Abstract
Purpose
We studied effects of diet-induced postmenopausal weight loss on gene expression and activity of proteins involved in lipogenesis and lipolysis in adipose tissue.
Methods
Fifty-eight postmenopausal women with overweight (BMI 32.5 ± 5.5) were randomized to eat an ad libitum Paleolithic-type diet (PD) aiming for a high intake of protein and unsaturated fatty acids or a prudent control diet (CD) for 24 months. Anthropometry, plasma adipokines, gene expression of proteins involved in fat metabolism in subcutaneous adipose tissue (SAT) and lipoprotein lipase (LPL) activity and mass in SAT were measured at baseline and after 6 months. LPL mass and activity were also measured after 24 months.
Results
The PD led to improved insulin sensitivity (P < 0.01) and decreased circulating triglycerides (P < 0.001), lipogenesis-related factors, including LPL mRNA (P < 0.05), mass (P < 0.01), and activity (P < 0.001); as well as gene expressions of CD36 (P < 0.05), fatty acid synthase, FAS (P < 0.001) and diglyceride acyltransferase 2, DGAT2 (P < 0.001). The LPL activity (P < 0.05) and gene expression of DGAT2 (P < 0.05) and FAS (P < 0.05) were significantly lowered in the PD group versus the CD group at 6 months and the LPL activity (P < 0.05) remained significantly lowered in the PD group compared to the CD group at 24 months.
Conclusions
Compared to the CD, the PD led to a more pronounced reduction of lipogenesis-promoting factors in SAT among postmenopausal women with overweight. This could have mediated the favorable metabolic effects of the PD on triglyceride levels and insulin sensitivity.
Keywords: Lipoprotein lipase, Obesity, Postmenopausal women, Diet, Fat metabolism
Introduction
Obesity, particularly abdominal obesity, is a major cause of morbidity and mortality. Among women, the prevalence of abdominal adiposity increases after menopause and is associated with an increased risk for metabolic disease [1].
Adipose tissue stores energy as triacylglycerols (TGs) in lipid droplets formed through lipogenesis, and fatty acids (FAs) are released from these stored TGs via lipolysis. Both processes are reportedly elevated in insulin-resistant individuals with obesity compared to insulin-sensitive individuals with obesity [2]. The cycle of lipid synthesis and degradation is required for the formation of diacylglycerols (DAGs) and free fatty acids (FFAs), which acts as regulatory ligands of nuclear receptors [3]. Elevated formation of FFAs and DAGs due to increased lipolysis in adipose tissue, may contribute to impaired intracellular insulin signaling, i.e., insulin resistance [2].
TGs in adipose tissue primarily originate from FAs released from TG-rich lipoproteins following lipoprotein lipase (LPL)-mediated intravascular lipolysis [4]. LPL is thus considered a gatekeeper enzyme to play an important role in the initiation and development of obesity [4]. Released FAs can enter adipocytes via either passive diffusion or through diffusion facilitated by the major transport protein CD36 [2].
Within a fat cell, FAs undergo a series of enzymatic reactions leading to their storage as TGs in lipid droplets. The final and likely rate-limiting step in TGs synthesis is catalyzed by diglyceride acyltransferase 2 (DGAT2) [5]. Fatty acid synthase (FAS) is an important factor in de novo lipogenesis in adipocytes, and is elevated in cases of obesity and in type 2 diabetes [6]. In cases of obesity, basal lipolysis may be elevated by increased production of pro-inflammatory factors such as TNF-α, increasing transcription of the rate-limiting enzyme adipose triglyceride lipase (ATGL) [7]. Moreover, lipolysis is controlled by a number of lipid droplet-associated proteins that influence droplet formation and stability [5]. In particular, perilipin1 is a key factor that protects TGs from hydrolysis by ATGL [7].
White adipose tissue is not only an energy-storage organ, but also an endocrine organ secreting a variety of adipokines, acting in locally or systemically ways. Adipokines, including leptin, adipsin and adiponectin, have endocrine effects on insulin sensitivity; and leptin also affects energy homeostasis. The secretions of these adipokines are affected by fat storage but the effect of macronutrient content in the diet is not well studied.
A recent study comparing before and after menopause demonstrated that postmenopausal women showed an increased tendency to store TGs in subcutaneous adipose tissue (SAT), associated with increased lipogenesis [8]. Thus, further studies regarding the putative reversibility of altered fat metabolism among postmenopausal women with overweight are of major interest.
We previously made a diet intervention with a 5-week ad libitum Paleolithic type diet (PD), characterized by a moderately increased intake of protein and high contents of monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs). This diet profoundly decreased abdominal obesity, blood lipid levels, and increased hepatic insulin sensitivity among postmenopausal women with obesity [9]. More recently we made a study on postmenopausal women with obesity, which revealed that a PD had sustained effects on circulating TG levels [10]. Moreover, a PD has also been reported to improve glucose sensitivity, lipid profiles, and blood pressure among healthy sedentary humans without concomitant weight loss [11].
Our hypothesis was that a diet-induced weight loss would affect the levels of adipokines, lipogenesis and lipolysis in postmenopausal women with obesity. We tested in this secondary analysis whether a PD with a high intake of unsaturated fat and a low intake of carbohydrates would have more pronounced beneficial effects on adipokines and key proteins in fat metabolism than a conventional prudent diet with a high carbohydrate content (CD).
Methods
Subjects and clinical protocol
A CONSORT flow diagram and additional details regarding inclusion criteria, dietary instructions, and procedures for anthropometry and dual-energy X-ray absorptiometry are described in a previous paper by Mellberg et al. [10].
Briefly, 70 postmenopausal women (age 60.5 ± 5.6 years) with overweight or obesity (BMI, 27–41 kg/m2) and normal fasting plasma glucose levels were randomized to an ad libitum Paleolithic-type diet (PD) or a prudent control diet (CD). Only women that had experienced at least 12 consecutive months without menstruation were included in the study. The CD followed the Nordic Nutrition Recommendations aimed to include 15 energy percent (E%) protein, 55 E% carbohydrates and 30 E% fat. The CD was based on high-fiber products, meat, fish, vegetables, fruits and low-fat dairy products. The PD aimed to include 30 E% protein, 30 E% carbohydrates, and 40 E% fat, with recommendations for a high intake of MUFAs and PUFAs, and a relatively low intake of carbohydrate. The PD was based on lean meat, fish, eggs, vegetables, fruits, berries, and nuts. Additional fat sources included avocado and rapeseed and olive oil used in food preparation and dressings. The PD excluded dairy products, cereals, added salt, and refined fats and sugar.
