Abstract
Purpose
Asthma in the elderly has different clinical features including more severe phenotypes with higher comorbidities. Epithelial cells are known to initiate innate/adaptive immune responses in asthmatic airways. We investigated clinical features and epithelial derived cytokine levels in elderly asthmatics compared to non-elderly asthmatics in a cross-sectional cohort of adult asthmatics in order to further understand its pathogenic mechanisms.
Methods
A total of 1,452 adult asthmatics were enrolled from a single tertiary hospital and were classified into 2 groups: 234 elderly (≥ 60 years at initial diagnosis) and 1,218 non-elderly (< 60 years at initial diagnosis) asthmatics. Asthma-related clinical parameters were compared between the 2 groups. Serum levels of epithelial cell-derived cytokines including interleukin (IL)-31, IL-33, IL-8, eotaxin-2, transforming growth factor beta 1 (TGF-β1) and periostin were measured by enzyme-linked immunosorbent assay.
Results
Significantly higher prevalence rates of late-onset asthma (onset age ≥ 40 years) and severe asthma, as well as the lower rate of atopy, blood/sputum eosinophil counts, total immunoglobulin E and eosinophil cationic protein levels were noted in elderly asthmatics compared to non-elderly asthmatics (P < 0.05, respectively). The forced expiratory volume in 1 second (FEV1, % predicted) level tended to be lower in elderly asthmatics (P = 0.07). In addition, serum IL-33 and IL-31 levels were significantly lower in elderly asthmatics, while no differences were found in the serum level of IL-8, eotaxin-2, TGF-β1 or periostin. Among elderly asthmatics, subjects with severe asthma had lower FEV1 (% predicted) value, but showed significantly higher serum levels of eotaxin-2 and TGF-β1, than those with non-severe asthma (P < 0.05 for each).
Conclusions
These findings suggest that age-related changes of epithelial cell-derived cytokines may affect clinical phenotypes and severity of elderly asthma: decreased levels of IL-33 and IL-31 may contribute to less Th2 phenotype, while increased levels of eotaxin-2 and TGF-β1 may contribute to severity.
Keywords: Asthma; older adults; epithelial cells; interleukin-33; interleukin-31; eotaxin-2, TGF-beta1
INTRODUCTION
The number of elderly people is increasing and the proportion of the elderly population in the US is projected to reach 25% of the population by 2050.1 The prevalence of asthma in the elderly is rapidly increasing worldwide from 3.6% to 17% and was reported at 7.1% in the Korean population.2,3,4 However, elderly asthmatics are underdiagnosed, suffer high morbidity and mortality with lower quality of life, which bring economic burden on healthcare systems due to increasing medical service expenses.5,6
Elderly asthma (EA) is considered to have different pathophysiologic mechanisms, depending on age-related changes in lung function and immune response, epigenetic factors, environmental modification, and various comorbidities; however, most of the previous studies have excluded elderly subjects.1,7 Aging is a strong factor that contributes to structural and physiological alterations in the airway, loss of lung function, senescence process of immune cells (immunosenescence) and modification of inflammatory response,1,8 which affects both innate and adaptive immunity.9 Previous studies demonstrated that elderly asthmatics showed lower atopy rate with poor control status.1,10 In an in vivo model, ovalbumin (OVA)-sensitized aged mice showed a lower level of serum total immunoglobulin E (IgE), less total cellularity with a lower eosinophil counts in bronchoalveolar lavage fluid (BALF), while BALF neutrophil counts were similar between the aged and young mice.8 These findings suggest that inflammatory mechanisms invovled in EA are different from those of non-elderly asthma (NEA), although airway inflammation in asthmatic airways is mainly consists of type-2 inflammation.8 Airway epithelial cells are the first line barrier that responds to external factors and produces various inflammatory mediators, which modulate innate and adaptive immune responses. Cytokines expressed in epithelial cells including interleukin (IL)-33 and IL-31 are known to regulate immune response in asthmatic airway. It is reported that IL-33/IL-31 axis is associated with type 2 inflammation cascades in asthma and allergic disease.11,12 In addition, other epithelial cell-derived cytokines such as periostin, transforming growth factor beta-1 (TGF-β1) and eotaxin-2 are known to lead airway inflammation and remodeling.13,14 However, the role of these cytokines in EA has not been demonstrated yet.
In the present study, we hypothesized that age-related changes in epithelial cells may affect immune responses of asthmatic airways that modify clinical phenotypes of EA. Serum levels of epithelial-derived cytokines were analyzed in association with clinical characteristics of EA in a cross-sectional cohort of adult asthmatics (with a retrospective design).
MATERIALS AND METHODS
Study subjects
We enrolled 692 normal controls (NCs) and a total of 1,452 asthmatic patients who had been treated in the Department of Allergy and Clinical Immunology, Ajou University Hospital (Suwon, Korea) for more than 2 years with standardized maintenance medications following the Global Initiative for Asthma (GINA) guideline.15 Of the total patients, 234 were classified as EA patients who were diagnosed and enrolled at age ≥ 60 years and the remaining were NEA patients (< 60 years). Previous studies have used different age limits defining EA patients as 55, 60 and 65 years; however, we chose the age cutoff at 60 years in this study. One hundred sixty-three (62.5%) patients were female. Their clinical characteristics were obtained from electronic medical records of each patient including age, sex, disease duration, smoking history, atopic status, asthma severity, sputum eosinophil and neutrophil count, total IgE level, asthma medication and exacerbation histories. Written informed consent forms were collected from the study subject. This study was approved by Ajou University Institutional Review Board (AJIRB-GEN-SMP-13-108).
