Abstract
The Achilles tendon (AT) moment arm transforms triceps surae muscle forces into a moment about the ankle which is critical for functional activities like walking. Moreover, the AT moment arm changes continuously during walking, as it depends on both ankle joint rotation and triceps surae muscle loading (presumably due to bulging of the muscle belly). Here, we posit that aging negatively effects the architecturally complex AT moment arm during walking, which thereby contributes to well-documented reductions in ankle moment generation during push-off. We used motion capture-guided ultrasound imaging to quantify instantaneous variations in the AT moment arms of young (23.9±4.3 years) and older (69.9±2.6 years) adults during walking, their dependence on triceps surae muscle loading, and their association with ankle moment generation during push-off. Older adults walked with 11% smaller AT moment arms and 11% smaller peak ankle moments during push-off than young adults. Moreover, as hypothesized, these unfavourable changes were significantly and positively correlated (r2=0.38, p<0.01). More surprisingly, aging attenuated load-dependent increases in AT moment arm (i.e., that between heel-strike and push-off at the same ankle angle); only young adults exhibited a significant increase in their AT moment arm due to triceps surae muscle-loading. Age-associated reductions in triceps surae volume or activation, and thus muscle bulging during force generation, may compromise the mechanical advantage of the AT during the critical push-off phase of walking in older adults. Thus, strategies to restore and/or improve locomotor performance in our aging population should consider these functionally important changes in musculoskeletal behavior.
Keywords: Gait, Plantarflexors, Triceps surae, Ultrasound, Sarcopenia, Elderly
Introduction
Reduced walking performance in old age, exemplified by shorter steps and slowed preferred speed, negatively effects health and independence (Studenski et al., 2011). While the mechanisms responsible for these changes are poorly understood, compromised plantarflexor (i.e., ankle extensor) moment generation during push-off appears to play an important role (Franz, 2016; Judge et al., 1996; Kerrigan et al., 1998; McGibbon, 2003; Winter et al., 1990). The Achilles tendon (AT) moment arm, the distance from the tendon’s line of action to the ankle joint center, transforms triceps surae (i.e., gastrocnemius and soleus) muscle contractile forces into a moment about the ankle, for example during functional activities like walking. Using motion capture-guided ultrasound imaging techniques, we (Rasske et al., 2017) recently discovered that the AT moment arm changes continuously during walking, potentially reflecting the combined effects of kinematic (i.e., joint angle) and kinetic (i.e., muscle loading) factors - the latter presumably an indirect effect of bulging during force generation (Arellano et al., 2016; Azizi et al., 2009). However, although functionally relevant, aging effects on these characteristics of the architecturally complex AT moment arm during walking have yet to be systematically explored.
Historically, in vivo measurements of the AT moment arm have been made during isolated ankle exercises and then assumed to apply to more dynamic activities such as walking (Fath et al., 2010, 2013; Hashizume et al., 2014; Leardini and O'Connor, 2002; Lee and Piazza, 2012; Maganaris, 2004; Manal et al., 2010; Olszewski et al., 2015). The convention established by many of these studies, and which has been widely adopted, is that the AT moment arm varies strictly as a function of ankle joint angle. This convention has broad implications; for example, current musculoskeletal modeling approaches use muscle-tendon geometry descriptions that vary based solely on joint kinematics (Arnold et al., 2010). Yet some studies have shown that the relation between AT moment arm and ankle joint rotation also exhibits a load dependence, varying significantly from rest with increasing muscle activity (Fath et al., 2013; Hashizume et al., 2014; Maganaris, 2004; Olszewski et al., 2015).
We recently discovered that load dependence in the AT moment arm may be functionally important; using a novel motion capture-guided ultrasound imaging technique designed to estimate the instantaneous AT moment arm during walking, we found evidence suggesting that muscle loading during push-off amplified the AT moment arm of young adults by 10% independent of ankle joint angle (Rasske et al., 2017). We suspect that muscle bulging during force generation systematically alters the AT line of action and thus its moment arm during walking. Indeed, Maganaris et al. (1998) reported that triceps surae muscle thickness (i.e., the distance between superficial and deep gastrocnemius and soleus aponeuroses) increases by as much as 47% during a plantarflexor maximum voluntary isometric contraction compared to rest (Maganaris et al., 1998). More specifically, it is anatomically plausible that bulging of the soleus under the gastrocnemius may have a larger effect on the AT line of action than bulging of the gastrocnemius itself, and Maganaris et al. (1998) report significant soleus thickening during force generation. Nevertheless, conceptually, for a given net muscle force generated during walking, load-dependent enhancement of the AT moment arm may subsequently enhance moment generation during push-off. Therefore, measuring aging effects on the AT moment arm during walking could provide direct insight into at least one mechanism underlying well-documented reductions in plantarflexor moment generation in old age.
