Abstract
Omega-3 fatty acids, one of the key building blocks of cell membranes, have been of particular interest to scientists for many years. However, only a small group of the most important omega-3 polyunsaturated fatty acids are considered. This full-length review presents a broad and relatively complete cross-section of knowledge about omega-3 monounsaturated fatty acids, polyunsaturates, and an outline of their modifications. This is important because all these subgroups undoubtedly play an important role in the function of organisms. Some monounsaturated omega-3s are pheromone precursors in insects. Polyunsaturates with a very long chain are commonly found in the central nervous system and mammalian testes, in sponge organisms, and are also immunomodulating agents. Numerous modifications of omega-3 acids are plant hormones. Their chemical structure, chemical binding (in triacylglycerols, phospholipids, and ethyl esters) and bioavailability have been widely discussed indicating a correlation between the last two. Particular attention is paid to the effective methods of supplementation, and a detailed list of sources of omega-3 acids is presented, with meticulous reference to the generally available food. Both the oral and parenteral routes of administration are taken into account, and the omega-3 transport through the blood-brain barrier is mentioned. Having different eating habits in mind, the interactions between food fatty acids intake are discussed. Omega-3 acids are very susceptible to oxidation, and storage conditions often lead to a dramatic increase in this exposure. Therefore, the effect of oxidation on their bioavailability is briefly outlined.
Keywords: Omega-3 fatty acids, chemistry, sources, bioavailability
1. Chemistry of Omega-3 Fatty Acids
Omega-3 fatty acids, called n-3 fatty acids or ω−3 fatty acids (n-3 FAs), are a heterogeneous group of fatty acids with a double bond between the third and fourth carbon atoms from the methyl end (from the ω−1 carbon atom). In general, we distinguish among them monounsaturated fatty acids (MUFAs; one double bond in carbon chain) and polyunsaturated fatty acids (PUFAs; more than one double bond in carbon chain). Conjugated fatty acids (CFAs) are a subset of PUFAs with at least one pair of conjugated double bonds [1], i.e., the double bonds are not separated by methylene bridges, but one single bond. We also mention some examples of modified omega-3 fatty acids like hydroxy fatty acids (HFAs), oxo fatty acids (keto fatty acids) and hydroperoxy fatty acid. Among the hydroxy fatty acids, we distinguish saturated or unsaturated fatty acids, consisting of a long unbranched carbon chain with a carboxyl group at one end and one or more hydroxy groups. Oxo or keto fatty acids are fatty acids having both a carboxy group and a ketonic or aldehydic group in the molecule. Hydroperoxy fatty acids, in turn, carry at least one hydroperoxy group (-OOH) in the molecule. Some authors find the terms long-chain (LC) n-3 PUFAs and omega-3 fatty acids identical in meaning [2], which can be misleading because “omega-3 fatty acids” is a broader term.
We assumed all fatty acids with a double bond at the ω−3 carbon atom to be omega-3 fatty acids. Omega-3 fatty acids show cis-trans isomerism with its extension to E-Z configuration [3]. We can speak of geometrical isomerism in the case of omega-3 fatty acids because two carbon atoms with sp2 hybridization connected by a double bond are linked to a hydrogen atom and group of atoms each. In order to determine the type of geometrical isomerism, at the beginning we choose the two most important substituents—one on the left, the second on the right of the double bond. In fatty acids, we have only one group (of atoms) on each side, because the two remaining binding sites occupies a hydrogen atom. In the cis-isomer these two groups are located on the same side of the reference plane (the plane passing through the atoms connected by a double bond and perpendicular to the plane in which these atoms and atoms directly associated with them are situated); in the trans-isomer they are in contrary positions [4]. The E-Z system is a bit more detailed. The mutual placement of the substituents is described by the Cahn-Ingold-Prelog (CIP) rule. The most important is the substituent whose atom directly connecting to the rest of the molecule (directly with the atom forming the double bond) has a higher atomic number (in the case of isotopes, a higher atomic mass). If in this position, in the substituents (on the right or left side of the double bond), there are identical atoms, then (to choose a substituent of greater importance) we take into account subsequent atoms, always choosing atoms with the highest atomic number. If a given atom is connected by multiple bonds, the bond should be replaced by the number of single bonds appropriate for its multiplicity—each atom present at a multiple binding must after transformation have a corresponding number of single bonds (C=C = 2 × C-C). The “E” configuration (from entgegen, German for “opposite”) means that two groups of higher CIP priority (one on the left, the second on the right from the double bond) are on opposite sides of the double bond (in the synperiplanar position). If those groups are on the same side of the double bond (in antyperiplanar position), configuration is defined as “Z” (from zusammen, German for “together”) [5]. For simplicity, according to many authors, we used the terms “cis” and “Z” as well as “trans” and “E” interchangeably. The cis-trans isomerism of fatty acids seems to play a particularly important role in shaping their chemical and biological activity, a good example of which are the various properties of conjugated fatty acid isomers [1].
Naturally occurring fatty acids usually have from four to 28 carbon atoms. However, many of them, especially those found in the brain, retina and spermatozoa, have a longer carbon chain [6,7,8]. Fatty acids can be divided, depending on the length of the carbon chain, into four basic groups:
Short-chain fatty acids (SCFAs), sometimes called volatile fatty acids (VFAs), contain from one to six carbon atoms (C1–6), formed as a result of the fermentation of carbohydrates by the gut microbiota in the digestive tract of mammals [9].
Medium-chain fatty acids (MCFAs) have from seven to 12 carbon atoms (C7–12) [10]; according to other sources eight to 14 carbon atoms [11].
Long-chain fatty acids (LCFAs) have from 14 to 18 carbon atoms (C14–18) and constitute the majority of fatty acids taken with food (diet) [12].
Very long-chain fatty acids (VLCFAs) have backbones containing more than 20 carbon atoms (C > 20) [13], or according to other authors no fewer than 20 carbon atoms (C ≥ 20) [12] or even more than 22 carbon atoms (C > 22) [14,15,16].
There can also be distinguished fatty acid subgroups, such as dietary long-chain saturated fatty acids (C ≥ 16) and long-chain polyunsaturated fatty acids (LCPUFAs/LC PUFAs; C ≥ 18). While dietary long-chain saturated fatty acids do not directly concern the subject of this article, they deserve to be distinguished in the general chemical classification due to the ease of incorporation into the adipose tissue, and therefore, nomen omen, special dietary significance [11]. Fatty acids with nine or fewer carbon atoms are in a liquid state at room temperature [10].
The most important, but small, group of fatty acids for humans are essential fatty acids (EFAs), which are necessary to maintain homeostasis, cannot be synthesized, or rather cannot be synthesized sufficiently by the organism, and must be supplied with food [17,18]. The significance of fatty acids in the animal diet was discussed by Osborne and Mendel [19] in 1920. In 1929 Burr and Burr [20] proved in their experiments on rats the ‘essential’ nature of some fatty acids. Some authors consider all PUFAs to be essential fatty acids [21] and determine linoleic acid (LA) and alpha-linolenic acid (ALA) as the most important [17,22], calling them “parent essential fatty acids” [23,24]. Others considered only arachidonic and linoleic acids as essential fatty acids because of their importance for the body’s growth and for maintaining the integrity of the skin [21]. The mammalian literature indicates 23 acids as essential, while the aquatic literature quotes only two EFAs—EPA and DHA. Considering the importance of ARA, we can take ARA, DHA and EPA as the most important long-chain PUFAs in mammals and fish [25]. Some animals can synthesize them using LA and ALA as precursors [25,26]. However, those precursors must be available in sufficient quantities [25]. Gurr and Harwood [27] detailed “essential nutrients” in contrast to “essential metabolites”. In the light of this assumption, “essential nutrients” are precursors of “essential metabolites” and cannot be synthesized by organisms for which they are “essential”. Notwithstanding, “essential” is a relative term—human and rats can synthesize LA and ALA from 16:2n-6 and 16:3n-3 contained in green vegetables, i.e., in microalgae, in conditions of defined substrate availability [28,29,30,31]. Considering that there is insufficient data showing that any individual PUFA is absolutely necessary during life, Cunnane divided essential fatty acids into ‘conditionally indispensable’ and ‘conditionally dispensable’ [32,33,34].
The most important data concerning chemistry and sources of omega-3 fatty acids are included in Table 1.
Table 1.
Omega-3 fatty acids—chemistry and sources.
