Abstract
Cathepsin B (CtsB) contributes to atherosclerosis and cancer progression by processing the extracellular matrix and promoting angiogenesis. Although CtsB was reported to promote and reduce angiogenesis, there is no mechanistic explanation that reconciles this apparent discrepancy. CtsB cleaves CD18 from the surface of immune cells, but its contribution to angiogenesis has not been studied. We developed an in vivo technique for visualization of immune cell transmigration from corneal vessels toward implanted cytokines. Wild-type (WT) leukocytes extravasated from limbal vessels, angiogenic stalks, and growing tip vessels and migrated toward the cytokines, indicating immune competence of angiogenic vessels. Compared to WT leukocytes, CtsB−/− leukocytes accumulated in a higher number in angiogenic vessels, but extravasated less toward the implanted cytokine. The accumulated CtsB−/− leukocytes in angiogenic vessels expressed more CD18. CD18−/− leukocytes extravasated later than WT leukocytes. However, once extravasated, CD18−/− leukocytes transmigrated more rapidly than their WT counterparts. These results suggest that, although CD18 facilitates efficient extravasation, outside of the vessel CD18 interaction with the extracellular matrix, it reduced transmigration velocity. Our results reveal an unexpected role for CtsB in leukocyte extravasation and transmigration, which advances our understanding of the complex contribution of CtsB to angiogenesis.—Nakao, S., Zandi, S., Sun, D., Hafezi-Moghadam, A. Cathepsin B-mediated CD18 shedding regulates leukocyte recruitment from angiogenic vessels.
Keywords: cornea, CD31, molecular imaging, ICAM-1, IL-1β
The role of immune cells in angiogenesis is well established (1). Infiltrating leukocytes release growth factors, such as VEGF, and chemokines that promote angiogenesis (2, 3). However, the contribution of angiogenic vessels to immune function is less well understood, partly because of the lack of suitable in vivo models.
Leukocyte accumulation in inflamed tissues follows a regulated sequence of events, which starts with an initial rolling of leukocytes on the activated endothelium, followed by firm adhesion, extravasation through the vessel wall, and subsequent transmigration to the extracellular matrix (4). Several specialized molecules fulfill well-defined functions in inflammatory leukocyte recruitment and angiogenesis (5, 6). The leukocyte integrin CD18 is a key contributor to firm adhesion through its interaction with endothelial intracellular adhesion molecule (ICAM)-1 as well as extracellular matrix molecules (7, 8).
VEGF-A induces angiogenesis and inflammatory leukocyte recruitment in various disorders (9, 10). Similarly, the cytokine IL-1β affects both angiogenesis and inflammation (2, 11–13). IL-1β is critically involved in the acute inflammatory response, activation, and chemotaxis of inflammatory and antigen presenting cells, up-regulation of adhesion molecules, and neovascularization (14).
Cathepsin B (CtsB) is a member of the Cts family lysosomal cysteine proteases with central roles in atherosclerosis and cancer (15). Secreted CtsB contributes to tumor progression by processing the extracellular matrix (16) and promoting angiogenesis. Joyce et al. (17) showed expression of CtsB in angiogenic vessels. Fukuda and Schmid-Schönbein (18) showed that leukocyte CtsB cleaves CD18 under physiologic shear values (1.5 and 5 dyn/cm2). It is unclear whether CtsB is pro- or antiangiogenic. In vitro, CtsB inhibition increases VEGF-A expression and promotes angiogenesis (19), whereas in vivo CtsB inhibition retards angiogenesis and tumorigenesis (17). This discrepancy motivated us to search for an explanation.
MATERIALS AND METHODS
Animals
All animal experiments were approved by the institutional animal care committee of Harvard Medical School and the Brigham and Women’s Hospital. Male 6–12-wk-old BALB/cN mice were purchased from Taconic (Germantown, NY, USA). Homozygous CtsB-deficient mice (CtsB−/−) and ITGB2/CD18-deficient mice (CD18−/−) on a C57BL/6 background and C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME, USA) were housed and cared for according to institutional guidelines. Six- to 12-wk-old mice were used for experiments.
Corneal micropocket assay in mice
Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg). Poly-HEMA pellets (0.3 µl, P3932; Millipore-Sigma, Billerica MA, USA) containing 30 ng IL-1β (401ML; R&D Systems, Minneapolis, MN, USA), were prepared and implanted in the corneas (20). IL-1β pellets were positioned at an ∼0.8–1 mm distance to the corneal limbus. After implantation, bacitracin ophthalmic ointment (Altana, Melville, NY, USA) was applied to each eye to prevent infection.
At 2, 4, or 6 d after implantation, digital images of the corneal vessels were obtained and recorded in OpenLab software, version 2.2.5 (Agilent Technologies), with standardized illumination and contrast.
Leukocyte transmigration rate assay
To visualize leukocyte extravasation, a new assay was developed (21). At the indicated times, the cytokine-implanted mice were anesthetized. For in vivo staining, 500 µl acridine orange (AO, 1 mg/ml) was injected intravenously. The AO concentration in the intravascular leukocytes and the endothelial cells significantly diminishes by 30 min after dye injection due to a washout effect.
The intravascular leukocytes rapidly take up the AO dye during the period of high plasma concentration and subsequently retained the dye. This results in the unique feature that the stained peripheral blood leukocytes that extravasate into the stroma can be distinguished from the pre-existing leukocytes in the corneal tissue, as has been shown in the retina (22, 23). This allowed us to follow a population of leukocytes that migrated toward the implanted cytokines at different time points after AO injections. In this study, we applied this technique to study the dynamics of leukocyte migration in the context of angiogenesis, by combining AO fluorography with the cytokine-induced corneal angiogenesis.
To stain the vascular endothelium and firmly adherent leukocytes, the animals were perfused with rhodamine-labeled concanavalin A lectin [10 µg/ml in PBS (pH 7.4) ConA; Vector Laboratories, Burlingame, CA, USA] at 30 min, 2, 6, and 10 h after AO injection. Under deep anesthesia, the chest cavity was opened. A small incision was made in the tip of the left ventricle with microsurgical scissors. Through the newly made incision, a 24-gauge perfusion needle was inserted into the left ventricle and gently advanced through the aortic valve to reach the ascending aorta. The perfusion needle was secured with a hemostat. Drainage was achieved through a small cut in the right atrium with microsurgical scissors. The animals were then perfused with 10 ml PBS to wash out the intravascular contents and unbound blood cells, 5 ml rhodamine-labeled ConA for staining, and subsequently 1 ml PBS to remove residual unbound ConA. Immediately after perfusion, the corneas were carefully removed and flatmounts were prepared with a mounting medium (TA-030-FM, Mountant Permafluor; Lab Vision Corp., Fremont, CA, USA). To observe transmigrated AO+ leukocytes in the cornea, it is necessary to image the cornea at higher magnification (×200) with a fluorescence microscope (DM RXA; Leica Microsystems, Deerfield, IL, USA). Digital images were obtained for documentation with a CCD camera (Dage-MTI, Michigan City, IN, USA). The contrast of the entire image was adjusted (Photoshop; Adobe, San Jose, CA, USA) to improve the distinction between AO+ cells and nonspecific background. In areas, where leukocytes were extensively clustered, a higher magnification in combination with changing the plane of focus provided a better distinction of individual cells within the clusters.