There are several reasons to suspect that aging decreases the AT moment arm and that these changes may be functionally meaningful. Most obvious, indirect evidence from the upper extremity implies that moment arms may decrease due to age-related reductions in muscle size via changes in the tendon line of action (Sugisaki et al., 2010). Cross-sectional comparisons of the triceps surae between young and older adults have revealed about a 20% decrease in muscle volume due to aging (Csapo et al., 2014). Additionally, Kim et al. (2011) found a proximal migration of the insertion of the AT onto the calcaneus with advancing age, which may influence its moment arm (Kim et al., 2011). Moreover, these changes may be functionally relevant; Lee and Piazza (2012) reported that smaller AT moment arms estimated during isolated ankle rotations correlated with slower walking speeds in some older adults (Lee and Piazza, 2012).
Therefore, our purpose was to estimate the instantaneous Achilles tendon moment arm in young and older adults during walking and to determine its relation to the peak moment generated by the plantarflexor muscles during push-off. We hypothesized that: (i) older adults would exhibit smaller AT moment arms than young adults during walking, and that (ii) the AT moment arm in walking would correlate with peak plantarflexor moment during push-off.
Methods
Subjects
Nine healthy young adults (age: 23.9 ± 4.3 years; height: 1.76 ± 0.15 m, mass: 70.3 ± 12.0 kg) and eight healthy older adults (age: 69.9 ± 2.6 years; height: 1.60 ± 0.11 m, mass: 64.7 ± 10.2 kg) participated in this study. Subjects provided written informed consent per the University of Wisconsin Health Sciences Institutional Review Board and completed a health questionnaire to assess the following exclusion criteria: BMI≥30, sedentary lifestyle, first degree family history of coronary artery disease, cigarette smoking, high blood pressure, high cholesterol, diabetes, orthopedic or neurological condition, taking medication that causes dizziness, or any unanticipated falls in the prior six months. Young adult data presented here were reanalyzed from a subset of previously published results. In this process, we excluded one young adult subject due to corrupted ground reaction force data.
Experimental protocol and measurements
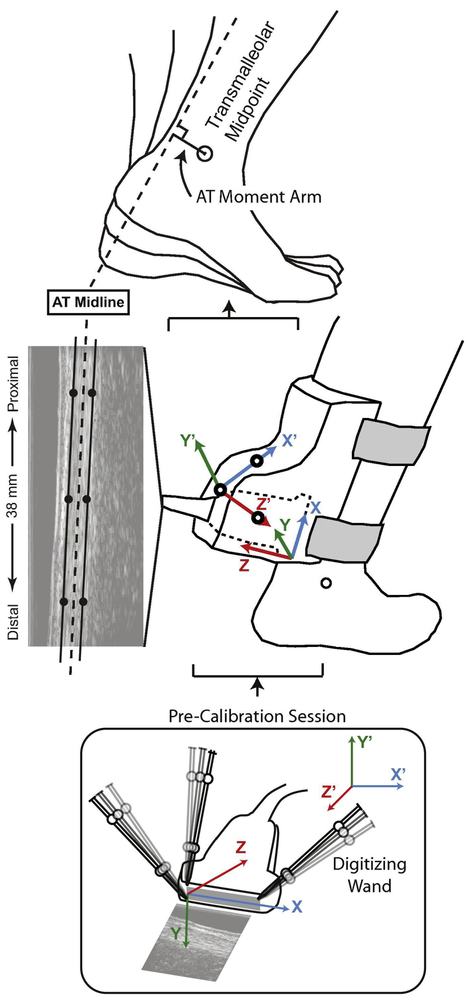
Prior to all testing, subjects walked for 6 min on a dual-belt, force measuring treadmill (Bertec, Columbus, OH) at 1.25 m/s to precondition their Achilles tendon (Hawkins et al., 2009) and allow their movement patterns to stabilize. Subjects then completed a 2 min walking trial at 1.25 m/s, during which we recorded bilateral ground reaction forces (GRF) at 2000 Hz. A custom orthotic positioned a 38 mm linear array transducer over the free AT (i.e. calcaneal insertion to soleus muscle-tendon junction) of subjects’ right leg, on average ~ cm superior to the calcaneal insertion (L14-5W/38, Ultrasonix, Richmond, BC) (Fig. 1). For each subject, we recorded ultrasound radiofrequency data from a longitudinal cross-section of the tendon at 77 frames/s over five strides. In synchrony, an 8-camera motion capture system (Motion Analysis, Corp., Santa Rosa, CA) operating at 200 Hz recorded the 3D trajectories of 17 anatomical markers and 14 tracking markers affixed via rigid clusters placed on subjects’ pelvis and right and left legs. An additional 3 markers defined the instantaneous position and orientation of the ultrasound orthotic.