Collection of clinical data
We analyzed age, sex, asthma duration, onset time, smoking status, atopy, asthma severity, blood eosinophil count, sputum eosinophil and neutrophil count, serum eosinophilic cationic protein (ECP), forced expiratory volume in 1 second (FEV1), and provocative concentration that caused a decrease in FEV1 (% predicted) of 20% on methacholine challenge test (methacholine PC20). Severe asthma was defined when patients are in uncontrolled asthma despite of maintaining combination inhalers composing medium/high-dose inhaled corticosteroids (ICS)/long-acting beta 2 agonist with ≥ 2 times of asthma exacerbations requiring systemic steroid (≥ 45 mg for 3 days) following the European Respiratory Society/American Thoracic Society (ERS/ATS) guideline.16 Atopy was defined as a positive result to at least 1 or more allergens on skin prick test with common inhalant allergens including tree mixture, grass mixture, mugwort, ragweed, cat fur, dog fur, Dermatophagoides pteronyssinus, Dermatophagoides farinae, and Alternaria spp. (Bencard, Bretford, UK). Total IgE and ECP levels were measured by the UniCAP system (ThermoFisher Scientific, Waltham, MA, USA). The serum levels of IL-8 (Endogen Inc., Woburn, MA, USA), eotaxin-2 (RayBio Inc., Norcross, GA, USA), TGF-β1 (R&D Systems, San Diego, CA, USA) and periostin (Shino test, Kanagawa, Japan),17 IL-33 and IL-31 (R&D Systems) were measured by enzyme-linked immunosorbent assay (ELISA). Serum samples were collected and frozen at −70°C and thawed before use. The spontaneous sputum samples were collected from the patients, and the total number of cell viability was determined with Trypan blue stain. The slides were prepared for cytological examination with Wright-Giemsa stain, and differential counts were determined by counting 100 leukocytes on each sputum slide. Sputum eosinophil/neutrophil counts were calculated as % of eosinophil or neutrophil number per 100 leukocytes counted.
Statistical analysis
Statistical analysis was performed using SPSS statistical software 20 (SPSS Inc, Chicago, CA, USA). Student's t-test was used to compare clinical parameters; Mann-Whitney U test was applied to compare serum cytokine levels. Pearson χ2 test was used to compare the prevalence rate of clinical parameters. Spearman rank correlation analysis was used to determine an association between the continuous parameters. Predictive values of serum eotaxin-2 and TGF-β1 levels were determined by receiver operating characteristic (ROC) analysis. P values < 0.05 were considered statistically significant. ROC analysis was performed to differentiate the severe phenotype from the non-severe phenotype among EA patients.
RESULTS
Comparison of clinical characteristics
The clinical characteristics of the study subjects are summarized in Table 1. Significantly higher prevalence rates of late-onset asthma (LOA, onset age ≥ 40 years old) and severe asthma were noted in EA patients than in NEA patients (Table 1). Asthma duration tended to be longer in EA patients than in NEA patients, although statistical significance was not reached (Table 1). Atopy rate, peripheral blood/sputum eosinophil counts, and serum ECP level were significantly lower in EA patients than in NEA patients, while sputum neutrophil count was significantly higher in EA patients (Table 1). When serum total IgE levels were log-transformed to correct the skewed distribution, it was significantly lower in EA than in NEA patients (Table 1). The baseline level of FEV1 (% predicted) was lower in EA patients than in NEA patients, although statistical significance was not reached (Table 1).
Table 1. Comparison of clinical characteristics between EA (age ≥ 60) and NEA (age < 60) patients.
| Parameters | EA (n = 234) | NEA (n = 1,218) | P values |
|---|---|---|---|
| Age (yr) | 65.87 ± 4.9 | 39.3 ± 11.7 | < 0.001 |
| Sex (female, %) | 69.6 | 61.0 | 0.012 |
| Duration (yr) | 7.8 ± 7.4 | 5.7 ± 10.2 | 0.07 |
| Disease onset (> 40 yr, %) | 94.9 | 33.3 | < 0.001 |
| Smoking (%) | 39.3 | 40.7 | 0.923 |
| Atopy (%) | 42.6 | 56.0 | 0.001 |
| Severity (severe %) | 26.6 | 19.9 | 0.046 |
| Blood eosinophil count (count/uL) | 257.3 ± 262.02 | 457.0 ± 820.4 | < 0.001 |
| Sputum eosinophil (%) | 15.5 ± 29.0 | 24.2 ± 32.4 | 0.018 |
| Sputum neutrophil (%) | 67.9 ± 32.4 | 56.6 ± 34.01 | 0.009 |
| Log total IgE (kU/L)* | 1.6 ± 1.05 | 1.83 ± 1.02 | 0.001 |
| ECP (ug/L) | 23.6 ± 24.8 | 36.3 ± 42.1 | < 0.001 |
| FEV1 (% predicted) | 81.6 ± 25.1 | 85.7 ± 21.6 | 0.07 |
| Log methacholine PC20 (mg/mL)* | 1.2 ± 14.05 | 1.1 ± 3.2 | 0.816 |
Values are given as number (%) for categorical variables and as mean ± SD for continuous variables. P values were applied by the Pearson χ2 test for categorical variables and Student's t-test for continuous variables.
