Abstract
Wheezing is one of the characteristic symptoms of asthma, but all preschool children with wheezing are not diagnosed with asthma. Preschool children are not cooperative enough to participate in spirometry and invasive tests. Thus, there is no conventional method to diagnose asthma in preschool children. We reviewed studies on non-invasive biomarkers for assessing asthma in preschool children. Specimens that can be easily obtained by non-invasive methods are blood, exhaled breath and urine. Eosinophils, eosinophil cationic protein and eosinophil-derived neurotoxin (EDN) in blood are helpful in evaluating eosinophilic inflammation of the airways. Exhaled breath contains nitric oxide, volatile organic compounds, various cytokines and mediators as analytical components. Fraction of exhaled nitric oxide has been used to assess the degree of eosinophil inflammation and has been standardized in school-age children and adults, but not yet in preschool children. Exhaled breath condensate (EBC) pH and various cytokines/mediators that are detected in EBC seem to be promising biomarkers for assessing asthma, but need more standardization and validation. There are several biomarkers useful for assessing asthma, but none are ideal. Some biomarkers need standardized methods of obtaining samples from uncooperative preschool children for clinical use and require sufficient validation. Recently, another activated eosinophil marker, serum EDN, has shown promising results as a biomarker for recurrent wheezing and asthma in preschool children.
Keywords: Wheezing; asthma; biomarkers; child, preschool; eosinophil-derived neurotoxin
INTRODUCTION
Asthma is a common, chronic inflammatory disease characterized by a history of respiratory symptoms such as wheezing, chronic cough, chest tightness and shortness of breath, and by various degrees of airflow obstruction. Asthma in adults and children over the age of 6 years is diagnosed by a history of asthma symptoms and by reversible airway obstruction revealed by spirometry. However, confirming asthma in preschool children is more challenging because wheezing (the main symptom of asthma) is a very common condition experienced by about half of children under 6 years of age,1 and preschool children are not cooperative enough to undergo spirometry. Thus, some countries2,3 provided guidelines for asthma diagnosis in preschool children, and Global Initiative for Asthma (GINA) guidelines suggest methods for predicting asthma. It is important to note that no globally accepted reliable methods to confirm pediatric asthma are currently available.4
The onset of asthma symptoms is possible at all ages, but usually occurs in childhood, with most asthmatics manifesting at preschool age.5 In addition, airway remodeling can occur in preschoolers,6 and early use of inhaled corticosteroids (ICS) may improve lung function7 and prevent acute exacerbations of asthma.8 Therefore, early prediction and management of asthma is important.
The asthma predictive index (API) was first proposed in 2000 and was based on Tucson birth cohort data.9 The API consists of 2 major criteria (parental history of a physician-diagnosed asthma and physician-diagnosed atopic dermatitis in the child) and 3 minor criteria (physician-diagnosed allergic rhinitis, wheezing apart from colds and eosinophilia). The presence of 1 major criterion or 2 minor criteria is associated with asthma. Although the API has been used clinically, it has a relatively low positive predictive value.10 In addition to the API, a number of asthma predicting models have been proposed over the past 20 years. Some models have high positive predictive values, while others have high negative predictive values, but none have both.11
Therefore, the biomarkers that can accurately diagnose and monitor asthma have been widely studied, and some have been included in clinical practice. The most commonly used biomarker media in preschool children are the blood, exhaled breath and urine.12,13,14,15,16 This review describes the biomarkers that can be used to assess asthma and monitor asthma control in preschool children.
BLOOD
Eosinophils
“Eosinophilic inflammation” is a well-known characteristic feature of asthma. Sputum eosinophilic inflammation is associated with pulmonary function and persistent airflow limitation in asthmatic children16 as wekk as airway remodeling in preschoolers with recurrent wheezing.17 Although the sputum eosinophil count is a widely-used biomarker for asthma diagnosis and management in adults and older children, sputum collection in preschool children is difficult. Wagener et al.18 suggested that the blood eosinophil counts were highly correlated with eosinophilic inflammation of the airways and were highly accurate in diagnosing eosinophilic inflammation of the sputum. In preschoolers, blood eosinophil count was closely correlated with bronchoalveolar lavage fluid (BALF) eosinophil count, but 68% of preschoolers did not produce a proper sputum specimen.19
Blood eosinophil counts correlate with the degree of airway inflammation and asthmatic activity. Nadif et al.20 found that blood eosinophilia (≥250/mm3) was related to frequent asthma attacks and nocturnal symptoms. Elevated blood eosinophil counts in infants with wheezing correlated with persistent wheezing at the school age.12 Just et al.21 followed up 219 infants with recurrent wheezing until they were 6 years old and found that 27% of them had persistent wheezing at 6 years of age, and the most important risk factor for persistent wheezing was blood eosinophilia (≥470/mm3). They suggested that the absence of eosinophilia could predict future remission of wheezing. Karakoc et al.22 measured blood eosinophils at 6 months, 6 years and 11 years in children included in the Tucson birth cohort and found that asthma was related to longitudinal blood eosinophil status and that this relationship was independent of atopy.22
Eosinophil cationic protein (ECP)
Eosinophil cationic protein is one of the 4 primary cationic proteins secreted by activated eosinophils, with the other 3 being major basic protein (MBP), eosinophil peroxidase (EPO) and eosinophil-derived neurotoxin (EDN).23 Of these proteins, ECP is the most widely studied clinical marker for eosinophil activity. ECP has been measured in plasma, serum, BALF, sputum, saliva, nasal lavage, nasal secretions, tears, urine and skin, with serum and sputum being well-established mediums of measurement.24 Blood ECP is elevated in various diseases; therefore, it is not useful as a diagnostic biomarker for asthma. However, it has been found helpful in assessing airway inflammation, asthma severity and compliance with anti-inflammatory treatment.24 Prehn et al.25 used serum ECP level in deciding the time to tail down anti-inflammatory therapy in asthmatic children followed-up over a period of 1 year. This strategy reduced emergency department visits or rescue medicine use during the study.25
However, clinical use of ECP is associated with several limitations. First, several factors, such as age, smoking, circadian rhythm, and seasonal variation can affect ECP levels.24 Secondly, the blood must be collected under strictly controlled and standardized conditions. Thirdly, serum ECP measurement does not offer advantages compared to blood eosinophil count measurement.26
EDN
EDN is 67% homologous to ECP. Similar to ECP, EDN is secreted only by eosinophils. Since MBP and ECP have high isoelectronic points, they are highly charged in the blood and stick to cell membranes or tube walls. This makes accurate measurement of their concentration in the blood difficult. In contrast, EDN has an isoelectronic point of 8.3, which is close to neutrality; therefore, accurate blood concentration measurement is possible.23 When 3 different amounts of purified EDN were added to several different sera and EDN concentrations were measured, the percentage of measured/added EDN concentrations ranged from 90% to 109%.27 Sedgwick et al.28 showed that the intracellular EDN level is increased in patients with asthma, while the ECP level is not.
