Abstract
Fragility fractures are increasingly recognized as a complication of both type 1 and type 2 diabetes, with fracture risk that increases with disease duration and poor glycemic control. Yet the identification and management of fracture risk in these patients remains challenging. This review explores the clinical characteristics of bone fragility in adults with diabetes and highlights recent studies that have evaluated bone mineral density (BMD), bone microstructure and material properties, biochemical markers, and fracture prediction algorithms (i.e., FRAX) in these patients. It further reviews the impact of diabetes drugs on bone as well as the efficacy of osteoporosis treatments in this population. We finally propose an algorithm for the identification and management of diabetic patients at increased fracture risk.
Keywords: Diabetes, Diabetes-related bone disease, Fracture, Osteoporosis
Epidemiology of diabetes and related fractures
Worldwide, one in 11 adults globally is estimated to have diabetes. The global prevalence of type 1 and type 2 diabetes in adults is currently estimated to be close to 425 million, with an expected increase to 629 million by 2045 [1]. In addition, there are an estimated 318 million adults with impaired glucose tolerance.
A meta-analysis including nearly 140,000 subjects with fractures reported a pooled relative risk (RR) of any fracture of 3.16 (95% CI 1.51–6.63; p = 0.002), hip fractures of 3.78 (95% CI 2.05–6.98; p < 0.001), and spine fractures of 2.88 (95% CI 1.71–4.82; p < 0.001) in type 1 diabetes [2]. The RR of a hip fracture in women with type 1 diabetes was 5.19 (95% CI 2.22–12.11, p < 0.001) compared to women without diabetes [2]. Weber et al. showed that increased risk of fractures extended across the life span, with hip fracture incidence occurring 10 to 15 years earlier in patients with type 1 diabetes than in those without [3].
An increased fracture risk has also been reported in some studies of type 2 diabetes [4, 5], but not others [6, 7]. In a middle-aged population of 33,000, diabetes was the strongest predictor of low-energy fracture in both men and women, with RR of 2.38 and 1.87, respectively [8]. In a meta-analysis of patients with type 2 diabetes, the summary RR of fractures at the hip in men was 2.8 [1.1, 6.6] and in women 2.1 [1.6. 2.7] [9].
When contrasting the risk of fractures in type 1 diabetes from type 2 diabetes, Vestergaard reported an odd ratio (OR) for hip fracture of 1.38 [1.2–1.6] in type 2 diabetes compared to 1.70 [1.3–2.2] in type 1 diabetes [4], although the latter was likely to be underestimated. Indeed meta-analyses published by Janhorbani [9] and Vestergaard [10] showed a stronger association and effect size for type 1 diabetes (RR 6.3 and 6.94 respectively) compared to type 2 diabetes (RR 1.7 and 1.38 respectively), in both men and women.
With an OR for osteoporotic fracture in patients with type 2 diabetes of about 1.5, only about 4% of the global osteoporotic fracture burden is statistically attributable to diabetes. However, considering the increasing prevalence of diabetes and the fact it may also be associated with greater risk for (injurious) falls [11], fragility fractures increasingly appear as a serious, yet neglected complication of this disease. Nevertheless, the link between diabetes and skeletal health receives only cursory attention in osteoporosis guidelines and even less in clinical diabetes guidelines [12].
Diabetes-related risk factors for fractures
Certain individuals with diabetes seem to be at greater risk of fracture than others. Hence, in type 2 diabetes, age and duration of diabetes are clearly important [4, 13–15]. In the cohort from Manitoba, Canada, consisting of men and women aged 40 years and older with or without diabetes (n = 6455/55′958), diabetes was a significant independent risk factor for major osteoporotic fractures (MOF) (hazard ratio (HR) 1.32; 95% CI 1.20–1.46). However, age significantly modified the effect of diabetes on hip fracture risk, with younger subjects having a higher relative risk, as the background risk rises in the overall population with aging (adjusted (a) HR age < 60, 4.67 [95% CI 2.76–7.89], age 60–69, 2.68 [1.77–4.04], age 70–79, 1.57 [1.20–2.04], age > 80, 1.42 [1. 10–1.99]; p interaction < 0.001) [15].
A study has suggested that fracture risk is not increased within the first 5 years of diabetes [16], while Ivers et al. observed high risk of any fracture only in patients with diabetes for at least 10 years [17]. A biphasic pattern has been proposed where fracture risk is in fact decreased in newly diagnosed type 2 diabetes, −which could be related to some protective effects of increased fat mass in these subjects–, and only increases significantly after 5 years [18]. In FRAX-adjusted analyses, only duration longer than 10 years was associated with a higher risk for MOF (HR 1.47, [1.30–1.66]) [19].
A meta-analysis in 2007 [10] did not reveal a clear association between fracture risk and glycemic control. However, recent observational and association studies reported increased fracture risk with worsening control as defined by glycated hemoglobin A1c (HbA1c) levels ≥ 7% [20, 21]. A clinical trial of glycemic control reported that maintaining a median A1c of 6.4% did not reduce fracture risk compared with a median A1c of 7.5% [22], but this trial could not assess effects of poor (> 8%) control on fracture risk. Diabetes has also been shown to be predictive of increased post-fracture mortality risk among patients with hip fractures [23, 24]. Consistent with the notion that longer duration and/or poor glycemic control could further increase fracture risk in diabetes, recent studies have shown that bone microstructural alterations are more prominent among diabetics with microvascular complications (see below) [25].
Impact of diabetes medication on fracture risk
The relationship between diabetes and bone fragility and therefore the identification of those individuals at increased risk of fracture is further complicated by the variable effects of diabetes medication on the skeleton (Table 1). Although there is no prospective trial on the effects of diabetes medication on bone fragility, results from observational and epidemiological studies and from adverse events in diabetes clinical trials have brought important insights into the potentially beneficial or deleterious effects of these medications on fracture risk.
Table 1.
Effects of diabetes medications on BMD and the risk of fracture in type 2 diabetes
| Medications | BMD | Risk of fracture |
|---|---|---|
| Metformin [4, 26] | =/↑ | ↓/= |
| Sulphonylureas [26] | NA | ↓/=/↑ |
| Thiazolidinediones [27, 28] | ↓↓/= | ↑↑/= |
| Incretins | ||
| GLP1 analogue [29] DPP4 inhibitor [30, 31] |
↑/= -- |
= ↓/= |
| SGLT2 inhibitors [32–34] | = | =/↑ |
| Insulin [35] | = | ↑ |
↑ increase, ↓ decrease, = unchanged, NA not available, GLP glucagon-like peptide, DPP4 dipeptidyl peptidase inhibitor 4, SGLT2 sodium/glucose cotransporter 2, BMD bone mineral density
Conventional medications
Observational studies have often reported an increased fracture risk in patients taking insulin [26]. Patients receiving insulin (and possibly insulin secretagogues) are at higher risk of fracture, through an indirect effect, in part because of hypoglycemia-induced falls [36, 37]. It is also possible that those on insulin suffer from diabetes for a longer duration and/or have a poorer glycemic control (i.e., over 5 years ago) with the disease-related complications (retinopathy, neuropathy) [38, 39] that could further contribute to falls and fracture risk.
In vitro studies have shown a positive effect of metformin on RUNX2 expression, improving, in turn, bone formation [38]. Clinical data confirmed either a neutral or a positive effect on fractures, making this widely used medication a safe option with regard to bone health [38].
Although in vitro data have not proved a direct effect of sulphonylureas on bone, epidemiological data have revealed an increased risk of fractures in treated patients. It has been hypothesized that the high risk of hypoglycemic events related to sulphonylureas may increase risk of falls and, by consequence, risk of fractures [38].
A number of both in vitro studies and clinical trials have proven that both rosiglitazone and pioglitazone treatment cause bone loss [40]. Thiazolidinediones (TZD) interact with peroxisome proliferator-activated receptor (PPAR)γ, which favors adipocyte differentiation at the expense of the osteoblast differentiation and regulates gene expression involved in adipogenesis, glucose homeostasis, and inflammation. A recent meta-analysis has pointed out that pioglitazone may play a negative role only in females with data not showing a significant risk for males. Current guidelines suggest avoiding pioglitazone in postmenopausal women or in men with other risk factors for bone fragility.
Newer medications
Both incretin mimetics, dipeptidyl peptidase-4 (DPP4) inhibitors and glucagon-like peptide-1 (GLP-1) analogs, have a safe skeletal profile in type 2 diabetes, likely because the positive effect of GLP-1 on bone formation and the low risk of hypoglycemic events [40].
Recent reports from the CANVAS study on sodium-glucose cotransporter 2 (SGLT2) inhibitors have indicated decreased bone density and higher risk of fractures in patients treated with canagliflozin [32]. The mechanisms for canaglifozine negative effects on bone are not entirely clear but SGLT2 inhibitors inhibit the proximal tubular reabsorption of glucose, while increasing phosphate reabsorption, thereby increasing serum phosphate levels that can be a trigger for PTH and increased bone turnover.
