Abstract
Background: This study aims to assess the specific difference of the health-related quality of life between people with Parkinson’s and non-Parkinson’s. Methods: A total of 1710 people were drawn from a prospective study with a smartphone-based survey named ‘100 for Parkinson’s’ to assess health-related quality of life. The EQ-5D-5L descriptive system and the EQ visual analogue scale were used to measure health-related quality of life and a linear mixed model was used to analyze the difference. Results: The mean difference of EQ-5D-5L index values between people with Parkinson’s and non-Parkinson’s was 0.15 (95%CI: 0.12, 0.18) at baseline; it changed to 0.17 (95%CI: 0.14, 0.20) at the end of study. The mean difference of EQ visual analogue scale scores between them increased from 10.18 (95%CI: 7.40, 12.96) to 12.19 (95%CI: 9.41, 14.97) from baseline to the end of study. Conclusion: Data can be captured from the participants’ own smart devices and support the notion that health-related quality of life for people with Parkinson’s is lower than non-Parkinson’s. This analysis provides useful evidence for the EQ-5D instrument and is helpful for public health specialists and epidemiologists to assess the health needs of people with Parkinson’s and indirectly improve their health status.
Keywords: Parkinson’s disease, EQ-5D-5L, EQ visual analogue scale, smartphone, prospective study
1. Introduction
Parkinson’s disease (PD) is a degenerative movement disorder affecting 1–2% of the population aged over 60 years and is the second most common neurodegenerative disorder after Alzheimer’s disease [1,2]. It is a universal disorder, with an estimated global incidence of 4.5–19 per 100,000 population per year and it is expected that the number of people with PD will double by 2030 [3]. In the United Kingdom (UK), the annual incidence of PD is 12 per 100,000 population [4]; in Sweden, it is 19.7 per 100,000 [5] and in Norway it is 12.6 per 100,000 [6]. The core motor features of PD comprise combinations of bradykinesia, resting tremor, rigidity, flexed posture, ‘freezing’ and loss of postural reflexes [7]. It is a disabling condition with significant impact on health-related quality of life (HRQoL) [8,9], as well as the non-motor symptoms of PD such as dementia, sleep disturbances, depression and falls [10]. HRQoL is a multidimensional and comprehensive construct that assesses the perceived impact of health or disease on the physical, mental and social functioning [11]. Generally, the EQ-5D is the most popular generic measure of health status for clinical and economic appraisal [12,13].
Traditionally, research data is collected by health care professionals in hospitals, clinics and the community. However, novel data sources have now emerged with the widespread use of mobile phones [14]. Software applications are increasingly being used to consent participants, determine the eligibility of participants, run study tasks and provide reliable data without the need for participants to travel to study sites. Examples are as follows: a UK smartphone study examines the association between weather and pain [15]; an asthma symptom-tracking app uses GPS tracking to provide air quality updates in users’ locations [16]; an app collects data on gait, voice changes, balance, dexterity, and memory in subjects with Parkinson’s disease [17]; an app uses participants’ physical activity and lifestyle information as well as surveys to evaluate risks of cardiovascular disease [18]. In addition, Mulhern et al. (2014) showed that completing the EQ-5D using mobile phones produced equivalent results to more traditional methods such as paper-and-pencil, but with added benefits such as lessening the burden of data entry [19]; Bot et al. (2016) emphasized that data collection via mobile phones have the advantage of large enrollment and repeated measurements on many individuals and ultimately may lead to quantification of the ebbs-and-flows of Parkinson symptoms [20].
However, to our best knowledge, there is no empirical evidence based on smartphone data capture to assess the health-related quality of life for people with Parkinson’s. It is well-known that people with Parkinson’s disease’s health-related quality of life is lower than the non-Parkinson’s, but no specific data have been reported on the magnitude of the difference. Public health specialists, epidemiologists, and policy makers need the data to assess the health needs of society and indirectly improve health status.
This paper aims to fill this gap by using the EQ-5D measure. There are two key strengths in general. Firstly, the data were drawn from one of the largest smartphone-powered studies which enabled symptom tracking for people with Parkinson’s. Secondly, it is the first study in UK to use the EQ-5D-5L descriptive system and the EQ visual analogue scale (EQ VAS) to show the specific difference of health-related quality of life for people with Parkinson’s and non-Parkinson’s.