For presentation of a larger region than can be imaged in higher magnification, micrographs from adjacent regions of the corneal tissues were positioned next to each other as also indicated in the legends of the figures. In each merged image, transmigrated AO+ leukocytes within the 0–300, 300–600, and 600–900 µm distances from the limbus area toward the implanted cytokine pellet, defined as zones 1–3 (Fig. 1B), were counted manually. Representative merged images from each condition were selected for the figures.
Figure 1.
In vivo leukocyte transmigration rate in IL-1β-implanted mouse cornea. A) In vivo imaging assay for quantification of leukocyte transmigration rate. B) Pellets containing IL-1β were implanted in the corneas of BALB/c mice. Composite photomicrographs of corneal neovascularization. Double staining of corneal flatmounts for leukocytes (CD45) and angiogenesis (ConA). AO+ traveling leukocytes in IL-1β-implanted corneas. Zone separation by the distance (300 µm) from the limbal vessels. To depict the corneal limbal vessels and the corneal stroma, including the site of pellet implantation at high resolution, adjacent micrographs were taken and merged in a mosaic fashion. Asterisk: implanted pellets. Scale bars, 100 µm.
To stain the vascular endothelium, we used 2 different techniques: perfusion with fluorescently labeled ConA and immunohistochemistry for CD31. Both ConA perfusion and CD31 immunohistochemistry stain the vasculature. In combination with the AO leukocyte fluorography, we used ConA to visualize the vessels, because ConA perfusion requires less procedural steps and therefore does not interfere with the AO assay. The staining of the vessels with the CD31Ab includes incubation steps with the primary and secondary antibodies and several washes, which could diminish the AO staining. A strength of CD31 immunohistochemistry is that it also stains immature or not-yet-perfused angiogenic vessels (24).
The current technique, involving ex vivo microscopy of the AO+ leukocytes, provides snapshots of the leukocyte locations. Although the locations of the leukocytes relative to the various vessels allowed quantification of the number of leukocytes in the various zones, it is not unequivocally conclusive from which vessels the individual leukocytes exited. To visualize the extravasation of the leukocytes from the various angiogenic vessels, our current imaging technique would have to be performed in vivo.
Whole-mount immunofluorescence
The animals’ eyes were enucleated and fixed with 4% paraformaldehyde for 30 min at 4°C. For whole-mount preparation the corneas were microsurgically exposed by removing other portions of the eye. Radial cuts were then made into the cornea. Tissues were washed with PBS, 3 times for 5 min, and then placed in methanol for 20 min. Tissues were incubated overnight at 4°C with anti-mouse CD31 mAb (5 µg/ml, 550274; BD Biosciences, San Diego, CA, USA) or anti-mouse CD45 mAb (40 µg/ml, 554875; BD Biosciences) diluted in PBS containing 10% goat serum and 1% Triton X-100. Tissues were washed 4 times for 20 min in PBS followed by incubation with Alexa Fluor 488 goat anti-rat IgG (20 µg/ml, A11006; Thermo Fisher Scientific) overnight at 4°C. Corneal flatmounts were prepared on glass slides using a mounting medium (TA-030-FM, PermaFluor aqueous mounting medium; Lab Vision Corporation). The flatmounts were examined by fluorescence microscopy and digital images were recorded using OpenLab software (version 2.2.5; Agilent Technologies) with standardized illumination and contrast. Angiogenesis was quantitatively analyzed by using a modification of the previously established protocols (25). In brief, photomicrographs of the CD31+ neovascular areas in each examined cornea were obtained in fluorescence microscopy (magnification: ×50; Leica Microsystems). Digital images were recorded using OpenLab software (version 2.2.5; Agilent Technologies) under standardized illumination and contrast. The images were converted into 2 gradations using Scion Image software (version 4.0.2; Scion Corp., Frederick, MD, USA). The area of the blood vessels was measured as the number of pixels and subsequently converted to surface area (mm2) using a scale for calibration.
Preparation of molecular imaging probes
We established the methodology for in vivo microsphere imaging (26–28), used in this study. In brief, carboxylated fluorescent or nonfluorescent microspheres (MS, 2 µm; Polysciences, Warrington, PA, USA) were covalently conjugated to protein G (Millipore-Sigma), using a carbodiimide-coupling kit (Polysciences) (6, 26, 29–31). Anti-mouse ICAM-1 Ab (553250; BD Biosciences), anti-mouse CD18 Ab (557437; BD Biosciences), or control goat IgG (AB-108-C; R&D Systems) was incubated with the microspheres at 0.4 mg/ml overnight at room temperature. Microspheres were washed in PBS with 1% BSA before use in vivo. Subsequently, 6 × 107 microspheres were injected into each mouse. The number of firmly adherent MSs in ConA+ blood vessels was counted manually by microscopy. Photomicrographs were obtained for presentation and documentation.
ELISA
Corneas with Poly-HEMA pellets alone or IL-1β implantation in C57BL/6 wild-type (WT) or CtsB−/− mice were harvested at d 3 after pellet implantation. Corneas were microsurgically isolated and placed in 100 ml of lysis buffer (mammalian cell lysis kit MCL1; Millipore-Sigma), supplemented with protease and phosphatase inhibitors (P2850, P5726, P8340; Millipore-Sigma), and sonicated at d 3 after cytokine pellet implantation. The lysate was centrifuged (12,000 rpm, 15 min, 4°C), and the supernatant was collected. The samples were lysed in 100 µl of a mammalian cell lysis kit and centrifuged. The supernatants were used in ELISA kits (R&D Systems) for mouse VEGF-A (MMV00).