In contrast, the limited available data for empagliflozin and dapagliflozin have not raised concerns for bone fragility [33]. A recent meta-analysis of 20 SGLT2 inhibitor trials actually has not confirmed an increased risk of fractures with dapagliflozin, empagliflozin, or canagliflozin [41]. More data are necessary to understand the effect of these new medications on bone health. Nevertheless, at the moment, empagliflozin and dapagliflozin may be preferred in diabetic patients with known bone fragility.
DXA and bone ultrasound
Most studies have shown that people with type 1 diabetes have lower bone mineral density (BMD) compared with healthy subjects [42]. It might be expected that obesity, which is a strong risk factor for type 2 diabetes, would protect against osteoporosis because of the known positive correlation between body mass index (BMI) and BMD. Indeed, type 2 diabetes is usually associated with a 5 to 10% higher areal BMD than healthy subjects [5, 10, 13, 43], though there is significant heterogeneity between studies [43]. The increase in BMD was more pronounced in younger men, in the presence of higher BMI and—perhaps surprisingly—higher HbA1c levels [43]. The higher BMD was predominantly a feature of the weight-bearing skeleton but not of nonweight-bearing sites such as the forearm [5]. However, the higher BMD noted in type 2 diabetes may also be independent of the increased skeletal loading as higher BMD persists even after adjustment for BMI in numerous cohort studies [43]. An Asian study also reported subjects with type 2 diabetes and hip fracture who are underweight, with a higher BMD compared to non-diabetic counterparts, suggesting other mechanisms for the higher BMD, such as persistent hyperglycemia related to insulin resistance [44].
This relatively higher BMD in those with type 2 diabetes implies that an even lower proportion of subjects with fracture will have a BMD T-score in the osteoporotic range (i.e., T-score ≤ −2.5) than among the non-diabetic population. Schwartz et al. showed that for a given T-score and age, the fracture risk was higher in type 2 diabetes patients compared to patients without type 2 diabetes [45]. Moreover, a T-score in a woman with diabetes is associated with hip fracture risk equivalent to a woman without diabetes with a T-score of approximately 0.5 units lower [45]. Nevertheless, data have clearly confirmed that while BMD systematically underestimates fracture risk, it still stratifies fracture risk in elderly patients with diabetes [46].
Some studies suggest that type 2 diabetes may also be associated with more rapid bone loss, which could also partially explain the increased rate of fractures. Schwartz et al. [47] found that older women with diabetes lose bone more rapidly than those without type 2 diabetes at many skeletal sites, but not the radius. Leslie et al. recently published that in a large registry-based study for Manitoba, women with diabetes had marginally greater BMD loss at the femoral neck but not at other sites compared to a control population without diabetes [48].
Contrarily to BMD, spine trabecular bone score (TBS) tends to be lower among diabetes patients than controls [49, 50]. Moreover, within the type 2 diabetes group, TBS was better in those with good glycemic control compared to those with poor glycemic control. Hence TBS was found to be a BMD-independent predictor of fracture and predicted fractures equally well in those with (aHR 1.27, 95% CI 1.10–1.46) and without diabetes (HR 1.31, 95% CI 1.24–1.38). To be noted, however, that the gradient of risk per 1 SD decrease in TBS remains less than for BMD in diabetic patients, whereas diabetes itself remains an independent risk factor for fractures even after adjustment for BMD and TBS [50]. Recent analyses indicate that TBS as evaluated on Hologic dual-energy x-ray absorptiometry (DXA) devices is inversely related to BMI and abdominal fat [51]. Whether TBS represents alterations of bone structure in diabetes therefore remains unknown.
There exist conflicting results with studies conducted using calcaneal ultrasound. In one study using quantitative ultrasound, speed of sound (SOS) measurements at the radius were significantly decreased in type 2 diabetes compared to controls [52], while another study reported that calcaneal SOS was not different between type 2 diabetes patients with prevalent vertebral fractures (VFs) compared to those without VFs [53].
Microarchitecture and bone quality
Since reduced BMD alone does not fully explain bone fragility, particularly not in type 2 diabetes, alteration in “bone quality” is being investigated using various techniques. With magnetic resonance imaging (MRI), Pritchard et al. measured larger holes in trabecular network of type 2 diabetes compared to controls at baseline [54]. Using HR-pQCT (Xtreme CT) at the distal radius and/or tibia, studies in postmenopausal women with or without diabetes suggest that there is a trend towards greater cortical porosity in type 2 diabetes compared to controls [55–57]. In 99 elderly women with type 2 diabetes and 954 age-matched controls from the Gothenburg Study, Nilsson et al. reported higher cortical porosity at the distal radius but not at the distal tibia in subjects with type 2 diabetes (+ 16%, p < 0.001) [58]. However, they did not find any other alteration in the trabecular or cortical microarchitecture nor decreased estimated bone strength among diabetics in this cohort [58]. Trabecular bone volume is more heterogeneous and is preserved or (apparently) increased [55], though the latter may arise from the trabecularization of the cortex [59]. Furthermore, the increased cortical porosity and larger trabecular heterogeneity is more evident in type 2 diabetes with fractures compared to type 2 diabetes without prevalent fractures [56]. In African-American women with diabetes, cortical porosity was reported to be 26% greater while cortical volumetric BMD (vBMD) was lower compared to controls [60]. A recent study of 52 subjects with type 2 diabetes, of whom 25 had microvascular disease demonstrated, such cortical deficits noted on HR-pQCT were only present in patients with the microvascular complications [25]. Higher cortical porosity in mid-cortical and periosteal layers in type 2 diabetes patients with prior fracture compared to type 2 diabetes without fractures suggests that these cortical sub-compartments might be sensitive to type 2 diabetes-induced toxicity and may reflect microvascular disease [61].
Bone strength estimated by microfinite element analysis (micro-FEA) was shown to be lower in type 2 diabetes compared to controls in association with increased cortical porosity at the distal radius [55, 58]. Furthermore, in type 2 diabetes with fractures, stiffness, failure load, and cortical load fraction were significantly decreased at the ultradistal and distal tibia compared to type 2 diabetes without fractures and this deficit is related to the higher cortical porosity [56]. However, it is unlikely that HR-pQCT will become sufficiently widely available for routine clinical purposes. DXA-derived surrogates for cortical bone volume and strength may provide additional information regarding cortical alterations in diabetes, as well as having potentially widespread accessibility.
Finally, few studies using microindentation of the tibia outer cortex have suggested that the estimated bone material strength index (BMSI) is decreased in type 2 diabetes compared to controls [58, 62], which could reflect alterations in collagen crosslinks by advanced glycation end products (AGEs) and in mineralization (also see below) [63]. These findings are consistent with the concept of “diabetoporosis” as previously suggested to characterize the bone fragility in this particular population [64].
Bone turnover: histomorphometry and serum markers
The gold standard for the study of bone turnover is quantitative bone histomorphometry. One of the best estimates of bone turnover rate is the bone formation rate divided by the surface referent (BFR/BS) and this has been shown to be decreased in diabetes at the cancellous, endocortical and intracortical surfaces by 70–80%. In two small studies, reductions in the mineralizing surface and the osteoblast surface (5 patients) and low bone formation (6 patients) have been reported [65, 66].
Most biochemical studies show that bone formation markers, procollagen type I N-terminal propeptide (PINP) and osteocalcin (OC) and the bone resorption markers c-telopeptide (CTX) and tartrate-resistant acid phosphatase 5b (TRAcP5b) activity are usually reduced in type 2 diabetes [38, 67, 68], whereas bone-specific alkaline phosphatase (BSAP) [69] and N-terminal telopeptide (NTX)/creatinine (Cr) [70, 71] are usually normal or slightly elevated.
It is noteworthy that in the context of a low bone turnover, the mechanisms for an apparent increase in the cortical porosity remain unexplained.
Other biochemical markers of bone fragility in diabetes
The bone content of pentosidine, the most abundant AGE [72, 73] in non-diabetics with hip fracture was greater than in those without hip fracture [74]. Bone pentosidine levels are related to the strength of the human vertebra, independent of BMD [73]. Increased levels of serum pentosidine, AGEs, and soluble receptors for advanced glycation end products (sRAGE) were reported in type 2 diabetes compared with controls [75, 76]. Serum pentosidine was associated with greater risk of vertebral fracture in patients with type 2 diabetes [77], while urinary pentosidine is associated with an increased risk of clinical and vertebral fractures [78, 79]. Serum endogenous secretory RAGE (esRAGE) was inversely related to the risk of vertebral fracture in type 2 diabetes and the effect was independent of BMD [80].
