Abstract
Adherence of pathogens to cellular targets is required to initiate most infections. Defining strategies that interfere with adhesion is important for the development of preventative measures against infectious diseases. As an adhesin to host extracellular matrix proteins and human keratinocytes, the trimeric autotransporter adhesin DsrA, a proven virulence factor of the Gram-negative bacterium Haemophilus ducreyi, is a potential target for vaccine development. A recombinant form of the N-terminal passenger domain of DsrA from H. ducreyi class I strain 35000HP, termed rNT-DsrAI, was tested as a vaccine immunogen in the experimental swine model of H. ducreyi infection. Viable homologous H. ducreyi was not recovered from any animal receiving four doses of rNT-DsrAI administered with Freund’s adjuvant at two-week intervals. Control pigs receiving adjuvant only were all infected. All animals receiving the rNT-DsrAI vaccine developed antibody endpoint titers between 3.5 and 5 logs. All rNT-DsrAI antisera bound the surface of the two H. ducreyi strains used to challenge immunized pigs. Purified anti-rNT-DsrAI IgG partially blocked binding of fibrinogen at the surface of intact H. ducreyi. Overall, immunization with the passenger domain of the trimeric autotransporter adhesin DsrA therefore accelerated clearance of H. ducreyi in experimental lesions, possibly by interfering with fibrinogen binding.
Keywords: Haemophilus ducreyi, trimeric autotransporter adhesin, DsrA, active protection
INTRODUCTION
The sexually transmitted pathogen Haemophilus ducreyi causes chancroid, a genital ulcer disease important for acquisition and transmission of HIV [1-3]. During experimental and natural infection, H. ducreyi co-localizes with neutrophils and fibrin in the dermis of the skin [4, 5]. H. ducreyi binds the precursor of fibrin, fibrinogen (Fg), using the lipoprotein FgbA [6] and the trimeric autotransporter adhesin (TAA) DsrA (Ducreyi serum resistance A) [7], proven virulence factors in experimental models of chancroid [8, 9]. DsrA is a multifunctional outer membrane protein also involved in binding fibronectin (Fn) [10], vitronectin (Vn) [9], HaCat keratinocytes [9] and mediating serum resistance in H. ducreyi [11, 12].
DsrA consists of a functional N-terminal passenger domain variable amongst TAA and a highly conserved C-terminal translocator domain (Fig. 1A) [13, 14]. Using a panel of DsrA proteins truncated only in the passenger domain [15], our laboratory showed that serum resistance and Fg, Fn and Vn binding by DsrA involves amino acids in the C-terminal section of the passenger domain [15] (Fig. 1A). Since DsrA has many attributes of a successful vaccine candidate, including surface expression, involvement in pathogenesis [8, 9], immunogenicity [15, 16], and eliciting surface-binding antisera [15], we sought to determine if a recombinant preparation of the passenger domain of the H. ducreyi DsrA protein from prototypical class I strain 35000HP (rNT-DsrAI) is protective against homologous and heterologous challenges in the experimental swine model of chancroid.
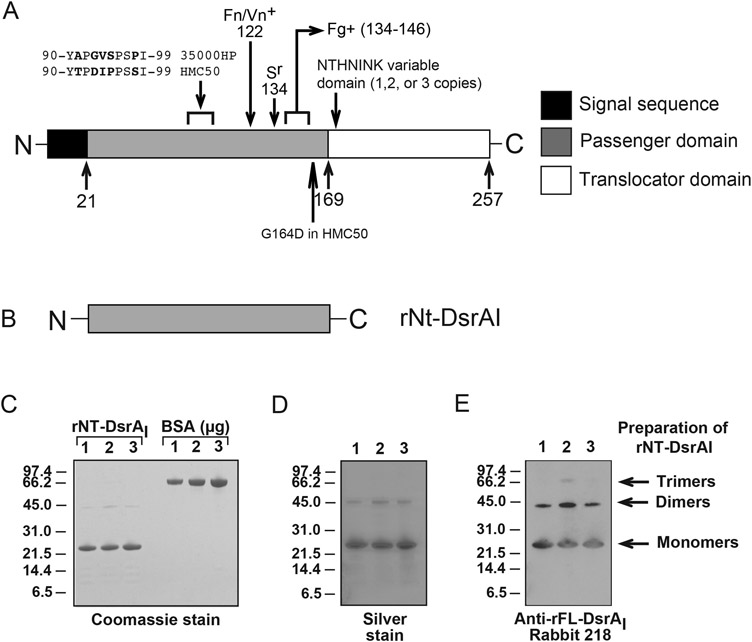
Figure 1. rNT-DsrAI as a vaccine immunogen against experimental chancroid.
A. Schematic representation of the DsrA protein showings its different domains, the sections involved in its different functions and the amino acids that vary between challenge strains. The arrows and numbers at the bottom of the cartoon indicate the amino acid at the end of each domain. Removal of the passenger domain of DsrA abrogates Fg, Vn and Fg binding and renders H. ducreyi serum sensitive in the presence of 50% normal human serum [7, 15]. These functions are regained (Fg+, Fn/Vn+, Sr) when the indicated C-terminal portions of the passenger domain of DsrA are expressed along with the translocator domain in an isogenic dsrA mutant strain [7, 15]. B. The passenger domain of rDsrAI, termed rNT-DsrAI, was used as the immunogen. C. Coomassie stained gel of the three preparations of rNT-DsrAI used in vaccination studies. Bovine serum albumin (BSA) was used to confirm concentration of the purified protein, in addition to determination of protein concentration using a commercially available kit. D. Silver stain of the same preparations in C. E. Western blot analysis of rNT-DsrAI preparations with a polyclonal Ab against full-length DsrA (rFL-DsrAI) [16]. Sr, serum resistance; Fn, fibronectin; Fg, fibrinogen; Vn, vitronectin; + indicates region of DsrA involved in binding to the particular protein.