| No | IUPAC Name | Common Name | Shorthand (Simplified Formula) | Molecular Formula | Formula/Structure | Molecular Weight | Sources [Refs.] |
|---|---|---|---|---|---|---|---|
| MONOUNSATURATED FATTY ACIDS (MUFAs) | |||||||
| 1 | (9Z)-dodec-9-enoic acid | 9-lauroleic acid/lauroleic acid | C12:1n-3 | C12H22O2 |
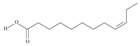
|
198.306 g/mol | 0.08% of fatty acid composition (total free fatty acids) of fresh and mature cheese samples of commercial (with full fat content) Manchego-type cheese and 0.05%/0.07% of the same cheese samples (fresh and mature) made from low-fat/full-fat milk with the addition of 2 g avocado oil per 100 mL of milk [35]; 0.31% of free fatty acids from bovine whey cream [36] |
| 2 | (9E)-dodec-9-enoic acid | - | C12:1n-3 | C12H22O2 |
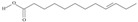
|
198.306 g/mol | in Peganum harmala fatty acids (concentration 0.31%) [37] |
| 3 | (11Z)-tetradec-11-enoic acid | - | C14:1n-3 | C14H26O2 |
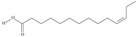
|
226.36 g/mol | a precursor of the sex pheromone component in Ostrinia scapulalis [38] |
| 4 | (11E)-tetradec-11-enoic acid | - | C14:1n-3 | C14H26O2 |
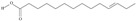
|
226.36 g/mol | 13.37% of leaf essential oil of Coriandrum sativum [39]; in Spodoptera littoralis [40] |
| 5 | (13E/Z)-hexadec-13-enoic acid | - | C16:1n-3 | C16H30O2 | undefined cis-trans isomerism | 254.414 g/mol | in microalgae—1.39%, 3.12%, 0.23%, 1.33% of Dunaliella tertiolecta, Platimonas viridis, Nefrochloris salina and Phaeodactylum tricornutum total fatty acids, respectively, also present in the gonads and larvae of oysters fed with the mentioned microalgae [41] |
| 6 | (13E)-hexadec-13-enoic acid | - | C16:1n-3 | C16H30O2 |

|
254.414 g/mol | in Brassica napus leaf discs—3.35%, 1.06%, 1.19% of total PG (phosphatidylglycerol), PA (phosphatidic acid) and PC (phosphatidylcholine) fatty acids, respectively, trace amounts in phosphatidylethanolamine (PE) were also found [42]; in the moth Spodoptera littoralis [43] |
| 7 | (13Z)-hexadec-13-enoic acid | - | C16:1n-3 | C16H30O2 |
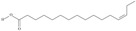
|
254.414 g/mol | in microalga Heterosigma carterae [44] |
| 8 | (15E/Z)-octadec-15-enoic acid | - | C18:1n-3 | C18H34O2 | undefined cis-trans isomerism | 282.468 g/mol | detected in pork (4.2 mg/100 g fatty acids of the fresh ham of adult pig, 4.8 mg/100 g fatty acids of the pork loin of adult pig) [45]) |
| 9 | (15Z)-octadec-15-enoic acid | - | C18:1n-3 | C18H34O2 |
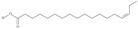
|
282.468 g/mol | detected in beef (0.217% (for the grass-fed beef samples) and 0.241% (for conventional beef) of total fatty acids mass) [46], cows milk (0.146–0.56 g/100 g of total fatty acids, dependently on applied diet) and butter [47], and in human blood (relative abundance for healthy male—0.04%) [48] and human milk (0.01–0.06% of total fatty acids of transitional and mature human milk from five regions in China [49], 0.02–0.29% by weight of total fatty acids in human milk samples collected from woman in the United States {the mean concetration for both (cis and trans) isomers—0.06%} [50]) |
| 10 | (15E)-octadec-15-enoic acid | - | C18:1n-3 | C18H34O2 |

|
282.468 g/mol | detected in cow’s milk (0.102–0.34 g/100 g of total fatty acids, dependently on applied diet) and butter [47], 0–0.15% by weight of total fatty acids in human milk samples collected from women in the United States (the mean concetration for both (cis and trans) isomers—0.06%) [50] |
| POLYUNSATURATED FATTY ACIDS (PUFAs) | |||||||
| 1 | (11Z,13Z)-hexadeca-11,13-dienoic acid | - | C16:1n-3 | C16H28O2 |
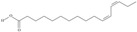
|
252.398 g/mol | in the (pheromone) gland extracts of the navel orangeworm, Amyelois transitella [51] |
| 2 | (7Z,10Z,13Z)-hexadeca-7,10,13-trienoic acid | roughanic acid | C16:3n-3 | C16H26O2 |
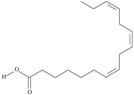
|
250.382 g/mol | as methyl ester: 0.11–0.12% of European wels catfish oil, 0.34–0.41% of common bleak oil (in Area (%)) [52]; 3, 1.1, 0.9, 6.6, 0.4, 4.1, 0.5, 0.5, 0.3, 0.1, 11.8% of Chinese cabbage, white cabbage, savoy, kale, red cabbage, brussels sprouts, cauliflower, kohlrabi, swede, cabbage lettuce, parsley fatty acid mass [53]—systematic names were placed in the line ‘α-linolenic acid’; in leaves of many conifer species, most in Larix leptolepus and Taxus baccata—4.8% and 5.8% of fatty acids of lives, respectively [54] |
| 3 | (4Z,7Z,10Z,13Z)-hexadeca-4,7,10,13-tetraenoic acid | - | C16:4n-3 | C16H24O2 |
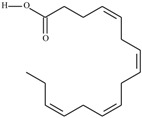
|
248.366 g/mol | as methyl 4,7,10,13-hexadecatetraenoate: 0.09–0.1% of Pontic shad oil, 0.08–0.16% of European wels catfish oil, 0.11–0.56% of common bleak oil [52] |
| 4 | (8Z,11Z,14Z)-heptadeca-8,11,14-trienoic acid | norlinolenic acid | C17:3n-3 | C17H28O2 |
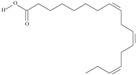
|
264.409 g/mol | in Salvia nilotica—0.4% of mixed esters and 2.3% of IV fraction (% by gas liquid chromatography) [55] |
| 5 | (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid | α-linolenic acid | C18:3n-3 | C18H30O2 |
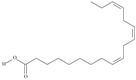
|
278.436 g/mol | 0.2% of black cumin (Nigella sativa) seed oil fatty acids [56]; 8% of Cardiospermum halicabum seed oil total fatty acids [57]; as methyl esters: 1.67% of Labeo rohita muscle tissue fatty acids, 1.39% of Cirrhinus mrigata muscle tissue fatty acids, 1.29% of Catla catla muscle tissue fatty acids [58]; in salted products of fish roe: 0.7% of Ikura (salmon), 0.4% of Tarako (pollock), 0.7% of Tobiko (flyingfish), 0.4% of Kazunoko (herring) total lipids [59]; 1.6–1.9% of salmon eggs total phospholipid fraction, 1–3.6% of salmon eggs total triacylglicerol fraction [60]; 1.1% of wild sardine (Sardina pilchardus) muscle total fatty acids (1% of captive sardine muscle total fatty acids), 0.7% of wild/captive sardine liver total fatty acids [61]; 0.61–0.83% for finwhale oils, 0.13–0.34% for seal oils [62]; 0.5% of tuna oil [63]; 0.5% of Epinephelus fasciatus muscle triacylglycerols, 0.1% of E. fasciatus PE and 0.1–0.2% of E. fasciatus PC, 0.1–0.5% of E. retouti muscle triacylglycerols, 0–0.1% of E. retouti PE and 0.1% of E. retouti PC [64]; 16.4 g/100 g of the milled chia seeds [65]; 21% of n-hexane extract of Senna italica aerial parts [66]; 40% of Chamaecyparis lawsoniana and 50% of Fokienia hodginsii total fatty acids [67]; 64.04% of chia (Salvia hispanica) seed oil fatty acids [68]; 0.25 g/100 g of total fatty acid methyl esters of milk fat from ewes, 2.57 g/100 g of total fatty acid methyl esters of milk fat from ewes fed diets with extruded linseed supplementation (12% linseed in dry matter) [69]; 59.5, 57, 46.4, 50, 39.8, 58.7, 48.9, 41.9, 50.8, 54.6, 41.7, 10, 6, 8.5, 5.9% of Chinese cabbage (Brassica chinensis), white cabbage (Brassica oleracea), savoy (Brassica oleracea var. sabauda), kale (Brassica oleracea convar. acephala var. sabellica), red cabbage (Brassica oleracea var. capitata), brussels sprouts (Brassica oleracea var. gemmifera), cauliflower (Brassica oleracea convar. botrytis var. botrytis), kohlrabi (Brassica oleracea convar. acephala var. gongylodes), swede (Brassica napus var. napobrassica), cabbage lettuce (Lactuca sativa var. capitata), parsley (Petroselinum crispum ssp. crispum), black salsify (Scorzonera hispanica), carrot (Daucus carota ssp. sativus), turnip-rooted parsley (Petroselinum crispum ssp. tuberosum), Florence fennel (Foeniculum vulgare var. azoricum) fatty acids mass, respectively; 8.7, 9.7, 11.5, 8.3, 10.2, 11.2, 9.3, 11, 12.3% of Chinese cabbage, white cabbage, savoy, kale, red cabbage, brussels sprouts, cauliflower, kohlrabi, swede, black salsify, cabbage lettuce, carrot, parsley, Florence fennel seed fatty acids mass, respectively [53]; in Trichosanthes kirilowii (33.77–38.66% of seed oils) [70]; in Linum usitatissimum (depending on the genotype it contains from 1.1 to 65.2% of total fatty acids in seeds) [71]; in paprika Capsicum annuum (29.93% of fresh pericarp fatty acids in Jaranda variety and 30.27% in Jariza variety) [72] |
| 6 | (9E,12E,15E)-octadeca-9,12,15-trienoic acid | linolenelaidic acid/elaidolinolenic acid | C18:3n-3 | C18H30O2 |

|
278.436 g/mol | in Turkish sage species—Salvia virgata, Salvia potentillifolia, Salvia recognita, Salvia tomentosa amounts to 0.4, 0.7, 1.1 and 1.4% of seed fatty acids, respectively [73]; 0.03% of tobacco seed oil fatty acids [74] |
| 7 | (9Z,11E,15Z)-octadeca-9,11,15-trienoic acid | rumelenic acid | C18:3n-3 | C18H30O2 |
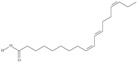
|
278.436 g/mol | in muscle samples from linseed oil-fed lambs [75]; 0.03 g/100 g of total fatty acid methyl esters of milk fat from ewes, 0.52 g/100 g of total fatty acid methyl esters of milk fat from ewes fed diets with extruded linseed supplementation (12% linseed in dry matter) [69] |
| 8 | (9Z,11E,15E)-octadeca-9,11,15-trienoic acid | - | C18:3n-3 | C18H30O2 |
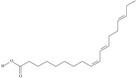
|