Figure 1.
We used ultrasound imaging and quantitative motion capture to estimate instantaneous variations in the AT moment arm in young and older subjects during treadmill walking at 1.25 m/s. In a pre-calibration session prior to human subjects testing, we used previously established techniques (Franz et al., 2015; Franz and Thelen, 2015; Rasske et al., 2017) and an instrumented wand to digitize three points on the face of the transducer and used those three points to establish the “probe-centered” coordinate system shown (x,y,z). Those three points were selected to identify the boundary elements of the linear array transducer (and thus of the edges of the ultrasound imaging) and a line orthogonal to that image. Accordingly, we established the transformation between ultrasound image coordinates and the coordinate system defined by reference markers placed on the custom orthotic securing the transducer (x’,y’,z’). Subsequent to this pre-calibration, those reference markers were never removed from the orthotic over the course of the study and remained identical for young and older subjects. We used this precalibration to transform the ultrasound, orthotic, and motion capture kinematics from walking trials into a common, “orthotic-centered” reference frame. Finally, we estimated the instantaneous AT moment arm over the gait cycle as the perpendicular distance from AT midline to the ankle joint center. Using motion capture, we estimated the right ankle “joint center” during walking using the transmalleolar midpoint.
Data analysis
Using previously published procedures (Rasske et al., 2017), we manually tracked the superficial and deep edges of the AT at three locations (proximal, middle, and distal) using B-mode images created from the RF data (0.297 mm × 0.019 mm pixels). Specifically, we identified three points each on the superficial and deep edges of the tendon, each separated by 10 mm along the length of the imaged tendon. We then defined the AT midline as the best fit line through points corresponding to the average of the superficial and deep edges at each location.
We filtered the marker trajectories and GRF data using 4th order low-pass Butterworth filters with a cut-off frequency of 6 Hz and 100 Hz, respectively. For each subject, we scaled a 7-segment, 18 degree-of-freedom model (Arnold et al., 2010) of the pelvis and legs to a standing calibration in which we identified functional spherical hip joint centers using leg circumduction tasks (Piazza et al., 2001). We then performed inverse kinematics and dynamics analyses using SIMM Dynamics Pipeline (Musculographics, Santa Rosa, CA) and SD/FAST (Parametric Technology, Waltham, MA) as described in detail by Silder et al. (Silder et al., 2008). Briefly, a global optimization inverse kinematics routine calculated pelvis and leg joint kinematics by minimizing the weighted sum of squared differences between measured and modelled marker positions. We derived subjects’ net ankle moment via inverse dynamics based on model kinematics, anthropometrics, and GRF measurements. We then estimated subject’s peak ankle extensor moment generation during push-off from the average of 25 consecutive strides. Finally, ankle joint moments were normalized to subject’s body mass.
In a pre-calibration session prior to human subjects testing, we used previously established techniques (Franz et al., 2015; Franz and Thelen, 2015; Rasske et al., 2017) and an instrumented wand to digitize three points on the face of the transducer and used those three points to establish a “probe-centered” coordinate system and the transformation between this coordinate system and that defined by reference markers placed on the custom orthotic securing the transducer (Fig. 1). This pre-calibration session established the coordinate transformation between the ultrasound images and the three markers defining the position and orientation of the ultrasound orthotic for use during walking trials. Subsequent to this pre-calibration, those reference markers were never removed from the orthotic over the course of the study and remained identical for young and older subjects. Finally. by transforming the ultrasound, orthotic, and motion capture kinematics into a common reference frame, we estimated the instantaneous AT moment arm over the gait cycle as the perpendicular distance from AT midline to the ankle joint center (Fig. 1) (Rasske et al., 2017). We defined the right ankle “joint center” during walking as the instantaneous transmalleolar midpoint. In our prior work, a Monte Carlo simulation assessed the sensitivity of these AT moment arm estimates to errors in identifying the tendon edges and found relatively small deviation (i.e. 1.6%) (Rasske et al., 2017).
