Abstract
Tea is one of the most popular beverages all over the world. Being an everyday drink for almost everyone, for centuries tea was considered safe and healthy. However, fungal contamination of tea at any stage of commodity production can pose a serious health hazard due to the accumulation of toxic secondary metabolites of moulds. Contemporary research revealed incidences of highly contaminated samples. Mycotoxin transfer from naturally contaminated raw tea into beverage was well studied for ochratoxin A only, and the possible leak of other mycotoxins is discussed. The results of several surveys were combined to evaluate aflatoxin B1 and ochratoxin A contamination levels in black tea and Pu-erh. Exposure estimate to aflatoxin B1 and ochratoxin A due to tea consumption was carried out based on these data. Average contamination level corresponds to the exposure of 3–40% (aflatoxin B1) and 5–24% (ochratoxin A) of mean overall estimates for different cluster diets. Lack of data does not allow the conclusion for the necessity of public health protection measures. It is necessary to perform representative studies of different kinds of tea for regulated mycotoxins at least. Contemporary techniques for analysis of mycotoxins in tea are summarised in the present review.
Keywords: tea, Pu-erh, Camellia sinensis, moulds, mycotoxins, occurrence, food safety, exposure, methods of determination
1. Introduction
Tea is an aromatic beverage commonly prepared by pouring hot or boiling water over cured leaves of the Camellia sinensis, an evergreen bush native to East Asia [1]. It is an everyday drink for almost everyone. A statistical study carried out in 2017 in the USA, the UK, and Germany showed 30% to 40% of respondents drink two to three cups of tea per day [2]. Tea is generally divided into categories based on fermentation degree. The most familiar kinds are black, white, oolong, green, and Pu-erh (post-fermented) tea. There are also teas flavored by the addition of mint, vanilla etc. and herbal teas, consisting of fruits and herbs, not Camellia sinensis. China, India, Kenya, Sri Lanka, and Turkey are the world’s largest tea producers. China and India accounted for about 43% and 22% of world tea production, respectively. According to the Food and Agriculture Organization of the United Nations Intergovernmental Group on tea, its consumption has increased by 4.5% annually over the last decade. By 2027, the world black tea production is projected to increase by an annual growth rate of 2.2% and green tea by 7.5% [3].
Consumption growth, especially of green tea, is believed to be accounted for by healthy lifestyle trends. Health benefits are associated with vitamins, microelements, essential oils, and polyphenols [4]. Two significant groups of tea polyphenols are catechins and flavonols. Catechins are abundant in less-fermented tea; epigallocatechin gallate may account for 50–80% of the total catechin (75–150 mg in a typical tea beverage) in tea. Flavonols are quercetin, kaempferol, myricetin, and their glycosides [5]. Tea polyphenols possess the bioactivity to affect the pathogenesis of some chronic diseases due to their antioxidant, anti-inflammatory, antiproliferative, antimutagenic, antibacterial and antiviral properties, protection against cardiovascular disease, hyperglycaemia, metabolic disorders, and some cancers [6,7]. However, a positive impact on health may be devalued by the presence of harmful contaminants, such as heavy metals, mycotoxins, and pesticide residues [6,8].
Mycotoxins are abiotic hazards produced by certain fungi that can grow on a variety of crops [9]. Mycotoxin production in tea can occur at any stage of its manufacturing: tea bush cultivation, harvest, processing, and storage. Poor agricultural practices, improper processing, drying, packaging, storage, and transport conditions promote fungal growth, increasing the risk of mycotoxin contamination. A subtropical climate, being favourable for tea cultivation, is also suitable for toxinogenic mould growth. Aflatoxins and ochratoxin A are one the most potent health hazards. Moreover, China and India—major tea producers—are in the list of the most aflatoxin affected countries [10]. It is reasonable that aflatoxins and ochratoxin A are the first candidates to be traced in tea. Most of the research concerns determination of the above mycotoxins. Their content in tea reached dozens and hundreds of ppb, respectively. Modern analytical techniques afford the development of multi-analyte and even multi-class methods. Recent issues aimed at multi-mycotoxin analyses revealed tea sample contamination with fumonisins, deoxynivalenol, and enniatins. The present study aims to summarise available data on fungi and mycotoxin occurrence in green, black, and Pu-erh tea. However, there is a distinct lack of representative and trustworthy issues to generate an accurate picture.
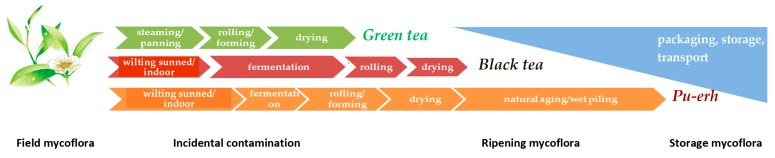
2. Moulds in Tea
Tea fungal contamination can occur at any stage of its production. Depending on the type, tea can be subjected to various mycoflora invasion, as shown in Figure 1. A wet and warm climate favorable for tea cultivation is also suitable for fungal growth. The principal genus identified in soils from subtropical tea plantations in China is Fusarium [11]. “Field” mycoflora is responsible for such mycotoxin production as deoxynivalenol and its derivatives, T-2 and HT-2 toxins, fumonisins etc. Subsequent steps of tea production, storage, and delivery to consumer impact contamination also [12]. Thirty-four fungal species were isolated from air, phyllosphere, and soil samples from a tea factory, including mycotoxin producing moulds (Aspergillus niger, A. flavus, A. fumigatus, and Fusarium lactis) [13]. Green tea manufacturing is the quickest: fresh leaves from plantations are subjected to immediate steaming or panning to deactivate oxidising enzymes and retain polyphenols. Steaming is followed by rolling and drying. Thus, green tea is expected to be the least contaminated at the processing stages. Compared to green, black tea production is more time consuming: wilting and fermentation steps are added to promote enzymatic oxidation and subsequent condensation of tea polyphenols into theaflavins and thearubigins [7,14].
Figure 1.
The common types of tea processing stages and associated mycoflora.
Mycological study of 40 black and green, loose and bagged tea samples from the Czech Republic revealed total fungal contamination [15], and similar results were obtained for 32 samples from Italy [16].
Aspergillus, Penicillium, Rhizopus, Eurotium, Cladosporium, and Trichoderma genera species were identified in tea, the first two dominating [15,16,17,18,19,20,21]. Aspergillus genera was usually represented by A. niger in black tea (Oman) [18]; black and green tea (Czech Republic) [15]; A. niger and A. tubingensis in green and black bagged tea (Italy) [16]; and A. niger, A. acidus, A. awamori, A. tubingensis, and A. carbonarius in herbal tea (Sweden) [22]. According to Rezacova and Kubatova, fermentation or plant origin did not affect tea fungal community, only storage mycoflora (saprobic fungi preferring dried food) was found [15]. Possible “field” mycoflora, even being not found in stored tea (approximately a year of proper storage results in mycelium loss [23]), can be traced by mycotoxins—its secondary metabolites. For example, detection of deoxynivalenol or enniatins in a tea sample is an indication of pre-harvest plant contamination by Fusarium species. Fresh leaf mycological examination could identify possible Camellia sinensis phytopathogens.