The first study to measure blood EDN levels included pediatric patients with asthma undergoing therapy with a fluticasone inhaler for 3 months.29 The blood EDN level was measured before and after treatment, and 1 month after the end of treatment. Blood EDN level did not show a significant correlation with pulmonary function, but decreased after fluticasone treatment and increased 1 month after cessation of fluticasone treatment.29 A study in adults reported a significant increase in blood EDN level in asymptomatic patients with asthma as soon as symptoms developed.30 Another study in adults reported a significant negative correlation between serum EDN levels and pulmonary function in patients with asthma.31
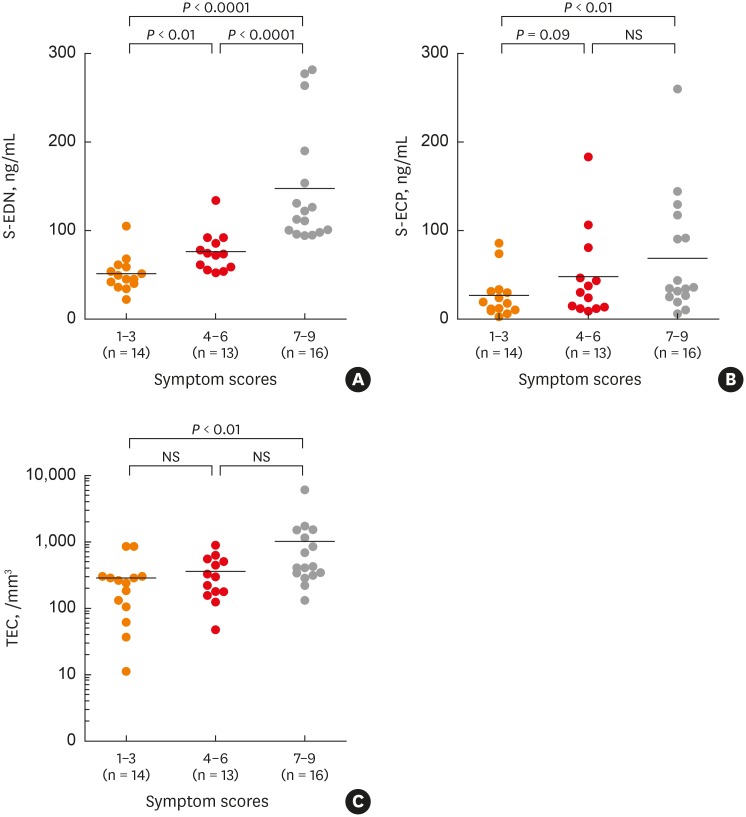
Serum EDN levels were measured in pediatric patients with atopic asthma or nonatopic asthma, and in normal controls. EDN levels increased only in patients with atopic asthma, and correlated with asthma severity.32 Another study in children measured serum EDN and ECP levels in asthmatic patients during acute and asymptomatic periods of asthma and compared obtained results with those measured in controls. Serum EDN levels decreased in asymptomatic patients compared to those in acute phase, and serum ECP levels tended to decrease in some patients, but increased in others. Both serum EDN and ECP levels were higher in asthmatic patients than in controls. Additionally, when asthmatic children were classified into mild, moderate and severe subgroups based on asthma severity, EDN levels were significantly different among the three groups, but ECP levels and eosinophil counts were not (Figure).33 In another study that measured serum EDN levels in young children with asthma and controls, the cutoff value for asthma diagnosis was 44.2 ng/mL, sensitivity was 81.3%, specificity was 87.1%, positive predictive value was 90.7%, and negative predictive value was 75.0% (Table).27
Figure. (A) Serum levels of EDN, (B) ECP, and (C) TECs, subgrouped according to asthma severity. Adapted from reference 33 with permission from “J Asthma”.
EDN, eosinophil derived neurotoxin; ECP, eosinophil cationic protein; TEC, total eosinophil count; NS, not significant.