278.436 g/mol | 0.01 g/100 g of total fatty acid methyl esters of milk fat from ewes, 0.21 g/100 g of total fatty acid methyl esters of milk fat from ewes fed diets with extruded linseed supplementation (12% linseed in dry matter) [69] |
| 9 | (5Z,9Z,12Z,15Z)-octadeca-5,9,12,15-tetraenoic acid | Coniferonic acid | C18:4n-3 | C18H28O2 |
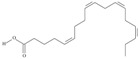
|
276.42 g/mol | found in many conifer species: 0.05% of Abies fraseri seed total fatty acids, 0.03% of A. nordmanniana ssp. nordmanniana seed total fatty acids, 0.05% of A. numidica seed total fatty acids, 0.06% of A. procera seed total fatty acids, 0.05% of Tsuga chinensis seed total fatty acids, 0.09% of Tsuga heterophylla seed total fatty acids, 1.78% of Pseudolarix amabilis seed total fatty acids [76]; 2% of Chamaecyparis lawsoniana and 2.8% of Fokienia hodginsii seed total fatty acids [67]; 11% of Abies vejarii Martínez leaf fatty acids [77] |
| 10 | (6Z,9Z,12Z,15Z)-octadeca-6,9,12,15-tetraenoic acid | stearidonic acid (SDA)/moroctic acid | C18:4n-3 | C18H28O2 |
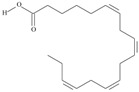
|
276.42 g/mol | in salted products of fish roe: 0.8% of Ikura (salmon), 0.8% of Tarako (pollock), 0.4% of Tobiko (flyingfish), 0.4% of Kazunoko (herring) total lipids [59]; 2.3% of wild sardine (Sardina pilchardus) muscle total fatty acids (1.5% of captive sardine muscle total fatty acids), 0.7% of wild/0.6% of captive sardine liver total fatty acids [61]; as methyl ester: 0.57–0.76% of common barbel oil, 1.52–2.04% of Pontic shad oil, 0.2–0.35% of European wels catfish oil, 0.28–0.59% of common bleak oil [52]; 0.41–0.71% for finwhale oils, 0.79–1.09% for seal oils (in weight per cent) [62]; 0–0.9% of Epinephelus retouti muscle triacylglycerols [64]; mean amounts of seeds total fatty acid methyl esters: 4.73% for Primulaceae, 4.99% for Boraginaceae, 0.36% for Hippophae rhamnoides, 0.77% for Cannabis sativa [78]; in Ribes nigrum and fish oils [77]; 14% and 16.2% of Echium humile ssp. pycnanthum seeds fatty acids, depending on the location [79] |
| 11 | (all-E/Z)-octadeca-9,11,13,15-tetraenoic acid | parinaric acid | C18:4n-3 | C18H28O2 | undefined cis-trans isomerism | 276.42 g/mol | 11.34% of the methanol extract of Spirogyra rhizoides [80] |
| 12 | (9Z,11E,13E,15Z)-octadeca-9,11,13,15-tetraenoic acid | α-parinaric acid (cis-parinaric acid) | C18:4n-3 | C18H28O2 |
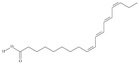
|
276.42 g/mol | 62% of Parinarium laurinum seed oil methyl esters [81]; 53.5% of Parinarium laurinum seed oil fatty acids [66]; 4% of Parinarium macrophyllum seed oil [82]; in Impatiens edgeworthii (48% of total fatty acids) [83]; in Impatiens glandulifera (42.5% of Impatiens oil) [84]; in the seed oil of Sebastiana brasiliensis (21.4, 32.5 and 35.1 peak area % of samples from National Park of Turvo, Santana da Boa Vista region and Cacapava do Sul region, respectively) [85]; 30% of Impatiens balsamina seed oil [86]; in Chrysobalanus icaco (10% of seed oil methyl esters) [81]; in evening primrose (Oenothera biennis) oil (3.5 × 10−5 mol/L) [87]; in stripped Borage (Borago officinalis) oil (1.6 × 10−4 mol/L) [82] |
| 13 | (9E,11E,13E,15E)-octadeca-9,11,13,15-tetraenoic acid | trans-parinaric acid (β-parinaric acid) | C18:4n-3 | C18H28O2 |

|
276.42 g/mol | “naturally occurring” [88]; in Impatiens spp. [89] |
| 14 | (9Z,15Z)-octadeca-9,15-dienoic acid | Mangiferic acid | C18:2n-3 | C18H32O2 |
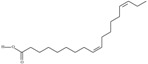
|
280.452 g/mol | 3.1–5.4% of fatty acids in pulp of mango fruit, 1% of mango peel total fatty acids (Mangifera indica L.) [90]; in muscle samples from linseed oil-fed lambs [75] |
| 15 | (all-E/Z)-octadeca-11,15-dienoic acid | - | C18:2n-3 | C18H32O2 | undefined cis-trans isomerism | 280.452 g/mol | in beef and mutton tallow—11E, 15E; 11Z(E), 15E(Z); 11Z, 15Z isomers [91]; 11E, 15Z isomer in muscle samples from linseed oil-fed lambs [75] |
| 16 | (12Z,15Z)-octadeca-12,15-dienoic acid | - | C18:2n-3 | C18H32O2 |
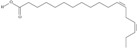
|
280.452 g/mol | in muscle samples from linseed oil-fed lambs [75] |
| 17 | (all-E/Z)-octadeca-10,15-dienoic acid | - | C18:2n-3 | C18H32O2 | undefined cis-trans isomerism | 280.452 g/mol | in beef and mutton tallow—10Z, 15Z; 10Z(E), 15E(Z) isomers [92] |
| 18 | (11Z,14Z,17Z)-icosa-11,14,17-trienoic acid | eicosatrienoic acid (ETE)/homo-alpha-linolenic acid | 20:3n-3 | C20H34O2 |
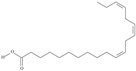
|
306.49 g/mol | In Sterculia urens seeds (2.96% of the total lipid) [91]; in Hibiscus sabdariffa seed oil (0.2% of total fatty acids) [93]; 2.22–6.59 mg/g of Platymonas subcordiformis dry weight and 24 to 42.04 mg/g of Porphyridium cruentum dry weight [94]; 3.97 g/kg soybean unit [95]; 6.78–8.73% of total fatty acids in Torreya grandis kernel oil [96]; 31.44% of Pittosporum undulatum seed oil total fatty acids [97]; 8.5% of total neutral lipids and 15.5% of free fatty acids of cork Phellodendron lavalei seeds [98]; 0.49 g/100g of Pinus halepensis seed oil total fatty acids [99]; 0.2% of black cumin (Nigella sativa) seed oil fatty acids [56]; 0.01–0.1% for finwhale oils, <0.01–0.03% for seal oils (in weight per cent) [62]; 3.3% of Ephedra gerardiana seed lipids and 2.2% of Cimicifuga racemosa seed oil [100]; 0.15% and 0.16% of ISA Brown and Arucana hen egg yolk total lipids, respectively [101]; 0.1, 0.2, 0.1, 0.2, 0.2, 0.2, 0.1% of white cabbage, kale, red cabbage, brussels sprouts, cauliflower, cabbage lettuce, parsley fatty acid mass; 0.1, 0.1, 0.1, 0.2, 0.1, 0.2, traces, 0.1, 0.1% of Chinese cabbage, white cabbage, savoy, kale, red cabbage, brussels sprouts, cauliflower, kohlrabi, swede seed fatty acid mass [53]—systematic names were placed in the line ‘α-linolenic acid’ |
| 19 | (8Z,11Z,14Z,17Z)-icosa-8,11,14,17-tetraenoic acid | eicosatetraenoic acid (ETA) | C20:4n-3 | C20H32O2 |
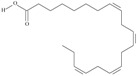
|
304.474 g/mol | in salted products of fish roe: 2.1% of Ikura (salmon), 0.6% of Tarako (pollock), 0.5% of Tobiko (flyingfish), 0.4% of Kazunoko (herring) total lipids [59]; 2.9–3.8% of salmon eggs total triacylglicerol fraction [48]; as methyl 8,11,14,17-eicosatetraenoate: 0.19–0.24% of common barbel oil, 0.82–1.44% of Pontic shad oil, 0.34–1% of European wels catfish oil, 0.17–0.35% of common bleak oil [52]; 0.35–0.78% for finwhale oils, 0.07–0.26% for seal oils (in weight per cent) [62]; 0.4–0.8% of Epinephelus retouti muscle triacylglycerols [64]; 0.06% of Agathis robusta seed lipids [67]; in lipids of bovine liver [102]; in herring (Clupea harrengus), mackerel (Scomber scombus) and capelin (Mallotus villosus) body oils in amounts of 5, 15 and 4 mg/g of fatty acids, respectively, in menhaden (Brevoortia spp.) oil and Indian oil sardine (Sardinella longiceps)—19 and 6 mg/g of fatty acids, respectively [103] |
| 20 | (5Z,11Z,14Z,17Z)-icosa-5,11,14,17-tetraenoic acid | juniperonic acid | C20:4n-3 | C20H32O2 |
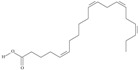
|
304.474 g/mol | 8.9 weight % of Biota orientalis seed oil [104]; 0.12–0.57% of Sargassum spp. total fatty acids [105]; 1% of Caltha palustris seed oil [106]; in many conifer species, most in Gnetum gnemon, Chaemaecyparis thyoides, Cephalotaxus sinensis, Taxus cuspidata, Pseudolarix amabilis, Araucaria angustifolia, Agathis australis, Zamia furfuracea—12.9, 7.7, 6.8, 6.8, 6.3, 6.3, 6.1, 6, % of leaf fatty acids, respectively [54,77] |
| 21 | (5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaenoic acid | eicosapentaenoic acid (EPA)/timnodonic acid | C20:5n-3 | C20H30O2 |
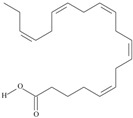
|
302.458 g/mol | 13% of Undaria pinnatifida essential oil composition [107]; 3.05 g/100 g (as methyl ester: 3.15% of fatty acids) muscle tissue of Labeo rohita, 2.51 g/100 g (as methyl ester: 2.51% of fatty acids) muscle tissue of Cirrhinus mrigata, 3.15 g/100 g (as methyl ester: 3.05% of fatty acids) muscle tissue of Catla catla [58]; in salted products of fish roe: 13.6% of Ikura (salmon), 18.8% of Tarako (pollock), 7% of Tobiko (flyingfish), 15% of Kazunoko (herring) total lipids [59]; 8.7–10.6% of salmon eggs total phospholipid fraction, 8.3–10.3% of salmon eggs total triacylglicerol fraction [60]; 13.6% of wild sardine (Sardina pilchardus) muscle total fatty acids (9.2% of captive sardine muscle total fatty acids), 6.5% of wild/7.2% of captive sardine liver total fatty acids [61]; as methyl ester: 2.32–3.41% of common barbel oil, 2.57–3.8% of Pontic shad oil, 2.41–6.1% of European wels catfish oil, 0.51–1.36% of common bleak oil [52]; 1.82–3.72% for finwhale oils, 6.37–8.12% for seal oils (in weight per cent) [62]; 1.8–3.5% of Variola louti muscle triacylglycerols [64]; 2% of Rhododendron sochadzeae leaves fatty acids (in hexan extract) [108] |
| 22 | (6Z,9Z,12Z,15Z,18Z)-henicosa-6,9,12,15,18-pentaenoic acid | heneicosapentaenoic acid (HPA) | C21:5n-3 | C21H32O2 |
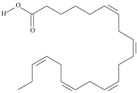
|
