Abstract
Rationale
Parkinson’s disease (PD) impairs working memory (WM)—the ability to maintain items in memory for short periods of time and manipulate them. There is conflicting evidence on the nature of the deficits caused by the disease, and the potential beneficial and detrimental effects of dopaminergic medication on different WM processes.
Objectives
We hypothesised that PD impairs both maintenance and manipulation of items in WM and dopaminergic medications improve this in PD patients but impair it in healthy older adults.
Methods
We tested 68 PD patients ON and OFF their dopaminergic medication, 83 healthy age-matched controls, and 30 healthy older adults after placebo and levodopa administration. We used the digit span, a WM test with three components (forwards, backwards, and sequence recall) that differ in the amount of manipulation required. We analysed the maximum spans and the percentage of lists correctly recalled, which probe capacity of WM and the accuracy of the memory processes within this capacity, respectively.
Results
PD patients had lower WM capacity across all three digit span components, but only showed reduced percentage accuracy on the components requiring manipulation (backwards and sequence spans). Dopaminergic medication did not affect performance in PD patients. In healthy older adults, levodopa did not affect capacity, but did impair accuracy on one of the manipulation components (sequence), without affecting the other (backwards).
Conclusions
This suggests that the deficit of maintenance capacity and manipulation accuracy in PD patients is not primarily a dopaminergic one and supports a potential “overdosing” of intact manipulation mechanisms in healthy older adults by levodopa.
Electronic supplementary material
The online version of this article (10.1007/s00213-018-5058-6) contains supplementary material, which is available to authorized users.
Keywords: Parkinson’s disease, Dopamine, Working memory, Levodopa, Short-term memory
Introduction
Working memory (WM) involves the maintenance and manipulation of elements held in memory for short periods of time. Early models suggested WM is composed of phonological and visuospatial storage components, and a central executive that manages attentional demands and the manipulation of stored elements (Baddeley 2012; Baddeley and Hitch 1974).
Parkinson’s disease (PD) has been associated with impaired WM, especially for visuospatial tasks or complex tasks requiring manipulation (Hoppe et al. 2000; Lewis et al. 2005; Werheid et al. 2002). Maintenance of WM is also impaired in PD (Fallon et al. 2017a), though a meta-analysis of 56 WM span studies suggested that verbal maintenance was reduced to a lesser extent than verbal manipulation, and that spatial WM was impaired the most (Siegert et al. 2008). Maintenance processes have been linked to parietal regions (Aboitiz et al. 2010; Müller and Knight 2006; Smith and Jonides 1997) and manipulation to prefrontal regions (Müller and Knight 2006; Smith and Jonides 1997). PD affects prefrontal regions before parietal (Braak et al. 2003), due to the greater dopaminergic innervation of the former (Yetnikoff et al. 2014), which could explain the greater deficits in manipulation processes.
Dopaminergic medication might improve both maintenance and manipulation in PD patients (Beato et al. 2008; Owen et al. 1997; Zokaei et al. 2015), though some tasks have found no effect of medication (Cooper et al. 1993; Fournet et al. 2000; Zokaei et al. 2015) or only shown benefits in specific subgroups such as patients with low baseline WM (Warden et al. 2016) or in patients with onset of motor symptoms on their left-side (Hanna-Pladdy et al. 2015). Importantly, the sample sizes of many of these studies are relatively small (n = 7–28), meaning they are only able to detect medium or large effect sizes. This may have contributed to the conflicting results.
Dopamine can also impair WM, perhaps due to an overdosing of intact areas of the brain (Cools and D’Esposito 2011). This is more often seen in healthy participants than patients (Bloemendaal et al. 2015; Fallon et al. 2017b), although benefits of dopamine are also seen (Fallon et al. 2017b; Luciana et al. 1989; Naef et al. 2017) as are no-effects (Linssen et al. 2012); this may reflect different optimal levels of dopamine for different functions within WM such as maintenance and manipulation. Interestingly, in one of these studies testing WM following administration of methylphenidate (a dopamine and noradrenaline reuptake inhibitor), both beneficial and deleterious effects were demonstrated in the same group of participants (Fallon et al. 2017b); methylphenidate improved distractor resistance on a spatial WM task, but impaired flexible updating of information held in WM. This suggests that the “overdose” effects reported in some studies may not be seen when using broad measures of WM but require specific measurement of the different underlying processes.
We hypothesised that PD would impair maintenance and manipulation of WM, and that dopaminergic medication would remediate the deficits in PD patients while “overdosing” and impairing WM in healthy older adults. We used a simple WM measure, the digit span (Wechsler 2008), which is commonly given in neuropsychological assessments. We used three variations of the digit span (forwards, backwards, sequence recall) with different contributions of maintenance and manipulation processes. This was given to a large sample of PD patients ON and OFF their normal dopaminergic medication and healthy age-matched controls (HC), and a separate group of healthy older adults after administration of placebo or levodopa.
