Abstract
Purpose
To determine whether disorganization of retinal inner layers (DRIL) on optical coherence tomography (OCT) is associated with ischemia on ultra-widefield fluorescein angiography (UWFFA) and with visual outcomes in eyes with acute, treatment-naïve central retinal vein occlusion (CRVO).
Design
Retrospective, single-institution, longitudinal cohort study.
Participants
Twenty-five consecutive patients with treatment-naïve CRVO and ≥ 1 year follow-up.
Methods
Two independent masked graders evaluated the extent of DRIL, ellipsoid zone disruption, external limiting membrane disruption, and other OCT parameters at the baseline, 6- month, 12-month, and final visits. Baseline UWFFA images were assessed for ischemic index values and foveal avascular zone (FAZ) enlargement.
Main Outcome Measures
Associations of DRIL with UWFFA findings and clinical outcomes including corrected visual acuity (VA).
Results
The median time to final follow-up was 24 months (range 12.1 – 43.9 months). Median DRIL extent at baseline was 765 µm (range 0 – 1000 µm). Eighteen of 25 eyes (72%) had some degree of DRIL at baseline, and 20 of 25 eyes (80%) had cystoid macular edema (CME). Neither the presence nor extent of DRIL at baseline was associated with presenting VA. In a cross-sectional analysis of each visit, extent of DRIL correlated with worse VA at both the 6-month (ρ = 0.656; p = 0.001) and final (ρ = 0.509; p = 0.016) visits. At final follow-up, DRIL extent was the OCT parameter most strongly correlated with baseline ischemic index (ρ = 0.418; p = 0.047) and baseline enlarged FAZ (p = 0.057) on UWFFA. On multivariate regression analysis, DRIL extent at final follow-up was the only OCT parameter associated with worse VA (p = 0.013) and remained significant when accounting for CME as a potential confounder.
Conclusions
Extent of DRIL was not associated with presenting VA in treatment-naïve eyes with acute CRVO. Following six months of follow-up however, DRIL extent correlated with worse VA and was predictive of worse VA throughout more than 2 years of follow-up. Ischemic features on UWFFA at baseline are predictive of the extent of DRIL development at final follow-up.
INTRODUCTION
Cystoid macular edema (CME) and macular ischemia are the most common causes of vision loss in central retinal vein occlusion (CRVO).1, 2 Several optical coherence tomography (OCT) features have been used as predictors of ischemia and visual acuity (VA) outcomes in CRVO and other retinal vascular diseases. External limiting membrane (ELM) integrity3, ellipsoid zone (EZ) integrity4, 5, hyperreflective foci (HRF)6, hyperreflective vertical lines3, prominent middle limiting membrane (p-MLM) sign,7 and paracentral acute middle maculopathy (PAMM)8 have all been evaluated as OCT-derived VA surrogates and markers of ischemia in CRVO.
Disorganization of the inner retinal layers (DRIL) is an OCT-derived biomarker that has been shown to be predictive of baseline VA as well as the VA after resolution of macular edema in both diabetes and uveitis.9–11 Mimouni et al. recently demonstrated that DRIL extent at baseline correlated with baseline VA and change in DRIL was predictive of VA improvement in a cohort of 136 eyes with RVO.12
The degree of enlargement of the foveal avascular zone (FAZ) and measurement of the area of non-perfusion by fluorescein angiography (FA) have been used to determine the degree of ischemia and predict VA outcomes in CRVO.13, 14 DRIL has been correlated with both enlarged FAZ and macular capillary non-perfusion in diabetic macular edema (DME) and is a reliable marker of ischemia.15, 16 However the correlation of DRIL with the severity of ischemia has been not been investigated in CRVO, a disease with a different pathophysiologic mechanism and natural history.
This study was designed to evaluate the correlation of DRIL and other OCT measures with the extent of ischemia on ultra-widefield fluorescein angiography (UWFFA) and visual outcomes in patients with acute treatment-naïve CRVO.
METHODS
Institutional Review Board approval was obtained through Duke University School of Medicine in Durham, North Carolina. All study-related procedures were performed in accordance with good clinical practice and applicable Food and Drug Administration regulations. The Duke University Investigational Review Board determined that informed consent was not necessary for this study because of its retrospective nature and absence of risks to participants involved. All research adhered to the tenets of the Declaration of Helsinki and all work using patient information was performed in compliance with the Health Insurance Portability and Accountability Act.
The Duke Enterprise Data Unified Content Explorer (DEDUCE) system was used to identify patients diagnosed with a CRVO (International Classification of Disease, Ninth Edition, code 362.37) between January 1st, 2009 and July 1st, 2016. Among these patients, those who were treatment-naïve at presentation, presented within 3 months of CRVO onset, had baseline UWFFA and spectral domain OCT (SD-OCT) images, and at least 1 year of follow-up were included. Baseline and follow-up demographic, clinical, and laboratory data were extracted from patient charts and analyzed. Multimodal imaging was reviewed as described below.
