Abstract
Background
Lysozymes and antibacterial peptides have been confirmed to protect humans against viral and bacterial infection, and accelerate wound healing. The study aimed to evaluate the effect of lysozyme-antimicrobial peptide fusion protein on the diabetic wound size reduction in streptozotocin (STZ)-induced diabetic rats.
Material/Methods
Diabetic rats were prepared via intraperitoneal injection of STZ, 70 mg/kg. A 2-cm circular incision with full thickness was made on the dorsum skin of the rats for preparation of diabetic wound model. The wounds were treated with the fusion protein or phosphate buffer saline.
Results
The fusion protein markedly accelerated wound healing, decreased levels of proinflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α, lipid peroxide (LPO) content, and expression of cyclooxygenase-2 (COX-2), and increased activities of antioxidant enzyme including superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) in serum, levels of pro-angiogenic cytokines such as vascular endothelial growth factor (VEGF) and intercellular adhesion molecule (ICAM-1), and expression of VEGF, FGF-2, p-ERK, and p-Akt protein in granulation.
Conclusions
The results of the present study suggested that the fusion protein accelerated wound healing by improving anti-inflammation and antioxidant, and increasing angiogenesis in granulation tissues of diabetic rats.
MeSH Keywords: Antimicrobial Cationic Peptides; Diabetes Mellitus, Experimental; Streptozocin; Wound Healing
Background
Diabetes mellitus is a chronic endocrine disorder in the world. Sustained diabetic state often results in vascular impairment which is called diabetic vascular complications [1,2]. Diabetic foot ulcer (DFU) is one of the most feared and costly diabetic vascular complications. DFU is closely related to peripheral vascular impairment resulting from hyperglycemia, and DFU further develops even when hyperglycemia is under control [3,4]. It is well known that hyperglycemia leads to the production of excessive reactive oxygen species (ROS) which impairs vessels [2]. It has been thought that diabetic vasculopathy results in peripheral microcirculation and coagulation failure, which further causes the decrease of blood supply in peripheral limbs. As a result, ischemic ulcers more easily occur in patients with diabetes, and are furthermore prone to infection [5]. Ischemia delays wound healing of DFU [6] and, together with infection, aggravates the progression of ulcers and consequently leads to amputation and death of many diabetic patients [7–9]. Therefore, it is beneficial to chronic diabetic wound healing that angiogenesis and vasculogenesis restore blood supply and provide nutrition and oxygen for wound tissues.
Lysozymes are ubiquitously basic antimicrobial enzymes which catabolize hydrolyzation of the β1–4 glyosidic bond in peptidoglycans between N-acetylglucosamine and N-acetylmuramic acid [10]. Lysozymes are widely found in various organisms including animals, plants, and bacteria [11]. Several studies have confirmed that lysozymes are involved in regulation of inflammation, therefore they are regarded as innate non-specific immune factors [12,13]. In fact, lysozymes are thought to be a potent defense which protects humans against viral and bacterial infection [12,14]. Furthermore, studies have shown that lysozymes play an important role in many pathological processes, such as alleviation of vascular disruption resulting from sepsis by suppressing the shedding of the endothelial cell protein C receptor [14], and the bronchodilator-based treatment scheme for chronic obstructive pulmonary disease (COPD) [15]. Antimicrobial peptides are small cationic peptides. These peptides have been found to possess powerful antimicrobial functions against Gram-positive and Gram-negative bacteria, fungi, parasites, and some viruses, and are considered as potential antibiotics drugs [16,17]. Similar to lysozymes, antimicrobial peptides are important constituents of the innate immune system, but are also the first-line of protection from infection [18,19]. Increasingly, studies have documented that antimicrobial peptides are implicated in many important physiological and pathological functions including mediation of inflammation and improvement of wound healing [20,21]. In addition to poor stability and lower expression, lysozymes and antimicrobial peptides possess hypofunctions. To enhance the beneficent effects of lysozymes and antimicrobial peptides, and their cooperation, they have been used to construct a fusion protein. One study showed that the fusion protein constructed with lysozymes and antimicrobial peptides increased anti-infection activities [22]. A recent report suggested that the lysozyme fusion protein may be useful for the future design and development of anti-HIV-1 therapeutic agents [23].
Lysozymes and antimicrobial peptides have been reported to improve wound healing of trauma injuries [12,21]. Previously, we constructed a lysozyme-antimicrobial peptide fusion gene by genetic recombination, and purified the fusion protein product, and the results have been patented (CN104961834A). Our results showed that the fusion protein exhibited antimicrobial activity. In the present study, we attempted to investigate the effect of the fusion protein on diabetic wound healing in STZ-induced diabetic rats, and further explore its possible mechanisms.
Material and Methods
Pentobarbital sodium, STZ, and a horseradish peroxidase-conjugated secondary antibody were purchased from Sigma (Sigma Chemical Co., St. Louis, MO, USA). Rabbit polyclonal antibodies β-actin, COX-2, vascular endothelial growth factor (VEGF), FGF-2, CD34, ERK1/2, p-ERK1/2, Akt, and p-Akt were purchased from Bio Basic Inc. (Canada). Lysozyme-antimicrobial peptide fusion protein was purchased from HebeiChuangyue Biotech. Co., Ltd. (egg-white lysozyme and active fragment of Drosophila melanogaster antimicrobial peptide genes were used to construct fusion genes and subclones) (Langfang, China).