MATERIALS AND METHODS
Bacterial strains and culture conditions
H. ducreyi strains are grouped in classes, class I or class II, based on variant outer membrane determinants [16-18]. Prototypical class I strain 35000HP, a human-passage isolate [19] of strain 35000 [20], is the source of the dsrA gene used for preparation of rNT-DsrAI. 35000HPΔdsrA (FX517) is an isogenic dsrA mutant of strain 35000HP [11]. Strain HMC50 is a class I H. ducreyi strain isolated in Jackson, MS [12]; the isogenic dsrA mutant of this strain is termed FX530 [12]. Strain HMC 112 is a class II H. ducreyi Bangladesh isolate [16, 21, 22]. Strain BE3145 was isolated from a cutaneous, non-genital chancroid lesion in a Samoan patient [23] and is a class I H. ducreyi strain, based on the amino acid sequence of DsrA. All strains were routinely sub-cultured on chocolate agar plates (CAP) supplemented with 5% FetalPlex (Gemini Bio-Products, West Sacramento, CA) and 1 X GGC (0.1% glucose, 0.001% glutamine, 0.026% cysteine) [24] at 34.5°C with 5% CO2. Escherichia coli strain BL21(DE3)pLysS (Life Technologies, Grand Island, NY [25]) was grown at 37°C in LB broth supplemented with ampicillin (100 μg/mL) and chloramphenicol (30 μg/mL) (Sigma-Aldrich, St. Louis, MO).
Expression and purification of rNT-DsrAI.
The nucleotide sequence encoding the passenger domain of dsrAI was amplified using PCR as previously described [16] and cloned into the pCRT7/CT-TOPO expression vector (Invitrogen, Carlsbad, CA), which contains a 6-histidine fusion tag. rNT-DsrAI was purified from a culture of BL21(DE3)pLysS expressing pUNCH1293 using nickel affinity chromatography as previously described [9, 16, 26], using the Prep Ease His-Tagged Protein Purification Maxi kit (Affimetrix, Cleveland, OH).
SDS-PAGE and Western blotting
Purified rNT-DsrAI was subjected to SDS-PAGE, followed by Coomassie or silver staining and Western blotting to determine purity of the protein, formation of multimers and to confirm its concentration and the absence of lipopolysaccharides (LPS), as previously described [16, 27]. Images were captured using the FluorChemE imager (Protein Simple, Santa Clara, CA). LPS content of rNT-DsrAI preparations was also determined using the Pyrogent 5000 LAL Assay kit (Lonza Inc., Allendale, NJ) following the manufacturer’s instructions and was found to be under detectable limits.
Animals studies
Three immunization experiments, approved by The Institutional Animal Care and Use Committee (IACUC), were performed at the North Carolina State University School of Veterinary Medicine (Raleigh, NC). Ten Yorkshire Cross pigs, obtained at three weeks of age, were immunized four times, two-weeks apart, with 250 μg of purified rNT-DsrAI in Freund’s complete (first immunization) and incomplete adjuvant (subsequent immunizations) (Sigma-Aldrich, St. Louis, MO), or adjuvant alone (control animals). Half-dose intramuscular immunizations were administered on both sides of the nuchal region of the neck. During challenge and biopsy procedures, pigs were sedated intramuscularly with 2 mg of ketamine HCl (Fort Dodge Laboratories, Fort Dodge, IA) and 2 mg of xylazine (Miles Laboratories, Shawnee Mission, KS) per kg of body weight. Animals were challenged one week (pig G) or two weeks after the final (fourth) rNT-DsrAI immunization with 10 μL of a bacterial suspension of strains 35000HP or HMC50 using a Multitest skin applicator (Lincoln Diagnostics, Decatur, IL) [21, 28]. The means (± standard deviations) of the inocula were as follows: 35000HP, 4.5 ± 0.32 × 103 CFU and 4.5 ± 0.23 × 102 CFU; HMC50, 5.1 ± 0.11 × 103 CFU and 3.6 ± 0.09 × 102 CFU. Six days after infection, 6 mm punch biopsies were removed from the ears and processed either for culture (GC broth) or histology (4% paraformaldehyde) as previously described [21, 28].
ELISA assays
Anti-rNT-DsrAI endpoint binding titer
rNT-DsrAI-specific serum Ab binding titers (endpoint) were determined by standard ELISA. Two-fold serial dilutions of test sera were performed in 384-well plates coated with rNT-DsrAI at 2.5 μg/mL and blocked for two hours at room temperature using bicarbonate buffer with 3% (w/v) non-fat dry milk. After an overnight incubation at 4°C, plates were washed 4 times with phosphate-buffered saline (PBS) plus 0.1% Tween-20. Anti-pig IgG alkaline phosphatase-conjugated antibody (Sigma, St. Louis, MO) was added to plates at a 1:10,000 dilution. Plates were then incubated at room temperature for two hours and washed four times. PNPP (p-Nitrophenyl Phosphate) substrate solution (Thermo Scientific, Rockford, IL) was added to the plates, which were incubated for 35 minutes at room temperature and read at an optical density (OD) of 405 nm using a Victor3 plate reader (Perkin Elmer, Waltham, MA). The baseline was set at three times the average plate background OD. Log endpoint titer (log10) is reported as the log of the reciprocal of the highest serum dilution at which the OD value was equal to or greater than baseline.