316.485 g/mol | as methyl 6,9,12,15,18-heneicosapentaenoate: 0.06–0.12% of common barbel oil, 0.07–0.08% of Pontic shad oil, 0.09% of European wels catfish oil [52]; 0.2–0.4% of diatom Skeletonema costatum total fatty acids, 0.1–0.2% of copepods Calanus and Centropages sp. total fatty acids, <0.1% of copepods Temora longicornia total fatty acids, 0.2–0.7% of euphausid Meganyctiphanes norvegica total fatty acids, 0.1–1.1% of euphausid Euphausia sp. total fatty acids, 0.1–0.3% of herring oil Clupea harengus total fatty acids, 0.19% of sturgeon oil Acipenser oxyrhynchus total fatty acids, 0.2–0.6% of mackerel oil Scomber scrombrus total fatty acids, 0.1–0.9% of fin whale (Balaenopterus physalus) blubber total fatty acids, 0.2% of dolphin (Tursiops truncatus) milk total fatty acids [109] |
| 23 | (13Z,16Z,19Z)-docosa-13,16,19-trienoic acid | docosatrienoic acid (DTrE) | C22:3n-3 | C22H38O2 |
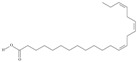
|
334.544 g/mol | in Lepidium sativium seed oil (47.66% of total fatty acids) [110]; trace amount in weed seed oil, largely charlock [111]; in salami-type sausage made of Baltic herring fillets, pork and lard (from < 0.2 to 0.3% during ripening) [112]; in shade dried neem (Azadirachta indica) flower powder present to an extent of 5.7% in the total lipid [113]; in eggs (2.92–3.23%), embryos (3.28–3.76%) and larvae (0–3.65%) of Oncorhynchus mykiss (from 0 to 3.76% of total fatty acids) [114]; in muscle tissue and liver of Diplodus vulgaris (0.8–11.6% of total fatty acids) [115] |
| 24 | (7Z,10Z,13Z,16Z,19Z)-docosa-7,10,13,16,19-pentaenoic acid | docosapentaenoic acid (DPA)/clupanodonic acid | C22:5n-3 | C22H34O2 |
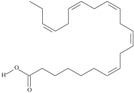
|
330.512 g/mol | as methyl esters: 1.45% of Labeo rohita muscle tissue fatty acids, 1.86% of Cirrhinus mrigata muscle tissue fatty acids, 1.09% of Catla catla muscle tissue fatty acids [58]; in salted products of fish roe: 5.6% of Ikura (salmon), 1.0% of Tarako (pollock), 2.8% of Tobiko (flyingfish), 1.3% of Kazunoko (herring) total lipids [59]; 6.3–8.1% of salmon eggs total phospholipid fraction, 4.9–6% of salmon eggs total triacylglicerol fraction [60]; 1.6% of wild sardine (Sardina pilchardus) muscle total fatty acids (2% of captive sardine muscle total fatty acids), 2.7% of wild/2.4% of captive sardine liver total fatty acids [61]; as methyl ester: 0.6–1.15% of common barbel oil, 0.44–0.83% of Pontic shad oil, 1.04–2.82% of European wels catfish oil, 0.06–0.23% of common bleak oil [52]; 1.07–2.28% for finwhale oils, 2.64–4.74% for seal oils (in weight per cent) [62]; 1.34% of tuna oil [51]; 1.9–3.3% of Variola louti muscle triacylglycerols [64]; 13.8/16.7% of wether/eve (lamb) raw meat fatty acids [116]; 0.07% and 1.6% of Hormosira banksii and Dictyota dichomota total fatty acids, respectively [117] |
| 25 | (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoic acid | docosahexaenoic acid (DHA)/cervonic acid | C22:6n-3 | C22H32O2 |
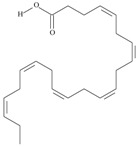
|
328.496 g/mol | 15.44 g/100 g (as methyl ester: 17.98% of fatty acids) muscle tissue of Labeo rohita, 18.07 g/100 g (as methyl ester: 8.07% of fatty acids) muscle tissue of Cirrhinus mrigata, 17.98 g/100 g (as methyl ester: 15.4% of fatty acids) muscle tissue of Catla catla [58]; in salted products of fish roe: 17.4% of Ikura (salmon), 22.2% of Tarako (pollock), 27.9% of Tobiko (flyingfish), 22.6% of Kazunoko (herring) total lipids [59]; 26–29.2% of salmon eggs total phospholipid fraction, 12.5–15.3% of salmon eggs total triacylglicerol fraction [60]; 14.8% of wild sardine (Sardina pilchardus) muscle total fatty acids (13.4% of captive sardine muscle total fatty acids), 12.8% of wild/17.1% of captive sardine liver total fatty acids [61]; as methyl ester: 1.36–2.5% of common barbel oil, 4.15–6.47% of Pontic shad oil, 1.44–3.83% of European wels catfish oil, 0.13–0.42% of common bleak oil [52]; 2.74–6.23% for finwhale oils, 5.14–8.22% for seal oils (in weight per cent) [62]; 14.8–19.4% of Epinephelus fasciatus musccle triacylglycerols [64] |
| 26 | (12Z,15Z,18Z,21Z)- tetracosa-12,15,18,21-tetraenoic acid | - | C24:4n-3 | C24H40O2 |
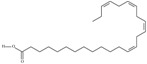
|
360.582 g/mol | 1.3% of Baltic herring (Clupea harengus) total fatty acids [118] |
| 27 | (9Z,12Z,15Z,18Z,21Z)-tetracosa-9,12,15,18,21-pentaenoic acid | - | C24:5n-3 | C24H38O2 |
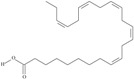
|
358.566 g/mol | 1.4% of Baltic herring total fatty acids [118] |
| 28 | (6Z,9Z,12Z,15Z,18Z,21Z)-tetracosa-6,9,12,15,18,21-hexaenoic acid | nisinic acid | C24:6n-3 | C24H36O2 |
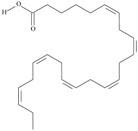
|
356.55 g/mol | 0.9% of Baltic herring total fatty acids [118] |
| 29 | (11Z,14Z,17Z,20Z,23Z)-hexacosa-11,14,17,20,23-pentaenoic acid | - | C26:5n-3 | C26H42O2 |
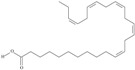
|
386.62 g/mol | 0.5% of of Baltic herring total fatty acids [118] |
| 30 | (8Z,11Z,14Z,17Z,20Z,23Z)-hexacosa-8,11,14,17,20,23-hexaenoic acid | - | C26:6n-3 | C26H40O2 |
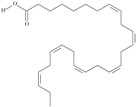
|
384.604 g/mol | 0.7% of Baltic herring total fatty acids [118] |
| 31 | (15Z,18Z,21Z,24Z,27Z)-triaconta-15,18,21,24,27-pentaenoic acid | - | C30:5n-3 | C30H50O2 |
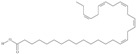
|
442.728 g/mol | as a methyl ester: 7% of Cliona celata total methyl esters [119] |
| 32 | (19Z,22Z,25Z,28Z,31Z)-tetratriaconta-19,22,25,28,31-pentaenoic acid | - | C34:5n-3 | C34H58O2 |

|
498.836 g/mol | 12.4% of the phospholipid fatty acids from Petrosia pellasarca [120] |
| 33 | (23Z,34Z)-heptatriaconta-23,34-dienoic acid | - | C37:2n-3 | C37H70O2 |

|
546.965 g/mol | in a CHCl3 extract of Iranian Rosa x damascena (Rosaceae) [121] |
| VERY LONG-CHAIN FATTY ACIDS WITH AN UNDEFINED POSITION OF DOUBLE BONDS | |||||||
| 34 | - | - | C24:4n-3 | C24H40O2 | - | 360.582 g/mol | in ram and bull spermatozoa [7] |
| 35 | - | - | C24:5n-3 | C24H38O2 | - | 358.566 g/mol | in bovine retina [8]; in ram spermatozoa [7] |
| 36 | - | - | C24:6n-3 | C24H36O2 | - | 356.55 g/mol | in bovine retina [8]; in ram spermatozoa [7] |
| 37 | - | - | C26:4n-3 | C26H44O2 | - | 388.636 g/mol | in ram spermatozoa [7] |
| 38 | - | - | C26:5n-3 | C26H42O2 | - | 386.62 g/mol | in ram spermatozoa [7] |
| 39 | - | - | C26:5n-3 | C26H42O2 | - | 386.62 g/mol | in bovine retina [8] |
| 40 | - | - | C26:6n-3 | C26H40O2 | - | 384.604 g/mol | in ram spermatozoa [7] |
| 41 | - | - | C26:6n-3 | C26H40O2 | - | 384.604 g/mol | in bovine retina [8] |
| 42 | - | - | C28:5n-3 | C28H46O2 | - | 414.674 g/mol | in bovine retina [8]; in ram and bull spermatozoa [7] |
| 43 | - | - | C28:6n-3 | C28H44O2 | - | 412.658 g/mol | in bovine retina [8]; in ram and bull spermatozoa [7] |
| 44 | - | - | C30:5n-3 | C30H50O2 | - | 442.728 g/mol | in bovine retina [8]; in ram and bull spermatozoa [7] |
| 45 | - | - | C30:6n-3 | C30H48O2 | - | 440.712 g/mol | in bovine retina [8]; in ram and bull spermatozoa [7] |
| 46 | - | - | C32:5n-3 | C32H54O2 | - | 470.782 g/mol | in bovine retina [8] |
| 47 | - | - | C32:6n-3 | C32H52O2 | - | 468.766 g/mol | in bovine retina [8]—main component of VLC-PUFA-PC of bovine retina [122]; in bull spermatozoa [7] |
| 48 | - | - | C32:7n-3 | C32H50O2 | - | 466.75 g/mol | in ram and bull spermatozoa [7] |
| 49 | - | - | C34:5n-3 | C34H58O2 | - | 498.836 g/mol | in bovine retina [8] |
| 50 | - | - | C34:6n-3 | C34H56O2 | - | 496.82 g/mol | in bovine retina [8]—main component of VLC-PUFA-PC of bovine retina [122]; in ram and bull spermatozoa [7] |
| 51 | - | - | C36:5n-3 | C36H62O2 | - | 526.89 g/mol | in bovine retina [8] |
| 52 | - | - | C36:6n-3 | C36H60O2 | - | 524.874 g/mol | in bovine retina [8] |
| 53 | - | - | C36:6n-3 | C36H60O2 | - | 524.874 g/mol | in normal human brain [122] |
| MODIFICATIONS OF OMEGA-3 FATTY ACIDS | |||||||
| 1 | (8Z)-5-oxo-11-hydroxyundec-8-enoic acid 11-O-glucoside | - | 5-oxo, 11-OH, 11-O-Glu-C11:1n-3 | C17H28O10 |
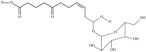
|
392.40 g/mol | in Youngia japonica [123] |
| 2 | (9Z)-12-oxododec-9-enoic acid | - | 12-oxo-C12:1n-3 | C12H20O3 |
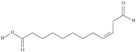
|
212.289 g/mol | present in many plants in large quantities, inter alia, in mature soybeans [124,125] |
| 3 | (9Z) -12-hydroxydodec-9-enoic acid | HDA | 12-OH-C12:1n-3 | C12H22O3 |
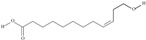
|
214.305 g/mol | widespread among plants [126,127,128] |
| 4 | (9Z,11E,15Z)-13-hydroperoxyoctadeca-9,11,15-trienoic acid | 13-LnHPO | 13-hydroperoxy-C18:3n-3 | C18H30O4 |
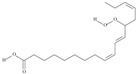
|
310.43 g/mol | in apple and tomato fruits [129] |
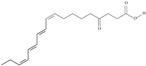
| 5 | (9Z,11E,13E,15Z)-4-oxooctadeca-9,11,13,15-tetraenoic acid | - | 4-oxo-C18:4n-3 | C18H26O3 |
|
290.403 g/mol | as a methyl ester—18% of Chrysobalanus icaco seed oil [81] |
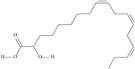
| 6 | (9Z,12Z,15Z)-2-hydroxyoctadeca-9,12,15-trienoic acid | 2-Hydroxy-linolenic acid/α-hydroxylinolenic acid | 2-OH-C18:3n-3 | C18H30O3 |
|
294.435 g/mol | 5.4% of Salvia nilotica seed oil [55] |
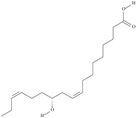
