Abstract
The aim of this study was to examine the relationship between main air pollutants and all cancer mortality by performing a meta-analysis. We searched PubMed, EMBASE (a biomedical and pharmacological bibliographic database of published literature produced by Elsevier), and the reference lists of other reviews until April 2018. A random-effects model was employed to analyze the meta-estimates of each pollutant. A total of 30 cohort studies were included in the final analysis. Overall risk estimates of cancer mortality for 10 µg/m3 per increase of particulate matter (PM)2.5, PM10, and NO2 were 1.17 (95% confidence interval (CI): 1.11–1.24), 1.09 (95% CI: 1.04–1.14), and 1.06 (95% CI: 1.02–1.10), respectively. With respect to the type of cancer, significant hazardous influences of PM2.5 were noticed for lung cancer mortality and non-lung cancer mortality including liver cancer, colorectal cancer, bladder cancer, and kidney cancer, respectively, while PM10 had harmful effects on mortality from lung cancer, pancreas cancer, and larynx cancer. Our meta-analysis of cohort studies indicates that exposure to the main air pollutants is associated with increased mortality from all cancers.
Keywords: air pollutants, cancer mortality, cohort study, meta-analysis
1. Introduction
The global level of particulate matter <2.5 μm in size (PM2.5) rose by 11.2% from 1990 (39.7 μg/m³) to 2015 (44.2 μg/m³), and exposure to PM2.5 was the fifth most common cause of death in 2015 globally, resulting in the deaths of 4.2 million people [1]. Ambient air pollutants were recently classified as lung carcinogens by the International Agency for Research on Cancer of the World Health Organization (WHO) and are considered as “the most extensive environmental carcinogens” [2].
To date, three meta-analyses [3,4,5] have examined the association between air pollution and lung cancer mortality; in them, a 10 µg/m3 increase in PM2.5 levels increased the risk of and mortality from cancer by 9%, 9%, and 7%, respectively. However, these three meta-analyses used both incidence and mortality data of lung cancer. Importantly, there is a difference between cancer incidence and mortality, because not all patients suffering from cancer will die from the disease [6]. Recent prospective cohort data collected from 623,048 participants over 22 years showed that a 4.4 μg/m3 increase in PM2.5 levels increased kidney and bladder cancer mortality rates by 14% and 13%, respectively [7]. Nitrogen dioxide (NO2) was also positively linked to increased mortality from colorectal cancer in this study (hazard ratio (HR) per 6.5 parts per billion (ppb): 1.06; 95% confidence interval (CI): 1.02–1.10).
At present, there are no reported quantitative meta-analyses on the association between ambient air pollution and mortality from all types of cancers. The current study addressed this gap by performing a meta-analysis of 30 cohort studies, as well as various subgroup analyses of the factors that might influence the results.
2. Materials and Methods
2.1. Data Sources and Searches
We searched PubMed and EMBASE (a biomedical and pharmacological bibliographic database of published literature produced by Elsevier) from October 1958 to April 2018 using common keywords related to air pollutants and cancer mortality. The keywords were “air pollution”, “air pollutants”, “particulate matter”, “nitrogen dioxide”, “sulfur dioxide”, and “ozone” for exposure factors and “cancer”, “malignancy”, and “carcinoma” for outcome factors. Additionally, we inspected the bibliographies of related articles and reviews to identify additional pertinent data.
2.2. Study Selection and Eligibility
We included observational articles that met the following criteria: (1) a prospective or retrospective cohort study; (2) examined the association between air pollution and mortality from any type of cancer; and (3) reported outcome measures with adjusted relative risk (RR) and 95% CI. When two or more analyses contained duplicated data or used the same participants, we included the more comprehensive analysis. We excluded the following: (1) in vivo and in vitro studies; (2) case reports, review articles, and letters; (3) studies on cancer incidence but not mortality; (4) studies with inconvertible data; and (5) studies assessing indoor, occupational, or accidental exposures to pollutants.
Using the selection criteria, three authors (H.B.K., J.Y.S., and B.P.) independently assessed the eligibility of the retrieved articles. Any disagreements among the evaluators were resolved by discussion with the help of a fourth author (Y.J.L.).
2.3. Data Extraction
Two authors (H.B.K. and B.P.) independently extracted the study characteristics from the eligible articles, which were then reviewed by a third author (Y.J.L). The extracted data included the name of the first author, publication year, type of cohort study, year in which the participants were enrolled, location of the study, means of quantifying exposure (e.g., degree of exposure, mean concentration of pollutants), number of cases, type and stage of cancer, adjusted confounding variables, and adjusted RR ratios and 95% CI.
2.4. Assessment of Methodological Quality
We used the Newcastle–Ottawa Scale (NOS) [8] to estimate the methodological quality of the studies included in our meta-analysis. The NOS is comprised of three subscales (selection of studies, comparability, and exposure), and its scores range from 0–9. There is no established cut-off point for high versus low quality; hence, we rated studies with higher than average scores as high-quality and analyzed all studies despite their score.
2.5. Main and Subgroup Analyses
The main analysis examined the association between long-term exposure to air pollutants and cancer mortality. Subgroup analyses assessed the effect of the following factors on cancer mortality: type of air pollutant, gender, geographical region, duration of cohort study, mean pollutant concentration according to WHO guidelines, type of cancer, stage of cancer, number of participants, methodological quality, and smoking status. Subgroup analyses were conducted separately for the two pollutants that most significantly impacted cancer mortality.
2.6. Statistical Analyses
Because most exposure-response meta-analyses consider the relationship between air pollution and disease mortality to be linear [9,10], our protocol also included standardized increments: a 10 μg/m3 increase in exposure to PM2.5; particulate matter <10 μm in size (PM10); NO2, nitrogen oxides (NOx), and sulfur dioxide (SO2); and a 10 ppb increase in exposure to ozone (O3). We recalculated the RR for the standardized increment for each pollutant by applying the following formula [11]:
where RR is the relative risk and ln is the log to the base e. If the RR was presented on a continuous scale as an interquartile range (IQR), we used the increment in IQR instead of the increments noted above.
To evaluate the association between air pollutants and cancer mortality, a pooled RR ratio and 95% CI was calculated from the adjusted RR ratio and 95% CI in each study. To test heterogeneity across studies, we used the Higgins I2 test to determine the percentage of total variation [12]. I2 was computed as follows:
| I2 = 100% × (Q − df)/Q |
where Q is Cochran’s heterogeneity statistic and df indicates the degrees of freedom. I2 values ranged from 0% (no observed heterogeneity) to 100% (maximal heterogeneity), with values >50% indicating substantial heterogeneity [12]. A random-effects model based on the DerSimonian and Laird method was used for calculating the overall RR and 95% CI values, because populations and methodologies differed among the studies [13].
We assessed publication bias using Begg’s funnel plot and Egger’s test [14]. When bias was present, the funnel plot showed asymmetry or Egger’s test had a p-value <0.05. We used Stata SE software, version 13.1 (StataCorp, College Station, TX, USA) for the statistical analyses.
