Abstract
The efficacy of second-line treatment in patients with epidermal growth factor receptor (EGFR) wild-type tumours is still debatable. We assessed the efficacy of a standard second-line chemotherapy compared with erlotinib in an individual patient data approach for meta-analysis. The primary endpoint was overall survival (OS), and secondary endpoint was progression-free survival (PFS). Both were compared by log-rank test. The ‘restricted mean survival time’ (RMST) was estimated in each study and the difference in mean survival time up to the last available time point was calculated. The Cox proportional hazards model was used on survival analyses to provide HRs, to adjust for confounding variables and to test possible interaction with selected factors. Three randomised trials comparing chemotherapy versus erlotinib were analysed, including 587 randomised patients. Overall, 74% of patients included in the original trials were considered. 464 deaths and 570 progressions or deaths were observed. Compared with erlotinib, chemotherapy was associated to a decreased risk of progression (29%; HR: 0.71, 95% CI: 0.60 to 0.84, p< 0.0001;) but with no statistical significant reduction in OS (HR: 0.89, 95% CI: 0.74 to 1.06; p<0.20). No heterogeneity was found in both analyses. Patients treated with chemotherapy gained an absolute 1.5 and 1.6 months, respectively, in PFS and lifetime (RMST 95% CI: PFS 0.49 to 2.44; OS 95% CI: −1.04 to 4.25). These results showed that patients without a constitutively activated EGFR had better PFS with chemotherapy rather than with erlotinib while no statistical difference was observed in OS.
Keywords: erlotinib, wild-type epidermal growth factor receptor (wt-EGFR), non-small cell lung cancer (NSCLC), independent patients’ data (IPD)
Introduction
Lung cancer is the leading cause of cancer death worldwide, and non-small cell lung cancer (NSCLC) accounts for approximately 80% of cases. Most patients presented with advanced disease at the time of diagnosis, with an estimated median overall survival (OS) inferior to 10 months.1
In 2004, two classes of somatic activating mutation s were described, for the first time, in the tyrosine kinase (TK) domain of the epidermal growth factor receptor gene (EGFR) in patients with advanced NSCLC. In vitro experiments showed that these molecular alterations determine the ligand independent activation of the EGFR pathway, thus making tumour cells sensitive to the small molecule epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), gefitinib.2 This finding has opened a new era in the treatment of lung cancer.
Thanks to the technological advances, several molecular alterations, amenable of targeted inhibition, have been discovered in NSCLC, particularly in the subgroup of patients with adenocarcinoma,3 substantially improving the first-line treatment options for metastatic lung cancer. Currently, patients who carry EGFR activating mutations or translocations in the anaplastic lymphoma kinase gene are treated upfront with their specific inhibitor, while chemotherapy remains the standard therapeutic choice for wild-type (WT) patients. While this distinction appears clear in first-line setting, for patients with EGFR WT or for individuals with undetermined mutation status, data from literature did not show a definite superiority of standard second-line chemotherapy regimens (docetaxel or pemetrexed) over TKIs. In the past years, several studies have been conducted, comparing EGFR-TKIs (erlotinib or gefitinib) to second-line chemotherapy in patients with NSCLC, independently of the EGFR genotype or focusing on WT patients only.4
Currently, for the treatment of patients with advanced NSCLC in the second-line setting regardless their mutational status, US Food and Drug Administration (FDA) approved, other than docetaxel and pemetrexed, the EGFR-TKI erlotinib, even though its role seems not to be appropriate for WT patients. This issue has been shown by several systematic reviews with meta-analysis that tried to pool together the aggregated data derived from these studies, just concluding that WT patients are not suitable for TKIs option.4–6
This came to an end when, in 2016, the FDA modified the indication for erlotinib (or treatment of NSCLC limiting its use to patients whose tumours have specific EGFR mutations).
Nevertheless, it is of great importance to obtain all the information needed even from those trial reporting results from subgroups. Possible imbalances between arms might be managed by appropriate analyses.
We tried in contacting all authors of relevant studies with the aim at performing an individual patient data (IPD) meta-analysis that could improve the quality of data and the type of analyses and produce more reliable results.