Post-fermented tea Pu-erh needs a dozen years of maturation to be considered ripened [24]. Wet piling is an alternative, affording to speed up the process. Regardless, the post-fermentation stage welcomes fungal contamination, and Pu-erh tea mycoflora attracts special attention. The common fungi isolated during the fermentation process of Pu-erh tea mainly belongs to Aspergillus, yeasts, Penicillium, Rhizopus, and Mucor [25]. The fungal and bacterial diversity of raw and ripened Pu-erh tea was well studied by Zhang [26]. The most abundant moulds found were A. niger (85% and 60% for 60 samples of ripe and raw Pu-erh), A. penicilloides, and A. cibarius. Ripening resulted in the occurrence of Penicillium species, such as P. brocae and P. citrinum [27]. Mogensen found A. acidus to be predominant in 10 samples of Pu-erh [21]. A. acidus and A. fumigatus frequency reached 50–80% in 36 Pu-erh samples studied by Haas et al., and Rhizomucor, Mycelia, Mucor, and Penicillium (P. citrinum, P. commune) fungal taxa were also detected [28].
3. Mycotoxins in Tea
Effects of tea consumption on nutrition and health were the focus of investigations over the last decades. Surprisingly, its safety was not paid so much attention. Abd El-Atya et al. reviewed tea contamination with pesticides, heavy metals, polycyclic aromatics, microorganisms, radionuclides, plant growth regulators, and mycotoxins. According to the authors, most contaminants leached into the tea brew are not detected or are found at a level lower than the regulatory limits and do not pose a public health hazard [6]. However, up to date research proved the problem of mycotoxins in tea is worth paying attention to, as shown in Table 1.
Table 1.
The occurrence of mycotoxins in tea.
| Tea | Country | Mycotoxin Positive/All Samples | Mycotoxin Content, μg/kg | LOD/LOQ, µg/kg | Ref. |
|---|---|---|---|---|---|
| Loose or Bricked Tea | |||||
| Black | Portugal | FBs: 16/18 | 80–280 (average *: 149) | 20/- | [19] |
| Turkey | FBs:5/51 | >LOD, <LOQ | 31/468 (FВ1), 103/1562 (FВ2) |
[29] | |
| Iran | AFL В1: 11/40 AFL В2: 2/40 AFLG1: 0/40 AFL G2: 3/40 Σ AFL: 11/40 |
average: 10 average: 12.1 |
1.0/- (AFL B1, G1), 0.2/- (AFL В2, G2) |
[30] | |
| Korea | AFL: 1/9 | 1.45 | -/- | [31] | |
| Russia | STC: 2/26 20 МТ: 0/26 |
0.4; 0.4 n.d. |
0.1-50/- | [32] | |
| Green | Turkey | FBs: 0/3 | n.d. | 31/468 (FВ1), 103/1562 (FВ2) |
[29] |
| Italy | AFL: 0/6 | n.d. | 0.5/- (AFL B1, G1) 0.2/- (AFL В2, G2) |
[20] | |
| Brazil | AFL: 1/9 | <LOQ | -/1 | [33] | |
| Russia | 21 МТ: 0/4 | n.d. | 0.1–50/- | [32] | |
| Germany | ОТА: 1/32 | 1.3 | -/- | [31] | |
| Red, White | Spain | AFL: 4/4 ** ОТА: 4/4 ** ZEN: 4/4 ** Т-2: 4/4 ** DON: 4/4 ** CIT: 3/4 ** FBs: 0/4 ** |
94.2–853.4 3.7–4.9 4.5–11.2 34.5–42.8 149.1–259.1 18.0–22.3 <LOD |
1.4 (ΣAFL) 0.025 (ОТА) 0.14 (ZEN) 0.28 (T-2) 14.8 (DON) 16.5 (CIT) 83 (FBs) |
[34] |
| White, Yellow, Green, Oolong, Black, Pu-erh, Herbal teas | Belgium, China | FВ1: 1/91 27 МТ: 0/91 |
76 n.d. |
2–122/- (raw tea), 0.4–46/-μg/L (beverage) |
[35] |
| Pu-erh | Austria | AFL В1, FBs:0/36, ОТА: 4/36 |
n.d. 0.65–94.7 (average: 3.08) |
1.7/- (Σ AFL), 10/- (FBs), 0.5/- (ОТА) |
[28] |
| China | AFL В1: 70/70 FBs: 70/70 ** Т-2: 70/70 ** DON: 70/70 ** |
0.02–8.5 16–499 5.2–47.7 357–2914 |
-/- AFL: HPLC-FLD (postcolumn deriv.) FBs, Т-2, DON: ELISA |
[36] | |
| China | AFL В1: 21/30 AFL В2: 4/30 AFLG1: 15/30 AFL G2: 3/30 |
0.4–15.1 0.1–6.3 0.4–19.0 9.9–56.6 |
-/- HPLC-FLD (precolumn deriv.) |
[37] | |
| Tea Bags | |||||
| Black | Italy | ОТА: 11/16 | 1.4–21.5 (average: 6.3) | 0.01 | [16] |
| Spain *** | 17 МТ: 0/12 | n.d. | LOD: 0.05–10 μg/L LOQ: 0.2–33 μg/L |
[38] | |
| Czech Republic | ОТА: 4/12 | 1.9–250 (average: 33.1) | LOD: 0.1, LOQ: 0.35 | [39] | |
| Green | Italy | ОТА: 14/16 | 0.1–20.0 (average: 7.2) | LOD: 0.01 | [16] |
| Spain *** | ENN В: 2/10 16 МТ: 0/10 |
~LOQ (~0.2 µg/L) n.d. |
LOD: 0.05–10 μg/L LOQ: 0.2–33 μg/L |
[38] | |
| Green + Mint | AFL В2: 6/8 AFL G1: 4/8 AFL G2: 4/8 15-acetyl DON: 2/8 13 МТ: 0/8 |
14.4–32.2 (average: 26) ~LOQ (~2.4 µg/L) 1.9–2.6 (average: 2.3) 60.5; 61 n.d. |
|||
| Red | 17 МТ: 0/14 | n.d. | |||
Notes: MT—mycotoxins, AFL—aflatoxin, FBs—fumonisins, FВ1—fumonisin В1, STC—sterigmatocystin, ОТА—ochratoxin А, DON—deoxynivalenol, ENN В—enniatin В, Т-2—Т-2 toxin, CIT—citrinin, ZEN—zearalenone; LOD—limit of detection, LOQ—limit of quantification, n.d.—not detected. * an average of all tested samples. ** sample contamination was examined by ELISA, the results are rather qualitative, than quantitative. *** mycotoxins were detected in brewed tea.
Most referenced articles describe the determination of the most toxic mycotoxins, such as aflatoxins and/or ochratoxin A, and fumonisins. Few studies focus on multi-mycotoxin contamination.