Throughout the entire intervention period, each group participated in a total of 12 group sessions led by dieticians. The group sessions gave information on the intervention diets and how to cook using recipes. They also included group discussions and information regarding dietary impacts on health and behavioral changes. During the first 6 months of the intervention, eight group sessions were held, followed by one group session every 3 months until the end of the intervention.
The present secondary analysis on fat metabolism included 58 women that had abdominal fat biopsies taken at baseline and after 6 months of dietary intervention. Dietary intake was assessed using 4-day (3 week days and 1 weekend day) estimated self-reported food records collected at baseline and monthly for 6 months. The reported food intake was converted to the estimates of energy and nutrient intake using the nutritional analysis package Dietist XP (version 3.0, Kost och Näringsdata AB, Bromma, Sweden) based on the food composition database of the Swedish National Food Administration (2008-03-06) [10].
Physical activity was measured using the Actiheart® monitor during a 7-day period, at baseline and at 6 months, concurrently with the self-reported food records. The study participants gave written informed consent, and the study was approved by the Regional Ethical Review Board at Umeå University. This trial was registered at clinicaltrials.gov as NCT00692536.
Blood samples were obtained after overnight fasting at baseline and 6 months. Plasma glucose and lipid levels were analyzed using a Vitros 5.1FS automated chemistry analyzer (Vitros Slides; Ortho-Clinical Diagnostics, Johnson & Johnson, NJ, USA). FFAs were determined in serum following the ACS-ACOD method using a NEFA-HR kit (Wako, Neuss, Germany). Insulin sensitivity was calculated applying the homeostasis model assessment for insulin resistance (HOMA-IR) [12]. SAT was obtained by needle aspiration under local anesthesia (Xylocaine 10 mg/mL; Astra Zeneca, Södertälje, Sweden), as previously described [13].
RNA extraction and real-time RT-PCR
Total RNA was extracted from SAT biopsies using the RNeasy® Lipid Tissue Mini kit and the RNA reversed transcribed using TaqMan® reverse transcription reagents as previously described [14]. Relative quantification real-time PCR was performed using an ABI Prism® 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) with Universal PCR Master Mix 2X (Roche Molecular Systems) and TaqMan gene expression assays (Applied Biosystems) for DGAT2 (Hs01045913_m1), FAS (Hs01005622_m1), LPL (Hs00173425_m1), ATGL (alias PNPLA2; Hs00386101_m1), Perilipin1 (alias PLIN; Hs00160173_m1), CD36 (Hs01567185_m1) and LRP10 (Hs00204094_m1). Reference genes were evaluated by comparing PPIA (Hs999999904_m1) and LRP10 within the full study cohort using the NormFinder algorithm, and calculated the %CV [15]. Accordingly, LRP10 appeared to be the most suitable gene. Accordingly, the expression levels of the target genes were normalized to LRP10. Due to the limited amounts of adipose tissue in the biopsies we could only analyze gene expression at baseline and after 6 months.
All samples from each subject were analyzed on the same plate in duplicate. To reduce interference from plate biases, subjects were paired and balanced according to diet, fat distribution, insulin sensitivity index, and blood pressure parameters. Samples/subjects were balanced and paired using a space-filling design from a principle component analysis model calculated based on the subjects’ baseline characteristics [16].
LPL activity and mass measurements
LPL activity and mass were measured in SAT as previously described [17]. The presented data are the mean values of three determinations. For LPL activity, 1 mU corresponds to the release of 1 nmol fatty acids per min. Samples taken at baseline, at 6 months and 24 months from the same individual were analyzed on the same day and in the same assay, to reduce inter-assay variability.
Analysis of adipokines in serum
Serum concentrations of leptin, adipsin, and adiponectin were determined using the Bio-Rad human diabetes kit (Hercules, CA, USA) following the manufacturer’s instructions, with the addition that all samples were centrifuged for 30 s at 11,000×g to remove any debris. All samples were assayed in duplicate and analyzed using the Luminex 200 Labmap system (Austin, TX, USA). Data were analyzed using Bio-Plex Manager software version 4.1.1 or 6.0 (Bio-Rad). Protein concentrations were interpolated from the appropriate standard curve. Mean %CV values were 4.0% for adiponectin, and 7.8% for adipsin and leptin.
Statistical analysis
PCA/OPLS
We performed further sample comparison modeling using a multivariate data analysis strategy to elucidate intervention-related effects on the whole fat metabolism profile. First, the data were inspected using principal components analysis (PCA) to detect potential outliers and clusters. Second, each individual’s sample collected after 6 months of intervention was subtracted from its baseline sample and missing data excluded. At last, we applied a variant of orthogonal partial least squares analysis (OPLS) [18], OPLS-effect projections (OPLS-EP) [19]. OPLS-EP extracts metabolic profiles based on paired analyses of individual effects, i.e., the dietary intervention effect. Because each subject acted as her own control, this strategy minimizes the influence from confounding factors, such as inter-individual variation [19]. The multivariate models were validated by calculating P values based on ANOVA from the cross-validated scores (CV-ANOVA). To ensure proper cross-validation groups and reduce the chance of creating an over-fitted model, special consideration was taken to keep the same participants in the same group. The multivariate confidence intervals presented here were based on jack-knifing [20]. OPLS-EP analyses were performed during weight loss (0–6 months) and weight maintenance (6–24 months).
Generalized estimating equations
General estimating equations (GEE) and linear regression analyses were performed using IBM SPSS Statistics for Mac, Version 22.0 (IBM Corp., Armonk, NY, USA). Data describing the anthropometrical and biochemical parameters are presented as mean ± SD. The sample size was estimated using power analysis based on changes in fat mass in a pilot study of postmenopausal women on a PD. It was estimated that 30 participants were needed in each group to achieve P < 0.05 with 80% power. The effects of diet over time were analyzed using separate multiple regression models, each including diet group, time, and the group-by-time interaction as predictors. Regression parameters were estimated using GEE, a method that tolerates some degree of between-group variance. An exchangeable correlation structure was used to model the dependence between repeated measurements within participants. Prior to analysis, dependent variables with a skewed distribution were transformed using natural logarithms. Outcome is presented as P values for the included factors and estimated marginal means, with corresponding 95% confidence intervals for each diet at each time-point. P value of < 0.05 was considered statistically significant.
Linear regression analysis
Univariate linear regression analyses were used to identify and characterize the relationship between a dependent variable and an independent variable. Outcome was presented using P values for the included factor and the coefficient of correlation R.
Results
A previous publication describes the results regarding anthropometry and metabolic functions, including circulating lipid levels and insulin sensitivity in all 70 participants [10]. This secondary analysis presents lipogenesis and lipolysis in adipose tissue in the 58 women with fat biopsies during the first 6 months of the study period. LPL mass and activity were analyzed at 24 months. Gene expression was not analyzed due to lack of fat tissue.
