Abstract
Household air pollution is estimated to be responsible for nearly three million premature deaths annually. Measuring fractional exhaled nitric oxide (FeNO) may improve the limited understanding of the association of household air pollution and airway inflammation. We evaluated the cross-sectional association of FeNO with exposure to household air pollution (24-h average kitchen and personal fine particulate matter and black carbon; stove type) among 139 women in rural Honduras using traditional stoves or cleaner-burning Justa stoves. We additionally evaluated interaction by age. Results were generally consistent with a null association; we did not observe a consistent pattern for interaction by age. Evidence from ambient and household air pollution regarding FeNO is inconsistent, and may be attributable to differing study populations, exposures, and FeNO measurement procedures (e.g., the flow rate used to measure FeNO).
Keywords: fractional exhaled nitric oxide, household air pollution, particulate matter
1. Introduction
Exposure to household air pollution from the combustion of solid fuels was estimated to be responsible for 2.5 million deaths and 77.2 million DALYS (disability-adjusted life years) in 2016 [1]. The incomplete combustion of solid fuels used for cooking and heating, such as wood or crop residue, emits extremely high levels of particulate matter (PM) and black carbon [2,3], among other pollutants [4]. The resulting household air pollution is associated with adverse health outcomes including chronic obstructive pulmonary disease (COPD), lung cancer, adult lower respiratory infections, cardiovascular disease [5,6] and adverse pregnancy outcomes [7]. Household air pollution is a modifiable exposure, and cleaner-burning cookstove technologies have demonstrated the potential to increase cooking efficiency and reduce human exposure to PM2.5 (fine particulate matter) by 50% or more [8].
The underlying mechanisms of pulmonary diseases associated with air pollution are not well understood; however, evidence suggests that exposure may result in increased reactive oxygen species and production of proinflammatory cytokines, leading to airway inflammation [9,10,11,12,13]. Previous household air pollution studies have focused on COPD, acute lower respiratory diseases, and forced expiratory volume; however, other pulmonary impacts, such as asthma and airway inflammation, have not been well studied [5].
Fractional exhaled nitric oxide (FeNO) measured in human breath is a non-invasive method to assess subclinical airway inflammation [14]. A measure of FeNO quantifies nitric oxide (NO) as an indication of eosinophilic inflammation initialized by cytokines and Type 2 helper cells (Th2) [15,16,17,18] and may provide unique information on airway inflammation to complement spirometry [19]. Acute and chronic exposure to air pollution may result in airway inflammation, which can potentially be quantified by measuring FeNO. Several previous studies have demonstrated that ambient air pollution exposure [20,21,22,23] and wood smoke exposure [24] are associated with increased levels of FeNO among healthy adults; other studies have observed null finding with ambient air pollution or woodsmoke and FeNO [25]. Strong evidence exists for association between exposure to household air pollution and respiratory disease such as COPD and lung cancer [26,27], while evidence for an association with asthma is inconclusive [28]. To our knowledge, only one study has assessed the association between household air pollution and FeNO, reporting a small increase in FeNO (2 ppb) immediately after cooking [29]. This study was limited in that it evaluated the association between household air pollution and FeNO using a measure of “stove type” as the exposure. Directly monitoring both kitchen and personal exposure to household air pollution for a 24-h period will improve models evaluating the association.
Although there is some evidence supporting the association of air pollution and increased FeNO among healthy adults, little is known about the association of household air pollution and FeNO. Measures of FeNO may provide insight into airway inflammation as a result of household air pollution studies. Our objective was to evaluate the cross-sectional association of exposure to household air pollution (measured personal concentrations and kitchen concentrations of air pollutants and stove type) with exhaled nitric oxide in adult women in rural Honduras using traditional and cleaner-burning Justa stoves; we additionally evaluated the effect modifying role of age on the observed relationship.
2. Materials and Methods
All study protocols were approved by the Colorado State Institutional Review Board. All study participants provided informed consent and received food items worth $5 U.S. dollars.
2.1. Study Setting
The study was conducted in nine communities surrounding the town of La Esperanza, Honduras. La Esperanza, located in the mountainous region of Western Honduras, is home to approximately 15,000 people. Our target population included all female primary cooks who used a traditional cookstove or a cleaner-burning Justa cookstove (See Figure 1). Traditional cookstoves in our population are typically self-built wood-burning stoves, with a metal griddle, large combustion chamber, and possibly a chimney. The cleaner-burning Justa stove is a common wood-burning cleaner-burning stove in Latin America with a rocket-elbow combustion chamber, chimney, and metal griddle suited to making tortillas.
Figure 1.
Typical traditional (left) and Justa (right) cookstoves in the homes of Honduran women.
2.2. Participants
The study team held local community meetings in villages surrounding La Esperanza and presented detailed information regarding the study to the community members. Researchers screened 500 households that attended the community meetings and expressed interest in the research project. We utilized convenience sampling to locate households who owned cleaner-burning Justa stoves, and identified nearby households that owned a traditional stove. In total, we visited 170 households from 9 February–30 April 2015. We recruited the female primary cook in each household to participate in the study. Enrollment criteria required that the primary cook be age 25–56, a non-smoker, not pregnant, and must have owned a traditional cookstove or Justa cookstove since at least 4 months prior to the interview. Upon visitation, eighteen of the 170 households were excluded as they did not have a female primary who meet the eligibility criteria. Two women chose not to participate in the study. We enrolled a total of 150 women into the study.
2.3. Exposure to Household Air Pollution
We assessed exposure to household air pollution using stove type (traditional or Justa) and by measuring 24-h average personal concentrations and kitchen concentrations of PM2.5 and black carbon. For kitchen concentrations, monitors for PM2.5 were placed in the kitchen between 76 and 127 cm above the stove, in the area where a woman cooks, and away from open windows and doors. For personal concentration measurements, we placed PM2.5 monitors in a small bag that each woman wore throughout the monitoring period, except when bathing or sleeping. Women were asked to place the bag next to them (on a table or hanging on the wall) when not wearing it. The inlet was clipped to the shoulder strap at the front of the woman’s chest near her breathing zone (Figure 2).
Figure 2.
Example placement for kitchen exposure measurements (left) and personal measurements (right).
PM2.5 was collected on 37-mm PTFE-coated glass fiber filters (FiberfilmTM T60A20, Pall Corporation, Port Washington, NY, USA). The filters were equilibrated at controlled temperature and relative humidity for at least 24-h and then pre-weighed at Colorado State University (CSU) using the microbalance (Mettler Toledo Microbalance, model MX5, resolution and repeatability of 1-ug). In the field laboratory, filters were placed into Triplex cyclones with a particle cut size of 2.5 µm (BGI by Mesa Labs, Butler, NJ, USA). Cyclones were attached to pumps (SKC AirCheck XR5000, SCKInc, Eighty Four, PA, USA) with a flow rate of 1.5 L/min. Pumps were pre-calibrated daily using a flow meter (DryCal Dc-Lite, Bios International, Mesa Labs, NJ, USA). We collected one filter blank every two weeks. After collection of the sample, filters were stored at −22 °C and then transported to CSU, equilibrated for temperature and relative humidity, and post-weighed. The 24-h average PM2.5 concentration was calculated from the change in filter mass adjusted for the average blank mass. We calculated the limit of detection (LOD) for PM2.5 as follows: average mass of blanks +3 times the standard deviation of the sample masses. All samples with a concentration less than the LOD (7 kitchen samples and 7 personal samples) were replaced with a value of LOD/√2. Due to a broken DryCal pump needed to calibrate the equipment used for PM2.5, we were unable to gather PM exposure measures from 41 houses. In addition, other samples were excluded from analysis due to; AirCheck pumps ran for less than 75% of the intended time (<18 h) (three personal and two kitchen samples); faulty post-weight (one personal sample); missing post-calibration data in the field (one kitchen sample).