Aflatoxins, ochratoxin A, and fumonisins were found in black tea samples. Aflatoxin B1 mean concentration achieved 10 µg/kg [30]. The extremely high concentration of ochratoxin A was found in black tea from the Czech Republic—up to 250 µg/kg [39]. Practically all investigated black tea samples from Portugal were contaminated with fumonisins at the level of hundreds of ppb [19]. At the same time, almost “clear” black tea samples were reported in Korea [31]. Our group studied the occurrence of 21 mycotoxins (aflatoxins В1, В2, G1, G2, ochratoxin A, zearalenone, fumonisins B1, B2, T-2 and HT-2 toxins, nivalenol, deoxynivalenol, 3 acetyl- and 15-acetyl deoxynivalenol, diacetoxyscirpenol, fusarenone X, α-и β-zearalenol, citrinin, sterigmatocystin) in 26 black and 4 green loose tea samples. Only two black tea samples were contaminated: sterigmatocystin was detected at near LOD (limit of detection) concentrations [32]. Green tea samples were the least contaminated. ELISA examination of four white and red tea samples from Spain revealed extremely high levels of aflatoxins, ochratoxin A, zearalenone, deoxynivalenol, T-2 toxin, and citrinin [34]. As far as mycotoxins were detected by the method exhibiting the propensity for cross-reactions and overestimation, these results we propose to consider qualitative.
Pu-erh tea, due to specific features of its manufacturing, is more contaminated than other kinds of tea. Aflatoxins, ochratoxin A, fumonisins, T-2 toxin, and deoxynivalenol were often found, especially in samples purchased in China. Contamination levels reached dozens and even hundreds of ppb. High levels of deoxynivalenol, fumonisins, and T-2 are doubtful because of the detection method used [36]. Thirty-six samples of Pu-erh tea purchased in the EU proved to be aflatoxin-free, whereas ochratoxin A occurred in 11% of samples, and contamination reached 94.7 µg/kg [28]. Multi-analyte analysis of 31 Pu-erh tea samples by HPLC-MS/MS revealed the presence of a wide spectrum of “unusual” mycotoxins, among them patulin, common for fruit matrixes. It was found in 9 of 15 raw samples (mean concentration is 1169 μg/kg) and in 2 of 16 ripened samples (915 μg/kg) and accounted for Penicillium citrinum [26].
The occurrence of ochratoxin A in bagged tea was up to 60%: mean ochratoxin A content in the samples from Italy was about 6 µg/kg for 16 samples of black tea and 7 µg/kg for 16 samples of green tea [16]. Pallares with colleagues studied contamination of tea bag infusions with 17 mycotoxins (aflatoxins, ochratoxin А, zearalenone, toxins Т-2 and НТ-2, deoxynivalenol and its acetyl derivates 3-acetyl- and 15-acetyl deoxynivalenol, nivalenol, enniatins A, A1, B, B1, and beauvericin). Raw tea was not tested. Beverages prepared from black and green tea bags were mycotoxin-free, except two green tea samples contaminated with enniatin B at near LOD levels. Aflatoxins В2 and G2 and trace amounts of aflatoxins G1 and 15-acetyl deoxynivalenol were found in brewed green tea with mint [38]. A high aflatoxin B1 concentration level was observed in linden and jasmine herbal tea [40,41].
Monbaliu with colleagues detected 27 mycotoxins (aflatoxins В1, В2, G1, and G2, ochratoxin А, zearalenone, fumonisins В1, В2, В3, toxins Т-2 and НТ-2, deoxynivalenol and its 3- and 15-acetyl derivates, de-epoxy-deoxynivalenol, diacetoxyscirpenol, nivalenol, neosolaniol, fusarenone Х, zearalenone, citrinin, sterigmatocystin, fumigaclavine, mycophenolic acid, paxillin, alternariol, alternariol monomethyl ether, altenuene) in 91 samples of different tea kinds and 76 µg/kg of fumonisin B1 was detected in 1 mix of Ceylon tea only [35].
4. Mycotoxin Transfer from Raw Tea into the Beverage
Several parameters define mycotoxin concentration in tea beverages: raw tea contamination level, mycotoxin thermal stability, and its ability to transfer from the matrix into aqueous infusions. Brewing is unable to degrade common mycotoxins [42,43] substantially. There are several water-soluble mycotoxins—aflatoxins (10–20 mg/mL), fumonisins (at least 20 mg/mL), zearalenone (0.02 µg/mL) [44], ochratoxin A (0.0004 mg/mL as acid, water-soluble as salt), T-2 toxin (~0.1 mg/mL), deoxynivalenol (55 mg/mL) [45]—that are able to be extracted from the matrix by water.
Mycotoxin transfer from naturally contaminated tea into beverage was studied for ochratoxin A only and corresponded to 35–40% in the case of raw black tea [39,46]. Transfer from bagged black and green tea was 34 ± 4% and 54 ± 15% [16]. Fruit tea brewing revealed a 10-fold lower leakage of mycotoxin, which accounted for a lower pH of the resulting infusion and presence of ochratoxin A in molecular form, that is almost water insoluble [39]. Aflatoxin transfer from artificially contaminated samples was found to be 28–33% [47]. Monbaliu et al. failed to detect fumonisin B1 in beverages after brewing of a naturally contaminated raw sample (76 µg/kg) despite high water solubility of fumonisins, and they attributed this fact to low sensitivity of the method [35]. Multi-mycotoxin analysis of tea prepared from tea bags revealed aflatoxins and 15-acetyl deoxynivalenol, as shown in Table 1 [38].
5. Exposure Assessment and Legislation
Aflatoxin B1 and ochratoxin A are the most toxic mycotoxins; their maximum levels in food commodities are set at ppb levels. To evaluate approximate dietary exposure to these contaminants due to tea consumption, we assumed the following: average daily tea consumption—3 cups per day, 2 g of raw tea is used to prepare 1 cup of tea, average body weight is 60 kg, ochratoxin A and aflatoxin average transfer from raw tea into beverage—30%. Average and maximum contamination levels, as shown in Table 2, were estimated based on combined data from several publications using lower bound approach. This exposure assessment is approximate, aimed to draw attention to the problem. Median contamination appeared to be below LOD, that is <0.5 µg/kg for modern methods of analysis. It corresponds to <0.02 ng/kg bw/day—<5% of the average of the lowest dietary exposure from staple food—for aflatoxin B1, and <0.11 ng/kg bw/week—<1%—for ochratoxin A. This impact can be considered as negligible. However, average contamination level results in 3–40% of aflatoxin B1 and 5–24% of ochratoxin A dietary intake compared to the lowest and the highest ends of the range of diet exposure. Compared to ochratoxin A provisional tolerable weekly intake, it corresponds to 0.7–1.7%. Exposure from tea calculated on the base of maximum mycotoxin content exceeds the highest average dietary exposure.
Table 2.