OPLS
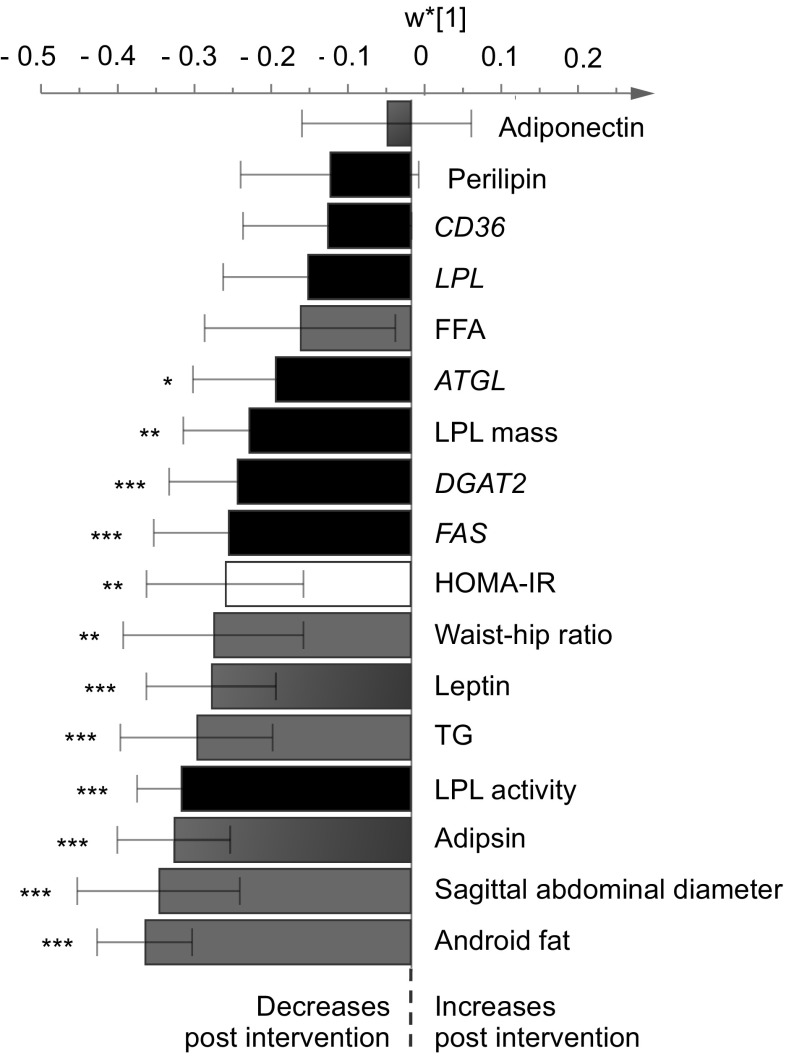
The OPLS analysis included the following variables: Fat distribution, blood lipids, insulin sensitivity, gene expression and activity levels of key proteins involved in lipogenesis and lipolysis, and adipokines (Fig. 1), at 6 months. We obtained a significant OPLS model only for the PD group (Fig. 1). However, an additional analysis including both groups in the same OPLS model revealed identical patterns of the included variables for both diets as for the PD group alone (data not shown). This suggests that the PD group response on the included variables is more pronounced as compared to the CD group and that no new information is to be found when including both groups in the same model.
Fig. 1.
A multivariate model of individual differences in postmenopausal women with overweight comparing samplings at baseline to those after 6 months of an ad libitum Paleolithic type diet (PD) intervention. Variables with negative axis values (multivariate OPLS loadings, w* [1]) are decreased after the intervention and vice versa for those with positive axis values. The shown confidence intervals are multivariate confidence interval, based on jack-knifing using a 95% confidence level. Bars labeled with stars are significantly altered after the intervention by means of two-sided paired t-tests, i.e. *P < 0.05, **P < 0.01, ***P < 0.001. ATGL adipose triglyceride lipase, DGAT2 diglyceride acyltransferase 2, FFAs free fatty acids, FAS fatty acid synthase, LPL lipoprotein lipase; TGs triacylglycerols
Sagittal abdominal diameter, android fat mass, and plasma TG levels decreased significantly, with android fat mass showing the most pronounced reduction. Insulin resistance (estimated by the HOMA-IR index) decreased significantly, with concomitant reductions of adipsin and leptin. With regards to lipogenesis and lipolysis, LPL activity showed the most pronounced reduction, followed by FAS, DGAT2, and LPL mass. We also detected a reduced expression of the ATGL gene, a key factor in intracellular lipolysis.
Generalized estimating equations
Anthropometric data
After 6 months of the intervention the PD group showed significantly larger reductions in body weight and sagittal abdominal diameter compared to the CD group (Table 1).
Table 1.