Healthy Sprague-Dawley (SD) rats weighing 220 g to 260 g were purchased from Nanjing Qinglongshan animal breeding ground (Nanjing, China) and raised in our standard animal facility with 22±2°C room temperature and 12-hour day/night alternate. All experimental procedures were handled according to Chinese Community guidelines for the use of experimental animals.
Induction of diabetes
Rats were randomly divided into 3 groups (n=12 per group): normal control (NORM-C) group, diabetes control (DIAT-C) group, and diabetes treated (DIAB-T) group. Rats from the DIAB-T group and the DIAT-C group were rendered diabetic by intraperitoneal injection with STZ (70 mg/kg freshly dissolved in 0.1 mol/L ice-cold sterile citrate buffer, pH 4.5; Sigma Aldrich) after an overnight fast, and animals in the NORM-C group were injected with citrate buffer. Three days after STZ administration, fasting blood glucose was determined for confirmation of diabetes. Rats with blood glucose concentration >300 mg/dL were diagnosed as diabetic. Rats were housed individually with standard pellet diet and free access to water.
Wound model and experimental design
After 2 weeks of diabetes, rats were anesthetized with 1.5% pentobarbital sodium by intraperitoneal injection (50 mg/kg). Wounds were created as described previously [24]. Dorsum skin was shaved for wounding. After disinfected with 75% ethanol, a circular excisional wound (2.0 cm in diameter) with full thickness was made on the dorsum skin per rats. The wounds from the DIAB-T rats were treated with fusion protein spray (0.05 mL/cm2, 50 μg/mL) twice per day, and the wounds from NORM-C and DIAT-C rats were treated with vehicle. At the end of experiment, blood samples were collected for determination of inflammatory cytokines and proangiogenic factors. The skin tissue in the wound area was harvested, and put into 4% paraformaldehyde for histopathological observation or preserved under −80°C for western blot analysis.
Wound closure
The wound area from each individual rat was traced. The wound sizes were recorded with a digital camera on day 0, 5, 10, 15, and 20 for measurement of wound closure. The wound area was determined via Image-Pro Plus 4.5 software. Wound closure was estimated in the wound from each rat. The rate of wound closure was assessed using the ratio of wound closure: the wound closure rate (%)=(initial area−measured area)/initial area×100%.
Determination of VEGF and ICAM-1
To estimate the level of proangiogenic factors in serum, vascular endothelial growth factor (VEGF), and intercellular adhesion molecule 1 (ICAM 1) were measured using VEGF and ICAM 1-specific ELISA kits (Hefei Bomei Biotechnology Co., Ltd., China). The levels of VEGF and ICAM 1 in serum were expressed as ng/L and pg/mL, respectively.
Analyses of inflammatory cytokines
Serum levels of inflammatory cytokines such as interleukin (IL)-6, and TNF-α were measured by ELISA kits (Hefei Bomei Biotechnology Co., Ltd., China) according to the manufacturer’s instructions. The levels of IL-6 and TNF-α in serum were expressed as ng/L, respectively.
Histopathology and Immunohistochemistry
At the end of experiment, wound samples (4 rats per group) were harvested for pathological study. Wound tissues were fixed in 4% paraformaldehyde. After dehydrated, fixed tissues were embedded in paraffin, and cut at a thickness of 5 μm onto slides for hematoxylin and eosin (H-E) and Masson trichrome staining. Histological slides were observed under light microscope. Histological features including morphology, epidermal regeneration, angiogenesis, and granulation tissue formation were observed in H-E stained slides. Masson trichrome stained slides were used to determine granulation tissue thickness. For immunohistochemistry of wound tissues, sections were deparaffinized for 2 hours. Then sections were immersed in EDTA buffer for antigenic retrieval, and incubated with hydrogen peroxide in methanol to inhibit endogenous peroxidase. After incubation with bovine serum albumin for blockade of non-specific binding, the sections were incubated rabbit polyclonal antibodies PDGF, CD34 primary antibody (Santa Cruz Biotechnology, USA) (1: 00) overnight at 4°C. The sections were rinsed with phosphate buffered saline, and then incubated with biotin-conjugated secondary antibody, washed, and incubated with streptavidin-horseradish peroxidase. The sections were imbedded in DBA, and counterstained with hematoxylin, and mounted. The number of small blood vessels was counted under high power field in sections stained with CD34.
Western blotting
Granulation tissues were homogenized and lysed in ice-cold lysis buffer (20 mmol/L Tris-HCl, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, and 1% Triton X-100) containing protease inhibitors (2 mmol/L sodium orthovanadate, 0.2 mmol/L PMSF, 2 μg/mL leupeptin, and 2 μg/mL aprotinin). After centrifuged at 13 000 g for 15 minutes at 4°C, the supernatant was collected. Equal amounts of denatured proteins in the supernatants were separated by 12% SDS-PAGE, and transferred to nitrocellulose membranes. Then the membranes were blocked in TBS-T (10 mmol/L Tris, 150 mmol/L NaCl, and 0.1% Tween20, pH 7.5) containing 5% skim milk for 2 hours. The membranes were incubated with rabbit polyclonal antibodies β-actin, COX-2, VEGF, FGF-2, ERK1/2, p-ERK1/2, Akt, and p-Akt (1: 500) (Bio Basic Inc., Canada) in TBS-T with 5% nonfat milk overnight at 4°C. After the membranes were incubated with anti-rabbit HRP-conjugated antibody (1: 10 000) in TBS-T, target proteins were determined via visualization with DAB (Bio Basic Inc., Canada).