SD-OCT image analysis
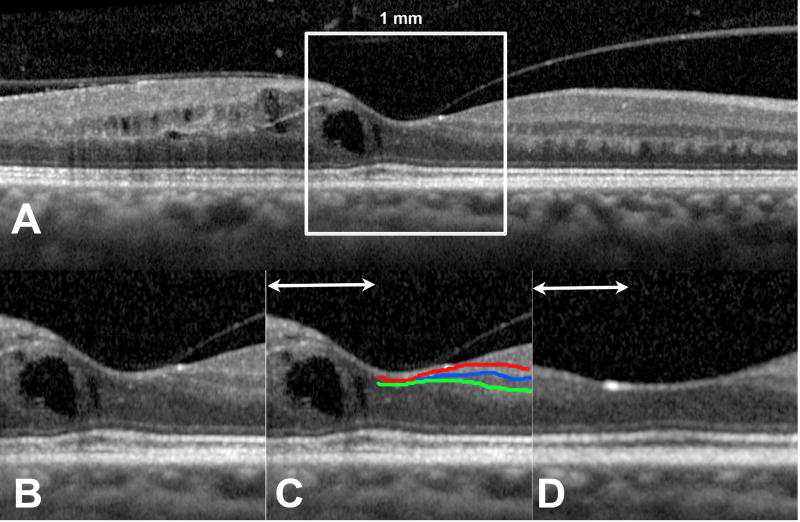
All patients underwent volume scans that encompassed a 25° × 30° region (Spectralis, Heidelberg Engineering, Carlsbad, CA) centered on the fovea. These were reviewed at baseline, 6-month, 12-month, and final visits in the study eye. The large majority of volume scans comprised 61-line raster B-scans and rarely 31-line raster B-scans. The central line scan centered on the foveal depression was identified, and the central 1 mm portion of this B-scan was analyzed by two masked graders (AST and DB). An overlay measuring 1000 µm (1 mm) centered on the foveal scan’s foveal depression was placed over the foveal scan to determine the central 1mm portion. DRIL was defined as the horizontal extent (µm) for which one or more boundaries between the inner retinal layers (ganglion cell layer and inner plexiform layer complex, inner nuclear layer and outer plexiform layer) were not separately identifiable (Figure 1). The central subfield thickness (CST), area of intraretinal cysts, and length of disruption of the ELM and EZ were measured. Total area of intraretinal cysts in the central 1 mm portion was calculated by tracing the outline of each individual cyst using the built-in caliper tool in the Spectralis software and calculating the sum of the area (mm2).
Figure 1. Representative optical coherence tomography images of a study patient.
Disorganization of retinal inner layers (DRIL) was evaluated in the central 1 mm of the line scan centered on the fovea (A). This 1 mm-wide portion (B) was evaluated for the boundaries between the ganglion cell layer-inner plexiform layer (red line), inner plexiform layer-inner nuclear layer (blue line), and inner nuclear layer-outer plexiform layer (green line). The horizontal extent of disruption of for which boundaries between any of these layers could not be identified was measured as DRIL (line with arrowheads). (C) An area of DRIL overlying a cyst of intraretinal fluid. Thirty months later, the fluid had resolved (D) but DRIL was still present.
Presence or absence of subretinal fluid (SRF) was noted, and subfoveal choroidal thickness was measured on enhanced depth images. Measurements between the two graders were compared and any value with a difference greater than 5% was jointly reviewed by both graders and a consensus value was determined. For values with less than a 5% difference, final values were obtained by averaging the two measurements.
UWFFA image analysis
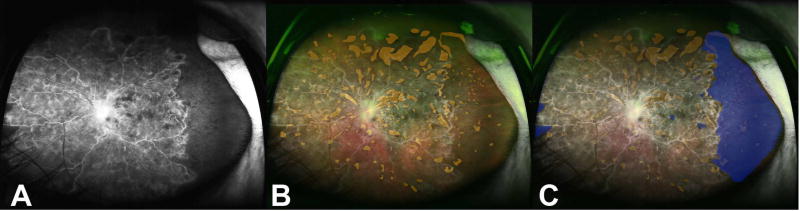
An early-phase, mid-phase, and late-phase UWFFA image obtained on the Optos 200 Tx camera (Optos Inc, Marlborough, MA) were reviewed for each study eye in a masked fashion by a single grader (AST). Images were reviewed for evidence of foveal avascular zone (FAZ) enlargement and evidence of retinal neovascularization. The extent of non-perfusion was quantified by calculating the ischemic index as has been previously described.17,18 Briefly, the mid-phase image was imported into Adobe Photoshop (Version CC 2017, Adobe Systems, San Jose, CA), where image contrast and brightness were adjusted to help clearly define the perfused/non-perfused junction. Next, areas of capillary non-perfusion were marked. The ischemic index (%) was calculated by measuring what percentage of imaged pixels representing the fundus was non-perfused (Figure 2). In eyes with a large amount of intra-retinal hemorrhage, the area of non-perfusion was determined by comparing the angiogram with the pseudocolor widefield photograph. The widefield pseudocolor photograph and angiogram were overlaid and areas corresponding to intraretinal hemorrhage were marked and these areas were excluded from ischemic index calculations.17 We did not have any eyes with vitreous hemorrhage that obscured the fundus vasculature.