Exposure estimate to aflatoxin B1 and ochratoxin A from tea.
| Mycotoxin | Tea | Data Numberof Samples, Origin | Contamination *, μg/kg | Exposure, ng/kg bw | Dietary Intake, ng/kg bw | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Mean | Max | Median | Mean | Max | |||||
| Aflatoxin B1 | Black | 40, Iran [30] 9, Korea [31] 26, Russia [32] |
<LOQ | 5.3 | 190 | per day | negligible | 0.16 | 5.7 | 0.4–2.6 [48] |
| Pu-erh | 36, Austria [28] 30, China [37] 70, China [36] |
1.6 | 2.6 | 15.1 | 0.05 | 0.08 | 0.45 | |||
| Ochratoxin A | Black | 12, Czech Republic [39] 26, Russia [32] 16 (bagged), Italy [16] |
<LOQ | 9.2 | 250 | per week | negligible | 1.93 | 52.5 | 8–17 [49] |
| Pu-erh | 36, Austria [28] | <LOQ | 3.8 | 94.7 | negligible | 0.80 | 19.9 | |||
* Lower bound approach was used to calculate means, i.e., samples below LOD or LOQ were considered as zero.
However, mycotoxins in tea are almost not regulated, except for several countries. National regulations concerning mycotoxins in tea have been established in Customs Union countries (Armenia, Belarus, Kazakhstan, Kyrgyzstan, and Russia) for aflatoxin B1 in raw tea—5 µg/kg [50], Argentina set limits for aflatoxin B1 and total aflatoxins in materials used for herbal tea infusions at 5 and 20 µg/kg, correspondingly [51]. Upper limits for such category as “all foods” have been set in Asian countries: in India for aflatoxin B1—30 µg/kg; in Sri Lanka—the same level for total aflatoxins; in Japan—10 µg/kg [52]; and in China—5–20 µg/kg depending on the food matrix [53]. Lack of occurrence data does not allow the conclusion for the necessity of public health protection measures. It is necessary to perform representative studies of different kinds of tea for regulated mycotoxins at least.
6. Methods of Mycotoxin Determination in Tea
Several parameters are defining an analytical procedure for mycotoxin determination in tea. First, one should decide whether to analyse raw material or prepared brewing. Permitted levels are set for raw material, while a consumer is much more interested in beverage safety. Recent studies are focused on both matrixes [16,39]. Regardless, the procedure involves sampling, extraction, extract purification, and mycotoxin determination. Raw tea sample preparation is analogous to the standard procedure of mycotoxin determination in food samples. The most common solvents used for extraction are methanol-water and acetonitrile-water, as shown in Table 3, with up to 80% of organics. Additional reagents are sometimes used for extraction, such as NaCl [20] or Tween-20 [47]. The choice of the clean-up method depends on the analysis objective: whether it is multi-determination or targeted analysis of several mycotoxins. In the latter case, immunoaffinity columns (IAC) are preferred due to their high specificity and maximal elimination of matrix components [20,28,30,39,41,47]. Conventional solid phase extraction (SPE) cartridges filled with an anion exchanger, such as amino silica, are appropriate for clean-up of weak acids (ochratoxin A, fumonisins) [19,29]. Liquid-liquid extraction with pH change is proposed for ochratoxin A determination; a combination of extraction and clean-up steps is possible due to a different water/organic phase solubility of molecular and anion forms of the analyte [39]. Comprehensive control of food commodities and requirement of occurrence data accumulation for a wide range of mycotoxins make HPLC coupled to mass spectrometry a method of choice. Sample treatment for multi-target determination should provide appropriate recoveries for compounds of different polarity (e.g., deoxynivalenol, logP = −0.7, enniatin B, logP = 6.5 [45]). At the same time, the matrix effect on electrospray ionisation (ESI) efficiency should be minimised or considered. “Dilute and shoot” procedure outcomes proved to be strongly dependent on the analyte/matrix combination [54]. QuEChERS approach (from Quick, Easy, Cheap, Effective, Rugged and Safe), combining extraction and clean-up, was initially developed for pesticide multi-residue analysis [55] and was adopted for a wide range of analytes and matrices [56]. QuEChERS, “dilute and shoot” with or without additional clean-up in dispersive, and pass through mode SPE were thoroughly validated for mycotoxin and pesticide analysis in green tea supplements [57], and raw tea [58] by HPLC coupled to high-resolution tandem mass spectrometric detection (HRMS/MS). Octadecyl silica gel (ODS) showed good potential as a sorbent for dispersive SPE. No clean-up procedure is also possible. It was used for detection of 30 mycotoxins in coffee samples: MeCN (1% acetic acid) extraction without further purification provided sufficient recovery, and matrix-matched calibration ensured accurate quantification of analytes [59].
Table 3.
Methods of mycotoxin determination in tea.
| Detection (Mycotoxin) | Tea | Publication. Year | Short Description | Sensitivity, μg/kg ** | Ref. | |
|---|---|---|---|---|---|---|
| Extraction/Clean up | HPLC/Detection | |||||
| TLC-FLD (AFL, ОТА, ZEN) | Herbal raw | 1998 | MeCN:Н2О (9:1, v/v), CHCl3 alkalinization/acidification |
TLC: silica gel toluene, ethyl acetate, formic acid (50 + 40 + 10 v/v). UV irradiation |
- | [17] |
| HPLC-FLD (FBs) | Black raw | 2001 | MeOH:Н2О (3:1, v/v), SPE: anion-exchange column |
Derivatisation (o-phthaldialdehyde), HPLC: ODS FLD, λEx/Em = 335 /440 nm |
31/468 (FВ1), 103/1562 (FВ2) |
[19] |
| HPLC-FLD (FBs) | Black, green raw | 2004 | MeOH:Н2О (3:1, v/v), SPE: anion-exchange column |
Derivatisation (o-phthaldialdehyde), HPLC: ODS FLD, λEx/Em = 338/455 нм |
LOD: ~30 (FВ1), ~470 (FВ2) |
[29] |