Immunohistochemistry
The eyes were harvested and snap frozen in optimal cutting temperature compound (Sakura Finetek, Ltd., Tokyo, Japan). Sections (10 µm) were prepared, air dried, and fixed in ice-cold acetone for 10 min. The sections were blocked with 3% nonfat dried milk bovine working solution (M7409; Millipore-Sigma) and stained with anti-mouse CD18 (10 µg/ml, 557437; BD Biosciences) and anti-CtsB (10 µg/ml, AF965; R&D Systems). After an overnight incubation, sections were washed and stained for 20 min with Alexa Fluor 488 goat anti-rat IgG (10 µg/ml, A11006; Thermo Fisher Scientific) and Alexa Fluor 647 rabbit anti-rabbit IgG (10 µg/ml, A21446; Thermo Fisher Scientific).
Western blot analysis
At d 3 after IL-1β implantation (30 ng), corneas were harvested and lysed in a mammalian cell lysis kit (MCL1; Millipore-Sigma). Lysates were subjected to SDS-PAGE and transferred to Immobilon membranes (Millipore-Sigma). Blots were incubated with anti-CtsB (0.1 µg/ml, AF965; R&D Systems), or anti-β-tubulin (1:1000, ab11308; Abcam, Cambridge, MA, USA) and visualized with a secondary antibody coupled to horseradish peroxidase (GE Healthcare) and ECL system.
Microfluidic studies
Leukocyte adhesion was analyzed using our microflow chamber assay (32). In brief, microfluidic chambers were coated with recombinant mouse P-selectin (5 µg/ml; R&D Systems) and recombinant mouse ICAM-1 (5 µg/ml; R&D Systems) at 4°C overnight. Coated chambers were connected to biocompatible polyester tubing (PE10; BD, Franklin Lakes, NJ, USA) at both ends. The tubing and microslides were incubated with 1% BSA (Millipore-Sigma) for 1 h to block nonspecific leukocyte interactions with the inner surfaces. WT and CtsB−/− blood was perfused through the chambers at 2.5 dyn/cm2. This shear rate was chosen to model the conditions in postcapillary venules (33), where most inflammatory leukocyte recruitment occurs. Subsequently, the chambers were perfused with PBS to wash out nonadhered leukocytes. The number of firmly adhered leukocytes was counted under an upright, fixed-stage intravital microscope (Leica Microsystems).
Statistical analysis
All values are expressed as mean ± sem. Data were analyzed by Student’s t test. Differences between the experimental groups were considered statistically significant when P < 0.05.
RESULTS
Cytokine-induced leukocyte extravasation and transmigration in angiogenesis
To investigate the impact of angiogenesis on immune function, we developed a new approach that combines cytokine-induced angiogenesis in the mouse cornea with specific visualization of extravasated leukocytes (Fig. 1A). IL-1β was implanted in the corneas of mice and AO was systemically applied to stain the nuclei of the transmigrating leukocytes. This method allowed visualization of transmigrating leukocytes moving toward the implanted pellet at different time points after leukocyte staining and from pre-existing normal or growing angiogenic vessels.
IL-1β caused angiogenesis from pre-existing limbal blood vessels starting 2 d after pellet implantation, and the tips of the angiogenic vessels reached the pellet by d 6 (Fig. 1B). To identify leukocytes in the cornea, we stained for the leukocyte marker CD45+ in IL-1β-implanted corneas. CD45+ cells were found around pre-existing blood vessels in untreated eyes, whereas more CD45+ leukocytes were in the cornea on d 2, 4, and 6, indicating tissue accumulation. To distinguish between pre-existing and newly extravasated leukocytes, we stained the intravascular cells with AO, whereas the pre-existing stromal leukocytes were quiescent. The combination of the corneal pocket assay with the AO staining allowed depiction of extravasated leukocytes from ConA-stained angiogenic vessels, which were characterized in the various zones of the cornea.
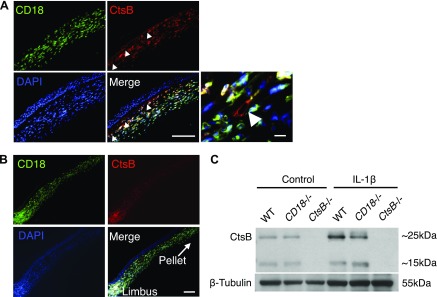
To examine CtsB and CD18 expression in IL-1β-dependent leukocyte transmigration, immunohistochemistry was performed. CD18+ leukocytes costained for CtsB (Fig. 2A). CtsB was also expressed in angiogenic blood vessels. These data indicate that both leukocytes and angiogenic vessels during inflammation express CtsB. CD18+ cells around the limbal angiogenic vessels strongly expressed CtsB (Fig. 2B). Western blot showed upregulation of CtsB expression in IL-1β-implanted corneas compared with controls (Fig. 2C). The blots showed 2 bands for CtsB at ∼25 and 15 kDa, consistent with prior reports using the same antibody (34). These data motivated us to quantify leukocyte transmigration in CtsB-deficient mice (CtsB−/−) in IL-1β-induced angiogenesis.
Figure 2.
CtsB expression in activated leukocytes and angiogenic vessels in the cornea. A, B) Double immunostaining of IL-1β-implanted cornea (d 3) with Abs against CD18, CtsB, and DAPI. A) Site of angiogenic vessels in the IL-1β-implanted cornea. (B) Site around the limbal vessels. Double-positive cells (red and green) are yellowish white. Arrowheads: CD18−CtsB+ vessels. C) Representative Western blots from untreated and IL-1β-implanted corneas (d 3) obtained from WT, CD18−/,− and CtsB−/− mice with α-CtsB Ab. CtsB−/− expressed no CtsB. CtsB was upregulated in IL-1β-implanted corneas of WT animals, not in CD18−/− mouse corneas. Scale bars, 50 µm.
In control eyes, very few AO+ leukocytes (<2 cells) were found in the immediate vicinity of the limbal vessels (zone 1), indicating a very low rate of constitutive leukocyte extravasation (Fig. 3A and Table 1).
Figure 3.