Changes of anthropometric data, serum lipids and adipokines in postmenopausal women with overweight at baseline and after 6 months of an intervention with an ad libitum Paleolithic-type diet (PD) or prudent control diet (CD)
| PD | Changes 0–6 months | CD | Changes 0–6 months | Model effect Diet by time |
|||
|---|---|---|---|---|---|---|---|
| Baseline | 6 months | P | Baseline | 6 months | P | P | |
| Age (years) | 60 ± 5.5 | 62 ± 5.7 | |||||
| Weight (kg) | 87 ± 10 | − 7.8 ± 4.5 | <0.001 | 87 ± 9.6 | − 3.9 ± 4.6 | < 0.001 | < 0.05 |
| Sagittal abdominal diameter (cm) | 22 ± 2.1 | − 4.3 ± 3.3 | <0.001 | 22 ± 2.1 | − 0.03 ± 1.9 | < 0.001 | < 0.001 |
| Waist/hip | 0.93 ± 0.07 | − 0.05 ± 0.07 | <0.001 | 0.94 ± 0.06 | − 2.4 ± 0.04 | < 0.001 | NS |
| Serum FFAs (mmol/L) | 0.49 ± 0.20 | − 0.06 ± 0.19 | NS | 0.52 ± 0.17 | − 0.06 ± 0.14 | < 0.05 | NS |
| Serum TGs (mmol/L) | 1.2 ± 0.53 | − 0.39 ± 0.41 | <0.001 | 1.3 ± 0.55 | − 0.11 ± 0.38 | NS | < 0.001 |
| Cholesterol in serum (mmol/L) | 5.9 ± 0.81 | − 0.66 ± 0.74 | < 0.001 | 5.6 ± 1.2 | − 0.38 ± 0.82 | 0.035 | NS |
| LDL-C (mmol/L) | 3.9 ± 0.76 | − 0.43 ± 0.58 | < 0.001 | 3.7 ± 1.1 | − 0.30 ± 0.63 | 0.037 | NS |
| HDL-C (mmol/L) | 1.5 ± 0.36 | − 0.07 ± 0.30 | NS | 1.3 ± 0.24 | − 0.04 ± 0.20 | NS | NS |
| HOMA-IR | 1.8 ± 1.1 | − 0.32 ± 1.3 | < 0.01 | 2.2 ± 1.0 | − 0.08 ± 1.3 | NS | NS |
| Adiponectin (mg/L) | 38 ± 13 | − 3.7 ± 7.0 | NS | 37 ± 12 | − 5.6 ± 8.1 | < 0.05 | NS |
| Leptin (ng/L) | 12 ± 5.0 | − 4.3 ± 5.1 | < 0.001 | 11 ± 4.4 | − 2.7 ± 2.7 | < 0.001 | NS |
| Adipsin (ng/L) | 340 ± 110 | − 100 ± 76 | < 0.001 | 330 ± 120 | − 110 ± 106 | < 0.001 | NS |
Data are shown as mean ± SD. n = 23–25 for the CD group; n = 32–33 for the PD group. Different n within a group is due to missing samples and different n between groups is due to a higher dropout rate in the CD group. Regression parameters were estimated by generalized estimating equations
FFAs free fatty acids, HDL-C high-density lipoproteins cholesterol, LDL-C low-density lipoprotein cholesterol, TGs triacylglycerols
Reported energy intake
The reported energy intake decreased similarly in both groups, while physical activity levels remained stable (Table 2). The reported intake of protein increased significantly more in the PD group compared to the CD group, but did not reach the target level of 30 E%. The PD group also reported a significantly higher intake of unsaturated FAs and cholesterol than the CD group (Table 2). Compared to baseline, the PD group reported a significantly decreased intake of carbohydrates, which was significantly lower than that reported by the CD group (Table 2). The intake ratio of fiber-to-carbohydrate increased in both groups and was more pronounced in the PD group compared to the CD group (Table 2). The reported intake of monosaccharides and disaccharides remained stable over time in both groups.
Table 2.
Changes of nutrient intake and physical activity in postmenopausal women with overweight at baseline and at 6 months of an intervention with an ad libitum Paleolithic-type diet (PD) or prudent control diet (CD)
| PD | CD | Model effect diet by time | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Change 0–6 months | P | Baseline | Change 0–6 months | P | P | |
| Energy intake (MJ/d) | 8.4 ± 1.5 | − 1.5 ± 1.5 | < 0.001 | 8.7 ± 1.6 | − 1.9 ± 1.5 | < 0.001 | NS |
| PAEE (MJ/d) | 3.2 ± 0.82 | − 0.10 ± 0.82 | NS | 3.3 ± 1.1 | − 0.08 ± 0.90 | NS | NS |
| Carbohydrate intake (E%) | 46 ± 4.1 | − 17.0 ± 5.7 | < 0.001 | 46 ± 4.5 | − 1.5 ± 5.9 | NS | < 0.001 |
| Mono- and disaccharide intake (E%) | 18 ± 5.7 | 0.73 ± 5.2 | NS | 20 ± 6.7 | − 1.2 ± 7.3 | NS | NS |
| Fiber intake (g)/carbohydrate intake (g) | 0.11 ± 0.02 | 0.09 ± 0.03 | < 0.001 | 0.10 ± 0.02 | 0.03 ± 0.03 | < 0.001 | < 0.001 |
| Fat intake (E%) | 34 ± 3.6 | 10 ± 6.7 | < 0.001 | 34 ± 3.8 | − 2.5 ± 4.8 | < 0.01 | < 0.001 |
| SFAs intake (E%) | 13 ± 2.1 | − 3.0 ± 3.2 | < 0.001 | 13 ± 2.0 | 1.8 ± 2.5 | < 0.001 | NS |
| MUFAs intake (E%) | 13 ± 1.9 | 7.9 ± 4.0 | < 0.001 | 13 ± 2.1 | − 1.3 ± 2.4 | < 0.01 | < 0.001 |
| PUFAs intake (E%) | 5.5 ± 1.3 | 4.2 ± 3.0 | < 0.001 | 5.4 ± 1.1 | 0.21 ± 1.8 | NS | < 0.001 |
| Cholesterol intake (E%) | 0.13 ± 0.03 | 0.29 ± 0.56 | < 0.001 | 0.15 ± 0.04 | 0.01 ± 0.06 | NS | < 0.001 |
| Protein intake (E%) | 17 ± 1.9 | 6.3 ± 2.9 | < 0.001 | 17 ± 2.5 | 1.9 ± 2.7 | < 0.001 | < 0.001 |
Data are presented as mean ± SD. n = 23–25 for the CD group; n = 32–33 for the PD group. Different n within a group is due to missing samples and different n between groups is due to a higher dropout rate in the CD group. The regression parameters were estimated by generalized estimating equations
MUFAs monounsaturated fatty acids, PAEE physical activity energy expenditure, PUFAs polyunsaturated fatty acids, SFAs saturated fatty acids
Circulating lipids and HOMA-IR
Serum TGs decreased significantly more in the PD group compared to the CD group (Table 1). Total serum cholesterol levels and LDL-cholesterol (LDL-C) decreased in both groups, without differences between groups (Table 1). The levels of HDL cholesterol and FFA remained stable in both groups (Table 1). The HOMA-IR index decreased significantly in the PD group, without significant difference between diet groups (Table 1).
Lipogenesis- and lipolysis promoting factors
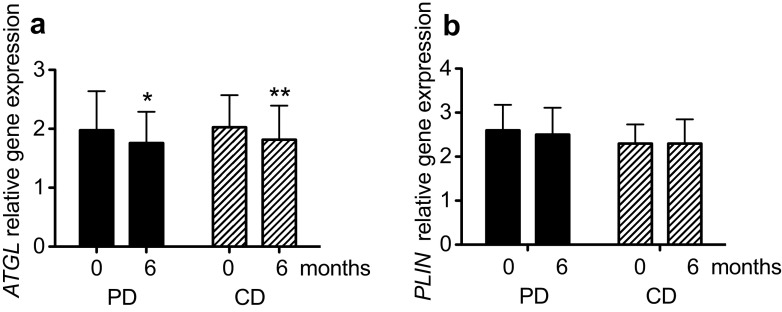
The gene expressions of LPL and CD36 were significantly decreased in the PD group, but no between-group differences were found (Figs. 2a, 3a). Expressions of DGAT2 and FAS decreased significantly more in the PD group compared to the CD group (P < 0.05 for both; Fig. 3b, c). The expression of ATGL decreased significantly in both groups, with no differences between groups (Fig. 4a). Perilipin1 mRNA levels were unchanged in both diet groups during the intervention (Fig. 4b).