Sclerostin, an inhibitor of the Wnt/β-catenin pathway and therefore an inhibitor of bone formation, was found to be significantly increased in type 2 diabetes compared to controls [81, 82]; and sclerostin levels have been shown to be positively correlated with fragility fractures in type 2 diabetes [83, 84]. Conversely, sclerostin levels were inversely associated with fracture risk in type 1 diabetes patients: the patients with the highest tertile of sclerostin had an 81% decreased risk of a fracture compared to the lowest tertile [85]. Whether any increase in circulating levels of sclerostin directly reflects an osteocytic dysfunction and/or is a marker of the vascular disease in type 2 diabetes patients remains unknown [86].
In this context, another new marker of osteocytic and periosteal cells activity may be of interest. Serum periostin and particularly its digested fragments have recently been associated with fracture risk in non-diabetes patients [87] and are currently under study in large diabetes population. In addition, serum microRNAs (miRNA) have been found to be altered in diabetes and that might explain some of the alterations in bone cell functions related to diabetes [88].
Anti-osteoporosis treatments in diabetic patients
No randomized clinical trials have directly evaluated the anti-fracture efficacy of osteoporosis treatment in diabetic patients; management is therefore largely empirical and derives from the good clinical practice and experience of the physician.
The clinical evidence regarding the efficacy of anti-osteoporosis treatments in diabetic patients is therefore provided by post hoc analyses in subgroups from randomized clinical trials that primarily enrolled osteoporosis patients and from a few observational studies (Table 2).
Table 2.
Effects of osteoporosis medications on BMD and the risk of fracture in type 2 diabetes
| Medications | BMD | Risk of fracture |
|---|---|---|
| Alendronate [89–91] | ↑ | NA/= |
| Etidronate [91] | NA | = |
| Risedronate [92] | ↑ | NA |
| Raloxifene [91, 93, 94] | NA | ↓/= |
| Denosumab | NA | NA |
| Teriparatide [95] | ↑ | = |
↑ increase, ↓ decrease, = unchanged, NA not available, BMD bone mineral density
In the Fracture Intervention Trial (FIT), postmenopausal women including diabetic participants with a femoral neck T-score < −1.6 were randomly treated with alendronate or placebo for 3 years. In a post hoc analysis, Keegan et al. [89] reported that diabetes did not alter the effect of alendronate on BMD gain vs placebo. Similarly, two relatively small observational studies showed than alendronate improved lumbar spine BMD but not hip BMD similarly in postmenopausal osteoporotic patients with and without diabetes [96, 97]. Data extracted from the Danish national prescription registry reported that diabetes, with or without complications, did not influence fracture risk in patients who adhered to alendronate [90]. Another Danish cohort study found no difference in the anti-fracture efficacy of alendronate or etidronate at the hip, lumbar spine, and forearm [91]. Furthermore, this study concluded that risk of hip fracture with these treatments was similar in type 1 diabetes, type 2 diabetes, and non-diabetic patients [91]. In osteoporotic Japanese women with diabetes in 3 phase III trials, risedronate treatment showed similar responses on lumbar spine BMD and bone markers between diabetic and non-diabetic patients [92]. There are no data regarding IV bisphosphonates (ibandronate, zoledronic acid) in diabetic patients; renal impairment may limit the utility of these therapies in diabetics. Data are not currently available regarding the anti-fracture efficacy of denosumab or the effects of discontinuation in those with diabetes. Considering that anti-resorptive treatments decrease bone turnover and increase the degree of mineralization, their effects on whole bone strength and fracture risk without low BMD remain to be ascertained.
In the MORE trial, univariate analysis showed a higher efficacy of raloxifene in reducing vertebral fracture risk in diabetic women compared to those without diabetes (p = 0.04) [93]. Anti-fracture efficacy of raloxifene was similar between patients with and without diabetes in the RUTH (Raloxifene Use for The Heart) trial and in a Danish cohort [91, 94].
Post hoc analyses of the DANCE study (Direct Analysis of Non-vertebral Fractures in the Community Experience) assessed the effects of teriparatide (20 μg/d SQ up to 24 months) on skeletal outcomes in patients with and without type 2 diabetes. Teriparatide treatment had a similar effect in diabetic vs non-diabetic persons on vertebral and total hip BMD. Interestingly, the effect on femoral neck was greater in the diabetic treated patients compared to those without diabetes. Incidence of non-vertebral fracture at 6 months was similar in both groups [95]. Nevertheless, because complicated diabetes could be associated with cortical porosity and teriparatide has been reported to increase cortical porosity [98], the effects of teriparatide on bone strength and fracture risk in severe diabetics remain to be specifically evaluated.
New and future osteoporosis medications
Abaloparatide may have potential in the treatment of bone fragility in diabetes as it can stimulate bone formation with a lesser increase in bone resorption. Romosozumab, an anti-sclerostin antibody, is currently under investigation as a new anabolic treatment [99] and has been shown to enhance bone mass and strength in diabetic animals [100]. Whether it could improve bone health in diabetics is of great interest. Recent signals of increased cardiovascular risk compared to alendronate raise safety concerns, especially in diabetic populations [101].
The above results obtained from observational studies and post hoc analyses are promising but ideally, the efficacy of osteoporotic treatments in diabetic patients should be demonstrated in prospective RCTs specifically recruiting patients with diabetes and fragility fractures or high fracture risk.
Management of bone fragility in adults with diabetes
Criteria to establish a diagnosis of osteoporosis are based on the presence of fragility fracture and/or a low BMD. These strict diagnostic criteria have to be differentiated from treatment thresholds. Since prior fracture predicts risk for future fracture as strongly in diabetic as in non-diabetic patients [15], treatment should be initiated when a patient with diabetes meets the intervention guidelines for the general population (Fig. 1). Otherwise, treatment should be considered at more favorable FRAX and BMD values in diabetic than in non-diabetic patients, as both BMD and FRAX may underestimate the risk of fracture in this population. Alternatively, FRAX estimates should be adjusted upwards in diabetics (see below).
Fig. 1.
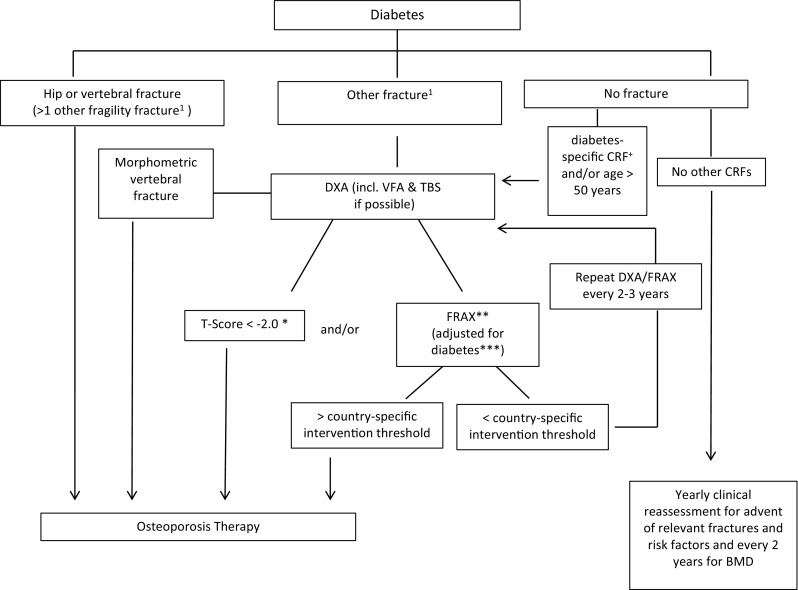
Fracture risk evaluation in patients with diabetes. * In diabetes, fracture risk at T-score < −2 equivalent for non-diabetes at T-score < −2.5 (see text). ** Depending on country-specific guidelines for therapies. *** For example, with TBS and/or “RA” – yes. + Diabetes-specific CRFs are listed in Table 3. 1In certain countries, humerus or pelvis fractures are also sufficient to initiate therapy; otherwise, more than non-vertebral non-hip fragility fracture could be required to initiate therapy; alternatively, a non-vertebral non-hip fragility fracture should prompt further exams to evaluate fracture risk
BMD intervention thresholds
If available, a BMD T-score < −2.5 at spine or hip in postmenopausal women and men over age 50 years confirms the diagnosis of osteoporosis and the need to consider pharmacotherapy, whether or not diabetes is present (Table 3). However, T-score BMD measured by DXA may underestimate fracture risk in patients with diabetes. Thus, a BMD intervention threshold at T-score − 2 at spine or hip could be considered appropriate (Fig. 1). Regrettably, this suggested adjustment and absolute cut-off although possibly appropriate in western populations may not be applicable to populations from Asia and the Middle East, where both age- and gender-adjusted BMD and fracture rates are lower than that in western counterparts but has not been shown specifically in the diabetes population.