2. Materials and Methods
2.1. Data Source
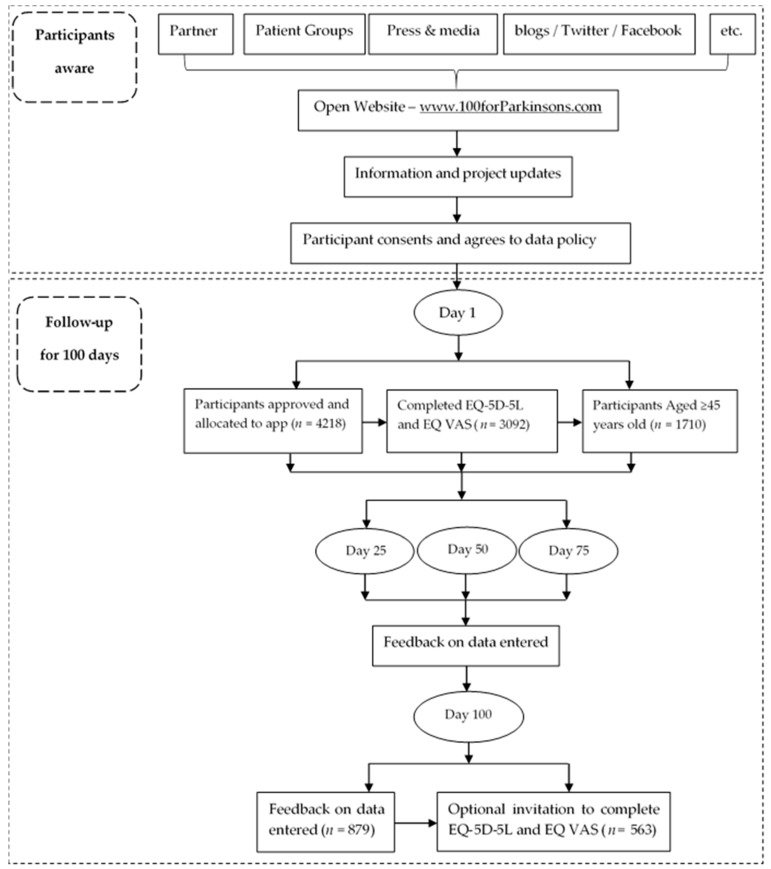
The data used for analysis constitutes one part of a dataset derived from the project named ‘100 for Parkinson’s’, an initiative of uMotif in conjunction with the Cure Parkinson’s Trust, Parkinson’s UK and the European Parkinson’s Disease Association. It was a smartphone-based study and followed the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines for reporting observational studies [21]. This project was a ‘public facing’ project, focused on engaging patients to capture data, with all participants being invited to use the uMotif data capture app on their own Android or iOS mobile phones and/or tablets for 100 consecutive days from the day they register to use the app. They then received summary feedback based on the data they entered at 25, 50, 75 and 100 days. Participants used the uMotif app to record a range of data including: daily symptoms, questionnaires (EQ-5D, PDQ-8, NMS30) diaries, medications, cognitive and motor testing games, biometric results and—for those with devices—the opportunity to connect to wearable devices (i.e., Fitbit, Pedometers, Apple watch). Participants became aware of the project via the general press and media, blogs, Twitter, Facebook, etc. People with Parkinson’s were the primary participant group; the group of non-Parkinson’s were mainly interested people from the general public visitors and were recruited from the open website (http://www.100forparkinsons.com). To be eligible, participants were adults aged over 18, with the ability to read, write, and speak in English at levels of proficiency necessary to function daily at their jobs and/or daily living, and had access to a smartphone and/or tablet on a daily basis. There were no exclusion criteria applied. A total of 4218 participants consented to take part in this project, patients from 62 countries expressed an interest in taking part. Among these respondents, this paper is UK only and based on data from participants who completed the EQ-5D questionnaire and aged more than 45 years old (n = 1710). At baseline, 1050 People with Parkinson’s and 660 non-Parkinson’s finished the EQ-5D instruments; at end of study (day 100), 301 people with Parkinson’s and 262 non-Parkinson’s finished the EQ-5D instruments (Figure 1).
Figure 1.
Participants flow chart of 100 for Parkinson’s project.
2.2. Research Ethics
The ‘100 for Parkinson’s’ study was conducted in accordance with the Declaration of Helsinki and received ethical approval from the Liverpool School of Tropical Medicine (No.15-050) and an ethics exemption from the New England IRB for participants from the USA (No.16-042). Consent was obtained by adapting E-Consent, a Participant Centered Consent (PCC) Toolkit developed by Sage Bionetworks. The process is similar to the standard consent process with the only difference being that a wet signature is not collected. All 100 for Parkinson’s participants provided e-consent for their de-identified data to be used in any future research projects that have been approved by the ‘100 for Parkinson’s Data Access Committee’. This current study received approval from the Data Access Committee for the analysis reported in this paper.
2.3. EQ-5D
EQ-5D is a standardized measure of health status developed by the EuroQol Group and includes the EQ-5D-5L descriptive system and the EQ visual analogue scale (EQ VAS) [22]. It is designed for self-completion, taking only a few minutes to complete. Firstly, the EQ-5D-5L asks patients to classify their health based on self-assessed levels of problems (no problems, slight problems, moderate problems, severe problems, and extreme problems or unable to perform) on five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. It contains 3125 possible health states defined by combining one level from each dimension, ranging from 11111 (full health) to 55555 (worst health). The numerals 1–5 have no arithmetic properties and should not be used as a cardinal score. A preference-based set of value set is used to calculate EQ-5D-5L index value from each health state because different country has a different value set according to the guidelines introduced by EuroQol Group. It is measured on a ‘0’ to ‘1’ scale where ‘0’ is defined as a health state equivalent to being dead and ‘1’ is full health. The EQ-5D-5L crosswalk value set for the United Kingdom was used, with index value ranges from −0.594 to 1 where ‘1’ is full health and −0.594 represents worst health [23]. Secondly, the EQ VAS asks respondents to rate their health on a visual analogue scale ranging from 0 (the worst imaginable health state) to 100 (the best imaginable health state). It is a quantitative measure to judge the health states of individual respondents. As sponsor of the ‘100 for Parkinson’s’ study, uMotif obtained approval from the EuroQol Research Foundation to use the EQ-5D instruments in the study.