Whole cell binding ELISA
Abs elicited to the rNT-DsrAI vaccine were examined for binding the surface of viable H. ducreyi strains 35000HP, 35000HPΔdsrA, and HMC50 using a whole cell binding ELISA as previously described [21, 28].
Flow cytometry
Flow cytometry was also used to measure binding of rNT-DsrAI Abs to the surface of viable H. ducreyi. IgG purified from rNT-DsrAI antisera using protein A/G agarose were labeled with FITC following the manufacturer’s instructions (Life Technologies, Grand Island, NY). FITC-labeled IgG were then mixed with a bacterial suspension (OD600=0.5 in GC broth) for 30 min at room temperature with mixing. Washed bacterial suspensions were analyzed using the Accuri™ C6 Flow Cytometer (BD Biosciences, San Jose, CA), recording one hundred thousand events with a 10,000 threshold.
Fibrinogen binding assay
Fg binding to the surface of viable H. ducreyi was performed as previously described [7], except that excess unlabeled anti-rNT-DsrAI IgG (1.25 mg) was added to the bacterial suspension prior to the addition of 5 μg of FITC-labeled Fg (FITC-Fg). Mean fluorescence intensity (MFI) were compared to that of a bacterial suspension not incubated with IgG competitor, defined as 100% binding to FITC-Fg.
Statistical analyses
Our primary outcome of interest was the mean across animals of the within-animal proportion of lesions with viable bacterial recovery for each strain and inoculum combination. Differences in bacterial recovery between animals vaccinated with rNT-DsrAI and animals vaccinated with adjuvant only were assessed using the exact version of the Mantel Haenszel mean score chi-square statistics [29]. Adjustments for multiple comparisons were made using a step down testing procedure [30], assessing the effect of the vaccine first within the 35000HP strain, then isolate HMC50. Testing was stopped at the first statistically insignificant result. Within each strain, a Hochberg procedure [31] was used to account for testing the effect of the vaccine at each of two doses. Results of the whole cell binding ELISA, flow cytometry and Fg blocking assay were analyzed using a non-paired t-test in Sigma Stat (version 3.5, Systat Software, San Jose, CA).
Accession numbers for nucleotide sequences
The Genbank accession numbers for the dsrA genes of the different strains discussed in this manuscript are as follows: 35000HP, AAP95674; HMC50, KF880962; BE3145, KF880963; HMC112, AAU12588.
RESULTS and DISCUSSION
The rNT-DsrAI immunogen is highly pure and forms multimers.
For use as an experimental immunogen, rNT-DsrAI (Fig. 1B) expressed from a plasmid in E. coli was purified using nickel affinity chromatography. Purity of rNT-DsrAI and its ability to form multimers was assessed by SDS-PAGE and Western blot, as trimer formation is important for structure and function in other TAAs [32-34]. The three preparations of rNT-DsrAI used for the vaccine trials were more than 95% pure by Coomassie staining, save a faint band around 45 kDa, assumed to be the dimer form of rNT-DsrAI (Fig. 1C). To ensure the absence of endotoxin and foreign proteins, rNT-DsrAI preparations were also subjected to silver staining. No band characteristic of E. coli LPS were found in either of the preparations, indicating the absence of measurable endotoxin beyond the level of detection for this assay (Fig. 1D). Absence of LPS was confirmed using a Limulus Amebocyte Lysate assay. The silver-stained gel also showed a 45-kDa band (Fig. 1D) similar to the one previously seen on the Coomassie-stained gel (Fig. 1C). To determine if this band represented multimers of DsrA, preparations of rNT-DsrAI were subjected to Western blotting using an antiserum to full-length rDsrA (rFL-DsrAI) [16]. As predicted, the 45-kDa band was reactive with polyclonal antisera to rFL-DsrAI, indicating that it is a multimer form of rDsrA (Fig. 1E). Taken together, these data confirm that rNT-DsrAI is highly pure, lacks detectable amounts of endotoxin or foreign protein, and forms multimers.
No viable homologous H. ducreyi was recovered from pigs receiving the rNT-DsrAI vaccine.
Animals were administered four doses of rNT-DsrAI in Freund’s adjuvant at two weeks intervals and challenged with two different class I H. ducreyi strains, 35000HP and HMC50, at two different doses (103 and 102 CFU). Prototypical class I strain 35000HP is the source of the dsrA gene for production of the recombinant immunogen. The DsrA protein from HMC50 exhibits 6 amino acid differences in the passenger domain and an extra NTHNINK repeat in the translocator domain, as compared to DsrA from strain 35000HP [11] (Fig. 1A). After receiving four 250-μg doses of rNT-DsrAI in Freund’s adjuvant, no viable homologous class I H. ducreyi strain 35000HP was recovered from any of the five vaccinated pigs (Table 1). Conversely, viable bacteria were cultured from all control animals receiving adjuvant only. The vaccine showed a significantly protective effect against infection with the 35000HP strain, with p-values of 0.0476 and 0.0079 for 103 CFU and 102 CFU, respectively. While all swine receiving adjuvant only were infected with H. ducreyi strain HMC50, with infection rates between 59 and 71%, viable H. ducreyi were only detected in lesions from three out of five rNT-DsrAI-immunized animals, with infection rates of 12-22% (Table 1 - p-values of 0.0873 and 0.0794 for 103 and 102 CFU inocula, respectively). Taken together, these results indicate that biweekly administration of four 250-μg doses of rNT-DsrAI in Freund’s adjuvant protects against a homologous challenge and trends toward protection against an heterologous infection.