Figure 2. Calculation of the ischemic index on ultra-widefield fluorescein angiography images.
(A) Representative ultra-widefield fluorescein angiogram (UWFFA) of an eye with a CRVO included in this study. (B) The ultra-widefield pseudocolor photograph was overlaid and areas of intraretinal hemorrhage were marked (orange). (C) Next, areas of hypofluorescence not corresponding to a hemorrhage (blue) were marked. The non-perfused pixels (blue) were measured and divided by the total number of imaged pixels representing the fundus and multiplied by 100 to calculate the ischemic index (%). Any pixels corresponding to areas of intraretinal hemorrhage (orange) were excluded from this calculation.
Statistical Analysis
Data was analyzed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA). Recorded corrected Early Treatment Diabetic Retinopathy (ETDRS) visual acuities were converted to logMAR visual acuity for the purposes of statistical analysis. Descriptive statistics were computed. Given that the data was not normally distributed, nonparametric tests were utilized. The relationship between continuous variables was assessed using Spearman’s rank correlation coefficient. The significance of the difference between two subgroups was assessed using the Wilcoxon rank sum test of difference among medians. Multivariate logistic regression for dichotomous outcomes and multivariate linear regression for continuous outcomes was performed.
P-values less than 0.05 were considered significant. Although we conducted multiple statistical tests, we did not apply the Bonferroni correction because it increases the risk of type II error which is not ideal given the exploratory nature of this study.
RESULTS
Patient characteristics
The cohort consisted of 25 eyes of 25 patients with a median patient age of 64 years at presentation (range 44 – 87 years) and median baseline logMAR VA of 0.60 (range 0 – 1.8). The median time to final follow-up was 24 months (range 12.1 – 43.9 months). From baseline to final follow-up, study eyes received a total of 242 anti-VEGF injections, 2 intravitreal steroid injections, and 3 sessions of panretinal laser photocoagulation. A total of 92 B-scans from 92 visits were graded. Inter-reader Pearson correlation coefficients ranged from 0.92 to 1.0 (0.92 for horizontal DRIL lengths, 1.0 for intraretinal cyst area, 0.97 for length of ELM disruption, and 0.98 for length of EZ disruption).
At baseline, 20 of the 25 eyes (80%) had CME on OCT. Baseline median CST as measured on OCT images was 447 µm (range 139 – 1085 µm) and median choroidal thickness was 235 µm (range 142 – 501 µm). Median DRIL extent at baseline was 735 µm (range 0 – 1000 µm). Additional baseline OCT parameters are shown in Table 1.
Table 1.
Baseline characteristics of study population
| Parameter | Mean | Median | Range |
|---|---|---|---|
| logMAR visual acuity | 0.65 | 0.60 | 0 – 1.82 |
| Central Subfield Thickness (µm) | 542.52 | 447 | 139 – 1085 |
| Subfoveal Choroidal Thickness (µm) | 253.88 | 235 | 142 – 501 |
| Area of Intraretinal Cysts (mm2) | 0.17 | 0.07 | 0 – 0.61 |
| Extent of Disorganization of the Inner Retinal Layers (DRIL) (µm) | 619.88 | 765 | 0 – 1000 |
| Extent of External Limiting Membrane Disruption (µm) | 428.08 | 0 | 0 – 1000 |
| Extent of Ellipsoid Zone Disruption (µm) | 427.84 | 0 | 0 – 1000 |
| Ischemic Index (%) | 17.70 | 16.13 | 0 – 44.70 |
Association of OCT parameters with visual acuity
Nonparametric bivariate correlation analysis was used to examine the relationship between the OCT parameters and VA at each visit in a cross-sectional manner. There was a significant correlation between the extent of DRIL and worse VA at both the 6-month (Spearman ρ = 0.656; p = 0.001) and final (Spearman ρ = 0.509; p = 0.016) visits. The extent of ELM disruption was the only OCT parameter that showed a significant correlation with worse VA across visits. The full results of this analysis are shown in Table 2.
Table 2.