Fig. 2.
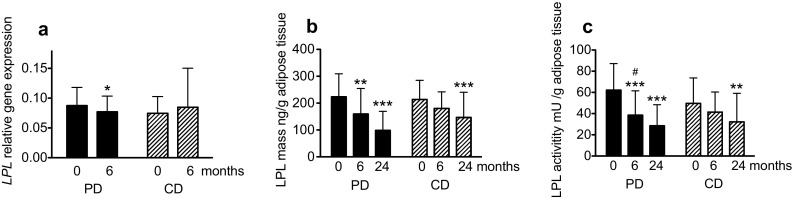
Lipoprotein lipase (LPL) relative gene expression (a), mass (b), and enzyme activity (c) in postmenopausal women with overweight after a 6-month and 24 month intervention with an ad libitum Paleolithic type diet (PD) or prudent control diet (CD) compared with at baseline. Data are presented as mean ± SD. n = 23–25 for the CD group; n = 32–33 for the PD group. Different n within a group is due to missing samples and different n between groups is due to a higher dropout rate in the CD group. The regression parameters were estimated using generalized estimating equations. Difference from baseline: *P < 0.05, **P < 0.01, ***P < 0.001; difference in change between groups (diet × time effect) # P < 0.05
Fig. 3.
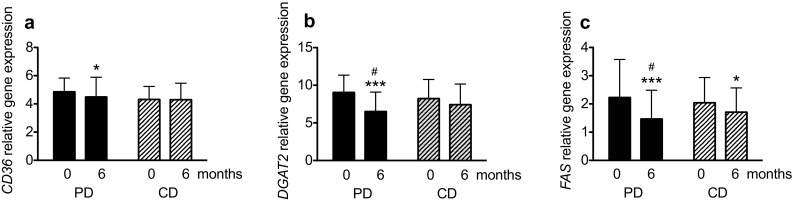
Relative expressions of CD36 (a), DGAT2 (b) and FAS (c), that encode proteins involved in lipogenesis in postmenopausal women with overweight after a 6-month intervention with an ad libitum Paleolithic-type diet (PD) or prudent control diet (CD) compared with at baseline. Data are presented as mean ± SD. n = 23–25 for the CD group; n = 32–33 for the PD group. Within-group differences in n were due to missing samples and between-group differences in n were due to a higher dropout rate in the CD group. The regression parameters were estimated using generalized estimating equations. Differences from baseline: *P < 0.05, **P < 0.01, ***P < 0.001; difference in change between groups (diet x time effect) # P < 0.05. DGAT2 diglyceride acyltransferase 2, FAS fatty acid synthase
Fig. 4.
Relative expressions of ATGL (a) and PLIN (b), that encode proteins involved in lipolysis in postmenopausal women with overweight after a 6-month intervention with an ad libitum Paleolithic-type diet (PD) or prudent control diet (CD) compared with at baseline. Data are presented as mean ± SD. n = 23–25 for the CD group; n = 32–33 for the PD group. Within-group differences in n were due to missing samples and between-group differences in n were due to a higher dropout rate in the CD group. The regression parameters were estimated using generalized estimating equations. Differences from baseline: *P < 0.05, **P < 0.01, ***P < 0.001. ATGL adipose triglyceride lipase, PLIN perilipin1
LPL mass, and activity levels decreased significantly in the PD group after 6 and 24 months, and there were significant differences in changes of LPL activity between the diet groups at both 6 and 24 months (P < 0.05 for both; Fig. 2b, c). Significant associations were found between the changed LPL activity and LPL mass at 24 months using linear regression analyses for both the PD group (R = 0.73, P = 0.001) and the CD group (R = 0.61, P = 0.019).
Adipokines in serum
Serum levels of leptin and adipsin decreased in both groups with no significant differences between groups (Table 1). Adiponectin levels decreased in the CD group, without differences between groups (Table 1).
Linear regression analysis
Using linear regression analyses, we tested possible associations between the main outcomes related to adipose tissue fat metabolism (i.e., LPL, DGAT2, FAS, CD36 and ATGL gene expressions; LPL mass and activity) and sagittal abdominal diameter, diet intake (i.e., carbohydrates and PUFAs) or HOMA-IR/insulin.
In the PD group, a significant association at 24 months was found between changes in sagittal abdominal diameter and LPL activity (R = 0.47, P < 0.05) and at 6 months between sagittal abdominal diameter and gen expression of DGAT2 (R = 0.48, P < 0.001), FAS (R = 0.48, P < 0.05), CD36 (R = 0.25, P < 0.05), LPL mass (R = 0.36, P < 0.05) and LPL activity (R = 0.58, P < 0.001). There were also significant associations between reported intake of carbohydrate and gene expression of DGAT2 (R = 0.38, P < 0.01), FAS (R = 0.27, P < 0.05), CD36 (R = 0.25, P < 0.05) and for LPL mass (R = 0.33, P < 0.05) and LPL activity (R = 0.44, P < 0.01) at 6 months in the PD group. Furthermore, significant associations at 6 months were found in the PD group between reported intake of PUFA and gene expression of DGAT2 (R = 0.42, P < 0.001), FAS (R = 0.34, P < 0.01), and for LPL activity (R = 0.34, P < 0.05). Significant associations between LPL mass and HOMA-IR (R = 0.38, P < 0.05) and circulating insulin levels (R = 0.42, P < 0.05) were found at 6 months in the PD group. No significant associations were found in the CD group.
Discussion
Previous studies have shown that an ad libitum PD with a high content of MUFA and PUFA, a relatively low intake of carbohydrate, and a high fiber-to-carbohydrate ratio can have major positive effects on metabolic balance, including increased glucose tolerance and decreased serum TGs [9, 10, 21, 22]. Our findings suggest that these beneficial effects of the PD can be partly mediated by decreased levels of lipogenesis-promoting factors in SAT. The OPLS analysis revealed that the most pronounced effects were on fat distribution factors, circulating TG levels, and adipose LPL activity within the PD group during the first 6 months of intervention.
