the gestational age studied.
Studies have suggested that increased fatty acid transport can also affect fetal growth (54). Although fatty acid transport was not directly measured, we used the relative amounts of essential fatty acids in the fetus and maternal plasma as an indication of transport because essential fatty acids are not synthesized in vivo (55). Fatty acid transport across the placenta, specifically transporter-mediated PUFA, did not appear to be decreased in the mice lacking apoA-I based on the ratio of PUFA in the maternal vs. fetal plasma.
PUFA concentrations were less in both the maternal and fetal circulations of the mice with low HDL levels, however. Because these fatty acids can mediate metabolism and inflammation, as shown in a recent clinical trial (56), we cannot rule out an effect of the fatty acids on placental function or fetal growth. In fact, intervention with certain PUFA can change the proteome of HDL to one that is more anti-inflammatory (57).
The other potential role of maternal cholesterol is a direct role on the fetus. Currently, there is no consensus as to how much maternally derived cholesterol is transported across the placenta (58). However, due to the relatively low fetal sterol synthesis rates early in gestation (59) and the direct relationship between maternal and fetal cholesterol in early, but not late, gestation (60), it is likely that maternally derived cholesterol enters the fetal circulation, where it could directly affect fetal metabolism, especially in the second trimester when there is a direct relationship between maternal and fetal cholesterol levels (60).
In summary, midgestation fetal size varied based on apoA-I gene dosage and plasma cholesterol concentrations. We focused on a potential effect of HDL on the transport of key nutrients as one of the primary routes by which fetal growth rates are affected. Although we did not find a definitive change in nutrient transport with different gene dosages, differences in nutrient transport trended to be increased in mice with the greatest cholesterol and apoA-I levels. Importantly, significant differences did occur when mice were grouped into those with or without apoA-I. Because there is growing evidence that HDL subpopulations exist with a variety of functions, future studies should address whether there is a relationship between HDL and fetal growth or placental function, vs. a correlation, and how HDL (and not progesterone or triglyceride concentrations) affects metabolism. It would also be necessary to determine if the distribution of HDL subpopulations differs between women with small newborn infants or those with preterm births. Using these results, we would hope to design a novel therapeutic target for women at risk of having LBW babies. Results from these types of studies might be of greatest benefit for individuals in resource-poor settings because women in these settings often have lower plasma cholesterol levels (61,–63) and a higher prevalence of LBW infants.
ACKNOWLEDGMENTS
The authors thank Dr. Debi Swertfeger (Cincinnati Children’s Hospital Medical Center) and Dr. Philip Howells (University of Cincinnati) for critical review of the manuscript, and Kathryn Owens (Cincinnati Children’s Hospital Medical Center) for technical assistance. This work was supported by the Bill and Melinda Gates Foundation (OPP1110668 to L.A.W.). The University of Cincinnati Mouse Metabolic Phenotype Center measured the fatty acid composition of the samples (U2C DK059630). The authors declare no conflicts of interest.
Glossary
- apo
apolipoprotein
- FPLC
fast performance liquid chromatography
- GLUT
glucose transporter
- HDL-C
HDL-cholesterol
- LBW
low birthweight
- MUFA
monounsaturated fatty acid
- PUFA
polyunsaturated fatty acid
- SFA
saturated fatty acid
- SNAT
sodium-coupled neutral amino acid transporter
AUTHOR CONTRIBUTIONS
S. L. Rebholz, J. T. Melchior, W. S. Davidson, H. N. Jones, and L. A. Woollett performed the research and analyzed the data; J. A. Welge performed statistics; J. T. Melchior, W. S. Davidson, H. N. Jones, A. M. Prentice, S. E. Moore, and L. A. Woollett reviewed the data; S. L. Rebholz, J. T. Melchior, W. S. Davidson, H. N. Jones, J. A. Welge, A. M. Prentice, S. E. Moore, and L. A. Woollett assisted in writing the manuscript.