2.4. Study Variables
The demographic data were self-reported by participants as part of the ‘100 for Parkinson’s’ study onboarding questionnaire completed within the uMotif data capture application. Participants in this study were drawn from two self-identified groups: People with Parkinson’s and non-Parkinson’s. They were followed from “day 1” to “day 100”, the EQ-5D questionnaires via smartphone were collected at the start of study and at the end of study, so the visit variable was categorized into two groups: at baseline and at end of study. Social-demographic characteristics considered in this study as confounding factors included gender (female or male), education (up to 16 years old means people stayed in school up to 16 years old, up to 18 years old means people stayed in school up to 18 years old, university/college/equivalent means the highest level of schooling person has completed), smoking status (No, never; No, but used to; Yes, occasionally/ weekly or Yes, daily) and alcohol use (No, never; Monthly or less; 2–4 times per month; 2–3 times per week; 4 or more times per week). In addition, two dummy variables were collected indicating whether participants had long-term health conditions diagnosed by a physician (such as hypertension, diabetes, heart problems, respiratory disease or cancer), and whether they live alone. These variables were selected based on the previous studies but constrained by the variables collected in the survey [24,25].
2.5. Statistical Analyses
The linear mixed model (LMM) was used to analyze the outcome variables, which includes both fixed and random effects. In this study, LMM was employed to analyze the difference of the HRQoL between people with Parkinson’s and non-Parkinson’s before and after 100 days when controlling for other confounding factors. The characteristics of the variables used are shown in Table 1. We estimated two mixed models for EQ-5D-5L value and EQ VAS score separately: Model 1 has group of participants (people with Parkinson’s and non-Parkinson’s), visit (at baseline and at end of study), and interaction between group and visit as fixed effects and subject as random effect. Model 2 is a model adding gender, education, smoking status, alcoholic drinking status, health condition and life style as covariates into model 1. From the above mixed models, the group differences in EQ-5D-5L value and EQ VAS score together with their 95%CIs at baseline and the end of the study were derived [26].
Table 1.
Characteristics of Parkinson’s and non-Parkinson’s participants at baseline (n = 1654).
| Variables | Parkinson’s | Non-Parkinson’s | χ2 | p | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Gender | 61.61 | <0.001 | ||||
| Female | 483 | 47.73 | 434 | 67.60 | ||
| Male | 529 | 52.27 | 208 | 32.40 | ||
| Education | 3.27 | 0.195 | ||||
| Up to 16 years old | 166 | 16.40 | 87 | 13.55 | ||
| Up to 18 years old | 109 | 10.77 | 64 | 9.97 | ||
| University/College/Equivalent | 737 | 72.83 | 491 | 76.48 | ||
| Do you smoke tobacco? | 1.36 | 0.714 | ||||
| No, never | 638 | 63.04 | 392 | 61.06 | ||
| No, but used to | 330 | 32.61 | 218 | 33.96 | ||
| Yes, occasionally/weekly | 21 | 2.08 | 13 | 2.02 | ||
| Yes, daily | 23 | 2.27 | 19 | 2.96 | ||
| How often do you have a drink containing alcohol? | 1.36 | 0.714 | ||||
| No, never | 155 | 15.32 | 65 | 10.12 | ||
| Monthly or less | 185 | 18.28 | 101 | 15.73 | ||
| 2–4 times per month | 197 | 19.47 | 136 | 21.18 | ||
| 2–3 times per week | 248 | 24.50 | 184 | 28.66 | ||
| 4 or more times per week | 227 | 22.43 | 156 | 24.31 | ||
| Do you have other long-term health conditions? | 3.35 | 0.067 | ||||
| No | 578 | 57.11 | 335 | 52.18 | ||
| Yes | 434 | 42.89 | 307 | 47.82 | ||
| Do you live alone? | 0.68 | 0.411 | ||||
| No | 876 | 86.56 | 547 | 85.20 | ||
| Yes | 136 | 13.44 | 95 | 14.80 | ||
All questionnaires were checked for missing data and outliers, and cleaned prior to data analysis. Mean (standard deviation) or frequency (proportions) were used to describe the characteristics of the study variables where appropriate. Differences in variables between people with Parkinson’s and Non-Parkinson’s were compared by chi-squared test. The statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA). A two-tailed p value < 0.05 was considered statistically significant.
3. Results
3.1. Characteristics of the Study Population
At baseline, a total of 1710 people aged more than 45 years old, including 1050 (61.40%) people with Parkinson’s and 660 (38.60%) non-Parkinson’s completed the EQ-5D instruments via their smartphone with a mean (standard deviation, SD) age of 62.62 (7.47) years old and 59.69 (7.17) years old for people with Parkinson’s and non-Parkinson’s, respectively. The basic characteristics were shown in Table 1. After 100 days, 301 (53.46%) people with Parkinson’s and 262 (46.54%) non-Parkinson’s completed the EQ-5D questionnaire again.