Methods
Ethical approval
Data are presented from several different studies running under different ethical approvals. Experiment 1 studies were approved by Frenchay and Southwest Central Bristol NHS RECs. Experiment 2 was approved by University of Bristol Faculty REC. All procedures were carried out in accordance with the relevant guidelines and regulations. All participants gave written informed consent, in accordance with the Declaration of Helsinki.
Participants
Experiment 1
Demographic details for all groups are presented in Table 1.
Table 1.
Demographics of participants tested in Experiments 1 and 2. Statistical comparisons are against HC from Experiment 1 (standard deviations in parentheses, range in square brackets). The older adults from Experiment 2 did not differ from the HC in Experiment 1 on any measure (p > .05). Three HC did not complete the MoCA
| Measure | PD Experiment 1 | HC Experiment 1 | HC Experiment 2 |
|---|---|---|---|
| Number | 68 | 83 | 30 |
| Age | 68.40 (6.53) [55–85] | 69.66 (8.49) [48–93] | 70.67 (6.83) [65–92] |
| Gender (M:F) | 49:19* | 39:44 | 14:16 |
| MoCA | 25.31* (2.88) [18–30] | 26.94 (2.37) [20–30] | 26.23 (3.15) [18–30] |
| MoCA > 25:25–21:< 21 | 37:26:5* | 60:19:1 | 18:10:2 |
| Years education | 13.70 (3.19) [9–25] | 14.65 (2.87) [7–21] | 14.33 (3.48) [10–24] |
| DASS | 22.91* (16.78) [4–64] | 14.07 (18.27) [0–94] | 11.27 (10.29) [1–39] |
| BIS | 54.04 (8.50) [38–69] | 50.33 (9.00) [31–77] | 48.50 (8.54) [31–66] |
| LARS | − 22.71* (4.81) [− 29 to − 13] | − 27.28 (4.57) [− 33 to − 16] | − 26.60 (5.54) [− 34 to − 14] |
| LDE | 605.41 (353.89) [150–1746] | ||
| Years symptoms | 5.79 (3.86) [1–18] | ||
| Years diagnosed | 4.63 (3.47) [1–14] | ||
| MDS-UPDRS-III ON | 27.88+ (13.36) [6–62] | ||
| MDS-UPDRS-III OFF | 32.85 (13.53) [7–69] |
MoCA, Montreal Cognitive Assessment; DASS, Depression, Anxiety and Stress Scale; BIS, Barratt Impulsivity Scale; LARS, Lille Apathy Rating Scale; LDE, levodopa dose equivalency; MDS-UPDRS-III, Movement Disorder Society Unified Parkinson’s Disease Rating Scale section III
*p < .05 for PD vs HC
+p < .05 for PD ON vs PD OFF
To generate a good sample size, we applied a robust, consistent protocol for testing digit span across several different studies. In total, we collected data from 68 PD patients and 83 HC who all performed the digit span (as well as other cognitive tasks dependent on the study). Patients with a diagnosis of idiopathic PD were recruited from neurology and movement disorder clinics at Southmead and Frenchay Hospitals in Bristol, UK. All were taking levodopa and/or dopamine receptor agonists, were not taking irreversible mono-amine oxidase inhibitors or acetylcholinesterase inhibitors, and did not have deep-brain stimulators implanted. They had no serious neurological disorders other than PD and had normal or corrected vision and hearing.
HC were recruited from our healthy volunteer database. They were 55 years or older, had no neurological disorders, were not taking dopaminergic medications, and had normal or corrected to normal vision and hearing.
Experiment 2
We recruited 35 healthy older (65+ years) adults from volunteer databases and Join Dementia Research databases. The same inclusion/exclusion criteria as for the HC group above were used, as well as contraindications and medical exclusions pertaining to the drugs administered (see Supplementary Materials 1 for full exclusion criteria). Two participants withdrew before completing one session, and three withdrew before completing both the drug and placebo session, leaving data from 30 participants analysed here.
Procedure
In the digit span, the experimenter reads aloud a list of digits at a rate of one per second and the participant must repeat the list back. All digits must be in the correct order for the list to be marked correct. The lists start at a length of two digits, and two lists of each length are read out. The list lengths increase by one digit until the participant gets both lists of the same length correct.
There are three components of the digit span: in the forwards span, the list must be recalled in the same order as said by the experimenter; in the backwards span, participants must repeat it in the reverse order to presentation order; in the sequence span, participants must recall the list in ascending numerical order. Forwards and sequence components present two lists of each length from 2 to 9 digits. The backwards component presents four lists of two digits length, then two lists from lengths of 3–8 digits. There are 16 lists in total for each component.