REFERENCES
- 1.Hviid A., Melbye M. (2007) The impact of birth weight on infectious disease hospitalization in childhood. Am. J. Epidemiol. 165, 756–761 [DOI] [PubMed] [Google Scholar]
- 2.Tofail F., Hamadani J. D., Ahmed A. Z., Mehrin F., Hakim M., Huda S. N. (2012) The mental development and behavior of low-birth-weight Bangladeshi infants from an urban low-income community. Eur. J. Clin. Nutr. 66, 237–243 [DOI] [PubMed] [Google Scholar]
- 3.Stein R. E., Siegel M. J., Bauman L. J. (2006) Are children of moderately low birth weight at increased risk for poor health? A new look at an old question. Pediatrics 118, 217–223 [DOI] [PubMed] [Google Scholar]
- 4.Raqib R., Alam D. S., Sarker P., Ahmad S. M., Ara G., Yunus M., Moore S. E., Fuchs G. (2007) Low birth weight is associated with altered immune function in rural Bangladeshi children: a birth cohort study. Am. J. Clin. Nutr. 85, 845–852 [DOI] [PubMed] [Google Scholar]
- 5.McCormick M. C. (1985) The contribution of low birth weight to infant mortality and childhood morbidity. N. Engl. J. Med. 312, 82–90 [DOI] [PubMed] [Google Scholar]
- 6.Barker D. J. (2005) The developmental origins of insulin resistance. Horm. Res. 64(Suppl 3), 2–7 [DOI] [PubMed] [Google Scholar]
- 7.Prentice A. M., Moore S. E. (2005) Early programming of adult diseases in resource poor countries. Arch. Dis. Child. 90, 429–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. (2014) Global Nutrition Targets 2025: Low birth weight policy brief. World Health Organization. Accessed September 22, 2017, at: www.who.int/nutrition/publications/globaltarget2025_policybrief_lbw/en/
- 9.Martin J. A., Hamilton B. E., Osterman M. J. K., Curtin S. C., Mathews T. J. (2015) Births: final data for 2013. In National Vital Statistics Reports, Vol. 64, pp. 1–65, National Center for Health Statistics, Hyattsville, MD, USA: [PubMed] [Google Scholar]
- 10.Whincup P. H., Kaye S. J., Owen C. G., Huxley R., Cook D. G., Anazawa S., Barrett-Connor E., Bhargava S. K., Birgisdottir B. E., Carlsson S., de Rooij S. R., Dyck R. F., Eriksson J. G., Falkner B., Fall C., Forsén T., Grill V., Gudnason V., Hulman S., Hyppönen E., Jeffreys M., Lawlor D. A., Leon D. A., Minami J., Mishra G., Osmond C., Power C., Rich-Edwards J. W., Roseboom T. J., Sachdev H. S., Syddall H., Thorsdottir I., Vanhala M., Wadsworth M., Yarbrough D. E. (2008) Birth weight and risk of type 2 diabetes: a systematic review. JAMA 300, 2886–2897 [DOI] [PubMed] [Google Scholar]
- 11.Rich-Edwards J. W., Stampfer M. J., Manson J. E., Rosner B., Hankinson S. E., Colditz G. A., Willett W. C., Hennekens C. H. (1997) Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. BMJ 315, 396–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barker D. J., Osmond C., Forsén T. J., Thornburg K. L., Kajantie E., Eriksson J. G. (2013) Foetal and childhood growth and asthma in adult life. Acta Paediatr. 102, 732–738 [DOI] [PubMed] [Google Scholar]
- 13.Barker D. J. (2006) Adult consequences of fetal growth restriction. Clin. Obstet. Gynecol. 49, 270–283 [DOI] [PubMed] [Google Scholar]
- 14.Kramer M. S. (2003) The epidemiology of adverse pregnancy outcomes: an overview. J. Nutr. 133(5Suppl 2), 1592S–1596S [DOI] [PubMed] [Google Scholar]
- 15.Valero De Bernabé J., Soriano T., Albaladejo R., Juarranz M., Calle M. E., Martínez D., Domínguez-Rojas V. (2004) Risk factors for low birth weight: a review. Eur. J. Obstet. Gynecol. Reprod. Biol. 116, 3–15 [DOI] [PubMed] [Google Scholar]