3.2. Main Results
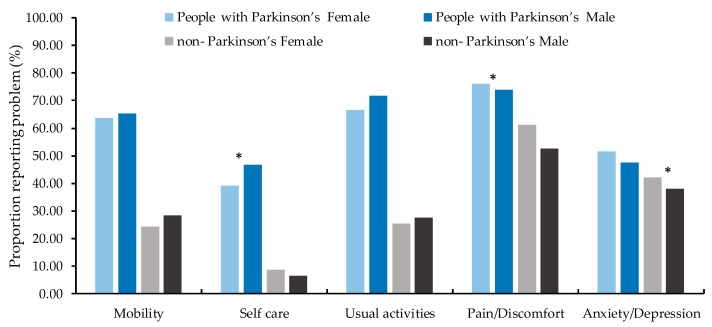
The health profile of participants at baseline is presented in Table 2 with the frequency of reported problems for each level by EQ-5D-5L dimension. People with Parkinson’s reported significantly (p ≤ 0.002) higher problems in mobility, self-care, usual activity, pain/discomfort and anxiety/depression than non-Parkinson’s. The most prevalent reported problems both for people with Parkinson’s and non-Parkinson’s were pain/discomfort: 74.86% and 58.48% respectively. Figure 2 showed for people with Parkinson’s, females had less frequency of reported problems on self-care (χ2 = 5.83, p = 0.016) but more frequency in pain/discomfort (χ2 = 4.33, p = 0.038) than males, there were no significant gender difference for mobility, usual activities and anxiety/depression (p > 0.05). For non-Parkinson’s, females reported more frequency of problem on anxiety/depression than males (χ2 = 8.54, p = 0.004) and there was no significant gender difference on other symptoms (p > 0.05).
Table 2.
The distribution of reported EQ-5D-5L levels 1 to 5 by dimension at baseline (n = 1710).
| Variables | Parkinson’s | Non-Parkinson’s | χ2 | p | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Mobility | 244.51 | <0.001 | ||||
| Level 1 | 374 | 35.62 | 489 | 74.09 | ||
| Level 2 | 424 | 40.38 | 120 | 18.18 | ||
| Level 3 | 190 | 18.10 | 32 | 4.85 | ||
| Level 4 | 56 | 5.33 | 18 | 2.73 | ||
| Level 5 | 6 | 0.57 | 1 | 0.15 | ||
| Self-care | 245.69 | <0.001 | ||||
| Level 1 | 597 | 56.86 | 607 | 91.97 | ||
| Level 2 | 347 | 33.05 | 35 | 5.31 | ||
| Level 3 | 87 | 8.29 | 9 | 1.36 | ||
| Level 4 | 17 | 1.62 | 8 | 1.21 | ||
| Level 5 | 2 | 0.18 | 1 | 0.15 | ||
| Usual Activity | 305.39 | <0.001 | ||||
| Level 1 | 323 | 30.75 | 487 | 73.79 | ||
| Level 2 | 465 | 44.29 | 124 | 18.79 | ||
| Level 3 | 202 | 19.24 | 32 | 4.85 | ||
| Level 4 | 47 | 4.48 | 12 | 1.82 | ||
| Level 5 | 13 | 1.24 | 5 | 0.7 | ||
| Pain/Discomfort | 86.51 | <0.001 | ||||
| Level 1 | 264 | 25.14 | 274 | 41.52 | ||
| Level 2 | 458 | 43.62 | 296 | 44.85 | ||
| Level 3 | 264 | 25.14 | 69 | 10.45 | ||
| Level 4 | 57 | 5.43 | 19 | 2.88 | ||
| Level 5 | 7 | 0.67 | 2 | 0.30 | ||
| Anxiety/Depression | 17.37 | 0.002 | ||||
| Level 1 | 531 | 50.57 | 390 | 59.09 | ||
| Level 2 | 366 | 34.85 | 205 | 31.06 | ||
| Level 3 | 127 | 12.10 | 59 | 8.94 | ||
| Level 4 | 23 | 2.19 | 4 | 0.61 | ||
| Level 5 | 3 | 0.29 | 2 | 0.30 | ||
level 1 response represents “no problems”, level 2 “slight problems”, level 3 “moderate problems”, level 4 “severe problems”, and level 5 “extreme problems” or “unable to perform.
Figure 2.
Frequency of reported problems for participants by gender at baseline (n = 1654). * means significant difference (p < 0.05) between two groups.