| 7 | (9Z,12R,15Z)-12-hydroxyoctadeca-9,15-dienoic acid | densipolic acid | 12-OH-C18:2n-3 | C18H32O3 |
|
296.451 g/mol | 2.1% of the seed oil of Lesquerella auriculata (this fatty acid is a major component of Lesquerella species seed oils from southeastern regions of the U.S.) [130]; the largest average content in the seeds of the eastern U.S. species: L. perforata, L. stonensis, L. densipila, L. lyrata, and L. lescuria—over 40% of total fatty acids [131]; in cv Linola 989 Linum usitatissimum (low linolenic flax) seeds at levels of 0.2 to 1% [132] |
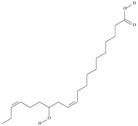
| 8 | (11Z,17Z)-14-hydroxyicosa-11,17-dienoic acid | auricolic acid | 14-OH-C20:2n-3 | C20H36O3 |
|
324.505 g/mol | 32% of the seed oil of Lesquerella auriculata [130]; the largest average content in the seeds of L. auriculata and L. densiflora—over 30% of total fatty acids [131] |
| 9 | (2E,6E,10Z)-7-ethyl-3,ll-dimethyl-2,6,10-tridecatrienoic acid (also as a methyl ester) | - | 3,11-diMe,7-Et-C13:3n-3 | C17H28O2 |
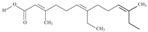
|
264.41 g/mol | converted to the juvenile hormon in the moth Hyalophora cecropia males [133] |
2. Sources of Omega-3 Fatty Acids
In the years 2003–2008 people in the US consumed, with food, on average 0.17 g/day (median intake 0.11 g/day) of long-chain omega-3 fatty acids (DHA, EPA and EPA equivalents (5% from conversion of ALA, 33% from conversion of SDA)), i.e., lower than the recommended 0.5 g/day. Among people consuming omega-3 fatty acids in the group below or at the 15th percentile of omega−3 FAs total consumption, the most important source of omega-3 acids were cereal products (36%), while in the group above or at the 85th percentile, the dominant source was fish (fish and mixtures; 71%—in contrast to the low-intake group, where the fish consumption was 1%). In both groups, the intake of seeds and nuts was low, and vegetables were consumed at 9% and 2%, respectively [134]. As can be easily seen, the share of omega-3 fatty acids in the diet is most affected by the supply of fish, because they are the primary source of EPA and DHA for humans [135]. This is due to the fact that the food of many fish are algae rich in EPA and DHA, and other organisms consuming algae, like fish or marine invertebrates [136,137]. Microalgae play a key role in the primary production of PUFAs and are their main source in seawater. Marine invertebrates are also an important and basic source of omega-3 PUFAs due to their ability to synthesize some of them de novo, for example, oyster Crassostrea gigas can produce EPA and DHA by consuming microalgae that do not contain both [137]. Seafood contains many valuable omega-3 acids. However, their frequent consumption exposes the human body to the neurotoxic effect of methyl mercury, which is especially harmful for the development of the central nervous system of the fetus [138]. Therefore, it is advisable to look for other sources of these fatty acids, and to include them in a balanced diet.
EPA occurs in large amounts in herring (15% of total lipids) [59], wild sardine (13.6% of muscle total FAs) [61] and pollock roe (18.8%) [59]. In plants, it is rather rare. Mention should be made of Undaria pinnatifida (13% of essential oil composition) [107] and Rhododendron sochadzeae (2% of leaf FA hexan extract) [108].
The main sources of DHA are flyingfish, herring, pollock and salmon roe (27.9%, 22.6%, 22.2%, and 17.4% of total lipids, respectively) [59], Cirrhinus mrigata (18.07 g/100 g muscle tissue FAs), and Catla catla (17.98 g/100 g muscle tissue FAs) [58]. The presence of this acid in the jackalberry (4.65 g FA per 100 g oil Diospyros mespiliformis) is noteworthy [139].
As mentioned above, both EPA and DHA are also synthesized from ALA using enzymes—desaturases and elongases [17,22,26,134], although in humans this process is insufficient—the conversion rate is 10–14% for men and women, respectively [136,140]. According to other authors, ALA is converted to EPA with a yield of 0.2 to 21% (in adult men 8%), and to DHA of zero to 9% (in women more than 9%) [141]. DHA can also be a substrate for the creation of EPA and vice versa (the conversion efficiency of EPA to DHA is < 0.1% in adult men) [141,142]. Moreover, ALA is a precursor of DPA as well [143].
α-Linolenic acid (ALA) is common in the higher plants and algae [22]. Rich sources of ALA also include Linum usitatissimum (depending on the genotype it contains from 1.1 to 65.2% of total fatty acids in seeds) [71], chia Salvia hispanica (64.04% of seed oil fatty acids and 16.4 g/100 g of milled chia seeds) [65,68], Trichosanthes kirilowii (33.77–38.66% of seed oils) [70], paprika Capsicum annuum (29.93% of fresh pericarp fatty acids in the Jaranda variety and 30.27% in the Jariza variety) [72], and many others. The content of ALA in fish is small, for example 1.1% in wild sardine (Sardina pilchardus) muscle total FAs [61].
8(Z),11(Z),14(Z)-Heptadecatrienoic acid, also called norlinolenic acid, was identified in Salvia nilotica [0.4% of mixed esters and 2.3% of IV fraction (% by gas liquid chromatography)] [55].
(all trans)-9,12,15-Octadecatrienoic acid (linolenelaidic acid) is present in Turkish sage species, and the weight per cent of seed FAs of Salvia virgata, Salvia potentillifolia, Salvia recognita, Salvia tomentosa amounts to 0.4, 0.7, 1.1, and 1.4%, respectively [73]. Nicotiana tabacum also contains linolenelaidic acid but in a minor amount, which is 0.03% of tobacco seed oil FAs [74].
(9Z,12Z,15Z)-2-Hydroxyoctadeca-9,12,15-trienoic acid (2-hydroxylinolenic acid, alpha-hydroxylinolenic acid) is a little known hydroxy acid found in Salvia nilotica (5.4% of Salvia nilotica seed oil) [55].
(11Z,14Z,17Z)-Icosa-11,14,17-trienoic acid (eicosatrienoic acid), sometimes named homo-alpha-linolenic acid, rarely occurs in animal sources—hen egg yolk contains this acid in the amount of only 0.15 – 0.16% of total lipids [101]. Much more was found in Torreya grandis kernel oil (6.78–8.73% of total FAs) [96] and cork Phellodendron lavalei seeds [15.5% of free fatty acids (FFAs)] [98], but the largest amount is contained in Pittosporum undulatum seed oil (31.44% of total FAs) [97].
Close derivatives of alpha-linolenic acid are two acids included in the minor polyenoic acids group [75]. Rumelenic acid (cis-9, trans-11, cis-15-octadecatrienoic acid) is formed by the isomerization of alpha-linolenic acid, while cis-9, trans-11, trans-15-octadecatrienoic acid is the isomer of rumelenic acid. Both acids are found in ruminants, for example in sheep’s milk [69,75].
Docosapentaenoic acid (DPA; (7Z,10Z,13Z,16Z,19Z)-docosa-7,10,13,16,19-pentaenoic acid), tradinionally called clupanodonic acid, shows structural similarity to EPA but has two more carbon atoms in the chain. Significant amounts are present in meat (13.8/16.7% of wether/ewe (lamb) raw meat FAs) [116], fish roe (5.5% of salmon roe total lipids [59], fish oils (1.04–2.82% of European wels catfish oil) [52], seal oil (2.64–4.74%) [62], and fish muscle (1.6% of wild sardine (Sardina pilchardus) muscle total fatty acids) [61]. DPA sporadically occurs in algae such as Hormosira banksii and Dictyota dichomota (0.07% and 1.6% of total FAs, respectively) [117].
Stearidonic acid (SDA; moroctic acid) is common in plants and fishes. Salmon roe contains a small SDA portion (0.8% of total lipids) [59]; a little more in wild sardine (2.3% of muscle total FAs) [61]. SDA occurs in many plants but in small amounts, except for plants from the families Primulaceae and Boraginaceae, where it can be found in relative big concentrations (mean amounts of 4.73 and 4.99% of seeds total FA methyl esters, respectively). Hippophae rhamnoides (Elaeagnaceae) seeds contain only 0.36% SDA [78]. Particularly noteworthy is Echium humile ssp. pycnanthum, in which seeds had SDA content estimated at up to 16.2% of total FAs [79].
(7Z,10Z,13Z)-Hexadeca-7,10,13-trienoic acid (roughanic acid) occurs in many plants and in fish oils, but usually in small amounts. As a result of reactions catalyzed by lipoxygenases, this is converted to oxygenated metabolites such as 7-, 8-, 10-, 11-, 13-, 14-hydroperoxides, and bis-allylic 9-hydroperoxide. However, hydroxy derivatives of roughanic acid are also present in plants rich in alpha-linolenic acid and not roughanic acid, including soybean and pea seedlings [144].
Omega-3 monounsaturated fatty acids are a small group, although with an undeniable role in nature—some such as (Z)-11-tetradecenoic acid, (E)-11-tetradecenoic acid and (10E,13Z)-10,13-heksadecadienoic acid are pheromone precursors in insects [38]. As contained in generally available food sources, 13Z-hexadecenoic acid, 15Z-octadecenoic acid, 9E-dodecenoic acid and 11E-tetradecenoic acid are especially worth noting. 15Z-octadecenoic acid, found in beef [46], pork [45], cows milk and butter [47], and mature human milk, is relatively common [49,50]. 11E-tetradecenoic acid occurs in Coriandrum sativum, whose leaf essential oil contains 13.37% of this acid [39].