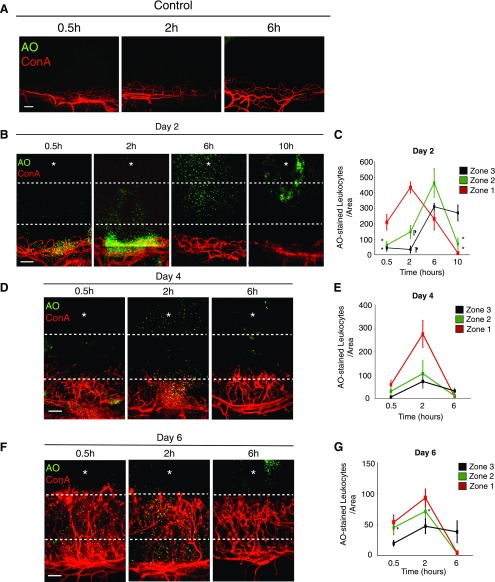
Ex vivo leukocyte transmigration rate during IL-1β-induced angiogenesis in the mouse cornea. To depict the corneal limbal vessels and the corneal stroma, including the site of pellet implantation at high resolution, composite corneal photomicrographs were generated by merging the digital images from adjacent regions of the cornea in a mosaic fashion. A) AO+ and Con A+ stains of resident leukocytes in the cornea of untreated mice at 0.5, 2, 6 h after AO injection. B) AO+ leukocytes and Con A+ angiogenic vessels in IL-1β-implanted corneas at the indicated time points after AO injection on d 2 after pellet implantation. C) Quantitation of the number of AO+ leukocytes in IL-1β-implanted corneas at the indicated time points after AO injection (n = 3–6). D, F) AO+ leukocytes and Con A+ angiogenic vessels in IL-1β-implanted corneas at the indicated times after AO injection on d 4 (D) and 6 (F) after pellet implantation. E, G) Quantitation of the number of AO+ leukocytes in IL-1β-implanted corneas at the indicated times after AO injection on d 4 (E) and 6 (G) (n = 3–6). *P < 0.05, ¶P < 0.01. Scale bars, 100 µm.
TABLE 1.
Quantitation of AO+ leukocytes in the 3 untreated corneal zones at 3 time points
| Zone | 0.5 h | 2 h | 6 h |
|---|---|---|---|
| 3 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0.8 ± 0.8 |
| 1 | 0.8 ± 1.1 | 2.0 ± 0.9 | 1.5 ± 1.5 |
On d 2 after IL-1β stimulation at 0.5 and 2 h after AO injection, AO+ leukocytes were observed around limbal vessels. Six hours after AO injection, most leukocytes infiltrated zones 2 and 3 around the implanted cytokine. At 10 h leukocytes accumulated around the cytokine pellet (Fig. 3B, C). On d 4 after IL-1β implantation, at 0.5 and 2 h after AO injection, ∼70% of AO+ leukocytes were observed in zone 1, even though the angiogenic tips had already reached zone 2 (Fig. 3D, E). In vivo imaging would be necessary to determine the source of individual leukocytes exiting the different vessels. At 6 h after AO injection, most leukocytes were in zone 3 (Fig. 3B–E).
On d 6 after IL-1β implantation, leukocyte transmigration appeared to occur from angiogenic stalks, as well as from the angiogenic tips (Fig. 3F, G). On d 2, 4, and 6, very few leukocytes remained in the vicinity of blood vessels by 6 h after AO injection (Fig. 3B, D, F).
Impact of CD18 in IL-1β-induced leukocyte transmigration and angiogenesis
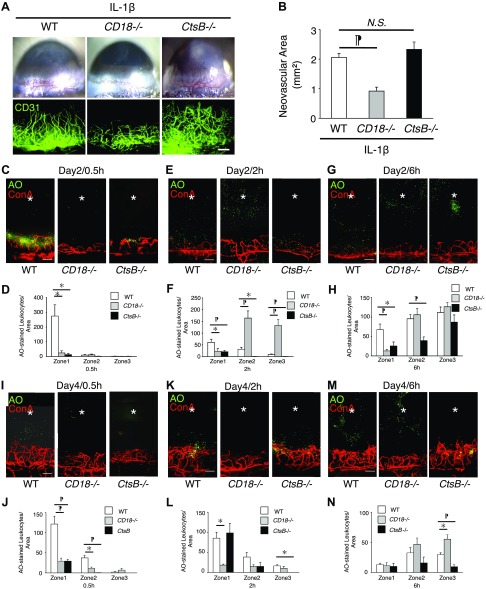
To examine the role of CD18 in IL-1β-induced angiogenesis, we implanted IL-1β in CD18−/− mice and stained blood vessels with anti-CD31 mAb. IL-1β-induced angiogenesis in CD18−/− mice was significantly less than in WT mice, when compared 6 d after implantation (Fig. 4A, B).
Figure 4.
Ex vivo leukocyte transmigration rate during IL-1β-induced angiogenesis in CD18−/− and CtsB−/− mice. To illustrate a large region of the corneal flatmount at higher resolution, composite micrographs were generated by merging the digital images from adjacent regions of the cornea in a mosaic fashion. A) Microscopic pictures of IL-1β-induced corneal neovascularization of WT, CD18−/−, and CtsB−/− mice. Double staining of corneal flatmounts for angiogenesis (CD31). B) Quantitative analysis of vascular area (n = 8–9). The neovascular area was determined by performing CD31 immunohistochemistry and measuring the vascular area. C–H) AO+ leukocytes and Con A+ angiogenic vessels in IL-1β-implanted corneas of CD18−/− and CtsB−/− mice at 0.5 (C), 2 (E), and 6 (G) h after AO injection on d 2 after pellet implantation. Quantitation of the number of AO+ leukocytes in IL-1β-implanted corneas at 0.5 (D), 2 (F) and 6 (H) h after AO injection on d 2. I–N) AO+ leukocytes and Con A+ angiogenic vessels in IL-1β-implanted corneas of CD18−/− and CtsB−/− mice at 0.5 (I), 2 (K) and 6 (M) h after AO injection on d 4 after the pellet implantation. Quantitation of the number of AO+ leukocytes in IL-1β-implanted corneas at 0.5 (J), 2 (L) and 6 (N) h after AO injection on d 4 (n = 4). *P < 0.05, ¶P < 0.01. Scale bars, 100 µm.
To investigate the role of CD18 in IL-1β-dependent leukocyte extravasation and transmigration, we injected AO intravenously in CD18−/− and WT mice on d 2 and 4 after implantation.
The number of AO+ leukocytes in zone 1 of CD18−/− mice was significantly less than in WT mice at 0.5 h on d 2 (Fig. 4C, D). At 2 h after AO injection, the number of leukocytes in zone 1 of CD18−/− mice was also significantly less than in WT (Fig. 4F). Surprisingly, the number of leukocytes in zones 2 and 3 of CD18−/− mice was significantly more than in WT at 2 h after AO injection, indicating that the CD18−/− leukocytes transmigrated faster than WT leukocytes, once they were in the corneal tissue. At 6 h after AO injection, the number of leukocytes in zone 1 of CD18−/− mice was significantly less than in WT (Fig. 4G, H). These data indicate that the significant contribution of CD18 to firm adhesion delayed extravasation of CD18−/− leukocytes from blood vessels. However, CD18 was not necessary for extravasation. Intriguingly, once CD18−/− leukocytes exited the vessels, they migrated through the stroma faster than WT leukocytes, suggesting that leukocyte CD18 impedes the leukocytes in their migration journey through the stroma.