We also identified factors that might affect the effect of erlotinib on OS and provide an unique opportunity to shed light on this important topic. The study allows for a deeper analysis of the pooled data of these three studies, adding the information that, based on the available markers and clinical factors, there is no subgroup of patients with WT NSCLC who can take advantages from a therapy based on erlotinib.
Materials and methods
Identification of eligible trials
On January 2015, we performed a comprehensive search to identify all phase III randomised trials comparing chemotherapy over erlotinib in second line.
We searched PubMed, Embase and the Cochrane Library for papers published until January 2015 with the following keywords: “erlotinib”, “TKI”, “lung neoplasms”, “NSCLC” and “Randomized Controlled Trial”.
We also searched the proceedings of the most important international meetings (American Society of Clinical Oncology, European Society of Medical Oncology, European Cancer Conference and World Conference on Lung Cancer) from 2005 onwards until January 2015.
Studies had to be closed to patient accrual and have at least one survival outcome (OS, progression-free survival (PFS)) to be included.
Studies were excluded if the percentage of genotyped patients was less than 50% to better generalise the results.
Data collection and database quality
For all patients enrolled, we requested individual data to the authors of each identified trial. Before we did the pooled analyses, we checked the data from each individual study and verified them for coherence with the original publications, and we discussed any discrepancies with the authors. Database quality was good for all the eligible studies. Data for survival analyses, and possible prognostic or predictive factors for all randomised patients were required and provided following an ‘ad hoc’ case report form.
Adverse events were not analysed because this issue is not related to subgroups only but it is a general aspect that affects the entire population.
Statistical analysis
The analyses were performed on all randomised patients meeting the inclusion criteria, according to the intention-to-treat principle. OS was defined as the time from randomisation until death. Patients still alive were censored at the date of last follow-up. PFS was defined as the time from randomisation until progression or death whichever occurred first. Patients alive without disease progression were censored at the date of last follow-up.
As primary analysis, OS and PFS curves were estimated by the Kaplan-Meier technique, and the null hypothesis of no difference between the two arms was assessed using the log-rank test stratified by trial. The Cox proportional hazards model was used on both survival analyses to provide an estimate of the HR, to adjust for confounding variables and to test possible interaction with selected factors.
As secondary analysis, the ‘restricted mean survival time’ (RMST), a model-free and censoring-independent parameter to quantify the treatment difference, was estimated in each singular study, and the difference between arms in mean survival time up to the last available time point were calculated. When the proportional hazards assumption is not valid, the standard maximum partial likelihood estimator of the HR approximates a parameter which is difficult, if not impossible, to interpret as the treatment contrast. Therefore, it is highly desirable to consider an estimable, model-free and censoring-independent parameter to quantify the treatment difference.7 8
This approach returns an easily interpretable, clinically meaningful summary of the survival function in the presence of censoring. The RMST is the average of all potential event times measured up to a time point t*, which can be estimated consistently by the area under the Kaplan-Meier curve over [0;t*].
For the pooled analysis, 1 year for PFS and 2 years for OS were used as last available time point mean and SD of RMST were the effect measure used for meta-analysis.
For all pooled analyses, the inverse variance method was used for weighting the results of each trial. A random effect model was considered because an a priori heterogeneity among studies was thought and a χ2 heterogeneity tests was used to test it. We also calculated the I2 statistic to measure inconsistency among trials.
If there were sufficient data available, we investigated whether any observed treatment effect was consistent or not across well-defined patient subgroups. These analyses were carried out on all trials and were stratified by trial. Then subgroup analyses were done within treatment categories identified as race, sex, histology and smoking status and K-ras mutation status.
χ2 tests for interaction or trend was used to test whether there was any evidence that types of patients benefit from chemotherapy.
All statistical analyses were carried out using SAS V.9.4 with a significant level of 0.05; all meta-analyses were performed with RevMan 5.3.9
Results
Overall 7 out of 133 studies were found to meet eligible criteria for randomised comparison of chemotherapy versus erlotinib in second-line treatments for NSCLC10–16 for which we contacted corresponding authors. The studies of Lee13 and Li16 were excluded because of phase II studies. No data were available for the study HORG14 and TITAN15 because authors never answered to our request.