Experiment 1
PD patients completed the three components (forwards, backwards, sequence) of the digit span once ON and once OFF medication (medication order randomised and counterbalanced), while HC completed it once. When coming OFF medication, PD patients were withdrawn from standard release dopaminergic medication for a minimum of 16 h and from long-lasting dopaminergic medications for a minimum of 24 h.
Experiment 2
This was a within-subjects double-blinded, placebo-controlled study. The participants completed both the drug and placebo conditions, in a randomised, counterbalanced order.
Healthy older adults received 10 mg/ml domperidone or a placebo, followed by 187.5 mg co-beneldopa (150 mg levodopa, 37.5 mg benserazide) or a second placebo. Neither participant nor experimenter knew on which visit the participant received the drug or placebo. Their heart rate and blood pressure were monitored before and after administration. After 1.5 h, they completed the digit span, along with other cognitive tests.
Data analysis
We used several scoring measures for the digit span to capture different sources of errors. The maximum span length correctly recalled gives a measure of the maximum capacity of a participant’s WM. This is calculated for each span component (forwards, backwards, and sequence) separately.
People can also make errors even before they have hit their capacity limit, which is not picked up by the maximum span measure. There are several measures sensitive to the number of errors in WM which reflect WM accuracy rather than capacity. The number of lists recalled correctly gives a simple count of these errors but is confounded by the fact that people with smaller capacities will exit the test earlier and thus not attempt as many lists as someone with a larger span. Therefore, we analysed the percentage of lists recalled correctly, which corrects for the number of lists attempted and gives a more reliable and accurate measure of the accuracy of WM (Conway et al. 2005; Friedman and Miyake 2005).
Assessing the percentage of digits recalled correctly rather than the lists may have even greater reliability and sensitivity as it captures extra information in the data (Friedman and Miyake 2005; Unsworth and Engle 2007). Unfortunately, the exact digits recalled were not consistently recorded for all participants; there are only digit error data for 45 PD and 52 HC from Experiment 1, and only for two participants from Experiment 2. These data are presented in Supplementary Materials 2 but are not reported here due to the much lower power and weaker effects.
As these measures are all calculated from the same set of data, we looked at the correlation matrix between them. There were weak or moderate correlations between most of the measures, although some of the correlations with the % digits correct measure were not significant (see Table S5 for values).
Q-Q plots showed that the spans were all approximately normal. Between-subject ANOVAs and t tests were used to compare PD patients and HC, and within-subject t tests to compare the effect of medications on PD patients and the effect of levodopa on healthy participants in Experiment 2. When comparing the three components, Bonferroni corrections were applied (α = 0.0167). Data were analysed using SPSS (IBM, version 23.0).
Data availability
Experiment 1 did not obtain consent to share individual participants’ data, so we are not able to publish or provide the data without further permission from our study sponsor.
Anonymised data from Experiment 2 are available from the University of Bristol’s data repository, data.bris, at 10.5523/bris.15du56inneqal1ys8rhzuhbmmu (Grogan et al. 2018).
Results
Experiment 1
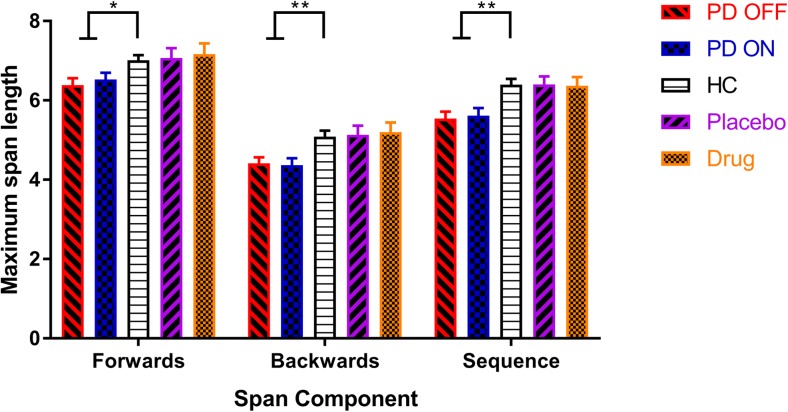
PD patients had lower capacities (maximum spans) for forwards (F (2, 216) = 4.572, p = .011, = .041), backwards (F (2, 216) = 6.590, p = .002, = .058), and sequence (F (2, 216) = 8.317, p < .001, = .072) components, with greater effect sizes in the manipulation spans (backwards and sequence spans; see Fig. 1 and Table 2). Paired t tests showed no significant differences between PD ON and OFF dopaminergic medication on any component (forwards: t (67) = 0.944, p = .348, d = 0.102; backwards: t (67) = − 0.309, p = .758, d = 0.032; sequence: t (67) = 0.456, p = .650, d = 0.049).