Black carbon concentrations were estimated based on the optical transmission of light through the air sampling filters [30] using a transmissometer (model OT-21, Magee Scientific, Berkeley, CA, USA). Transmission data were converted to mass concentrations based on published mass-absorption values for combustion aerosol [31] and corrected for a filter loading artefact that leads to an underestimation of the estimation at high sample loading [32]. The limit of detection was estimated to be 0.86 µg/m3, which corresponds to three times the standard deviation of 54 blank samples (additional blank filters were used from field sampling campaigns conducted within the same year to estimate the reference values for the transmissometer since pre-sampling transmission data were not collected on sample filters). Values below the LOD (kitchen: n = 3; personal: n = 10) were substituted by LOD/√2. Detailed information on black carbon is available as supplementary materials.
2.4. Fractional Exhaled Nitric Oxide
FeNO was measured at a flow rate of 50 mL/s within a range of 5 to 300 ppb using a NIOX MINO (Version 9, Aerocrine AB, Solna, Sweden). Each participant completed the FeNO measurement on the morning after the 24-h exposure assessment. Participants stood upright, emptied their lungs, inhaled steadily through the NIOX MINO, and then exhaled at a slow and steady rate for 10 s. Two participants had a value for the FeNO below the limit of detection (LOD) of 5 ppb; we replaced these values with the limit of detection divided by the √2. There were no values above 300 ppb. Additional health outcomes, including blood pressure and diabetes, were evaluated in our study population and published elsewhere [33,34].
2.5. Covariate Assessment
Questionnaires in Spanish were administered to obtain demographic data and anthropometric information in the homes of participants. Responses were entered into the electronic data collection system, Open Data Kit [35]. As indicators of socioeconomic status, we measured the number of beds per person in the household, years of formal education, electricity availability, number of assets (cars, bikes, motorbikes, televisions, radios, refrigerators, sewing machines, electricity), and a dietary-diversity score for each participant. To determine an individual’s dietary-diversity score, women were asked to report all food they had eaten in the previous 24-h period including the number of portions [36]. The final dietary-diversity score was a sum of the number of food groups a woman had eaten at least one portion of in the past 24 h, ranging from 1 to 10. Elevation of the household was measured using Maps.me, a cell-phone GPS application (My.com B.V., Version 6.5.3).
The body mass index (BMI) (kg/m2) and waist-to-hip ratio was measured for each woman as measures of obesity. Women self-reported symptoms of nose irritation, cough, mucus, and difficulty breathing at the time of our visit. Women were noted to have exposure to second-hand smoke if there were any smokers in the household. Physical activity was assessed by obtaining information on the number of hours per day and number of days per week women performed particular tasks (cut and carried wood, ground corn, washed clothes, milked cows, worked in the field, carried heavy items or children, walked normally, walked uphill, and sat relaxing). The number of hours dedicated to each activity was multiplied by a corresponding metabolic equivalent (MET) and summed over a week to evaluate overall physical effort [37].
2.6. Statistical Analysis
Data were analyzed using SAS® software version 9.4 (SAS Institute, Inc., Cary, NC, USA). We used descriptive statistics, such as frequencies and relative frequencies, to summarize the exposure measures, health measures, and covariates for all participants who had a FeNO value. We calculated Wilcoxon rank sum tests and Chi-square tests to evaluate statistical differences of covariates between our traditional and cleaner-burning Justa stove users.
We calculated Spearman correlation coefficients for the kitchen and personal PM2.5 and kitchen and personal black carbon. We used linear regression to assess the association between age and FeNO and height and FeNO. In addition, we evaluated the association between several self-reported health symptoms (cough, chest tightness, current mucus, and difficulty breathing) and FeNO in separate linear regression models adjusting for a-priori confounders age, height, waist-to-hip ratio, BMI, dietary-diversity score, number of assets owned, and years of education.
We used separate linear regression models to evaluate the association between stove type and FeNO, and between each of the four measured pollutants (kitchen PM2.5, personal PM2.5, kitchen black carbon, and personal black carbon) and FeNO. FeNO and pollutant measurements were natural log transformed to meet model assumptions of residual normality. We chose potential confounders based on a-priori knowledge [38,39]. These included age, height, two measures of obesity (BMI and waist-to-hip ratio), and years of education (less than 6 years or 6 or more years) number of assets, and dietary-diversity score). We evaluated the crude association between FeNO and the various options for confounding by obesity and socio-economic status. Among the obesity and socio-economic status (SES) options, the variable with the strongest crude association with FeNO was chosen for inclusion in the model. All final models were adjusted for age, height, waist-to-hip ratio, and dietary diversity score [40].
We also assessed additive interaction FeNO and age utilizing a dichotomous age variable (less than 40 years and older or equal 40 years old) by including a multiplication term of age and exposure in the both crude and adjusted models [41]. We evaluated the significance of the interaction term with p-value of 0.1. We also conducted the analysis after removing five participants who reported exposure to second-hand smoke and after removing persons who reported having a respiratory symptom at the time of measurement (difficulty breathing, sore throat, mucus, tight chest, or cough). Finally, we removed the upper and lower 5% of FeNO values to assess the sensitivity of the model results to extreme values.
3. Results
A total of 150 women completed the study; 139 had FeNO measurements, 98 had complete data for measurements of FeNO, fine particulate matter, and black carbon. Baseline population characteristics are presented in Table 1. The average age of women in the study was 37.1 years (SD: 9.1), average BMI was 25.8 kg/m2 (SD: 4.2), and about half the population (n = 66) had less than 6 years of education. All covariates were similar between to the two stove type groups with the exception of elevation. (Tables S1 and S2 compare the demographic data of the population included in analysis and those excluded due to missing FeNO or exposure).
Table 1.
Population characteristics among nonsmoking primary female cooks using traditional or cleaner-burning Justa stoves, rural Honduras (n = 139).