Contribution of CtsB to leukocyte recruitment in angiogenesis
CtsB−/− mice showed less AO+ leukocyte transmigration than WT in IL-1β-induced angiogenesis, 2 d after implantation and at 0.5, 2, and 6 h after AO injection (Fig. 4C–H). However, some CtsB−/− leukocytes transmigrated into zone 3 at 6 h after AO injection (Fig. 4G, H). On d 4 at 0.5 h CtsB−/− AO+ cells were seen to be associated with the limbal and angiogenic vasculature. Although some of these cells appeared outside of the vessels, they did not transmigrate as far as WT leukocytes in the corneal tissue (Fig. 4I, J). The number of CtsB−/− leukocytes was less than WT leukocytes in zone 3 at 2 and 6 h (Fig. 4K–N). These data indicate that most CtsB−/− leukocytes stayed in zone 1 at 2 h.
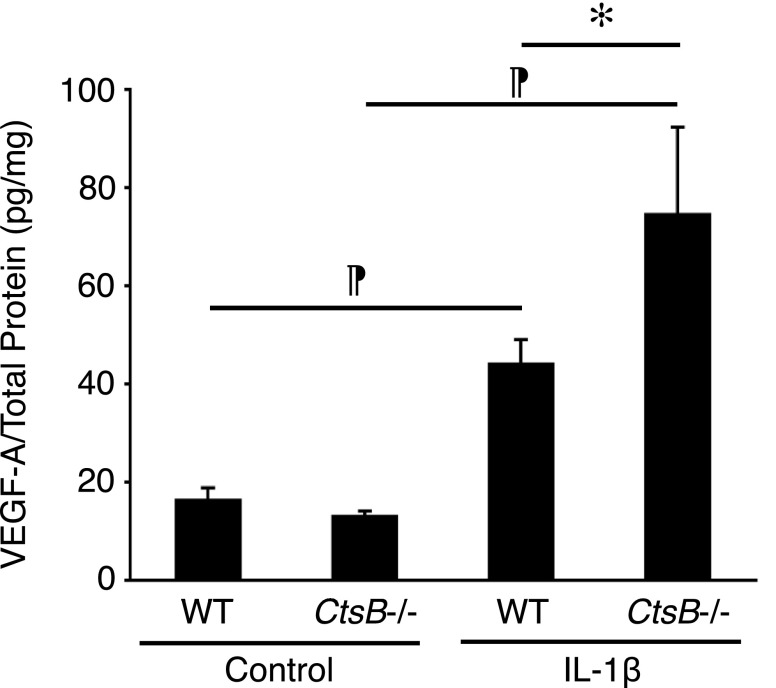
Despite the leukocyte transmigration deficit in CtsB−/− mice, IL-1β-induced angiogenesis did not differ from WT (Fig. 4A, B). Because CtsB enzymatically degrades VEGF-A (19), we measured VEGF-A in the IL-1β-implanted corneas. VEGF-A was significantly higher in IL-1β-implanted CtsB−/− corneas than in WT corneas (Fig. 5).
Figure 5.
VEGF-A expression in IL-1β-induced angiogenesis of CtsB−/− mice. The average protein levels of VEGF-A in IL-1β-implanted corneas of WT and CtsB−/− mice measured by ELISA (d 3) (n = 4–12). *P < 0.05, ¶P < 0.01.
Role of CtsB in IL-1β-induced angiogenesis and leukocyte adhesion
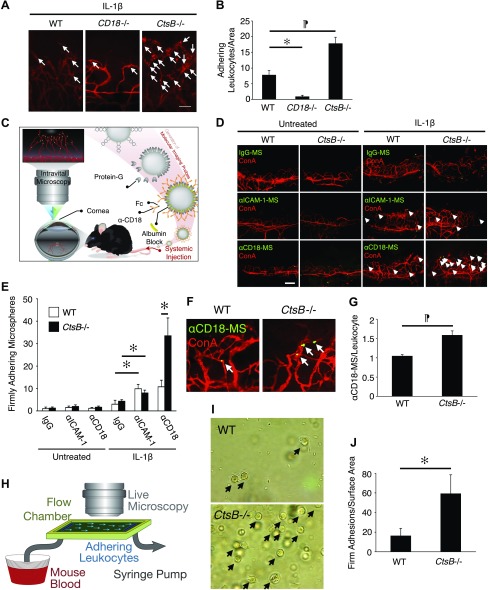
To understand, how CtsB regulates leukocyte recruitment in IL-1β-induced angiogenesis, we quantified the adherent leukocytes in IL-1β-induced angiogenesis of CtsB−/− and WT mice using ConA staining. The number of firmly adherent leukocytes in IL-1β-induced angiogenic vessels was significantly higher in CtsB−/− mice and lower in CD18−/− mice compared to WT (P = 0.009 and 0.02, respectively; Fig. 6A, B). To understand the underlying cause of the higher firm leukocyte adhesions in CtsB−/− mice, we quantified ICAM-1 and CD18 in IL-1β-induced angiogenic vessels, using our in vivo molecular imaging technique (Fig. 6C). The number of adherent α-ICAM-1- or α-CD18-conjugated MSs was significantly higher in IL-1β-induced angiogenic vessels than in normal pre-existing blood vessels, whereas there was no difference in the adhesion of the control MSs (Fig. 6D, E). The number of α-CD18-conjugated MSs but not α-ICAM-1-conjugated MS in IL-1β-induced angiogenesis of CtsB−/− mice was significantly higher than in WT mice (Fig. 6D, E), suggesting higher expression of CD18 in CtsB−/− leukocytes. To quantitatively compare the level of CD18 expression in CtsB−/− leukocytes with WT, we measured the ratio of α-CD18-conjugated MSs per firmly adherent leukocyte (MS/Leu) in CtsB−/− leukocytes compared to WT. CtsB−/− leukocytes showed a significantly higher MS:Leu ratio than WT mice, indicative of higher CD18 expression in these leukocytes (Fig. 6F, G).
Figure 6.