Table. Accuracy of serum EDN as a diagnostic tool for asthma in children.
| Asthma group* (n = 48) | Control group (n = 31) | Total (n = 79) | |
|---|---|---|---|
| Positive* | 39 | 4 | 43 |
| Negative* | 9 | 27 | 36 |
| Total | 48 | 31 | 79 |
Sensitivity: 39/(39 + 9) = 81.3%, specificity: 27/(27 + 4) = 87.1%, positive predictive value: 39/(39 + 4) = 90.7%, and negative predictive value: 27/(27 + 9) = 75.0%.
EDN, eosinophil-derived neurotoxin.
*Test results: K® EDN ELISA Kit. Adapted from reference 27 with permission from “Allergol Int”.
We reported a previous study performed on infants admitted with respiratory syncytial virus bronchiolitis and treated with montelukast or placebo for 3 months and followed-up for 1 year. In the montelukast treatment group, serum EDN levels and the incidence of wheezing decreased.13 In addition, serum EDN levels at 3 months (end of treatment) correlated significantly with the total number of wheezing episodes at 12 months in the montelukast group (r = 0.531, P < 0.001) and in the placebo group (r = 0.720, P < 0.0001).34
Recently, we have reported another study on preschool children sensitized to house dust mite, with recurrent wheezing and serum EDN levels > 53 ng/mL. This study cohort was divided into 2 groups: one group was treated with budesonide inhalation and the other group was treated with montelukast for 3 months. The number of asthma control days increased similarly in both groups, while repeatedly measured serum EDN levels were significantly reduced only in the montelukast-treated group.35
In conclusion, serum EDN levels require further investigation as an asthma diagnostic tool, but can be used as a biomarker for treatment and monitoring of asthma.
Periostin
Periostin is expressed in periosteum and osteocytes, and elevated during growth. Its levels can also increase in other inflammatory diseases or tumors.36 Several pediatric studies reported no differences in serum periostin concentrations between allergic patients and controls.37 Conversely, one study did find that children with asthma had higher serum periostin levels than controls (76.0 [65.0–91.8] vs. 71.0 [57.5–80.0] ng/mL; P = 0.017].
However, the importance of this finding in terms of diagnostics is controversial, given that the ranges in each group overlapped considerably.38
EXHALED BREATH
Exhaled breath contains various components. Depending on the collection method, it can be classified into 3 domains: fraction of exhaled nitric oxide (FeNO), volatile organic compounds (VOCs), and exhaled breath concentrate (EBC).
FeNO
Nitric oxide is one of the intrinsic regulators that not only plays a role in normal physiological processes such as vasodilation, bronchodilation, and neurotransmission, but is also important in acute and chronic inflammation due to increased production during inflammation.39 Measurement of FeNO is a easy, safe, simple and noninvasive method to evaluate type 2 or allergic airway inflammation. It is the most extensively studied asthma biomarker and FeNO use in routine asthma management is increasing.
Recently, clinical applications of FeNO in asthmatic children have been well documented.40 FeNO measurement is recommended for detecting eosinophilic airway inflammation, determining the likelihood of steroid responsiveness, monitoring the degree of inflammation in patients already diagnosed with asthma and evaluating anti-inflammatory treatment adherence in poorly controlled patient.40
FeNO measurement in preschool children and infants is not as well standardized as in older children, and a variety of measurement techniques have been developed.41 Several cross-sectional studies reported increased FeNO levels in preschool-aged patients with probable asthma.42,43 Debley et al.44 measured FeNO and pulmonary function using the raised-volume rapid thoracic compression technique in children with a mean age of 15.8 months and reevaluated the lung function test 6 months later. FeNO levels were significantly higher in bronchodilator responsive patients. In addition, high FeNO levels were significantly positively correlated with systemic steroid use due to wheezing and negatively correlated with lung function reduction after 6 months.44
Van der Heijden et al.45 reported a mean value of FeNO measured by an offline method in preschool children aged 1-5 years of 7.1 ppb (2.8-11.5 ppb), and found no difference in age, height or weight, which are known as confounders.
Singer et al.46 reported that the probability of asthma diagnosis in school age increased when FeNO level was high in infancy and that the odds ratio of asthma occurrence increased by 2.44 for every 5 ppb of FeNO measured during infancy.46 Another study suggested that FeNO in 3- to 5-year-old preschoolers could distinguish between non-wheezers and wheezers on the basis of 6 ppb and between recurrent wheezers and non-recurrent wheezers on the basis of 10 ppb.47
In conclusion, FeNO measurement should be used to assess the degree of eosinophil inflammation rather than to diagnose asthma. In preschool children, this method is not yet standardized, and more research is needed.