Conjugated fatty acids still require detailed research, as some of them (conjugated linoleic acid) have antitumor activity and a number of properties that give benefits in the fight against hypertension, obesity and diabetes [1,145]. Conjugated omega-3 fatty acids, important from the point of view of their availability in food, are alpha- and beta-parinaric acid. Trans-parinaric acid, also called beta-parinaric acid [88], has all the bonds in the trans configuration. In the case of cis-parinaric acid (alpha-parinaric acid [146]), however, the name is somewhat misleading, because its isomerism can be described as cis-trans-trans-cis [147]. Parinaric acid, due to its natural ability of fluorescence and high susceptibility to free radicals, is used (as cis-parinaric acid) as an indicator of lipid peroxidation in cell membranes [148,149,150,151,152,153,154] and (as trans-parinaric acid) to assess the effect of temperature change on cell membranes [88,155]. Some organisms are able to convert nonconjugated fatty acids into conjugated counterparts [156]. In addition to tetraenoic parinaric acid, in plants mainly conjugated trienoic acids are found with 18 and 17 carbon atoms. Acetylenic and dienoic ones may have a different number of methylene bridges [157]. Parinarium laurinum seeds are the richest in alpha-parinaric acid sources (62% of seed oil methyl esters) [81].
Very long-chain fatty acids (VLCFAs; C > 22) play many important roles in the body, including modulating the functions of neutrophils. While their activity decreases with increasing carbon chain length, they remain efficient superoxide production activators in neutrophils, much better than N-formylmethionyl-leucyl-phenylalanine (FMLP) [15]. VLCFAs also have the ability to regulate PKC activity [16]. However, this still requires further research, especially in vivo. Their predisposed location in the body is also interesting—they are found in large amounts in the human brain [7,122,158,159], bovine retina [8,122], ram, bull, boar, and human spermatozoa [7,14] as well as in rat testicles [160], cultures of mouse spermatides and spermatocytes [161], and human vascular endothelial cells culture [162]. The VLC n-3 FAs acids have been found in dipolyunsaturated phosphatydylcholines of bovine retina and sphingomyelin of ram spermatozoa, as well as in bull spermatozoa [7,8,14]. In the normal human brain, the presence of VLC n-3 hexaenoic acids (36:6) was also found, but in smaller amounts than omega-6 fatty acids [122]. Tetratriaconta-19,22,25,28,31-pentaenoic acid (34:5) has been described in Marine Sponge (Petrosia pellasarca) [120].
There are numerous modifications of omega-3 fatty acids, and many of them play the role of hormones or compounds produced in stressful situations in plants. We only mention a few examples that occur in dietary sources. The most important seem to be the previously mentioned 2-hydroxylinolenic acid and two hydroxy acids occurring in plants of the genus Lesquerella—densipolic and auricolic acid [130,163,164,165,166]. One of them, densipolic acid, was found in Linum usitatissimum [132]. Another example is (9Z)-12-hydroxy-9-dodecenoic acid (HDA), which is one of the main products of the lipoxygenase pathway, widespread among plants [126,127,128]. Additionally, oxo fatty acids are prevalent in the plant world. For instance, 12-oxo-cis-9-dodecenoic acid is one of the reaction products catalyzed by the fatty acid hydroperoxide lyase, present in many plants, in large quantities, inter alia, in mature soybeans [124,125]. Table 1 lists the acids belonging to all of the groups discussed above.
3. Bioavailability
Bioavailability is a relative term, which can refer to both the speed of absorption and the quantity of the substance absorbed. The speed can be understood as the rate at which the substance is absorbed from the gastrointestinal tract and reaches the portal system. Absorption of the substance occurs in the gastrointestinal tract only to a certain extent, depending on many factors. The extent of absorption and the speed of substance transport to the portal circulation describe the bioavailability in the narrower sense. Traditionally, bioavailability can also be considered in a broader context, taking into account the amount of substance that reaches the systemic circulation or the place of physiological destiny (activity) [167]. This broader approach is particularly important when considering the effect of metabolic processes and excretion on the transport of substances from the portal circulation. Not all of the absorbed substance reaches the systemic circulation or tissue compartment consistent with the physiological destination. This difference in amount is very important from the point of view of pharmacokinetics and dietary planning.
Fatty acids may be present in the body as free fatty acids, bound to glycerol, to form triacylglycerol (TG), diacylglycerol (DAG) or monoacylglycerol (MAG), or to form a composition of membrane phospholipids. In naturally occurring TG molecules, LC PUFA occupies the second position [167]. In the phospholipids of cell membranes the latter position is competed by EPA and DHA with arachidonic acid, and if necessary, they are released by the enzyme phospholipase A2 and are used to synthesise eicosanoids [136]. Otherwise, in the human brain, VLCFAs (C34–38) are attached to the skeleton of glycerol (glycerol moiety), which in phospholipids are located in the sn-1 position [122].
In fish and fish oils, LC omega-3 PUFAs are mainly found as triacylglycerides and free fatty acids [167,168]. In Krill oil phospholipids are also an important fraction of these fatty acids (30–65% of EPA and DHA), mainly phosphatidylcholine [167,169,170,171]. EPA and DHA represent approximately 18% and 12%, respectively, of the content of naturally occurring fish oils [167]. However, due to the transesterification process, oil blends often contain much more of both EPA and DHA. This process is related to the substitution of the removed glycerol backbone with ethanol, resulting in the formation of ethylesters (EE), which can then be converted to re-esterified TG (rTG)-ethanol is enzymatically removed, resulting in free fatty acids being released, then attached by enzymes back to the glycerol backbone [167,168]. An example of a drug in which the content of EPA (DHA) is increased as a result of transesterification is Lovaza [168,172]. Another method to increase the content of EPA and DHA has been used in Epanova. Glycerol is removed and replaced with a hydrogen atom, which, in combination with the released fatty acid, forms a carboxylic acid. Ethylesters (of which Lovaza is composed) require the hydrolysis of the ester bond by pancreatic lipase before they release the free fatty acids that can be absorbed in the small intestine. This step is not required by the carboxylic acids. Interestingly, EPA and DHA-EE are also absorbed unchanged. However, this form accounts for < 1% of the total pool of EPA and DHA in circulation after ingestion of omega-3 acids EE [168].
In rTG, LC PUFAs take up not mainly the sn-2 position (which takes place in natural TG), but can also (simultaneously) be bound in the position of sn-1 or sn-3 with equal paradigmicity. rTG particles frequently contain two LC PUFAs—then the probability of binding EPA and DHA in the sn-1 or sn-3 position is higher than in the sn-2 position [167]. According to Schuchardt, [167] binding of LC omega-3 PUFA to glycerol in the sn-1/3 position facilitates the lipase hydrolysis of the bond, thus increasing bioavailability. According to Dyerberg, [173] the presence of MAG and DAG in rTG mixtures increases the absorption of LC PUFAs in the intestine due to the easier formation of micelles. Bandarra [174], in turn, based on the results of his research on hamsters, proves that the location of DHA in the sn-2 position increases the absorption of this acid in the intestine and its incorporation into tissues. However, a certain limitation of Bandarra’s study is that the author used a commercially available fish oil, which is known only to be rich in DHA with an unspecified binding site with the glycerol backbone—we do not know what part of DHA is associated in positions other than sn-2.
3.1. Methods of Measuring the Bioavailability of Omega-3 Fatty Acids
We can measure omega-3 FAs concentration in plasma, serum, blood cells and lymph. The content of FAs in the plasma reflects the short to medium-long supply of fatty acids in the diet, while the concentration of fatty acids in the blood cells is usually a good indicator of long-term bioavailability [167,175]. As far as the long-chain omega-3 fatty acids are concerned, it is possible to measure many markers that indicate the presence of DHA in a specific form, but only one (the level of phospholipid EPA in plasma) that is useful for determining the level of EPA [167,176]. Admittedly, erythrocyte EPA is a weak dose-dependent indicator of LC omega-3 PUFAs substitution at normal dietary levels, however (sum of), erythrocyte EPA and DHA concentration seems to be, as will be mentioned below, a relatively good indicator of long-term bioavailability and also reflects the content of LC omega-3 PUFAs in non-blood tissues [167,177]. In Browning’s study, [177] EPA + DHA-PC (in the case of sudden changes in intake) and platelet/mononuclear cells EPA + DHA (in the case of long-term consumption assessment) were considered biomarkers that best represent the intake of fish with high fat content in a typical UK population (1–4 servings a week).
3.2. Chemical Binding
The bioavailability of omega-3 fatty acids can also be expressed by calculating different coefficients, the most important of which seem to be omega-3 index, Cmax and AUCt (area under the (concentration-time) curve), the modification of which is the incremental area under the curve (iAUCt) [169].
The omega-3 index is defined as the proportion of the sum of EPA and DHA content in the total fatty acid content in the erythrocyte (erythrocyte membrane), and is expressed as a percentage [167,178,179]. It is a good indicator of long-term bioavailability (from the last 80–120 days [179]) due to the long lifetime of erythrocytes and their high number in the blood. Plasma content of EPA and DHA, as well as many other fatty acids, weakly correlates or does not correlate at all with the levels of these acids in the erythrocyte membrane [179]. The omega-3 index is also a good indicator of the incorporation of fatty acids into tissues, and this applies not only to gastrointestinal tissues, but also to the myocardium, liver, and kidney [167,180]. It was established that the supply of long-chain omega-3 fatty acids at the level of 1 g/d may result in an increase in the omega-3 index by two percentage points over eight weeks [181]. The omega-3 index is influenced by many factors, such as smoking, physical activity (the intensity of which decreases the omega-3 index), and genes [178].
The maximum levels of EPA and DHA in plasma (Cmax) can be determined five–nine h after administration, while the persistent levels of EPA and DHA in plasma are achieved within two weeks of daily supplementation. The half-life of EPA and DHA after repeated administration is 37 h and 48 h, respectively [170].
The bioavailability of omega-3 fatty acids varies depending on the type of chemical binding (lipid structure), and can be ranked as follows: PL > rTG > TG > FFA > EE [167,178,179,182,183]. Both EE and rTG are not natural components of dietary oils, but they are created in the process of their chemical modification, which is called transesterification. As a result, highly concentrated oils containing up to 90% of EPA and DHA can be obtained [167]. The hierarchical order presented above does not, however, reflect reality to the extent that some authors would like it to. In their trial Kohler et al. [178] proved that the bioavailability of EPA and DHA in the form of phospholipids does not have to be greater than the bioavailability of these acids in the form of triglycerides—the bioavailability of EPA and DHA in krill meal was comparable to their bioavailability in fish oil, despite a slightly higher total fat content in krill meal. Yurko-Mauro et al. [184] achieved similar results—krill oil containing almost 44% of phospholipids showed bioavailability similar to the bioavailability of fish oil, both in the form of triglycerides and ethyl esters. This indicates the (great) share of non-chemical binding (and total fat content) factors in shaping the bioavailability of omega-3 fatty acids [169,178,185]. One explanation may be the observation of Nordoy, Reference [186], who noticed similarly good absorption of omega-3 fatty acids (including EPA and DHA) in the form of TG and EE if they are administered as a component of fish oil in equivalent amounts. Both krill oil, and to a lesser extent, krill meal and fish oil, which are rich in fat.