EA, elderly asthma; NEA, non-elderly asthma; ECP, eosinophil cationic protein; FEV1, forced expiratory volume in 1 second; Ig, immunoglobulin; methacholine PC20, concentration of methacholine to induce a 20% decline in FEV1%; SD, standard deviation.
*Log-transformed data were shown.
Immunologic features
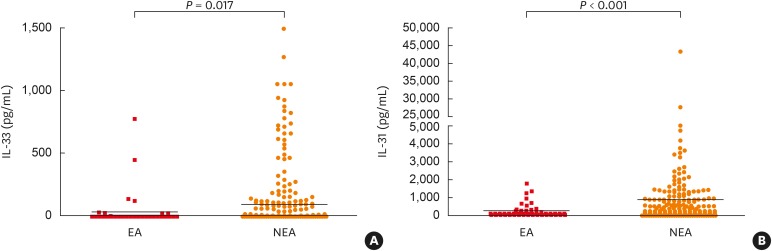
The serum levels of eotaxin-2 (1,234.7 ± 877.2 vs. 942.5 ± 594.1 pg/mL, P < 0.001), TGF-β1 (30.2 ± 15.3 vs. 25.5 ± 11.2 ng/mL, P = 0.001), and periostin (80.6 ± 39.4 vs. 57.3 ± 29.6 ng/mL, P < 0.001) were significantly higher in asthmatic patients than NCs, while no differences were found in terms of IL-8 (60.9 ± 32.7 vs. 32.7 ± 66.8 pg/mL, P = 0.420), IL-33 (72.2 ± 209.5 vs. 73.1 ± 185.1 pg/mL, P = 0.705) or IL-31 (734.5 ± 2,933.6 vs. 843.3 ± 1,686.6 pg/mL, P = 0.120) levels. When they were compared between EA and NEA patients, significantly lower levels of serum IL-31 and IL-33 were found in EA patients than in NEA patients (Table 2, Fig. 1), while no difference were found in serum IL-8, eotaxin-2, TGF-β1 and periostin levels were not significantly different between 2 groups (Table 2).
Table 2. Comparison of serum cytokine and chemokine levels between EA patients, NEA patients, ENCs and NENCs.
| Cytokines | EA (n = 234) | NEA (n = 1,218) | ENC (n = 25) | NENC (n = 647) | P value | ||
|---|---|---|---|---|---|---|---|
| EA vs. NEA | EA vs. ENC | ENC vs. NENC | |||||
| IL-8 (pg/mL) | 47.0 ± 167.8 | 64.1 ± 322.4 | 31.58 ± 25.6 | 32.9 ± 68.7 | 0.328 | 0.054 | 0.069 |
| IL-33 (pg/mL) | 24.5 ± 113.9 | 82.3 ± 223.4 | 9.78 ± 25.5 | 80.5 ± 194.3 | 0.017 | 0.456 | 0.612 |
| IL-31 (pg/mL) | 233.0 ± 730.7 | 846.6 ± 3214 | 349.3 ± 762.8 | 899.1 ± 1,754.1 | < 0.001 | 0.257 | 0.286 |
| Eotaxin-2 (pg/mL) | 1,305.9 ± 1,007.1 | 1,221.5 ± 854.3 | 867.8 ± 349.9 | 951.5 ± 617.5 | 0.814 | 0.290 | 0.732 |
| TGF-β1 (ng/mL) | 28.8 ± 15.3 | 30.3 ± 15.3 | 31.4 ± 4.37 | 25.09 ± 11.5 | 0.536 | 0.707 | 0.08 |
| Periostin (ng/mL) | 77.2 ± 42.4 | 81.5 ± 38.8 | 78.8 ± 10.9 | 55.3 ± 30.3 | 0.185 | 0.517 | 0.042 |
Values are given as mean ± SD for continuous variables. P values were applied by Mann-Whitney U test.
EA, elderly asthma; NEA, non-elderly asthma; ENC, elderly normal control; NENC, non-elderly normal control; IL, interleukin; TGF-β1, transforming growth factor beta 1; SD, standard deviation.
Fig. 1. Comparison of serum levels of IL-33 and IL-31 between EA and NEA patients. Serum cytokine levels were measured by ELISA. Each mean value is represented and P values were analyzed by the Mann-Whitney U test. P < 0.05 was considered significantly different.
IL, interleukin; EA, elderly asthma; NEA, non-elderly asthma; ELISA, enzyme-linked immunosorbent assay.