- 16.Lager S., Powell T. L. (2012) Regulation of nutrient transport across the placenta. J. Pregnancy 2012, 179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brett K. E., Ferraro Z. M., Yockell-Lelievre J., Gruslin A., Adamo K. B. (2014) Maternal-fetal nutrient transport in pregnancy pathologies: the role of the placenta. Int. J. Mol. Sci. 15, 16153–16185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero R., Dey S. K., Fisher S. J. (2014) Preterm labor: one syndrome, many causes. Science 345, 760–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan T. K. (2016) Role of the placenta in preterm birth: a review. Am. J. Perinatol. 33, 258–266 [DOI] [PubMed] [Google Scholar]
- 20.Mudd L. M., Holzman C. B., Evans R. W. (2015) Maternal mid-pregnancy lipids and birthweight. Acta Obstet. Gynecol. Scand. 94, 852–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulkarni S. R., Kumaran K., Rao S. R., Chougule S. D., Deokar T. M., Bhalerao A. J., Solat V. A., Bhat D. S., Fall C. H., Yajnik C. S. (2013) Maternal lipids are as important as glucose for fetal growth: findings from the Pune Maternal Nutrition Study. Diabetes Care 36, 2706–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sattar N., Greer I. A., Galloway P. J., Packard C. J., Shepherd J., Kelly T., Mathers A. (1999) Lipid and lipoprotein concentrations in pregnancies complicated by intrauterine growth restriction. J. Clin. Endocrinol. Metab. 84, 128–130 [DOI] [PubMed] [Google Scholar]
- 23.Edison R. J., Berg K., Remaley A., Kelley R., Rotimi C., Stevenson R. E., Muenke M. (2007) Adverse birth outcome among mothers with low serum cholesterol. Pediatrics 120, 723–733 [DOI] [PubMed] [Google Scholar]
- 24.Wadsack C., Tabano S., Maier A., Hiden U., Alvino G., Cozzi V., Hüttinger M., Schneider W. J., Lang U., Cetin I., Desoye G. (2007) Intrauterine growth restriction is associated with alterations in placental lipoprotein receptors and maternal lipoprotein composition. Am. J. Physiol. Endocrinol. Metab. 292, E476–E484 [DOI] [PubMed] [Google Scholar]
- 25.Boersma E. R. (1980) Serum lipids in maternal/cord blood pairs from normal and low birthweight infants in Dar es Salaam, Tanzania. Acta Paediatr. Scand. 69, 747–751 [DOI] [PubMed] [Google Scholar]
- 26.Pecks U., Brieger M., Schiessl B., Bauerschlag D. O., Piroth D., Bruno B., Fitzner C., Orlikowsky T., Maass N., Rath W. (2012) Maternal and fetal cord blood lipids in intrauterine growth restriction. J. Perinat. Med. 40, 287–296 [DOI] [PubMed] [Google Scholar]
- 27.Mitra S., Misra S., Nayak P. K., Sahoo J. P. (2012) Effect of maternal anthropometry and metabolic parameters on fetal growth. Indian J. Endocrinol. Metab. 16, 754–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misra V. K., Trudeau S., Perni U. (2011) Maternal serum lipids during pregnancy and infant birth weight: the influence of prepregnancy BMI. Obesity (Silver Spring) 19, 1476–1481 [DOI] [PubMed] [Google Scholar]
- 29.Clausen T., Burski T. K., Øyen N., Godang K., Bollerslev J., Henriksen T. (2005) Maternal anthropometric and metabolic factors in the first half of pregnancy and risk of neonatal macrosomia in term pregnancies. A prospective study. Eur. J. Endocrinol. 153, 887–894 [DOI] [PubMed] [Google Scholar]
- 30.Friis C. M., Qvigstad E., Paasche Roland M. C., Godang K., Voldner N., Bollerslev J., Henriksen T. (2013) Newborn body fat: associations with maternal metabolic state and placental size. PLoS One 8, e57467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Retnakaran R., Ye C., Hanley A. J., Connelly P. W., Sermer M., Zinman B., Hamilton J. K. (2012) Effect of maternal weight, adipokines, glucose intolerance and lipids on infant birth weight among women without gestational diabetes mellitus. CMAJ 184, 1353–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crume T. L., Shapiro A. L., Brinton J. T., Glueck D. H., Martinez M., Kohn M., Harrod C., Friedman J. E., Dabelea D. (2015) Maternal fuels and metabolic measures during pregnancy and neonatal body composition: the healthy start study. J. Clin. Endocrinol. Metab. 100, 1672–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McConihay J. A., Honkomp A. M., Granholm N. A., Woollett L. A. (2000) Maternal high density lipoproteins affect fetal mass and extra-embryonic fetal tissue sterol metabolism in the mouse. J. Lipid Res. 41, 424–432 [PubMed] [Google Scholar]