| Total (n = 139) | Traditional (n = 72) | Justa (n = 67) | Test Value | |
|---|---|---|---|---|
| n (%) or Mean (SD); Range | n (%) or Mean (SD); Range | n (%) or Mean (SD); Range | (p-Value) * | |
| Age (years) | 37.1 (9.1); 25–56 | 38.3 (9.9); 25–56 | 35.9 (7.9); 25–56 | 0.29 |
| Height (meters) | 1.45 (0.05); 1.37–1.59 | 1.45 (0.05); 1.37–1.59 | 1.45 (0.04); 1.37–1.56 | 0.40 |
| Waist-to-hip ratio | 0.87 (0.06); 0.74–1.10 | 0.88 (0.06); 0.74–1.09 | 0.87 (0.05); 0.77–0.99 | 0.08 |
| Body mass index (kg/m2) | 25.8 (4.2); 17.6–37.5 | 25.5 (4.4); 17.5–37.5 | 26.2 (3.8); 18.2–33.6 | 0.24 |
| Physical activity (MET) 1 | 212 (103); 31–542 | 216 (110); 31–542 | 209 (95); 46–444 | 0.82 |
| Elevation (meters) | 1916 (107); 1729–2157 | 1896 (98); 1736–2152 | 1938 (112); 1729–2157 | 0.01 |
| Beds per person 2 | 0.52 (0.18); 0.2–1.0 | 0.50 (0.17); 0.2–1.0 | 0.55 (0.19); 0.25–1.0 | 0.15 |
| Diet diversity score 3 | 6.1 (1.7); 2–10 | 6.1 (1.7); 3–10 | 5.9 (1.6); 2–10 | 0.62 |
| Years of education | ||||
| Less than six years | 66 (48.1%) | 38 (53.5%) | 28 (42.4%) | 0.26 |
| Six or more years | 71 (51.8%) | 33 (46.5%) | 38 (57.6%) | |
| Number of assets 4 | ||||
| Less than two | 67 (48.5%) | 38 (53.5%) | 34 (51.0%) | 0.87 |
| Two or more | 71 (51.5%) | 33 (46.5%) | 33 (49.0%) | |
| Years spent cooking with biomass | 25.6 (9.9); 7–50 | 26.6 (10.8); 7–49 | 24.5 (8.8); 9–50 | 0.38 |
| Self-reported exposure to secondhand smoke | 5 (3.6%) | 5 (3.6%) | 0 (0%) | - |
| Fractional exhale nitric oxide (ppb) | 17.9 (12.1); 3.5–95 | 17.4 (10.8); 3.5–62 | 18.5 (13.4); 5–95 | 0.64 |
PPB, parts per billion; SD, Standard Deviation. 1 Physical Activity: The sum of metabolic equivalents including the following self-reported activities: cut wood, grind corn, wash clothes, milk the cow, work in the field, carry a heavy weight, and walk normally outside the house. For each activity the number of hours per week was calculated and multiplied with the corresponding metabolic equivalent (MET) from the Compendium of Physical Activities (Ainsworth et al. 2015). 2 Total n = 137; Traditional = 71; Justa = 66. 3 Dietary-diversity score: The sum of the number of food categories consumed in the past 24-h (10 categories); used as an indicator of socioeconomic status (Savy et al. 2006). 4 Number of assets (Total n = 138, Traditional = 71). Assets include cars, bikes, motorbikes, televisions, radios, refrigerators, sewing machines, electricity. * Wilcoxon rank sum test for continuous variables or chi-square p-value for categorical variables.
Fractional exhaled nitric oxide values ranged from 3.5 ppb to 95 ppb, with a mean of 17.9 ppb (standard deviation [SD]:12.1) and median of 15.0 ppb. Among traditional stove users, mean FeNO was 17.4 ppb (SD: 10.8) and the median was 14.5 ppb, while Justa stove users had a mean FeNO of 18.5 ppb (SD: 13.4; p = 0.64) and median 16.0 ppb. Of the 11 women who did not complete the FeNO measurement, eight women attempted the measurement but were not successful in maintaining their exhaled breath after more than eight attempts. In addition, three women did not attempt the FeNO measurement due to recent surgery or stroke. The 11 women excluded from the analysis had similar exposures to the rest of the sample population (See Supplementary Table S1).
The four 24-h continuous pollutant measures were strongly correlated. Within pollutants, there was a positive correlation between kitchen concentrations and personal concentrations to PM2.5 (0.80) and kitchen and personal black carbon (0.77). PM2.5 and black carbon exposures were correlated among kitchen measurements (0.89) and personal measurements (0.78). Twenty-four hour average concentrations of each pollutant are shown in Table 2. As expected, kitchen PM2.5 was higher than personal PM2.5 with means of 254 µg/m3 (SD: 329) and 100 µg/m3 (SD: 70) respectively. The same pattern holds for kitchen and personal black carbon. In addition, women who owned traditional stoves were exposed to higher concentrations of each of the two pollutants than women who owned Justa stoves (p-value ≤ 0.01 for all four exposure concentrations).
Table 2.
24-h average kitchen and personal fine particulate matter and black carbon concentrations, traditional and Justa stove users, rural Honduras. PM2.5: fine particulate matter.
| All Participants | Traditional Stove Users | Justa Stove Users | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Min | 25th | Median | 75th | Max | n | Min | 25th | Median | 75th | Max | n | Min | 25th | Median | 75th | Max | |
| 24-h average kitchen PM2.5 (µg/m3) | 98 | 18 | 61 | 116 | 369 | 1654 | 58 | 18 | 90 | 172 | 448 | 1654 | 40 | 18 | 37 | 68 | 150 | 1134 |
| 24-h average personal PM2.5 (µg/m3) | 98 | 18 | 48 | 80 | 138 | 346 | 59 | 18 | 62 | 112 | 154 | 346 | 39 | 18 | 39 | 52 | 81 | 174 |
| 24-h average kitchen Black Carbon (µg/m3) | 98 | 1 | 8 | 18 | 78 | 1172 | 58 | 1 | 14 | 44 | 113 | 1172 | 40 | 1 | 4 | 11 | 15 | 469 |
| 24-h average personal Black Carbon (µg/m3) | 98 | 1 | 4 | 7 | 17 | 123 | 58 | 1 | 6 | 14 | 32 | 123 | 40 | 1 | 1 | 4 | 8 | 47 |
We did not observe associations between age or height and FeNO (Table 3). Several self-reported respiratory symptoms were associated with increasing FeNO (Table 3). For example, after adjusting for age, height, waist-to-hip ratio, BMI, number of assets, years of education (<6 or ≥6 years), and dietary-diversity score, women who reported having a cough at the time of the assessment had a 78.8% higher FeNO level than those who did not report a cough (95% CI: 38.8%, 130.2%). Similarly, women who reported having current mucus had a 47.4% higher FeNO level (95% CI: 11.2%, 95.2%) than those who did not report current mucus.
Table 3.
Estimated crude and adjusted percentage difference in fractional exhaled nitric oxide in relation to measures of current, self-reported symptoms among traditional and Justa stove users, rural Honduras.
| n | Crude Percent Difference in FeNO | 95% CI | n | Adjusted Percent Difference in FeNO | 95% CI | |
|---|---|---|---|---|---|---|
| Age (years) 1 | 139 | <0.1 | (−0.2, 0.3) | 136 | 0.1 | (−0.2, 0.4) |
| Height (meters) 2 | 139 | −3.79 | (−39.51, 53.02) | 136 | −0.25 | (−37.59, 59.44) |
| Cough 3 | ||||||
| No | 118 | ref | 115 | |||
| Yes | 21 | 78.8 | (38.8, 130.2) | 21 | 78.8 | (37.5, 132.5) |
| Chest Tightness 3 | ||||||
| No | 128 | ref | 125 | |||
| Yes | 11 | 17.6 | (−17.9, 68.3) | 11 | 24.5 | (−14.5, 81.5) |
| Mucus 3 | ||||||
| No | 121 | ref | 118 | |||
| Yes | 18 | 47.4 | (11.2, 95.3) | 18 | 52.4 | (13.4, 104.8) |
| Difficulty Breathing 3 | ||||||
| No | 129 | ref | 126 | |||
| Yes | 10 | 42.1 | (−2.0, 105.9) | 10 | 39.4 | (−5.1, 104.8) |
Cl: Confidence interval; PM2.5: fine particulate matter. 1 Model was adjusted for height, waist-to-hip ratio, body mass index, dietary-diversity score, years of education (<6 or ≥6 years), and number of assets (<2 or ≥2) (Assets include cars, bikes, motorbikes, televisions, radios, refrigerators, sewing machines, electricity). 2 Model was adjusted for age, waist-to-hip ratio, body mass index, dietary-diversity score, years of education (<6 or ≥6 years), and number of assets (<2 or ≥2) (Assets include cars, bikes, motorbikes, televisions, radios, refrigerators, sewing machines, electricity). 3 Exhaled nitric oxide was log-transformed. Categorical variable beta coefficients were entered into the formula (e^β − 1)*100). The estimates for the categorical measures of exposure can be interpreted as the percent difference in FeNO when comparing those who had the health system to those who did not.