Table 3.
Risk factors for fractures in diabetes
| Common risk factors | |
| FRAX CRF* | |
| Low BMD | |
| Recurrent falls | |
| Disease-specific risk factors | |
| Diabetes duration > 5 years | |
| Diabetes medication: insulin, TZDs, possibly SGLT2 inhibitors | |
| HbA1c > 7% | |
| Microvascular complications: peripheral and autonomic neuropathy, retinopathy, nephropathy |
CRF clinical risk factor, BMD bone mineral density, TZD thiazolidinedione, SGL2 sodium-glucose cotransporter 2, Hb1Ac glycated hemoglobin A1c
*Age, sex, weight, height, previous fracture, family history of hip fracture, current smoking, glucocorticoid, rheumatoid arthritis, alcohol, BMD
Moreover, diabetic patients with prominent BMD loss upon two consecutive measurements (i.e., > = 5% after 2 years) and when measurements are close to the intervention threshold might be considered for treatment (Fig. 1).
FRAX®
Conventional clinical risk factors (CRFs) can be employed to identify patients with diabetes at increased fracture risk (Table 3), although risk assessment tools like FRAX do not fully capture these increased risks and thus systematically underestimate the risk of osteoporosis-related fractures in patients with type 2 diabetes [45, 102]. Hence, for a given FRAX score, fracture risk was actually higher in type 2 diabetes patients compared to patients without type 2 diabetes [45]. Diabetes has been shown to be a significant predictor of subsequent major osteoporotic fracture even after correcting for those CRFs included in risk assessment tools like FRAX [102]. The TBS adjustment to FRAX will capture some of the excess fracture risk associated with type 2 diabetes [50, 103].
Since type 2 diabetes confers an increased risk of fracture that is independent of conventional CRFs, it has been proposed that type 2 diabetes be considered for inclusion in future iterations of FRAX [102]. It has been estimated that the fracture risk in diabetes calculated with FRAX is equivalent to adding 10 years of age or reducing the BMD T-score by 0.5 SD [45]. One option is to substitute rheumatoid arthritis (RA) with type 2 diabetes in FRAX. We are of the opinion that such a FRAX adjustment for type 2 diabetes can be clinically useful despite limitations, and we recommend that FRAX be employed to assess fracture risk in type 2 diabetes by substituting RA with type 2 diabetes [104] (Fig. 1).
General measures: lifestyle intervention
Lifestyle intervention is always recommended in patients with diabetes and it is the basis of any clinical guidelines. However, weight loss is associated with both muscle and bone loss that may increase the risk of bone fragility and sarcopenia [105]. Sarcopenia and sarcopenic-obesity are risk factors for falls, and frailty and should be prevented by an adequate protein intake and weight-bearing exercise [106, 107]. Physical activity helps to prevent bone loss during a weight loss program and is associated with decreased sclerostin [108] with improvement in quality of life [109] even in the elderly. Other non-pharmacological measures such as avoidance of smoking and limitation in alcohol intake (< 3 units per day) always remain important.
At diagnosis, serum 25-hydroxy-vitamin D levels have been found to be lower in patients with type 1 diabetes than in age-matched controls [110]. Lower levels of vitamin D are associated with type 2 diabetes as well [111], mostly in the obese and insulin-resistant states. Although the benefits of vitamin D supplementation on bone have not been demonstrated in diabetics, by analogy with the non-diabetic population a daily vitamin D intake of 800 IU/day may be recommended, although it may not be sufficient in type 2 diabetes and progressive higher doses could be required to achieve optimal serum levels (30 ng/ml). An adequate calcium intake (preferably from diet) (1000 mg/day) is recommended as well.
Glycemic control
A strong association between complications of diabetes and fracture risk has been documented [4, 7, 13]. The established higher propensity for falls in the individual with diabetes [14, 112, 113] probably also contributes to the increased fracture risk observed in this population. Peripheral neuropathy, retinopathy and any visual impairment, recent fall history, tendency to hypoglycemia, hypotension, and autonomic neuropathy should be noted and where possible corrected (Table 3).
Tight glycemic control (HbA1c 6.5–6.9%) was associated with the lowest risk of fracture in a large cohort of elderly patients with diabetes [114]. However, both hypoglycemia and hyperglycemia are associated with increased risk of fractures and falls [11], though probably via different mechanisms. Therefore, mostly in the elderly, a less stringent glycemic control in order to avoid risk of hypoglycemic events (and consequently of falls) has been proposed [115] and recently recommended by EASD/ADA guidelines [116].
Anti-diabetic treatments such as thiazolidinediones should be avoided in diabetics with bone fragility [27]. Canagliflozin, but not necessarily all SGLT2 inhibitors, should also probably be avoided in these patients [32]. Medications with a neutral or favorable effect on bone metabolism, such as metformin and incretin-based treatments, should be the preferred treatment [38, 40].
Osteoporosis treatment
At this time and in the absence of strong evidence against, bisphosphonates remain the first choice for osteoporosis treatment in diabetic patients. Although there are no specific data on the efficacy of denosumab in diabetic patients, this may be a preferred option in diabetic patients who are older and/or have a declining renal function. However, the use and potential benefit of anti-resorptive drugs in patients with type 2 diabetes characterized by near normal BMD and/or normal or low bone turnover markers, whose bone fragility may mostly result from poor bone material properties, remains unproven and of potential concern. In this context, teriparatide, and in the future abaloparatide or romosozumab, present a potential interest.
Conclusion
Patients with diabetes are at increased risk of fragility fractures. While the pathophysiology of bone fragility in these patients is not entirely clear, it is likely multifactorial. Longitudinal studies have established that FRAX and BMD T-score predict fracture risk in those with type 2 diabetes but both require adjustment for diabetes to avoid underestimation of risk. The optimal approach to management of patients with diabetes has not yet been established based on prospective clinical studies. Hence, our currently proposed algorithm should be considered as a consensus among some experts which may change over time as more evidence will be gathered. Data would suggest that if a patient has indication for therapy based on criteria developed for non-diabetes patients, these patients should be treated with osteoporosis drugs. In absence of established osteoporosis though, these medications may be used with caution though, as the effects of these drugs in situations where bone fragility is mainly due to alterations in bone quality remain to be thoroughly evaluated. Future studies should continue to evaluate the structural determinants (microstructure, material properties, …) of bone fragility and refine the fracture prediction algorithms by including disease-specific determinants of fracture (Table 3). New trials will have to prospectively investigate the efficacy and safety of osteoporosis treatment in diabetics with and without low aBMD.
Acknowledgements
We are grateful to the Committee of Scientific Advisors of the International Osteoporosis Foundation for their review and endorsement of this paper. This study was supported by an unrestricted grant from MSD. The sponsor did not have any role in preparation, review or approval of the manuscript.
Bone and Diabetes Working Group of IOF
SL Ferrari, B Abrahamsen, K Akesson, MSM Ardawi, M Chandran, C Cooper, R Eastell, G El-Hajj Fuleihan, R Josse, DL Kendler, M Kraenzlin, WD Leslie, A Mithal, N Napoli, A Suzuki, AV Schwartz.
Compliance with ethical standards
Conflict of interest
S Ferrari has received research grants, honoraria, and/or consultancies from Amgen, UCB, MSD, Labatec, Agnovos. B Abrahamsen has received research contracts with Novartis and UCB with funds paid to the institution. D Kendler has received research grants, honoraria, and/or consultancies from Amgen, Eli Lilly, AstraZeneca, Pfizer. R Eastell has received research grants from Alexion, Amgen Inc., and Ultragenyx, and consulting fees from Immunodiagnostic Systems, GlaxoSmithKline, and Amgen Inc. A Suzuki has received research grants and/or honoraria from Astellas, Chugai, Daiichi-Sankyo, Kyowa-Hakko Kirin, MSD, Novo Nordisk, Ono, Pfizer, Taisho Toyama, Tanabe-Mitsubishi and Takeda. R Josse has received consultancy fees and speaker honoraria from Amgen, Lilly, Merck. K Akesson has received lecture fees or consultancies from MSD, UCB, Amgen, Eli Lilly and Sandoz. A Schwartz has received a research grant from Hologic and consulting fees from Amgen. N Napoli has received consulting fees from Lilly and Amgen. G El-Hajj Fulehain, M Chandran, DD Pierroz, M Kraenzlin have no disclosure.