Association of clinical parameters and cytokine levels according to the severity in EA
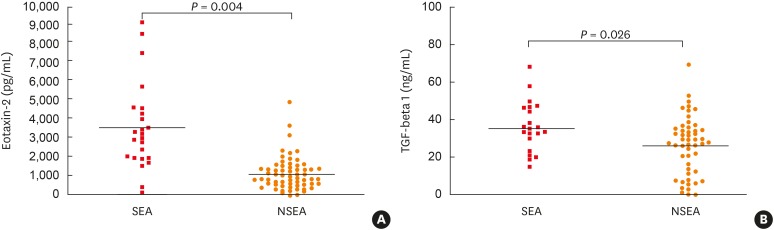
EA patients were classified into severe and non-severe eosinophilic asthma (SEA and NSEA). Although there were no significant differences in clinical variables including disease duration, atopy profile, or blood/sputum eosinophils between 2 groups, SEA patients had a significantly lower FEV1 (% predicted) level with a higher prevalence of smoking rate than NSEA patients (Table 3). Significant higher serum levels of eotaxin-2 and TGF-β1 were noted in SEA patients than in NSEA patients (Table 4, Fig. 2). When the cutoff value of serum eotaxin-2 level discriminating SEA from NSEA was determined at 1,181.6 pg/mL, the ROC curve analysis showed 66.7% sensitivity and 60.3% specificity (area under the curve [AUC] = 0.700; 95% confidence interval [CI], 0.575–0.826; P = 0.004, Supplementary Fig. S1). As for the serum TGF-β1 level, the cutoff value that discriminating SEA from NSEA patients was determined at 32.5 ng/mL with 68.2% sensitivity and 66.7% specificity (AUC = 0.664; 95% CI, 0.526–0.801; P = 0.026). There was a positive correlation between the serum eotaxin-2 and TGF-β1 levels in EA patients (r = 0.401; P < 0.001, Supplementary Fig. S2).
Table 3. Comparison of clinical and immunologic findings between SEA and NSEA patients.
| Parameters | SEA (n = 46) | NSEA (n = 127) | P values |
|---|---|---|---|
| Age (yr) | 65.3 ± 4 | 66.18 ± 4.9 | 0.293 |
| Sex (female, %) | 71.7 | 70.9 | 0.911 |
| Duration (yr) | 10.9 ± 8.6 | 7.1 ± 7.14 | 0.092 |
| Disease onset (> 40 yr, %) | 93.3 | 95.1 | 0.786 |
| Smoking (%) | 68.2 | 29.7 | 0.006 |
| Atopy (%) | 34.1 | 48.2 | 0.119 |
| Blood eosinophil count (count/uL) | 270.3 ± 218.5 | 257.0 ± 274.1 | 0.823 |
| Sputum eosinophil (%) | 13.4 ± 30.1 | 16.2 ± 29.1 | 0.722 |
| Sputum neutrophil (%) | 71.6 ± 34.2 | 66.7 ± 32.1 | 0.592 |
| Log Total IgE (kU/L)* | 1.5 ± 1.03 | 1.94 ± 0.86 | 0.028 |
| ECP (ug/L) | 29.3 ± 24.5 | 21.9 ± 25.0 | 0.202 |
| FEV1 (% predicted) | 61.09 ± 25.1 | 90.2 ± 21.9 | < 0.001 |
| Log methacholine PC20 (mg/mL)* | 0.59 ± 0.6 | 0.8 ± 0.49 | 0.225 |
| Nasal polyp (%) | 11.1 | 29 | 0.122 |
| CRS (%) | 60 | 50 | 0.428 |
Values are given as number (%) for categorical variables and as mean ± SD for continuous variables. P values were applied by the Pearson χ2 test for categorical variables and Student's t-test for continuous variables.
SEA, severe elderly asthma; NSEA, non-severe elderly asthma; Ig, immunoglobulin; ECP, eosinophil cationic protein; FEV1, forced expiratory volume in 1 second; methacholine PC20, concentration of methacholine to induce a 20% decline in FEV1; SD, standard deviation; CRS, chronic rhinosinusitis.
*Log-transformed data were shown.
Table 4. Comparison of serum cytokine and chemokine levels between SEA and NSEA patients.
| Cytokines | SEA (n = 46) | NSEA (n = 127) | P values |
|---|---|---|---|
| IL-8 (pg/mL) | 39.1 ± 58.0 | 50.7 ± 195.4 | 0.563 |
| IL-33 (pg/mL) | 8.89 ± 32.04 | 30.1 ± 130.6 | 0.873 |
| IL-31 (pg/mL) | 262.9 ± 512.7 | 227.8 ± 801.1 | 0.527 |
| Eotaxin-2 (pg/mL) | 1,763.2 ± 1,149.6 | 1,106.05 ± 877.2 | 0.004 |
| TGF-β1 (ng/mL) | 34.9 ± 15.1 | 26.3 ± 14.9 | 0.026 |
| Periostin (ng/mL) | 75.78 ± 31.7 | 77.7 ± 46.3 | 0.946 |
Values are given as mean ± SD for continuous variables. P values were applied by Mann-Whitney U test.
SEA, severe elderly asthma; NSEA, non-severe elderly asthma; IL, interleukin; TGF-β1, transforming growth factor beta 1; SD, standard deviation.