Statistical analysis
Values were expressed as mean ±SD. Statistical differences were performed using unpaired Student t-test or one-way analysis of variance (ANOVA) followed by Bonferroni correction/Dunn test. A value of P<0.05 was considered statistically significant. The analysis was performed with SPSS, 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Characteristics of diabetic rats
After administration of STZ, rats exhibited an increase in levels of blood glucose, and blood glucose concentration remained above 300 mg/dL in rats treated with STZ throughout the experiment. Persistent hyperglycemia resulted in the typical diabetic characteristics including reduced body weight, increased water consumption and urinary volume. At the end of the experiment, survival rates were 12 out of 12 in the NORM-C group, 8 out of 12 in the DIAB-C, and 9 out of 12 in the DIAB-C.
Fusion protein accelerates wound healing
The areas of wounds were measured for determining the wound contraction rate in the diabetic rats. In this experiment, the wounds from the diabetic rats presented with inflammatory features, such as swelling and festering, but the contraction rate of the diabetic wounds showed no obvious differences in the subsequent 2 days compared with the NORM-C rats. Treatment of fusion protein reduced inflammatory features of the diabetic wounds, and significantly promoted wound closure compared with DIAT-C rat wounds on day 5 (P<0.01) and after 5 days (P<0.01) (Table 1). Wounds treated with the fusion protein were almost healed after 21 days, and wound reduction was up to 85%.
Table 1.
Effect of fusion protein on rate of wound closure.
| Day 5(%) | Day 10(%) | Day 15(%) | Day 20(%) | |
|---|---|---|---|---|
| NORM-C | 17.24±1.65 | 46.57±3.05 | 79.22±3.47 | 90.41±3.54 |
| DIAB-C | 12.36±1.32** | 27.34±2.81** | 49.56±3.50** | 74.79±3.32** |
| DIAB-T | 15.53±1.95## | 38.04±3.29## | 65.18±4.37## | 85.53±3.94## |
Results were expressed as mean ± standard error of the mean of the values. The number of animals per group was 8 for measure of wound closure. The wound closure rate (%)=(initial area−measured area)/initial area×100%. NORM-C – normal control; DIAB-C – diabetes control; DIAB-T – diabetes treated with the fusion protein.
P<0.01 versus NORM-C;
P<0.01 versus DIAB-C.
Histological analysis of wound tissues
Observation of wound healing showed that treatment of the fusion protein alleviated inflammation, and improved wound healing compared with the DIAB-C rat wounds. The skin structure was clearly observed in the wounds from the NORM-C and DIAB-T rats, but more epidermis nipples were observed in the wounds of the DIAB-C rats.
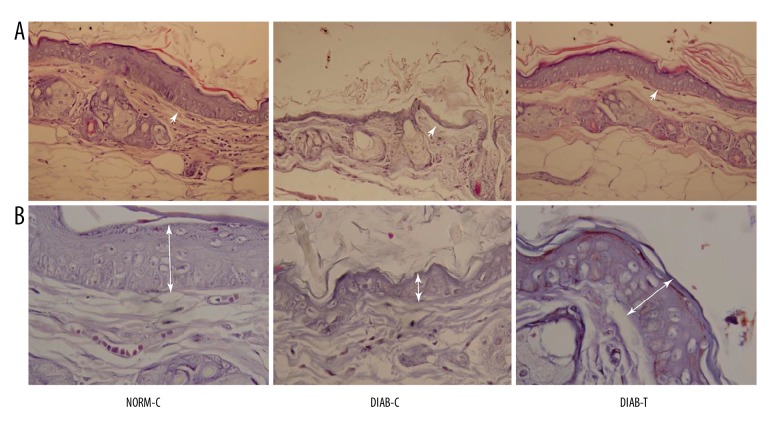
Tissue sections indicated that stratified epithelium and hair follicles were clearly observed in the wounds from the NORM-C and DIAB-T rats. Collagen of wound tissues were regularly arranged in the DIAB-T rats compared with the DIAB-C rats. On day 20, epidermal cells were fully regenerated in the wounds from the DIAB-T rats and recovered wound tissues. Features of re-epithelialization, such as thicker epidermal layer and connective tissue, were more evident in DIAB-T rats than in DIAB-C rats (Figure 1A). Furthermore, more new capillaries and deposited collagen fibers were observed in granulation tissue from the DIAB-T rats when compared with the DIAB-C rats (Figure 1A).
Figure 1.
Morphological change in the wound tissue of each group. Photomicrographs of (A) hematoxylin and eosin (H-E) staining at 400× and (B) Masson staining at 1000×. NORM-C rats showed re-epithelialization around the wound. The wound of the DIAB-C rats presented inflammatory cell infiltration. Re-epithelialization was observed in wounds of the DIAB-T rats. NORM-C – normal control; DIAB-C – diabetes control; DIAB-T – diabetes treated with the fusion protein.
In addition, we determined the thickness of granulation tissues through Masson trichrome staining to evaluate granulation tissue formation. The results showed that the granulation tissues in the DIAB-T rats were thicker compared to the DIAB-C rats (Figure 1B).