Crude and adjusted linear regression results for household air pollution are presented in Table 4. Given the natural log-transformed dependent variables (FeNO) and the natural log-transformed independent continuous pollution exposure variables, we present the results for continuous exposures as the percent increase or decrease in FeNO for a 25% increase in pollutant exposure. Overall results for the continuous pollution measurements were consistent with a null association. For example, a 25% higher personal PM2.5 concentration was associated with a 0.8% higher FeNO level (95% CI: −3.1–4.9. The estimates for categorical stove type are presented as a percent increase in FeNO as compared to the reference group (Justa stove). Again, the results were consistent with a null association. In Table 4 we also present a sensitivity analysis removing the top and bottom 5% of FeNO values. The results demonstrate that the adjusted models were somewhat sensitive to the highest and lowest FeNO values, but overall, it is difficult to determine the impact, given the wide confidence intervals.
Table 4.
Estimated crude and adjusted 1 percentage difference in fractional exhaled nitric oxide in relation to measures of exposure to household air pollution (per 25% increase in 24-h average measured pollution, or by stove type) among traditional and Justa stove users, rural Honduras.
| n | Crude Percent Difference in FeNO | 95% CI | n | Adjusted Percent Difference in FeNO 1 | 95% CI | |
|---|---|---|---|---|---|---|
| 24-h average kitchen PM2.5 (µg/m3) 2 | 98 | 0.3 | (−2.0, 2.7) | 84 | 0.5 | (−2.0, 3.1) |
| 24-h average personal PM2.5 (µg/m3) 2 | 98 | 0.8 | (−3.1, 4.9) | 85 | 0.8 | (−3.4, 5.2) |
| 24-h average kitchen Black Carbon (µg/m3) 2 | 98 | −0.1 | (−1.8, 1.6) | 84 | −0.1 | (−1.9, 1.8) |
| 24-h average personal Black Carbon (µg/m3) 2 | 98 | <0.0 | (−2.1, 1.9) | 84 | −0.2 | (−2.4, 2.0) |
| Stove Type 3 | 139 | 136 | ||||
| Justa | 67 | ref | 65 | ref | ||
| Traditional | 72 | −6.5 | (−22.9, 13.6) | 71 | −6.1 | (−23.5, 15.3) |
Cl: Confidence interval; PM2.5: fine particulate matter. 1 Models were adjusted for age, height, waist-to-hip ratio, body mass index, dietary-diversity score, years of education (<6 or ≥6 years), and number of assets (<2 or ≥2) (Assets include cars, bikes, motorbikes, televisions, radios, refrigerators, sewing machines, electricity). 2 Exhaled nitric oxide and measured pollution were both log transformed. Beta coefficients were entered into the formula ((1.25^β) − 1) and multiplied by 100. We can interpret the estimate of the continuous pollution exposures as a percent increase in exhaled nitric oxide for each 25% increase in exposure. Example: There is a 0.4% higher FeNO level with a 25% higher kitchen PM2.5 concentration. 3 Exhaled nitric oxide was log-transformed. Categorical variable beta coefficients were entered into the formula (e^β − 1)*100). The estimates for the categorical measures of exposure can be interpreted as the percent difference in FeNO when comparing traditional stove to the reference (Justa stove).
In our interaction analysis, presented in Table 5, participants who were 40 years or older tended to have a larger percent increase in FeNO in relation to exposure to household air pollution compared to the younger participants, although the evidence for interaction was not strong. The additional sensitivity analyses had similar results (results not presented).
Table 5.
Estimates for effect modification by age (dichotomized at the median value of 40 years) for the percentage difference in fractional exhaled nitric oxide in relation to measures of exposure to household air pollution (per 25% increase in 24-h average measured pollution, or by stove type) among traditional and Justa stove users, rural Honduras 1.
| n | Adjusted Percent Difference 1 | 95% CI | p-Value for Interaction | |
|---|---|---|---|---|
| 24-h average kitchen PM2.5 (µg/m3) 2 | 0.7 | |||
| Age < 40 | 65 | <0.1 | (−3.3, 3.4) | |
| Age ≥ 40 | 31 | 1.0 | (−3.0, 5.1) | |
| 24-h average personal PM2.5 (µg/m3) 2 | 0.2 | |||
| Age < 40 | 66 | −0.9 | (−5.7, 4.3) | |
| Age ≥ 40 | 31 | 5.2 | (−3.3, 14.3) | |
| 24-h average kitchen Black Carbon (µg/m3) 2 | 0.8 | |||
| Age < 40 | 65 | −0.3 | (−2.6, 2.1) | |
| Age ≥ 40 | 31 | 0.2 | (−2.7, 3.2) | |
| 24-h average personal Black Carbon (µg/m3) 2 | 0.7 | |||
| Age < 40 | 65 | −0.5 | (−3.1, 2.3) | |
| Age ≥ 40 | 31 | 0.5 | (−3.3, 4.7) | |
| Stove Type 3 (traditional compared to Justa [ref]) | ||||
| Traditional | ||||
| Age < 40 | 72 | −12.2 | (−31.9, 13.1) | 0.3 |
| Age ≥ 40 | 67 | 11.1 | (−22., 59.2) |
Cl: Confidence interval; PM2.5: fine particulate matter. 1 All models adjusted for age, height, waist-to-hip ratio, body mass index, dietary-diversity score, years of education (<6 or ≥6 years), and number of assets (<2 or ≥2) (Assets include cars, bikes, motorbikes, televisions, radios, refrigerators, sewing machines, electricity). 2 Exhaled nitric oxide and measured pollution are both log transformed. Beta coefficients were entered into the formula ((1.25^β) − 1) and multiplied by 100. We can interpret the estimate of the continuous pollution exposures as a percent increase in exhaled nitric oxide for each 25% increase in exposure. Example: There is a 0.2% higher FeNO level with a 25% higher kitchen PM2.5 concentration among women less than 40 years old. 3 Exhaled nitric oxide was log-transformed. Categorical variable beta coefficients were entered into the formula (e^β − 1)*100). The estimates for the categorical measures of exposure can be interpreted as the percent difference in FeNO when comparing a specific stove type to the reference (traditional stove).
4. Discussion
Our study provided a unique opportunity to examine associations between measures of exposure to household air pollution and FeNO, a measure of airway inflammation. We add to the limited body of evidence investigating these associations among adults; in particular, our study is, to our knowledge, the first to examine the association of FeNO and household air pollution among healthy adult women using direct exposure measurements of PM2.5 and black carbon.