TABLE 1.
Recovery of viable H. ducreyi from ear skin biopsies of pigs immunized with adjuvant only or 4 doses of rNT-DsrAI administered at 2-week intervals
| Adjuvant only | rNT-DsrAI + adjuvant | ||||||
|---|---|---|---|---|---|---|---|
|
H. ducreyi+ biopsies / total # of biopsies* |
H. ducreyi+ biopsies / total # of biopsies |
||||||
| Strain | Experiment | Pig ID |
103 CFU | 102 CFU | Pig ID |
103 CFU | 102 CFU |
| 35000HP | 1 | A | 6/6 | 6/6 | C | 0/6 | 0/6 |
| B | 3/5 | 1/6 | D | 0/6 | 0/6 | ||
| 2 | F | 4/5 | 2/3 | G | 0/4 | 0/3 | |
| 3 | I | 2/5 | 1/4 | K | 0/4 | 0/4 | |
| J | 0/5 | 2/4 | L | 0/4 | 0/4 | ||
| TOTAL | 15/26 (58%)a | 12/23 (52%)b | 0/24 (0%)a | 0/23 (0%)b | |||
| HMC50 | 1 | A | 3/3 | 3/3 | C | 0/3 | 0/3 |
| B | 1/3 | 1/3 | D | 1/3 | 0/3 | ||
| 2 | F | 0/2 | 2/3 | G | 0/3 | 0/3 | |
| 3 | I | 3/4 | 1/4 | K | 1/4 | 1/5 | |
| J | 5/5 | 3/4 | L | 0/4 | 3/4 | ||
| TOTAL | 12/17 (71%)c | 10/17 (59%)d | 2/17 (12%)c | 4/18(22%)d | |||
The following p-values reference the lowercase letters in the table:
p=0.0476
p=0.0079
p=0.0873
p=0.0794 (Exact Mantel Haenszel mean score chi-square test; EXACT MHCHI option in SAS PROC FREQ).
The total # of biopsies reflects the number of inoculated sites
To assess disease severity of lesions six days after infection, biopsies were microscopically scored on a scale of 1 to 5, where 1 indicates normal skin and 5, an ulcer [21, 28]. The mean lesion scores between control animals and those receiving the rNT-DsrAI vaccine were not statistically different for either strain or inocula (data not shown). Thus, even though the immune response elicited to the rNT-DsrAI vaccine cleared viable H. ducreyi from the infection site, it did not modify disease outcome. Overall, these findings suggest that the rNT-DsrAI vaccine accelerated the clearance of viable bacteria from experimental lesions.
rNT-DsrAI administration induced a rapid and sustained humoral response.
To study the humoral immune response elicited to the rNT-DsrAI vaccine, log endpoint titers of antisera from rNT-DsrAI-immunized animals, determined using purified rNT-DsrAI immunogen in a standard ELISA, were graphed over the vaccine trial timeline in a scatter plot according to weeks after initial vaccination. All animals responded as expected to the immunogen, with rapid increases in titers after most immunizations (Fig. 2). Endpoint titers of antisera from animals immunized with the rNT-DsrAI vaccine on day of infection (week 8 for all except pig G, which is week 7) varied from 3.5 to 5 logs, indicating that all animals mounted a robust humoral immune response against the experimental rNT-DsrAI vaccine.
Figure 2. Log endpoint binding titers from adjuvant-only and rNT-DsrAI-immunized animals.
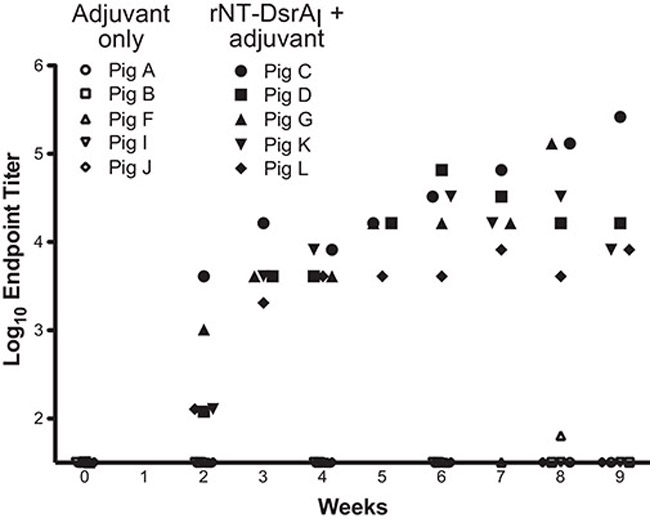
Sera collected weekly from control (adjuvant only) and rNT-DsrAI-immunized pigs were assayed for reactivity to rNT-DsrAI by ELISA. Shown are log endpoint titers, as defined in Materials and Methods, for each antiserum at each time point.
Post immune sera bind denatured and native class I DsrA proteins at the surface of H. ducreyi strains.