3. Results
3.1. Eligible Studies
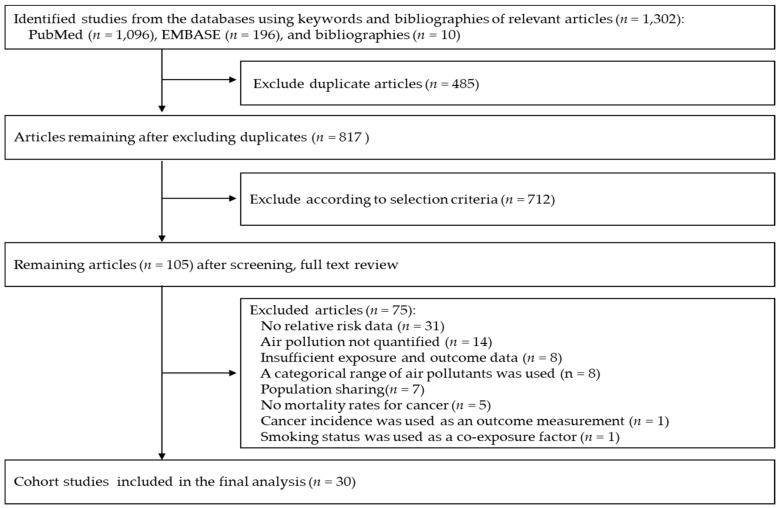
The abstracts of a total of 1302 articles were identified in the initial investigation of two databases and by hand-searching relevant bibliographies. After excluding 485 duplicated articles, two of the authors independently surveyed the eligibility of all studies and excluded an additional 712 articles that did not meet the predetermined inclusion criteria (Figure 1). Finally, the full texts of the remaining 105 articles were inspected, of which 75 articles were excluded for the following reasons: no RR data (n = 31), air pollution not quantified (n = 14), insufficient exposure and outcome data (n = 8), a categorical range of air pollutants was used (n = 8), population sharing (n = 7), no mortality rates for cancer (n = 5), cancer incidence was used as an outcome measurement (n = 1), and smoking status was used as a co-exposure factor (n = 1). The remaining 30 cohort studies were included in the meta-analysis [7,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. All cohort studies were prospective except the study by Ancona et al. [35], which was retrospective.
Figure 1.
Flow diagram for identification of relevant studies.
3.2. Characteristics of Studies Included in the Final Analysis
Table 1 shows the general characteristics of the 30 cohort studies included in our meta-analysis. All studies were published between 1999 and 2017 and together comprised >36,077,332 participants. In studies reporting age, the mean age of the participants was 57.3 years (range: 0–120 years). Regarding the type of cancer, most of them concerned lung cancer, while some of them involved all types. Mostly, the selected studies were conducted in the United States (n = 10), the Netherlands (n = 3), and China (n = 3). Adjusted variables of each study were presented in Table A1.
Table 1.
General characteristics of the cohort studies included in the final analysis (n = 30).
| References (Publication Year) | Type of Cohort Study | Country | Years Enrolled | Number of Cases | Cancer Site | Definition of Pollutant Exposure (Incremental Increase) | RR (95% CI) | Quality Assessment (Newcastle–Ottawa Stars) |
|---|---|---|---|---|---|---|---|---|
| Abbey et al. (1999) [15] | Prospective | USA | 1977–1992 | 29 cases | Lung | PM10 24.08 µg/m3 increase | 3.36 (1.57–7.19) | 8 |
| Hoek et al. (2002) [16] | Prospective | Netherlands | 1986–1994 | 244 cases | Non-lung | NO2 30 µg/m3 increase | 1.08 (0.63–1.85) | 9 |
| Nafstad et al. (2004) [17] | Prospective | Norway | 1972–1998 | 382 cases | Lung | NOx 10 µg/m3 increase | 1.11 (1.03–1.19) | 8 |
| Filleul et al. (2005) [18] | Prospective | France | 1974–2000 | 178 cases | Lung | NO2 10 µg/m3 increase | 1.48 (1.05–2.06) | 9 |
| Boldo et al. (2006) [19] | Prospective | Spain | 1999–2003 | 1901 cases | Lung | PM2.5 15 µg/m3 increase | 1.14 (1.04–1.23) | 5 |
| Brunekreef et al. (2009) [20] | Prospective | Netherlands | 1987–1996 | 1935 cases | Lung | PM2.5 10 µg/m3 increase | 1.06 (0.82–1.38) | 8 |
| McKean-Cowdin et al. (2009) [21] | Prospective | USA | 1982–1988 | 1284 cases | Brain | PM2.5 10 µg/m3 increase | 0.91 (0.74–1.11) | 8 |
| Cao et al. (2010) [22] | Prospective | China | 1991–2000 | 624 cases | Lung | SO2 10 µg/m3 increase | 1.04 (1.02–1.06) | 8 |
| Poppe CA et al. (2011) [23] | Prospective | USA | 1983–1988 | 3194 cases | Lung | PM2.5 10 µg/m3 increase | 1.14 (1.04–1.23) | 8 |
| Hart et al. (2011) [24] | Prospective | USA | 1985–2000 | 800 cases | Lung | PM2.5 4 µg/m3 increase | 1.02 (0.95–1.10) | 6 |
| Katanoda et al. (2011) [25] | Prospective | Japan | 1983–1992 | 518 cases | Lung | PM2.5 10 µg/m3 increase | 1.24(1.12–1.37) | 8 |
| Lipsett et al. (2011) [26] | Prospective | USA | 1996–2005 | 234 cases | Lung | PM2.5 10 µg/m3 increase | 0.95 (0.70–1.28) | 8 |
| Lepeule et al. (2012) [27] | Prospective | USA | 1974–2009 | 350 cases | Lung | PM2.5 10 µg/m3 increase | 1.37 (1.07–1.75) | 9 |
| Hales et al. (2013) [28] | Prospective | New Zealand | 1996–1998 | 1686 cases | Lung | PM10 1 µg/m3 increase | 1.02 (1.00–1.03) | 8 |
| Hu et al. (2013) [29] | Prospective | USA | 1999–2009 | 255,128 women | Breast | PM10 10 µg/m3 increase | 1.13 (1.02–1.25) | 6 |
| Carey et al. (2013) [30] | Prospective | United Kingdom | 2003–2007 | 5273 cases | Lung | PM2.5 1.9 µg/m3 increase | 1.04 (0.99–1.09) | 6 |