Leukocyte adhesion in IL-1β-induced angiogenesis of CtsB−/− mice. To illustrate a large region of the corneal flatmount at higher resolution, composite micrographs were generated by merging the digital images from adjacent regions of the cornea in a mosaic fashion. A) Representative micrographs of flatmounted IL-1β-implanted corneas of WT, CD18−/−, and CtsB−/− mice at 4 d after pellet implantation. Firmly adherent leukocytes in the corneal neovasculature were visualized by perfusion with ConA. Arrows: firmly adherent leukocytes in the inflamed corneal neovasculature. B) The average number of firmly adherent leukocytes in the inflamed corneal neovasculature of WT, CD18−/−, and CtsB−/− mice (n = 4–6). WT, 7.8 ± 1.5/area; CD18−/−, 0.8 ± 0.5; and CtsB−/−, 17.8 ± 2.1. C) Experimental design of our in vivo molecular imaging assay. D) α-IgG, α-ICAM-1 mAb or α-CD18 mAb–conjugated microspheres (MSs) (green) and rhodamine-conjugated ConA (red). Arrows: firmly adherent MSs in blood vessels. E) Quantitation of the number of MSs in corneal vessels of untreated and IL-1β-implanted eyes (d 4; n = 4–7). F) Ex vivo visualization of accumulated MSs in corneal vessels: α-CD18 mAb–conjugated MSs and rhodamine-conjugated ConA. Arrows: adherent MSs. G) Quantification of the firmly adherent MSs in corneal vessels. H) The design of the microfluidic mouse leukocyte adhesion experiments. I) Representative micrographs of firmly adherent leukocytes (arrows) from WT and CtsB−/− mice. J) Quantitation of the number of firmly adherent leukocytes in WT and CtsB−/− mice at a shear stress of 2.5 dyn/cm2. *P < 0.05, ¶P < 0.01. Scale bars, 100 µm.
To investigate the functional consequence of lack of CtsB on CD18 expression of leukocytes under dynamic flow conditions, we investigated leukocyte adhesion in our mouse microflow chamber system (Fig. 6H). CtsB−/− leukocytes adhered significantly more than WT, confirming the importance of CtsB-mediated CD18 shedding for leukocyte release under flow (2.5 dyn/cm2; Fig. 6I, J).
DISCUSSION
Inflammation and angiogenesis broadly interface, sharing several cellular and molecular mediators (5). A key molecule in both processes is CtsB, the role of which in angiogenesis requires further investigation. Intriguingly, under different conditions CtsB both promotes and suppresses angiogenesis. Joyce et al. (17) found CtsB to be proangiogenic, having a higher activity during tumorigenesis. In vivo, CtsB inhibition retards tumorigenesis and vascular growth (17). In contrast, Im et al. (19) found CtsB to be antiangiogenic in vitro, limiting endothelial tube formation through degradation of the endogenous VEGF-A.
To better understand how CtsB acts both as a pro- and antiangiogenic, we revisited the role of CtsB in inflammation and angiogenesis. In a novel model that combines inflammatory leukocyte recruitment and angiogenesis, we studied cytokine-induced angiogenesis concomitant to leukocyte extravasation and found that IL-1β-induced angiogenesis does not differ in WT and CtsB−/− animals. Based on prior reports, a higher (19) or lower (17) level of angiogenesis would have been expected.
At the same time, we found higher levels of VEGF-A in IL-1β-implanted corneas of CtsB−/− compared with WT animals. This result is in line with Im et al. (19) who showed proteolytic degradation of VEGF-A by CtsB in angiogenesis. Yet despite higher VEGF-A levels, cytokine-induced angiogenesis in CtsB−/− mice did not differ from that in WT animals, suggesting an important contribution of CtsB to angiogenesis, as outlined in Joyce et al. (17).
Although the contribution of leukocytes in vascular growth is well established, how angiogenesis regulates immune response is beginning to be understood. In our unique in vivo cytokine migration assay, we studied the dynamics of immune cell recruitment from angiogenic vessels. At every examined time point, a significant number of WT leukocytes extravasated and migrated through the corneal tissue toward the implanted IL-1β. The leukocyte extravasation appeared to take place from the angiogenic vessels that were closer to the cytokine pellet, but also through the angiogenic stalks and the pre-existing corneal limbal vessels. Future in vivo imaging studies will determine from what type of vessels each emigrated cell originated.
Compared to WT, a larger number of CtsB−/− leukocytes adhered to the vessel wall, but extravasated significantly less than WT leukocytes and their extravasation was delayed. To investigate the higher leukocyte accumulations and less efficient extravasation of CtsB−/− leukocytes, we used our molecular imaging technique for in vivo quantitation of cell surface molecules. In all examined animals, molecular imaging revealed a significantly higher number of adherent α-ICAM-1- or α-CD18-conjugated imaging probes in IL-1β-induced angiogenic vessels than in normal pre-existing blood vessels.
In vivo expression of endothelial ICAM-1 was comparable in WT and CtsB−/− mice, whereas significantly more leukocyte-specific CD18 was expressed in CtsB−/− mice. This suggests that, in CtsB−/− mice, leukocytes firmly bind to the endothelial surfaces; however, these leukocytes do not extravasate and migrate toward the implanted cytokine. Our conclusion that firmly adherent CtsB−/− leukocytes have greater CD18 expression in vivo was based on binding of targeted imaging probes. A challenge in investigating the impact of CtsB in leukocyte CD18 expression is that CtsB fulfills its proteolytic function under shear conditions (18). Therefore, we used the microsphere binding technique to quantitate CD18 expression in situ. Fukuda and Schmid-Schönbein (18) addressed the challenge, by exposing rat peripheral blood leukocytes to shear in the cone-and-plate assay.
Our data further showed a time delay for extravasation of CD18−/− leukocytes compared with WT leukocytes from blood vessels into the tissues; however, once in the stroma, the extravasated CD18−/− leukocytes migrated toward the cytokine pellet, indicating that CD18 was not essential for tissue migration. Indeed, CD18+ cells around limbal vessels of IL-1β-induced corneas strongly expressed CtsB, suggesting active proteolytic processing of the CD18 molecules in leukocytes that are about to commence migration. Both leukocytes and angiogenic endothelium express CtsB (35–37). An open question remains the source of CtsB in cleavage of CD18 from leukocytes under shear.
CtsB cleaves CD18 under shear (18). This effect could mean that the higher CD18 levels we find in CtsB−/− leukocytes are related to the lack of CD18 shedding, when binding to endothelial ICAM-1 under shear (18). CD18−/− mice showed less corneal angiogenesis than WT, but CD18−/− leukocytes did not show deficits in stromal accumulation and migration. A possible explanation is the significantly higher peripheral blood leukocyte counts in CD18−/− mice (38). Another possibility is that CD18 deficit differentially affects accumulation of macrophage subtypes, as M2 macrophages significantly contribute to corneal angiogenesis (2, 11, 12).