Fig. 2. Comparison of serum levels of eotaxin-2 and TGF-β1 according to severity in elderly asthma patients. Severe asthma was defined according to International ERS/ATS guideline. Serum cytokine levels were measured by ELISA. Each mean value is represented and P values were analyzed by the Mann-Whitney U test. P < 0.05 was considered significantly different.
TGF-β1, transforming growth factor beta 1; ERS/ATS, European Respiratory Society/American Thoracic Society; ELISA, enzyme-linked immunosorbent assay; SEA, severe elderly asthma; NSEA, non-severe elderly asthma.
DISCUSSION
In the present study, we characterized the clinical phenotypes of EA and compared serum epithelial-derived cytokines between EA and NEA. EA has been found to present more severe phenotypes (higher prevalence of severe asthma and lower FEV1 [% predicted]) with less type 2 inflammation, but had lower levels of serum IL-33/IL-31 than NEA. Moreover, SEA patients had higher serum levels of eotaxin-2 and TGF-β1 than NSEA. Based on these findings, we suggest that changes of epithelial cell-derived cytokines (serum IL-33, IL-31, eotaxin-2 and TGF-β1) may contribute to present less type 2, but severe phenotypes of EA.
Asthma is characterized by airway inflammation associated with reversible airflow obstruction and airway hyperresponsiveness, which can be exacerbated by allergic and non-allergic triggers including respiratory viral infections.15 Distinct clinical phenotypes of EA are characterized by LOA, fixed airway obstruction and high prevalence of comorbid diseases, which are referred as to intrinsic asthma (lower atopy rate).18,19 LOA has been recognized as an asthma phenotype representing greater airway obstruction and more frequent exacerbations followed by lung function decline.19,20 Non-Th2 type of LOA in association with aging was reported.21 In addition, respiratory viral infections, the most common asthma exacerbation factors could precipitate LOA, which immunosenescence in elderly patients could enhance virus-induced airway inflammation and asthma exacerbations.21,22,23 It is known that symptoms in EA are more difficult to control and less respond to conventional anti-inflammatory agent such as ICS, and that EA is a less eosinophilic type24,25,26 and can be exacerbated by various comorbid conditions.3,10,27 Moreover, less type 2 inflammation may shift to Th1/Th17 inflammation in EA: allergen-challenged older mice had greater mucous cell metaplasia with higher expression of interferon-γ than younger mice, showing a high bias towards a Th1 response.28 Sputum neutrophil counts were higher in EA patients29; ICS resistant type 2-low asthmatic patients showed increased expression of IL-17, which contribute to develop severe asthma with increased mucus secretion and greater airway obstruction.30 In the present study, the most EA patients had LOA, and had lower atopy rate, lower sputum eosinophilia but higher neutrophilia; they have higher prevalence of severe asthma and lower lung function. In addition, diagnosis of EA is challenging due to cognitive impairment, stagnancy, limitation in performing basic pulmonary tests and comorbidities.1 These findings suggest that EA patients present less type 2, but severe phenotype (due to the aging process and comorbid conditions); therefore, early diagnosis and careful monitoring are essential to achieve control status and to prevent lung function decline.
Airway epithelial cells are the first barrier in the airway which initiates innate/adaptive immune responses. Aging decreases airway ciliary clearance and induces disarrangement of airway cell microtubules.31,32 IL-33 (mainly expressed in epithelial cells) and its receptor ST2 have been found to be a key cytokine to derive type 2-mediated eosinophilic inflammation.33,34 IL-31 (extensively expressed in activated Th2 cells and partly in monocytes, dendritic cells, basophils and mast cells) and its receptor expressed on immune cells including bronchial epithelial cells play a role in Th2-mediated airway inflammation in the asthmatic airway; IL-31/its receptor levels expressed in bronchial tissue were positively correlated with disease severity or other Th2 cytokine expression in asthmatic airway.35,36,37 Moreover, a close link (synergic responses) between IL-33 and IL-31 was suggested in allergic airway inflammation.11,38 In the present study, EA patients had significantly lower levels of IL-33 and IL-31 with lower atopy rate and less eosinophilia compared to NEA patients, suggesting that age-related changes of epithelial cells may reduce expression of IL-33 in the airway of EA, thereby reducing IL-31 expression, which may contribute to develop less atopic/less type 2 phenotype in EA.
There have been several studies suggesting that increased levels eotaxin-2 and TGF-β1 are associated with asthma severity and control status.14,39 Eotaxin-2 mainly released from airway epithelial cells is a chemoattractant factor for eosinophils.40,41 TGF-β1, which is also released from airway epithelial cells, plays a critical role in epithelial cell apoptosis, subepithelial fibrosis and mucus hypersecretion in the process of airway inflammation/remodeling.39,41,42 In the present study, serum levels of eotaxin-2 and TGF-β1 were significantly higher in SEA than in NSEA patients with a significant correlation was between these 2 cytokines (no significant correlation with serum periostin or total eosinophil count). These findings suggest that eotaxin-2 released from airway epithelial cells plays a key role to attract eosinophils, and that TGF-β1 could lead to fixed airway obstruction and lung function decline in the asthmatic airway of severe EA. We also found no significant difference in serum periostin level between SEA and NSEA. Since serum periostin has been known as a serum biomarker for representing the phenotype of severe asthma in adult asthma cohorts,43,44,45 this conflicting finding may be derived from that serum periostin is a biomarker of type-2 inflammation; EA has less-atopic phenotype. Smoking has been suggested to be a significant risk factor for severe asthma in a Korean adult asthmatic cohort.46 Elderly asthmatics who are smokers are at higher risk of progression into asthma-chronic obstructive pulmonary disease overlap syndrome.47,48 In the present study, the prevalence of smoking history (current/ex-smokers) was significantly higher in SEA patients than in NSEA patients, suggesting that smoking is a risk factor for severe asthma in EA patients.