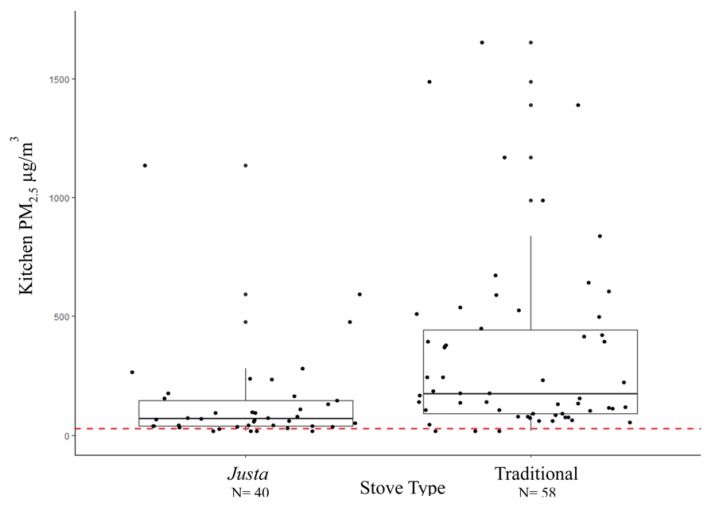
We observed that although traditional stove users were exposed to an average of 40% higher levels of fine particulate matter compared to Justa stove users, the 24-h measures of pollutant exposure for both stove users surpassed the concentration guideline for PM2.5 of 25 µg/m3 24-h average set by the World Health Organization (WHO) [42] (Figure 3). It is critical that cleaner-burning cookstove exposure levels are reduced to be as close as possible to the WHO guidelines in order observe population-level improvements on health outcomes. The median FeNO levels observed in our population are slightly higher (14.5 ppb among traditional stove users and 16.0 ppb among Justa stove users) than the levels seen in a similar study among a sample of women in Peru using biomass burning cookstoves (10 ppb among traditional stove users and 10.5 ppb among cleaner-burning stove users) [29]. Pollard et al. reported a non-meaningful 2 ppb increase in exhaled nitric oxide among all participants in rural households immediately after a cooking event; however, they did not directly measure pollutant concentrations.
Figure 3.
24-h average kitchen PM2.5 concentrations, Traditional and Justa Stove Users, Rural Honduras (n = 98). PM2.5: fine particulate matter. Top and bottom lines of rectangle represent the 75th and 25th percentiles. The middle line represents the median. The top whisker denotes the value of the 3rd quartile plus 1.5 times the IQR. The bottom whisker denotes the value of the 1st quartile minus 1.5 times the IQR. The red line indicates the World Health Organization (WHO) 24-h guideline of 25 µg/m3.
Overall, we did not observe evidence supporting the hypothesis that increased exposure to household air pollution was linked to airway inflammation as measured by FeNO, after adjusting for potential confounders. The evidence from studies on associations of ambient air pollution with FeNO has been inconsistent. For example, although positive associations have been demonstrated between increased levels of ambient PM2.5 and black carbon and FeNO among healthy adults [20,43,44], several studies reported null results similar to ours [25,45]. Additionally, several chamber and panel studies of direct exposure to wood smoke and FeNO have also reported results that do not support an association. [46,47,48,49]. There were no differences in the association of measured household air pollution and FeNO among women less than 40 years old and among those 40 years old and older.
FeNO measured at a constant flow rate has been highlighted as a simple, reproducible, non-invasive biomarker of eosinophilic airway inflammation for use in air pollution studies. The inconsistent results observed in the literature may be due in part to the technology available to assess FeNO. Varying the flow rate at which FeNO measurements are collected may allow researchers to partition the source of nitric oxide into two distinct anatomical regions: the proximal and distal airways [50,51]. The current ATS standard is for a flow rate of 50 mL/s, providing information from the proximal airways [14,52,53]. FeNO measured or calculated for a higher flow rate, such as 270 mL/s (FeNO270), may be associated with airway inflammation from the distal compartment [51,54].
Previous studies examining the association between air pollution and FeNO at different flow rates have reported inconsistent results for the proximal and distal airways, indicating potential mechanistic differences of exposure [24,49]. For example, a cohort study among 5841 Swedish adults explored the association between ozone and PM10 on FeNO measured at 50 mL/s and 270 mL/s, respectively [55]. The authors observed no clear effect of PM10 on either measure of FeNO; however, after adjusting for other pollutants, they observed an interquartile range increase in 120-h ozone was associated with a 5.1% (95% CI: 1.7%–8.5%) higher FeNO270 measurement and associated (although not significantly) with a 3.6% (−0.4% to 3.4%) higher FeNO50 [55]. In addition, a wood smoke chamber study by Barregard et al. 2008 reported a net increase in FeNO270 from distal airways three hours post exposure, but no increase in FeNO50 from proximal airways [24].
Our study is limited to the approximation of eosinophilic inflammation in the proximal airways measured by a flow rate of 50 mL/s (FeNO50). It may be useful for future studies to assess FeNO associated with the distal airways, because PM2.5 is small enough to deposit in the distal airways and alveoli [56,57]. Assessing inflammation that may have a stronger expected association to the distal airways would require measurements of exhaled NO at a different flow rate. For example, the semi-portable electrochemical analyser Hypair FeNO mediansoft Exp’air (50, 100, 150 mL/s), could be used in the field setting.
The cross-sectional nature of the study design may limit our ability to establish that exposure preceded airway inflammation. We attempted to address this potential limitation by including only women who had been using a cleaner-burning cookstove for more than four months (average length of Justa stove ownership was just under two years). Selection bias could have occurred in our study if those who were enrolled in the study were different—with respect to both exposure and disease—to those who did not participate. For example, if women self-selected into the study because they were exposed to high levels of household air pollution and had poor health, our study results may over exaggerate the association of household air pollution and FeNO. We do not suspect selection bias played a large role in our study, as women were unaware of our health outcome, FeNO; therefore, our population is likely to be similar to the target population with respect to disease.
Potential exposure measurement error is likely to be non-differential with respect to measured FeNO values. Likewise, disease measurement error is likely non-differential with respect to measured exposure. Any measurement error of the exposure could have resulted in a bias towards the null, while measurement error in our continuous outcome would not result in bias. The results are limited, and may not be generalizable to other populations, especially those outside Central America, or to populations using different stove and fuel combinations.
Although we had a relatively small sample size, the association between FeNO and self-reported respiratory symptoms indicates that we may have accurately captured airway inflammation as it relates to the presence of symptoms [39,58]. While we did not see an association between FeNO and age or FeNO and height, as we would have expected based on previous literature, [38,39,58,59,60], this may be due to the relatively small range of age and height in our study. For example, our study population only covered adults age 25–56 (mean 37.1 years, SD: 9.1 years). In addition, we observed very little variability in height (mean: 1.45 m, SD: 0.05 m), which may have influenced our inability to capture an association between FeNO and height. Our crude and adjusted linear regression models were not substantially different, indicating that measured confounding was likely not a concern in our study; however, measurement error or residual confounding, for example by SES, may still exist. FeNO can be impacted by diet, particularly green, leafy vegetable consumption [61]. Although a dietary recall survey conducted in our study population was not specific enough to capture this intake, overall report of vegetable consumption was low (median intake, 0 servings). Finally, we did not include questions in our demographic and health survey that would provide insight into the asthma, atopy, or genetic variations among participants in order to assess effect modification. No participants, however, reported taking any asthma medication. Asthma or atopy status and genetic variations are thought to modify the effect of air pollution on FeNO [56,62,63]; similar effect modification could be expected in household air pollution. We did not evaluate former smoking among our study population, and cannot determine if former smoking may contribute to the relatively low FeNO levels observed in our study. Smoking however, is uncommon among the rural female population in Honduras.