| HPLC (AFL) | Herbal, green raw | 2007 | MeOH:Н2О (80:20, v/v), NaCl, SPE: IAC |
HPLC: ODS Post-column derivatisation (Br2, Cobra cell) FLD, λEx/Em = 365/435 nm |
LOD: 0.5 (AFL B1, G1) 0.2 (AFL В2, G2) |
[20] |
| ELISA (ОТА, FBs, ΣAFL, ZEN, Т-2, DON, CIT) | Red, white raw | 2009 |
AFL, ZEN, Т-2, DON: MeCN:Н2О (84:16, v/v), SPE: IAC (AFL + ZEN, DON + Т-2) FBs: MeCN:Н2О (50:50, v/v), SPE: IAC CIT: H3PO4:CH2Cl2, SPE: polyamide column ОТА: CH2Cl2 alkalinisation/acidification |
ELISA λ = 450 nm |
LOD: 0.025 (ОТА) 83 (FBs) 1.4 (ΣAFL) 0.14 (ZEN) 0.28 (T-2) 14.8 (DON) 16.5 (CIT) |
[34] |
| UHPLC-MS/MS (27 Mycotoxins) | White, green, yellow, black, oolong, Pu-erh raw and beverage | 2010 | Ethyl acetate: formic acid (99:1, v/v), SPE: amino-и ODS |
UHPLC: ODS MS/MS: electrospray, positive polarity, internal standards |
LOD: 2–122 (raw tea), 0.4–46 μg/L (beverage) |
[35] |
| HPLC (AFL) | Black raw | 2012 | MeCN:MeOH:Н2О (10:6:4, v/v), 8% Tween-20. SPE: IAC |
HPLC: ODS Post-column photochemical derivatization, FLD, λEx/Em = 360/440 nm |
LOD: 0.3 μg/L (AFL B1, G1) 0.15 μg/L (AFL В2, G2) |
[47] |
| HPLC-FLD (AFL) | Green raw | 2012 | Acetone:Н2О (85:15) SPE: IAC |
HPLC: ODS Post-column derivatisation (Br2, Cobra cell) FLD, λEx/Em = 360/435 nm |
LOQ: 1 | [33] |
| ELISA, HPLC, HPLC-MS (AFL, ZEN, OTA) | Pu-erh raw | 2013 | AFL:MeOH:Н2О (70:30, v/v) + Tween-20, SPE: IAC FBs: MeCN:MeOH:Н2О (1:1:2 v/v), SPE: IAC ОТА: MeСN:Н2О (80:20, v/v), SPE: IAC |
AFL: HPLC: ODS Post-column derivatization (Br2, Cobra cell) FLD, λEx/Em = 362/440 nm FBs: HPLC: ODS, MS/MS ОТА: HPLC: ODS FLD, λEx/Em = 333/460 nm |
LOD: 1.7 (Σ AFL), 10 (FBs), 0.5 (ОТА) |
[28] |
| ELISA (AFL) | Herbal raw | 2013 | MeOH:Н2О (70:30, v/v) | ELISA | LOD: 1.7 (Σ AFL), 1.0 (AFL B1) |
[40] |
| HPLC-FLD (AFL) | Black raw | 2013 | MeOH:Н2О (80:20, v/v) + NaCl, SPE: IAC |
HPLC: ODS Post-column derivatization (Br2, Cobra cell) FLD, λEx/Em = 360/435 nm |
LOD: 1.0 (AFL B1, G1), 0.2 (AFL В2, G2) |
[30] |
| HPLC-FLD (ОТА) | Black raw, beverage | 2014 | Raw: CHCl3 + alkalinisation/acidification SPE: IAC Beverages: SPE: phenyl silica gel |
HPLC: ODS FLD, λEx/Em = 333/465 nm |
LOD: 0.1 LOQ: 0.35 |
[39] |
| HPLC (AFL В1)ELISA (FВ1, DON, Т-2) | Pu-erh raw | 2014 | MeOH:Н2О (70:30, v/v), SPE: IAC (AFL B1) |
FВ1, DON, Т-2: ELISA AFL В1: HPLC: ODS Post-column derivatisation (iodine), FLD |
- | [36] |
| HPLC, ELISA (AFL) | Pu-erh raw | 2015 | MeCN:Н2О (84:16, v/v), SPE |
ELISA HPLC: ODS Precolumn derivatisation (TFA) FLD, λEx/Em = 360/440 nm |
- calibration curve lowest level corresponds to 1 |
[37] |
| HPLC-HRMS/MS (55 Mycotoxins) | Raw tea | 2015 | QuEChERS dispersive SPE: ODS |
HPLC: ODS HRMS/MS, electrospray |
1–1000 | [58] |
| HPLC-FLD (AFL) | Herbal raw | 2016 | MeOH:Н2О (6:4, v/v) with NaCl, SPE: IAC |
HPLC: ODS Post-column derivatisation (Br2, Cobra cell analogue) FLD, λEx/Em = 362/440 nm |
LOD: 0.1 (AFL B1, G1) 0.02 (AFL В2, G2) |
[41] |
| HPLC-MS/MS, (16 Mycotoxins) | Black, green, red, green + mint beverage | 2017 | Dispersive liquid-liquid microextraction by MeCN-ethyl acetate and MеОН-chloroform | HPLC: ODS MS/MS: electrospray, positive polarity, matrix match calibration |
LOD: 0.05–10 μg/L LOQ: 0.2–33 μg/L |
[38] |
| HPLC-MS/MS, (22 Mycotoxins) | Black, green | 2018 | MeCN: Н2О:acetic acid (79:20:1) | HPLC: ODS MS/MS: ESI, positive polarity |
LOD: 0.1–50 | [32] |
| HPLC-FLD (ОТА) | Tea bags | 2018 | MeOH/NaHCO3aq. 1%, (70/30) SPE: IAC |
HPLC: ODS FLD, λEx/Em = 333/466 nm Precolumn derivatisation (BF3) for positive findings confirmation Internal standard: diflunisal |
LOD: 0.01 | [16] |
| HPLC-HRMS/MS (4 Mycotoxins Tested) | Green tea raw, brew | 2018 | -QuEChERS without dSPE (raw) -MeCN, Н2О, MgSO4, NaCit, 5-fold dilution prior analysis (brew) -MeCN, Н2О, formic acid (brew) |
HPLC: ODS HRMS/MS, electrospray |
FBs—n.d. LOQ (DON) = 500 LOQ (OTA) = 10 |
[60] |
* TLC—thin layer chromatography, SPE—solid-phase extraction, dSPE—dispersive SPE, IAC—immunoaffinity column, ODS—octadecyl silica gel, FLD—fluorimetric detection, λEx/Em—excitation/emission wavelength, TFA—trifluoroacetic acid, ESI—electrospray ionisation, HRMS/MS—high-resolution tandem mass spectrometric detection. **—for HPLC-MS/MS method validation most of the authors follow SANTE requirements since 2015: SANTE 11945/2015 and its later upgrade SANTE/11813/2017 [61].
Reverse-phase HPLC coupled with specific detectors is a method of choice for mycotoxin analysis. Despite not being “up to date”, FLD provide sufficiently sensitive, selective, and low-cost detection of aflatoxins and ochratoxin A. Multi-mycotoxin analysis supposes applying of MS/MS technology: dozens of mycotoxins can be determined in a single run [32,38]. The current trend is the multi-class detection of food contaminants, such as mycotoxins and pesticides [57,58], or mycotoxins, pesticides, packaging, and process-induced contaminants [60] by HRMS/MS technique. The latter research, being not very successful in mycotoxins′ detection, is also worth paying attention to due to the thorough analysis of parameters affecting method performance. The crucial point of LC-MS with ESI is analyte ionization suppression/enhancement in the presence of matrix components. Tea exhibits the strongest matrix effect, which manifests itself, in a changing fragmentation pattern of mycotoxin ochratoxin A [58]. Matrix effect can be accounted for by such major co-extracts as polyphenols, caffeine, and amino acids [60]. For example, the average caffeine content in tea is about 3.5% [62], that exceeds a minor contaminate concentration (e.g., 10 µg/kg) by over 106 times. Matrix-matched calibrations [38,60] or internal standards [35] are usually used to overcome the problem.