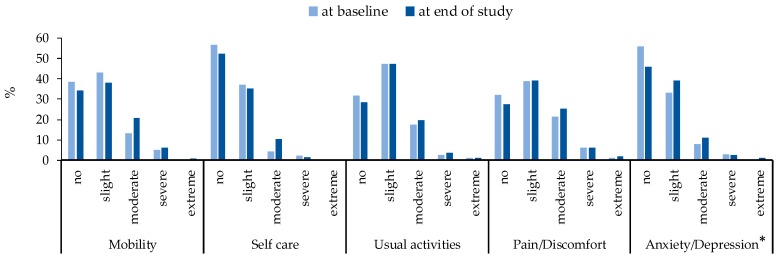
Table 3 summarized the EQ-5D-5L index values and EQ VAS scores at baseline and the end of study separately. Overall, the EQ-5D-5L values decreased significantly from 0.70 at baseline to 0.66 at the end of study for Parkinson’s (p = 0.042) and the EQ VAS scores decreased from 73.40 at baseline to 72.13 at the end of study for Parkinson’s without statistical support (p = 0.855), but they seem more stable for non-Parkinson’s (p > 0.05). According to Figure 3, for people with Parkinson’s, the percentage for no problems for anxiety/depression was decreased from baseline to the end of study while increased for slight and moderate problems (χ2 = 9.91, p = 0.042), there were no significant changes for mobility(χ2 = 8.91, p = 0.064), self-care (χ2 = 9.25, p = 0.055), usual activities(χ2 = 1.24, p = 0.871) and pain/discomfort(χ2 = 2.98, p = 0.562). Table 4 showed the mean differences of EQ-5D-5L values and EQ VAS scores between people with Parkinson’s and non-Parkinson’s at baseline and the end of study separately by four LMMs (before and after adjusting for subjects’ gender, education, smoking status, alcoholic drinking status, health condition and life style). After adjusting, the EQ-5D-5L value (confidence interval: CI) for people with Parkinson’s was 0.15 (95%CI: 0.12, 0.18) lower than non-Parkinson’s at baseline; the difference raised to 0.17 (95%CI: 0.14, 0.20) at the end of study. The mean difference of EQ VAS scores between people with Parkinson’s and non-Parkinson’s also increased from 10.18 (95%CI: 7.40, 12.96) to 12.19 (95%CI: 9.41, 14.97) from baseline to the end of study. This suggests the health-related quality of life for people with Parkinson’s was worse than non-Parkinson’s and that the difference between them increased with Parkinson’s disease progression.
Table 3.
Summary of EQ-5D-5L values and EQ visual analogue scale (VAS) scores at baseline and the end of study (n = 563).
| Summary | EQ-5D-5L | p | EQ-VAS | p | ||
|---|---|---|---|---|---|---|
| At Baseline | At End of Study | At Baseline | At End of Study | |||
| Parkinson’s | 0.042 | 0.855 | ||||
| Mean | 0.70 | 0.66 | 73.40 | 72.13 | ||
| SD | 0.19 | 0.22 | 17.87 | 18.62 | ||
| Median | 0.73 | 0.70 | 78.00 | 76.00 | ||
| Q1 | 0.62 | 0.58 | 62.00 | 65.00 | ||
| Q3 | 0.84 | 0.78 | 86.00 | 85.00 | ||
| Non-Parkinson’s | 0.346 | 0.960 | ||||
| Mean | 0.83 | 0.82 | 82.66 | 83.58 | ||
| SD | 0.16 | 0.19 | 14.66 | 12.26 | ||
| Median | 0.84 | 0.84 | 86.00 | 86.00 | ||
| Q1 | 0.75 | 0.74 | 79.00 | 77.00 | ||
| Q3 | 1.00 | 1.00 | 92.50 | 93.00 | ||
SD: standard deviation; Q1 is equal to the 25th percentile of data; Q3 is equal to the 75th percentile of data.
Figure 3.
Changes of EQ-5D-5L from baseline to end of study for people with Parkinson’s (n = 301). * means significant difference (p < 0.05) among different level of anxiety/depression.
Table 4.
The mean differences of EQ-5D-5L values and EQ VAS scores between people with Parkinson’s and non-Parkinson’s: Results from linear mixed model analysis (n = 563).
| EQ-5D | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean Difference | 95%CI | p | Mean Difference | 95%CI | p | |||
| Lower | Upper | Lower | Upper | |||||
| EQ-5D-5L values (Non-Parkinson’s vs. Parkinson’s) | ||||||||
| Baseline | 0.13 | 0.10 | 0.17 | <0.001 | 0.15 | 0.12 | 0.18 | <0.001 |
| End of study | 0.16 | 0.12 | 0.18 | <0.001 | 0.17 | 0.14 | 0.20 | <0.001 |
| EQ VAS scores (Non-Parkinson’s vs. Parkinson’s) | ||||||||
| Baseline | 9.25 | 6.56 | 11.95 | <0.001 | 10.18 | 7.40 | 12.96 | <0.001 |
| End of study | 11.45 | 8.76 | 14.14 | <0.001 | 12.19 | 9.41 | 14.97 | <0.001 |
Linear mixed model was used to estimate the group differences at baseline and the end of study. In model 1, group of participants, visit, and group*visit are treated as fixed effects and subject as random effect. Model 2 is based on model 1 plus age of participants, gender, education, smoking status, alcoholic drinking status, health condition and life style. CI: Confidence Interval.
4. Discussion
The EuroQol Group, which developed and owns the copyright on the EQ-5D, recommends that both the descriptive system of EQ-5D-5L and the EQ VAS should be used in the assessment of quality of life [27]. To the best of our knowledge, this is the first smartphone based prospective study reporting specific data on the difference of validity of EQ-5D-5L and EQ VAS between people with Parkinson’s and non-Parkinson’s in UK. Furthermore, 100 for Parkinson’s is the largest ever dataset gathered from Parkinson’s patients.