Statistical analysis
First, we assessed whether older adults exhibited smaller moment arms during walking using a mixed, two-way factorial analysis of variance (ANOVA) to test for significant main effects of and interactions between age (between subject factor, old vs. young) and gait cycle phase (within subject effect, 20% bins) on estimates of the AT moment arm. Second, we more specifically tested for aging effects on load-dependence of the AT moment arm independent of ankle joint rotation as follows. We extracted for each subject the AT moment arm (i) at heel-strike, an instant of relatively negligible load on the Achilles tendon, and (ii) at the instant the ankle passed through the same ankle angle during push-off Here, a second mixed, two-way factorial ANOVA tested for main effects of and interactions between age and muscle loading. For main effects and interaction terms from each ANOVA, we used an alpha level of 0.05 to define significance and report partial eta squared (ηp2) values for effect sizes. We then performed four planned pairwise comparisons using a Bonferroni adjusted alpha level for significance to correct for multiple testing (p=0.05/4 = 0.0125). First, we performed two planned post-hoc paired-samples t-tests to determine the effect of muscle loading on the AT moment arm (i.e., that at an identical ankle angle at heel-strike vs. during push-off) in young and in older subjects. Second, we performed independent-samples t-tests to assess aging effects on: (i) peak ankle moment and (ii) the AT moment arm at the instant of peak ankle moment. Here, we report Cohen’s d effect sizes for each pairwise comparison. Finally, we calculated correlation coefficients between the AT moment arm at the instant of peak ankle moment and the peak ankle moment during push-off using data pooled from all subjects.
Results
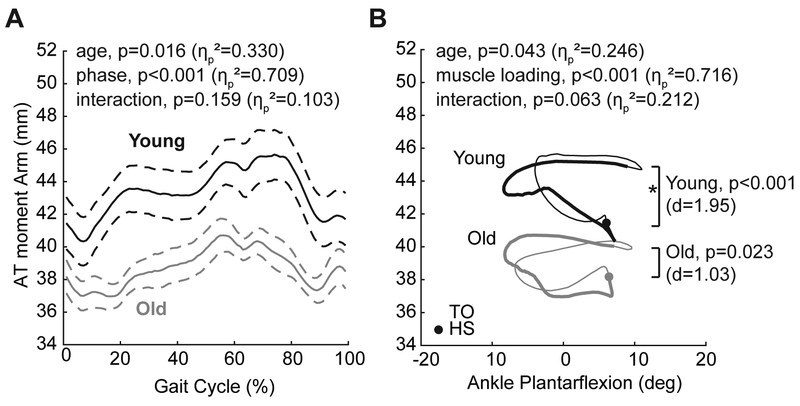
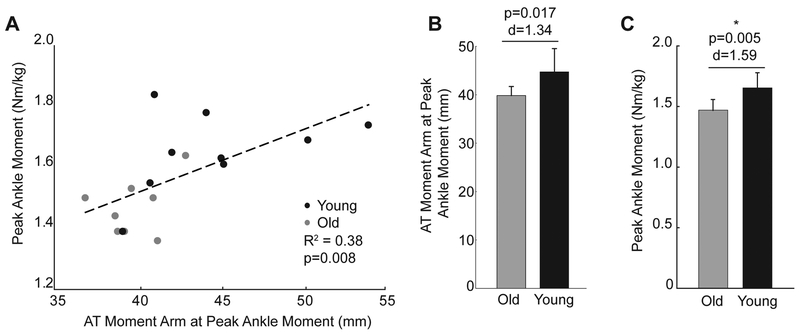
In both older and young adults, the AT moment arm progressively increased through stance phase (Fig. 2A), and did not vary strictly as a function of ankle joint angle (Fig. 2B). We did not find a significant interaction between age and gait phase on the AT moment arm (p=0.159, ηp2=0.103). However, the AT moment arms of older adults were 11% (i.e., 5.17 mm) smaller on average than young adults during walking (age main effect, p=0.016, ηp2=0.330, Fig. 2A), an effect that remained significant even when controlling for subject height as a covariate. Further, aging tended to attenuate the load-dependent increase in the AT moment arm between heel-strike and push-off at the same ankle angle (interaction, p=0.063, ηp2=0.212, Fig. 2B). Specifically, using a Bonferroni-adjusted level of significance, we found a significant effect of muscle loading on the AT moment arm in young (mean difference: 3.25±1.67 mm [i.e., +8%], p<0.001, d=1.95) but not in older adults (mean difference: 1.65±1.61 mm, p=0.023, d=1.03) (Fig. 2B). Older adults also walked with 11% smaller peak ankle moments on average during push-off than young adults (young: 1.65±0.13 Nm/kg, older: 1.47±0.09 Nm/kg, p=0.005, d=1.59). Finally, AT moment arm at the instant of peak ankle moment, a value that tended to be less in older than in young subjects (young: 44.7±4.8 mm, older: 39.8±1.9 mm, p=0.017. d=1.34), significantly and positively correlated with peak ankle moment during push-off (r2=0.38, p=0.008) (Fig. 3). However, the load-dependent increase in the AT moment (i.e., that between heel-strike and push-off at the same ankle angle) was independent of peak AT moment arm magnitude (r2=0.08, p=0.260) and did not itself correlate with peak ankle moment during push-off (r2=0.14, p=0.142).
Figure 2.