Few studies were focused on tea brew analysis for the reason of mycotoxin transfer estimation [16,39,46] or analytical method development [35,38,60]. Mycotoxin extraction from brewing was carried out by means of IAC (ochratoxin A targeted analysis) [28,39] or dispersive liquid-liquid microextraction (16 mycotoxins, recovery from 66% to 127%) [38] or liquid-liquid extraction and “dilute and shoot” approach (proved to be suitable for ochratoxin A detection) [60]. Hence, the authors followed their preference while preparing tea brew, in short, 1.5–2 g of ground raw tea sample was put in contact with 150–250 mL of boiling water for 3–8 min [16,35,38,39] or Turkish traditional brewing [46]; it is reasonable to propose standard procedure [60,63].
7. Conclusions
Camellia sinensis is cultivated and processed in subtropics preferentially. Warm and wet climate also suits mould growth. However, no field fungi were reported in the samples from the local markets in Europe. Storage mycoflora was identified in black and green tea samples. Pu-erh post-fermented tea is characterized by ripening mycoflora. Aspergillus and Penicillium genera species dominate in both.
Thus, most of the mycotoxin assays were aimed at aflatoxin or ochratoxin A determination. Average aflatoxin B1 concentration raised to 10 μg/kg, ochratoxin A—to 33 μg/kg Modern techniques make possible multi-mycotoxin analysis. Deoxynivalenol and its acetyl derivatives, fumonisins, and enniatin B were found in raw tea samples, supporting the idea of field fungal invasion. Tea beverage safety depends on raw tea contamination level and ability of the mycotoxin to transfer from loose tea into brewing. It was well studied for ochratoxin A only. Compared to raw tea, 30–50% of ochratoxin A was found in the beverage. Aflatoxins and deoxynivalenol leak into brewing also. Extreme toxicity of aflatoxins and occasional occurrence of highly contaminated samples highlight evaluation of their transfer from naturally contaminated samples essential.
Approximate exposure estimates to aflatoxin B1 and ochratoxin A due to tea consumption were carried out based on the combined results of several black and Pu-erh tea surveys. Average contamination level corresponds to the exposure of 3–40% (aflatoxin B1) and 5–24% (ochratoxin A) of mean overall estimates for different cluster diets.
Lack of reliable occurrence data does not allow the conclusion for the necessity of public health protection measures. It is necessary to perform representative studies of different kinds of tea for regulated mycotoxins at least.
Acknowledgments
The authors are grateful for the support of Russian Science Foundation (“Emergent mycotoxins in food of plant origin: development of methods of determination, survey of occurrence, species-specific characterization of toxigenic molds, creation of regulatory”. Project number 18-16-00077).
Author Contributions
I.S. and M.K. performed literature analysis and wrote manuscript, V.T. provided its critical revision.
Funding
This research and the APC was funded by Russian Science Foundation grant number 18-16-00077.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
The review summarises data on fungi and mycotoxins detected in various kinds of tea. Mycotoxins transfer from raw tea into the beverage, and possible impact from dietary exposure to aflatoxin B1 and ochratoxin A is discussed. A modern analytical procedure survey is the foundation for the creation of an appropriate method for the determination of mycotoxins in tea.
References
- 1.Diby L., Kahia J., Kouamé C., Aynekulu E. Tea, Coffee, and Cocoa. Encycl. Appl. Plant Sci. 2017;3:420–425. doi: 10.1016/B978-0-12-394807-6.00179-9. [DOI] [Google Scholar]
- 2.The Statistics Portal. [(accessed on 22 August 2018)]; Available online: https://www.statista.com/statistics/695779/average-daily-tea-consumption-by-country/
- 3.CCP: TE 18/CRS1 Committee on the Commodity Problems Intergovernmental Group on Tea Twenty-Third Session Hangzhou, the People’s Republic of China, 17–20 May 2018. Current Market Situation and Medium-Term Outlook. [(accessed on 20 August 2018)]; Available online: http://www.fao.org/docrep/meeting/018/K7538E.pdf.
- 4.Sereshti H., Samadi S., Jalali-Heravi M. Determination of volatile components of green, black, oolong and white tea by optimised ultrasound-assisted extraction-dispersive liquid-liquid microextraction coupled with gas chromatography. J. Chromatogr. A. 2013;1280:1–8. doi: 10.1016/j.chroma.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 5.Khan N., Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007;81:519–533. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abd El-Atya A.M., Choia J.-H., Musfiqur R.M.D., Kim S.-W., Tosun A., Shima J.-H. Residues and contaminants in tea and tea infusions. Food Addit. Contam. 2014;31:1794–1804. doi: 10.1080/19440049.2014.958575. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L., Zhang Z., Zhou Y., Ling T., Wan X. Chinese dark teas: Postfermentation, chemistry and biological activities. Food Res. Intern. 2013;53:600–607. doi: 10.1016/j.foodres.2013.01.016. [DOI] [Google Scholar]
- 8.Li X., Zhang Z., Li P., Zhang Q., Zhang W., Ding X. Determination for major chemical contaminants in tea (Camellia sinensis) matrices: A review. Food Res. Intern. 2013;53:649–658. doi: 10.1016/j.foodres.2012.12.048. [DOI] [Google Scholar]
- 9.Marin S., Ramos A.J., Cano-Sancho G., Sanchis V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013;60:218–237. doi: 10.1016/j.fct.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 10.Klingelhöfer D., Zhu Y., Braun M., Bendels M.H.K., Brüggmann D., Groneberg D.A. Aflatoxin—Publication analysis of a global health threat. Food Control. 2018;89:280–290. doi: 10.1016/j.foodcont.2018.02.017. [DOI] [Google Scholar]
- 11.Wang L.-M., Huang D.-F., Fang Y., Wang F., Li F.-L., Liao M. Soil fungal communities in tea plantation after 10 years of chemical vs. integrated fertilization. Chil. J. Agric. Res. 2017;77:355–364. doi: 10.4067/S0718-58392017000400355. [DOI] [Google Scholar]