At the baseline, our study found the mean EQ-5D-5L values for people with Parkinson’s was 0.70, it was 0.83 for non-Parkinson’s. Because of the difference due to variation in categories of diseases, the population sampled or the value sets employed [28], we could not compare the mean EQ-5D-5L values for people in England with people in other places all over the world; however, we can find the difference of EQ VAS scores between them. In this study, The mean EQ VAS scores at the baseline was 73.40 for people with Parkinson’s, higher than stroke patients without coma (49.5) [29] and patients suffering from urinary incontinence (53.91) [30]; For non-Parkinson’s, the mean EQ VAS scores were 82.66, which were lower than the healthy people in Germany (91.5) [31] and Vietnam (87.4) [32] but higher than the EQ VAS scores in South Australian (78.6) [24], Spain (75.0), Italy (77.1), Korea (79.5) [33]. These conclusions show the health-related quality of life for people with Parkinson’s and non-Parkinson’s in the UK may not be low among patients with chronic disease and healthy populations in other countries.
Parkinson’s disease is the second most frequent neurodegenerative diseases and has progressive damage not only to motor symptoms but also to mental symptoms [34]. Our study finds the anxiety/depression for people with Parkinson’s became more serious after 100 days and quality of life for them became poorer. This is consistent with research on anxiety/depression may reduce quality of life in people with Parkinson’s disease because depression and anxiety may make patients have poorer response to receive treatment, more severe symptoms and higher functional impairment [35,36]. Therefore anxiety/depression should be considered in the future intervention strategies for people with Parkinson’s disease. In addition, our study estimated the specific difference of EQ-5D-5L values and EQ VAS scores between people with Parkinson’s and non-Parkinson’s after adjusting for confounders to make this conclusion generalizable. It is significant for clinicians and Parkinson’s carers or supporters to understand the condition of health-related quality of life for people with Parkinson’s with reference data. Public health specialists and epidemiologists may use the specific data to reflect the level of health-related quality of life for people with Parkinson’s and assess the health needs of them. In short, such data could be used by various stakeholders, to indirectly improve the health status of people with Parkinson’s.
For people with Parkinson’s, the prevalence of the most frequently reported EQ-5D-5L health state in our study was pain and discomfort, which was not surprising, considering that pain is a common symptom in Parkinson’s disease and can be found in two thirds of patients [37,38]. In addition, pain and discomfort was also the most frequently reported EQ-5D-5L health state for non-Parkinson’s participants which is similar to studies using traditional data collection surveys in England [39], South Australian [24], Germany [31] and Poland [40]. This equivalence indicates that a smartphone based prospective study may provide a viable alternative to traditional data collection methods. Furthermore, the ceiling effect was not high in this study with only 7.60% of people with Parkinson’s reporting a perfect health status, which is a lower ceiling effect than seen in a diabetic sample (nearly 1/3 of total sample) [41].
The current study has several limitations. Firstly, this is an observational study and the determinants of health-related quality of life included in this study are limited by the pre-specified questions in the surveys. There could be some potential unobserved confounding factors we did not control for in the linear mixed model. Secondly, EQ-5D questionnaires were administered in the fixed order, this may affect the proportion of missing answers. The desirable solution would be to present instruments in a random order. A third limitation is that the diagnosis of Parkinson’s was self-identified, without a formal diagnosis from a physician. A fourth limitation is the lack of control of the severity of Parkinson’s symptoms. A fifth limitation is our study is a prospective study and the follow-up bias inevitably influences the sample at the end of study. A final limitation is our study reports the changes of health-related quality of life for people with Parkinson’s based on quantitative study, more evidences based on the qualitative studies and randomized controlled trial are needed to clarify the functional change in the next research steps.
5. Conclusions
In summary, the smartphone based prospective study provides a viable data collection method to show that the health-related quality of life for people with Parkinson’s and non-Parkinson’s in the UK may not be low among patients with chronic disease and healthy population all over the world. The health-related quality of life for people with Parkinson’s is lower than non-Parkinson’s and the gap becomes more evident at the end of a prospective study. However, this is only a statistically significant change based on quantitative data; more evidence from qualitative studies are needed to clarify the functional change for people with Parkinson’s in the next research steps. This study provides useful literature on the EQ-5D, although the finding of health differences is to be expected. In addition, the data are helpful for understanding how low the health-related quality of life for people with Parkinson’s is compared to healthy population and are useful for public health specialists and epidemiologists to assess the health needs and indirectly improve their health status.
Acknowledgments
We express our appreciation to the Nesta/Cabinet Office Centre for Social Action Innovation Fund, a growing number of patient groups (the Cure Parkinson’s Trust and Parkinson’s UK, the European Parkinson’s Disease Association), uMotif Limited and global sponsor partners for their support.
Author Contributions
Conceptualization, D.W., B.H. and A.H.; Data curation, B.H. and I.W.; Formal analysis, X.F. and D.W.; Investigation, D.W., B.H., R.C., A.H., H.M. and I.W.; Writing—original draft, X.F., D.W., B.H., M.F.J., G.B. and R.C.; Writing—review and editing, X.F., D.W., M.F.J. and A.H.