This study has some limitations. First, the number of elderly asthmatics, especially that of severe asthmatics, is relatively small. Secondly, this is a retrospective study based on electronic medical record and we could not monitor Asthma Control Test (ACT) scores of each patients with anti-asthmatic medications when enrolled. Thirdly, we measured cytokine levels only in serum, not sputum sample or BALF samples. Finally, we could not confirm the function of cytokines studied using in vitro and in vivo models. Further studies are needed to elucidate mechanisms by which epithelial cell function and cytokines are modified and alter airway inflammation with age in EA.
In conclusion, age-related changes of epithelial cell-derived cytokines may affect clinical phenotypes and severity of EA.
ACKNOWLEDGMENTS
This study was supported by a grant (HI16C0992) from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
ROC curves of serum eotaxin-2 level for predicting the phenotype of severe asthma. Serum eotaxin-2 level was quantified by ELISA. Serum eotaxin-2 optimal cutoff for discriminating severe elderly asthma from non-sever elderly asthma was 1,181.6 pg/mL. The sensitivity and specificity were 66.7% and 60.3%, respectively, with an AUC value of 0.700 (95% CI, 0.575–0.826; P = 0.004). The sensitivity and specificity were calculated according to optimal cutoff value. P < 0.05 was considered as significantly different.
Serum level of eotaxin-2 and TGF-β1 were positively correlated in elderly asthma patients. Serum cytokines were quantified by ELISA. Spearmann rank correlation (r) was used to analyze the correlation and P < 0.05 was considered significantly different.
References
- 1.Hanania NA, King MJ, Braman SS, Saltoun C, Wise RA, Enright P, et al. Asthma in the elderly: Current understanding and future research needs--a report of a National Institute on Aging (NIA) workshop. J Allergy Clin Immunol. 2011;128:S4–S24. doi: 10.1016/j.jaci.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillman A, Douglass JA. Asthma in the elderly. Asia Pac Allergy. 2012;2:101–108. doi: 10.5415/apallergy.2012.2.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park J, Kim TB, Joo H, Lee JS, Lee SD, Oh YM. Diseases concomitant with asthma in middle-aged and elderly subjects in Korea: a population-based study. Allergy Asthma Immunol Res. 2013;5:16–25. doi: 10.4168/aair.2013.5.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park SY, Kim JH, Kim HJ, Seo B, Kwon OY, Chang HS, et al. High prevalence of asthma in elderly women: findings from a Korean national health database and adult asthma cohort. Allergy Asthma Immunol Res. 2018;10:387–396. doi: 10.4168/aair.2018.10.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parameswaran K, Hildreth AJ, Chadha D, Keaney NP, Taylor IK, Bansal SK. Asthma in the elderly: underperceived, underdiagnosed and undertreated; a community survey. Respir Med. 1998;92:573–577. doi: 10.1016/s0954-6111(98)90311-0. [DOI] [PubMed] [Google Scholar]
- 6.Moorman JE, Mannino DM. Increasing U.S. asthma mortality rates: who is really dying? J Asthma. 2001;38:65–71. doi: 10.1081/jas-100000023. [DOI] [PubMed] [Google Scholar]
- 7.Yáñez A, Cho SH, Soriano JB, Rosenwasser LJ, Rodrigo GJ, Rabe KF, et al. Asthma in the elderly: what we know and what we have yet to know. World Allergy Organ J. 2014;7:8. doi: 10.1186/1939-4551-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathur SK, Nyenhuis SM. Changes in immune function in asthma in the elderly. Aging Health. 2009;5:551–559. doi: 10.2217/ahe.09.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray MA, Chotirmall SH. The impact of immunosenescence on pulmonary disease. Mediators Inflamm. 2015;2015:692546. doi: 10.1155/2015/692546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang EK, Jin HJ, Nam YH, Shin YS, Ye YM, Nahm DH, et al. The predictors of poorly controlled asthma in elderly. Allergy Asthma Immunol Res. 2012;4:270–276. doi: 10.4168/aair.2012.4.5.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Salvo E, Ventura-Spagnolo E, Casciaro M, Navarra M, Gangemi S. IL-33/IL-31 axis: a potential inflammatory pathway. Mediators Inflamm. 2018;2018:3858032. doi: 10.1155/2018/3858032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chai R, Liu B, Qi F. The significance of the levels of IL-4, IL-31 and TLSP in patients with asthma and/or rhinitis. Immunotherapy. 2017;9:331–337. doi: 10.2217/imt-2016-0131. [DOI] [PubMed] [Google Scholar]