Nonparametric bivariate analysis of visual acuity and OCT parameters at all visits
| VA at Baseline Visit |
VA at 6-Month Visit |
VA at 12-Month Visit |
VA at Final Visit | |||||
|---|---|---|---|---|---|---|---|---|
| OCT Parameter |
Spearman ρ |
P Value |
Spearman ρ |
P Value |
Spearman ρ |
P Value |
Spearman ρ |
P Value |
| Central Subfield Thickness | 0.443 | 0.026 | −0.035 | 0.882 | −0.179 | 0.423 | 0.006 | 0.978 |
| Subfoveal Choroidal Thickness | −0.329 | 0.107 | −0.307 | 0.1760 | −0.056 | 0.803 | −0.281 | 0.204 |
| Area of Intraretinal Cysts | 0.628 | <0.001 | 0.223 | 0.332 | 0.088 | 0.697 | 0.189 | 0.399 |
| Extent of DRIL | 0.3635 | 0.0741 | 0.656 | 0.001 | 0.280 | 0.206 | 0.509 | 0.016 |
| Extent of ELM Disruption | 0.559 | 0.004 | 0.440 | 0.046 | 0.437 | 0.043 | 0.537 | 0.010 |
| Extent of EZ Disruption | 0.605 | 0.001 | 0.545 | 0.012 | 0.399 | 0.066 | 0.537 | 0.010 |
OCT: Optical coherence tomography; DRIL: Disorganization of retinal inner layers; ELM: External limiting membrane; EZ: Ellipsoid zone; VA = corrected visual acuity
Next, the relationship between the measured OCT parameters at the baseline visit and VA at subsequent visits was analyzed. Both the extent of ELM disruption and the extent of EZ disruption at baseline correlated with worse VA at all future visits (Table 3). A similar analysis was conducted examining measured OCT parameters at the 6-month visit and VA at subsequent follow-up visits. The extent of DRIL at the 6-month visit was the only OCT parameter that predicted worse VA at both the 12-month visit (Spearman ρ = 0.617; p = 0.003) as well as the final visit (Spearman ρ = 0.591; p = 0.010) (Table 4).
Table 3.
Analysis of baseline OCT and ultra-widefield fluorescein angiography findings and visual acuity at follow-up visits
| VA at 6-Month Visit |
VA at 12-Month Visit |
VA at Final Visit | ||||
|---|---|---|---|---|---|---|
| Parameter | Spearman ρ |
P Value |
Spearman ρ |
P Value |
Spearman ρ | P Value |
| Central Subfield Thickness | 0.470 | 0.027 | 0.311 | 0.148 | 0.564 | 0.006 |
| Subfoveal Choroidal Thickness | −0.174 | 0.438 | −0.032 | 0.884 | −0.365 | 0.095 |
| Area of Intraretinal Cysts | 0.392 | 0.072 | 0.356 | 0.095 | 0.596 | 0.003 |
| Extent of DRIL | 0.294 | 0.184 | 0.229 | 0.294 | 0.266 | 0.232 |
| Extent of ELM Disruption | 0.490 | 0.021 | 0.414 | 0.049 | 0.586 | 0.004 |
| Extent of EZ Disruption | 0.603 | 0.003 | 0.500 | 0.015 | 0.624 | 0.002 |
| Ischemic Index | 0.318 | 0.149 | 0.424 | 0.045 | 0.372 | 0.088 |
| Enlarged FAZ‡ | --- | 0.028 | --- | 0.041 | --- | 0.003 |
Wilcoxon Rank Sum two-tailed test of significance. OCT: Optical coherence tomography; VA: Best-corrected visual acuity; DRIL: Disorganization of retinal inner layers; ELM: External limiting membrane; EZ: Ellipsoid zone; FAZ: Foveal avascular zone
Table 4.
Nonparametric bivariate analysis of 6-month OCT parameters and visual acuity at follow-up visits
| VA at 12-Month Visit | VA at Final Visit | |||
|---|---|---|---|---|
| OCT Parameter | Spearman ρ | P Value | Spearman ρ | P Value |
| Central Subfield Thickness | −0.208 | 0.365 | −0.059 | 0.815 |
| Subfoveal Choroidal Thickness | −0.323 | 0.154 | −0.462 | 0.054 |
| Area of Intraretinal Cysts | 0.146 | 0.528 | 0.181 | 0.472 |
| Extent of DRIL | 0.617 | 0.003 | 0.591 | 0.010 |
| Extent of ELM Disruption | 0.401 | 0.071 | 0.395 | 0.105 |
| Extent of EZ Disruption | 0.517 | 0.016 | 0.417 | 0.085 |
OCT: Optical coherence tomography; VA: Corrected visual acuity; DRIL: Disorganization of retinal inner layers; ELM: External limiting membrane; EZ: Ellipsoid zone
Multivariate regression analysis was then performed to examine the relationship between measured OCT parameters and VA. At baseline, none of the OCT parameters were found to be significantly associated with VA at final follow-up. At the final follow-up, DRIL was the only OCT parameter to be associated with VA (p = 0.013). To determine if the presence of CME could be a confounder, this analysis was repeated controlling for CME at final follow-up (Supplement Table 1), and the extent of DRIL at final follow-up remained the only OCT parameter associated with worse VA at final follow-up (p = 0.024).
Next, the multivariate models were reduced using a stepwise regression by which the least significant variable was removed at each step until only significant variables remained. For baseline OCT parameters, the only remaining variable to be associated with worse VA at final follow-up was baseline CST (p = 0.004). For OCT parameters at final follow-up, CST (p < 0.001), extent of ELM disruption (p < 0.001), and extent of DRIL (p = 0.006) were associated with worse VA at final follow-up.