Effect of fusion protein on inflammation
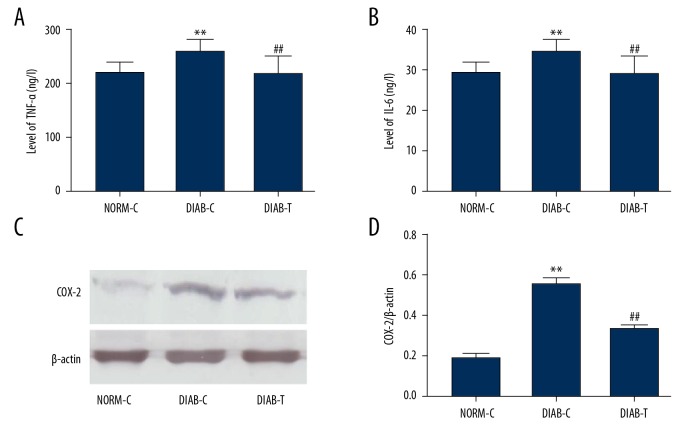
These results indicated that the diabetic state significantly enhanced serum levels of TNF-α (P<0.01) and IL-6 (P<0.01) in DIAT-C rats when compared to the NORM-C rats after 21 days (Figure 2A, 2B). However, treatment with fusion protein significantly reduced levels of TNF-α (P<0.05) and IL-6 (P<0.05) compared with DIAT-C rats (Figure 2A, 2B). In addition, histological observation indicated that infiltrated leukocytes were scarcely observed in the granulation tissue from the DIAB-T rats (Figure 1A).
Figure 2.
Effect of fusion protein on inflammatory cytokines in serum, and expression of COX-2. (A) Tumor necrosis factor α (TNF-α); (B) Interleukin 6 (IL-6); (C) western blot of cyclooxygenase 2 (COX-2); and (D) quantitative analysis of cyclooxygenase 2 (COX-2). Results were expressed as mean ± standard error of the mean of the values. The number of animals per group was 8 for determination of TNF-α, IL-6 content, and 6 for COX-2. NORM-C – normal control; DIAB-C – diabetes control; DIAB-T – diabetes treated with the fusion protein. ** P<0.01 versus NORM-C; # P<0.05, ## P<0.01 versus DIAB-C.
Furthermore, COX-2, an inflammatory mediator, was determined by western blotting. The result showed that the expression of COX-2 protein was upregulated in DIAB-C rats compared with the NORM-C rats, and that treatment with fusion protein downregulated the expression of COX-2 protein (P<0.01) (Figure 2C, 2D).
Effect of fusion protein on antioxidation
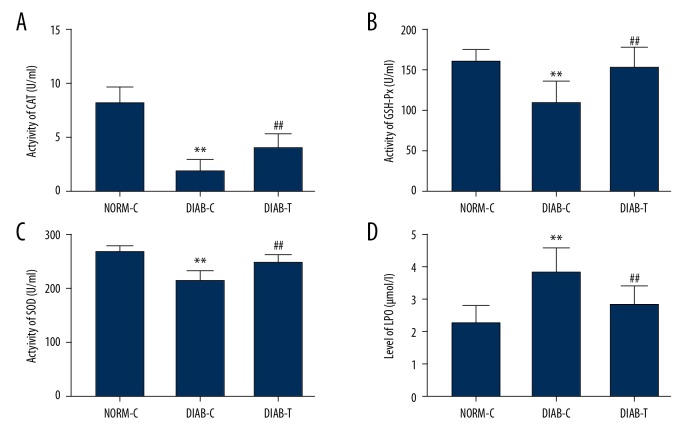
Serum content of lipid peroxide (LPO) was significantly enhanced in DIAB-C rats compared with NORM-C rats (P<0.01), and sustained hyperglycemia reduced activities of superoxide dismutase (SOD) (P<0.01), CAT (P<0.01), and glutathione peroxidase (GSH-Px) (P<0.01) (Figure 3). Treatment with fusion protein elevated the activities of SOD (P<0.01), CAT (P<0.01), and GSH-Px (P<0.01), and decreased LPO content compared to DIAB-C rats (P<0.01) (Figure 3).
Figure 3.
Fusion protein improved antioxidation in diabetic rats. (A) catalase (CAT); (B) glutathione peroxidase (GHS-Px); (C) superoxide dismutase (SOD); and (D) lipid peroxidase (LPO). Results were expressed as mean ± standard error of the mean of the values. The number of animals per group was 8 for determination of CAT, GHS-Px, SOD activity, LPO content. NORM-C – normal control; DIAB-C – diabetes control; DIAB-T – diabetes treated with the fusion protein. ** P<0.01 versus NORM-C; ## P<0.01 versus DIAB-C.