| Cesaroni et al. (2013) [31] | Prospective | Italy | 2001–2010 | 12,208 cases | Lung | PM2.5 10 µg/m3 increase | 1.05 (1.01–1.10) | 8 |
| Heinrich et al. (2013) [32] | Prospective | Germany | 1990-2008 | 41 cases | Lung | PM10 7 µg/m3 increase | 1.84 (1.23–2.74) | 8 |
| Yorifuji et al. (2013) [33] | Prospective | Japan | 1999–2009 | 116 cases | Lung | NO2 10 µg/m3 increase | 1.20(1.03–1.40) | 8 |
| Fischer et al. (2015) [34] | Prospective | Netherlands | 2004–2011 | 53,735 cases | Lung | PM10 10 µg/m3 increase | 1.26 (1.21–1.30) | 8 |
| Ancona et al. (2015) [35] | Retrospective | Italy | 2001–2010 | 2196 cases | All | PM10 27 µg/m3 increase | 1.04 (0.92–1.17) | 8 |
| Chen et al. (2016) [36] | Prospective | China | 1998–2009 | 140 cases | Lung | PM10 10 µg/m3 increase | 1.05 (1.03–1.06) | 9 |
| Eckel et al. (2016) [37] | Prospective | USA | 1988–2009 | 352,053 cases | Lung | PM2.5 5.3 µg/m3 increase | 1.15 (1.14–1.16) | 7 |
| Weichenthal et al. (2016) [38] | Prospective | Canada | 1991–2009 | 3200 cases | Lung | PM2.5 10 µg/m3 increase | 1.05 (1.00–1.10) | 7 |
| Wong et al. (2016) [39] | Prospective | Hong Kong | 1998–2011 | 4531 cases | All | PM2.5 10 µg/m3 increase | 1.22 (1.11–1.34) | 8 |
| Cohen et al. (2016) [40] | Prospective | Israel | 1992–2013 | 105 cases | All | NOx 10 ppb increase | 1.08 (0.93–1.26) | 9 |
| Guo et al. (2017) [41] | Prospective | China | 1990–2009 | 315,530 cases | Lung | PM2.5 10 µg/m3 increase | 1.08 (1.07–1.09) | 5 |
| Pun et al. (2017) [42] | Prospective | USA | 2000–2008 | 255,544 cases | All | PM2.5 10 µg/m3 increase | 1.11 (1.09–1.12) | 7 |
| Deng et al. (2017) [43] | Prospective | USA | 2000–2009 | 20,221 cases | Liver | PM2.5 10 µg/m3 increase | 1.18 (1.16–1.20) | 8 |
| Turner et al. (2017) [7] | Prospective | Canada | 1982–2004 | 43,320 cases | Non-lung | NO2 6.5 ppb increase | 1.06 (1.02–1.10) | 8 |
Abbreviations: CI, confidence interval; NO, nitrogen oxides; PM, particulate matter; ppb, parts per billion; RR, relative risk.
Ten studies used fixed-site monitor measurements for the exposure assessment method, while 17 studies used modeling-based assessment methods such as land-use regression or air dispersion models. All studies except three [29,31,35] were funded by public/governmental organizations or independent scientific foundations. The NOS scores of the studies ranged from 5 to 9; the average score was 7.7. The number of high-quality studies (NOS score ≥ 8) was 21. Data were extracted from the general population in all studies except four, which were conducted on breast cancer patients [29], lung cancer patients [37], patients with myocardial infarction [40], and liver cancer patients [43], respectively.
3.3. Overall Meta-Estimates and Publication Bias
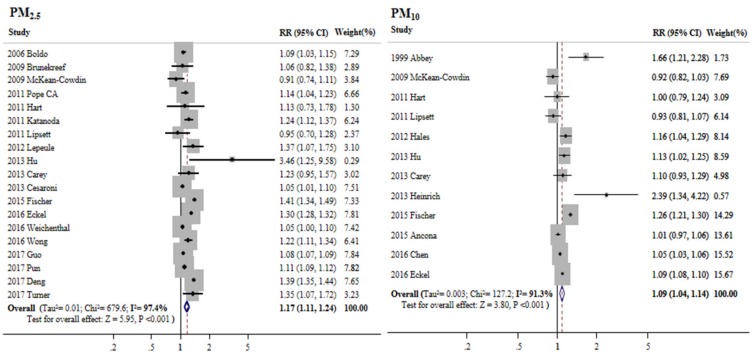
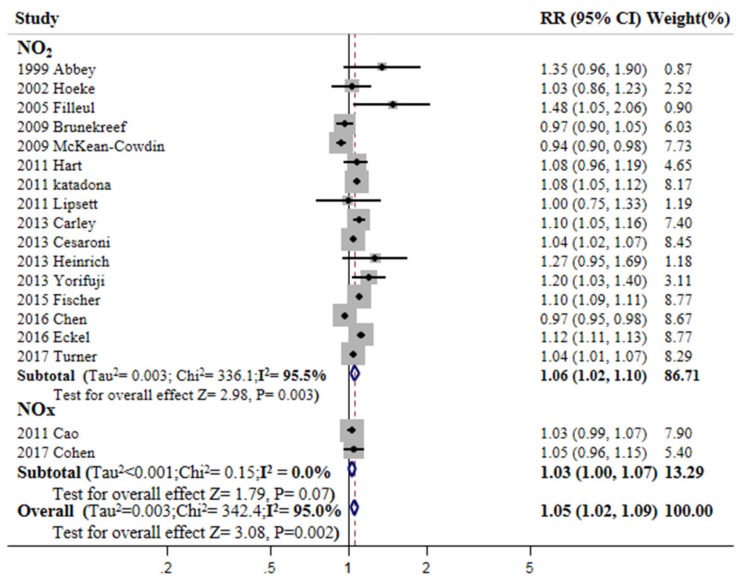
All-cancer mortality significantly correlated with long-term exposure to PM2.5 (RR: 1.17; 95% CI: 1.11–1.24; I2: 97.4%), PM10 (RR: 1.09; 95% CI: 1.04–1.14; I2: 45.7%) (Figure 2), and NO2 (RR: 1.06; 95% CI: 1.02–1.10; I2: 95.5%) (Figure 3). Significant, although less strong, mortality associations were also observed for NOx (RR: 1.03; 95% CI: 1.00–1.07; I2: 0.0%) and SO2 (RR: 1.03; 95% CI: 1.00–1.05; I2: 56.6%). Pooled data for NO2 and NOx indicated that air pollutants composed of nitrogen compounds significantly increased the risk of cancer mortality (RR: 1.05; 95% CI: 1.02–1.09; I2: 95.0%). Exposure to O3 reduced the risk estimate, albeit not to a significant extent (RR: 0.98; 95% CI: 0.90–1.07; I2: 74.5%; not shown in figure). In Table A2, a stratified analysis showed no publication bias in terms of the results for PM2.5, PM10, and NO2 (Egger’s test for asymmetry: p = 0.40, 0.68, and 0.41, respectively; Begg’s funnel plots were all symmetrical).
Figure 2.
Mortality from cancer according to long-term exposure to particulate matter (PM) in a random-effects meta-analysis of observational studies. RR, relative risk; CI, confidence interval (RR and 95% CI are for a 10 μg/m3 increase in PM2.5 and PM10).
Figure 3.
Mortality from cancer according to long-term exposure to nitrogen dioxide (NO2) and nitrogen oxides (NOx) in a random-effects meta-analysis of observational studies. RR, relative risk; CI, confidence interval (RR and 95% CI are for a 10 μg/m3 increase in NO2 and NOx).