Association of ultra-widefield fluorescein angiography parameters with visual acuity
Baseline UWFFA were analyzed and the calculated median ischemic index was 16.3 % (range 0 – 44.7%). Additionally, 26% of patients were determined to have an enlarged FAZ on baseline UWFFA. The correlation between these baseline measures of ischemia and VA at each visit was examined. A larger ischemic index at baseline was associated with worse VA at both the baseline (Spearman ρ = 0.456; p = 0.022) as well as the 12-month (Spearman ρ = 0.424; p = 0.045) visits. The presence of an enlarged FAZ on baseline UWFFA was associated with worse VA at every visit (p < 0.05 for all visits).
Association of ultra-widefield fluorescein angiography with OCT parameters
Further analysis was conducted to determine the relationship between baseline measures of ischemia on UWFFA and OCT parameters at the baseline visit and at final follow-up. At baseline, the extent of ELM disruption was the only OCT parameter that was associated with both a larger ischemic index (Spearman ρ = 0.440; p = 0.028) and enlarged FAZ (p = 0.018). At final follow-up, the extent of DRIL was the OCT parameter most strongly correlated with baseline ischemic index (Spearman ρ = 0.418; p = 0.047) as well as with baseline enlarged FAZ (p = 0.057, Wilcoxon rank sum test) (Table 5).
Table 5.
Relationship between measured OCT parameters at each visit, baseline ischemic index, and presence of baseline enlarged FAZ.
| Baseline Visit | 6 Month Visit | 12 Month Visit | Final Visit | |||||
|---|---|---|---|---|---|---|---|---|
| OCT Parameter |
Ischemic Index† |
Enlarged FAZ‡ |
Ischemic Index† |
Enlarged FAZ‡ |
Ischemic Index† |
Enlarged FAZ‡ |
Ischemic Index† |
Enlarged FAZ‡ |
| Central Subfield Thickness | 0.366 (p = 0.072) | 0.046 | −0.162 (p = 0.483) | 0.890 | −0.356 (p = 0.104) | 0.564 | 0.092 (p = 0.685) | 0.331 |
| Subfoveal Choroidal Thickness | 0.148 (p = 479) | 0.599 | −0.132 (p = 0.568) | 0.459 | −0.215 (p = 0.336) | 0.934 | 0.190 (p = 0.396) | 0.533 |
| Area of Intraretinal Cysts | 0.183 (p = 0.382) | 0.012 | −0.014 (p = 0.952) | 0.669 | −0.193 (p = 0.390) | 0.735 | 0.371 (p = 0.089) | 0.138 |
| Extent of DRIL | 0.087 (p = 0.678) | 0.235 | 0.360 (p = 0.109) | 0.100 | 0.222 (p = 0.321) | 0.595 | 0.418 (p = 0.047) | 0.057 |
| Extent of ELM Disruption | 0.440 (p = 0.028) | 0.018 | 0.138 (p = 0.550) | 0.482 | 0.008 (p = 0.970) | 0.817 | 0.061 (p = 0.789) | 0.185 |
| Extent of EZ Disruption | 0.327 (p = 0.111) | 0.060 | 0.161 (p = 0.486) | 0.339 | 0.190 (p = 0.397) | 0.851 | 0.061 (p = 0.789) | 0.185 |
Spearman correlation coefficient (p value);
Wilcoxon Rank Sum two-tailed test of significance.
OCT: Optical coherence tomography; FAZ: Foveal avascular zone; DRIL: Disorganization of retinal inner layers; ELM: External limiting membrane; EZ: Ellipsoid zone
DISCUSSION
In this investigation, we found that in treatment-naive eyes with acute CRVO, the extent of DRIL was associated with worse VA at the 6-month and subsequent visits following presentation. The ischemic index on baseline UWFFA was also predictive of the final extent of DRIL development. Notably, DRIL was the only OCT feature that was associated with worse VA at final follow-up. At the baseline visit, however, the extent of DRIL was neither correlated with baseline measures of ischemia nor predictive of future VA. The baseline OCT parameter most strongly associated with final VA was CST.