The analysis of the three remaining studies included all 587 patients randomised into the trials (number of erlotinib/chemotherapy: all 303/284; TAILOR10109/110; DELTA12 109/90; PROSE11 85/84); overall, 74% of patients included in the trials were considered (TAILOR 219/222: 99%; DELTA 199/301: 66%; PROSE 169/302: 62%).
Table 1 describes the characteristics of evaluated patients per study group.
Table 1.
Baseline characteristics of included studies
| TAILOR | PROSE | DELTA | ||||||||||
| Chemo: 110 | Erl: 109 | Chemo: 84 | Erl: 85 | Chemo: 90 | Erl: 109 | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Age median (minimum– maximum) | 65 (33–81) | 64 (39–78) | 64 (42–77) | 67 (47–81) | 67 (36–85) | 66 (37–82) | ||||||
| Gender | ||||||||||||
| Male | 73 | 66.4 | 77 | 70.6 | 60 | 71.4 | 59 | 69.4 | 68 | 75.6 | 85 | 78.0 |
| Female | 37 | 33.6 | 32 | 29.4 | 24 | 28.6 | 26 | 30.6 | 22 | 24.4 | 24 | 22.0 |
| Smoke habits | ||||||||||||
| Never smoker | 30 | 27.3 | 19 | 17.4 | 7 | 8.3 | 9 | 10.6 | 14 | 15.6 | 22 | 20.2 |
| Ex smoker | 52 | 47.3 | 55 | 50.5 | 51 | 60.7 | 54 | 63.5 | 68 | 75.6 | 82 | 75.2 |
| Current smoker | 28 | 25.5 | 35 | 32.1 | 26 | 31.0 | 22 | 25.9 | 8 | 8.9 | 5 | 4.6 |
| Histology | ||||||||||||
| Squamous | 21 | 19.1 | 25 | 22.9 | 12 | 14.3 | 21 | 24.7 | 19 | 21.1 | 22 | 20.2 |
| Adenocarcinoma | 74 | 67.3 | 69 | 63.3 | 58 | 69.0 | 50 | 58.8 | 58 | 64.4 | 72 | 66.1 |
| Bronchioloalveolar | 2 | 1.8 | 3 | 2.8 | 1 | 1.2 | 2 | 2.4 | NA | NA | NA | NA |
| Large cell | 2 | 1.8 | NA | NA | 4 | 4.8 | 6 | 7.1 | 4 | 4.4 | 7 | 6.4 |
| Mixed | 1 | 0.9 | 1 | 0.9 | NA | NA | NA | NA | NA | NA | NA | NA |
| Other | 6 | 5.5 | 8 | 7.3 | 7 | 8.3 | 5 | 5.9 | 9 | 10.0 | 8 | 7.3 |
| Not known | 4 | 3.6 | 3 | 2.8 | 2 | 2.4 | 1 | 1.2 | NA | NA | NA | NA |
| Performance status | ||||||||||||
| 0 | 53 | 48.2 | 52 | 47.7 | 43 | 51.2 | 46 | 54.1 | 38 | 42.2 | 52 | 47.7 |
| 1 | 50 | 45.4 | 48 | 44.0 | 36 | 42.9 | 34 | 40.0 | 49 | 54.5 | 52 | 47.7 |
| 2 | 7 | 6.4 | 9 | 8.3 | 5 | 5.9 | 5 | 5.9 | 3 | 3.3 | 5 | 4.6 |
Chemo, chemotherapy; Erl, erlotinib; NA, not applicable.
Overall, a good balancing of baseline characteristics was achieved between treatments. Analysis across studies showed that DELTA study had a proportion of male higher than the TAILOR and PROSE, and a lower proportion of smokers.
TAILOR had as primary objective whether patients with EGFR WT could benefit from treatment with docetaxel or erlotinib. DELTA, in which an Asian population was enrolled, wanted to show the superiority of erlotinib over docetaxel in second-third line, in patients not selected for EGFR mutation status. PROSE, had a different conception yet, as it wanted to demonstrate that a proteomic signature could select patients either for chemotherapy or erlotinib. Moreover, in both DELTA and PROSE studies, a subgroup analysis, testing the interaction between EGFR mutations and treatment, was preplanned, and the majority of the enrolled patients was EGFR WT.