To determine specificity of the humoral response elicited to the rNT-DsrAI vaccine, we performed Western blot analysis against cellular lysates from the two challenge strains, an isogenic dsrA mutant, the recently isolated cutaneous chancroid strain BE3145 [23] and class II H. ducreyi strain HMC112 (Fig. 3). Abs elicited to rNT-DsrAI immunization recognized denatured homologous and heterologous DsrA proteins on Western blot (Fig. 3). Antisera from pigs C, D, G and K bound multimers of class I and class II DsrA from strain HMC112 (Fig. 3). Of note, reactivity of antisera from one of the rNT-DsrAI-immunized animals, pig L, was lower than the others; this reflects the lower Ab titers observed in this pig (Fig. 2). Taken together, these data show that all rNT-DsrAI antisera recognize denatured DsrA from homologous and heterologous H. ducreyi strains, suggesting that the humoral response elicited to the rNT-DsrAI recognizes linear epitopes of different DsrA proteins.
Figure 3. rNT-DsrAI antisera bind to homologous and heterologous denatured DsrA proteins.
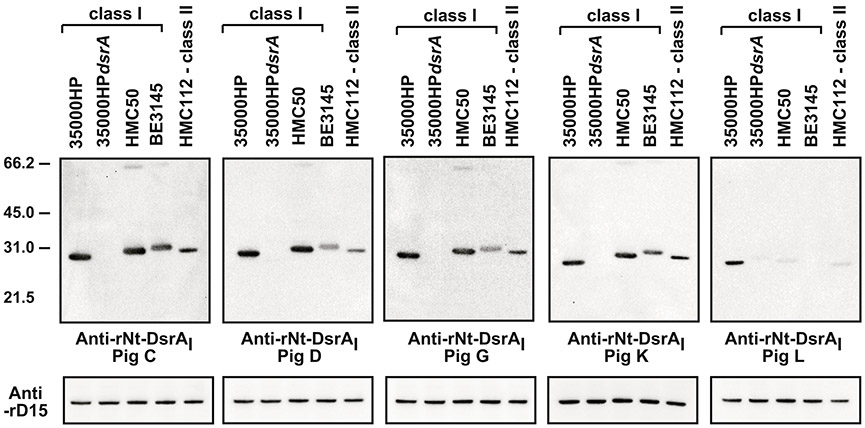
Total cellular proteins (from approximately 1 × 107 CFU) from the indicated strains were subjected to SDS-PAGE and Western blotting with the indicated polyclonal Abs at 1:10,000. All blots were incubated and developed concurrently. Blots were stripped and re-incubated with anti-rD15 [37] to show equal loading. Ponceau S staining and protein concentration were also used to determine equal loading in each lane (data not shown). Shown are representative blots from at least 2 independent experiments. The results presented in this figure reflect the reactivity of anti- rNT-DsrAI obtained one week after the 4th immunization, on the day of infection.
Using two different methods, a whole cell binding ELISA and flow cytometry, we tested the reactivity of rNT-DsrAI antisera from vaccinated pigs to the surface of H. ducreyi. Antisera from adjuvant controls did not bind any of the strains tested (pig A in Fig. 4B and data not shown). Furthermore, none of the rNT-DsrAI antisera reacted to an isogenic dsrA mutant strain (35000HPdsrA) (Fig. 4), corroborating our findings from Western analysis that the rNT-DsrAI vaccine elicited a humoral immune response specific to DsrA (Fig. 3). rNT-DsrAI antisera bound the surface of homologous strain 35000HP at similar levels (Fig. 4A and 4B), except for antiserum from pig L, which reacted significantly less than that of pig G, as measured by flow cytometry (Fig 4B). When measured against heterologous strain HMC50, all rNT-DsrAI antisera bound to the bacterial surface; however, reactivity of pig L antiserum to viable HMC50 was statistically lower than the antisera from all the other animals (Fig. 4). Significant differences also existed between antiserum from pigs C, D, G and K when surface binding was measured by flow cytometry.
Figure 4. rNT-DsrAI antisera bind the surface of class I H. ducreyi strains 35000HP and HMC50.
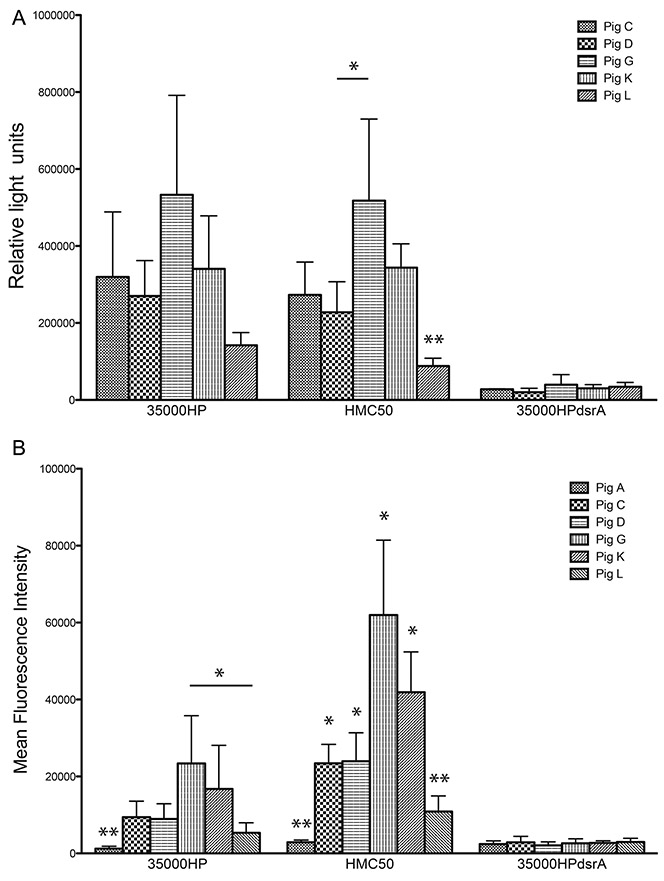
Antisera from the five rNT-DsrAI-vaccinated pigs were analyzed for binding to the bacterial cell surface using a whole cell-binding ELISA (A) and flow cytometry (B). A. Reactivity (mean ± standard deviation of at least three independent experiments) of antisera to homologous strain 35000HP, heterologous strains HMC50, and isogenic dsrA mutant FX517 (35000HPdsrA). B. Binding of FITC-labeled rNT-DsrAI antisera IgG (from individual pigs C, D, G, K or L) and one animal receiving adjuvant only (pig A) to the surface of H. ducreyi, expressed as mean fluorescent intensity (MFI). Shown are means ± standard deviations of the MFI obtained from 4 independent experiments. For HMC50, all bars are statistically different from one another, except for pigs C and D, and G and K. The results presented in this figure reflect the reactivity of anti- rNT-DsrAI obtained one week after the 4th immunization, on the day of infection. * p < 0.05; ** p < 0.05 as compared to all other bars in the same group, obtained using a unpaired t-test.