3.4. Subgroup Analyses of the Association between PM2.5 and Cancer Mortality
The significant relationship between PM2.5 and cancer mortality was very similar in the subgroup analyses stratified by gender, geographical region, follow-up period, mean levels of pollutant concentration, stage of cancer, number of participants, methodological quality, and smoking status.
As shown in Table 2, long-term exposure to PM2.5 increased mortality from liver cancer, colorectal cancer, bladder cancer, and kidney cancer, as well as mortality from lung cancer. There was a similar association between PM2.5 and mortality from non-lung cancer (RR: 1.16, 95% CI: 1.04–1.30) when compared with mortality from lung cancer (RR: 1.14, 95% CI: 1.07–1.21). In addition, early stage cancer was more prominent in relation to air pollution and cancer mortality (RR: 1.81, 95% CI: 1.63–2.01 for localized state; RR: 1.47, 95% CI: 1.36–1.59 for regional state; and RR: 1.17, 95% CI: 1.05–1.30, for metastatic state, respectively).
Table 2.
Particulate matter and cancer mortality in the subgroup meta-analysis of cohort studies by various factors. WHO, World Health Organization.
| Subgroups | PM2.5 | PM10 | ||||
|---|---|---|---|---|---|---|
| No. of Studies | Summary RR (95% CI) | I2 (%) | No. of Studies | Summary RR (95% CI) | I2 (%) | |
| Gender | ||||||
| Male only | 5 | 1.14 (1.00, 1.29) | 80.5 | 4 | 1.06 (0.93, 1.22) | 69.1 |
| Female only | 6 | 1.13 (1.05, 1.21) | 32.0 | 6 | 1.03 (0.92, 1.15) | 72.3 |
| Male and Female | 16 | 1.18 (1.11, 1.25) | 97.8 | 6 | 1.10 (1.05, 1.16) | 94.9 |
| Region | ||||||
| America | 11 | 1.18 (1.08, 1.29) | 97.2 | 6 | 1.05 (1.05. 1.23) | 76.5 |
| Europe | 5 | 1.16 (1.00, 1.35) | 94.9 | 4 | 1.18 (0.99, 1.41) | 95.3 |
| Asia | 3 | 1.17 (1.05, 1.30) | 85.1 | 1 | 1.05 (1.03, 1.06) | NA |
| Follow-up period | ||||||
| <10 years | 10 | 1.17 (1.07, 1.27) | 96.3 | 4 | 1.11 (0.96, 1.29) | 89.6 |
| ≥10 years | 9 | 1.19 (1.07, 1.32) | 98.1 | 9 | 1.06 (1.03, 1.09) | 82.1 |
| Mean levels of pollutant concentration according to the WHO guideline | ||||||
| Below the standard | 4 | 1.20 (1.04, 1.39) | 98.3 | 1 | 1.16 (1.04, 1.29) | NA |
| Above the standard | 12 | 1.18 (1.09, 1.28) | 91.1 | 9 | 1.09 (1.04, 1.15) | 93.1 |
| Types of cancer | ||||||
| Lung cancer | 14 | 1.14 (1.07, 1.21) | 97.1 | 9 | 1.07 (1.03, 1.11) | 83.3 |
| Cancers other than lung cancer | 5 | 1.16 (1.04, 1.30) | 90.9 | 3 | 1.05 (0.99, 1.11) | 44.1 |
| Brain cancer | 2 | 1.00 (0.84, 1.19) | 36.1 | 2 | 0.93 (0.83, 1.03) | 0.0 |
| Lymphatic & hematopoietic cancer | 2 | 1.06 (0.90, 1.25) | 10.6 | 1 | 1.04 (0.93, 1.16) | NA |
| Breast cancer | 3 | 1.60 (0.94, 2.72) | 83.4 | 2 | 1.06 (0.93, 1.21) | 64.6 |
| Liver cancer | 2 | 1.29 (1.06, 1.58) | 67.8 | 1 | 1.11 (0.84, 1.46) | NA |
| Pancreas cancer | 1 | 0.96 (0.91, 1.02) | NA | 1 | 1.05 (1.04, 1.28) | NA |
| Larynx cancer | 1 | 1.09 (0.66, 1.79) | NA | 1 | 1.27 (1.06, 1.54) | NA |
| Stomach cancer | 2 | 1.17 (0.83, 1.65) | 73.4 | 1 | 0.99 (0.84, 1.16) | NA |
| Colorectal cancer | 2 | 1.08 (1.00, 1.17) | 0.0 | 1 | 0.87 (0.71, 1.07) | NA |
| Bladder cancer | 1 | 1.32 (1.07, 1.60) | NA | 1 | 1.17 (0.88, 1.57) | NA |
| Kidney cancer | 1 | 1.35 (1.07, 1.72) | NA | 1 | 1.03 (0.84, 1.26) | NA |
| Stage of cancer | ||||||
| Localized | 3 | 1.81 (1.63, 2.01) | 74.0 | 2 | 1.20 (1.12, 1.28) | 45.1 |
| Regional | 3 | 1.47 (1.36, 1.59) | 55.2 | 2 | 1.12 (1.11, 1.13) | 0.0 |
| Metastasis | 3 | 1.17 (1.05, 1.30) | 71.2 | 2 | 1.08 (1.02, 1.14) | 49.3 |
| No. of participants | ||||||
| Small (<100,000) [15,16,17,18,22,24,25,27,32,33,35,36,39,40] | 5 | 1.22 (1.15, 1.30) | 0.0 | 6 | 1.05 (0.97, 1.13) | 77.0 |
| Large (>100,000) [7,19,20,21,23,28,29,30,31,34,37,38,41,42,43] | 14 | 1.17 (1.10, 1.24) | 98.1 | 6 | 1.11 (1.02, 1.21) | 92.8 |
| Methodological quality | ||||||
| Low quality (<8) | 9 | 1.14 (1.06, 1.22) | 98.1 | 4 | 1.09 (1.08, 1.10) | 0.0 |
| High quality (≥8) | 10 | 1.20 (1.08, 1.33) | 93.5 | 8 | 1.10 (1.01, 1.21) | 94.2 |
| Smoking status | ||||||
| Non-smokers | 3 | 1.14 (1.01, 1.28) | 0.0 | 1 | 1.66 (1.22, 2.28) | NA |
| Ex-smokers | 3 | 1.47 (1.17, 1.84) | 51.4 | |||
| Current smokers | 2 | 1.33 (1.20, 1.49) | 0.0 | |||
NA, not applicable; PM, particulate matter; RR, relative risk; WHO, world health organization.
3.5. Subgroup Analyses of the Association between PM10 and Cancer Mortality
Long-term exposure to PM10 significantly correlated with cancer mortality in subgroup analyses stratified by mean pollutant concentration, cancer stage, methodological quality, and smoking status. As shown in Table 2, it increased the mortality rate in pancreas cancer, larynx cancer, and lung cancer. However, PM10 was not related to mortality from cancers other than lung cancer, in contrast to PM2.5. Similar to PM2.5, PM10 best correlated with mortality in early-stage cancer.