Contributor Information
S.L. Ferrari, Email: serge.ferrari@unige.ch
on behalf of the Bone and Diabetes Working Group of IOF:
S.L. Ferrari, B. Abrahamsen, K. Akesson, M.S.M. Ardawi, M. Chandran, C. Cooper, R. Eastell, G. El-Hajj Fuleihan, R. Josse, D.L. Kendler, M. Kraenzlin, W.D. Leslie, A. Mithal, N. Napoli, A. Suzuki, and A.V. Schwartz
References
- 1.International Diabetes Federation (2017) IDF diabetes atlas - Eighth Edition
- 2.Shah VN, Shah CS, Snell-Bergeon JK. Type 1 diabetes and risk of fracture: meta-analysis and review of the literature. Diabet Med. 2015;32:1134–1142. doi: 10.1111/dme.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber DR, Haynes K, Leonard MB, Willi SM, Denburg MR. Type 1 diabetes is associated with an increased risk of fracture across the life span: a population-based cohort study using The Health Improvement Network (THIN) Diabetes Care. 2015;38:1913–1920. doi: 10.2337/dc15-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48:1292–1299. doi: 10.1007/s00125-005-1786-3. [DOI] [PubMed] [Google Scholar]
- 5.Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, Robbins J, Rodriguez BL, Johnson KC, Margolis KL. Risk of fracture in women with type 2 diabetes: the Women's Health Initiative Observational Study. J Clin Endocrinol Metab. 2006;91:3404–3410. doi: 10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- 6.Fraser LA, Pritchard J, Ioannidis G, Giangegorio LM, Adachi JD, Papaioannou A, Leslie WD. Clinical risk factors for fracture in diabetes: a matched cohort analysis. J Clin Densitom. 2011;14:416–421. doi: 10.1016/j.jocd.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oei L, Zillikens MC, Dehghan A, Buitendijk GHS, Castano-Betancourt MC, Estrada K, Stolk L, Oei EHG, van Meurs JBJ, Janssen JAMJL, Hofman A, van Leeuwen JPTM, Witteman JCM, Pols HAP, Uitterlinden AG, Klaver CCW, Franco OH, Rivadeneira F. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the Rotterdam Study. Diabetes Care. 2013;36:1619–1628. doi: 10.2337/dc12-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmberg AH, Johnell O, Nilsson PM, Nilsson J, Berglund G, Akesson K. Risk factors for fragility fracture in middle age. A prospective population-based study of 33,000 men and women. Osteoporos Int. 2006;17:1065–1077. doi: 10.1007/s00198-006-0137-7. [DOI] [PubMed] [Google Scholar]
- 9.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166:495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- 10.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta-analysis. Osteoporos Int. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 11.Johnston SS, Conner C, Aagren M, Ruiz K, Bouchard J. Association between hypoglycaemic events and fall-related fractures in Medicare-covered patients with type 2 diabetes. Diabetes Obes Metab. 2012;14:634–643. doi: 10.1111/j.1463-1326.2012.01583.x. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain JJ, Kalyani RR, Leal S, Rhinehart AS, Shubrook JH, Skolnik N, Herman WH. Treatment of type 1 diabetes: synopsis of the 2017 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med. 2017;167:493–498. doi: 10.7326/M17-1259. [DOI] [PubMed] [Google Scholar]
- 13.de Liefde I, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int. 2005;16:1713–1720. doi: 10.1007/s00198-005-1909-1. [DOI] [PubMed] [Google Scholar]
- 14.Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Bauer DC, Tylavsky FA, de Rekeneire N, Harris TB, Newman AB. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Intern Med. 2005;165:1612–1617. doi: 10.1001/archinte.165.14.1612. [DOI] [PubMed] [Google Scholar]
- 15.Leslie WD, Morin SN, Lix LM, Majumdar SR. Does diabetes modify the effect of FRAX risk factors for predicting major osteoporotic and hip fracture? Osteoporos Int. 2014;25:2817–2824. doi: 10.1007/s00198-014-2822-2. [DOI] [PubMed] [Google Scholar]
- 16.Kanis JA. Assessment of osteoporosis at the primary health care level. World Health Organization Scientific Group. WHO Collaborating Centre for Metabolic Bone Diseases: University of Sheffield; 2008. [Google Scholar]
- 17.Ivers RQ, Cumming RG, Mitchell P, Peduto AJ. Diabetes and risk of fracture: The Blue Mountains Eye Study. Diabetes Care. 2001;24:1198–1203. doi: 10.2337/diacare.24.7.1198. [DOI] [PubMed] [Google Scholar]
- 18.Leslie WD, Lix LM, Prior HJ, Derksen S, Metge C, O'Neil J. Biphasic fracture risk in diabetes: a population-based study. Bone. 2007;40:1595–1601. doi: 10.1016/j.bone.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Majumdar SR, Leslie WD, Lix LM, Morin SN, Johansson H, Oden A, McCloskey EV, Kanis JA. Longer duration of diabetes strongly impacts fracture risk assessment: The Manitoba BMD Cohort. J Clin Endocrinol Metab. 2016;101:4489–4496. doi: 10.1210/jc.2016-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider AL, Williams EK, Brancati FL, Blecker S, Coresh J, Selvin E. Diabetes and risk of fracture-related hospitalization: the Atherosclerosis Risk in Communities Study. Diabetes Care. 2013;36:1153–1158. doi: 10.2337/dc12-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li CI, Liu CS, Lin WY, Meng NH, Chen CC, Yang SY, Chen HJ, Lin CC, Li TC. Glycated hemoglobin level and risk of hip fracture in older people with type 2 diabetes: a competing risk analysis of Taiwan Diabetes Cohort Study. J Bone Miner Res. 2015;30:1338–1346. doi: 10.1002/jbmr.2462. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz AV, Margolis KL, Sellmeyer DE, Vittinghoff E, Ambrosius WT, Bonds DE, Josse RG, Schnall AM, Simmons DL, Hue TF, Palermo L, Hamilton BP, Green JB, Atkinson HH, O'Connor PJ, Force RW, Bauer DC. Intensive glycemic control is not associated with fractures or falls in the ACCORD randomized trial. Diabetes Care. 2012;35:1525–1531. doi: 10.2337/dc11-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu F, Jiang C, Shen J, Tang P, Wang Y. Preoperative predictors for mortality following hip fracture surgery: a systematic review and meta-analysis. Injury. 2012;43:676–685. doi: 10.1016/j.injury.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Huang YF, Shyu YI, Liang J, Chen MC, Cheng HS, Wu CC. Diabetes and health outcomes among older Taiwanese with hip fracture. Rejuvenation Res. 2012;15:476–482. doi: 10.1089/rej.2011.1308. [DOI] [PubMed] [Google Scholar]
- 25.Shanbhogue VV, Hansen S, Frost M, Jorgensen NR, Hermann AP, Henriksen JE, Brixen K. Compromised cortical bone compartment in type 2 diabetes mellitus patients with microvascular disease. Eur J Endocrinol. 2016;174:115–124. doi: 10.1530/EJE-15-0860. [DOI] [PubMed] [Google Scholar]
- 26.Napoli N, Strotmeyer ES, Ensrud KE, Sellmeyer DE, Bauer DC, Hoffman AR, Dam TTL, Barrett-Connor E, Palermo L, Orwoll ES, Cummings SR, Black DM, Schwartz AV. Fracture risk in diabetic elderly men: the MrOS study. Diabetologia. 2014;57:2057–2065. doi: 10.1007/s00125-014-3289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu ZN, Jiang YF, Ding T. Risk of fracture with thiazolidinediones: an updated meta-analysis of randomized clinical trials. Bone. 2014;68:115–123. doi: 10.1016/j.bone.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ. 2009;180:32–39. doi: 10.1503/cmaj.080486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su B, Sheng H, Zhang M, Bu L, Yang P, Li L, Li F, Sheng C, Han Y, Qu S, Wang J. Risk of bone fractures associated with glucagon-like peptide-1 receptor agonists' treatment: a meta-analysis of randomized controlled trials. Endocrine. 2015;48:107–115. doi: 10.1007/s12020-014-0361-4. [DOI] [PubMed] [Google Scholar]
- 30.Monami M, Dicembrini I, Antenore A, Mannucci E. Dipeptidyl peptidase-4 inhibitors and bone fractures: a meta-analysis of randomized clinical trials. Diabetes Care. 2011;34:2474–2476. doi: 10.2337/dc11-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosenzon O, Wei C, Davidson J, Scirica BM, Yanuv I, Rozenberg A, Hirshberg B, Cahn A, Stahre C, Strojek K, Bhatt DL, Raz I. Incidence of fractures in patients with type 2 diabetes in the SAVOR-TIMI 53 Trial. Diabetes Care. 2015;38:2142–2150. doi: 10.2337/dc15-1068. [DOI] [PubMed] [Google Scholar]