- 13.Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, et al. Roles of epithelial cell-derived periostin in TGF-β activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci U S A. 2010;107:14170–14175. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman JM, Naik C, Holguin F, Ray A, Ray P, Trudeau JB, et al. Epithelial eotaxin-2 and eotaxin-3 expression: relation to asthma severity, luminal eosinophilia and age at onset. Thorax. 2012;67:1061–1066. doi: 10.1136/thoraxjnl-2012-201634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Global Initiative for Asthma. Global strategy for asthma management and prevention [Internet] place unknown: Global Initiative for Asthma; 2018. Available from: http://www.ginasthma.org. [Google Scholar]
- 16.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto M, Hoshino T, Kitasato Y, Sakazaki Y, Kawayama T, Fujimoto K, et al. Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur Respir J. 2011;37:1119–1127. doi: 10.1183/09031936.00059810. [DOI] [PubMed] [Google Scholar]
- 18.Baptist AP, Hao W, Karamched KR, Kaur B, Carpenter L, Song PX. Distinct asthma phenotypes among older adults with asthma. J Allergy Clin Immunol Pract. 2018;6:244–249.e2. doi: 10.1016/j.jaip.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathur SK. Allergy and asthma in the elderly. Semin Respir Crit Care Med. 2010;31:587–595. doi: 10.1055/s-0030-1265899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim MA, Shin SW, Park JS, Uh ST, Chang HS, Bae DJ, et al. Clinical characteristics of exacerbation prone adult asthmatics identified by cluster analysis. Allergy Asthma Immunol Res. 2017;9:483–490. doi: 10.4168/aair.2017.9.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirano T, Matsunaga K. Late-onset asthma: current perspectives. J Asthma Allergy. 2018;11:19–27. doi: 10.2147/JAA.S125948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moskwa S, Piotrowski W, Marczak J, Pawełczyk M, Lewandowska-Polak A, Jarzębska M, et al. Innate immune response to viral infections in primary bronchial epithelial cells is modified by the atopic status of asthmatic patients. Allergy Asthma Immunol Res. 2018;10:144–154. doi: 10.4168/aair.2018.10.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim CK, Callaway Z, Gern JE. Viral infections and associated factors that promote acute exacerbations of asthma. Allergy Asthma Immunol Res. 2018;10:12–17. doi: 10.4168/aair.2018.10.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barua P, O'Mahony MS. Overcoming gaps in the management of asthma in older patients: new insights. Drugs Aging. 2005;22:1029–1059. doi: 10.2165/00002512-200522120-00004. [DOI] [PubMed] [Google Scholar]
- 25.Song WJ, Jee YK. More effective strategies are needed for elderly asthmatics in real-world practice. Allergy Asthma Immunol Res. 2015;7:419–420. doi: 10.4168/aair.2015.7.5.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trinh HK, Ban GY, Lee JH, Park HS. Leukotriene receptor antagonists for the treatment of asthma in elderly patients. Drugs Aging. 2016;33:699–710. doi: 10.1007/s40266-016-0401-2. [DOI] [PubMed] [Google Scholar]
- 27.Choi GS, Shin YS, Kim JH, Choi SY, Lee SK, Nam YH, et al. Prevalence and risk factors for depression in Korean adult patients with asthma: is there a difference between elderly and non-elderly patients? J Korean Med Sci. 2014;29:1626–1631. doi: 10.3346/jkms.2014.29.12.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Busse PJ, Zhang TF, Srivastava K, Schofield B, Li XM. Effect of ageing on pulmonary inflammation, airway hyperresponsiveness and T and B cell responses in antigen-sensitized and -challenged mice. Clin Exp Allergy. 2007;37:1392–1403. doi: 10.1111/j.1365-2222.2007.02775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busse PJ, Birmingham JM, Calatroni A, Manzi J, Goryachokovsky A, Fontela G, et al. Effect of aging on sputum inflammation and asthma control. J Allergy Clin Immunol. 2017;139:1808–1818.e6. doi: 10.1016/j.jaci.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson D, Humbert M, Buhl R, Cruz AA, Inoue H, Korom S, et al. Revisiting type 2-high and type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy. 2017;47:161–175. doi: 10.1111/cea.12880. [DOI] [PubMed] [Google Scholar]
- 31.Ho JC, Chan KN, Hu WH, Lam WK, Zheng L, Tipoe GL, et al. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med. 2001;163:983–988. doi: 10.1164/ajrccm.163.4.9909121. [DOI] [PubMed] [Google Scholar]
- 32.Svartengren M, Falk R, Philipson K. Long-term clearance from small airways decreases with age. Eur Respir J. 2005;26:609–615. doi: 10.1183/09031936.05.00002105. [DOI] [PubMed] [Google Scholar]