PM10, unlike PM2.5, did not adversely affect mortality rates in men, women, patients in Europe, patients with follow-up periods <10 years, and a small study size.
4. Discussion
Our meta-analysis of 30 cohort studies involved >1.0 million cases in 14 countries and hence provided sufficient statistical power. It showed that ambient air pollution significantly correlated with cancer mortality in analyses including all participants, as well as those stratified for various factors. Among the pollutants examined, PM2.5, PM10, or NO2 were most strongly associated with cancer mortality, whereas O3 was not significantly associated.
The deleterious effects of air pollution on survival were not limited to the lungs, but also included non-lung organs, especially in cancer patients exposed to PM2.5. Evidence from several in vivo studies suggests that particulate pollutants can travel to the liver, kidneys, and brain [44,45,46]. Our study indicates that air pollution is more strongly linked to cancer mortality in early-stage patients than those in later stages. Although many clinicians presume that the opposite is true, current research shows that patients in earlier stages of cancer may require more education regarding air pollution exposure prevention.
How air pollution increases cancer mortality rates is unclear, but two mechanisms have been proposed. The first mechanism involves DNA damage due to oxidative stress. Reactive oxygen species cause oxidative stress and are generated in response to PM [47]. Nitrogen pollutants can exacerbate the effects of oxidative stress on the progression of breast, prostate, colorectal, cervical, and other cancers [48]. Exposure to SO₂ is extremely harmful, as it induces oxidative stress in many organs [49]. Undue oxidative stress in cancer cells may seriously affect survival outcomes by promoting cell proliferation, genetic instability, and mutations [50]. In a prospective cohort study from the United States that included 30,239 Caucasian and African-American participants, there was a significant association between an oxidative stress and cancer mortality [51].
The second mechanism involves inflammation. In an in vitro study, inhaled gaseous and particulate pollutants increased the production of proinflammatory cytokines such as interleukin (IL)-6 and IL-8 [52]. In a cohort panel study conducted in the United States, exposure to NOx and PM increased plasma IL-6 levels over a 12-week period [53]. The poor prognosis of gastric cancer and non-Hodgkin’s lymphoma has been linked to excessive amounts of the proinflammatory cytokines tumor necrosis factor and IL-1, respectively [54,55]. Furthermore, the production of tumor-associated macrophages, which occurs during the inflammatory reaction, is a sign of an exacerbated cancer state [56]. Thus, inflammation caused by exposure to air pollution may result in cancer mortality.
Unlike the other pollutants in our study, O3 did not significantly impact lung cancer and brain cancer survival. Similarly, in the meta-analysis conducted by Atkinson et al. on lung cancer only [57], there was no association between long-term exposure to O3 and lung cancer mortality (RR: 0.95; 95% CI: 0.83–1.08; I2:: 55%). Nonetheless, evaluating this relationship is challenging. O3 is comprised of a combination of noxious air elements termed the “photochemical cocktail”, and its mechanisms of formation and destruction differ from those of other pollutants [58].
The key strengths of our meta-analysis are its inclusion of all cancer types, its separation of cancer mortality from cancer incidence, and its coverage of more countries and cases than previous studies. It also included more factors in its subgroup analyses than did the three previous meta-analyses that assessed the association between air pollution and lung cancer risk [3,4,5]. Furthermore, unlike previous studies, it examined the impact of air pollution on non-lung cancer mortality as well as lung cancer mortality. Overall, it provided the most comprehensive information to date on the mortality risk of cancer patients exposed to the main air pollutants.
The limitations of the current study include (1) no distinction between urban and rural areas; (2) considerable heterogeneity as indicated by the Higgins I2 values; (3) no information about indoor air pollution caused by heating, cooking, and passive smoking; (4) inclusion of only one or two studies in most cancer subgroups (lung and breast cancers were the exceptions); and (5) no data on confounding factors such as physical activity, X-ray testing, and radon exposure [59].
5. Conclusions
Our data showing a robust association between air pollution and all-cancer mortality have important implications for public health. This association applied to almost all of the pollutants examined in the study and was strongest for particulate pollutants in the regions wherein their mean concentrations were below standard levels. Similarly, a recent cohort study in the United States with >60 million participants found that exposure to PM2.5 increased all-cause mortality rates at concentrations below the present national limits [60]. Hence, rigorous environmental health policies are needed to keep air pollution levels, and consequently cancer mortality rates, as low as possible. Additionally, our results show that different types of PM increase the mortality rates for different types of non-lung cancers (PM2.5: liver, colorectal, bladder, and kidney; PM10: pancreas and larynx); hence, they may act via different mechanisms. Future research should focus on the association between certain types of pollutants and mortality from organ- and type-specific cancers.
Acknowledgments
The authors would like to express gratitude to Sun-Young Kim for providing necessary materials and helpful comments on the article.
Appendix A
Table A1.
Adjusted variables of each study.
| Study | Adjusted Variables |
|---|---|
| Abbey et al. (1999) [15] | Education, smoking status, and alcohol use |
| Hoek et al. (2002) [16] | Age, sex, smoking status, education, occupation, SEP, BMI, alcohol consumption, total fat intake, vegetable consumption, and fruit consumption |
| Nafstad et al. (2004) [17] | Age, education, smoking habits, leisure-time physical activity, occupation, and risk groups for cardiovascular diseases |
| Filleul et al. (2005) [18] | Age; sex; smoking habits; educational level; BMI; and occupational exposure to dust, gases, and fumes |
| Boldo et al. (2006) [19] | Not available |
| Brunekreef et al. (2009) [20] | Age, sex, and smoking status |
| McKean-Cowdin et al. (2009) [21] | Age, sex, race, education level, number of colds in the past year, family history of brain cancer, previous radium treatment, number of head/neck X-rays, and use of vitamins |
| Cao et al. (2010) [22] | Age, sex, BMI, physical activity, education, smoking status, age at starting to smoke, years smoked, cigarettes per day, alcohol intake, and hypertension |