Our results show that failure to proteolytically cleave CD18 as in CtsB−/− leukocytes impedes recruitment. Lack of CD18 shedding could maintain the binding of the leukocytes to endothelial ICAM-1, preventing them from leaving the vessel. To address this hypothesis, we studied adhesion of CtsB−/− leukocytes to immobilized ICAM-1 in microfluidic chambers (31). We found significantly more CtsB−/− than WT leukocytes adhering to the immobilized ICAM-1 at the same shear, reinforcing the importance of shedding for mobility of the adherent leukocytes (39).
This work provides mechanistic evidence of the contribution of CtsB to angiogenesis and inflammatory cell migration in tissues and offers an explanation that can reconcile the discrepancy between the pro- and antiangiogenic roles of CtsB.
ACKNOWLEDGMENTS
The authors thank Rebecca Garland and Aliaa Barakat (Molecular Biomarkers Nano-Imaging Laboratory, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School) for preparation of the manuscript. Rebecca Garland passed away on August 11, 2017. The authors are deeply saddened by her departure and she will be much missed. This work was supported by the U.S. National Institutes of Health (NIH) Impact Award, National Institute of Diabetes and Digestive and Kidney Diseases Grant DK108238-01 (to A.H.-M.); Juvenile Diabetes Research Fund (JDRF) Innovation award (to A.H.-M.); an overseas Research Fellowship Award from Bausch & Lomb; a Fellowship Award from the Japan Eye Bank Association and Tear Film and Ocular Surface Society; a Young Investigator Fellowship (to S.N., under the mentorship of A.H.-M.); and grants from the Japan Society for the Promotion of Science (JSPS) KAKENHI, Grant-in-Aid for Young Scientists (A)25713057 (to S.N.) and Grant-in-Aid for Scientific Research (C)17K11456 (to S.N.). The authors declare no conflicts of interest.
Glossary
- AO
acridine orange
- ConA
concanavalin A lectin
- CtsB
cathepsin B
- MS
microsphere
- WT
wild type
AUTHOR CONTRIBUTIONS
S. Nakao and A. Hafezi-Moghadam designed the research and organized the experiments; S. Nakao, S. Zandi, D. Sun, and A. Hafezi-Moghadam performed research and analyzed data; and S. Nakao, S. Zandi, and A. Hafezi-Moghadam wrote the paper.
REFERENCES
- 1.Wels J., Kaplan R. N., Rafii S., Lyden D. (2008) Migratory neighbors and distant invaders: tumor-associated niche cells. Genes Dev. 22, 559–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakao S., Kuwano T., Tsutsumi-Miyahara C., Ueda S., Kimura Y. N., Hamano S., Sonoda K. H., Saijo Y., Nukiwa T., Strieter R. M., Ishibashi T., Kuwano M., Ono M. (2005) Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. J. Clin. Invest. 115, 2979–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nozawa H., Chiu C., Hanahan D. (2006) Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc. Natl. Acad. Sci. USA 103, 12493–12498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorokin L. (2010) The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 10, 712–723 [DOI] [PubMed] [Google Scholar]
- 5.Avraamides C. J., Garmy-Susini B., Varner J. A. (2008) Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 8, 604–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakao S., Zandi S., Hata Y., Kawahara S., Arita R., Schering A., Sun D., Melhorn M. I., Ito Y., Lara-Castillo N., Ishibashi T., Hafezi-Moghadam A. (2011) Blood vessel endothelial VEGFR-2 delays lymphangiogenesis: an endogenous trapping mechanism links lymph- and angiogenesis. Blood 117, 1081–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond M. S., Springer T. A. (1993) A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J. Cell Biol. 120, 545–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishida S., Yamashiro K., Usui T., Kaji Y., Ogura Y., Hida T., Honda Y., Oguchi Y., Adamis A. P. (2003) Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat. Med. 9, 781–788 [DOI] [PubMed] [Google Scholar]
- 9.Detmar M., Brown L. F., Schön M. P., Elicker B. M., Velasco P., Richard L., Fukumura D., Monsky W., Claffey K. P., Jain R. K. (1998) Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J. Invest. Dermatol. 111, 1–6 [DOI] [PubMed] [Google Scholar]
- 10.Nakao S., Arima M., Ishikawa K., Kohno R., Kawahara S., Miyazaki M., Yoshida S., Enaida H., Hafezi-Moghadam A., Kono T., Ishibashi T. (2012) Intravitreal anti-VEGF therapy blocks inflammatory cell infiltration and re-entry into the circulation in retinal angiogenesis. Invest. Ophthalmol. Vis. Sci. 53, 4323–4328 [DOI] [PubMed] [Google Scholar]
- 11.Nakao S., Hata Y., Miura M., Noda K., Kimura Y. N., Kawahara S., Kita T., Hisatomi T., Nakazawa T., Jin Y., Dana M. R., Kuwano M., Ono M., Ishibashi T., Hafezi-Moghadam A. (2007) Dexamethasone inhibits interleukin-1beta-induced corneal neovascularization: role of nuclear factor-kappaB-activated stromal cells in inflammatory angiogenesis. Am. J. Pathol. 171, 1058–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakao S., Noda K., Zandi S., Sun D., Taher M., Schering A., Xie F., Mashima Y., Hafezi-Moghadam A. (2011) VAP-1-mediated M2 macrophage infiltration underlies IL-1β- but not VEGF-A-induced lymph- and angiogenesis. Am. J. Pathol. 178, 1913–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakao S., Zandi S., Lara-Castillo N., Taher M., Ishibashi T., Hafezi-Moghadam A. (2012) Larger therapeutic window for steroid versus VEGF-A inhibitor in inflammatory angiogenesis: surprisingly similar impact on leukocyte infiltration. Invest. Ophthalmol. Vis. Sci. 53, 3296–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garlanda C., Dinarello C. A., Mantovani A. (2013) The interleukin-1 family: back to the future. Immunity 39, 1003–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lutgens S. P., Cleutjens K. B., Daemen M. J., Heeneman S. (2007) Cathepsin cysteine proteases in cardiovascular disease. FASEB J. 21, 3029–3041 [DOI] [PubMed] [Google Scholar]