Differences in endpoint Ab titers (Fig. 2) and reactivity of rNT-DsrAI antisera to denatured and native DsrA (Fig. 2-4) may be explained by the use of animals with variable genetic background [35]. Variation of the immune response to the vaccine is therefore expected, similar to what might be seen in a human population after an immunization. The lower titer of antiserum from pig L obtained in the ELISA (Fig. 2) was reflected in results from Western blot and whole cell binding ELISA, with reduced reactivity in both instances (Fig. 3 and 4, respectively). Of note, pigs whose sera had lower endpoint titers on day of infection (pigs D, K and L with titers between 5000 and 50000; Fig. 2), although protected against the homologous challenge, had breakthrough infection with heterologous strains HMC50 (Table 1). Furthermore, antisera from pig L, which had the lowest titer and was the least reactive to the surface of H. ducreyi of all rNT-DsrAI antisera, was from the animal with the highest infection rate, with most sites from which viable heterologous strains were recovered (3 out of a total of 8 lesions yielded viable HMC50, Table 1). Taken together, these findings suggest that high titers and/or high affinity/avidity of the Ab population elicited to the rNT-DsrAI vaccine might be necessary for complete protection against an heterologous H. ducreyi strain.
rNT-DsrAI antisera partially block binding of Fg to H. ducreyi.
In search of a mechanism of protection of the rNT-DsrAI vaccine, we examined the bactericidal and opsonic capabilities of Abs elicited to rNT-DsrAI. At a final concentration of 25% with 25% normal human serum, none of the rNT-DsrAI antiserum promoted killing of H. ducreyi (data not shown). These same antisera also did not kill H. ducreyi strain 35000HP in the presence of purified human neutrophils (data not shown). Attempts to use swine neutrophils in opsonophagocytic assays were not successful.
Preliminary results examining blocking of Fn, Fg and Vn binding to the surface of H. ducreyi by rNT-DsrAI antisera using the Western blot approach revealed only limited interference (data not shown); however, a significant reduction of Fg binding to the surface of viable bacteria using purified IgG was observed with 80% (4 out of 5) of the rNT-DsrAI antisera using a recently developed flow cytometry method [7], for both 35000HP and HMC50 H. ducreyi strains (Fig. 5). These data suggest that the humoral immune response elicited to the rNT-DsrAI vaccine may partially interfere with binding of Fg to the surface of H. ducreyi; however, since Fg blocking by anti- rNT-DsrAI is similar for both strains 35000HP and HMC50, this activity does not appear to explain the difference in protection between the H. ducreyi strains tested. Because Fg is the precursor of fibrin, and H. ducreyi has been shown to co-localize with fibrin in both natural and experimental chancroid [5, 36], it is tempting to speculate that the rNT-DsrAI vaccine may prevent interaction of H. ducreyi with fibrin in the experimental lesion. This hypothesis remains to be tested.
Fig. 5. rNT-DsrAI antisera partially block binding of Fg to the surface of H. ducreyi.
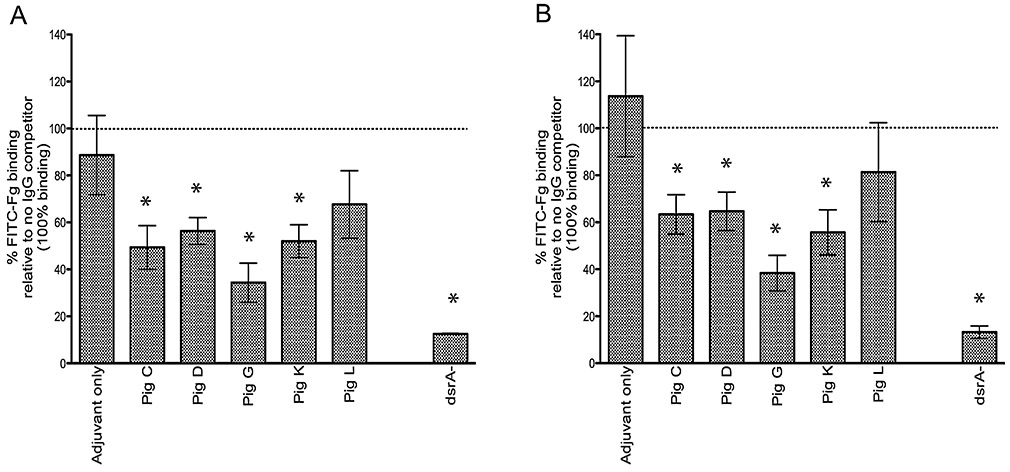
Suspensions of H. ducreyi strains 35000HP (A) and HMC50 (B) were incubated with IgG purified from rNT-DsrAI antisera prior to addition of FITC-Fg (5 μg). The MFI of washed bacterial suspensions was then measured using flow cytometry and compared to the MFI of a strain not incubated with anti-rNT-DsrAI IgG (no IgG competitor, defined as 100% binding – dotted line). IgG from animals receiving adjuvant only was used as a negative control. dsrA-, FITC-Fg binding by an isogenic dsrA mutant (FX517 for strain 35000HP in A, FX530 for strain HMC50 in B). Shown are means ± standard deviations of 3 experiments performed on three consecutive days, which reflect the reactivity of anti- rNT-DsrAI obtained one week after the 4th immunization, on the day of infection. * p < 0.05 using a non-paired t-test, as compared to incubation with IgG from antisera of animals receiving adjuvant only.