5. Conclusions
In conclusion, we did not observe evidence of increased eosinophilic airway inflammation from exposure to household air pollution. Further research is needed to assess the utility of FeNO in the field of household air pollution. Future studies may find it useful to consider measurements of this biomarker at differing airflow rates, in well-designed longitudinal studies.
Acknowledgments
We are grateful to Gloribel Bautista Cuellar and Jonathan Stack for their fieldwork and contributions to data collection.
Supplementary Materials
The following are available online at http://www.mdpi.com/1660-4601/15/11/2544/s1, Table S1: Population characteristics of nonsmoking primary female cooks in rural Honduras included in the study and participants excluded due to missing FeNO data, Table S2: Population characteristics of nonsmoking primary female cooks in rural Honduras included in the study and participants excluded due to missing exposure data (PM2.5), Table S3: Estimated crude and adjusted percentage difference in fractional exhaled nitric oxide in relation to measures of exposure to household air pollution (per 25% increase in 24-h average measured pollution, or by stove type) among traditional and Justa stove users, rural Honduras with (Top and Bottom 5% removed).
Author Contributions
Conceptualization, M.L.C., A.M.B., J.V., S.A., A.O.P. and J.L.P.; Data curation, M.L.B.-C., M.L.C., S.R. and B.N.Y.; Formal analysis, M.L.B.-C. and M.L.C.; Funding acquisition, M.L.C., J.V. and J.L.P.; Investigation, M.L.B.-C., M.L.C., S.R., B.N.Y. and J.L.P.; Methodology, M.L.C., A.M.B., J.V., S.J.R., A.W., C.L. and J.L.P.; Project administration, M.L.C., S.R., B.N.Y. and J.L.P.; Resources, J.V. and S.A.; Supervision, M.L.C., J.V. and J.L.P.; Validation, M.L.C., J.R.B., R.B., T.L.N., J.V., S.R., A.W., C.L., N.G., C.Q., K.K. and J.L.P.; Writing-original draft, M.L.B.-C.; Writing-review & editing, M.L.C., S.R., B.N.Y., A.M.B., J.B., R.B., T.N., J.V., S.J.R., A.W., C.L., N.G., C.Q., K.K. and J.L.P.
Funding
This research was funded by National Institute of Environmental Health Sciences of the National Institutes of Health under award number R21ES022810. Content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
Author Anibal Osorto works for AHDESA, the manufacturer of the Justa stove that was evaluated in this project. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Gakidou E., Afshin A., Abajobir A.A., Abate K.H., Abbafati C., Abbas K.M., Abd-Allah F., Abdulle A.M., Abera S.F., Aboyans V., et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1345–1422. doi: 10.1016/S0140-6736(17)32366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roden C. a., Bond T.C., Conway S., Osorto Pinel A.B., MacCarty N., Still D. Laboratory and field investigations of particulate and carbon monoxide emissions from traditional and improved cookstoves. Atmos. Environ. 2009;43:1170–1181. doi: 10.1016/j.atmosenv.2008.05.041. [DOI] [Google Scholar]
- 3.Jetter J., Zhao Y., Smith K.R., Khan B., Yelverton T., Decarlo P., Hays M.D. Pollutant emissions and energy efficiency under controlled conditions for household biomass cookstoves and implications for metrics useful in setting international test standards. Environ. Sci. Technol. 2012;46:10827–10834. doi: 10.1021/es301693f. [DOI] [PubMed] [Google Scholar]
- 4.Naeher L.P., Brauer M., Lipsett M., Zelikoff J.T., Simpson C.D., Koenig J.Q., Smith K.R. Woodsmoke Health Effects: A Review. Inhal. Toxicol. 2007;19:67–106. doi: 10.1080/08958370600985875. [DOI] [PubMed] [Google Scholar]
- 5.Smith K.R., Bruce N., Balakrishnan K., Adair-Rohani H., Balmes J., Chafe Z., Dherani M., Hosgood H.D., Mehta S., Pope D., et al. Millions dead: How do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annu. Rev. Public Health. 2014;35:185–206. doi: 10.1146/annurev-publhealth-032013-182356. [DOI] [PubMed] [Google Scholar]
- 6.Forouzanfar M.H., Afshin A., Alexander L.T., Anderson H.R., Bhutta Z.A., Biryukov S., Brauer M., Burnett R., Cercy K., Charlson F.J., et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amegah A.K., Quansah R., Jaakkola J.J.K. Household air pollution from solid fuel use and risk of adverse pregnancy outcomes: A systematic review and meta-analysis of the empirical evidence. PLoS ONE. 2014;9:e113920. doi: 10.1371/journal.pone.0113920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruce N., Pope D., Rehfuess E., Balakrishnan K., Adair-Rohani H., Dora C. WHO indoor air quality guidelines on household fuel combustion: Strategy implications of new evidence on interventions and exposure–risk functions. Atmos. Environ. 2015;106:451–457. doi: 10.1016/j.atmosenv.2014.08.064. [DOI] [Google Scholar]
- 9.Bernstein J.A., Alexis N., Barnes C., Bernstein I.L., Bernstein J.A., Nel A., Peden D., Diaz-Sanchez D., Tarlo S.M., Williams P.B. Health effects of air pollution. J. Allergy Clin. Immunol. 2004;114:1116–1123. doi: 10.1016/j.jaci.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Anderson J.O., Thundiyil J.G., Stolbach A. Clearing the Air: A Review of the Effects of Particulate Matter Air Pollution on Human Health. J. Med. Toxicol. 2012;8:166–175. doi: 10.1007/s13181-011-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly F.J., Fussell J.C. Air pollution and airway disease. Clin. Exp. Allergy. 2011;41:1059–1071. doi: 10.1111/j.1365-2222.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 12.Holgate S.T., Sandström T., Frew A.J., Stenfors N., Nördenhall C., Salvi S., Blomberg A., Helleday R., Söderberg M. Health effects of acute exposure to air pollution. Part I: Healthy and asthmatic subjects exposed to diesel exhaust. Res. Rep. Health Eff. Inst. 2003;112:1–30. [PubMed] [Google Scholar]
- 13.van Eeden S.F., Tan W.C., Suwa T., Mukae H., Terashima T., Fuji T., Qui D., Vincent R., Hogg J.C. Cytokines Involved in the Systemic Inflammatory Response Induced by Exposure to Particulate Matter Air Pollutants (PM 10) Am. J. Respir. Crit. Care Med. 2001;164:826–830. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- 14.Dweik R., Boggs P., Erzurum S., Irvin C. An Official ATS Clinical Practice Guideline: Interpretation of Exhaled Nitric Oxide Levels (FENO) for. Am. J. Respir. Crit. Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fahy J. V Eosinophilic and neutrophilic inflammation in asthma: Insights from clinical studies. Proc. Am. Thorac. Soc. 2009;6:256–259. doi: 10.1513/pats.200808-087RM. [DOI] [PubMed] [Google Scholar]