Fig. 1.
The mean WM capacity (maximum spans) for PD patients ON and OFF dopamine, HC, and healthy older participants on levodopa and placebo on each component of the digit span (SEM bars). PD patients had lower capacities than HC for all span components, but there were no effects of dopamine for PD patients or healthy older adults for any component. *p < .01667 (Bonferroni-corrected threshold), **p < .001667
Table 2.
Effect sizes and p values from group comparisons of digit span measures. Between-subject one-way ANOVAs were used to compare HC vs PD ON vs PD OFF, while paired t tests were used to compare PD ON vs PD OFF and Drug vs Placebo. Bonferroni corrections were applied at a significance threshold of α = .01667
| Comparison | Measure | Forwards | Backwards | Sequence | |||
|---|---|---|---|---|---|---|---|
| d | p value | d | p value | d | p value | ||
| HC vs PD ON vs PD OFF | Max span | 0.414 | 0.011358* | 0.496 | 0.001667** | 0.557 | 0.000331** |
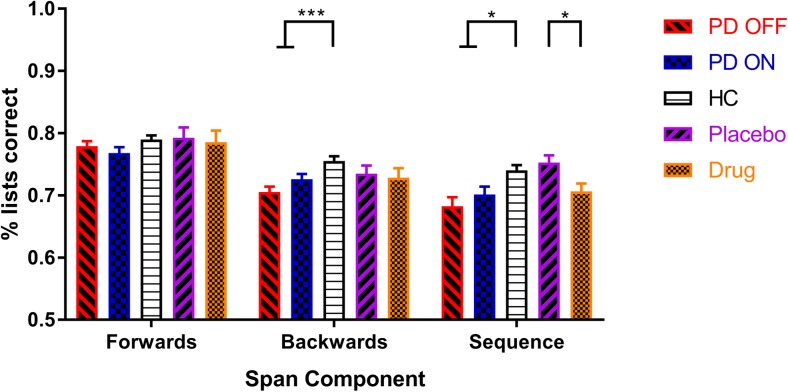
| % lists correct | 0.263 | 0.164839 | 0.578 | 0.000167*** | 0.487 | 0.001975* | |
| PD ON vs PD OFF | Max span | 0.102 | 0.348482 | 0.032 | 0.758273 | 0.049 | 0.650100 |
| % lists correct | 0.147 | 0.220807 | 0.281 | 0.048302 | 0.167 | 0.155641 | |
| Drug vs placebo | Max span | 0.070 | 0.609901 | 0.051 | 0.757700 | 0.028 | 0.902247 |
| % lists correct | 0.072 | 0.654236 | 0.085 | 0.654438 | 0.689 | 0.006718* | |
*p < .0167
**p < .00167
***p < .000167
PD patients had lower accuracy (i.e., lower percentage of lists correct) than HC only for the manipulation components (see Fig 2; backwards: F (2, 216) = 9.060, p = .0002, = .077; sequence: F (2, 216) = 6.410, p = .0020, = .056) but not for the forwards component (F (2,216) = 1.818, p = .1648, = .017). Dopaminergic medication did not affect the accuracy for any component (forwards: t (67) = − 1.236, p = .2208, d = 0.147; backwards: t (67) = 2.011, p = .0483, d = 0.281; sequence: t (67) = 1.436, p = .1556, d = 0.167).
Fig. 2.
The mean WM accuracy (percentage of lists correct) for each group, on each digit span component (SEM bars). PD patients had lower accuracy scores for the backwards and sequence components but not the forwards component. Dopamine did not affect accuracy in PD patients, but levodopa did decrease accuracy on the sequence component for healthy older adults. *p < .01667 (Bonferroni-corrected threshold), ***p < .0001667
As PD patients had lower capacity on the forwards component but did not have lower accuracy, we compared these two measures directly to see whether we could conclude that PD only affected capacity and did not affect accuracy (this conclusion is not possible from one significant effect and one non-significant effect). We also applied this comparison to the other two components. We converted capacity into a percentage to be on the same scale as the percentage of lists correct and ran a mixed ANOVA (within-subject factor: measure type; between-subject factor: group (PD or HC)). The forwards component had a significant measure * group interaction (F (1, 217) = 6.511, p = .011, = .029) that passed the Bonferroni-corrected threshold (α = .01667), while backwards (F (1, 217) = 4.641, p = .032, = .021) and sequence (F (1, 217) = 4.984, p = .027, = .022) did not. This suggests that PD affects the capacity and accuracy differently for the forwards component, but not the backwards or sequence components.
Post-hoc tests
We performed post-hoc exploratory tests to examine the types of errors people were making. The different types of errors made were scored using a method adapted from Woods et al. (2011a; see Supplementary Materials 3 for procedure and examples). In brief, the number of different types of order errors (swaps and permutations) and number errors (substitutions, omissions, intrusions) was scored. These were converted to percentages by dividing them by the number of digits attempted and multiplying by 100. These were not analysed for Experiment 2 as there were only two participants with all digits recorded (see “Methods” section).