In TAILOR and DELTA, only patients with histological diagnosis were enrolled, while PROSE allowed to enter also patients with cytological diagnosis. Furthermore, in both TAILOR and PROSE, the CT scan evaluation was performed every 8 weeks, while in DELTA, the radiological assessment was performed every month for the first 4 months, and then every 2 months. Moreover, while in TAILOR and PROSE, docetaxel was administered at the dose of 75 mg/m2 every 21 days or at 35 mg/m2 on days 1, 8, 15, every 28 days, patients enrolled in DELTA received 60 mg/m2 every 21 days. Finally, while tumour samples from patients enrolled in TAILOR and DELTA were analysed according to the Sanger method, those from patients recruited in DELTA were genotyped by a highly sensitive PCR method, the PCR-invader method, peptide nucleic acid-locked nucleic acid PCR clamp method.
PFS and overall survival analysis
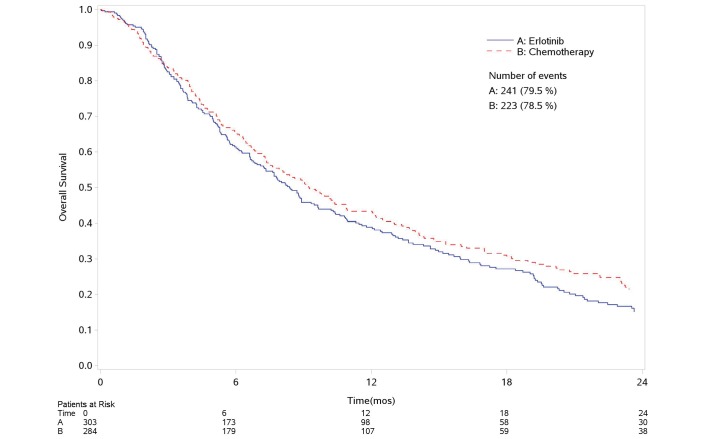
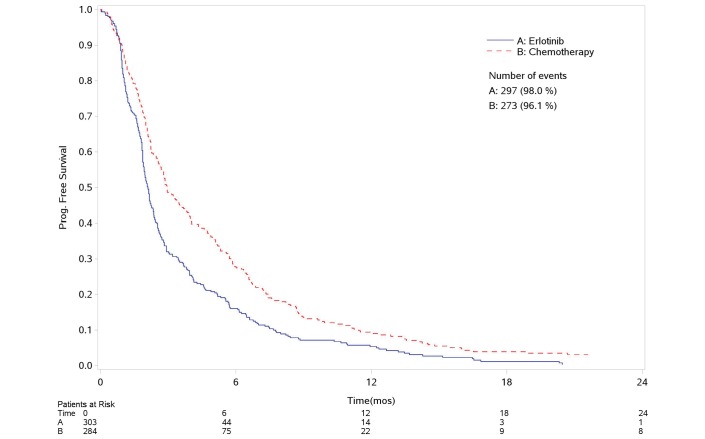
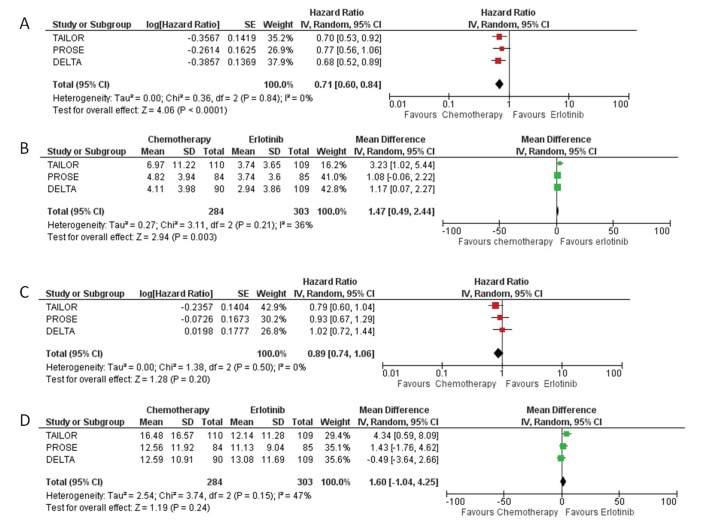
Figure 1 and figure 2 report the survival curves for OS and PFS, respectively. Four hundred and sixty-four deaths and 570 progressions or deaths were observed. Compared with erlotinib, chemotherapy was associated to a decreased risk of progression (29%) but with no reduction in OS (HR-PFS: 0.71, 95% CI: 0.60 to 0.84, p<0.0001; HR-OS: 0.89, 95% CI: 0.74 to 1.06; p<0.2, figure 3A,C). No heterogeneity was found in both analyses.