A: Group mean (standard error) AT moment arm over an average gait cycle for young and older adults. B: Group mean AT moment arm versus ankle joint angle for young and older adults. Asterisks (*) indicate an effect of muscle loading on the AT moment arm, significant only for young subjects using a Bonferroni-adjusted level of significance (i.e., p<0.0125). Specifically, we assessed load dependence via pairwise comparisons between the AT moment arm: (i) at heel-strike, an instant of relatively negligible load on the Achilles tendon, and (ii) at the instant the ankle passed through the same ankle angle during push-off.
Figure 3.
A: AT moment arm at the instant of the peak ankle moment positively and significantly correlated with peak ankle moment generation during the push-off phase of walking. B: Group mean (standard deviation) AT moment arm at the instant of peak ankle moment in young and older subjects. C: Group mean (standard deviation) peak ankle moment in young and older subjects. Asterisk (*) indicates statistical significance using a Bonferonni-adjusted alpha level of 0.0125.
Discussion
The net mechanical output from the propulsive plantarflexor muscles during the push-off phase of walking decreases with advancing age (Winter et al., 1990). The mechanisms governing these changes have largely remained elusive and are most certainly multifactorial. However, prior evidence from isolated ankle rotations has suggested possible associations between smaller AT moment arms and slower walking speeds in some older adults (Lee and Piazza, 2012). Here we used in vivo ultrasound imaging to estimate aging effects on the Achilles tendon moment arm during walking and its association with decrements in the peak ankle moment during push-off. As hypothesized, older adults exhibited significantly smaller AT moment arms on average during walking than young adults. Aging also attenuated the load-dependent increase in the AT moment arm during the stance phase of walking (i.e., that between heel-strike and push-off at the same ankle angle). Indeed, only young adults exhibited a significant increase in their AT moment arm due to triceps surae muscle-loading. Finally, and also as hypothesized, we found that smaller AT moment arms correlated with diminished peak ankle moments in older versus young adults. As we elaborate in more detail below, we suspect that age-associated reductions in triceps surae muscle size, and consequently muscle bulging during force generation, may underlie these functionally relevant changes in musculoskeletal behavior during walking in older adults.
Although only moderately associated with larger reductions in muscle-force generating capacity (Clark and Manini, 2012), aging does bring concomitant reductions in triceps surae muscle mass (i.e., sarcopenia) and muscle volume. Cross-sectional comparisons between older and young adults revealed, for example, 30%, 13%, and 16% reductions in medial gastrocnemius, lateral gastrocnemius, and soleus muscle volumes, respectively (Csapo et al., 2014). Moreover, indirect evidence using magnetic resonance imaging of the triceps brachii suggests that muscle cross-sectional areas can systematically alter distal free tendon lines of action and thereby increase muscle-tendon moment arms (Sugisaki et al., 2010; Sugisaki et al., 2015). Although we did not measure muscle architecture in our study participants, we suspect that the smaller AT moment arms in older adults arise at least in part from their smaller triceps surae muscle cross-sectional areas compared to young adults.
Decrements in muscle size in older adults may also explain the smaller increase in their AT moment arm with the rise in plantarflexor force generation during the stance phase compared to young adults. Indeed, we previously proposed that muscle bulging during force generation could contribute to load-dependent enhancement of the AT moment arm during walking, at least in young adults (Rasske et al., 2017). This purported mechanism is in line with prior measurements of the AT moment during isolated contractions. For example, Hashizume et al. (2014) found significantly larger AT moment arms at 30% maximum voluntary contraction than at rest at a given joint angle (Hashizume et al., 2014). Those authors interpreted their results to suggest that muscle contraction yields a prominent posteromedial displacement of the line of action of the Achilles tendon force. Accordingly, even for the same activation, older adults appear unable to take advantage of an increase in the mechanical advantage of the triceps surae muscles via load-dependent enhancement of the AT moment arm. We also note that there is an alternative, or perhaps related, explanation for the diminished load-dependent enhancement of the AT moment arm during walking in older adults. Holt et al. (2016) recently found that triceps surae muscle gearing (i.e., the ratio of muscle shortening to fiber shortening), and thus fiber rotation, is lost in old age (Holt et al., 2016). Accordingly, a smaller gear ratio in older triceps surae muscles could diminish muscle bulging during force generation and thus restrict load-dependent changes in the AT line of action during walking.