Many studies, however, maintain that krill oil, especially when very rich in phospholipids, is characterized by extremely high bioavailability, significantly higher than triglyceride-rich fish oil [170,187,188,189]. Those also find an understandable explanation. Between the omega-3 PUFAs bound to phospholipids and those linked to triglycerides, there is some difference associated with predestination to a specific type of blood transport and metabolism in the liver. DHA-TG is preferentially assigned to LDL-PL, while DHA-PL is preferentially assigned to HDL-PL. It was also found that omega-3 PUFAs, including DHA, in the form associated with phospholipids, are more intensely embedded in tissues. This is probably due to the better availability of LC n-3 PUFA-PL acids contained in krill oil than those present in fish oil LC n-3 PUFA-TG for liver beta-oxidation pathways [189]. Krill oil inhibits de novo lipogenesis, but enhances fatty oxidation [170].
3.3. Brain Transport
Omega-3 acids are incorporated into the cell membrane of many organs and tissues, above all the heart, nervous tissue and retina [167]. Oral supplementation with omega-3 PUFAs increases the content of these acids in the cerebrospinal fluid [190]. Efficient passage of the blood-brain barrier, however, requires carrier particles—in the case of DHA, it is 1-lyso, 2-docosahexaenoyl-glycerophosphocholine (LysoPC-DHA), which increases intracerebral DHA transport up to 10-fold. It is a brain-specific particle and does not facilitate the transport of DHA to the heart, liver or kidney, although detailed studies are required in humans. Carriers (transporting DHA to the brain) with potentially better properties are synthesized, an example of which is obtaining of AceDoPC (1-acetyl,2-docosahexaenoyl-glycerophosphocholine) [191].
3.4. Parenteral Administration
Most of the studies, especially those based on humans, which serve to determine the bioavailability of omega-3 acids, apply to their oral administration. It is difficult to fully validate the parenteral administration of omega-3 fatty acids in relation to the healthy population, because this method of supply is reserved mainly for patients undergoing intensive therapy, both adults and preterm infants [142,192,193]. In addition, it is worth noting that parenteral administration of mixtures based on fish oil may lead to biochemical liver damage and even the progression of fibrosis in this organ [194].
Al-Taan et al. [195] conducted a study on 20 patients awaiting the surgical removal of colorectal metastases from liver cancer. These individuals had normal liver function tests and plasma lipid levels within the reference range. The aim of the study was to assess the content of fatty acids in plasma phosphatidylcholine and erythrocytes during and after intravenous infusion of oil emulsion. Phosphatidylcholine (PC) is the main phospholipid that can be found in the circulation (blood) and erythrocyte (membrane) during and shortly after intravenous infusion of the oil emulsion. Parenteral administration of DHA and EPA lipid emulsion allowed a rapid and significant increase in their blood levels (EPA/DHA in plasma PC and EPA in erythrocytes). However, EPA levels returned to their initial values five–12 days after the end of the infusion. Not only Al-Taan, but also Browning [177], suggested a quicker turnover of EPA than DHA in cells, and thus this effect occurs with both oral and intravenous supply, with a different time of administration. The fact of a relatively short infusion of oil emulsion is also significant.
3.5. Matrix Effect and Emulsification
Many authors emphasize the key role of the fat content in food in the absorption of omega-3 acids (‘the matrix effect’ [167,179]) and suggest the necessity of introducing a recommendation to consume formulations containing omega-3 fatty acids with high-fat food [167,178,196]. Schuchardt underscores the lack of the expected cardioprotective effect in the German population supplementing omega-3 PUFAs during breakfast due to the relatively low-fat typical German breakfast [167]. Similarly, American society is in the habit of eating a low-fat breakfast, which in their case contains only 16% of the fat consumed during the day [197]. In addition, the fat content of food is sometimes inversely proportional to the amount of omega-3 fatty acids it contains. For example, high-fat plants may have few omega-3 fatty acids—Entandrophragma angolense—a potential health benefit food source has fat estimated as 59.43% of fresh weight. However, 33.29 weight % of Entandrophragma seed oil contains oleic acid and only 0.2 weight % is alpha-linolenic acid [139,198]. Chia, on the other hand, contains oil in the amount of 27 g per 100 g of seeds, of which 64.04% is ALA and only 14.98% are saturated fatty acids [68]. Soybean contains slightly less fat. However, the high content of linoleic acid and the unfavourable omega-6/omega-3 ratio make it a food of dubious health value, especially because it has been shown that induction of obesity in mice with soybean oil is possible [95,139,199].
The fat contained in the diet stimulates the pancreas to secrete fat-digesting enzymes and the gallbladder to eject bile that contains bile salts, which emulsify fats and activate pancreatic lipase [196]. However, it is recommended that persons with a high cardiovascular risk should reduce the supply of animal fat. On the other hand, substituting animal fat with vegetable fat is not necessarily a good solution, taking into account eating habits, ubiquitous overweight and often low content of omega-3 fatty acids in rich-fat plants. These people benefit from the achievements of nanotechnology, among which Cavazos-Garduno [200] mentions nanoparticles, nanospheres, nanocapsules, solid lipid nanoparticles (SLN), self-emulsifying drug delivery systems (SEDDS) and nanoemulsions, which significantly improve the absorption of fatty acids in a low-fat environment. Research indicates that particles smaller than 0.2 micrometers are absorbed better.
The self-microemulsifying delivery system (SMEDS) contains LC n-3 FA-EE and a number of compounds that are emulsified in the stomach without the need for a high-fat meal [196]. It has been known for many years that emulsified omega-3 acids are characterized by equally good bioavailability in poor and high fat environments [196,201]. Qin analysis [196] indicates that the absorption of DHA + EPA EE contained in SMEDS preparations is 6 times higher than DHA + EPA EE alone. To confirm the effectiveness of SMEDS technology, long-term studies are needed to analyze the embedding process of the above-mentioned fatty acids in the structure of cell membranes, especially erythrocytes.
The benefits of emulsification were also described by Puri, [138]. He used similar self-nanoemulsifying drug delivery systems (SNEDDS).
Absorption of omega-3 fatty acid ethylesters can also be enhanced through the use of Advanced Lipid Technologies (ALT). ALT is, according to the manufacturer, a special lipophilic system that, regardless of the supply of food and the fat content thereof, increases the bioavailability of lipid-based compounds, including omega-3 fatty acid ethyl esters, generating the spontaneous formation of micelles. An example of a preparation equipped with this component is SC401 (DHA and EPA ethylesters + Advanced Lipid Technologies). In the supply of low-fat food, the intake of SC401 was associated with significantly higher values demonstrating the bioavailability of DHA and EPA than Lovaza (nearly 2-fold higher Cmax and 3-fold higher AUC(0-last)).
Free fatty acids are another example of a formula well absorbed with a low fat content in the diet. Epanova bioavailability in conditions of low fat supply is much greater than with Lovaza (AUC(0−t) for Epanova is four times higher than for Lovaza), which in turn, consumed with high-fat food, has similar bioavailability as SC401 accepted in conditions of low food supply [201].
Vegetarians and vegans will also be able to use the emulsification technique in line with their own lifestyle, as the results of the latest research indicate the possibility of using vegetable proteins from pea, lentil, and faba bean as emulsifiers [202]. Moreover, oil-in-water nanoemulsions are constructed using vegetarian oils (algae and flaxseed oil) [202,203].
A very common method of the incorporation of nanoemulsion into food is its transformation, most often into the form of powder, using microencapsulation [204]. Microencapsulation stabilizes the oil mixture, protects it against oxidation, and eliminates the phase difference often present when mixed with food [181]. Microencapsulation of fish oil increases the bioavailability of omega-3 PUFAs to a value very close to the bioavailability of these acids in meals rich in fish oil in liquid form [205]. Emulsification increases the bioavailability of LC omega-3 PUFAs by increasing the efficiency of incorporation into triacylgliceryde-rich lipoproteins [206]. Emulsifiers also modify the expression of genes responsible for the transport of fatty acids in enterocytes [143]. Emulsification (pre-emulsification) increases the absorption of LC PUFAs, including DHA, EPA, and ALA, but does not have the effect on the absorption of fatty acids having shorter chain length and fewer unsaturated bonds [143,207,208].
Of course, emulsification elevates not only the bioavailability of omega-3 acids in the blood, but also in the lymph. Emulsifiers used in food are part of this mechanism. For example, soya lecithin added to flaxseed oil increases the number and size of ALA-rich chylomicrons produced by enterocytes. However, another emulsifier, sodium caseinate, significantly reduces the absorption of ALA in the intestine. This is probably related to the effect on the expression of the FABP2 gene, which is involved in the transport of fatty acids in enterocytes. It was noted that the presence of soy lecithin in the emulsion was accompanied by high expression of this gene, whereas the use of sodium caseinate was associated with a decrease in FABP2 gene expression [143]. This process requires careful research on humans, because it is likely that the effect of emulsifiers on gene expression should be considered during food production, especially when we expect a specific healing effect.
4. Interactions between Long-Chain Polyunsaturated Fatty Acids
Linoleic acid (LA), which is omega-6 fatty acid, competes with ALA for enzymes converting alpha-linolenic acid to EPA, DPA, and DHA. LA is (similarly to arachidonic acid), inter alia, the precursor of eicozanoids, while DHA, DPA, and EPA can be transformed into anti-inflammatory agents. [25,26,209]. Changes in the supply of omega-6 PUFAs affect the plasma concentration of omega-3 fatty acids. This applies to both non-esterified omega-3 fatty acids and those associated with phospholipids, triglycerides, and cholesteryl esters. Taha et al. [209] showed that reducing the supply of omega-6 PUFAs for 12 weeks increases the concentration of omega-3 PUFAs in the plasma. In addition, the simultaneous increase in the supply of omega-3 PUFAs for a dozen weeks further elevates the concentration of these acids in the plasma and lowers the level of arachidonic acid. An important interaction of omega-6 PUFAs with omega-3 PUFAs is the production of non-native thromboxanes and leukotrienes [170]. This probably results, among others, from the substrate competition for the catalytic center of desaturases and elongases, and the reduction of arachidonic acid production with a sufficiently high supply of n-3 PUFAs.