Fusion protein stimulates angiogenesis in diabetic wounds
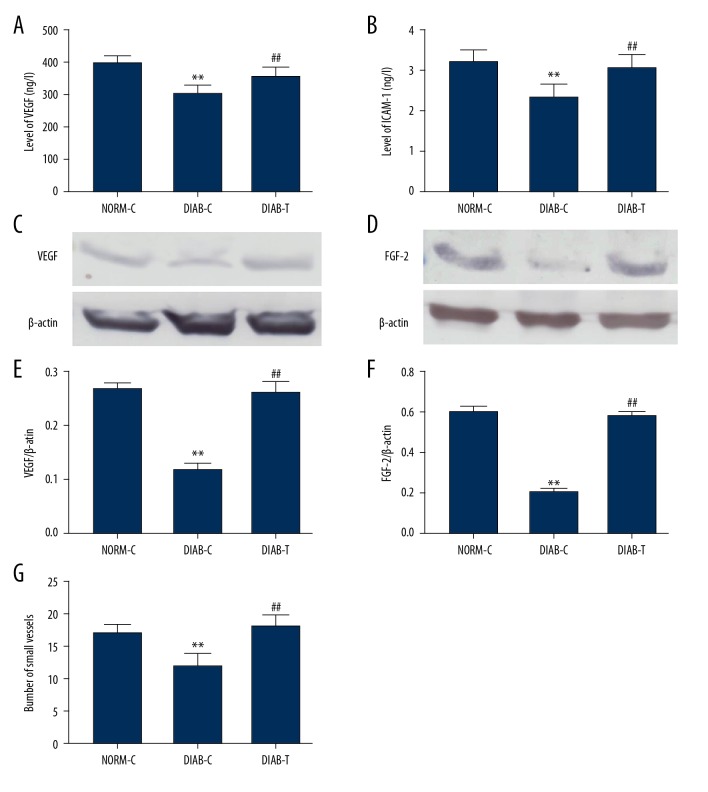
The small vessels were observed under a light microscope, and counted in granulation tissues. The result showed a decrease in the number of small vessels in DIAB-C rats compared with NORM-C rats, and fusion protein increased the number of small vessels in granulation tissue from DIAB-T rats (P<0.01) (Figure 4G).
Figure 4.
Fusion protein accelerated angiogenesis in wound tissue. (A) Serum levels of VEGF; (B) serum levels of ICAM-1; (C) expression of VEGF; (D) relative levels of VEGF; (E) expression of FGF-2; (F) relative levels of FGF-2; (G) number of small vessels, the small vessels were counted via CD34-positive staining. Results were expressed as mean ± standard error of the mean of the values. The number of animals per group was 8 for determination of VEGF, ICAM-1 content in serum, and 6 for expression of VEGF, and FGF-2. NORM-C – normal control; DIAB-C – diabetes control; DIAB-T – diabetes treated with the fusion protein. ** P<0.01 versus NORM-C; ## P<0.01 versus DIAB-C.
We also determined cytokines involved in angiogenesis. The results showed an increase in serum levels of VEGF (P<0.01) and ICAM-1 (P<0.01) (Figure 4A, 4B), and upregulation of VEGF and FGF-2 in granulation tissues from DIAB-T rats compared with the DIAB-C rats (P<0.01) (Figure 4C, 4D).
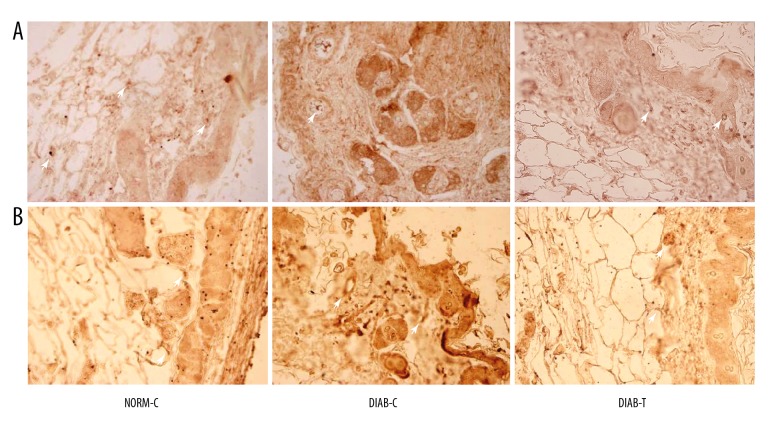
Analysis of immunohistochemistry indicated that the fusion protein increased PDGF expression in the granulation tissue (Figure 3A). CD34 characterized the angiogenic phenotypical features, CD34 expression in the wounds was detected via immunohistochemistry. As shown in Figure 3B, the fusion protein increased CD34 expression in the wounds.
Effect of fusion protein on expression of p-ERK and p-Akt proteins
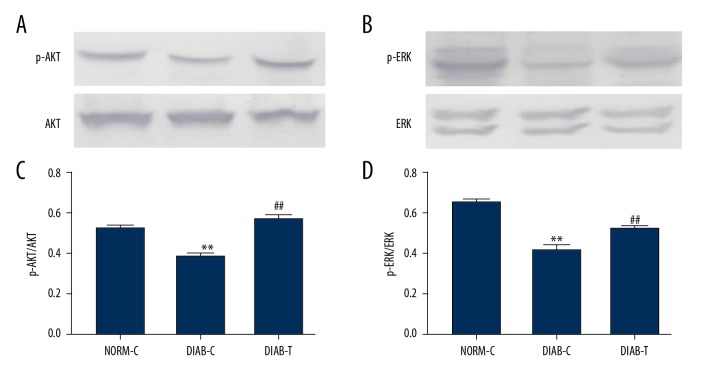
To further explore the possible mechanism of fusion protein effect, expressions of phosphorylated ERK (p-ERK) and AKT (p-Akt) were determined in granulation tissue. The results showed that p-ERK and p-AKT were increased in DIAB-T rats compared with DIAB-C rats (P<0.01) (Figure 6).
Figure 6.
Effect of fusion protein on p-Akt and p-ERK1/2 expression. (A) Expression of p-AKT; (B) relative levels of p-AKT; (C) expression of p-ERK 1/2; and (D) relative levels of p-ERK 1/2. Results were expressed as mean ± standard error of the mean of the values. The number of animals per group was 6 for expression of p-AKT, and p-ERK 1/2. ** P<0.01 versus NORM-C; ## P<0.01 versus DIAB-C.