- 32.Watts NB, Bilezikian JP, Usiskin K, Edwards R, Desai M, Law G, Meininger G. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101:157–166. doi: 10.1210/jc.2015-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ljunggren O, Bolinder J, Johansson L, Wilding J, Langkilde AM, Sjostrom CD, Sugg J, Parikh S. Dapagliflozin has no effect on markers of bone formation and resorption or bone mineral density in patients with inadequately controlled type 2 diabetes mellitus on metformin. Diabetes Obes Metab. 2012;14:990–999. doi: 10.1111/j.1463-1326.2012.01630.x. [DOI] [PubMed] [Google Scholar]
- 34.Bilezikian JP, Watts NB, Usiskin K, Polidori D, Fung A, Sullivan D, Rosenthal N. Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J Clin Endocrinol Metab. 2016;101:44–51. doi: 10.1210/jc.2015-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monami M, Cresci B, Colombini A, Pala L, Balzi D, Gori F, Chiasserini V, Marchionni N, Rotella CM, Mannucci E. Bone fractures and hypoglycemic treatment in type 2 diabetic patients: a case-control study. Diabetes Care. 2008;31:199–203. doi: 10.2337/dc07-1736. [DOI] [PubMed] [Google Scholar]
- 36.Kachroo S, Kawabata H, Colilla S, Shi L, Zhao Y, Mukherjee J, Iloeje U, Fonseca V. Association between hypoglycemia and fall-related events in type 2 diabetes mellitus: analysis of a U.S. commercial database. J Manag Care Spec Pharm. 2015;21:243–253. doi: 10.18553/jmcp.2015.21.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallander M, Axelsson KF, Nilsson AG, Lundh D, Lorentzon M. Type 2 diabetes and risk of hip fractures and non-skeletal fall injuries in the elderly: a study from the Fractures and Fall Injuries in the Elderly Cohort (FRAILCO) J Bone Miner Res. 2017;32:449–460. doi: 10.1002/jbmr.3002. [DOI] [PubMed] [Google Scholar]
- 38.Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL, IOF Bone Diabetes Working G Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13:208–219. doi: 10.1038/nrendo.2016.153. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz AV, Vittinghoff E, Sellmeyer DE, Feingold KR, Rekeneire N, Strotmeyer ES, Shorr RI, Vinik AI, Odden MC, Park SW, Faulkner KA, Harris TB, for the Health, Aging, and Body Composition Study Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care. 2008;31:391–396. doi: 10.2337/dc07-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palermo A, D'Onofrio L, Eastell R, Schwartz AV, Pozzilli P, Napoli N. Oral anti-diabetic drugs and fracture risk, cut to the bone: safe or dangerous? A narrative review. Osteoporos Int. 2015;26:2073–2089. doi: 10.1007/s00198-015-3123-0. [DOI] [PubMed] [Google Scholar]
- 41.Ruanpeng Darin, Ungprasert Patompong, Sangtian Jutarat, Harindhanavudhi Tasma. Sodium-glucose cotransporter 2 (SGLT2) inhibitors and fracture risk in patients with type 2 diabetes mellitus: A meta-analysis. Diabetes/Metabolism Research and Reviews. 2017;33(6):e2903. doi: 10.1002/dmrr.2903. [DOI] [PubMed] [Google Scholar]
- 42.Hough FS, Pierroz DD, Cooper C, Ferrari SL, IOF CSA Bone and Diabetes Working Group Mechanisms in endocrinology: mechanisms and evaluation of bone fragility in type 1 diabetes mellitus. Eur J Endocrinol. 2016;174:R127–R138. doi: 10.1530/EJE-15-0820. [DOI] [PubMed] [Google Scholar]
- 43.Ma L, Oei L, Jiang L, Estrada K, Chen H, Wang Z, Yu Q, Zillikens MC, Gao X, Rivadeneira F. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27:319–332. doi: 10.1007/s10654-012-9674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmberg AH, Nilsson PM, Nilsson JA, Akesson K. The association between hyperglycemia and fracture risk in middle age. A prospective, population-based study of 22,444 men and 10,902 women. J Clin Endocrinol Metab. 2008;93:815–822. doi: 10.1210/jc.2007-0843. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE, Donaldson MG, Cauley JA, Harris TB, Koster A, Womack CR, Palermo L, Black DM, Study of Osteoporotic Fractures (SOF) Research Group. Osteoporotic Fractures in Men (MrOS) Research Group. Health, Aging, and Body Composition (Health ABC) Research Group Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305:2184–2192. doi: 10.1001/jama.2011.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schacter GI, Leslie WD. DXA-based measurements in diabetes: can they predict fracture risk? Calcif Tissue Int. 2017;100:150–164. doi: 10.1007/s00223-016-0191-x. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz AV, Ewing SK, Porzig AM, et al. Diabetes and change in bone mineral density at the hip, calcaneus, spine, and radius in older women. Front Endocrinol (Lausanne) 2013;4:62. doi: 10.3389/fendo.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leslie WD, Morin SN, Majumdar SR, Lix LM. Effects of obesity and diabetes on rate of bone density loss. Osteoporos Int. 2018;29:61–67. doi: 10.1007/s00198-017-4223-9. [DOI] [PubMed] [Google Scholar]
- 49.Caffarelli C, Giambelluca A, Ghini V, Francolini V, Pitinca MDT, Nuti R, Gonnelli S. In Type-2 diabetes subjects trabecular bone score is better associated with carotid intima-media thickness than BMD. Calcif Tissue Int. 2017;101:404–411. doi: 10.1007/s00223-017-0297-9. [DOI] [PubMed] [Google Scholar]
- 50.Leslie WD, Aubry-Rozier B, Lamy O, Hans D, Manitoba Bone Density P. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602–609. doi: 10.1210/jc.2012-3118. [DOI] [PubMed] [Google Scholar]
- 51.Mazzetti G, Berger C, Leslie WD, Hans D, Langsetmo L, Hanley DA, Kovacs CS, Prior JC, Kaiser SM, Davison KS, Josse R, Papaioannou A, Adachi JR, Goltzman D, Morin SN, CaMos Research Group Densitometer-specific differences in the correlation between body mass index and lumbar spine trabecular bone score. J Clin Densitom. 2017;20:233–238. doi: 10.1016/j.jocd.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Tao B, Liu JM, Zhao HY, Sun LH, Wang WQ, Li XY, Ning G. Differences between measurements of bone mineral densities by quantitative ultrasound and dual-energy X-ray absorptiometry in type 2 diabetic postmenopausal women. J Clin Endocrinol Metab. 2008;93:1670–1675. doi: 10.1210/jc.2007-1760. [DOI] [PubMed] [Google Scholar]
- 53.Yamaguchi T, Yamamoto M, Kanazawa I, Yamauchi M, Yano S, Tanaka N, Nitta E, Fukuma A, Uno S, Sho-no T, Sugimoto T. Quantitative ultrasound and vertebral fractures in patients with type 2 diabetes. J Bone Miner Metab. 2011;29:626–632. doi: 10.1007/s00774-011-0265-9. [DOI] [PubMed] [Google Scholar]
- 54.Pritchard JM, Giangregorio LM, Atkinson SA, Beattie KA, Inglis D, Ioannidis G, Punthakee Z, Adachi JD, Papaioannou A. Association of larger holes in the trabecular bone at the distal radius in postmenopausal women with type 2 diabetes mellitus compared to controls. Arthritis Care Res (Hoboken) 2012;64:83–91. doi: 10.1002/acr.20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burghardt AJ, Kazakia GJ, Sode M, de Papp AE, Link TM, Majumdar S. A longitudinal HR-pQCT study of alendronate treatment in postmenopausal women with low bone density: relations among density, cortical and trabecular microarchitecture, biomechanics, and bone turnover. J Bone Miner Res. 2010;25:2558–2571. doi: 10.1002/jbmr.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patsch JM, Burghardt AJ, Yap SP, Baum T, Schwartz AV, Joseph GB, Link TM. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res. 2013;28:313–324. doi: 10.1002/jbmr.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Compston JE, Flahive J, Hosmer DW, Watts NB, Siris ES, Silverman S, Saag KG, Roux C, Rossini M, Pfeilschifter J, Nieves JW, Netelenbos JC, March L, LaCroix AZ, Hooven FH, Greenspan SL, Gehlbach SH, Díez-Pérez A, Cooper C, Chapurlat RD, Boonen S, Anderson FA, Jr, Adami S, Adachi JD, for the GLOW Investigators Relationship of weight, height, and body mass index with fracture risk at different sites in postmenopausal women: the Global Longitudinal study of Osteoporosis in Women (GLOW) J Bone Miner Res. 2014;29:487–493. doi: 10.1002/jbmr.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nilsson AG, Sundh D, Johansson L, Nilsson M, Mellstrom D, Rudang R, Zoulakis M, Wallander M, Darelid A, Lorentzon M. Type 2 diabetes mellitus is associated with better bone microarchitecture but lower bone material strength and poorer physical function in elderly women: a population-based study. J Bone Miner Res. 2017;32:1062–1071. doi: 10.1002/jbmr.3057. [DOI] [PubMed] [Google Scholar]