VOCs
Exhaled breath contains thousands of VOCs that are the results of physiologic or pathologic processes in the human body.48 Thus, VOC analysis in the exhaled breath can be useful for assessing the condition of the body, especially the lungs, as it can detect disease-specific VOCs and is suitable for research purposes. Electronic noses can be used to identify patterns of various VOCs mixtures called “breathprints” and to analyze exhaled air in order to differentiate different disease phenotypes. Electronic noses allow direct measurement, and are fast, portable, suitable and inexpensive for clinical practice.49
Although few studies have been conducted in children, several of them have shown differences between VOCs in pediatric patients with asthma and normal controls.50 Robroeks et al.51 investigated whether asthma exacerbations in children could be predicted by exhaled VOC profiles. A total of 40 children with asthma were observed for 1 year, and FeNO, VOCs profiles in exhaled breath, lung function test, and symptoms were determined every 2 months. Combining 6 different exhaled VOCs could predict asthma exacerbation (correct classification 96%, sensitivity 100% and specificity 93%), while FeNO and lung function did not predict asthma exacerbation.51 Smolinska et al.52 investigated exhaled VOCs in 252 children aged 2-6 years from the “Asthma DEtection and Monitoring (ADEM) ” cohort. The authors selected 17 VOCs that discriminated asthmatic children from transient wheezers. The correct prediction rate was 80%. They showed that a VOCs profile could discriminate among asthmatic, transient wheezing and healthy children.52,53 In addition, they investigated whether EBC, VOCs, inflammatory gene expression and lung function could predict asthma development in preschool wheezers. They followed-up 202 preschool recurrent wheezers (aged 2-4 years) until 6 years of age. They concluded that adding information on VOCs and inflammatory gene expression to the API could improve the accuracy of asthma diagnosis in preschoolers.54
EBC
EBC is obtained by cooling and condensing exhaled breath through contact with a cold condenser.55 It contains water vapor and volatile and non-volatile components from airway lining fluid. Recommendations for standardization of sampling, analyzing and reporting of data were published in 2017 by the European Respiratory Society Task Force team.55 Several types of molecules, such as hydrogen ion, molecules related to oxidative stress and eicosanoids, were included.
EBC pH reflects airway acidification. Many studies have shown that EBC pH was lower in children with asthma than in controls, while some studies found no difference.56 In a study, although no significant overall differences in EBC pH were found between asthmatic children and controls, a significantly lower pH was detected in asthmatic children with forced expiratory volume in 1 second < 80% of predicted (P = 0.03).57 Another studies showed that low EBC pH was associated with asthma exacerbations.58 These results suggest that lower EBC pH may be a useful biomarker for severe or uncontrolled asthma.
The role of NO products in asthmatic airway inflammation is well known. Aldehydes and lipid hydroperoxides are derived from the oxygenation of membrane phospholipids and polyunsaturated fatty acids.
Eicosanoids are heterogeneous metabolites of arachidonic acid involved in asthmatic inflammation. Several studies showed no significant differences in prostaglandins levels, except for 8-isoprostane, between asthmatic children and controls.56 Leukotriene B4 (LTB4) is a potent neutrophil chemoattractant. Several studies showed that LTB4 was elevated59 in asthmatic children, regardless of atopy.60 The cysteinyl-leukotriens (cys-LTs) (LTC4, LTD4 and LTE4) are powerful bronchoconstrictors and proinflammatory mediators. EBC cys-LTs levels were elevated in asthmatic children, and were associated with asthma severity and exacerbations.59
Cytokines of the Th2 pathway have been evaluated as biomarkers in EBC. Interleukin (IL)-4, IL-5 and interferon (IFN)-γ were elevated in asthmatic children compared to controls.
Only a few studies evaluated EBC in pre-school children.61 One study evaluated LTB4, LTE4, 8-isoprostane and nitrite/nitrate levels in the EBC of healthy preschool children and preschool children with wheezing, and showed increased LTB4, LTE4 and nitrite levels in children with wheezing episodes, but no difference in 8-isoprostant and nitrate concentrations.61 A prospective study investigated whether inflammatory markers in EBC and pre- and post-bronchodilator interrupter resistance assessed at preschool age were associated with wheezing phenotypes at school age, and demonstrated that 5-year-old children with persistent wheezing already had elevated exhaled inflammatory markers by preschool age.62 Another prospective study evaluated changes in EBC biomarkers (pH, IL-1α, IL-2, IL-4, IL-5, IL-10, IFN-γ, sICAM and CCL-11) before and after ICS treatment for 8 weeks in preschool children aged 2-4 years, and the presence of asthma was evaluated at 6 years of age. They concluded that EBC biomarkers could not predict asthma diagnosis.63 A study that evaluated whether cytokines in EBC could predict an ICS response in preschool wheezers showed that EBC cytokines failed to predict ICS response.64 Another study showed that the feasibility of collecting EBC varied depending on sampling methods in pre-school children.65 In conclusion, although EBC is a promising method for non-invasive evaluation of airway inflammation, more research and standardization in pre-school children are needed.
URINE ANALYSIS
Urine is an attractive test medium for pre-school children because it can be easily obtained, regardless of age. EDN and LTB4 are the most extensively studied urine biomarkers for asthma, and research using “-omics” has recently been increasing.
In a recent meta-analysis, Klonoff-Cohen and Polavarapu66 evaluated urine EDN as a diagnostic tool for asthma in children. The standardized mean difference (SMD) between acute asthmatics and healthy controls was 1.94 (confidence interval [CI], 1.67-2.22), and the SMD between asymptomatic asthmatics and controls was 1.58 (CI, 1.27-1.88). They suggested the following urine EDN cutoff values for asthmatics and healthy children: 134-140 µg/mmol creatinine (Cr) for acute asthmatics; 65-75 µg/mmol Cr for acute asthmatics after treatment; and 56–61 µg/mmol Cr for healthy controls. However, they also acknowledged several study limitations. First, they could not evaluate the sensitivity and specificity. Secondly, the studies included in the meta-analysis used different diagnostic and severity criteria. Thirdly, EDN-measuring methods and equipment were different. Finally, measurements were not made repeatedly after treatment as several studies were cross-sectional. Thus, it is too early to apply the proposed EDN cutoff points in clinical practice.
Two birth cohort studies evaluated whether urinary LTE4 measurements predicted asthma development.67,68 One reported that elevated urinary LTE4 levels at 2 years of age were associated with higher risk of developing asthma by the age 3 years.67 The other reported that LTE4 levels at the age of 1 month were not associated with the development of asthma at 6 years of age.68 Therefore, urinary LTE4 is not yet considered as a biomarker for asthma diagnosis or prediction.