- 34.Kramer M. S., Kahn S. R., Dahhou M., Otvos J., Genest J., Platt R. W., Evans R. W. (2013) Maternal lipids and small for gestational age birth at term. J. Pediatr. 163, 983–988 [DOI] [PubMed] [Google Scholar]
- 35.Warnick G. R., Albers J. J. (1978) A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J. Lipid Res. 19, 65–76 [PubMed] [Google Scholar]
- 36.Gordon S. M., Deng J., Lu L. J., Davidson W. S. (2010) Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. J. Proteome Res. 9, 5239–5249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.González Fernández-Niño S. M., Smith-Moritz A. M., Chan L. J., Adams P. D., Heazlewood J. L., Petzold C. J. (2015) Standard flow liquid chromatography for shotgun proteomics in bioenergy research. Front. Bioeng. Biotechnol. 3, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones H. N., Woollett L. A., Barbour N., Prasad P. D., Powell T. L., Jansson T. (2009) High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 23, 271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNamara R. K., Able J., Jandacek R., Rider T., Tso P. (2009) Inbred C57BL/6J and DBA/2J mouse strains exhibit constitutive differences in regional brain fatty acid composition. Lipids 44, 1–8 [DOI] [PubMed] [Google Scholar]
- 40.Mark P. J., Smith J. T., Waddell B. J. (2006) Placental and fetal growth retardation following partial progesterone withdrawal in rat pregnancy. Placenta 27, 208–214 [DOI] [PubMed] [Google Scholar]
- 41.He S., Allen J. C. Jr., Malhotra R., Østbye T., Tan T. C. (2016) Association of maternal serum progesterone in early pregnancy with low birth weight and other adverse pregnancy outcomes. J. Matern. Fetal Neonatal Med. 29, 1999–2004 [DOI] [PubMed] [Google Scholar]
- 42.Wu L., de Bruin A., Saavedra H. I., Starovic M., Trimboli A., Yang Y., Opavska J., Wilson P., Thompson J. C., Ostrowski M. C., Rosol T. J., Woollett L. A., Weinstein M., Cross J. C., Robinson M. L., Leone G. (2003) Extra-embryonic function of Rb is essential for embryonic development and viability. Nature 421, 942–947 [DOI] [PubMed] [Google Scholar]
- 43.Gordon S. M., Hofmann S., Askew D. S., Davidson W. S. (2011) High density lipoprotein: it’s not just about lipid transport anymore. Trends Endocrinol. Metab. 22, 9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah A. S., Tan L., Long J. L., Davidson W. S. (2013) Proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J. Lipid Res. 54, 2575–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heinecke J. W. (2010) The protein cargo of HDL: implications for vascular wall biology and therapeutics. J. Clin. Lipidol. 4, 371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kontush A., Chapman M. J. (2012) High-Density Lipoproteins. Structure, Metabolism, Function, and Therapeutics, John Wiley & Sons, Inc., Hoboken, NJ, USA [Google Scholar]
- 47.Knopp R. H., Bergelin R. O., Wahl P. W., Walden C. E. (1985) Relationships of infant birth size to maternal lipoproteins, apoproteins, fuels, hormones, clinical chemistries, and body weight at 36 weeks gestation. Diabetes 34(Suppl 2), 71–77 [DOI] [PubMed] [Google Scholar]