DRIL is thought to represent disruptions of synaptic connections of amacrine, bipolar, and horizontal cells within the inner retina, although this remains to be confirmed histologically.9 Although DRIL has been reported in diabetic retinopathy, retinal vascular disease, and uveitis, it is not yet known whether DRIL represents a generic sign of tissue damage, is specific to the underlying process, or is due to the mechanical stresses of the macular edema itself..9–11, 15, 16, 18 Pelosini and colleagues proposed that the bipolar axons can snap and cause loss of visual signal processing if macular edema causes stretching beyond their elastic limits, which would support the possibility that inner retinal changes such as DRIL may be explained by this process.20 However, it has also been reported that DRIL resolution patterns in DME are predictive of subsequent VA, independent of macular edema resolution patterns, which suggests that the underlying pathophysiology of DRIL may not be due the mechanical stresses of edema alone.10
DRIL has been associated with both capillary non-perfusion and an enlarged FAZ in DME.15, 16, 18, 19 20 The association between macular ischemia and poor VA is also well established.21 CRVO results in anatomic neurodegenerative changes in the inner retina, and it is likely that DRIL represents an inner retinal circulation compromise and a vascular insult. Animal studies that have shown that disruption of the inner retinal laminations promotes abnormal vascular growth and retinal bleeding also suggest the association of DRIL with ischemic sequelae and poor visual outcomes.22
We found that DRIL at the 6-month, but not the baseline, visit was associated with future VA. Additionally, baseline ischemic parameters on UWFFA correlated with the extent of DRIL at the final but not baseline visit. These findings may be attributed to the fact that the ischemic insult is acute and usually more severe in CRVO compared to DME and thus the full extent of DRIL may not manifest at the time of presentation and may be partially masked by CME. Capillary non-perfusion and downstream secondary retinal structural alterations likely contribute to the extent of DRIL. Following an acute ischemic-reperfusion event, there is an initial structural and functional disruption of the inner retinal layers followed by a second wave of apoptosis and remodeling of the inner retina with corresponding functional changes that occur weeks after the inciting event.23 Laser-induced CRVO models also show an acute phase with marked edema, followed by atrophy of the inner layers of the retina in the chronic phase.24 Ongoing neurodegenerative changes have also been shown in animal models of acute cerebral ischemia up to 4 weeks following the initial event.25 DRIL persisting long term may represent irreversible damage to the inner retinal layers.
Mimouni and colleagues explored the relationship between DRIL and VA in 136 eyes with branch retinal vein occlusion (BRVO), hemiretinal vein occlusion (HRVO), and CRVO and found that the baseline DRIL extent correlated with baseline VA, and changes in DRIL were predictive of changes in VA after three monthly bevacizumab injections.12 However, more than two-thirds of their cohort (69%) was comprised of patients with BRVO or HRVO, and individual relationships of DRIL across RVO subtypes were not explored. As the underlying pathophysiologic mechanisms and severity of ischemia are different across the RVO subtypes, the findings may not be representative of CRVO alone.26 Balaratnasingam et al. evaluated FAZ size as measured by OCT angiography (OCTA) as a potential prognostic marker in a cohort of 95 eyes with DME, CRVO, and BRVO.15 They found a significant correlation between FAZ size and DRIL extent, though this was a cross-sectional study and included only 11 patients with CRVO. In a small sample of 13 RVO eyes of which 4 were CRVO, Radwan et al. did not observe a correlation between DRIL and baseline VA.10
In addition to DRIL, other OCT-derived markers have been evaluated in RVO but only a few specifically in CRVO. The p-MLM sign has been reported in 28% of eyes with acute CRVO and has been associated with worse initial and final VA as well as ischemic CRVO.7 Additionally, PAMM has been observed in CRVO and other retinal vascular conditions and is indicative of ischemia in the deeper capillary bed of the inner retina.27 However, there has been no correlation between p-MLM and PAMM and other clinical measures.28
Limitations of this study include those inherent to its retrospective nature and small sample size. Some prior studies of DRIL have examined the change in OCT parameters and how they related to change in future VA. These analyses were conducted in the present study; however, they did not reveal significant findings, which may also indicate that these analyses were underpowered due to the small sample size. Further analysis is also needed to determine the role (if any) of concurrent CME as a confounder when grading DRIL.
In quantifying retinal surface area of UWFFA images, there are challenges in correcting for the warping produced when the retina, a nearly spherical surface, is projected onto a two-dimensional plane for viewing and analysis. Different approaches have been described to correct this including a stereographic projection method to correct distortion,29 a method that been validated using the retinal prosthesis as an “intraocular ruler”.30 We did not correct for the nonlinear peripheral distortion in our UWFFA images while calculating the ischemic index. This may have been one of the possible reasons for the low correlation between ischemic features and visual acuity and OCT parameters in this study. However, studies have shown good correlation between ischemic index measurements on such warped images and measured areas of non-perfusion on the corresponding dewarped images.31
Another limitation is that we did not adjust for normal perfused retina in our series. Singer et al. underlined the necessity of the exclusion of normal peripheral non-perfused retina, which can be considered as physiologic non-perfusion, when assessing the total area for the calculation of ischemic index using montaged images.32 There is variation in UWF images across subjects and even in the same subject during follow-up examinations. The median age in our cohort was 64 years, and Singer et al. reported that normal perfused retina was smaller in the subjects 60 years of age and older than in younger subjects, in all quadrants. This suggests that there may be a need to adjust the reference for normal perfused retinal area depending on the age and this may have altered some of the OCT-ischemic index correlations.
Strengths of our investigation include evaluation of DRIL as an OCT-derived surrogate in a cohort of CRVO patients in a real-world setting using widely adopted imaging techniques across a long follow-up period. Data were reviewed by independent masked graders with excellent agreement. Unique to this study is the correlation of retinal ischemia severity as measured on UWFFA and DRIL.
In conclusion, in treatment-naïve eyes with acute CRVO, development of DRIL is significantly associated with ischemia as well as VA through ≥1-year follow-up. While this data adds support to the use of DRIL as an imaging biomarker in CRVO, further investigation with prospective data collection is required to validate our findings and to further elucidate the role of DRIL in influencing clinical outcomes in eyes with CRVO.