Figure 1.
Overall survival. Kaplan-Meier curve. mos, months.
Figure 2.
Progression-free survival. Kaplan-Meier curve. mos, months.
Figure 3.
Forest plot of combined HRs for progression-free survival (A); forest plot of combined difference in mean (RMSD) for progression-free survival (B); forest plot of combined HRs for overall survival (C); forest plot of combined difference in mean (RMSD) for overall survival (D). IV, inverse of variance; RMSD, root-mean-square deviation.
Patients treated with chemotherapy gained an absolute 1.5 and 1.6 months, respectively, in progression-free survival and lifetime (RMST 95% CI: PFS 0.49 to 2.44; OS 95% CI: −1.04 to 4.25).
In this case, we found a moderate heterogeneity (36% and 47%, respectively, for PFS and OS) likely because this type of analysis is affected by extreme values (figure 3B,D).
OS results were confirmed after adjustment by age, gender, smoking habit, performance status, histotype and K-RAS (HR-OS: 0.87, 95% CI: 0.72 to 1.04; p<0.137) (table 2).
Table 2.
Multivariable analysis of prognostic factors for overall survival
| Factor | Ref. Level | HR | 95% CI | P values | |
| Treatment | Chemotherapy | Erlotinib | 0.87 | 0.72 to 1.04 | 0.137 |
| Age (years) | Continous | 1.00 | 0.99 to 1.01 | 0.690 | |
| Gender | Female | Male | 0.73 | 0.58 to 0.93 | 0.011 |
| Smoke habits | Former | Current | 1.08 | 0.85 to 1.36 | 0.643 |
| Never | 0.95 | 0.68 to 1.34 | |||
| Histology | Non-squamous | Squamous | 0.87 | 0.69 to 1.11 | 0.258 |
| Performance status | >0 | 0 | 1.74 | 1.49 to 2.03 | <0.001 |
| K-ras* | Mutated | Non-mutated | 1.28 | 0.99 to 1.65 | 0.066 |
Analysis is stratified by study.
*Evaluated only in TAILOR and PROSE trials.
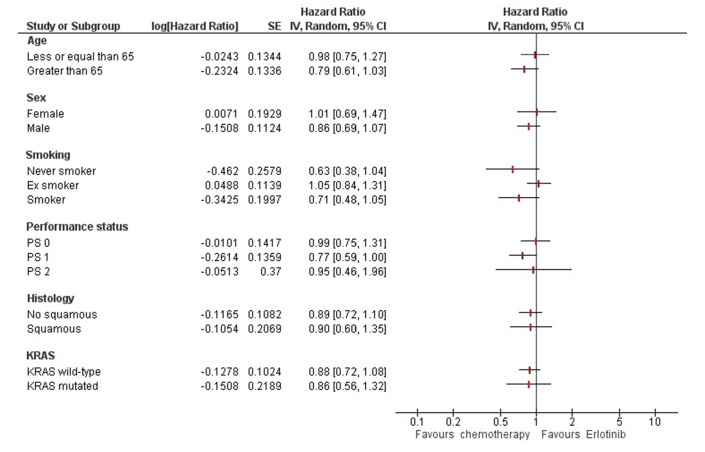
Among the factors evaluated for possible prognostic effect on survival, only gender (female) and performance status (0) were found to be associated to a better prognosis (table 2). No factors resulted associated to a differential effect of therapy (figure 4).
Figure 4.
Factors associated to differential treatment effect for overall survival. IV, inverse of variance; PS, performance status.
Discussion
In the last years, the question about treating EGFR WT NSCLC patients with erlotinib has been the focus of much debate.