Funding
This research received no external funding. The APC was funded by uMotif Limited.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Nussbaum R.L., Ellis C.E. Alzheimer’s Disease and Parkinson’s Disease. N. Engl. J. Med. 2003;348:1356–1364. doi: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- 2.Lewitt P.A. Levodopa for the Treatment of Parkinson’s Disease. N. Engl. J. Med. 2008;359:2468–2476. doi: 10.1056/NEJMct0800326. [DOI] [PubMed] [Google Scholar]
- 3.Dorsey E.R., Constantinescu R., Thompson J.P., Biglan K.M., Holloway R.G., Kieburtz K., Marshall F.J., Ravina B.M., Schifitto G., Siderowf A. Projected Number of People with Parkinson Disease in the Most Populous Nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 4.Duncan G.W., Khoo T.K., Coleman S.Y., Brayne C., Yarnall A.J., O’Brien J.T., Barker R.A., Burn D.J. The Incidence of Parkinson’s Disease in the North-East of England. Age Ageing. 2014;43:257–263. doi: 10.1093/ageing/aft091. [DOI] [PubMed] [Google Scholar]
- 5.Linder J., Stenlund H., Forsgren L. Incidence of Parkinson’s Disease and Parkinsonism in Northern Sweden: A Population-Based Study. Mov. Disord. 2010;25:341–348. doi: 10.1002/mds.22987. [DOI] [PubMed] [Google Scholar]
- 6.Alves G., Müller B., Herlofson K., Hogenesch I., Telstad W., Aarsland D., Tysnes O.B., Larsen J.P. Incidence of Parkinson’s Disease in Norway: The Norwegian Parkwest Study. J. Neurol. Neurosurg. Psychiatry. 2009;80:851. doi: 10.1136/jnnp.2008.168211. [DOI] [PubMed] [Google Scholar]
- 7.Fahn S. Description of Parkinson’s Disease as a Clinical Syndrome. Ann. N. Y. Acad. Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 8.Schrag A., Marjan J., Niall Q. What Contributes to Quality of Life in Patients with Parkinson’s Disease? J. Neurol. Neurosurg. Psychiatry. 2000;69:289. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skorvanek M., Martinez-Martin P., Kovacs N., Zezula I., Rodriguez-Violante M., Corvol J.C., Taba P., Seppi K., Levin O., Schrag A.E. Relationship between the Mds-Updrs and Quality of Life in Parkinson’s Disease. J. Neurol. Sci. 2015;353:87–91. doi: 10.1016/j.jns.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhuri K.R., Healy D.G., Schapira A.H. Non-Motor Symptoms of Parkinson’s Disease: Diagnosis and Management. Lancet Neurol. 2017;5:235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Martin P. What is quality of life and how do we measure it? Relevance to Parkinson’s disease and movement disorders. Mov. Disord. 2017;32:382–392. doi: 10.1002/mds.26885. [DOI] [PubMed] [Google Scholar]
- 12.Brazier J., Ratcliffe J., Saloman J., Tsuchiya A. Measuring and Valuing Health Benefits for Economic Evaluation. [(accessed on 10 November 2016)]; http://dx.doi.org/10.1093/med/9780198725923.001.0001 Available online:
- 13.Brooks R. A new facility for the measurement of health-related quality of life. Health Policy. 1990;3:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 14.OFcom Facts and Figures. [(accessed on 3 July 2015)]; Available online: http://media.ofcom.org.uk/facts/
- 15.Druce K.L., McBeth J., van der Veer S.N., Selby D.A., Vidgen B., Georgatzis K., Hellman B., Lakshminarayana R., Chowdhury A., Schultz D.M., et al. Recruitment and Ongoing Engagement in a UK Smartphone Study Examining the Association Between Weather and Pain: Cohort Study. JMIR Mhealth Uhealth. 2017;5:e168. doi: 10.2196/mhealth.8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Respiratory Institute Asthma Mobile Health Study. [(accessed on 15 September 2015)]; Available online: http://apps.icahn.mssm.edu/asthma/
- 17.University of Rochester Medical Center Apple Highlights Parkinson’s App with URMC Ties. [(accessed on 9 March 2015)]; Available online: https://www.urmc.rochester.edu/news/story/4268/apple-highlights-parkinsons-app-with-urmc-ties.aspx.
- 18.Stanford Medicine My-heart Counts iPhone Application. [(accessed on 15 September 2015)]; Available online: https://med.stanford.edu/myheartcounts.html.