| Poppe CA et al. (2011) [23] | Age, sex, smoking status, education, marital status, BMI, alcohol consumption, occupational exposures, and diet |
| Hart et al. (2011) [24] | Age, calendar year, decade of hire, region of residence, race, ethnicity, census region of residence, the healthy worker survivor effect, and years of work in each of the job groups |
| Katanoda et al. (2011) [25] | Age, sex, smoking status, pack-years, smoking status of family members living together, daily green and yellow vegetable consumption, daily fruit consumption, and use of indoor charcoal or briquette braziers for heating |
| Lipsett et al. (2011) [26] | Age, race, smoking status, total pack-years, BMI, marital status, alcohol consumption, second-hand smoke exposure at home, dietary fat, dietary fiber, dietary calories, physical activity, menopausal status, hormone therapy use, family history of MI or stroke, blood pressure medication, aspirin use, and contextual variables (income, income inequality, education, population size, racial composition, and unemployment) |
| Lepeule et al. (2012) [27] | Age, sex, time in the study, BMI, education, and smoking history |
| Hales et al. (2013) [28] | Age, sex, ethnicity, social deprivation, income, education, smoking history, and ambient temperature |
| Hu et al. (2013) [29] | Age, race, marital status, cancer stage, year diagnosed, education, income, and accessibility to medical resources |
| Carey et al. (2013) [30] | Age, sex, smoking, BMI, and education |
| Cesaroni et al. (2013) [31] | Sex, marital status, place of birth, education, occupation, and SEP |
| Heinrich et al. (2013) [32] | Educational level and smoking history |
| Yorifuji et al. (2013) [33] | Age, sex, smoking category, BMI, hypertension, diabetes, financial capability, and area mean income |
| Fischer et al. (2015) [34] | Age, sex, marital status, region of origin, standardized household income, and neighborhood social status |
| Ancona et al. (2015) [35] | Age, gender, education, occupation, civil status, area-based SEP index, and outdoor nitrogen dioxide (NO2) concentration |
| Chen et al. (2016) [36] | Age, gender, marital status, education, BMI, smoking status, alcohol consumption, occupational exposures, and leisure exercise |
| Eckel et al. (2016) [37] | Age, sex, race/ethnicity, marital status, education index, SEP, rural-urban commuting area, distance to primary interstate highway, histology at diagnosis, year of diagnosis, and initial treatment |
| Weichenthal et al. (2016) [38] | Age, sex, aboriginal ancestry, visible minority status, immigrant status, marital status, highest level of education, employment status, occupational classification, and household income |
| Wong et al. (2016) [39] | Age, gender, BMI, smoking status, exercise frequency, education level, and personal monthly expenditure |
| Cohen et al. (2016) [40] | Age, sex, ethnicity, SEP, obesity at baseline, and smoking status |
| Guo et al. (2017) [41] | None |
| Pun et al. (2017) [42] | Race, smoking, diabetes, BMI, alcohol consumption, asthma, and median income |
| Deng et al. (2017) [43] | Age, sex, race/ethnicity, marital status, SEP, RUCA, distance to primary interstate highway, month and year of diagnosis, and initial treatments |
| Turner et al. (2017) [7] | Age, race/ethnicity, gender, education, marital status, BMI, smoking status, passive smoking, vegetable/fruit/fiber consumption, fat consumption, alcohol consumption, industrial exposures, occupation dirtiness index, and 1990 ecological covariates |
Abbreviations: BMI, body mass index; MI, myocardial infarction; RUCA, rural–urban commuting area; SEP, socio-economic position.
Table A2.
Assessment of publication bias using Begg’s funnel plot and Egger’s test.
| Air Pollutants | p-Value from Egger’s Test | Begg’s Funnel Plot |
|---|---|---|
| PM2.5 | 0.40 | Symmetry |
| PM10 | 0.68 | Symmetry |
| NO2 | 0.41 | Symmetry |
Abbreviations: NO2, nitrogen dioxide; PM, particulate matter.
Author Contributions
Y.-J.L. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; Study concept and design, Y.-J.L. and H.B.K.; Acquisition, analysis, or interpretation of data, Y.-J.L., B.P., H.B.K., and J.-Y.S.; Drafting of the manuscript, H.B.K; Critical revision of the manuscript for important intellectual content, Y.-J.L.; Statistical analysis, Y.-J.L. and H.B.K.
Fundings
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cohen A.J., Brauer M., Burnett R., Anderson H.R., Frostad J., Estep K., Balakrishnan K., Brunekreef B., Dandona L., Dandona R., et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study. Lancet. 2017;389:1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loomis D., Grosse Y., Lauby-Secretan B., El Ghissassi F., Bouvard V., Benbrahim-Tallaa L., Guha N., Baan R., Mattock H., Straif K. International Agency for Research on Cancer Monograph Working Group IARC. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013;14:1262–1263. doi: 10.1016/S1470-2045(13)70487-X. [DOI] [PubMed] [Google Scholar]
- 3.Hamra G.B., Guha N., Cohen A., Laden F., Raaschou-Nielsen O., Samet J.M., Vineis P., Forastiere F., Saldiva P., Yorifuji T., et al. Outdoor particulate matter exposure and lung cancer: A systematic review and meta-analysis. Environ. Health Perspect. 2014;122:906–911. doi: 10.1289/ehp/1408092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui P., Huang Y., Han J., Song F., Chen K. Ambient particulate matter and lung cancer incidence and mortality: A meta-analysis of prospective studies. Eur. J. Public Health. 2015;25:324–329. doi: 10.1093/eurpub/cku145. [DOI] [PubMed] [Google Scholar]
- 5.Yang W.S., Zhao H., Wang X., Deng Q., Fan W.Y., Wang L. An evidence-based assessment for the association between long-term exposure to outdoor air pollution and the risk of lung cancer. Eur. J. Cancer Prev. 2016;25:163–172. doi: 10.1097/CEJ.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 6.Smittenaar C.R., Petersen K.A., Stewart K., Moitt N. Cancer incidence and mortality projections in the UK until 2035. Br. J. Cancer. 2016;115:1147–1155. doi: 10.1038/bjc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner M.C., Cohen A., Jerrett M., Gapstur S.M., Diver W.R., Pope C.A., 3rd, Krewski D., Beckerman B.S., Samet J.M. Ambient Air Pollution and Cancer Mortality in the Cancer Prevention Study II. Environ. Health Perspect. 2017;125:087013. doi: 10.1289/EHP1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 9.Mustafic H., Jabre P., Caussin C., Murad M.H., Escolano S., Tafflet M., Périer M.C., Marijon E., Vernerey D., Empana J.P., et al. Main air pollutants and myocardial infarction: A systematic review and meta-analysis. JAMA. 2012;307:713–721. doi: 10.1001/jama.2012.126. [DOI] [PubMed] [Google Scholar]