We found that order errors were increased in PD patients only for the backwards span (p = .0004), not forwards (p = .3473) or sequence spans (p = .1187). There were no differences between PD ON and OFF (p > .1). Other types of errors (substitution, omission, intrusion) were not different between the groups (p > .05) or medication conditions (p > .0167).
We also examined the influence of cognitive function (and other factors) on the PD results. PD patients with a large range of MoCA scores were included in the analysis, and we found no correlation between MoCA and the difference in digit span measures ON and OFF medication. We also split the participants into high and low cognitive function groups (MoCA ≥ 26) and ran ANOVAs with this included as a between-subject factor. No interactions between this factor and group or medication state were found.
We ran similar analyses for the duration of disease, UPDRS scores, levodopa dose equivalency, laterality of motor symptoms, and other questionnaire scores mentioned in Table 1, but found no significant associations (p > .0167, see Supplementary Materials 2 for details).
Experiment 2
Looking at the healthy older adults given levodopa and placebo, levodopa did not affect WM capacity for any component (forwards: t (29) = 0.516, p = .610, d = 0.070; backwards: t (29) = 0.311, p = .758, d = .051; sequence: t (29) = − 0.0124, p = .920, d = 0.028) (see Fig 1 and Table 2).
However, levodopa did decrease the accuracy for the sequence component (t (29) = − 2.919, p = .007, d = 0.689), though not for the forwards (t (29) = − 0.453, p = .654, d = 0.072) or backwards (t (29) = − 0.452, p = .654, d = 0.085) components (see Fig 2). No effects of weight-adjusted levodopa dose were seen (see Supplementary Materials 2).
Only two participants from Experiment 2 had their error responses recorded, so the percentage of digits correct were not analysed for Experiment 2.
Discussion
PD patients had lower average WM capacity than HC for each component of the digit span, as well as lower average accuracy for backwards and sequence components. A post-hoc analysis also revealed that PD patients made more transposition errors on the backwards component of the digit span. Dopaminergic medication did not affect performance on any component or measure in PD patients. In healthy older adults, levodopa did not affect capacity, but did decrease the accuracy for the sequence component.
PD patients were only worse on the maintenance component (forwards digit span) when measuring the maximum span length recalled, not the percentage of lists correct. However, for the manipulation components (backwards and sequence spans) PD impaired the maximum capacity and percentage of lists correct similarly. The two measures were moderately correlated, with the forwards component showing the strongest correlation between capacity and accuracy. Despite this, the forwards component was the only component to have a significant group * measure interaction, suggesting that only this component was differently affected by PD.
This distinction suggests that the two measures are tapping into distinct processes during WM—the capacity and the accuracy. It also suggests that PD impairs the capacity for maintenance and manipulation processes, but only reduces accuracy of manipulation processes. This aligns with previous literature which has suggested that while PD does lead to more decay of precision of items held in memory (Fallon et al. 2017a; Zokaei et al. 2015), there are greater deficits when manipulation is required (Lewis et al. 2005) and that this is due to increased number of errors (Fallon et al. 2017a).
Alternatively, the difference in the measures may simply reflect poorer sensitivity of the accuracy measure. However, previous literature suggests that the percentage accuracy scores actually have greater sensitivity and reliability than simpler measures such as the maximum span length (Conway et al. 2005; Friedman and Miyake 2005), which would argue against this view.
The general deficit in WM capacity across components could reflect a reduction in the number of items that can be maintained in WM having a knock-on effect onto the manipulation components in the backwards and sequence components. The three components are not completely independent measures as shown by the moderate correlations between them (see Supplementary Materials 2). If PD reduces the number of items a person can hold in their memory, then this would also reduce the maximum spans possible in the backwards and sequence components. This is unlikely to be the sole driver of this deficit however, as backwards and sequence components had lower mean maximum spans than the forwards component, meaning they were not hitting the ceiling imposed by the maintenance capacity and that there is an extra source of error in these manipulation components.
If PD harms WM but dopaminergic medication does not improve it, then a non-dopaminergic pathology is suspected. PD patients have alterations to many neuromodulatory systems including noradrenaline, acetylcholine, and serotonin (Jellinger 1991; Scatton et al. 1983), which may underlie the deficit. Alternatively, it could be a dopaminergic pathology, but simply one that is too severe to be repaired by dopamine replacement therapy, although this seems unlikely given that motor symptoms, usually seen before cognitive changes, are still helped by dopaminergic medication, as evident in the reduced UPDRS scores in PD patients when ON medication.