Recently, several studies reported on metabolomic analysis for asthma diagnosis,69 prediction of asthma development,14,15 and prediction of response to corticosteroid treatment.70 A cross-sectional study reported that nuclear magnetic resonance spectroscopy-derived metabolite profiles of urine differentiated children with stable asthma from children with unstable asthma or controls with 94% accuracy.69 Carraro et al. 14 recruited preschool children with recurrent wheezing and followed them for 3 years and analyzed urine samples by ultra-performance liquid chromatography mass spectrometry and identified metabolic variables to discriminate early-onset asthma from transient wheezing (area under curve = 0.98).
These results suggest that metabolomic analysis can be a useful method for finding urine biomarker or combinations of urine metabolites for asthma diagnosis.
CONCLUSION
Many biomarkers for the diagnossis and monitoring asthma have been studied — some are already being used in clinical settings, while others require further studies.
FeNO has been the most extensively studied and used in clinical practice, but no standardized method for measuring it in preschool children is currently available. Urinary EDN and LTE4 levels have been widely studied as candidates for asthma biomarkers. However, their clinical use is subject to several limitations.
Blood ECP has been attracting much attention as a method of diagnosing eosinophilic inflammation, but it is not superior to blood eosinophil counts. Some biomarkers need standardized methods of obtaining samples from uncooperative preschool children for clinical use and require sufficient
Recently, another activated eosinophil marker, serum EDN, has shown promising results as a biomarker for recurrent wheezing and asthma in preschool children.
ACKNOWLEDGMENTS
This study was supported in party by “The Characterization Research 2017” from Inje University.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 2.Arakawa H, Hamasaki Y, Kohno Y, Ebisawa M, Kondo N, Nishima S, et al. Japanese guidelines for childhood asthma 2017. Allergol Int. 2017;66:190–204. doi: 10.1016/j.alit.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Ducharme FM, Dell SD, Radhakrishnan D, Grad RM, Watson WT, Yang CL, et al. Diagnosis and management of asthma in preschoolers: a Canadian Thoracic Society and Canadian Paediatric Society position paper. Can Respir J. 2015;22:135–143. doi: 10.1155/2015/101572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Initiative for Asthma. Global strategy for asthma management and prevention [Internet] place unknown: Global Initiative for Asthma; 2018. [cite 2018 Jun 6]. Available from: www.ginasthma.org. [Google Scholar]
- 5.Punekar YS, Sheikh A. Establishing the sequential progression of multiple allergic diagnoses in a UK birth cohort using the General Practice Research Database. Clin Exp Allergy. 2009;39:1889–1895. doi: 10.1111/j.1365-2222.2009.03366.x. [DOI] [PubMed] [Google Scholar]
- 6.Saglani S, Payne DN, Zhu J, Wang Z, Nicholson AG, Bush A, et al. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med. 2007;176:858–864. doi: 10.1164/rccm.200702-212OC. [DOI] [PubMed] [Google Scholar]
- 7.Agertoft L, Pedersen S. Effects of long-term treatment with an inhaled corticosteroid on growth and pulmonary function in asthmatic children. Respir Med. 1994;88:373–381. doi: 10.1016/0954-6111(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 8.Chen YZ, Busse WW, Pedersen S, Tan W, Lamm CJ, O'Byrne PM. Early intervention of recent onset mild persistent asthma in children aged under 11 yrs: the Steroid Treatment As Regular Therapy in early asthma (START) trial. Pediatr Allergy Immunol. 2006;17(Suppl 17):7–13. doi: 10.1111/j.1600-5562.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 9.Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–1406. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 10.Savenije OE, Kerkhof M, Koppelman GH, Postma DS. Predicting who will have asthma at school age among preschool children. J Allergy Clin Immunol. 2012;130:325–331. doi: 10.1016/j.jaci.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Smit HA, Pinart M, Antó JM, Keil T, Bousquet J, Carlsen KH, et al. Childhood asthma prediction models: a systematic review. Lancet Respir Med. 2015;3:973–984. doi: 10.1016/S2213-2600(15)00428-2. [DOI] [PubMed] [Google Scholar]
- 12.Gaillard EA, McNamara PS, Murray CS, Pavord ID, Shields MD. Blood eosinophils as a marker of likely corticosteroid response in children with preschool wheeze: time for an eosinophil guided clinical trial? Clin Exp Allergy. 2015;45:1384–1395. doi: 10.1111/cea.12535. [DOI] [PubMed] [Google Scholar]