Discussion
In the present study, we investigated the effects of the lysozyme-antimicrobial peptide fusion protein on diabetic wound healing. The data showed that sustained hyperglycemia delayed wound healing in diabetic rats. Treatment of the fusion protein accelerated diabetic wound healing, reduced levels of pro-inflammatory factors including TNF-α and IL-6, enhanced antioxidant, and improved angiogenesis in granulation tissues from wound of diabetic rats.
It is well known that wound healing is a complex pathophysiological process involving various physiological events such as inflammation, re-epithelialization, angiogenesis, and granulation tissue formation. Formation of granulation tissue plays a critical role in wound healing, and is closely related to blood vessels [25]. The existing evidence has shown that oxidative stress and inflammatory response can cause vessel injury and impair vessel formation by reducing proangiogenic factors and angiogenesis signaling [26–29]. In the present study, we investigated the effect of the fusion protein on diabetic wound healing, and explored its possible mechanisms including changes in oxidative stress, inflammation, expression of proangiogenic factors, and angiogenesis signaling. The results indicated that the fusion protein promoted diabetic wound healing, and the number of vessels was increased in wound sections stained with H-E. Lysozymes and antimicrobial peptides have been reported to improve wound healing and angiogenesis [15,20]. Therefore, our study results suggest that wound healing of lysozymes and antimicrobial peptides occurred with fusion protein.
Lysozymes and antimicrobial peptides, innate non-specific immune factors, have been reported to be involved in regulation of inflammation [14]. Various studies have shown that excessive or persistent releases of proinflammatory cytokines, such as IL-6 and TNF-α, increase generation of proteases which impairs wound tissue and hampers wound contraction by increasing the risk of wound infection in patients with diabetes [26,30]; and the reduction of inflammation is beneficial to diabetic wound healing [31]. In our present study, treatment with the fusion protein decreased levels of proinflammatory cytokines, such as IL-6 and TNF-α, and downregulated expression of cyclooxygenase-2 (COX-2) protein, an important enzyme which mediates inflammatory response [32]. Oxidative stress often leads to depletion of anti-oxidase including SOD, CAT, and GSH-Px, and an increase in lipid peroxidation product. Therefore, oxidative stress is considered to be involved in and play an important role in diabetes and its complications [33]. In our study, we found that the fusion protein enhanced the activity of SOD, CAT, and GSH-Px, and decreased the content of lipid peroxide (LPO), which suggested that the fusion protein reduced oxidative stress. It has been widely known that oxidative stress resulting from hyperglycemia is a primary pathogenesis of diabetic vascular complications, including DFU [27,34,35]. It has been reported that the reduction of oxidative stress decreases inflammation [36], and improves healing of DFU [27,35]. Our study results indicated that the healing associated with fusion protein treatment to diabetic wounds may be associated with its alleviation of vascular impairment by reducing inflammation and oxidative stress, further increasing the blood supply in wounds.
Angiogenesis failure and vasculopathy play a critical role in occurrence and development of diabetes and its complications. Angiogenesis failure reduced granulation tissue formation and delayed diabetic wound healing [37]. Various studies have shown that hyperglycemia reduces VEGF and FGF-2 expression in myocardial tissue, wound tissue, and the peripheral limb in diabetic animals [28,29,38], which decreases blood perfusion via impairing angiogenesis [39,40]. Key proangiogenic factors, such as VEGF and FGF-2, are implicated in angiogenesis by improving migration and proliferation of endothelial cells [29,41,42], and therefore used to treat diabetic wound [41]. Furthermore, studies have documented that signaling impairment of these factors, rather than their expression, disrupts angiogenesis and diabetic wound repair [43,44]. Our results showed that the fusion protein increased phosphorylation of Akt and ERK1/2. Akt and ERK1/2 are activated by phosphorylation. Activated Akt and ERK1/2 are involved in mediation of angiogenesis by improving cell proliferation and survival, and activity of NOS (nitric oxide synthase) [45–49]. Previous studies indicated that lysozymes and antimicrobial peptides promote wound healing of trauma injuries [15,20], although the mechanism was not documented. The present study suggested that fusion protein improved angiogenesis by regulating angiogenesis signaling.
In the present study, we explored the effects of the fusion protein on diabetic wound healing and some possible mechanisms, but the preliminary results needed to be further confirmed in long-term studies, and more detailed molecular mechanisms, such as cell migration and proliferation; in addition, the changes in signaling pathway affecting angiogenesis should be further elucidated.
Conclusions
In conclusion, our data supported that the fusion protein presented inflammatory effects and increased anti-oxidation, and improved angiogenesis in granulation tissue of diabetic wounds. These beneficial effects of the fusion protein may play an important role in diabetic wound healing. However, there were some limitations in this study. First, the study was limited by the small sample size. Second, some the mechanisms, such as neuropathy, were not illuminated in our study. Finally, time was relatively short from preparation of wound to treatment with the fusion protein so that features of diabetic ulcer could not be fully shown. Our results suggested that fusion protein may be a potential therapeutic agent against ischemic wounds, and further studies are required and should pay attention to these noted study limitations.
Figure 5.
Immunohistochemistry analysis of the granulation tissues (400×). (A) Staining of PDGF, PDGF-positive staining showed expression of proangiogenic factor; and (B) staining of CD34, CD34-positive staining was used to observe the small vessels in granulation tissues. NORM-C – normal control; DIAB-C – diabetes control; DIAB-T – diabetes treated with the fusion protein.
Acknowledgments
The authors thank Dr. Jinlong Chen for providing the fusion protein.