Finally, as hypothesized, we found that age-related reductions in peak plantarflexor moment generated during push-off positively correlated with smaller moment arms in older adults. These findings agree well with those of Baxter et al. (2014), who used magnetic resonance imaging to reveal that both the AT moment arm and plantarflexor muscle volume correlated with net plantarflexor moments during isolated contractions in young subjects (Baxter and Piazza, 2014). In fact, those authors suggested that the AT moment arm is at least as important as muscle size in governing ankle plantarflexion strength. The age-related reduction in plantarflexor performance during walking has been implicated in governing slower preferred walking speeds in older adults, which ultimately predicts their health and independence (Hardy et al., 2007; Studenski et al., 2011). Here, our findings implicate smaller AT moment arms, and thus diminished functional transmission of triceps surae muscle forces, as functionally relevant in this biomechanical cascade of events independent of changes in muscle strength. Indeed, our findings may help to explain previously reported correlations between smaller AT moment arms and slower preferred speeds in slow walking older adults (Lee and Piazza, 2012).
In addition to their functional relevance to age-related mobility impairment, our results also have important implications for computational modeling and simulation of human movement. Current musculoskeletal models assume kinematically-derived moment arms for the gastrocnemius and soleus (Arnold et al., 2010), yet we continue to see a load-dependent behavior in these architectural parameters during walking. Moreover, the present study extends these observations to further reveal that this load-dependence is complex and age-dependent, presumably governed by changes in muscle size and muscle bulging. Although the functional implications have yet to be systematically explored, models without load-dependent variations in muscle-tendon moment arms remain fundamentally incomplete. For example, even when muscle volume and maximum moment-generating capacity are drawn from the same subjects (Holzbaur et al., 2007a; Holzbaur et al., 2007b), models using these data (Arnold et al., 2010; Saul et al., 2015) often require an artificially inflated peak muscle stress (i.e., specific tension) of 50.8 N/cm2, well beyond physiological values (Close, 1972; Erskine et al., 2011; Harber and Trappe, 2008; Lannergren and Westerblad, 1987), to resolve architectural and kinetic information measured in vivo. These limitations in model development highlight our incomplete scientific understanding of the physiology of moment-generation in vivo with broad implications.
We acknowledge several important limitations of our study. First, we estimated the ankle joint center based on anatomical landmarks, an approach sensitive to marker placement errors and which may simplify the ankle joint kinematics during locomotion. We note two limitations related to our use of the transmalleolar midpoint to estimate the ankle joint center. First, this estimate may differ from that identified using the finite helical axis during ankle joint rotation (Lundberg et al., 1989). Siston et al. (2005) quantitatively compared error magnitudes among five different anatomically-based methods (including the transmalleolar midpoint) and two kinematically-derived methods (i.e., model fits to estimate functional center of rotation) of determining the ankle joint center compared to reference, ground truth data obtained from subject-specific MRI (Siston et al., 2005). Ultimately, those authors concluded that their most accurate and sophisticated kinematically-derived model fit was statistically equivalent in error magnitude to the anatomically-based approach we use here. More specifically, error magnitudes ranged from 2-4 mm on average. Nevertheless, second, an equally relevant consideration is that age-related changes to muscle force-generating capacity and the stiffness of peri- and intra-articular ankle joint structures could shift the finite helical axis of the ankle joint compared to young adults. Because these methodological decisions could have affected our estimates of AT moment arm variations and aging affects thereof, our outcomes and interpretations should thus be interpreted within this context while motivating next logical steps in this line of research. As another potential source of error, a single investigator manually identified the edges of the AT from cine B-mode images. We quantitatively evaluated this limitation in our previously published work and found that simulated manual tracking errors were more than four times smaller than the average variation in the AT moment arm during walking (Rasske et al., 2017). Moreover, the accuracy of our coordinate transformations, and thus the conversion of the tendon’s line of action to 3D coordinates for co-registration, rests on the resolution of our motion capture system to accurately estimate the positions of the probe tracking markers. We also did not measure subjects’ gastrocnemius and soleus muscle size nor bulging during force generation, which will be important to include in future mechanistic studies. Moreover, we interpret changes in the AT moment arm has functionally meaningful in the context of triceps surae mechanical advantage. However, these predictions come with certain assumptions, for example that age-related changes to the input moment arm (i.e., the distance between the center of pressure of the ground reaction force vector and the ankle joint center) do not necessarily offset the reported changes in the Achilles tendon moment arm. Finally, we report absolute magnitudes of the AT moment arm and its load dependence during walking and did not normalize these values to subject anthropometrics. Previous work has revealed little evidence that AT moment arms predictably scale to anthropometric measurements (Waugh et al., 2011) or correlate to body height (Hashizume et al., 2014). Indeed, as in these prior studies, we found no significant correlation between AT moment arm and subject height (p=0.15).
In summary, our findings reveal that age-related reductions in peak ankle moment during push-off are correlated with smaller AT moment arms in older adults measured in vivo during walking. Age-associated reductions in triceps surae muscle volume, and thus muscle bulging during force generation, may underlie these changes. Our results provide a potential biomechanical basis for earlier associations between AT moment arm and preferred speed in slow walking older adults (Lee and Piazza, 2012). In addition, we provide further evidence of complex, load-dependent variations in the AT moment arm during walking that may be relevant in governing triceps surae mechanical performance. Accordingly, strategies to restore and/or improve locomotor performance in our aging population should consider these functionally important changes in musculoskeletal behavior.