More interesting is the apparent sparing of maintenance accuracy from the WM manipulation accuracy deficit caused by PD. This could suggest that the underlying processes accounting for errors on the spans is different when manipulation of the items is required, as PD seems to reduce the maximum number of items that can be maintained in WM, without increasing errors made. To explain this, we invoke the multicompartment model of WM (Baddeley 2003), which posits a phonological loop for storage of items, and a central executive that mediates manipulation of items stored. We propose that PD impairs the capacity of the phonological loop, without increasing errors in storage under this limit, and also impairs the central executive’s ability to interact with items stored.
Our exploratory analysis of the types of errors made found that PD patients made more order errors than HC for the backwards component only. Looking for order errors in the sequence component does not make much sense as the participants recalled the digits in numerical order, so did not have to remember the position of the digits during presentation. Order errors in this component were very rare, and likely reflect something entirely different to a transposition error in the other two components. We believe that this pattern of results suggests that PD patients make more order errors when manipulation is required, but that this is hidden when they recall the digits in numerical order. This could suggest that PD patients are more prone to “misbinding” items and locations when manipulation is required. This would contradict several studies examining misbinding in PD patients using continuous measures of error, which find PD patients do not differ in the amount of misbinding to HC (Fallon et al. 2017a; Zokaei et al. 2014, 2015).
Alternatively, it is possible that patients are actually “over-binding”, which prevents them from reversing the order of those items when reversing the entire list. The majority of the order errors were “swap errors” where two digits are swapped (e.g. the correct answer 12345 becomes 12435). In the backwards component (where the presented list would have been 54321), this could be either because the participants have swapped the items around and then reversed the list, or they reversed the list except for those two items which “stuck” in their relative order.
The pattern of results from Experiment 2 suggests that levodopa does not affect the maximum capacity of any of the digit span components but may reduce the accuracy only for the sequence span. This induced deficit supports the dopamine overdose hypothesis which posits that dopaminergic drugs will overdose intact functioning in the brain, leading to impairments (Cools and D’Esposito 2011). That manipulation accuracy was reduced by levodopa corroborates reports that methylphenidate impairs the flexible updating of WM information (Fallon et al. 2017b), which would be needed in the sequence span. However, this effect should be interpreted with caution; unlike the pattern of effects seen in PD patients, this one is isolated. There was no impairment on the other manipulation component (the backwards span). Therefore, while it may be that levodopa does impair manipulation accuracy of WM items, it is also possible that this is simply an artefact or false positive.
There are several drawbacks in using the digit span that should be considered. As only two lists of each length are presented, it provides a very noisy measure of performance. Adapted versions presented via computer are available which use repeated presentations of list lengths, and do not exit when they fail to recall the lists, but instead decrease the length and then increase it back up if they recall that one correctly (Woods et al. 2011b). This step-up/step-down procedure is more sensitive to people’s maximum capacities. Computerised assessment would also rule out any variability induced by slightly different speaking speeds, accents, volume, and diction, from different experimenters, which may have affected performance. Other computerised tasks are available which provide analogue error measures on WM, which have shown far greater sensitivity than the digit span (Zokaei et al. 2015). Work with these tasks has suggested that WM capacity may not be determined by the number of discrete “slots” for information but rather by the allocation of a shared capacity resource (Bays et al. 2009; Schneegans and Bays 2016). Future work with these more sensitive tasks will be able to separate out the specific WM processes impaired by PD and affected by dopamine. These could be used to test predictions from this study, such as PD impairs WM maintenance and manipulation capacity but not maintenance accuracy, while dopamine does not remediate this deficit in PD but may “overdose” manipulation accuracy in healthy controls.
In summary, PD impaired maximum capacity of maintenance spans, and the capacity and accuracy of manipulation spans. Despite this, PD patients show no benefit of dopaminergic medication on maintenance or manipulation of WM, suggesting that the deficit is not wholly dopaminergic. Levodopa also did not affect WM capacity in healthy older adults, but may have decreased accuracy on manipulation span, although this effect should be viewed with caution.
Electronic supplementary material
1 The exclusion criteria for Experiment 2, based on the drugs given to participants. (DOCX 17 kb)
2 Supplementary analyses including the percentage of digits correct, means and standard deviations of summary measures, and correlations between digit span measures and additional measures. (DOCX 968 kb)
3 Procedure for scoring error types on the digit span, and analysis of these error types. (DOCX 454 kb)
Acknowledgements
The authors thank Bristol Research into Alzheimer’s and Care of the Elderly charity (BRACE) for the use of their building, all the patients and participants who took part in this research, and the funders (Wellcome [097081/Z/11/Z] and BRACE [BRI17/15]).