- 12.Luo Y., Liu X., Li J. Updating techniques on controlling mycotoxins—A review. Food Control. 2018;89:123–132. doi: 10.1016/j.foodcont.2018.01.016. [DOI] [Google Scholar]
- 13.Dutta B.K., Dutta S., Nath P.K. Mycotoxin production potential of mycoflora in tea. In: Jain N.K., Rahman F., Baker P., editors. Economic Crisis in Tea Industry. Studium Press; New Delhi, India: 2008. pp. 221–232. [Google Scholar]
- 14.Soni R.P., Katoch M., Kumar A., Ladohiya R., Verma P. Tea: Production, Composition, Consumption and its Potential an Antioxidant and Antimicrobial Agent. Intl. J. Food. Ferment. Technol. 2015;5:95–106. doi: 10.5958/2277-9396.2016.00002.7. [DOI] [Google Scholar]
- 15.Řezacova V., Kubatova A. Saprobic microfungi in tea based on Camellia sinensis and on other dried herbs. Czech Mycol. 2005;57:79–89. [Google Scholar]
- 16.Carraturo F., De Castro O., Troisi J., De Luca A., Masucci A., Cennamo P., Trifuoggi M., Aliberti F., Guida M. Comparative assessment of the quality of commercial black and green tea using microbiology analyses. BMC Microbiol. 2018;18:4. doi: 10.1186/s12866-017-1142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halt M. Moulds and mycotoxins in herb tea and medicinal plants. Eur. J. Epidemiol. 1998;14:269–274. doi: 10.1023/A:1007498613538. [DOI] [PubMed] [Google Scholar]
- 18.Elshafie A.E., Al-Lawatia T., Al-Bahry S. Fungi associated with black tea and tea quality in the Sultanate of Oman. Mycopathologia. 1999;145:89–93. doi: 10.1023/A:1007034729467. [DOI] [PubMed] [Google Scholar]
- 19.Martins M.L., Martins H.M., Bernardo F. Fumonisins B 1 and B 2 in Black Tea and Medicinal Plants. J. Food Prot. 2001;64:1268–1270. doi: 10.4315/0362-028X-64.8.1268. [DOI] [PubMed] [Google Scholar]
- 20.Romagnoli B., Menna V., Gruppioni N., Bergamini C. Aflatoxins in spices, aromatic herbs, herb-teas and medicinal plants marketed in Italy. Food Control. 2007;18:697–701. doi: 10.1016/j.foodcont.2006.02.020. [DOI] [Google Scholar]
- 21.Mogensen J.M., Varga J., Thrane U., Frisvad J.C. Aspergillus acidus from Pu-erh tea and black tea does not produce ochratoxin A and fumonisin B2. Intern. J. Food Microbiol. 2009;132:141–144. doi: 10.1016/j.ijfoodmicro.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Storari M., Dennert F.G., Bigler L., Gessler C., Broggini G.A.L. Isolation of mycotoxins producing black Aspergilli in herbal teas available on the Swiss market. Food Control. 2012;26:157–161. doi: 10.1016/j.foodcont.2012.01.026. [DOI] [Google Scholar]
- 23.Minaeva L.P. (Federal Research Centre of Nutrition, Biotechnology and Food Safety, Moscow, Russia). Personal communication. 2018.
- 24.Zhao Z.-J., Pan Y.-Z., Liu Q.-J., Li X.-H. Exposure assessment of lovastatin in Pu-erh tea. Int. J. Food Microbiol. 2013;164:26–31. doi: 10.1016/j.ijfoodmicro.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Z.J., Hu X.C., Liu Q.J. Recent advances on the fungi of Pu-erh ripe tea. Intern. Food Res. J. 2015;22:1240–1246. [Google Scholar]
- 26.Zhang Y., Skaar I., Sulyok M., Liu X., Rao M., Taylor J.W. The Microbiome and Metabolites in Fermented Pu-erh Tea as Revealed by High-Throughput Sequencing and Quantitative Multiplex Metabolite Analysis. PLoS ONE. 2016;11:e0157847. doi: 10.1371/journal.pone.0157847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Z.J., Tong H.R., Zhou L., Wang E.X., Liu Q.J. Fungal colonisation of Pu-erh tea in Yunnan. J. Food Safety. 2010;30:769–784. doi: 10.1111/j.1745-4565.2010.00240.x. [DOI] [Google Scholar]
- 28.Haas D., Pfeifer B., Reiterich C., Partenheimer R., Reck B., Buzina W. Identification and quantification of fungi and mycotoxins from Pu-erh tea. Int. J. Food Microbiol. 2013;166:316–322. doi: 10.1016/j.ijfoodmicro.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Omurtag G.Z., Yazicioglu D. Determination of fumonisin B1 and B2 in herbal tea and medicinal plants in Turkey by high-performance liquid chromatography. J. Food Prot. 2004;67:1782–1786. doi: 10.4315/0362-028X-67.8.1782. [DOI] [PubMed] [Google Scholar]
- 30.Pouretedal Z., Mazaheri M. Aflatoxins in black tea in Iran. Food Addit. Contam. Part B. 2013;6:127–129. doi: 10.1080/19393210.2013.764551. [DOI] [PubMed] [Google Scholar]
- 31.Weidenbörner M. Mycotoxins in Foodstuffs. 2nd ed. Springer Science + Business Media; New York, NY, USA: 2013. p. 483. [Google Scholar]
- 32.Chaly Z.A., Sedova I.B., Kiseleva M.G. Occurrence of mycotoxins in tea. Prob. Nutr. 2018;87:199–200. [Google Scholar]
- 33.Prado G., Altoé A.F., Gomes T.C.B., Leal A.S., Morais V.A.D., Oliveira M.S., Ferreira M.B., Gomes M.B., Paschoal F.N., Souza R.V., et al. Occurrence of aflatoxin B1 in natural products. Braz. J. Microbiol. 2012;43:1428–1435. doi: 10.1590/S1517-83822012000400026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santos L., Marin S., Sanchis V., Ramos A.J. Screening of mycotoxin multi contamination in medicinal and aromatic herbs sampled in Spain. J. Sci. Food Agric. 2009;89:1802–1807. doi: 10.1002/jsfa.3647. [DOI] [Google Scholar]
- 35.Monbaliu S., Wu A., Zhang D., Van Peteghem C., De Saeger S. Multimycotoxin UPLC−MS/MS for tea, herbal infusions and the derived drinkable products. J. Agric. Food Chem. 2010;58:12664–12671. doi: 10.1021/jf1033043. [DOI] [PubMed] [Google Scholar]
- 36.Wu J.-Y., Yang G.-Y., Chen J.-L., Li W.-X., Li J.-T., Fu C.-X., Jiang G.-F., Zhu W. Investigation for Pu-Erh Tea Contamination Caused by Mycotoxins in a Tea Market in Guangzhou. J Basic Appl. Sci. 2014;10:349–356. [Google Scholar]
- 37.Li W.G., Xu K.L., Xiao R., Yin G.F., Liu W.W. Development of an HPLC-based method for the detection of aflatoxins in Pu-erh tea. Int. J. Food Prop. 2015;18:842–848. doi: 10.1080/10942912.2014.885043. [DOI] [Google Scholar]
- 38.Pallarés N., Font G., Mañes J., Ferrer E. Multimycotoxin LC-MS/MS analysis in tea beverages after dispersive liquid-liquid microextraction (DLLME) J. Agric. Food Chem. 2017;65:10282–10289. doi: 10.1021/acs.jafc.7b03507. [DOI] [PubMed] [Google Scholar]