Acknowledgements
We gratefully acknowledge Drs. Darryl Thelen and Katherine Saul for their helpful comments, particularly regarding the implications of our findings for the development and use of musculoskeletal modeling and simulation.
Funding
This work was supported by the National Institutes of Health (grant numbers F32AG044904, R01AG051748).
Footnotes
Conflicts of Interest Statement
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arellano CJ, Gidmark NJ, Konow N, Azizi E, Roberts TJ, 2016. Determinants of aponeurosis shape change during muscle contraction. Journal of biomechanics 49, 1812–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold EM, Ward SR, Lieber RL, Delp SL, 2010. A model of the lower limb for analysis of human movement. Annals of biomedical engineering 38, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi E, Halenda GM, Roberts TJ, 2009. Mechanical properties of the gastrocnemius aponeurosis in wild turkeys. Integrative and comparative biology 49, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter JR, Piazza SJ, 2014. Plantar flexor moment arm and muscle volume predict torque-generating capacity in young men. Journal of applied physiology 116, 538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BC, Manini TM, 2012. What is dynapenia? Nutrition 28, 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close RI, 1972. Dynamic properties of mammalian skeletal muscles. Physiological reviews 52, 129–197. [DOI] [PubMed] [Google Scholar]
- Csapo R, Malis V, Sinha U, Du J, Sinha S, 2014. Age-associated differences in triceps surae muscle composition and strength - an MRI-based cross-sectional comparison of contractile, adipose and connective tissue. BMC musculoskeletal disorders 15, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine RM, Jones DA, Maffulli N, Williams AG, Stewart CE, Degens H, 2011. What causes in vivo muscle specific tension to increase following resistance training? Experimental physiology 96, 145–155. [DOI] [PubMed] [Google Scholar]
- Fath F, Blazevich AJ, Waugh CM, Miller SC, Korff T, 2010. Direct comparison of in vivo Achilles tendon moment arms obtained from ultrasound and MR scans. Journal of applied physiology 109, 1644–1652. [DOI] [PubMed] [Google Scholar]
- Fath F, Blazevich AJ, Waugh CM, Miller SC, Korff T, 2013. Interactive effects of joint angle, contraction state and method on estimates of achilles tendon moment arms. Journal of applied biomechanics 29, 241–244. [DOI] [PubMed] [Google Scholar]
- Franz JR, 2016. The age-associated reduction in propulsive power generation in walking. Exercise and sport sciences reviews 44, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR, Slane LC, Rasske K, Thelen DG, 2015. Non-uniform in vivo deformations of the human Achilles tendon during walking. Gait & posture 41, 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR, Thelen DG, 2015. Depth-dependent variations in Achilles tendon deformations with age are associated with reduced plantarflexor performance during walking. J Appl Physiol 119, 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harber M, Trappe S, 2008. Single muscle fiber contractile properties of young competitive distance runners. Journal of applied physiology 105, 629–636. [DOI] [PubMed] [Google Scholar]
- Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA, 2007. Improvement in usual gait speed predicts better survival in older adults. Journal of the American Geriatrics Society 55, 1727–1734. [DOI] [PubMed] [Google Scholar]
- Hashizume S, Iwanuma S, Akagi R, Kanehisa H, Kawakami Y, Yanai T, 2014. The contraction-induced increase in Achilles tendon moment arm: a three-dimensional study. Journal of biomechanics 47, 3226–3231. [DOI] [PubMed] [Google Scholar]
- Hawkins D, Lum C, Gaydos D, Dunning R, 2009. Dynamic creep and pre-conditioning of the Achilles tendon in-vivo. Journal of biomechanics 42, 2813–2817. [DOI] [PubMed] [Google Scholar]
- Holt NC, Danos N, Roberts TJ, Azizi E, 2016. Stuck in gear: age-related loss of variable gearing in skeletal muscle. The Journal of experimental biology 219, 998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbaur KR, Delp SL, Gold GE, Murray WM, 2007a. Moment-generating capacity of upper limb muscles in healthy adults. Journal of biomechanics 40, 2442–2449. [DOI] [PubMed] [Google Scholar]
- Holzbaur KR, Murray WM, Gold GE, Delp SL, 2007b. Upper limb muscle volumes in adult subjects. Journal of biomechanics 40, 742–749. [DOI] [PubMed] [Google Scholar]
- Judge JO, Davis RB 3rd, Ounpuu S, 1996. Step length reductions in advanced age: the role of ankle and hip kinetics. The journals of gerontology. Series A, Biological sciences and medical sciences 51, M303–312. [DOI] [PubMed] [Google Scholar]