Supplementary Material
In treatment naïve CRVO eyes, disorganization of retinal inner layers was associated with visual-acuity through over two years of follow-up and its extent was influenced by severity of ischemic index on the baseline wide-field angiogram
Acknowledgments
Financial Support: Ronald G. Michels Foundation (AST). NIH Core Grant for Vision Research EY05722 and the Unrestricted RPB Grant from Research to Prevent Blindness Inc. (both to Duke University Department of Ophthalmology).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: Retina Society Annual Meeting, October 5–8, 2017, Boston, Massachusetts and American Society of Retina Specialists Annual Meeting, July 20–25, 2018, Vancouver, British Columbia
Conflict of Interest: No conflicting relationship exists for any author.
References
- 1.Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–41. discussion 141-3. [PMC free article] [PubMed] [Google Scholar]
- 2.McIntosh RL, Rogers SL, Lim L, et al. Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117:1113–1123. e15. doi: 10.1016/j.ophtha.2010.01.060. [DOI] [PubMed] [Google Scholar]
- 3.Hasegawa T, Masuda N, Ogata N. Highly reflective line in optical coherence tomography images of eyes with macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2015;159:925–33. e1. doi: 10.1016/j.ajo.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 4.Shin HJ, Chung H, Kim HC. Association between integrity of foveal photoreceptor layer and visual outcome in retinal vein occlusion. Acta Ophthalmol. 2011;89:e35–40. doi: 10.1111/j.1755-3768.2010.02063.x. [DOI] [PubMed] [Google Scholar]
- 5.Ota M, Tsujikawa A, Murakami T, et al. Foveal Photoreceptor Layer in Eyes with Persistent Cystoid Macular Edema Associated with Branch Retinal Vein Occlusion. American Journal of Ophthalmology. 2008;145:273–280. e1. doi: 10.1016/j.ajo.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Mo B, Zhou HY, Jiao X, Zhang F. Evaluation of hyperreflective foci as a prognostic factor of visual outcome in retinal vein occlusion. Int J Ophthalmol. 2017;10:605–612. doi: 10.18240/ijo.2017.04.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko J, Kwon OW, Byeon SH. Optical coherence tomography predicts visual outcome in acute central retinal vein occlusion. Retina. 2014;34:1132–41. doi: 10.1097/IAE.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 8.Ghasemi Falavarjani K, Phasukkijwatana N, Freund KB, et al. En Face Optical Coherence Tomography Analysis to Assess the Spectrum of Perivenular Ischemia and Paracentral Acute Middle Maculopathy in Retinal Vein Occlusion. Am J Ophthalmol. 2017;177:131–138. doi: 10.1016/j.ajo.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Sun JK, Radwan SH, Soliman AZ, et al. Neural Retinal Disorganization as a Robust Marker of Visual Acuity in Current and Resolved Diabetic Macular Edema. Diabetes. 2015;64:2560–70. doi: 10.2337/db14-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radwan SH, Soliman AZ, Tokarev J, Zhang L, van Kuijk FJ, Koozekanani DD. Association of Disorganization of Retinal Inner Layers With Vision After Resolution of Center-Involved Diabetic Macular Edema. JAMA Ophthalmol. 2015;133:820–5. doi: 10.1001/jamaophthalmol.2015.0972. [DOI] [PubMed] [Google Scholar]
- 11.Grewal DS, O'Sullivan ML, Kron M, Jaffe GJ. Association of Disorganization of Retinal Inner Layers With Visual Acuity In Eyes With Uveitic Cystoid Macular Edema. Am J Ophthalmol. 2017;177:116–125. doi: 10.1016/j.ajo.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Mimouni M, Segev O, Dori D, Geffen N, Flores V, Segal O. Disorganization of the Retinal Inner Layers as a Predictor of Visual Acuity in Eyes With Macular Edema Secondary to Vein Occlusion. Am J Ophthalmol. 2017;182:160–167. doi: 10.1016/j.ajo.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Natural history and clinical management of central retinal vein occlusion. The Central Vein Occlusion Study Group. Arch Ophthalmol. 1997;115:486–91. doi: 10.1001/archopht.1997.01100150488006. [DOI] [PubMed] [Google Scholar]
- 14.Parodi MB, Visintin F, Della Rupe P, Ravalico G. Foveal avascular zone in macular branch retinal vein occlusion. Int Ophthalmol. 1995;19:25–8. doi: 10.1007/BF00156415. [DOI] [PubMed] [Google Scholar]