Abbreviations
- PD
Parkinson’s disease
- WM
working memory
- HC
healthy control
- MoCA
Montreal Cognitive Assessment
- DASS
Depression, Anxiety and Stress Scale
- BIS
Barratt Impulsivity Scale
- LARS
Lille Apathy Rating Scale
- LDE
levodopa dose equivalency
- MDS-UPDRS-III
Movement Disorder Society Unified Parkinson’s Disease Rating Scale section III
Compliance with ethical standards
Conflict of interests
The authors declare that they have no conflict of interest.
References
- Aboitiz F, Aboitiz S, García RR. The phonological loop. Curr Anthropol. 2010;51(s1):S55–S65. doi: 10.1086/650525. [DOI] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nature Reviews. Neuroscience. 2003;4(10):829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: theories, models, and controversies. Annual Review of Psychologyn. 2012;63:1–29. doi: 10.1146/annurev-psych-120710-100422. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Hitch G. Working memory. In: Bower GH, editor. The psychology of learning and motivation. New York: Academic Press; 1974. pp. 47–89. [Google Scholar]
- Bays PM, Catalao RFG, Husain M. The precision of visual working memory is set by allocation of a shared resource. J Vis. 2009;9(10):7–7. doi: 10.1167/9.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato R, Levy R, Pillon B, Vidal C, Tezenas S, Montcel D, Cardoso F. Working memory in Parkinson’s disease patients: clinical features and response to levodopa. Arq Neuropsiquiatr. 2008;66(2):147–151. doi: 10.1590/S0004-282X2008000200001. [DOI] [PubMed] [Google Scholar]
- Bloemendaal M, van Schouwenburg MR, Miyakawa A, Aarts E, D’Esposito M, Cools R. Dopaminergic modulation of distracter-resistance and prefrontal delay period signal. Psychopharmacology. 2015;232(6):1061–1070. doi: 10.1007/s00213-014-3741-9. [DOI] [PubMed] [Google Scholar]
- Braak Heiko, Tredici Kelly Del, Rüb Udo, de Vos Rob A.I, Jansen Steur Ernst N.H, Braak Eva. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging. 2003;24(2):197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: a methodological review and user’s guide. Psychon Bull Rev. 2005;12(5):769–786. doi: 10.3758/BF03196772. [DOI] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69(12):e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J a, Sagar HJ, Sullivan EV. Short-term memory and temporal ordering early Parkinson’s disease: effects of disease chronicity and medication. Neuropsychologia. 1993;31(9):933–949. doi: 10.1016/0028-3932(93)90149-T. [DOI] [PubMed] [Google Scholar]
- Fallon SJ, Mattiesing RM, Muhammed K, Manohar S, Husain M. Fractionating the neurocognitive mechanisms underlying working memory: independent effects of dopamine and Parkinson’s disease. Cereb Cortex. 2017;27(12):5727–5738. doi: 10.1093/cercor/bhx242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon SJ, van der Schaaf ME, ter Huurne N, Cools R. The neurocognitive cost of enhancing cognition with methylphenidate: improved distractor resistance but impaired updating. J Cogn Neurosci. 2017;29(4):652–663. doi: 10.1162/jocn_a_01065. [DOI] [PubMed] [Google Scholar]
- Fournet N, Moreaud O, Roulin JL, Naegele B, Pellat J. Working memory functioning in medicated Parkinson’s disease patients and the effect of withdrawal of dopaminergic medication. Neuropsychology. 2000;14(2):247–253. doi: 10.1037/0894-4105.14.2.247. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. Comparison of four scoring methods for the reading span test. Behav Res Methods. 2005;37(4):581–590. doi: 10.3758/BF03192728. [DOI] [PubMed] [Google Scholar]
- Grogan J, Isotalus H, & Coulthard E (2018) DARet1 digit span data. 10.5523/bris.15du56inneqal1ys8rhzuhbmmu
- Hanna-Pladdy B, Pahwa R, Lyons KE. Paradoxical effect of dopamine medication on cognition in Parkinson’s disease: relationship to side of motor onset. J Int Neuropsychol Soc. 2015;21:259–270. doi: 10.1017/S1355617715000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe CD, Müller UD, Werheid KD, Thöne AD, von Cramon YD. Digit ordering test: clinical, psychometric, and experimental evaluation of a verbal working memory test. Clin Neuropsychol. 2000;14(1):38–55. doi: 10.1076/1385-4046(200002)14:1;1-8;FT038. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Pathology of Parkinson’s disease: changes other than the nigrostriatal pathway. Mol Chem Neuropathol. 1991;14:153–197. doi: 10.1007/BF03159935. [DOI] [PubMed] [Google Scholar]