The greatly reduced LPL activity in SAT in the PD group was paralleled by a decreased LPL mass. The trend was similar in the CD group, but less pronounced compared to the PD group. Earlier studies of the impact of weight loss on LPL activity in SAT show discordant results [23, 24]. In support of our present data, prior studies have shown reduced adipose tissue LPL activity after surgically induced weight loss in individuals with morbidly obesity [25–27]. Additionally, a study of postmenopausal women with overweight or obesity after a 6-month hypocaloric dietary intervention demonstrated that decreased LPL activity was associated with reductions in abdominal adiposity, total cholesterol, LDLs and TGs [28]. Accordingly, the PD group in the present study showed a more pronounced decrease in LPL activity, associated with greater reductions of abdominal adiposity compared to the CD group. The association between changes of LPL activity and sagittal abdominal diameter in the PD group was verified by both the linear regression analysis and multivariate analysis via OPLS. LPL in adipose tissue is predominantly regulated at the post-translational level by nutritional factors, such as fasting, glucose and insulin [4, 29, 30]. Insulin has effects also at the level of LPL gene transcription [31]. The significant decrease in LPL protein mass may be a consequence of the lower intake of carbohydrate and circulating insulin, which were significantly associated to LPL mass in the PD group at 6 months.
The PD group also showed a significantly decreased expression of CD36, which could reduce FA uptake and utilization [32]. The expression of CD36 is upregulated by insulin, and the expression in adipose tissue is also upregulated in obesity and in type 2 diabetes patients [33]. Genetic studies have revealed that variations within the CD36 locus are associated with metabolic dysfunction through effects on whole-body adiposity [34]. From a metabolic perspective, partial CD36 deficiency is associated with a beneficial phenotype, such that the CD36 reduction in the PD group was associated with decreased abdominal adiposity at 6 months in the PD group [35]. Furthermore, the expression of the important lipogenic enzyme DGAT2 decreased significantly more in the PD group compared to the CD group. Gene expression of DGAT2 is downregulated in adipocytes by weight reduction in humans and is regulated by nutritional factors such as glucose and PUFAs [36–38]. Carbohydrate and PUFA intake were associated with DGAT2 gene expression in the PD group at 6 months and may explain the more pronounced decreased DGAT2 expression found in this study group.
Both diet groups showed decreased expression of the lipogenic enzyme FAS. De novo lipogenesis through FAS in the liver is upregulated by glucose, fructose and insulin, and are downregulated by a high-fat diet and possibly by PUFAs [6]. The expression of FAS in the adipose tissue was more strongly influenced in the PD group than in the CD group, possibly due to the reduced carbohydrate content and/or increased PUFA content in the PD, and/or by the decreased circulating insulin levels in the PD group [6]. This is supported by the association between intake of PUFA and carbohydrates to FAS expression at 6 months in the PD group. Notably, the regulatory responsiveness of FAS in adipose tissue is less pronounced than in liver [6].
Both diet groups showed decreased expression of the ATGL gene, indicating reduced intracellular lipolysis of stored TGs. This finding is consistent with earlier weight-loss studies in humans with obesity following a hypocaloric diet, suggesting that weight loss per se determines this reduction of gene expression [39, 40]. Activation and recruitment of lipases such as ATGL to the lipid droplet surface are regulated by perilipin 1 [41]. We did not find any changes in perilipin 1 gene expression. The explanation for this unaltered expression may be regulation on the protein level by phosphorylation. Importantly, an overall reduction of basal lipolysis in adipose tissue may lower the risks of ectopic fat storage and reduced insulin sensitivity in other tissues, such as skeletal muscle [42].
The PD group showed an improved metabolic situation manifested by increased insulin sensitivity, as indicated by a decreased HOMA-IR index, and lower circulating leptin and adipsin levels. Leptin represses food intake and increases energy expenditure, and is elevated in women with a metabolic syndrome, likely due to leptin resistance or “hypothalamic leptin insufficiency” [43, 44]. Increased levels of adipsin are reported in postmenopausal women with obesity, and have been suggested to be important for the development of a metabolic syndrome in this patient group [43]. While earlier studies have reported stable or increased adiponectin levels during weight reduction in postmenopausal women [45, 46], the CD group in our present study showed a significant decrease in serum adiponectin levels. The differences between groups may be due to different intake of FFAs that may affect PPARγ, an obligatory transcription factor for adiponectin. Notably, we analyzed total adiponectin levels in blood, while the high-molecular-weight form of adiponectin is considered the most etiologically important component with regards to metabolic effects [47].
Strengths and limitations
The strengths of this study include a relatively low total dropout rate and the relatively long intervention time. The higher dropout rate in the CD group, largely due to lack of motivation, may have influenced the between-group differences in weight reduction and body composition. Since LPL activity and mass was expressed per g adipose tissue, and the fat cell size is expected to decrease on weight loss due to the reduced content of TG, we may have underestimated the reduction of LPL in adipose tissue. There was no energy restriction but the energy intake decreased in both groups equally at 6 months. A constant energy intake is preferable when evaluating the impact of the macronutrient change on metabolism. Finally, the present study participants were relatively healthy. Future interventions should include subjects with different degrees of metabolic dysfunction, and also include measurements of fat cell size.
Conclusion
Our present results show that a PD, high in PUFAs and low in carbohydrates, has a more pronounced effect on adipose tissue lipid metabolism than a CD by reducing gene expression of DGAT2 and FAS at 6 months and decreasing LPL activity at 24 months despite similar weight loss. This is linked to improved insulin sensitivity at 6 months and a more pronounced reduction of circulating TGs, suggesting that a PD may be a promising tool to decrease cardiovascular risk in healthy postmenopausal women with overweight.
Acknowledgements
This study was supported by grants from The Swedish Council for Working Life and Social Research (2006–0699), the Swedish Research Council (12237 and 2015–02942), the Swedish Heart and Lung Foundation (20130684), the County Council of Västerbotten, and Umeå University, Sweden. Inger Arnesjö, Katarina Iselid, and Monica Holmgren assisted with the health screening of the study participants, and provided technical assistance. Sara Carlsson measured lipoprotein lipase activity and mass as part of her work for her sixth semester project at the Medical School, and Solveig Nilsson provided technical assistance. Susanne Sandberg informed the participants about the diets and analyzed their food records. Fredrik Jonsson provided statistical advice. TO, BL, CL, and MR designed the study; MR, CM, TO, BL, CB, EW, and EM collected the data; CB and EC analyzed the data; and CB, EC, GO, and TO were responsible for writing the manuscript. All authors read and approved the final manuscript. CB had primary responsibility for the final content.
Ethical standards
The study participants gave written informed consent prior to their inclusion in the study. The study was approved by the Regional Ethical Review Board at Umeå University and has, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. This trial was registered at clinicaltrials.gov as NCT00692536.
Conflict of interest
Gunilla Olivecrona is shareholder and board member in Lipigon Pharmaceuticals AB. The other authors have no conflict of interest.