- 13.Kim CK, Choi J, Kim HB, Callaway Z, Shin BM, Kim JT, et al. A randomized intervention of montelukast for post-bronchiolitis: effect on eosinophil degranulation. J Pediatr. 2010;156:749–754. doi: 10.1016/j.jpeds.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Carraro S, Bozzetto S, Giordano G, El Mazloum D, Stocchero M, Pirillo P, et al. Wheezing preschool children with early-onset asthma reveal a specific metabolomic profile. Pediatr Allergy Immunol. 2018;29:375–382. doi: 10.1111/pai.12879. [DOI] [PubMed] [Google Scholar]
- 15.Chiu CY, Lin G, Cheng ML, Chiang MH, Tsai MH, Su KW, et al. Longitudinal urinary metabolomic profiling reveals metabolites for asthma development in early childhood. Pediatr Allergy Immunol. 2018;29:496–503. doi: 10.1111/pai.12909. [DOI] [PubMed] [Google Scholar]
- 16.Lee YJ, Lee HH, Choi BS, Jee HM, Kim KW, Sohn MH, et al. Association between eosinophilic airway inflammation and persistent airflow limitation. J Asthma. 2013;50:342–346. doi: 10.3109/02770903.2013.776074. [DOI] [PubMed] [Google Scholar]
- 17.Castro-Rodriguez JA, Saglani S, Rodriguez-Martinez CE, Oyarzun MA, Fleming L, Bush A. The relationship between inflammation and remodeling in childhood asthma: a systematic review. Pediatr Pulmonol. 2018;53:824–835. doi: 10.1002/ppul.23968. [DOI] [PubMed] [Google Scholar]
- 18.Wagener AH, de Nijs SB, Lutter R, Sousa AR, Weersink EJ, Bel EH, et al. External validation of blood eosinophils, FENO and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015;70:115–120. doi: 10.1136/thoraxjnl-2014-205634. [DOI] [PubMed] [Google Scholar]
- 19.Jochmann A, Artusio L, Robson K, Nagakumar P, Collins N, Fleming L, et al. Infection and inflammation in induced sputum from preschool children with chronic airways diseases. Pediatr Pulmonol. 2016;51:778–786. doi: 10.1002/ppul.23366. [DOI] [PubMed] [Google Scholar]
- 20.Nadif R, Siroux V, Oryszczyn MP, Ravault C, Pison C, Pin I, et al. Heterogeneity of asthma according to blood inflammatory patterns. Thorax. 2009;64:374–380. doi: 10.1136/thx.2008.103069. [DOI] [PubMed] [Google Scholar]
- 21.Just J, Nicoloyanis N, Chauvin M, Pribil C, Grimfeld A, Duru G. Lack of eosinophilia can predict remission in wheezy infants? Clin Exp Allergy. 2008;38:767–773. doi: 10.1111/j.1365-2222.2008.02966.x. [DOI] [PubMed] [Google Scholar]
- 22.Karakoc F, Remes ST, Martinez FD, Wright AL. The association between persistent eosinophilia and asthma in childhood is independent of atopic status. Clin Exp Allergy. 2002;32:51–56. doi: 10.1046/j.0022-0477.2001.01273.x. [DOI] [PubMed] [Google Scholar]
- 23.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 24.Koh GC, Shek LP, Goh DY, Van Bever H, Koh DS. Eosinophil cationic protein: is it useful in asthma? A systematic review. Respir Med. 2007;101:696–705. doi: 10.1016/j.rmed.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Prehn A, Seger RA, Torresani T, Molinari L, Sennhauser FH. Evaluation of a clinical algorithm involving serum eosinophil cationic protein for guiding the anti-inflammatory treatment of bronchial asthma in childhood. Pediatr Allergy Immunol. 2000;11:87–94. doi: 10.1111/j.1399-3038.2000.00079.x. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Marcos L, Edwards J, Kennington E, Aurora P, Baraldi E, Carraro S, et al. Priorities for future research into asthma diagnostic tools: a PAN-EU consensus exercise from the European asthma research innovation partnership (EARIP) Clin Exp Allergy. 2018;48:104–120. doi: 10.1111/cea.13080. [DOI] [PubMed] [Google Scholar]
- 27.Kim CK, Callaway Z, Park JS, Kwon E. Utility of serum eosinophil-derived neurotoxin (EDN) measurement by ELISA in young children with asthma. Allergol Int. 2017;66:70–74. doi: 10.1016/j.alit.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Sedgwick JB, Vrtis RF, Jansen KJ, Kita H, Bartemes K, Busse WW. Peripheral blood eosinophils from patients with allergic asthma contain increased intracellular eosinophil-derived neurotoxin. J Allergy Clin Immunol. 2004;114:568–574. doi: 10.1016/j.jaci.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 29.Hoekstra MO, Grol MH, Hovenga H, Bouman K, Stijnen T, Koëter GH, et al. Eosinophil and mast cell parameters in children with stable moderate asthma. Pediatr Allergy Immunol. 1998;9:143–149. doi: 10.1111/j.1399-3038.1998.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 30.Morioka J, Kurosawa M, Inamura H, Nakagami R, Mizushima Y, Omura Y, et al. Increased END/EPX in ongoing asthma. Allergy. 2000;55:1203–1204. doi: 10.1034/j.1398-9995.2000.00858.x. [DOI] [PubMed] [Google Scholar]
- 31.Badar A, Hussain MM, Saeed W, Aslam M. Correlation of eosinophil derived neurotoxin with airway resistance in asthmatics. J Pak Med Assoc. 2010;60:97–101. [PubMed] [Google Scholar]
- 32.Kim KW, Lee KE, Kim ES, Song TW, Sohn MH, Kim KE. Serum eosinophil-derived neurotoxin (EDN) in diagnosis and evaluation of severity and bronchial hyperresponsiveness in childhood asthma. Lung. 2007;185:97–103. doi: 10.1007/s00408-006-0054-8. [DOI] [PubMed] [Google Scholar]