Footnotes
Source of support: This study was supported by the Army Key Project (NO. BJN14C001)
Conflict of Interest
None.
References
- 1.Newton KM, Wagner EH, Ramsey SD, et al. The use of automated data to identify complications and comorbidities of diabetes: A validation study. J Clin Epidemiol. 1999;52(3):199–207. doi: 10.1016/s0895-4356(98)00161-9. [DOI] [PubMed] [Google Scholar]
- 2.Zguira MS, Vincent S, Le Douairon Lahaye S, et al. Intense exercise training is not effective to restore the endothelial NO-dependent relaxation in STZ-diabetic rat aorta. Cardiovasc Diabetol. 2013;12:32. doi: 10.1186/1475-2840-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 4.Tomita M, Kabeya Y, Okisugi M, et al. Diabetic microangiopathy is an independent predictor of incident diabetic foot ulcer. J Diabetes Res. 2016;2016 doi: 10.1155/2016/5938540. 5938540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuttolomondo A, Casuccio A, Guercio G, et al. Arterial stiffness, endothelial and cognitive function in subjects with type 2 diabetes in accordance with absence or presence of diabetic foot syndrome. Cardiovasc Diabetol. 2017;16(1):2. doi: 10.1186/s12933-016-0483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang ES, Basu A, O’Grady M, et al. Projecting the future diabetes population size and related costs for the U.S. Diabetes Care. 2009;32(12):2225–29. doi: 10.2337/dc09-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johannesson A, Larsson GU, Ramstrand N, et al. Incidence of lower-limb amputation in the diabetic and nondiabetic general population: A 10-year population-based cohort study of initial unilateral and contralateral amputations and reamputations. Diabetes Care. 2009;32(2):275–80. doi: 10.2337/dc08-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan MB, Hess TM, Bartle B, et al. Diabetic foot ulcer severity predicts mortality among veterans with type 2 diabetes. J Diabetes Complications. 2017;31(3):556–61. doi: 10.1016/j.jdiacomp.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loupa CV, Meimeti E, Voyatzoglou E, et al. Successful nonsurgical therapy of a diabetic foot osteomyelitis in a patient with peripheral artery disease with almost complete radiological restoration. BMC Res Notes. 2018;11(1):579. doi: 10.1186/s13104-018-3694-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callewaert L, Michiels CW. Lysozymes in the animal kingdom. J Biosci. 2010;35(1):127–60. doi: 10.1007/s12038-010-0015-5. [DOI] [PubMed] [Google Scholar]
- 11.Subroto T, Sufiati S, Beintema JJ. Papaya (Carica papaya) lysozyme is a member of the family 19 (basic, class II) chitinases. J Mol Evol. 1999;49(6):819–21. doi: 10.1007/pl00000075. [DOI] [PubMed] [Google Scholar]
- 12.Nakimbugwe D, Masschalck B, Deckers D, et al. Cell wall substrate specificity of six different lysozymes and lysozyme inhibitory activity of bacterial extracts. FEMS Microbiol Lett. 2006;259(1):41–46. doi: 10.1111/j.1574-6968.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- 13.Ragland SA, Criss AK. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017;13(9):e1006512. doi: 10.1371/journal.ppat.1006512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim KH, Park SJ, Lee YJ, et al. Inhibition of UVB-induced skin damage by exopolymers from Aureobasidium pullulans SM-2001 in hairless mice. Basic Clin Pharmacol Toxicol. 2015;116(2):73–86. doi: 10.1111/bcpt.12288. [DOI] [PubMed] [Google Scholar]
- 15.Fukuchi Y, Tatsumi K, Inoue H, et al. Prevention of COPD exacerbation by lysozyme: A double-blind, randomized, placebo-controlled study. Int J Chron Obstruct Pulmon Dis. 2016;11:831–38. doi: 10.2147/COPD.S103105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilchie AL, Wuerth K, Hancock RE. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol. 2013;9(12):761–68. doi: 10.1038/nchembio.1393. [DOI] [PubMed] [Google Scholar]
- 17.Fjell CD, Hiss JA, Hancock RE, et al. Designing antimicrobial peptides: Form follows function. Nat Rev Drug Discov. 2011;11(1):37–51. doi: 10.1038/nrd3591. [DOI] [PubMed] [Google Scholar]
- 18.Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414(6862):454–57. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 19.Mahlapuu M, Hakansson J, Ringstad L, et al. Antimicrobial peptides: An emerging category of therapeutic agents. Front Cell Infect Microbiol. 2016;6:194. doi: 10.3389/fcimb.2016.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koczulla AR, Bals Antimicrobial peptides: Current status and therapeutic potential. Drugs. 2003;63(4):389–406. doi: 10.2165/00003495-200363040-00005. [DOI] [PubMed] [Google Scholar]
- 21.Feng XL, Zhou B, Cao RB, et al. Immunomodulatory roles and functional analysis of pre-B lymphocyte DT40 cells with the bursal-derived BSP-II treatment. Peptides. 2012;36(2):292–98. doi: 10.1016/j.peptides.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Moon JY, Kim SY, Kim WK, et al. Protective efficacy of a Salmonella Typhimurium ghost vaccine candidate constructed with a recombinant lysozyme-PMAP36 fusion protein in a murine model. Can J Vet Res. 2017;81(4):297–303. [PMC free article] [PubMed] [Google Scholar]