- 16.Barnes P.J., Dweik R.A., Gelb A.F., Gibson P.G., George S.C., Grasemann H., Pavord I.D., Ratjen F., Silkoff P.E., Taylor D.R., et al. Exhaled nitric oxide in pulmonary diseases a comprehensive review. Chest. 2010;138:682–692. doi: 10.1378/chest.09-2090. [DOI] [PubMed] [Google Scholar]
- 17.Possa S.S., Leick E.A., Prado C.M., Martins M.A., Tibério I.F.L.C. Eosinophilic inflammation in allergic asthma. Front. Pharmacol. 2013;4:46. doi: 10.3389/fphar.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zamora R., Vodovotz Y., Billiar T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000;6:347–373. doi: 10.1007/BF03401781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harnan S.E., Tappenden P., Essat M., Gomersall T., Minton J., Wong R., Pavord I., Everard M., Lawson R. Measurement of exhaled nitric oxide concentration in asthma: A systematic review and economic evaluation of NIOX MINO, NIOX VERO and NObreath. Health Technol. Assess. 2015;19:1–330. doi: 10.3310/hta19820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang W., Wang G., Lu S.-E., Kipen H., Wang Y., Hu M., Lin W., Rich D., Ohman-Strickland P., Diehl S.R., et al. Inflammatory and oxidative stress responses of healthy young adults to changes in air quality during the Beijing Olympics. Am. J. Respir. Crit. Care Med. 2012;186:1150–1159. doi: 10.1164/rccm.201205-0850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Amsterdam J.G., Verlaan B.P., Van Loveren H., Elzakker B.G., Vos S.G., Opperhuizen A., Steerenberg P.A. Air pollution is associated with increased level of exhaled nitric oxide in nonsmoking healthy subjects. Arch. Environ. Health. 1999;54:331–335. doi: 10.1080/00039899909602496. [DOI] [PubMed] [Google Scholar]
- 22.Xu T., Hou J., Cheng J., Zhang R., Yin W., Huang C., Zhu X., Chen W., Yuan J. Estimated individual inhaled dose of fine particles and indicators of lung function: A pilot study among Chinese young adults. Environ. Pollut. 2018;235:505–513. doi: 10.1016/j.envpol.2017.12.074. [DOI] [PubMed] [Google Scholar]
- 23.Shi J., Chen R., Yang C., Lin Z., Cai J., Xia Y., Wang C., Li H., Johnson N., Xu X., et al. Association between fine particulate matter chemical constituents and airway inflammation: A panel study among healthy adults in China. Environ. Res. 2016;150:264–268. doi: 10.1016/j.envres.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Barregard L., Sällsten G., Andersson L., Almstrand A.-C., Gustafson P., Andersson M., Olin A.-C. Experimental exposure to wood smoke: Effects on airway inflammation and oxidative stress. Occup. Environ. Med. 2008;65:319–324. doi: 10.1136/oem.2006.032458. [DOI] [PubMed] [Google Scholar]
- 25.Strak M., Boogaard H., Meliefste K., Oldenwening M., Zuurbier M., Brunekreef B., Hoek G. Respiratory health effects of ultrafine and fine particle exposure in cyclists. Occup. Environ. Med. 2010;67:118–124. doi: 10.1136/oem.2009.046847. [DOI] [PubMed] [Google Scholar]
- 26.Torres-Duque C., Maldonado D., Perez-Padilla R., Ezzati M., Viegi G. Biomass fuels and respiratory diseases: A review of the evidence. Proc. Am. Thorac. Soc. 2008;5:577–590. doi: 10.1513/pats.200707-100RP. [DOI] [PubMed] [Google Scholar]
- 27.Po J.Y.T., FitzGerald J.M., Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: Systematic review and meta-analysis. Thorax. 2011;66:232–239. doi: 10.1136/thx.2010.147884. [DOI] [PubMed] [Google Scholar]
- 28.Schei M.A., Hessen J.O., Smith K.R., Bruce N., McCracken J., Lopez V. Childhood asthma and indoor woodsmoke from cooking in Guatemala. J. Expo. Anal. Environ. Epidemiol. 2004;14 doi: 10.1038/sj.jea.7500365. [DOI] [PubMed] [Google Scholar]
- 29.Pollard S.L., Williams D.L., Breysse P.N., Baron P.A., Grajeda L.M., Gilman R.H., Miranda J.J., Checkley W. A cross-sectional study of determinants of indoor environmental exposures in households with and without chronic exposure to biomass fuel smoke. Environ. Health. 2014;13:12. doi: 10.1186/1476-069X-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen A.D.A., Rosen H., Novakov T. The aethalometer—An instrument for the real-time measurement of optical absorption by aerosol particles. Sci. Total Environ. 1984;36:191–196. doi: 10.1016/0048-9697(84)90265-1. [DOI] [Google Scholar]
- 31.Chylek P., Ramaswamy V., Cheng R., Pinnick R.G. Optical properties and mass concentration of carbonaceous smokes. Appl. Opt. 1981;20:2980–2985. doi: 10.1364/AO.20.002980. [DOI] [PubMed] [Google Scholar]
- 32.Kirchstetter T.W., Novakov T. Controlled generation of black carbon particles from a diffusion flame and applications in evaluating black carbon measurement methods. Atmos. Environ. 2007;41:1874–1888. doi: 10.1016/j.atmosenv.2006.10.067. [DOI] [Google Scholar]
- 33.Rajkumar S., Clark M.L., Young B.N., Benka-Coker M.L., Bachand A.M., Brook R.D., Nelson T.L., Volckens J., Reynolds S.J., L’Orange C., et al. Exposure to Household Air Pollution from Biomass-Burning Cookstoves and HbA1c and Diabetic Status among Honduran Women. Indoor Air. 2018:768–776. doi: 10.1111/ina.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young B.N., Clark M.L., Rajkumar S., Benka-Coker M.L., Bachand A., Brook R.D., Nelson T.L., Volckens J., Reynolds S., L’Orange C., et al. Exposure to household air pollution from biomass cookstoves and blood pressure among women in rural honduras: A cross-sectional study. Indoor Air. 2018:1–13. doi: 10.1111/ina.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunette W., Sundt M., Dell N., Chaudhri R., Breit N., Borriello G. Open Data Kit 2.0: Expanding and Refining Information Services for Developing Regions; Proceedings of the 14th Workshop on Mobile Computing Systems and Applications; Jekyll Island, GA, USA. 26–27 February 2013. [Google Scholar]
- 36.Arps S. Socioeconomic status and body size among women in Honduran Miskito communities. Ann. Hum. Biol. 2011;38:508–519. doi: 10.3109/03014460.2011.564206. [DOI] [PubMed] [Google Scholar]
- 37.Ainsworth B.E., Haskell W.L., Herrmann S.D., Meckes N., Bassett D.R., Tudor-Locke C., Greer J.L., Vezina J., Whitt-Glover M.C., Leon A.S. 2011 compendium of physical activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 38.Olin A.-C. Height, Age, and Atopy Are Associated With Fraction of Exhaled Nitric Oxide in a Large Adult General Population Sample. CHEST J. 2006;130:1319. doi: 10.1378/chest.130.5.1319. [DOI] [PubMed] [Google Scholar]
- 39.Dressel H., de la Motte D., Reichert J., Ochmann U., Petru R., Angerer P., Holz O., Nowak D., Jörres R.A. Exhaled nitric oxide: Independent effects of atopy, smoking, respiratory tract infection, gender and height. Respir. Med. 2008;102:962–969. doi: 10.1016/j.rmed.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Savy M., Martin-Prével Y., Traissac P., Eymard-Duvernay S., Delpeuch F. Dietary diversity scores and nutritional status of women change during the seasonal food shortage in rural Burkina Faso. J. Nutr. 2006;136:2625–2632. doi: 10.1093/jn/136.10.2625. [DOI] [PubMed] [Google Scholar]