- 33.Kim CK, Callaway Z, Fletcher R, Koh YY. Eosinophil-derived neurotoxin in childhood asthma: correlation with disease severity. J Asthma. 2010;47:568–573. doi: 10.3109/02770901003792833. [DOI] [PubMed] [Google Scholar]
- 34.Kim CK, Seo JK, Ban SH, Fujisawa T, Kim DW, Callaway Z. Eosinophil-derived neurotoxin levels at 3 months post-respiratory syncytial virus bronchiolitis are a predictive biomarker of recurrent wheezing. Biomarkers. 2013;18:230–235. doi: 10.3109/1354750X.2013.773078. [DOI] [PubMed] [Google Scholar]
- 35.Kim CK, Callaway Z, Park JS, Nishimori H, Ogino T, Nagao M, et al. Montelukast reduces serum levels of eosinophil-derived neurotoxin in preschool asthma. Allergy Asthma Immunol Res. 2018;10:686–697. doi: 10.4168/aair.2018.10.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Idolazzi L, Ridolo E, Fassio A, Gatti D, Montagni M, Caminati M, et al. Periostin: the bone and beyond. Eur J Intern Med. 2017;38:12–16. doi: 10.1016/j.ejim.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Inoue Y, Izuhara K, Ohta S, Ono J, Shimojo N. No increase in the serum periostin level is detected in elementary school-age children with allergic diseases. Allergol Int. 2015;64:289–290. doi: 10.1016/j.alit.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Song JS, You JS, Jeong SI, Yang S, Hwang IT, Im YG, et al. Serum periostin levels correlate with airway hyper-responsiveness to methacholine and mannitol in children with asthma. Allergy. 2015;70:674–681. doi: 10.1111/all.12599. [DOI] [PubMed] [Google Scholar]
- 39.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 40.Song WJ, Kwon JW, Kim EJ, Lee SM, Kim SH, Lee SY, et al. Clinical application of exhaled nitric oxide measurements in a Korean population. Allergy Asthma Immunol Res. 2015;7:3–13. doi: 10.4168/aair.2015.7.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 42.van der Valk RJ, Caudri D, Savenije O, Koppelman GH, Smit HA, Wijga AH, et al. Childhood wheezing phenotypes and FeNO in atopic children at age 8. Clin Exp Allergy. 2012;42:1329–1336. doi: 10.1111/j.1365-2222.2012.04010.x. [DOI] [PubMed] [Google Scholar]
- 43.Moeller A, Diefenbacher C, Lehmann A, Rochat M, Brooks-Wildhaber J, Hall GL, et al. Exhaled nitric oxide distinguishes between subgroups of preschool children with respiratory symptoms. J Allergy Clin Immunol. 2008;121:705–709. doi: 10.1016/j.jaci.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Debley JS, Stamey DC, Cochrane ES, Gama KL, Redding GJ. Exhaled nitric oxide, lung function, and exacerbations in wheezy infants and toddlers. J Allergy Clin Immunol. 2010;125:1228–1234.e13. doi: 10.1016/j.jaci.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Heijden HH, Brouwer ML, Hoekstra F, van der Pol P, Merkus PJ. Reference values of exhaled nitric oxide in healthy children 1–5 years using off-line tidal breathing. Pediatr Pulmonol. 2014;49:291–295. doi: 10.1002/ppul.22796. [DOI] [PubMed] [Google Scholar]
- 46.Singer F, Luchsinger I, Inci D, Knauer N, Latzin P, Wildhaber JH, et al. Exhaled nitric oxide in symptomatic children at preschool age predicts later asthma. Allergy. 2013;68:531–538. doi: 10.1111/all.12127. [DOI] [PubMed] [Google Scholar]
- 47.Sayão LB, de Britto MC, Burity E, Rattes C, Reinaux CM, Fink J, et al. Exhaled nitric oxide as a diagnostic tool for wheezing in preschool children: a diagnostic accuracy study. Respir Med. 2016;113:15–21. doi: 10.1016/j.rmed.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Amann A, Costello BL, Miekisch W, Schubert J, Buszewski B, Pleil J, et al. The human volatilome: volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J Breath Res. 2014;8:034001. doi: 10.1088/1752-7155/8/3/034001. [DOI] [PubMed] [Google Scholar]
- 49.Fens N, van der Schee MP, Brinkman P, Sterk PJ. Exhaled breath analysis by electronic nose in airways disease. Established issues and key questions. Clin Exp Allergy. 2013;43:705–715. doi: 10.1111/cea.12052. [DOI] [PubMed] [Google Scholar]
- 50.Caldeira M, Barros AS, Bilelo MJ, Parada A, Câmara JS, Rocha SM. Profiling allergic asthma volatile metabolic patterns using a headspace-solid phase microextraction/gas chromatography based methodology. J Chromatogr A. 2011;1218:3771–3780. doi: 10.1016/j.chroma.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 51.Robroeks CM, van Berkel JJ, Jöbsis Q, van Schooten FJ, Dallinga JW, Wouters EF, et al. Exhaled volatile organic compounds predict exacerbations of childhood asthma in a 1-year prospective study. Eur Respir J. 2013;42:98–106. doi: 10.1183/09031936.00010712. [DOI] [PubMed] [Google Scholar]
- 52.Smolinska A, Klaassen EM, Dallinga JW, van de Kant KD, Jobsis Q, Moonen EJ, et al. Profiling of volatile organic compounds in exhaled breath as a strategy to find early predictive signatures of asthma in children. PLoS One. 2014;9:e95668. doi: 10.1371/journal.pone.0095668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van de Kant KD, van Berkel JJ, Jöbsis Q, Lima Passos V, Klaassen EM, van der Sande L, et al. Exhaled breath profiling in diagnosing wheezy preschool children. Eur Respir J. 2013;41:183–188. doi: 10.1183/09031936.00122411. [DOI] [PubMed] [Google Scholar]