- 59.Bala Y, Bui QM, Wang XF, Iuliano S, Wang Q, Ghasem-Zadeh A, Rozental TD, Bouxsein ML, Zebaze RM, Seeman E. Trabecular and cortical microstructure and fragility of the distal radius in women. J Bone Miner Res. 2015;30:621–629. doi: 10.1002/jbmr.2388. [DOI] [PubMed] [Google Scholar]
- 60.Yu EW, Putman MS, Derrico N, Abrishamanian-Garcia G, Finkelstein JS, Bouxsein ML. Defects in cortical microarchitecture among African-American women with type 2 diabetes. Osteoporos Int. 2015;26:673–679. doi: 10.1007/s00198-014-2927-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heilmeier U, Cheng K, Pasco C, Parrish R, Nirody J, Patsch JM, Zhang CA, Joseph GB, Burghardt AJ, Schwartz AV, Link TM, Kazakia G. Cortical bone laminar analysis reveals increased midcortical and periosteal porosity in type 2 diabetic postmenopausal women with history of fragility fractures compared to fracture-free diabetics. Osteoporos Int. 2016;27:2791–2802. doi: 10.1007/s00198-016-3614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Furst JR, Bandeira LC, Fan WW, Agarwal S, Nishiyama KK, McMahon DJ, Dworakowski E, Jiang H, Silverberg SJ, Rubin MR. Advanced glycation endproducts and bone material strength in type 2 diabetes. J Clin Endocrinol Metab. 2016;101:2502–2510. doi: 10.1210/jc.2016-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamaguchi T, Sugimoto T. Bone metabolism and fracture risk in type 2 diabetes mellitus. Bonekey Rep. 2012;1:36. doi: 10.1038/bonekey.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferrari S. Diabetes and bone. Calcif Tissue Int. 2017;100:107–108. doi: 10.1007/s00223-017-0234-y. [DOI] [PubMed] [Google Scholar]
- 65.Manavalan JS, Cremers S, Dempster DW, Zhou H, Dworakowski E, Kode A, Kousteni S, Rubin MR. Circulating osteogenic precursor cells in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97:3240–3250. doi: 10.1210/jc.2012-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. Bone loss and bone turnover in diabetes. Diabetes. 1995;44:775–782. doi: 10.2337/diab.44.7.775. [DOI] [PubMed] [Google Scholar]
- 67.Starup-Linde J. Diabetes, biochemical markers of bone turnover, diabetes control, and bone. Front Endocrinol (Lausanne) 2013;4:21. doi: 10.3389/fendo.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reyes-Garcia R, Rozas-Moreno P, Lopez-Gallardo G, Garcia-Martin A, Varsavsky M, Aviles-Perez MD, Munoz-Torres M. Serum levels of bone resorption markers are decreased in patients with type 2 diabetes. Acta Diabetol. 2013;50:47–52. doi: 10.1007/s00592-011-0347-0. [DOI] [PubMed] [Google Scholar]
- 69.Shu A, Yin MT, Stein E, Cremers S, Dworakowski E, Ives R, Rubin MR. Bone structure and turnover in type 2 diabetes mellitus. Osteoporos Int. 2012;23:635–641. doi: 10.1007/s00198-011-1595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanazawa I, Yamaguchi T, Yamauchi M, Yamamoto M, Kurioka S, Yano S, Sugimoto T. Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int. 2011;22:187–194. doi: 10.1007/s00198-010-1184-7. [DOI] [PubMed] [Google Scholar]
- 71.Zhou Y, Li Y, Zhang D, Wang J, Yang H. Prevalence and predictors of osteopenia and osteoporosis in postmenopausal Chinese women with type 2 diabetes. Diabetes Res Clin Pract. 2010;90:261–269. doi: 10.1016/j.diabres.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 72.Vashishth D. The role of the collagen matrix in skeletal fragility. Curr Osteoporos Rep. 2007;5:62–66. doi: 10.1007/s11914-007-0004-2. [DOI] [PubMed] [Google Scholar]
- 73.Viguet-Carrin S, Roux JP, Arlot ME, Merabet Z, Leeming DJ, Byrjalsen I, Delmas PD, Bouxsein ML. Contribution of the advanced glycation end product pentosidine and of maturation of type I collagen to compressive biomechanical properties of human lumbar vertebrae. Bone. 2006;39:1073–1079. doi: 10.1016/j.bone.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 74.Saito M, Fujii K, Mori Y, Marumo K. Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int. 2006;17:1514–1523. doi: 10.1007/s00198-006-0155-5. [DOI] [PubMed] [Google Scholar]
- 75.Kerkeni M, Saidi A, Bouzidi H, Ben Yahya S, Hammami M. Elevated serum levels of AGEs, sRAGE, and pentosidine in Tunisian patients with severity of diabetic retinopathy. Microvasc Res. 2012;84:378–383. doi: 10.1016/j.mvr.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 76.Ng ZX, Chua KH, Iqbal T, Kuppusamy UR. Soluble receptor for advanced glycation end-product (sRAGE)/pentosidine ratio: a potential risk factor determinant for type 2 diabetic retinopathy. Int J Mol Sci. 2013;14:7480–7491. doi: 10.3390/ijms14047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:1013–1019. doi: 10.1210/jc.2007-1270. [DOI] [PubMed] [Google Scholar]
- 78.Schwartz AV, Garnero P, Hillier TA, Sellmeyer DE, Strotmeyer ES, Feingold KR, Resnick HE, Tylavsky FA, Black DM, Cummings SR, Harris TB, Bauer DC, Health, Aging, and Body Composition Study Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:2380–2386. doi: 10.1210/jc.2008-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tanaka S, Kuroda T, Saito M, Shiraki M. Urinary pentosidine improves risk classification using fracture risk assessment tools for postmenopausal women. J Bone Miner Res. 2011;26:2778–2784. doi: 10.1002/jbmr.467. [DOI] [PubMed] [Google Scholar]
- 80.Yamamoto M, Yamaguchi T, Yamauchi M, Sugimoto T. Low serum level of the endogenous secretory receptor for advanced glycation end products (esRAGE) is a risk factor for prevalent vertebral fractures independent of bone mineral density in patients with type 2 diabetes. Diabetes Care. 2009;32:2263–2268. doi: 10.2337/dc09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gennari L, Merlotti D, Valenti R, Ceccarelli E, Ruvio M, Pietrini MG, Capodarca C, Franci MB, Campagna MS, Calabrò A, Cataldo D, Stolakis K, Dotta F, Nuti R. Circulating sclerostin levels and bone turnover in type 1 and type 2 diabetes. J Clin Endocrinol Metab. 2012;97:1737–1744. doi: 10.1210/jc.2011-2958. [DOI] [PubMed] [Google Scholar]
- 82.Ardawi MS, Akhbar DH, Alshaikh A, Ahmed MM, Qari MH, Rouzi AA, Ali AY, Abdulrafee AA, Saeda MY. Increased serum sclerostin and decreased serum IGF-1 are associated with vertebral fractures among postmenopausal women with type-2 diabetes. Bone. 2013;56:355–362. doi: 10.1016/j.bone.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 83.Yamamoto M, Yamauchi M, Sugimoto T. Elevated sclerostin levels are associated with vertebral fractures in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013;98:4030–4037. doi: 10.1210/jc.2013-2143. [DOI] [PubMed] [Google Scholar]
- 84.Heilmeier U, Carpenter DR, Patsch JM, Harnish R, Joseph GB, Burghardt AJ, Baum T, Schwartz AV, Lang TF, Link TM. Volumetric femoral BMD, bone geometry, and serum sclerostin levels differ between type 2 diabetic postmenopausal women with and without fragility fractures. Osteoporos Int. 2015;26:1283–1293. doi: 10.1007/s00198-014-2988-7. [DOI] [PubMed] [Google Scholar]
- 85.Starup-Linde J, Lykkeboe S, Gregersen S, Hauge EM, Langdahl BL, Handberg A, Vestergaard P. Bone structure and predictors of fracture in type 1 and type 2 diabetes. J Clin Endocrinol Metab. 2016;101:928–936. doi: 10.1210/jc.2015-3882. [DOI] [PubMed] [Google Scholar]