- 15.Balaratnasingam C, Inoue M, Ahn S, et al. Visual Acuity Is Correlated with the Area of the Foveal Avascular Zone in Diabetic Retinopathy and Retinal Vein Occlusion. Ophthalmology. 2016;123:2352–2367. doi: 10.1016/j.ophtha.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson L, Ramu J, Triantafyllopoulou I, et al. Diagnostic accuracy of disorganization of the retinal inner layers in detecting macular capillary non-perfusion in diabetic retinopathy. Clin Exp Ophthalmol. 2015;43:735–41. doi: 10.1111/ceo.12557. [DOI] [PubMed] [Google Scholar]
- 17.Tsui IKA, Havunjian MA, Hubschman S, Heilweil G, Prasad PS, Oliver SC, Yu F, Bitrian E, Hubschman JP, Friberg T, Schwartz SD. Ischemic index and neovascularization in central retinal vein occlusion. Retina. 2011;31:105–10. doi: 10.1097/IAE.0b013e3181e36c6d. [DOI] [PubMed] [Google Scholar]
- 18.Dodo Y, Murakami T, Uji A, Yoshitake S, Yoshimura N. Disorganized retinal lamellar structures in nonperfused areas of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015;56:2012–20. doi: 10.1167/iovs.14-15924. [DOI] [PubMed] [Google Scholar]
- 19.Spaide RF. Volume-Rendered Optical Coherence Tomography of Diabetic Retinopathy Pilot Study. Am J Ophthalmol. 2015;160:1200–10. doi: 10.1016/j.ajo.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Yeung L, Lima VC, Garcia P, Landa G, Rosen RB. Correlation between spectral domain optical coherence tomography findings and fluorescein angiography patterns in diabetic macular edema. Ophthalmology. 2009;116:1158–67. doi: 10.1016/j.ophtha.2008.12.063. [DOI] [PubMed] [Google Scholar]
- 21.Arend O, Wolf S, Harris A, Reim M. The relationship of macular microcirculation to visual acuity in diabetic patients. Arch Ophthalmol. 1995;113:610–4. doi: 10.1001/archopht.1995.01100050078034. [DOI] [PubMed] [Google Scholar]
- 22.Simmons AB, Merrill MM, Reed JC, Deans MR, Edwards MM, Fuerst PG. Defective Angiogenesis and Intraretinal Bleeding in Mouse Models With Disrupted Inner Retinal Lamination. Invest Ophthalmol Vis Sci. 2016;57:1563–77. doi: 10.1167/iovs.15-18395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmid H, Renner M, Dick HB, Joachim SC. Loss of inner retinal neurons after retinal ischemia in rats. Invest Ophthalmol Vis Sci. 2014;55:2777–87. doi: 10.1167/iovs.13-13372. [DOI] [PubMed] [Google Scholar]
- 24.Ebneter A, Agca C, Dysli C, Zinkernagel MS. Investigation of retinal morphology alterations using spectral domain optical coherence tomography in a mouse model of retinal branch and central retinal vein occlusion. PLoS One. 2015;10:e0119046. doi: 10.1371/journal.pone.0119046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang F, Xing S, He M, et al. Nogo-A is associated with secondary degeneration of substantia nigra in hypertensive rats with focal cortical infarction. Brain Res. 2012;1469:153–63. doi: 10.1016/j.brainres.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 26.Jaulim A, Ahmed B, Khanam T, Chatziralli IP. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina. 2013;33:901–10. doi: 10.1097/IAE.0b013e3182870c15. [DOI] [PubMed] [Google Scholar]
- 27.Rahimy E, Sarraf D, Dollin ML, Pitcher JD, Ho AC. Paracentral acute middle maculopathy in nonischemic central retinal vein occlusion. Am J Ophthalmol. 2014;158:372–380. e1. doi: 10.1016/j.ajo.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 28.Browning DJ, Punjabi OS, Lee C. Assessment of ischemia in acute central retinal vein occlusion from inner retinal reflectivity on spectral domain optical coherence tomography. Clin Ophthalmol. 2017;11:71–79. doi: 10.2147/OPTH.S122683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Croft DEvHJ, Wykoff CC, Clifton D, Verhoek M, Fleming A, Brown DM. Precise montaging and metric quantification of retinal surface area from ultra-widefield fundus photography and fluorescein angiography. Ophthalmic Surg Lasers Imaging Retina. 2014;45:312–7. doi: 10.3928/23258160-20140709-07. [DOI] [PubMed] [Google Scholar]
- 30.Sagong M, van Hemert J, Olmos de Koo LC, Barnett C, Sadda SR. Assessment of accuracy and precision of quantification of ultra-widefield images. Ophthalmology. 2015;122:864–6. doi: 10.1016/j.ophtha.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Tan CS, Chew MC, Van hemert J, Singer MA, Bell D, Sadda SR. Measuring the precise area of peripheral retinal non-perfusion using ultra-widefield imaging and its correlation with the ischaemic index. Br J Ophthalmol. 2016;100(2):235–9. doi: 10.1136/bjophthalmol-2015-306652. [DOI] [PubMed] [Google Scholar]
- 32.Singer M, Sagong M, van Hemert J, Kuehlewein L, Bell D, Sadda SR. Ultra-widefield Imaging of the Peripheral Retinal Vasculature in Normal Subjects. Ophthalmology. 2016;123:1053–9. doi: 10.1016/j.ophtha.2016.01.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.