- 54.Klaassen EM, van de Kant KD, Jöbsis Q, van Schayck OC, Smolinska A, Dallinga JW, et al. Exhaled biomarkers and gene expression at preschool age improve asthma prediction at 6 years of age. Am J Respir Crit Care Med. 2015;191:201–207. doi: 10.1164/rccm.201408-1537OC. [DOI] [PubMed] [Google Scholar]
- 55.Horváth I, Barnes PJ, Loukides S, Sterk PJ, Högman M, Olin AC, et al. A European Respiratory Society technical standard: exhaled biomarkers in lung disease. Eur Respir J. 2017;49:1600965. doi: 10.1183/13993003.00965-2016. [DOI] [PubMed] [Google Scholar]
- 56.Thomas PS, Lowe AJ, Samarasinghe P, Lodge CJ, Huang Y, Abramson MJ, et al. Exhaled breath condensate in pediatric asthma: promising new advance or pouring cold water on a lot of hot air? A systematic review. Pediatr Pulmonol. 2013;48:419–442. doi: 10.1002/ppul.22776. [DOI] [PubMed] [Google Scholar]
- 57.Ratnawati MJ, Morton J, Henry RL, Thomas PS. Exhaled breath condensate nitrite/nitrate and pH in relation to pediatric asthma control and exhaled nitric oxide. Pediatr Pulmonol. 2006;41:929–936. doi: 10.1002/ppul.20469. [DOI] [PubMed] [Google Scholar]
- 58.Brunetti L, Francavilla R, Tesse R, Strippoli A, Polimeno L, Loforese A, et al. Exhaled breath condensate pH measurement in children with asthma, allergic rhinitis and atopic dermatitis. Pediatr Allergy Immunol. 2006;17:422–427. doi: 10.1111/j.1399-3038.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 59.Baraldi E, Carraro S, Alinovi R, Pesci A, Ghiro L, Bodini A, et al. Cysteinyl leukotrienes and 8-isoprostane in exhaled breath condensate of children with asthma exacerbations. Thorax. 2003;58:505–509. doi: 10.1136/thorax.58.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montuschi P, Martello S, Felli M, Mondino C, Barnes PJ, Chiarotti M. Liquid chromatography/mass spectrometry analysis of exhaled leukotriene B4 in asthmatic children. Respir Res. 2005;6:119. doi: 10.1186/1465-9921-6-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caballero S, Martorell A, Escribano A, Belda J. Markers of airway inflammation in the exhaled breath condensate of preschool wheezers. J Investig Allergol Clin Immunol. 2013;23:7–13. [PubMed] [Google Scholar]
- 62.van de Kant KD, Jansen MA, Klaassen EM, van der Grinten CP, Rijkers GT, Muris JW, et al. Elevated inflammatory markers at preschool age precede persistent wheezing at school age. Pediatr Allergy Immunol. 2012;23:259–264. doi: 10.1111/j.1399-3038.2011.01244.x. [DOI] [PubMed] [Google Scholar]
- 63.Klaassen EM, van de Kant KD, Jöbsis Q, Høvig ST, van Schayck CP, Rijkers GT, et al. Symptoms, but not a biomarker response to inhaled corticosteroids, predict asthma in preschool children with recurrent wheeze. Mediators Inflamm. 2012;2012:162571. doi: 10.1155/2012/162571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van de Kant KD, Koers K, Rijkers GT, Lima Passos V, Klaassen EM, Mommers M, et al. Can exhaled inflammatory markers predict a steroid response in wheezing preschool children? Clin Exp Allergy. 2011;41:1076–1083. doi: 10.1111/j.1365-2222.2011.03774.x. [DOI] [PubMed] [Google Scholar]
- 65.Rosias PP, Robroeks CM, van de Kant KD, Rijkers GT, Zimmermann LJ, van Schayck CP, et al. Feasibility of a new method to collect exhaled breath condensate in pre-school children. Pediatr Allergy Immunol. 2010;21:e235–44. doi: 10.1111/j.1399-3038.2009.00909.x. [DOI] [PubMed] [Google Scholar]
- 66.Klonoff-Cohen H, Polavarapu M. Eosinophil protein X and childhood asthma: a systematic review and meta-analysis. Immun Inflamm Dis. 2016;4:114–134. doi: 10.1002/iid3.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiu CY, Tsai MH, Yao TC, Tu YL, Hua MC, Yeh KW, et al. Urinary LTE4 levels as a diagnostic marker for IgE-mediated asthma in preschool children: a birth cohort study. PLoS One. 2014;9:e115216. doi: 10.1371/journal.pone.0115216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chawes BL, Bønnelykke K, Bisgaard H. Elevated eosinophil protein X in urine from healthy neonates precedes development of atopy in the first 6 years of life. Am J Respir Crit Care Med. 2011;184:656–661. doi: 10.1164/rccm.201101-0111OC. [DOI] [PubMed] [Google Scholar]
- 69.Saude EJ, Skappak CD, Regush S, Cook K, Ben-Zvi A, Becker A, et al. Metabolomic profiling of asthma: diagnostic utility of urine nuclear magnetic resonance spectroscopy. J Allergy Clin Immunol. 2011;127:757–764.e1. doi: 10.1016/j.jaci.2010.12.1077. [DOI] [PubMed] [Google Scholar]
- 70.Park YH, Fitzpatrick AM, Medriano CA, Jones DP. High-resolution metabolomics to identify urine biomarkers in corticosteroid-resistant asthmatic children. J Allergy Clin Immunol. 2017;139:1518–1524.e4. doi: 10.1016/j.jaci.2016.08.018. [DOI] [PubMed] [Google Scholar]