- 16.Berquin I. M., Sloane B. F. (1996) Cathepsin B expression in human tumors. Adv. Exp. Med. Biol. 389, 281–294 [DOI] [PubMed] [Google Scholar]
- 17.Joyce J. A., Baruch A., Chehade K., Meyer-Morse N., Giraudo E., Tsai F. Y., Greenbaum D. C., Hager J. H., Bogyo M., Hanahan D. (2004) Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell 5, 443–453 [DOI] [PubMed] [Google Scholar]
- 18.Fukuda S., Schmid-Schönbein G. W. (2003) Regulation of CD18 expression on neutrophils in response to fluid shear stress. Proc. Natl. Acad. Sci. USA 100, 13152–13157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Im E., Venkatakrishnan A., Kazlauskas A. (2005) Cathepsin B regulates the intrinsic angiogenic threshold of endothelial cells. Mol. Biol. Cell 16, 3488–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakao S., Hafezi-Moghadam A. (2016) The corneal micropocket assay: a model of angiogenesis and lymphangiogenesis. Methods Mol. Biol. 1430, 311–316 [DOI] [PubMed] [Google Scholar]
- 21.Nakao S., Zandi S., Faez S., Kohno R., Hafezi-Moghadam A. (2012) Discontinuous LYVE-1 expression in corneal limbal lymphatics: dual function as microvalves and immunological hot spots. FASEB J. 26, 808–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishiwaki H., Ogura Y., Kimura H., Kiryu J., Honda Y. (1995) Quantitative evaluation of leukocyte dynamics in retinal microcirculation. Invest. Ophthalmol. Vis. Sci. 36, 123–130 [PubMed] [Google Scholar]
- 23.Miyahara S., Kiryu J., Miyamoto K., Katsuta H., Hirose F., Tamura H., Musashi K., Honda Y., Yoshimura N. (2004) In vivo three-dimensional evaluation of leukocyte behavior in retinal microcirculation of mice. Invest. Ophthalmol. Vis. Sci. 45, 4197–4201 [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi M., Nakao S., Arita R., Kaizu Y., Arima M., Zhou Y., Kita T., Yoshida S., Kimura K., Isobe T., Kaneko Y., Sonoda K. H., Ishibashi T. (2016) Vascular normalization by ROCK inhibitor: therapeutic potential of ripasudil (K-115) eye drop in retinal angiogenesis and hypoxia. Invest. Ophthalmol. Vis. Sci. 57, 2264–2276 [DOI] [PubMed] [Google Scholar]
- 25.Rogers M. S., Birsner A. E., D’Amato R. J. (2007) The mouse cornea micropocket angiogenesis assay. Nat. Protoc. 2, 2545–2550 [DOI] [PubMed] [Google Scholar]
- 26.Sun D., Nakao S., Xie F., Zandi S., Schering A., Hafezi-Moghadam A. (2010) Superior sensitivity of novel molecular imaging probe: simultaneously targeting two types of endothelial injury markers. FASEB J. 24, 1532–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie F., Sun D., Schering A., Nakao S., Zandi S., Liu P., Hafezi-Moghadam A. (2010) Novel molecular imaging approach for subclinical detection of iritis and evaluation of therapeutic success. Am. J. Pathol. 177, 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun D., Nakao S., Xie F., Zandi S., Bagheri A., Kanavi M. R., Samiei S., Soheili Z. S., Frimmel S., Zhang Z., Ablonczy Z., Ahmadieh H., Hafezi-Moghadam A. (2014) Molecular imaging reveals elevated VEGFR-2 expression in retinal capillaries in diabetes: a novel biomarker for early diagnosis. FASEB J. 28, 3942–3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hafezi-Moghadam A., Ley K. (1999) Relevance of L-selectin shedding for leukocyte rolling in vivo. J. Exp. Med. 189, 939–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyahara S., Almulki L., Noda K., Nakazawa T., Hisatomi T., Nakao S., Thomas K. L., Schering A., Zandi S., Frimmel S., Tayyari F., Garland R. C., Miller J. W., Gragoudas E. S., Masli S., Hafezi-Moghadam A. (2008) In vivo imaging of endothelial injury in choriocapillaris during endotoxin-induced uveitis. FASEB J. 22, 1973–1980 [DOI] [PubMed] [Google Scholar]
- 31.Garland R. C., Sun D., Zandi S., Xie F., Faez S., Tayyari F., Frimmel S. A., Schering A., Nakao S., Hafezi-Moghadam A. (2011) Noninvasive molecular imaging reveals role of PAF in leukocyte-endothelial interaction in LPS-induced ocular vascular injury. FASEB J. 25, 1284–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hafezi-Moghadam A., Thomas K. L., Cornelssen C. (2004) A novel mouse-driven ex vivo flow chamber for the study of leukocyte and platelet function. Am. J. Physiol. Cell Physiol. 286, C876–C892 [DOI] [PubMed] [Google Scholar]
- 33.Ruggeri Z. M. (1993) Mechanisms of shear-induced platelet adhesion and aggregation. Thromb. Haemost. 70, 119–123 [PubMed] [Google Scholar]
- 34.Shree T., Olson O. C., Elie B. T., Kester J. C., Garfall A. L., Simpson K., Bell-McGuinn K. M., Zabor E. C., Brogi E., Joyce J. A. (2011) Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 25, 2465–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinha A. A., Gleason D. F., Staley N. A., Wilson M. J., Sameni M., Sloane B. F. (1995) Cathepsin B in angiogenesis of human prostate: an immunohistochemical and immunoelectron microscopic analysis. Anat. Rec. 241, 353–362 [DOI] [PubMed] [Google Scholar]
- 36.Strojnik T., Kos J., Zidanik B., Golouh R., Lah T. (1999) Cathepsin B immunohistochemical staining in tumor and endothelial cells is a new prognostic factor for survival in patients with brain tumors. Clin. Cancer Res. 5, 559–567 [PubMed] [Google Scholar]
- 37.Coffelt S. B., Tal A. O., Scholz A., De Palma M., Patel S., Urbich C., Biswas S. K., Murdoch C., Plate K. H., Reiss Y., Lewis C. E. (2010) Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res. 70, 5270–5280 [DOI] [PubMed] [Google Scholar]
- 38.Mizgerd J. P., Kubo H., Kutkoski G. J., Bhagwan S. D., Scharffetter-Kochanek K., Beaudet A. L., Doerschuk C. M. (1997) Neutrophil emigration in the skin, lungs, and peritoneum: different requirements for CD11/CD18 revealed by CD18-deficient mice. J. Exp. Med. 186, 1357–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hafezi-Moghadam A., Thomas K. L., Prorock A. J., Huo Y., Ley K. (2001) L-selectin shedding regulates leukocyte recruitment. J. Exp. Med. 193, 863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]