When TAILOR, DELTA and PROSE were designed, the predictive role of EGFR activating mutations was not yet clear, and the therapeutic options in second-line setting were weak, based only on single agent chemotherapy or erlotinib, but with any criteria to select population.
With the aim to compare the efficacy of standard second-line chemotherapy and erlotinib in EGFR WT NSCLC patients, we performed this IPD analysis. The three studies selected, although from different rational and objectives, shared the same study design. Despite these differences, results were similar, being the PFS in favour of chemotherapy in EGFR WT patients. Unfortunately, only results from TAILOR showed a longer OS in the chemotherapy arm. It is important to emphasise that TAILOR did not allow the cross-over treatment in further lines and that only taxane naive patients were included, while PROSE and DELTA allowed previous taxane therapy. Furthermore, pemetrexed was an option for the second-line chemotherapy only in the PROSE study. These differences are potentially responsible of differing results of these three trials, even though these aspects did not reflect in heterogeneity when data were analysed using the common method.
Heterogeneity appears when results are presented as mean difference. This may be due to the fact that the effect measure suffers from extreme values.
The strength of this meta-analysis is that the results give us a more accurate estimate of the benefit of chemotherapy compared with erlotinib.
Having all the row data allowed for a very sensitive analysis of factors potentially associated to survival. Based on this analysis, we can conclude that there is no evidence of predictive role of investigated factors, and only performance status and gender results to be associated with prognosis. This was already the primary endpoint of PROSE trial with the hypothesis of demonstrating the existence of a significant interaction between the proteomic test and the treatment. Unfortunately, this was the only trial with such a design.
The weakness lies in the fact that not all trials with the required characteristics were included in this analysis because on one hand we did not receive answer from all the contacted authors, but on the other hand we arbitrary chose a minimum percentage of genotyped patients (>50%) to be included in the analysis.
A generalisation of these results is not easy to achieve even because patient characteristics among studies differed remarkably, that is, in the PROSE study there were considerable number of squamous and KRAS mutated patients; DELTA has a totally Asian population, while the others Caucasian.
Is this meta-analysis enough to say that no longer erlotinib should not be given in patients with EGFR WT? Since all three studies show that even a small percentage of patients despite the absence of EGFR mutations will benefit from erlotinib, research should focus on this question. One hypothesis is to evaluate if they are EGFR mutated patients and the Sanger’s sequencing is not enough sensitive to recognise them or if they are patients with other alterations that somehow also activate the EGFR pathway. For instance, EGFR-kinase domain duplication (KDD) recently was identified and documented that a patient with metastatic lung adenocarcinoma harbouring the EGFR-KDD derived significant antitumour response from treatment with the EGFR inhibitor afatinib. However, we are confident that in patients with good Eastern Cooperative Oncology Group performance status and able to tolerate chemotherapy in second line, based on these findings, a chemotherapeutic treatment should be preferred before using EGFR-TKI. Another key question is whether all this discussion will end with the new arrival of immunotherapy and combination of docetaxel with nintedanib or ramucirumab? The answer is no. In fact, it is already clear that the innovative therapies are not applicable for all patients. Therefore, it is possible that the treatment of the lung cancer in the future will have a therapeutic algorithm much more complex than now. Patients will be tested for a much greater number of alterations including all the new discovered activating mutations. In principle, it is possible that for most activating mutations there will be their specific correspondent inhibitor. A fraction of patients who do not have an activating mutation will be candidate for this promising new class of drugs targeting the immune checkpoints and the remaining ones still for chemotherapy alone or in combination with new agents.
The future is very promising with many treatment options unimaginable when TAILOR, DELTA and PROSE had been drawn, but the old chemotherapy remains a part of therapeutic choices alone or in combination. We currently have much more therapeutic opportunities, and the appropriate question is what are the considerations that must be included in decision process to propose the best therapeutic option for the single patient. The therapeutic choice will not only be defined by promising survival outcomes of the novel therapeutic approaches, but it will be determined by patients’ comorbidities, biomolecular and radiological tumour characteristics and by overall patients’ preference.
Acknowledgments
This paper is dedicated to the memory of Irene Floriani.
Footnotes
Contributors: Conception or design of the work; drafting the article; critical revision of the article; final approval of the version to be published: All authors. Data collection: VT and MC. Data analysis and interpretation: MC and VT.