References
- 1.Toth MJ, Tchernof A, Sites CK, Poehlman ET. Menopause-related changes in body fat distribution. Ann N Y Acad Sci. 2000;904:502–506. doi: 10.1111/j1749-6632.2000.tb06506.x. [DOI] [PubMed] [Google Scholar]
- 2.Gauthier MS, Perusse JR, Lavoie ME, Sladek R, Madiraju SR, Ruderman NB, Coulombe B, Prentki M, Rabasa-Lhoret R. Increased subcutaneous adipose tissue expression of genes involved in glycerolipid-fatty acid cycling in obese insulin-resistant versus—sensitive individuals. J Clin Endocrinol Metab. 2014;99(12):E2518-2528. doi: 10.1210/jc.2014-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masoodi M, Kuda O, Rossmeisl M, Flachs P, Kopecky J. Lipid signaling in adipose tissue: connecting inflammation and metabolism. Biochim Biophys Acta. 2015;1851(4):503–518. doi: 10.1016/j.bbalip.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab. 2009;297(2):E271-288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 5.Sturley SL, Hussain MM. Lipid droplet formation on opposing sides of the endoplasmic reticulum. J Lipid Res. 2012;53(9):1800–1810. doi: 10.1194/jlr.R028290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ameer F, Scandiuzzi L, Hasnain S, Kalbacher H, Zaidi N. De novo lipogenesis in health and disease. Metabolism. 2014;63(7):895–902. doi: 10.1016/j.metabol.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Arner P, Langin D. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol Metab. 2014;25(5):255–262. doi: 10.1016/j.tem.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Santosa S, Jensen MD. Adipocyte fatty acid storage factors enhance subcutaneous fat storage in postmenopausal women. Diabetes. 2013;62(3):775–782. doi: 10.2337/db12-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryberg M, Sandberg S, Mellberg C, Stegle O, Lindahl B, Larsson C, Hauksson J, Olsson T. A Palaeolithic-type diet causes strong tissue-specific effects on ectopic fat deposition in obese postmenopausal women. J Intern Med. 2013;274(1):67–76. doi: 10.1111/joim.12048. [DOI] [PubMed] [Google Scholar]
- 10.Mellberg C, Sandberg S, Ryberg M, Eriksson M, Brage S, Larsson C, Olsson T, Lindahl B. Long-term effects of a Palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. 2014;68(3):350–357. doi: 10.1038/ejcn.2013.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frassetto LA, Schloetter M, Mietus-Synder M, Morris RC, Jr, Sebastian A. Metabolic and physiologic improvements from consuming a paleolithic, hunter-gatherer type diet. Eur J Clin Nutr. 2009;63(8):947–955. doi: 10.1038/ejcn.2009.4. [DOI] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.Stomby A, Simonyte K, Mellberg C, Ryberg M, Stimson RH, Larsson C, Lindahl B, Andrew R, Walker BR, Olsson T. Diet-induced weight loss has chronic tissue-specific effects on glucocorticoid metabolism in overweight postmenopausal women. Int J Obesity. 2015;39(5):814–819. doi: 10.1038/ijo.2014.188. [DOI] [PubMed] [Google Scholar]
- 14.Evans J, Goedecke JH, Soderstrom I, Buren J, Alvehus M, Blomquist C, Jonsson F, Hayes PM, Adams K, Dave JA, Levitt NS, Lambert EV, Olsson T. Depot- and ethnic-specific differences in the relationship between adipose tissue inflammation and insulin sensitivity. Clin Endocrinol. 2011;74(1):51–59. doi: 10.1111/j.1365-2265.2010.03883.x. [DOI] [PubMed] [Google Scholar]
- 15.Gabrielsson BG, Olofsson LE, Sjogren A, Jernas M, Elander A, Lonn M, Rudemo M, Carlsson LM. Evaluation of reference genes for studies of gene expression in human adipose tissue. Obes Res. 2005;13(4):649–652. doi: 10.1038/oby.2005.72. [DOI] [PubMed] [Google Scholar]
- 16.Thysell E, Chorell E, Svensson MB, Jonsson P, Antti H. Validated and predictive processing of gas chromatography-mass spectrometry based metabolomics data for large scale screening studies, diagnostics and metabolite pattern verification. Metabolites. 2012;2(4):796–817. doi: 10.3390/metabo2040796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruge T, Sukonina V, Myrnas T, Lundgren M, Eriksson JW, Olivecrona G. Lipoprotein lipase activity/mass ratio is higher in omental than in subcutaneous adipose tissue. Eur J Clin Invest. 2006;36(1):16–21. doi: 10.1111/j.1365-2362.2006.01584.x. [DOI] [PubMed] [Google Scholar]
- 18.Trygg J, Wold S. Orthogonal projections to latent structures (O-PLS) J Chemometr. 2002;16(3):119–128. doi: 10.1002/cem.695. [DOI] [Google Scholar]
- 19.Jonsson P, Wuolikainen A, Thysell E, Chorell E, Stattin P, Wikström P, Antti H. Constrained randomization and multivariate effect projections improve information extraction and biomarker pattern discovery in metabolomics studies involving dependent samples. Metabolomics. 2015 doi: 10.1007/s11306-015-0818-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley E, Gail G. A leisurely look at the bootstrap, the jack-knife, and cross-validation. Am Stat. 1983;37:36–48. [Google Scholar]
- 21.Jonsson T, Granfeldt Y, Ahren B, Branell UC, Palsson G, Hansson A, Soderstrom M, Lindeberg S. Beneficial effects of a paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009 doi: 10.1186/1475-2840-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manheimer EW, van Zuuren EJ, Fedorowicz Z, Pijl H. Paleolithic nutrition for metabolic syndrome: systematic review and meta-analysis. Am J Clin Nutr. 2015 doi: 10.3945/ajcn.115.113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor-necrosis-factor in human adipose-tissue—regulation by obesity, weight-loss, and relationship to lipoprotein-lipase. J Clin Invest. 1995;95(5):2111–2119. doi: 10.1172/Jci117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauriege P, Imbeault P, Doucet E, Lacaille M, Langin D, Almeras N, Despres JP, Tremblay A. Weight loss and regain in obese individuals: a link with adipose tissue metabolism indices? J Physiol Biochem. 2013;69(3):497–505. doi: 10.1007/s13105-013-0237-8. [DOI] [PubMed] [Google Scholar]
- 25.Pardina E, Lecube R, Llamas R, Catalán R, Galard R, Fort JM, Allende H, Vargas V, Baena-Fustegueras JA, Peinado-Onsurbe J. Lipoprotein lipase but not hormone-sensitive lipase activities achieve normality after surgically induced weight loss in morbidly obese patients. Obes Surg. 2009;19(8):1150–1158. doi: 10.1007/s11695-009-9853-3. [DOI] [PubMed] [Google Scholar]