In conclusion, our vaccine trial shows that administration of four 250-μg doses of a recombinant form of the passenger domain of the TAA DsrA administered bi-weekly in Freund’s adjuvant is an effective immunogen against experimental H. ducreyi infection, as it protected against a homologous challenge and provided partial protection against infection with a heterologous H. ducreyi strain. The humoral immune response developed to this vaccine was high titered and recognized native DsrA at the surface of viable H. ducreyi. Surface-binding Abs elicited to the rNT-DsrAI vaccine partially blocked binding of Fg at the surface of H. ducreyi. Taken together, these data indicate that the rNT-DsrAI vaccine elicits a specific humoral response against DsrA at the surface of H. ducreyi that may interfere with Fg binding.
HIGHLIGHTS.
The passenger domain of DsrA (rNT-DsrA) was tested as a vaccine against chancroid
rNT-DsrA protected against a homologous challenge
High titered DsrA antisera bound the surface of viable Haemophilus ducreyi
Anti-DsrA IgG partially blocked fibrinogen binding by viable bacteria
Protection by the rNT-DsrA vaccine may involve a novel mechanism
ACKNOWLEDGEMENTS
This work was supported by the Southeastern Sexually Transmitted Infections Cooperative Research Center funded by the US National Institutes of Health (U19-AI031496). Some of the research was performed in the Regional Biocontainment Laboratory at Duke, which received partial support for construction from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (UC6-AI058607).
Abbreviations:
- TAA
Trimeric autotransporter adhesion
- Fg
fibrinogen
- Fn
fibronectin
- Vn
vitronectin
- rNT-DsrAI
passenger domain of the DsrA protein from class I H. ducreyi strain 35000HP
- Abs
antibodies
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing personal or financial interests.
REFERENCES
- [1].Trees DL, Morse SA. Chancroid and Haemophilus ducreyi: An update. Clin Microbiol Rev. 1995;8:357–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bong CT, Bauer ME, Spinola SM. Haemophilus ducreyi: clinical features, epidemiology, and prospects for disease control. Microbes Infect. 2002;4:1141–8. [DOI] [PubMed] [Google Scholar]
- [3].Lewis DA. Chancroid: clinical manifestations, diagnosis, and management. Sex Transm Infect. 2003;79:68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bauer ME, Goheen MP, Townsend CA, Spinola SM. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infection & Immunity. 2001;69:2549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bauer ME, Townsend CA, Ronald AR, Spinola SM. Localization of Haemophilus ducreyi in naturally acquired chancroidal ulcers. Microbes Infect. 2006;8:2465–8. [DOI] [PubMed] [Google Scholar]
- [6].Bauer ME, Townsend CA, Doster RS, Fortney KR, Zwickl BW, Katz BP, et al. A fibrinogen-binding lipoprotein contributes to the virulence of Haemophilus ducreyi in humans. J Infect Dis. 2009;199:684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fusco WG, Elkins C, Leduc I. Trimeric Autotransporter DsrA Is a Major Mediator of Fibrinogen Binding in Haemophilus ducreyi. Infect Immun. 2013;81:4443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bong CT, Throm RE, Fortney KR, Katz BP, Hood AF, Elkins C, et al. DsrA-deficient mutant of Haemophilus ducreyi is impaired in its ability to infect human volunteers. Infect Immun. 2001;69:1488–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cole LE, Kawula TH, Toffer KL, Elkins C. The Haemophilus ducreyi serum resistance antigen DsrA confers attachment to human keratinocytes. Infect Immun. 2002;70:6158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Leduc I, White CD, Nepluev I, Throm RE, Spinola SM, Elkins C. Outer membrane protein DsrA is the major fibronectin-binding determinant of Haemophilus ducreyi. Infect Immun. 2008;76:1608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Elkins C, Morrow KJ Jr., Olsen B Serum resistance in Haemophilus ducreyi requires outer membrane protein DsrA. Infect Immun. 2000;68:1608–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Abdullah M, Nepluev I, Afonina G, Ram S, Rice P, Cade W, et al. Killing of dsrA mutants of Haemophilus ducreyi by normal human serum occurs via the classical complement pathway and is initiated by immunoglobulin M binding. Infect Immun. 2005;73:3431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cotter SE, Surana NK, St Geme JW 3rd. Trimeric autotransporters: a distinct subfamily of autotransporter proteins. Trends Microbiol. 2005;13:199–205. [DOI] [PubMed] [Google Scholar]
- [14].Lyskowski A, Leo JC, Goldman A. Structure and biology of trimeric autotransporter adhesins. Adv Exp Med Biol. 2011;715:143–58. [DOI] [PubMed] [Google Scholar]