- 48.Naksen W., Prapamontol T., Mangklabruks A., Chantara S., Thavornyutikarn P., Srinual N., Panuwet P., Ryan P. B., Riederer A. M., Barr D. B. (2015) Associations of maternal organophosphate pesticide exposure and PON1 activity with birth outcomes in SAWASDEE birth cohort, Thailand. Environ. Res. 142, 288–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fielding C. J., Fielding P. E. (2004) Membrane cholesterol and the regulation of signal transduction. Biochem. Soc. Trans. 32, 65–69 [DOI] [PubMed] [Google Scholar]
- 50.Melchior J. T., Street S. E., Andraski A. B., Furtado J. D., Sacks F. M., Shute R. L., Greve E. I., Swertfeger D. K., Li H., Shah A. S., Lu L. J., Davidson W. S. (2017) Apolipoprotein A-II alters the proteome of human lipoproteins and enhances cholesterol efflux from ABCA1. J. Lipid Res. 58, 1374–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Regal J. F., Gilbert J. S., Burwick R. M. (2015) The complement system and adverse pregnancy outcomes. Mol. Immunol. 67, 56–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones H. N., Powell T. L., Jansson T. (2007) Regulation of placental nutrient transport: a review. Placenta 28, 763–774 [DOI] [PubMed] [Google Scholar]
- 53.Jansson T., Aye I. L., Goberdhan D. C. (2012) The emerging role of mTORC1 signaling in placental nutrient-sensing. Placenta 33(Suppl 2), e23–e29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herrera E., Ortega-Senovilla H. (2014) Lipid metabolism during pregnancy and its implications for fetal growth. Curr. Pharm. Biotechnol. 15, 24–31 [DOI] [PubMed] [Google Scholar]
- 55.Chambaz J., Ravel D., Manier M. C., Pepin D., Mulliez N., Bereziat G. (1985) Essential fatty acids interconversion in the human fetal liver. Biol. Neonate 47, 136–140 [DOI] [PubMed] [Google Scholar]
- 56.Haghiac M., Yang X. H., Presley L., Smith S., Dettelback S., Minium J., Belury M. A., Catalano P. M., Hauguel-de Mouzon S. (2015) Dietary omega-3 fatty acid supplementation reduces inflammation in obese pregnant women: a randomized double-blind controlled clinical trial. PLoS One 10, e0137309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burillo E., Mateo-Gallego R., Cenarro A., Fiddyment S., Bea A. M., Jorge I., Vázquez J., Civeira F. (2012) Beneficial effects of omega-3 fatty acids in the proteome of high-density lipoprotein proteome. Lipids Health Dis. 11, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woollett L. A. (2008) Where does fetal and embryonic cholesterol originate and what does it do? Annu. Rev. Nutr. 28, 97–114 [DOI] [PubMed] [Google Scholar]
- 59.Baardman M. E., Erwich J. J., Berger R. M., Hofstra R. M., Kerstjens-Frederikse W. S., Lütjohann D., Plösch T. (2012). The origin of fetal sterols in second-trimester amniotic fluid: endogenous synthesis or maternal-fetal transport? Am. J. Obstet. Gynecol. 207, 202.e19–202.e25 [DOI] [PubMed] [Google Scholar]
- 60.Napoli C., D’Armiento F. P., Mancini F. P., Postiglione A., Wiztum J. L., Palumbo G., Palinski W. (1997) Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia: intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J. Clin. Invest. 100, 2680–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kohler I. V., Anglewicz P., Kohler H. P., McCabe J. F., Chilima B., Soldo B. J. (2012) Evaluating health and disease in Sub-Saharan Africa: minimally invasive collection of plasma in the Malawi Longitudinal Study of Families and Health (MLSFH). Genus 68, 1–27 [PMC free article] [PubMed] [Google Scholar]
- 62.Moore S. E., Halsall I., Howarth D., Poskitt E. M., Prentice A. M. (2001) Glucose, insulin and lipid metabolism in rural Gambians exposed to early malnutrition. Diabet. Med. 18, 646–653 [DOI] [PubMed] [Google Scholar]
- 63.Udoh A. E., Ndem E. D., Itam E. H., Odigwe C. O., Archibong E. (1994) Serum cholesterol profile of some Nigerian pregnant women. Acta Med. Hung. 50, 75–81 [PubMed] [Google Scholar]