- 26.Ferrer R, Pardina E, Rossell J, Baena-Fustegueras JA, Lecube A, Balibrea JM, Caubet E, Gonzalez O, Vilallonga R, Fort JM, Peinado-Onsurbe J. Decreased lipases and fatty acid and glycerol transporter could explain reduced fat in diabetic morbidly obese. Obesity. 2014;22(11):2379–2387. doi: 10.1002/Oby.20861. [DOI] [PubMed] [Google Scholar]
- 27.Imbeault P, Almeras N, Richard D, Despres JP, Tremblay A, Mauriege P. Effect of a moderate weight loss on adipose tissue lipoprotein lipase activity and expression: existence of sexual variation and regional differences. Int J Obes Relat Metab Disord. 1999;23(9):957–965. doi: 10.1038/sj.ijo.0801025. [DOI] [PubMed] [Google Scholar]
- 28.Nicklas BJ, Rogus EM, Berman DM, Dennis KE, Goldberg AP. Responses of adipose tissue lipoprotein lipase to weight loss affect lipid levels and weight regain in women. Am J Physiol Endocrinol Metab. 2000;279(5):E1012-1019. doi: 10.1152/ajpendo.2000.279.5.E1012. [DOI] [PubMed] [Google Scholar]
- 29.Olivecrona G. Role of lipoprotein lipase in lipid metabolism. Curr Opin Lipidol. 2016;27(3):233–241. doi: 10.1097/Mol.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 30.Kersten S. Physiological regulation of lipoprotein lipase. Biochim Biophys Acta Mol Cell Biol Lipids. 2014;1841(7):919–933. doi: 10.1016/j.bbalip.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Ruge T, Sukonina V, Kroupa O, Makoveichuk E, Lundgren M, Svensson MK, Olivecrona G, Eriksson JW. Effects of hyperinsulinemia on lipoprotein lipase, angiopoietinlike protein 4, and glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1 in subjects with and without type 2 diabetes mellitus. Metabol Clin Exp. 2012;61(5):652–660. doi: 10.1016/j.metabol.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Pepino MY, Kuda O, Samovski D, Abumrad NA. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu Rev Nutr. 2014;34:281–303. doi: 10.1146/annurev-nutr-071812-161220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonen A, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ. The fatty acid transporter FAT/CD36 is upregulated in subcutaneous and visceral adipose tissues in human obesity and type 2 diabetes. Int J Obes (Lond) 2006;30(6):877–883. doi: 10.1038/sj.ijo.0803212. [DOI] [PubMed] [Google Scholar]
- 34.Heni M, Mussig K, Machicao F, Machann J, Schick F, Claussen CD, Stefan N, Fritsche A, Haring HU, Staiger H. Variants in the CD36 gene locus determine whole-body adiposity, but have no independent effect on insulin sensitivity. Obesity (Silver Spring) 2011;19(5):1004–1009. doi: 10.1038/oby.2010.251. [DOI] [PubMed] [Google Scholar]
- 35.Love-Gregory L, Abumrad NA. CD36 genetics and the metabolic complications of obesity. Curr Opin Clin Nutr Metab Care. 2011;14(6):527–534. doi: 10.1097/MCO.0b013e32834bbac9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meegalla RL, Billheimer JT, Cheng D. Concerted elevation of acyl-coenzyme A:diacylglycerol acyltransferase (DGAT) activity through independent stimulation of mRNA expression of DGAT1 and DGAT2 by carbohydrate and insulin. Biochem Biophys Res Commun. 2002;298(3):317–323. doi: 10.1016/S00006-291X(02)02466-X. [DOI] [PubMed] [Google Scholar]
- 37.Hirata T, Unoki H, Bujo H, Ueno K, Saito Y. Activation of diacylglycerol O-acyltransferase 1 gene results in increased tumor necrosis factor-alpha gene expression in 3T3-L1 adipocytes. FEBS Lett. 2006;580(21):5117–5121. doi: 10.1016/j.febslet.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 38.Mangravite LM, Dawson K, Davis RR, Gregg JP, Krauss RM. Fatty acid desaturase regulation in adipose tissue by dietary composition is independent of weight loss and is correlated with the plasma triacylglycerol response. Am J Clin Nutr. 2007;86(3):759–767. doi: 10.1093/ajcn/86.3.759. [DOI] [PubMed] [Google Scholar]
- 39.Koppo K, Valle C, Siklova-Vitkova M, Czudkova E, de Glisezinski I, van de Voorde J, Langin D, Stich V. Expression of lipolytic genes in adipose tissue is differentially regulated during multiple phases of dietary intervention in obese women. Physiol Res. 2013;62(5):527–535. doi: 10.33549/physiolres.932483. [DOI] [PubMed] [Google Scholar]
- 40.Jocken JWE, Langin D, Smit E, Saris WHM, Valle C, Hul GB, Holm C, Arner P, Blaak EE. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J Clin Endocr Metab. 2007;92(6):2292–2299. doi: 10.1210/jc.2006-1318. [DOI] [PubMed] [Google Scholar]
- 41.Arner P, Langin D. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrin Met. 2014;25(5):255–262. doi: 10.1016/j.tem.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Magkos F, Fabbrini E, Conte C, Patterson BW, Klein S. Relationship between adipose tissue lipolytic activity and skeletal muscle insulin resistance in nondiabetic women. J Clin Endocr Metab. 2012;97(7):E1219-E1223. doi: 10.1210/Jc.2012-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chedraui P, Perez-Lopez FR, Escobar GS, Palla G, Montt-Guevara M, Cecchi E, Genazzani AR, Simoncini T, Research Group for the Omega Women. ’. s Health P Circulating leptin, resistin, adiponectin, visfatin, adipsin and ghrelin levels and insulin resistance in postmenopausal women with and without the metabolic syndrome. Maturitas. 2014;79(1):86–90. doi: 10.1016/j.maturitas.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80–84. doi: 10.1016/j.cca.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Abbenhardt C, McTiernan A, Alfano CM, Wener MH, Campbell KL, Duggan C, Foster-Schubert KE, Kong A, Toriola AT, Potter JD, Mason C, Xiao L, Blackburn GL, Bain C, Ulrich CM. Effects of individual and combined dietary weight loss and exercise interventions in postmenopausal women on adiponectin and leptin levels. J Intern Med. 2013;274(2):163–175. doi: 10.1111/joim.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryan AS, Nicklas BJ, Berman DM, Elahi D. Adiponectin levels do not change with moderate dietary induced weight loss and exercise in obese postmenopausal women. Int J Obes Relat Metab Disord. 2003;27(9):1066–1071. doi: 10.1038/sj.ijo.0802387. [DOI] [PubMed] [Google Scholar]
- 47.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, Imai Y, Nagai R, Kadowaki T. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29(6):1357–1362. doi: 10.2337/Dc05-1801. [DOI] [PubMed] [Google Scholar]