- 39.Malir F., Ostry V., Pfohl-Leszkowicz A., Toman J., Bazin I., Roubal T. Transfer of Ochratoxin A into Tea and Coffee Beverages. Toxins. 2014;6:3438–3453. doi: 10.3390/toxins6123438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hacıbekiroğlu I., Kolak U. Aflatoxins in various food from Istanbul, Turkey. Food Addit. Contam. Part B. 2013;6:260–264. doi: 10.1080/19393210.2013.813080. [DOI] [PubMed] [Google Scholar]
- 41.Tosun H., GünçErgönül P., FatmaUçok E. Occurrence of aflatoxins (B1, B2, G1, G2) in herbal tea consumed in Turkey. J. Verbr. Lebensm. 2016;11:265–269. doi: 10.1007/s00003-016-1032-6. [DOI] [Google Scholar]
- 42.Feuell A.J. Aflatoxin in groundnuts. Part 9: Problems of detoxification. Trop Sci. 1996;8:61–70. [Google Scholar]
- 43.Kabak B. The fate of mycotoxins during thermal food processing. J. Sci. Food Agric. 2009;89:549–554. doi: 10.1002/jsfa.3491. [DOI] [Google Scholar]
- 44.Chemical and physical characteristics of the principal mycotoxins. IARC Sci. Publ. 2012;158:31–38. [PubMed] [Google Scholar]
- 45.PubChem Compound. [(accessed on 28 August 2018)]; Available online: https://pubchem.ncbi.nlm.nih.gov/compound/
- 46.Toman J., Malir F., Ostry V., Kilic M.A., Roubal T., Grosse Y., Pfohl-Leszkowicz A. Transfer of ochratoxin A from raw black tea to tea infusions prepared according to the Turkish tradition. J. Sci. Food Agric. 2017;98:261–265. doi: 10.1002/jsfa.8464. [DOI] [PubMed] [Google Scholar]
- 47.Viswanath P., Nanjegowda D.K., Govindegowda H., Dattatreya A.M., Siddappa V. Aflatoxin determination in black tea (Camellia sinensis)—Status and development of a protocol. J. Food Saf. 2012;32:13–21. doi: 10.1111/j.1745-4565.2011.00339.x. [DOI] [Google Scholar]
- 48.Benford D., Leblanc J.-C., Setzer R.W. Application of the margin of exposure (MoE) approach to substances in food that are genotoxic and carcinogenic Example: Aflatoxin B1 (AFB1) Food Chem. Toxicol. 2010;48:34–41. doi: 10.1016/j.fct.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization (WHO) WHO Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Ochratoxin A. WHO; Geneva, Switzerland: 2008. [(accessed on 10 September 2018)]. Available online: http://apps.who.int/food-additives-contaminants-jecfa-database/PrintPreview.aspx?chemID=1905. [Google Scholar]
- 50.Technical Regulations of the Customs Union TR CU 021/2011 On Food Safety. [(accessed on 10 September 2018)];2011 Available online: http://www.eurexcert.com/TRCUpdf/TRCU-0021-On-food-safety.pdf.
- 51.Zhang L., Dou X.-W., Zhang C., Logrieco A.F., Yang M.-H. A Review of Current Methods for Analysis of Mycotoxins in Herbal Medicines. Toxins. 2018;10:65. doi: 10.3390/toxins10020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anukul N., Vangnai K., Mahakarnchanakul W. Significance of regulation limits in mycotoxin contamination in Asia and risk management programs at the national level. J. Food Drug Anal. 2013;21:227–241. doi: 10.1016/j.jfda.2013.07.009. [DOI] [Google Scholar]
- 53.China Food Safety National Standard Maximum Levels of Mycotoxins in Food (GB 2761-2017) [(accessed on 29 October 2018)];2017 Available online: https://gain.fas.usda.gov/Recent%20GAIN%20Publications/China%20Releases%20Standard%20for%20 Maximum%20Levels%20of%20Mycotoxins%20in%20Foods%20_Beijing_China%20-%20Peoples%20Republic%20of_5-9-2018.pdf.
- 54.Malachová A., Sulyok M., Beltrán E., Berthiller F., Krska R. Optimization and validation of a quantitative liquid chromatography-tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J. Chromatogr. A. 2014;1362:145–156. doi: 10.1016/j.chroma.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 55.Lehotay S.J. Determination of pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate: Collaborative study. J AOAC Int. 2007;90:485–520. [PubMed] [Google Scholar]
- 56.González-Curbelo M.Á., Socas-Rodríguez B., Herrera-Herrera A.V., González-Sálamo J., Hernández-Borges J., Rodríguez-Delgado M.Á. Evolution and applications of the QuEChERS method. TrAC Trend. Anal. Chem. 2015;71:169–185. doi: 10.1016/j.trac.2015.04.012. [DOI] [Google Scholar]
- 57.Martínez-Domínguez G., Romero-González R., Garrido A.F. Multi-class methodology to determine pesticides and mycotoxins in green tea and royal jelly supplements by liquid chromatography coupled to Orbitrap high-resolution mass spectrometry. Food Chem. 2016;197:907–915. doi: 10.1016/j.foodchem.2015.11.070. [DOI] [PubMed] [Google Scholar]
- 58.Dzuman Z., Zachariasova M., Veprikova Z., Godula M., Hajslova J. Multi-analyte high-performance liquid chromatography coupled to high-resolution tandem mass spectrometry method for control of pesticide residues, mycotoxins, and pyrrolizidine alkaloids. Anal. Chim. Acta. 2015;863:29–40. doi: 10.1016/j.aca.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 59.Reichert B., de Kok A., Pizzutti I.R., Scholten J., Cardoso C.D., Spanjer M. Simultaneous determination of 117 pesticides and 30 mycotoxins in raw coffee, without clean-up, by LC-ESI-MS/MS analysis. Anal. Chim. Acta. 2018;1004:40–50. doi: 10.1016/j.aca.2017.11.077. [DOI] [PubMed] [Google Scholar]
- 60.Cladiere M., Delaporte G., Le Roux E., Camel V. Multi-class analysis for simultaneous determination of pesticides, mycotoxins, process-induced toxicants and packaging contaminants in tea. Food Chem. 2018;242:113–121. doi: 10.1016/j.foodchem.2017.08.108. [DOI] [PubMed] [Google Scholar]
- 61.Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed. [(accessed on 29 October 2018)]; SANTE/11813/2017 Supercedes SANTE/11945/2015 Implemented by 01/01/2018. Available online: https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2017-11813.pdf.
- 62.Komes D., Horžić D., Belščak A., Kovačević Ganič K., Baljak A. Determination of Caffeine Content in Tea and Maté Tea by Using Different Methods. Czech J. Food Sci. 2009;27:213–216. doi: 10.17221/612-CJFS. [DOI] [Google Scholar]
- 63.International Organization for Standardization ISO 3103:1980—Tea—Preparation of Liquor for use in Sensory Tests (p. 4) [(accessed on 29 October 2018)];1980 Available online: https://www.iso.org/standard/8250.html.