- [15].Leduc I, Olsen B, Elkins C. Localization of the domains of the Haemophilus ducreyi trimeric autotransporter DsrA involved in serum resistance and binding to the extracellular matrix proteins fibronectin and vitronectin. Infect Immun. 2009;77:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].White CD, Leduc I, Olsen B, Jeter C, Harris C, Elkins C. Haemophilus ducreyi Outer membrane determinants, including DsrA, define two clonal populations. Infect Immun. 2005;73:2387–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Post DM, Gibson BW. Proposed second class of Haemophilus ducreyi strains show altered protein and lipooligosaccharide profiles. Proteomics. 2007;7:3131–42. [DOI] [PubMed] [Google Scholar]
- [18].Post DM, Munson RS Jr., Baker B, Zhong H, Bozue JA, Gibson BW. Identification of genes involved in the expression of atypical lipooligosaccharide structures from a second class of Haemophilus ducreyi. Infect Immun. 2007;75:113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Al-Tawfiq JA, Thornton AC, Katz BP, Fortney KR, Todd KD, Hood AF, et al. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J Infect Dis. 1998;178:1684–7. [DOI] [PubMed] [Google Scholar]
- [20].Hammond GW, Lian CJ, Wilt JC, Ronald AR. Antimicrobial susceptibility of Haemophilus ducreyi. Antimicrobial Agents & Chemotherapy. 1978;13:608–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fusco WG, Afonina G, Nepluev I, Cholon DM, Choudhary N, Routh PA, et al. Immunization with the Haemophilus ducreyi hemoglobin receptor HgbA with adjuvant monophosphoryl lipid A protects swine from a homologous but not a heterologous challenge. Infect Immun. 2010;78:3763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wood GE, Dutro SM, Totten PA. Target cell range of the Haemophilus ducreyi hemolysin and its involvement in invasion of human epithelial cells. Infect Immun. 1999;67:3740–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ussher JE, Wilson E, Campanella S, Taylor SL, Roberts SA. Haemophilus ducreyi causing chronic skin ulceration in children visiting Samoa. Clin Infect Dis. 2007;44:e85–7. [DOI] [PubMed] [Google Scholar]
- [24].Dutro SM, Wood G, Totten P. Prevalence of, antibody response to, and immunity induced by Haemophilus ducreyi hemolysin. Infect Immun. 1999;67:3317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Studier FW, Rosenberg AH, Dunn JJ, Dubendorff JW. Use of T7 RNA polymerase to direct expression of cloned genes. Method Enzymol. 1990;185:60–89. [DOI] [PubMed] [Google Scholar]
- [26].Patterson K, Olsen B, Thomas C, Norn D, Tam M, Elkins C. Development of a rapid immunodiagnostic test for Haemophilus ducreyi. J Clin Microbiol. 2002;40:3694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Leduc I, Richards P, Davis C, Schilling B, Elkins C. A novel lectin, DltA, is required for expression of a full serum resistance phenotype in Haemophilus ducreyi. Infect Immun. 2004;72:3418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Leduc I, Fusco WG, Choudhary N, Routh PA, Cholon DM, Almond GW, et al. Passive immunization with a polyclonal antiserum to the hemoglobin receptor of Haemophilus ducreyi confers protection against a homologous challenge in the experimental swine model of chancroid. Infect Immun. 2011;79:3168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stokes ME, Davis CS, Koch GG. Categorical Data Analysis Using SAS. 3 ed: SAS Institute Inc.; 2012. [Google Scholar]
- [30].Williams DA. A test for differences between treatment means when several dose levels are compared with a zero dose control. Biometrics. 1971;27:103–17. [PubMed] [Google Scholar]
- [31].Hochberg Y A sharper Bonferroni procedure for multiple test of significance. Biometrika. 1988;75:800–2. [Google Scholar]
- [32].Cotter SE, Surana NK, Grass S, St Geme JW 3rd. Trimeric autotransporters require trimerization of the passenger domain for stability and adhesive activity. J Bacteriol. 2006;188:5400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schutz M, Weiss EM, Schindler M, Hallstrom T, Zipfel PF, Linke D, et al. Trimer stability of YadA is critical for virulence of Yersinia enterocolitica. Infect Immun. 2010;78:2677–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mikula KM, Leo JC, Lyskowski A, Kedracka-Krok S, Pirog A, Goldman A. The translocation domain in trimeric autotransporter adhesins is necessary and sufficient for trimerization and autotransportation. J Bacteriol. 2012;194:827–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cole LE, Toffer KL, Fulcher RA, San Mateo LR, Orndorff PE, Kawula TH. A humoral immune response confers protection against Haemophilus ducreyi infection. Infect Immun. 2003;71:6971–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bauer ME, Spinola SM. Localization of Haemophilus ducreyi at the pustular stage of disease in the human model of infection. Infection & Immunity. 2000;68:2309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Thomas KL, Leduc I, Olsen B, Thomas CE, Cameron DW, Elkins C. Cloning, overexpression, purification, and immunobiology of an 85-kilodalton outer membrane protein from Haemophilus ducreyi. Infect Immun. 2001;69:4438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]