- 33.Sjöberg LC, Nilsson AZ, Lei Y, Gregory JA, Adner M, Nilsson GP. Interleukin 33 exacerbates antigen driven airway hyperresponsiveness, inflammation and remodeling in a mouse model of asthma. Sci Rep. 2017;7:4219. doi: 10.1038/s41598-017-03674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY. IL-33 exacerbates eosinophil-mediated airway inflammation. J Immunol. 2010;185:3472–3480. doi: 10.4049/jimmunol.1000730. [DOI] [PubMed] [Google Scholar]
- 35.Ip WK, Wong CK, Li ML, Li PW, Cheung PF, Lam CW. Interleukin-31 induces cytokine and chemokine production from human bronchial epithelial cells through activation of mitogen-activated protein kinase signalling pathways: implications for the allergic response. Immunology. 2007;122:532–541. doi: 10.1111/j.1365-2567.2007.02668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai T, Wu D, Li W, Chen M, Yi Z, Huang D, et al. Interleukin-31 expression and relation to disease severity in human asthma. Sci Rep. 2016;6:22835. doi: 10.1038/srep22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu W, Luo R, Chen Y, Sun C, Wang J, Zhou L, et al. Interleukin-31 promotes helper T cell type-2 inflammation in children with allergic rhinitis. Pediatr Res. 2015;77:20–28. doi: 10.1038/pr.2014.151. [DOI] [PubMed] [Google Scholar]
- 38.Vocca L, Di Sano C, Uasuf CG, Sala A, Riccobono L, Gangemi S, et al. IL-33/ST2 axis controls Th2/IL-31 and Th17 immune response in allergic airway diseases. Immunobiology. 2015;220:954–963. doi: 10.1016/j.imbio.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Al-Alawi M, Hassan T, Chotirmall SH. Transforming growth factor β and severe asthma: a perfect storm. Respir Med. 2014;108:1409–1423. doi: 10.1016/j.rmed.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Menzies-Gow A, Ying S, Sabroe I, Stubbs VL, Soler D, Williams TJ, et al. Eotaxin (CCL11) and eotaxin-2 (CCL24) induce recruitment of eosinophils, basophils, neutrophils, and macrophages as well as features of early- and late-phase allergic reactions following cutaneous injection in human atopic and nonatopic volunteers. J Immunol. 2002;169:2712–2718. doi: 10.4049/jimmunol.169.5.2712. [DOI] [PubMed] [Google Scholar]
- 41.Ben-Yehuda C, Bader R, Puxeddu I, Levi-Schaffer F, Breuer R, Berkman N. Airway eosinophil accumulation and eotaxin-2/CCL24 expression following allergen challenge in BALB/c mice. Exp Lung Res. 2008;34:467–479. doi: 10.1080/01902140802220625. [DOI] [PubMed] [Google Scholar]
- 42.Makinde T, Murphy RF, Agrawal DK. The regulatory role of TGF-β in airway remodeling in asthma. Immunol Cell Biol. 2007;85:348–356. doi: 10.1038/sj.icb.7100044. [DOI] [PubMed] [Google Scholar]
- 43.Kim MA, Izuhara K, Ohta S, Ono J, Yoon MK, Ban GY, et al. Association of serum periostin with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2014;113:314–320. doi: 10.1016/j.anai.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Izuhara K, Ohta S, Ono J. Using periostin as a biomarker in the treatment of asthma. Allergy Asthma Immunol Res. 2016;8:491–498. doi: 10.4168/aair.2016.8.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parulekar AD, Atik MA, Hanania NA. Periostin, a novel biomarker of Th2-driven asthma. Curr Opin Pulm Med. 2014;20:60–65. doi: 10.1097/MCP.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 46.Kim TB, Park CS, Bae YJ, Cho YS, Moon HB COREA Study Group. Factors associated with severity and exacerbation of asthma: a baseline analysis of the cohort for reality and evolution of adult asthma in Korea (COREA) Ann Allergy Asthma Immunol. 2009;103:311–317. doi: 10.1016/S1081-1206(10)60530-3. [DOI] [PubMed] [Google Scholar]
- 47.Sano H, Iwanaga T, Nishiyama O, Sano A, Higashimoto Y, Tomita K, et al. Characteristics of phenotypes of elderly patients with asthma. Allergol Int. 2016;65:204–209. doi: 10.1016/j.alit.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Kim YK, Kim SH, Tak YJ, Jee YK, Lee BJ, Kim SH, et al. High prevalence of current asthma and active smoking effect among the elderly. Clin Exp Allergy. 2002;32:1706–1712. doi: 10.1046/j.1365-2222.2002.01524.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ROC curves of serum eotaxin-2 level for predicting the phenotype of severe asthma. Serum eotaxin-2 level was quantified by ELISA. Serum eotaxin-2 optimal cutoff for discriminating severe elderly asthma from non-sever elderly asthma was 1,181.6 pg/mL. The sensitivity and specificity were 66.7% and 60.3%, respectively, with an AUC value of 0.700 (95% CI, 0.575–0.826; P = 0.004). The sensitivity and specificity were calculated according to optimal cutoff value. P < 0.05 was considered as significantly different.
Serum level of eotaxin-2 and TGF-β1 were positively correlated in elderly asthma patients. Serum cytokines were quantified by ELISA. Spearmann rank correlation (r) was used to analyze the correlation and P < 0.05 was considered significantly different.