- Lewis SJG, Slabosz A, Robbins TW, Barker RA, Owen AM. Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson’s disease. Neuropsychologia. 2005;43(6):823–832. doi: 10.1016/j.neuropsychologia.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Linssen AMW, Vuurman EFPM, Sambeth A, Riedel WJ. Methylphenidate produces selective enhancement of declarative memory consolidation in healthy volunteers. Psychopharmacology. 2012;221(4):611–619. doi: 10.1007/s00213-011-2605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Depue RA, Arbisi P, Leon A. Facilitation of working memory i n humans by a D2 dopamine receptor agonist. J Cogn Neurosci. 1989;4(1):58–68. doi: 10.1162/jocn.1992.4.1.58. [DOI] [PubMed] [Google Scholar]
- Müller NG, Knight RT. The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience. 2006;139(1):51–58. doi: 10.1016/j.neuroscience.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Naef M, Müller U, Linssen A, Clark L, Robbins TW, Eisenegger C. Effects of dopamine D2/D3 receptor antagonism on human planning and spatial working memory. Transl Psychiatry. 2017;7(4):e1107. doi: 10.1038/tp.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Iddon JL, Hodges JR, Summers BA, Robbins TW. Spatial and non-spatial working memory at different stages of Parkinson’s disease. Neuropsychologia. 1997;35(4):519–532. doi: 10.1016/S0028-3932(96)00101-7. [DOI] [PubMed] [Google Scholar]
- Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res. 1983;275(2):321–328. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- Schneegans S, Bays PM. No fixed item limit in visuospatial working memory. Cortex. 2016;83:181–193. doi: 10.1016/j.cortex.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert RJ, Weatherall M, Taylor KD, Abernethy DA. A meta-analysis of performance on simple span and more complex working memory tasks in Parkinson’s disease. Neuropsychology. 2008;22(4):450–461. doi: 10.1037/0894-4105.22.4.450. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Working memory: a view from neuroimaging. Cogn Psychol. 1997;33:5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. On the division of short-term and working memory: an examination of simple and complex span and their relation to higher order abilities. Psychol Bull. 2007;133(6):1038–1066. doi: 10.1037/0033-2909.133.6.1038. [DOI] [PubMed] [Google Scholar]
- Warden C, Hwang J, Marshall A, Fenesy M, Poston KL. The effects of dopamine on digit span in Parkinson’s disease. J Clin Mov Disord. 2016;3:5. doi: 10.1186/s40734-016-0033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale—fourth edition (WAIS–IV) San Antonio, TX: NCS Pearson; 2008. [Google Scholar]
- Werheid K, Hoppe C, Thöne A, Müller U, Müngersdorf M, von Cramon DY. The adaptive digit ordering test: clinical application, reliability, and validity of a verbal working memory test. Arch Clin Neuropsychol. 2002;17(6):547–565. doi: 10.1016/S0887-6177(01)00134-2. [DOI] [PubMed] [Google Scholar]
- Woods DL, Herron TJ, Yund EW, Hink RF, Kishiyama MM, Reed B. Computerized analysis of error patterns in digit span recall. J Clin Exp Neuropsychol. 2011;33(7):721–734. doi: 10.1080/13803395.2010.550602. [DOI] [PubMed] [Google Scholar]
- Woods DL, Kishiyama MM, Yund EW, Herron TJ, Edwards B, Poliva O, Reed B. Improving digit span assessment of short-term verbal memory. J Clin Exp Neuropsychol. 2011;33(1):101–111. doi: 10.1080/13803395.2010.493149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetnikoff L, Lavezzi HN, Reichard RA, Zahm DS. An update on the connections of the ventral mesencephalic dopaminergic complex. Neuroscience. 2014;282:23–48. doi: 10.1016/j.neuroscience.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zokaei N, Burnett Heyes S, Gorgoraptis N, Budhdeo S, Husain M. Working memory recall precision is a more sensitive index than span. J Neuropsychol. 2015;9:319–329. doi: 10.1111/jnp.12052. [DOI] [PubMed] [Google Scholar]
- Zokaei N, Mcneill A, Proukakis C, Beavan M, Jarman P, Korlipara P, et al. Visual short-term memory deficits associated with GBA mutation and Parkinson’s disease. Brain. 2014;137(8):2303–2311. doi: 10.1093/brain/awu143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1 The exclusion criteria for Experiment 2, based on the drugs given to participants. (DOCX 17 kb)
2 Supplementary analyses including the percentage of digits correct, means and standard deviations of summary measures, and correlations between digit span measures and additional measures. (DOCX 968 kb)
3 Procedure for scoring error types on the digit span, and analysis of these error types. (DOCX 454 kb)
Data Availability Statement
Experiment 1 did not obtain consent to share individual participants’ data, so we are not able to publish or provide the data without further permission from our study sponsor.
Anonymised data from Experiment 2 are available from the University of Bristol’s data repository, data.bris, at 10.5523/bris.15du56inneqal1ys8rhzuhbmmu (Grogan et al. 2018).