Funding: TAILOR trial was supported by the Agenzia Italiana del Farmaco (AIFA), code: FARM6F5JER. PROSE trial was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy; the Italian Ministry of Health; and Biodesix. DELTA trial was sponsored by the National Hospital Organization, an independent administrative agency in Japan.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Goldstraw P, Crowley J, Chansky K, et al. on behalf of the International Association for the Study of Lung Cancer International Staging Committee and Participating Institutions. J Thorac Oncol 2007;2:706–14.17762336 [Google Scholar]
- 2.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497–500. 10.1126/science.1099314 [DOI] [PubMed] [Google Scholar]
- 3.Rosell R, Karachaliou N. Large-scale screening for somatic mutations in lung cancer. Lancet 2016;387:1354–6. 10.1016/S0140-6736(15)01125-3 [DOI] [PubMed] [Google Scholar]
- 4.Vale CL, Burdett S, Fisher DJ, et al. Should tyrosine kinase inhibitors be considered for advanced non-small-cell lung cancer patients with wild type EGFR? Two systematic reviews and meta-analyses of randomized trials. Clin Lung Cancer 2015;16:173–82. 10.1016/j.cllc.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao N, Zhang XC, Yan HH, et al. Efficacy of epidermal growth factor receptor inhibitors versus chemotherapy as second-line treatment in advanced non-small-cell lung cancer with wild-type EGFR: a meta-analysis of randomized controlled clinical trials. Lung Cancer 2014;85:66–73. 10.1016/j.lungcan.2014.03.026 [DOI] [PubMed] [Google Scholar]
- 6.Li N, Yang L, Ou W, et al. Meta-analysis of EGFR tyrosine kinase inhibitors compared with chemotherapy as second-line treatment in pretreated advanced non-small cell lung cancer. PLoS One 2014;9:e102777 10.1371/journal.pone.0102777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol 2013;13:152 10.1186/1471-2288-13-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.COBRA. On the restricted mean event time in survival analysis. http://biostats.bepress.com/harvardbiostat/paper156
- 9.Review Manager (RevMan). [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. [Google Scholar]
- 10.Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 2013;14:981–8. 10.1016/S1470-2045(13)70310-3 [DOI] [PubMed] [Google Scholar]
- 11.Gregorc V, Novello S, Lazzari C, et al. Predictive value of a proteomic signature in patients with non-small-cell lung cancer treated with second-line erlotinib or chemotherapy (PROSE): a biomarker-stratified, randomised phase 3 trial. Lancet Oncol 2014;15:713–21. 10.1016/S1470-2045(14)70162-7 [DOI] [PubMed] [Google Scholar]
- 12.Kawaguchi T, Ando M, Asami K, et al. Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol 2014;32:1902–8. 10.1200/JCO.2013.52.4694 [DOI] [PubMed] [Google Scholar]
- 13.Lee DH, Lee JS, Kim SW, et al. Three-arm randomised controlled phase 2 study comparing pemetrexed and erlotinib to either pemetrexed or erlotinib alone as second-line treatment for never-smokers with non-squamous non-small cell lung cancer. Eur J Cancer 2013;49:3111–21. 10.1016/j.ejca.2013.06.035 [DOI] [PubMed] [Google Scholar]
- 14.Karampeazis A, Voutsina A, Souglakos J, et al. Pemetrexed versus erlotinib in pretreated patients with advanced non-small cell lung cancer: a Hellenic Oncology Research Group (HORG) randomized phase 3 study. Cancer 2013;119:2754–64. 10.1002/cncr.28132 [DOI] [PubMed] [Google Scholar]
- 15.Ciuleanu T, Stelmakh L, Cicenas S, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol 2012;13:300–8. 10.1016/S1470-2045(11)70385-0 [DOI] [PubMed] [Google Scholar]
- 16.Li N, Ou W, Yang H, et al. A randomized phase 2 trial of erlotinib versus pemetrexed as second-line therapy in the treatment of patients with advanced EGFR wild-type and EGFR FISH-positive lung adenocarcinoma. Cancer 2014;120:1379–86. 10.1002/cncr.28591 [DOI] [PubMed] [Google Scholar]