- Kerrigan DC, Todd MK, Della Croce U, Lipsitz LA, Collins JJ, 1998. Biomechanical gait alterations independent of speed in the healthy elderly: evidence for specific limiting impairments. Archives of physical medicine and rehabilitation 79, 317–322. [DOI] [PubMed] [Google Scholar]
- Kim PJ, Martin E, Ballehr L, Richey JM, Steinberg JS, 2011. Variability of insertion of the Achilles tendon on the calcaneus: an MRI study of younger subjects. J Foot Ankle Surg 50, 41–43. [DOI] [PubMed] [Google Scholar]
- Lannergren J, Westerblad H, 1987. The temperature dependence of isometric contractions of single, intact fibres dissected from a mouse foot muscle. The Journal of physiology 390, 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leardini A, O'Connor JJ, 2002. A model for lever-arm length calculation of the flexor and extensor muscles at the ankle. Gait & posture 15, 220–229. [DOI] [PubMed] [Google Scholar]
- Lee SS, Piazza SJ, 2012. Correlation between plantarflexor moment arm and preferred gait velocity in slower elderly men. Journal of biomechanics 45, 1601–1606. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Svensson OK, Nemeth G, Selvik G, 1989. The axis of rotation of the ankle joint. J Bone Joint Surg Br 71, 94–99. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, 2004. Imaging-based estimates of moment arm length in intact human muscle-tendons. European journal of applied physiology 91, 130–139. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Baltzopoulos V, Sargeant AJ, 1998. In vivo measurements of the triceps surae complex architecture in man: implications for muscle function. The Journal of physiology 512 (Pt 2), 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manal K, Cowder JD, Buchanan TS, 2010. A hybrid method for computing achilles tendon moment arm using ultrasound and motion analysis. Journal of applied biomechanics 26, 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGibbon CA, 2003. Toward a better understanding of gait changes with age and disablement: neuromuscular adaptation. Exercise and sport sciences reviews 31, 102–108. [DOI] [PubMed] [Google Scholar]
- Olszewski K, Dick TJ, Wakeling JM, 2015. Achilles tendon moment arms: the importance of measuring at constant tendon load when using the tendon excursion method. Journal of biomechanics 48, 1206–1209. [DOI] [PubMed] [Google Scholar]
- Piazza SJ, Okita N, Cavanagh PR, 2001. Accuracy of the functional method of hip joint center location: effects of limited motion and varied implementation. Journal of biomechanics 34, 967–973. [DOI] [PubMed] [Google Scholar]
- Rasske K, Thelen DG, Franz JR, 2017. Variation in the human Achilles tendon moment arm during walking. Computer methods in biomechanics and biomedical engineering 20, 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul KR, Hu X, Goehler CM, Vidt ME, Daly M, Velisar A, Murray WM, 2015. Benchmarking of dynamic simulation predictions in two software platforms using an upper limb musculoskeletal model. Computer methods in biomechanics and biomedical engineering 18, 1445–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silder A, Heiderscheit B, Thelen DG, 2008. Active and passive contributions to joint kinetics during walking in older adults. Journal of biomechanics 41, 1520–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siston RA, Daub AC, Giori NJ, Goodman SB, Delp SL, 2005. Evaluation of methods that locate the center of the ankle for computer-assisted total knee arthroplasty. Clin Orthop Relat Res 439, 129–135. [DOI] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J, 2011. Gait speed and survival in older adults. Jama 305, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugisaki N, Wakahara T, Miyamoto N, Murata K, Kanehisa H, Kawakami Y, Fukunaga T, 2010. Influence of muscle anatomical cross-sectional area on the moment arm length of the triceps brachii muscle at the elbow joint. Journal of biomechanics 43, 2844–2847. [DOI] [PubMed] [Google Scholar]
- Sugisaki N, Wakahara T, Murata K, Miyamoto N, Kawakami Y, Kanehisa H, Fukunaga T, 2015. Influence of muscle hypertrophy on the moment arm of the triceps brachii muscle. Journal of applied biomechanics 31, 111–116. [DOI] [PubMed] [Google Scholar]
- Waugh CM, Blazevich AJ, Fath F, Korff T, 2011. Can Achilles tendon moment arm be predicted from anthropometric measures in pre-pubescent children? Journal of biomechanics 44, 1839–1844. [DOI] [PubMed] [Google Scholar]
- Winter DA, Patla AE, Frank JS, Walt SE, 1990. Biomechanical walking pattern changes in the fit and healthy elderly. Physical therapy 70, 340–347. [DOI] [PubMed] [Google Scholar]