- 19.Mulhern B., O’Gorman H., Rotherham N., Brazier J. Comparing the measurement equivalence of EQ-5D-5L across different modes of administration. Health Qual. Life Outcomes. 2014;17:191. doi: 10.1186/s12955-015-0382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bot B.M., Suver C., Neto E.C., Kellen M., Klein A., Bare C., Doerr M., Pratap A., Wilbanks J., Dorsey E.R., et al. The mPower study, Parkinson disease mobile data collected using ResearchKit. Sci. Data. 2016;3:160011. doi: 10.1038/sdata.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P., STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. BMJ. 2017;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herdman M., Gudex C., Lloyd A., Janssen M.F., Kind P., Parkin D., Bonsel G., Badia X. Development and Preliminary Testing of the New Five-Level Version of Eq-5d (Eq-5d-5l) Qual. Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Hout B., Janssen M.F., Feng Y.S., Kohlmann T., Busschbach J., Golicki D., Lloyd A., Scalone L., Kind P., Pickard A.S. Interim Scoring for the Eq-5d-5l: Mapping the Eq-5d-5l to Eq-5d-3l Value Sets. Value Health. 2012;15:708–715. doi: 10.1016/j.jval.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 24.McCaffrey N., Kaambwa B., Currow D.C., Ratcliffe J. Health-related quality of life measured using the EQ-5D-5L: South Australian population norms. Health Qual. Life Outcomes. 2016;14:133. doi: 10.1186/s12955-016-0537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jecmenica-Lukic M.V., Pekmezovic T.D., Petrovic I.N., Dragasevic N.T., Kostić V.S. Factors associated with deterioration of health-related quality of life in multiple system atrophy: 1-year follow-up study. Acta Neurol. Belg. 2018;11:1–7. doi: 10.1007/s13760-018-0962-4. [DOI] [PubMed] [Google Scholar]
- 26.Wang D., Clayton T., A Bakhai A. In: Interaction Clinical Trials: A Practical Guide to Design, Analysis and Reporting. Wang D., Bakhai A., editors. Remedica; Chicago, IL, USA: 2006. pp. 305–316. ISBN-10 1901346722, ISBN-13 9781901346722. [Google Scholar]
- 27.EQ-5D EQ-5D-5L|About. [(accessed on 18 April 2017)]; Available online: https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/
- 28.Parkin D., Rice N., Devlin N. Statistical analysis of EQ-5D profiles: Does the use of value sets bias inference? Med. Decis. Mak. 2010;5:556. doi: 10.1177/0272989X09357473. [DOI] [PubMed] [Google Scholar]
- 29.Golicki D., Niewada M., Buczek J., Karlinska A., Kobayashi A., Janssen M.F., Pickard A.S. Validity of EQ-5D-5L in stroke. Qual. Life Res. 2015;4:845–850. doi: 10.1007/s11136-014-0834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garciagordillo M.A., Colladomateo D., Olivares P.R., Adsuar J.C. Application of Eq-5d-5l Questionnaire in Patients Suffering from Urinary Incontinence. Actas Urol. Esp. 2016;7:457–462. doi: 10.1016/j.acuro.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Hinz A., Kohlmann T., Stöbel-Richter Y., Zenger M., Brähler E. The quality of life questionnaire EQ-5D-5L: Psychometric properties and normative values for the general German population. Qual. Life Res. 2014;2:443–447. doi: 10.1007/s11136-013-0498-2. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen L.H., Tran B.X., Hoang L.Q.N., Tran T.T., Latkin C.A. Quality of life profile of general Vietnamese population using EQ-5D-5L. Health Qual. Life Outcomes. 2017;15:199. doi: 10.1186/s12955-017-0771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szende A., Janssen B., Cabases J. Population Norms for the EQ-5D. In: Szende A., Janssen B., Cabases J., editors. Self-Reported Population Health: An International Perspective Based on EQ-5D. Springer; Dordrecht, The Netherlands: 2014. p. 21. [Google Scholar]
- 34.Lees A.J., Hardy J., Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 35.Pimenta M., Moreira D., Nogueira T., Silva C., Pinto E.B., Valenca G.T., Almeida L.R.S. Anxiety Independently Contributes to Severity of Freezing of Gait in People with Parkinson’s Disease. J. Neuropsychiatry Clin. Neurosci. 2018;9:6. doi: 10.1176/appi.neuropsych.17090177. [DOI] [PubMed] [Google Scholar]
- 36.Dissanayaka N.N., Sellbach A., Silburn P.A., O’Sullivan J.D., Marsh R., Mellick G.D. Factors associated with depression in Parkinson’s disease. J. Affect Disord. 2011;132:82–88. doi: 10.1016/j.jad.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Sophie M., Ford B. Management of pain in Parkinson’s disease. CNS Drugs. 2012;11:937. doi: 10.1007/s40263-012-0005-2. [DOI] [PubMed] [Google Scholar]
- 38.Ford B. Pain in Parkinson’s disease. Mov. Disord. 2010;S1:S98–S103. doi: 10.1002/mds.22716. [DOI] [PubMed] [Google Scholar]
- 39.Feng Y., Devlin N., Herdman M. Assessing the health of the general population in England: How do the three- and five-level versions of EQ-5D compare? Health Qual. Life Outcomes. 2015;13:171. doi: 10.1186/s12955-015-0356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Golicki D., Niewada M. EQ-5D-5L Polish population norms. Arch. Med. Sci. 2017;1:191–200. doi: 10.5114/aoms.2015.52126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collado M.D., García G.M.A., Olivares P.R., Adsuar J.C. Normative Values of Eq-5d-5l for Diabetes Patients from Spain. Nutr. Hosp. 2015;32:1595–1602. doi: 10.3305/nh.2015.32.4.9605. [DOI] [PubMed] [Google Scholar]