- 86.Morales-Santana S, Garcia-Fontana B, Garcia-Martin A, Rozas-Moreno P, Garcia-Salcedo JA, Reyes-Garcia R, Munoz-Torres M. Atherosclerotic disease in type 2 diabetes is associated with an increase in sclerostin levels. Diabetes Care. 2013;36:1667–1674. doi: 10.2337/dc12-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pepe J, Bonnet N, Herrmann FR, Biver E, Rizzoli R, Chevalley T, Ferrari SL. Interaction between LRP5 and periostin gene polymorphisms on serum periostin levels and cortical bone microstructure. Osteoporos Int. 2018;29:339–346. doi: 10.1007/s00198-017-4272-0. [DOI] [PubMed] [Google Scholar]
- 88.Heilmeier U, Hackl M, Skalicky S, Weilner S, Schroeder F, Vierlinger K, Patsch JM, Baum T, Oberbauer E, Lobach I, Burghardt AJ, Schwartz AV, Grillari J, Link TM. Serum miRNA signatures are indicative of skeletal fractures in postmenopausal women with and without type 2 diabetes and influence osteogenic and Adipogenic differentiation of adipose tissue-derived mesenchymal stem cells in vitro. J Bone Miner Res. 2016;31:2173–2192. doi: 10.1002/jbmr.2897. [DOI] [PubMed] [Google Scholar]
- 89.Keegan T. H.M., Schwartz A. V., Bauer D. C., Sellmeyer D. E., Kelsey J. L. Effect of Alendronate on Bone Mineral Density and Biochemical Markers of Bone Turnover in Type 2 Diabetic Women: The Fracture Intervention Trial. Diabetes Care. 2004;27(7):1547–1553. doi: 10.2337/diacare.27.7.1547. [DOI] [PubMed] [Google Scholar]
- 90.Abrahamsen B, Rubin KH, Eiken PA, Eastell R. Characteristics of patients who suffer major osteoporotic fractures despite adhering to alendronate treatment: a National Prescription registry study. Osteoporos Int. 2013;24:321–328. doi: 10.1007/s00198-012-2184-6. [DOI] [PubMed] [Google Scholar]
- 91.Vestergaard P, Rejnmark L, Mosekilde L. Are antiresorptive drugs effective against fractures in patients with diabetes? Calcif Tissue Int. 2011;88:209–214. doi: 10.1007/s00223-010-9450-4. [DOI] [PubMed] [Google Scholar]
- 92.Inoue D, Muraoka R, Okazaki R, Nishizawa Y, Sugimoto T. Efficacy and safety of Risedronate in osteoporosis subjects with comorbid diabetes, hypertension, and/or dyslipidemia: a post hoc analysis of phase III trials conducted in Japan. Calcif Tissue Int. 2016;98:114–122. doi: 10.1007/s00223-015-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnell O, Kanis JA, Black DM, Balogh A, Poor G, Sarkar S, Zhou C, Pavo I. Associations between baseline risk factors and vertebral fracture risk in the Multiple Outcomes of Raloxifene Evaluation (MORE) Study. J Bone Miner Res. 2004;19:764–772. doi: 10.1359/JBMR.040211. [DOI] [PubMed] [Google Scholar]
- 94.Ensrud KE, Stock JL, Barrett-Connor E, Grady D, Mosca L, Khaw KT, Zhao Q, Agnusdei D, Cauley JA. Effects of raloxifene on fracture risk in postmenopausal women: the Raloxifene Use for the Heart Trial. J Bone Miner Res. 2008;23:112–120. doi: 10.1359/jbmr.070904. [DOI] [PubMed] [Google Scholar]
- 95.Schwartz AV, Pavo I, Alam J, Disch DP, Schuster D, Harris JM, Krege JH. Teriparatide in patients with osteoporosis and type 2 diabetes. Bone. 2016;91:152–158. doi: 10.1016/j.bone.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 96.Dagdelen S, Sener D, Bayraktar M. Influence of type 2 diabetes mellitus on bone mineral density response to bisphosphonates in late postmenopausal osteoporosis. Adv Ther. 2007;24:1314–1320. doi: 10.1007/BF02877778. [DOI] [PubMed] [Google Scholar]
- 97.Iwamoto J, Sato Y, Uzawa M, Takeda T, Matsumoto H. Three-year experience with alendronate treatment in postmenopausal osteoporotic Japanese women with or without type 2 diabetes. Diabetes Res Clin Pract. 2011;93:166–173. doi: 10.1016/j.diabres.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 98.Hansen S, Hauge EM, Beck Jensen JE, Brixen K. Differing effects of PTH 1-34, PTH 1-84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQCT. J Bone Miner Res. 2013;28:736–745. doi: 10.1002/jbmr.1784. [DOI] [PubMed] [Google Scholar]
- 99.Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, Zerbini CAF, Milmont CE, Chen L, Maddox J, Meisner PD, Libanati C, Grauer A. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 100.Hamann C, Rauner M, Hohna Y, et al. Sclerostin antibody treatment improves bone mass, bone strength, and bone defect regeneration in rats with type 2 diabetes mellitus. J Bone Miner Res. 2013;28:627–638. doi: 10.1002/jbmr.1803. [DOI] [PubMed] [Google Scholar]
- 101.Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417–1427. doi: 10.1056/NEJMoa1708322. [DOI] [PubMed] [Google Scholar]
- 102.Giangregorio LM, Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA. FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res. 2012;27:301–308. doi: 10.1002/jbmr.556. [DOI] [PubMed] [Google Scholar]
- 103.McCloskey EV, Oden A, Harvey NC, Leslie WD, Hans D, Johansson H, Kanis JA. Adjusting fracture probability by trabecular bone score. Calcif Tissue Int. 2015;96:500–509. doi: 10.1007/s00223-015-9980-x. [DOI] [PubMed] [Google Scholar]
- 104.Leslie WD, Rubin MR, Schwartz AV, Kanis JA. Type 2 diabetes and bone. J Bone Miner Res. 2012;27:2231–2237. doi: 10.1002/jbmr.1759. [DOI] [PubMed] [Google Scholar]
- 105.Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Armamento-Villareal R, Aguirre L, Napoli N, Shah K, Hilton T, Sinacore DR, Qualls C, Villareal DT. Changes in thigh muscle volume predict bone mineral density response to lifestyle therapy in frail, obese older adults. Osteoporos Int. 2014;25:551–558. doi: 10.1007/s00198-013-2450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scott D, Seibel M, Cumming R, Naganathan V, Blyth F, Le Couteur DG, Handelsman DJ, Waite LM, Hirani V. Sarcopenic obesity and its temporal associations with changes in bone mineral density, incident falls, and fractures in older men: The Concord Health and Ageing in Men Project. J Bone Miner Res. 2017;32:575–583. doi: 10.1002/jbmr.3016. [DOI] [PubMed] [Google Scholar]
- 108.Armamento-Villareal R, Sadler C, Napoli N, Shah K, Chode S, Sinacore DR, Qualls C, Villareal DT. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res. 2012;27:1215–1221. doi: 10.1002/jbmr.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Napoli N, Shah K, Waters DL, Sinacore DR, Qualls C, Villareal DT. Effect of weight loss, exercise, or both on cognition and quality of life in obese older adults. Am J Clin Nutr. 2014;100:189–198. doi: 10.3945/ajcn.113.082883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pozzilli P., Manfrini S., Crinò A., Picardi A., Leomanni C., Cherubini V., Valente L., Khazrai M., Visalli N. Low Levels of 25-hydroxyvitamin D3and 1,25-dihydroxyvitamin D3in Patients with Newly Diagnosed Type 1 Diabetes. Hormone and Metabolic Research. 2005;37(11):680–683. doi: 10.1055/s-2005-870578. [DOI] [PubMed] [Google Scholar]
- 111.Hurskainen AR, Virtanen JK, Tuomainen TP, Nurmi T, Voutilainen S. Association of serum 25-hydroxyvitamin D with type 2 diabetes and markers of insulin resistance in a general older population in Finland. Diabetes Metab Res Rev. 2012;28:418–423. doi: 10.1002/dmrr.2286. [DOI] [PubMed] [Google Scholar]
- 112.Berlie HD, Garwood CL. Diabetes medications related to an increased risk of falls and fall-related morbidity in the elderly. Ann Pharmacother. 2010;44:712–717. doi: 10.1345/aph.1M551. [DOI] [PubMed] [Google Scholar]
- 113.Schwartz AV, Hillier TA, Sellmeyer DE, Resnick HE, Gregg E, Ensrud KE, Schreiner PJ, Margolis KL, Cauley JA, Nevitt MC, Black DM, Cummings SR. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care. 2002;25:1749–1754. doi: 10.2337/diacare.25.10.1749. [DOI] [PubMed] [Google Scholar]
- 114.Conway BN, Long DM, Figaro MK, May ME. Glycemic control and fracture risk in elderly patients with diabetes. Diabetes Res Clin Pract. 2016;115:47–53. doi: 10.1016/j.diabres.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pozzilli P, Leslie RD, Chan J, De Fronzo R, Monnier L, Raz I, Del Prato S. The A1C and ABCD of glycaemia management in type 2 diabetes: a physician's personalized approach. Diabetes Metab Res Rev. 2010;26:239–244. doi: 10.1002/dmrr.1092. [DOI] [PubMed] [Google Scholar]
- 116.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]