- 41.Clark M.L., Peel J.L. Perspectives in Household Air Pollution Research: Who Will Benefit from Interventions? Curr. Environ. Health Rep. 2014:250–257. doi: 10.1007/s40572-014-0021-0. [DOI] [Google Scholar]
- 42.World Health Organization . Air Quality Guidelines: Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide. Volume 3 World Health Organization; Geneva, Switzerland: 2006. [Google Scholar]
- 43.Adamkiewicz G., Ebelt S., Syring M., Slater J., Speizer F.E., Schwartz J., Suh H., Gold D.R. Association between air pollution exposure and exhaled nitric oxide in an elderly population. Thorax. 2004;59:204–209. doi: 10.1136/thorax.2003.006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubowsky Adar S., Adamkiewicz G., Gold D.R., Schwartz J., Coull B.A., Suh H. Ambient and microenvironmental particles and exhaled nitric oxide before and after a group bus trip. Environ. Health Perspect. 2007;115:507–512. doi: 10.1289/ehp.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoda Y., Otani N., Sakurai S., Shima M. Acute effects of summer air pollution on pulmonary function and airway inflammation in healthy young women. J. Epidemiol. 2014;24:312–320. doi: 10.2188/jea.JE20130155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riddervold I., Bønløkke J., Olin A., Grønborg T., Schlünssen V., Skogstrand K., Hougaard D., Massling A., Sigsgaard T. Effects of wood smoke particles from wood-burning stoves on the respiratory health of atopic humans. Part. Fibre Toxicol. 2012;9:12. doi: 10.1186/1743-8977-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sehlstedt M., Dove R., Boman C., Pagels J., Swietlicki E., Löndahl J., Westerholm R., Bosson J., Barath S., Behndig A.F., et al. Antioxidant airway responses following experimental exposure to wood smoke in man. Part. Fibre Toxicol. 2010;7:21. doi: 10.1186/1743-8977-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muala A., Rankin G., Sehlstedt M., Unosson J., Bosson J.A., Pourazar J., Nystrom R., Boman C., Blomberg A. Effects of experimental wood smoke exposure in healthy human subjects. Am. J. Respir. Crit. Care Med. 2013;187:A3504. [Google Scholar]
- 49.Stockfelt L., Sallsten G., Olin A.-C., Almerud P., Samuelsson L., Johannesson S., Molnar P., Strandberg B., Almstrand A.-C., Bergemalm-Rynell K., et al. Effects on airways of short-term exposure to two kinds of wood smoke in a chamber study of healthy humans. Inhal. Toxicol. 2012;24:47–59. doi: 10.3109/08958378.2011.633281. [DOI] [PubMed] [Google Scholar]
- 50.Tsoukias N.M., George S.C. A two-compartment model of pulmonary nitric oxide exchange dynamics. J. Appl. Physiol. 1998;85:653–666. doi: 10.1152/jappl.1998.85.2.653. [DOI] [PubMed] [Google Scholar]
- 51.Eckel S.P., Salam M.T. Single high flow exhaled nitric oxide is an imperfect proxy for distal nitric oxide. Occup. Environ. Med. 2013;70:519–520. doi: 10.1136/oemed-2013-101458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hogman M., Stromberg S., Schedin U., Frostell C., Hedenstierna G., Gustafson L.E. Nitric oxide from the human respiratory tract efficiently quantified by standardized single breath measurements. Acta Physiol. 1997;159:345–346. doi: 10.1046/j.1365-201X.1997.00101.x. [DOI] [PubMed] [Google Scholar]
- 53.Silkoff P.E., Mcclean P.A., Slutsky A.S., Furlott H.G., Hoffstein E., Wakita S., Chapman K.R., Szalai J.P., Zamel N. Marked flow-dependence of exhaled nitric oxide using a new technique to exclude nasal nitric oxide. Am. J. Respir. Crit. Care Med. 1997;155:260–267. doi: 10.1164/ajrccm.155.1.9001322. [DOI] [PubMed] [Google Scholar]
- 54.George S.C., Hogman M., Permutt S., Silkoff P.E. Modeling pulmonary nitric oxide exchange. J. Appl. Physiol. 2004;96:831–839. doi: 10.1152/japplphysiol.00950.2003. [DOI] [PubMed] [Google Scholar]
- 55.Modig L., Dahgam S., Olsson D., Nyberg F., Wass K., Forsberg B., Olin A.-C. Short-term exposure to ozone and levels of exhaled nitric oxide. Epidemiology. 2014;25:79–87. doi: 10.1097/EDE.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 56.Salam M.T., Byun H.-M., Lurmann F., Breton C.V., Wang X., Eckel S.P., Gilliland F.D. Genetic and epigenetic variations in inducible nitric oxide synthase promoter, particulate pollution, and exhaled nitric oxide levels in children. J. Allergy Clin. Immunol. 2012;129:232–239.e7. doi: 10.1016/j.jaci.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brauer M., Avila-Casado C., Fortoul T.I., Vedal S., Stevens B., Churg A. Air pollution and retained particles in the lung. Environ. Health Perspect. 2001;109:1039–1043. doi: 10.1289/ehp.011091039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olin A.C., Rosengren A., Thelle D.S., Lissner L., Torén K. Increased fraction of exhaled nitric oxide predicts new-onset wheeze in a general population. Am. J. Respir. Crit. Care Med. 2010;181:324–327. doi: 10.1164/rccm.200907-1079OC. [DOI] [PubMed] [Google Scholar]
- 59.Olin A.C., Alving K., Torén K. Exhaled nitric oxide: Relation to sensitization and respiratory symptoms. Clin. Exp. Allergy. 2004;34:221–226. doi: 10.1111/j.1365-2222.2004.01888.x. [DOI] [PubMed] [Google Scholar]
- 60.Pignatti P., Spanevello A. Towards a practical clinical use of fractioned exhaled nitric oxide levels in chronic cough. Ann. Transl. Med. 2016;4:357. doi: 10.21037/atm.2016.08.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bjermer L., Alving K., Diamant Z., Magnussen H., Pavord I., Piacentini G., Price D., Roche N., Sastre J., Thomas M., et al. Current evidence and future research needs for FeNO measurement in respiratory diseases. Respir. Med. 2014;108:830–841. doi: 10.1016/j.rmed.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 62.Salam M.T., Bastain T.M., Rappaport E.B., Islam T., Berhane K., Gauderman W.J., Gilliland F.D. Genetic variations in nitric oxide synthase and arginase influence exhaled nitric oxide levels in children. Allergy. 2011;66:412–419. doi: 10.1111/j.1398-9995.2010.02492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salam M.T., Lin P.C., Eckel S.P., Gauderman W.J., Gilliland F.D. Inducible nitric oxide synthase promoter haplotypes and residential traffic-related air pollution jointly influence exhaled nitric oxide level in children. PLoS ONE. 2015;10:e0145363. doi: 10.1371/journal.pone.0145363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.