- 10.Shah A.S., Langrish J.P., Nair H., McAllister D.A., Hunter A.L., Donaldson K., Newby D.E., Mills N.L. Global association of air pollution and heart failure: A systematic review and meta-analysis. Lancet. 2013;382:1039–1048. doi: 10.1016/S0140-6736(13)60898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang W.S., Wang X., Deng Q., Fan W.Y., Wang W.Y. An evidence-based appraisal of global association between air pollution and risk of stroke. Int. J. Cardiol. 2014;175:307–313. doi: 10.1016/j.ijcard.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 12.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 13.Borenstein M., Hedges L.V., Higgins J.P., Rothstein H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 14.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbey D.E., Nishino N., McDonnell W.F., Burchette R.J., Knutsen S.F., Lawrence Beeson W., Yang J.X. Long-term inhalable particles and other air pollutants related to mortality in nonsmokers. Am. J. Respir. Crit. Care Med. 1999;159:373–382. doi: 10.1164/ajrccm.159.2.9806020. [DOI] [PubMed] [Google Scholar]
- 16.Hoek G., Brunekreef B., Goldbohm S., Fischer P., van den Brandt P.A. Association between mortality and indicators of traffic-related air pollution in the Netherlands: A cohort study. Lancet. 2002;360:1203–1209. doi: 10.1016/S0140-6736(02)11280-3. [DOI] [PubMed] [Google Scholar]
- 17.Nafstad P., Håheim L.L., Wisløff T., Gram F., Oftedal B., Holme I., Hjermann I., Leren P. Urban air pollution and mortality in a cohort of Norwegian men. Environ. Health Perspect. 2004;112:610–615. doi: 10.1289/ehp.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filleul L., Rondeau V., Vandentorren S., Le Moual N., Cantagrel A., Annesi-Maesano I., Charpin D., Declercq C., Neukirch F., Paris C., et al. Twenty five year mortality and air pollution: Results from the French PAARC survey. Occup. Environ. Med. 2005;62:453–460. doi: 10.1136/oem.2004.014746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boldo E., Medina S., LeTertre A., Hurley F., Mücke H.G., Ballester F., Aguilera I., Eilstein D., Apheis Group Apheis: Health impact assessment of long-term exposure to PM2.5 in 23 European cities. Eur. J. Epidemiol. 2006;21:449–458. doi: 10.1007/s10654-006-9014-0. [DOI] [PubMed] [Google Scholar]
- 20.Brunekreef B., Beelen R., Hoek G., Schouten L., Bausch-Goldbohm S., Fischer P., Armstrong B., Hughes E., Jerrett M., van den Brandt P. Effects of long-term exposure to traffic-related air pollution on respiratory and cardiovascular mortality in the Netherlands: The NLCS-AIR study. Res. Rep. Health Eff. Inst. 2009;139:5–71. [PubMed] [Google Scholar]
- 21.McKean-Cowdin R., Calle E.E., Peters J.M., Henley J., Hannan L., Thurston G.D., Thun M.J., Preston Martin S. Ambient air pollution and brain cancer mortality. Cancer Causes Control. 2009;20:1645–1651. doi: 10.1007/s10552-009-9412-1. [DOI] [PubMed] [Google Scholar]
- 22.Cao J., Yang C., Li J., Chen R., Chen B., Gu D., Kan H. Association between long-term exposure to outdoor air pollution and mortality in China: A cohort study. J. Hazard. Mater. 2011;186:1594–1600. doi: 10.1016/j.jhazmat.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 23.Pope C.A., 3rd, Burnett R.T., Turner M.C., Cohen A., Krewski D., Jerrett M., Gapstur S.M., Thun M.J. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: Shape of the exposure-response relationships. Environ. Health Perspect. 2011;119:1616–1621. doi: 10.1289/ehp.1103639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hart J.E., Garshick E., Dockery D.W., Smith T.J., Ryan L., Laden F. Long-term ambient multipollutant exposures and mortality. Am. J. Respir. Crit. Care Med. 2011;183:73–78. doi: 10.1164/rccm.200912-1903OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katanoda K., Sobue T., Satoh H., Tajima K., Suzuki T., Nakatsuka H., Takezaki T., Nakayama T., Nitta H., Tanabe K., et al. An association between long-term exposure to ambient air pollution and mortality from lung cancer and respiratory diseases in Japan. J. Epidemiol. 2011;21:132–143. doi: 10.2188/jea.JE20100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipsett M.J., Ostro B.D., Reynolds P., Goldberg D., Hertz A., Jerrett M., Smith D.F., Garcia C., Chang E.T., Bernstein L. Long-term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am. J. Respir. Crit. Care Med. 2011;184:828–835. doi: 10.1164/rccm.201012-2082OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lepeule J., Laden F., Dockery D., Schwartz J. Chronic exposure to fine particles and mortality: An extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environ. Health Perspect. 2012;120:965–970. doi: 10.1289/ehp.1104660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hales S., Blakely T., Woodward A. Air pollution and mortality in New Zealand: Cohort study. J. Epidemiol. Community Health. 2012;66:468–473. doi: 10.1136/jech.2010.112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu H., Dailey A.B., Kan H., Xu X. The effect of atmospheric particulate matter on survival of breast cancer among US females. J. Epidemiol. Community Health. 2012;66:468–473. doi: 10.1007/s10549-013-2527-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carey I.M., Atkinson R.W., Kent A.J., van Staa T., Cook D.G., Anderson H.R. Mortality associations with long-term exposure to outdoor air pollution in a national English cohort. Am. J. Respir. Crit. Care Med. 2013;187:1226–1233. doi: 10.1164/rccm.201210-1758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cesaroni G., Badaloni C., Gariazzo C., Stafoggia M., Sozzi R., Davoli M., Forastiere F. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ. Health Perspect. 2013;121:324–331. doi: 10.1289/ehp.1205862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinrich J., Thiering E., Rzehak P., Krämer U., Hochadel M., Rauchfuss K.M., Gehring U., Wichmann H.E. Long-term exposure to NO2 and PM10 and all-cause and cause-specific mortality in a prospective cohort of women. Occup. Environ. Med. 2013;70:179–186. doi: 10.1136/oemed-2012-100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yorifuji T., Kashima S., Tsuda T., Ishikawa-Takata K., Ohta T., Tsuruta K., Doi H. Long-term exposure to traffic-related air pollution and the risk of death from hemorrhagic stroke and lung cancer in Shizuoka, Japan. Sci. Total Environ. 2013;443:397–402. doi: 10.1016/j.scitotenv.2012.10.088. [DOI] [PubMed] [Google Scholar]
- 34.Fischer P.H., Marra M., Ameling C.B., Hoek G., Beelen R., de Hoogh K., Breugelmans O., Kruize H., Janssen N.A., Houthuijs D. Air Pollution and Mortality in Seven Million Adults: The Dutch Environmental Longitudinal Study (DUELS) Environ. Health Perspect. 2015;123:697–704. doi: 10.1289/ehp.1408254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ancona C., Badaloni C., Mataloni F., Bolignano A., Bucci S., Cesaroni G., Sozzi R., Davoli M., Forastiere F. Mortality and morbidity in a population exposed to multiple sources of air pollution: A retrospective cohort study using air dispersion models. Environ. Res. 2015;137:467–474. doi: 10.1016/j.envres.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 36.Chen X., Zhang L.W., Huang J.J., Song F.J., Zhang L.P., Qian Z.M., Trevathan E., Mao H.J., Han B., Vaughn M., et al. Long-term exposure to urban air pollution and lung cancer mortality: A 12-year cohort study in Northern China. Sci. Total Environ. 2016;571:855–861. doi: 10.1016/j.scitotenv.2016.07.064. [DOI] [PubMed] [Google Scholar]