- 23.Jin Q, Chen H, Wang X, et al. The effects of the recombinant CCR5 T4 lysozyme fusion protein on HIV-1 Infection. PLoS One. 2015;10(7):e0131894. doi: 10.1371/journal.pone.0131894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lizotte F, Pare M, Denhez B, et al. PKCdelta impaired vessel formation and angiogenic factor expression in diabetic ischemic limbs. Diabetes. 2013;62(8):2948–57. doi: 10.2337/db12-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murthy S, Gautam MK, Goel S, et al. Evaluation of in vivo wound healing activity of Bacopa monniera on different wound model in rats. Biomed Res Int. 2013;2013 doi: 10.1155/2013/972028. 972028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–29. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fadini GP, Albiero M, Menegazzo L, et al. The redox enzyme p66Shc contributes to diabetes and ischemia-induced delay in cutaneous wound healing. Diabetes. 2010;59(9):2306–14. doi: 10.2337/db09-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chou E, Suzuma I, Way KJ, et al. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: A possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002;105(3):373–79. doi: 10.1161/hc0302.102143. [DOI] [PubMed] [Google Scholar]
- 29.Lu D, Zhang L, Wang H, et al. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) enhances engraftment and angiogenesis of mesenchymal stem cells in diabetic hindlimb ischemia. Diabetes. 2012;61(5):1153–59. doi: 10.2337/db11-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ingram JR, Cawley S, Coulman E, et al. Levels of wound calprotectin and other inflammatory biomarkers aid in deciding which patients with a diabetic foot ulcer need antibiotic therapy (INDUCE study) Diabet Med. 2018;35(2):255–61. doi: 10.1111/dme.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuttolomondo A, Maida C, Pinto A. Diabetic foot syndrome: Immune-inflammatory features as possible cardiovascular markers in diabetes. World J Orthop. 2015;6(1):62–76. doi: 10.5312/wjo.v6.i1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilroy DW, Colville-Nash PR, Willis D, et al. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5(6):698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 33.Vincent AM, Brownlee M, Russell JW. Oxidative stress and programmed cell death in diabetic neuropathy. Ann NY Acad Sci. 2002;959:368–83. doi: 10.1111/j.1749-6632.2002.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 34.Brownlee M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes. 2005;54(6):1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 35.Wang GG, Li W, Lu XH, et al. Taurine attenuates oxidative stress and alleviates cardiac failure in type I diabetic rats. Croat Med J. 2013;54(2):171–79. doi: 10.3325/cmj.2013.54.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song YS, Park CM. Luteolin and luteolin-7-O-glucoside strengthen antioxidative potential through the modulation of Nrf2/MAPK mediated HO-1 signaling cascade in RAW 264.7 cells. Food Chem Toxicol. 2014;65:70–75. doi: 10.1016/j.fct.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 37.Andersen CA, Roukis TS. The diabetic foot. Surg Clin North Am. 2007;87(5):1149–77. x. doi: 10.1016/j.suc.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Frank S, Hubner G, Breier G, et al. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem. 1995;270(21):12607–13. doi: 10.1074/jbc.270.21.12607. [DOI] [PubMed] [Google Scholar]
- 39.Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet. 2003;361(9368):1545–51. doi: 10.1016/S0140-6736(03)13169-8. [DOI] [PubMed] [Google Scholar]
- 40.Biscetti F, Straface G, De Cristofaro R, et al. High-mobility group box-1 protein promotes angiogenesis after peripheral ischemia in diabetic mice through a VEGF-dependent mechanism. Diabetes. 2010;59(6):1496–505. doi: 10.2337/db09-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeshita S, Rossow ST, Kearney M, et al. Time course of increased cellular proliferation in collateral arteries after administration of vascular endothelial growth factor in a rabbit model of lower limb vascular insufficiency. Am J Pathol. 1995;147(6):1649–60. [PMC free article] [PubMed] [Google Scholar]
- 42.Roskoski R., Jr VEGF receptor protein-tyrosine kinases: Structure and regulation. Biochem Biophys Res Commun. 2008;375(3):287–91. doi: 10.1016/j.bbrc.2008.07.121. [DOI] [PubMed] [Google Scholar]
- 43.Tanii M, Yonemitsu Y, Fujii T, et al. Diabetic microangiopathy in ischemic limb is a disease of disturbance of the platelet-derived growth factor-BB/protein kinase C axis but not of impaired expression of angiogenic factors. Circ Res. 2006;98(1):55–62. doi: 10.1161/01.RES.0000197842.38758.45. [DOI] [PubMed] [Google Scholar]
- 44.Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, et al. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15(11):1298–306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaul YD, Seger R. The MEK/ERK cascade: From signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773(8):1213–26. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Li F, Chen C, Chen SM, et al. ERK signaling mediates long-term low concentration 3,3′-diindolylmethane inhibited nasopharyngeal carcinoma growth and metastasis: An in vitro and in vivo study. Oncol Rep. 2016;35(2):955–61. doi: 10.3892/or.2015.4428. [DOI] [PubMed] [Google Scholar]
- 47.Farese RV, Sajan MP, Standaert ML. Insulin-sensitive protein kinases (atypical protein kinase C and protein kinase B/Akt): Actions and defects in obesity and type II diabetes. Exp Biol Med (Maywood) 2005;230(9):593–605. doi: 10.1177/153537020523000901. [DOI] [PubMed] [Google Scholar]
- 48.Bellacosa A, Kumar CC, Di Cristofano A, et al. Activation of AKT kinases in cancer: Implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 49.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116(Pt 7):1175–86. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]