- 37.Eckel S.P., Cockburn M., Shu Y.H., Deng H., Lurmann F.W., Liu L., Gilliland F.D. Air pollution affects lung cancer survival. Thorax. 2016;71:891–898. doi: 10.1136/thoraxjnl-2015-207927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weichenthal S., Crouse D.L., Pinault L., Godri-Pollitt K., Lavigne E., Evans G., van Donkelaar A., Martin R.V., Burnett R.T. Oxidative burden of fine particulate air pollution and risk of cause-specific mortality in the Canadian Census Health and Environment Cohort (CanCHEC) Environ. Res. 2016;146:92–99. doi: 10.1016/j.envres.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Wong C.M., Tsang H., Lai H.K., Thomas G.N., Lam K.B., Chan K.P., Zheng Q., Ayres J.G., Lee S.Y., Lam T.H., et al. Cancer Mortality Risks from Long-term Exposure to Ambient Fine Particle. Cancer Epidemiol. Biomark. Prev. 2016;25:839–845. doi: 10.1158/1055-9965.EPI-15-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen G., Levy I., Yuval, Kark J.D., Levin N., Broday D.M., Steinberg D.M., Gerber Y. Long-term exposure to traffic-related air pollution and cancer among survivors of myocardial infarction: A 20-year follow-up study. Eur. J. Prev. Cardiol. 2017;24:92–102. doi: 10.1177/2047487316669415. [DOI] [PubMed] [Google Scholar]
- 41.Guo Y., Zeng H., Zheng R., Li S., Pereira G., Liu Q., Chen W., Huxley R. The burden of lung cancer mortality attributable to fine particles in China. Sci. Total Environ. 2017;579:1460–1466. doi: 10.1016/j.scitotenv.2016.11.147. [DOI] [PubMed] [Google Scholar]
- 42.Pun V.C., Kazemiparkouhi F., Manjourides J., Suh H.H. Long-Term PM2.5 Exposure and Respiratory, Cancer, and Cardiovascular Mortality in Older US Adults. Am. J. Epidemiol. 2017;186:961–969. doi: 10.1093/aje/kwx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng H., Eckel S.P., Liu L., Lurmann F.W., Cockburn M.G., Gilliland F.D. Particulate matter air pollution and liver cancer survival. Int. J. Cancer. 2017;141:744–749. doi: 10.1002/ijc.30779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kreyling W.G., Semmler-Behnke M., Seitz J., Scymczak W., Wenk A., Mayer P., Takenaka S., Oberdörster G. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal. Toxicol. 2009;21:55–60. doi: 10.1080/08958370902942517. [DOI] [PubMed] [Google Scholar]
- 45.Elder A., Gelein R., Silva V., Feikert T., Opanashuk L., Carter J., Potter R., Maynard A., Ito Y., Finkelstein J., et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 2006;114:114,1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pery A.R., Brochot C., Hoet P.H., Nemmar A., Bois F.Y. Development of a physiologically based kinetic model for 99m-technetium-labelled carbon nanoparticles inhaled by humans. Inhal. Toxicol. 2009;21:1099–1107. doi: 10.3109/08958370902748542. [DOI] [PubMed] [Google Scholar]
- 47.Møller P., Danielsen P.H., Karottki D.G., Jantzen K., Roursgaard M., Klingberg H., Jensen D.M., Christophersen D.V., Hemmingsen J.G., Cao Y., et al. Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat. Res. Rev. Mutat. Res. 2014;762:133–166. doi: 10.1016/j.mrrev.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Kruk J., Aboul-Enein H.Y. Reactive Oxygen and Nitrogen Species in Carcinogenesis: Implications of Oxidative Stress on the Progression and Development of Several Cancer Types. Mini Rev. Med. Chem. 2017;17:904–919. doi: 10.2174/1389557517666170228115324. [DOI] [PubMed] [Google Scholar]
- 49.Zhu M., Du J., Liu A.D., Holmberg L., Tang C., Jin H. Effect of endogenous sulfur dioxide in regulating cardiovascular oxidative stress. Histol. Histopathol. 2014;29:1107–1111. doi: 10.14670/HH-29.1107. [DOI] [PubMed] [Google Scholar]
- 50.Kang D., Hamasaki N. Mitochondrial oxidative stress and mitochondrial DNA. Clin. Chem. Lab. Med. 2003;41:1281–1288. doi: 10.1515/CCLM.2003.195. [DOI] [PubMed] [Google Scholar]
- 51.Kong S.Y., Goodman M., Judd S., Bostick R.M., Flanders W.D., McClellan W. Oxidative balance score as predictor of all-cause, cancer, and noncancer mortality in a biracial US cohort. Ann. Epidmiol. 2015;25:256–262. doi: 10.1016/j.annepidem.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veranth J.M., Moss T.A., Chow J.C., Labban R., Nichols W.K., Walton J.C., Watson J.G., Yost G.S. Correlation of in vitro cytokine responses with the chemical composition of soil-derived particulate matter. Environ. Health Perspect. 2006;114:341–349. doi: 10.1289/ehp.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X., Staimer N., Gillen D.L., Tjoa T., Schauer J.J., Shafer M.M., Hasheminassab S., Pakbin P., Vaziri N.D., Sioutas C., et al. Associations of oxidative stress and inflammatory biomarkers with chemically characterized air pollutant exposures in an elderly cohort. Environ. Res. 2016;150:306–319. doi: 10.1016/j.envres.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Omar E.M., Carrington M., Chow W.H., McColl K.E., Bream J.H., Young H.A., Herrera J., Lissowska J., Yuan C.C., Rothman N., et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 55.Warzocha K., Ribeiro P., Bienvenu J., Roy P., Charlot C., Rigal D., Coiffier B., Salles G. Genetic polymorphisms in the tumor necrosis factor locus influence non-Hodgkin’s lymphoma outcome. Blood. 1998;91:3574–3581. [PubMed] [Google Scholar]
- 56.Duncan L.M., Richards L.A., Mihm M.C., Jr. Increased mast cell density in invasive melanoma. J. Cutan. Pathol. 1998;25:11–15. doi: 10.1111/j.1600-0560.1998.tb01683.x. [DOI] [PubMed] [Google Scholar]
- 57.Atkinson R.W., Butland B.K., Dimitroulopoulou C., Heal M.R., Stedman J.R., Carslaw N., Jarvis D., Heaviside C., Vardoulakis S., Walton H., et al. Long-term exposure to ambient ozone and mortality: A quantitative systematic review and meta-analysis of evidence from cohort studies. BMJ Open. 2016;6:e009493. doi: 10.1136/bmjopen-2015-009493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chardon B., Host S., Lefranc A., Millard F., Gremy I. What exposure indicator should be used to study the short-term respiratory health effect of photochemical air pollution? A case study in the Paris metropolitan area (2000–2003) Environ. Risques Sante. 2007;6:345–353. [Google Scholar]
- 59.Bräuner E.V., Andersen Z.J., Andersen C.E., Pedersen C., Gravesen P., Ulbak K., Hertel O., Loft S., Raaschou-Nielsen O. Residential radon and brain tumour incidence in a Danish cohort. PLoS ONE. 2013;8:e74435. doi: 10.1371/journal.pone.0074435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Q., Wang Y., Zanobetti A., Wang Y., Koutrakis P., Choirat C., Dominici F., Schwartz J.D. Air Pollution and Mortality in the Medicare Population. N. Engl. J. Med. 2017;376:2513–2522. doi: 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]