Based on Wood’s critical analysis, [185], it can be concluded that an increase in EPA level and also in a certain degree of DHA can probably be achieved by reducing the supply of linoleic acid and/or increasing the intake of alpha-linolenic acid, although the extent of the change is small. It is suggested to maintain the ratio of omega-6/omega-3 consumption in the range from 1/5 to 1/10, and ideally, as in Japanese society, from 1/2 to 1/4 [141,210]. In turn, for breastfeeding mothers, this ratio should not exceed 10 [211]. Meanwhile, the diets of Western societies are characterized by a clear advantage of the supply of omega-6 fatty acids and the ratio of n-6/n-3 shaping in the range of 15/1 to 16.7/1 [212]. The discussion about the preferred ratio of consumption of omega-6 to omega-3 fatty acids has been going on for many years and still has not produced convincing results. Researchers are wondering about the legitimacy of returning to an “ancestral diet”, which is attributed to the balanced consumption of both groups of fatty acids at the level of 1:1 [213,214,215,216]. Attention is also paid to certain health benefits that may result from a strictly defined omega-6 to omega-3 ratio, as exemplified by the postulated beneficial effect of the 4:1 ratio on the functioning of the nervous system or reduction of tumor cell proliferation in patients with colorectal cancer who they were nourishing themselves, keeping the ratio 2.5:1 in the diet [212,217,218,219]. The above authors indicate the desirability of consuming up to four times more omega-6 fatty acids than omega-3 and this value depends on the disease under consideration. Regardless of the symptoms or lack thereof, most researchers postulated not exceeded intakes omega6/omega-3 at 10 [211,213]. In light of eating habits of the societies of many highly developed countries, a certain compromise seems to be current recommendations for the consumption of omega-6 and omega-3 fatty acids, which shape the omega-6/omega-3 index in the range from 7:1 to 11:1 [220].
Kohler et al. [178] studied the bioavailability of DHA and EPA added in the form of ethyl esters to sausages with similar ALA content. Both the Omega-3 index and the percentage of EPA and DHA in erythrocytes increased significantly more in the verum group than in the control (placebo) group, in which they remained almost unchanged. The content of DPA similarly increased in both groups. The absence of a significant increase in EPA and DHA in the control group, despite ALA supplementation in both groups, is not surprising, as ALA is converted to EPA and DHA to a very small extent [136,140,141]. The difference in the increase in EPA, DHA and DPA levels in the control group may be due to the different conversion efficiency of ALA to the above-mentioned acids, although this is not the only reason, because these acids may also be converted into each other. Interestingly, the ALA level increased slightly in the control group (p = 0.038), while in the treatment group it decreased slightly (p = 0.141). Despite the small difference in these ALA levels and the lack of statistical significance of ALA decrease in the treatment group, the explanation of large differences in ALA levels after supplementation between individual participants requires careful research on the metabolic interactions of individual omega-3 fatty acids.
5. Lipid Oxidation
LC omega-3 PUFAs are sensitive to oxidation [206,221]. Some studies report increased levels of peroxidized lipids in fish oil capsules, and as their regular consumption may adversely affect health, this problem needs to be considered [206]. One of the ways of protecting omega-3 acids against oxidation is the microencapsulation of oils using WPI-GA complex coacervates and spray dried microcapsules [221]. One of the less expensive alternative methods is ionic gelation using Salvia hispanica mucus in combination with sodium alginate and calcium [222]. The effect of oxidation on the bioavailability of omega-3 fatty acids is not clear. According to Staprans and Naruszewicz [223,224], the consumption of peroxidized vegetable oils increases the number of peroxidized lipids in chylomicrons. Ottestad [206], on the other hand, reports that consumption of oxidised cod liver oil has no effect on the incorporation of omega-3 PUFAs into lipoproteins rich in triacylglycerides.
6. Conclusions
Over recent years, there has been significant progress in research on the bioavailability of omega-3 fatty acids. We know more and more of their sources and we understand better the importance of their chemical structure and the form of supplementation. People consume omega-3 fatty acids mainly with fish, and to a lesser extent with cereal products and meat. Some of them, however, do not appear in the abovementioned products, or their presence is unexplored. In addition, the content of individual acids in plants depends on the place of their occurrence, and in animals on the type of diet. Despite the high availability of seafood, which is rich in many omega-3 acids, the authors recognize the need to constantly search for new sources of omega-3 fatty acids due to the risk of excessive exposure to mercury methyl and other toxic compounds present in fishing areas. In addition, some acids, such as coniferonic or juniperonic acid, occur mainly in plants that are not widely used in consumption, or are even poisonous, but can be used in the pharmaceutical industry and medicinal chemistry. This is important because of the many reasons for finding omega-3 fatty acids and their derivatives, valuable active substances that may be important in the development of new drugs and therapeutic regimens. Opinions about the bioavailability of omega-3 fatty acids are divided. Some believe that phospholipid-bound acids are absorbed better, as well as more intensely incorporated into tissues than those associated in triglyceride form, due to the specificity of blood transport and better accessibility of beta-oxidation pathways. Others, however, are of the opinion that factors unrelated to the type of chemical binding play a key role, and the fat content in food is decisive. In addition, promising effects seem to result from the use of special lipophilic systems and nano(micro)emulsions. However, the type of emulsifier used should be taken into account, as some influence the expression of the gene involved in the transport of fatty acids in enterocytes in various ways. Fatty acids affect each other’s metabolism in the body, which is of dietary importance. Several times higher consumption of omega-6 than omega-3 fatty acids is recommended by most researchers. Omega-3 fatty acids, like all fatty acids, are highly susceptible to oxidation. There are methods that disrupt this process and may be used in the production of supplements, but the significance of the process itself for bioavailability is unclear.
It is hoped that reading the above review encourages scientists to carry out further research, which will dispel these and many other doubts.
Abbreviations
The following abbreviations are used in this manuscript:
| 13-LnHPO | (9Z,11E,15Z)-13-Hydroperoxyoctadeca-9,11,15-trienoic acid |
| ALA | alpha-Linoleic acid |
| ALT | Advanced Lipid Technologies |
| ARA | Arachidonic acid |
| AUCt | Area under the (concentration-time) curve |
| CFA | Conjugated fatty acid |
| Cmax | Maximum concentration |
| DAG | Diacylglycerol |
| DHA | (4Z,7Z,10Z,13Z,16Z,19Z)-Docosa-4,7,10,13,16,19-hexaenoic acid (cervonic acid) |
| DPA | (7Z,10Z,13Z,16Z,19Z)-Docosa-7,10,13,16,19-pentaenoic acid (clupanodonic acid) |
| DTrE | (13Z,16Z,19Z)-Docosa-13,16,19-trienoic acid |
| EE | Ethyl esters |
| EFA | Essential fatty acid |
| EPA | (5Z,8Z,11Z,14Z,17Z)-Icosa-5,8,11,14,17-pentaenoic acid (timnodonic acid) |
| ETA | (8Z,11Z,14Z,17Z)-Icosa-8,11,14,17-tetraenoic acid |
| ETE | (11Z,14Z,17Z)-Icosa-11,14,17-trienoic acid (homo-alpha-linolenic acid) |
| FA | Fatty acid |
| FFA | Free fatty acid |
| FMLP | N-Formylmethionyl-leucyl-phenylalanine |
| HDA | (9Z)-12-Hydroxydodec-9-enoic acid |
| HDL | High-density lipoprotein |
| HFA | Hydroxy fatty acid |
| HPA | (6Z,9Z,12Z,15Z,18Z)-Henicosa-6,9,12,15,18-pentaenoic acid |
| iAUCt | Incremental area under the (concentration-time) curve |
| LA | Linoleic acid |
| LC | Long-chain |
| LDL | Low-density lipoprotein |
| MAG | Monoacylglycerol |
| MCFA | Medium-chain fatty acid |
| MUFA | Monounsaturated fatty acid |
| PA | Phosphatidic acid |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| PG | Phosphatidylglycerol |
| PKC | Protein kinase C |
| PL | Phospholipids |
| PUFA | Polyunsaturated fatty acid |
| rTG | Re-esterified TG |
| SC401 | DHA and EPA ethylesters + Advanced Lipid Technologies |
| SCFA | Short-chain fatty acid |
| SDA | Stearidonic acid |
| SEDDS | Self-emulsifying drug delivery system |
| SLN | Solid lipid nanoparticles |
| SMEDS | Self-microemulsifying delivery system |
| SNEDDS | Self-nanoemulsifying drug delivery system |
| TG | Triacylglycerol (Triacylglyceride, Triglyceride) |
| VFA | Volatile fatty acid |
| VLCFA | Very long-chain fatty acid |
| WPI-GA | Whey protein isolate-gum arabic (complex) |
Funding
This research received no external funding
Conflicts of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Nagao K., Yanagita T. Conjugated fatty acids in food and their health benefits. J. Biosci. Bioeng. 2005;100:152–157. doi: 10.1263/jbb.100.152. [DOI] [PubMed] [Google Scholar]
- 2.Scorletti E., Byrne C.D. Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu. Rev. Nutr. 2013;33:231–248. doi: 10.1146/annurev-nutr-071812-161230. [DOI] [PubMed] [Google Scholar]
- 3.Chatgilialoglu C., Ferreri C., Melchiorre M., Sansone A., Torreggiani A. Lipid geometrical isomerism: From chemistry to biology and diagnostics. Chem. Rev. 2014;114:255–284. doi: 10.1021/cr4002287. [DOI] [PubMed] [Google Scholar]
- 4.IUPAC . Compendium of Chemical Terminology—The “Gold Book”. 2nd ed. Blackwell Scientific Publications; Oxford, UK: 1997. [(accessed on 10 December 2017)]. cis-trans isomers. Available online: http://goldbook.iupac.org/html/C/C01093.html. [Google Scholar]
- 5.IUPAC . Compendium of Chemical Terminology—The “Gold Book”. 2nd ed. Blackwell Scientific Publications; Oxford, UK: 1997. [(accessed on 10 December 2017)]. E, Z . Available online: http://goldbook.iupac.org/html/E/E01882.html. [Google Scholar]
- 6.IUPAC . Compendium of Chemical Terminology—The “Gold Book”. 2nd ed. Blackwell Scientific Publications; Oxford, UK: 1997. [(accessed on 10 December 2017)]. Fatty Acids. Available online: http://goldbook.iupac.org/html/F/F02330.html. [Google Scholar]
- 7.Poulos A., Sharp P., Johnson D., White I., Fellenberg A. The occurrence of polyenoic fatty acids with greater than 22 carbon atoms in mammalian spermatozoa. Biochem. J. 1986;240:891–895. doi: 10.1042/bj2400891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aveldaño M.I., Sprecher H. Very long chain (C24 to C36) polyenoic fatty acids of the n-3 and n-6 series in dipolyunsaturated phosphatidylcholines from bovine retina. J. Biol. Chem. 1987;262:1180–1186. [PubMed] [Google Scholar]
- 9.Rombeau J.L., Kripke S.A., Settle R.G. Short-chain fatty acids production, absorption, metabolism, and intestinal effects. In: Kritchevsky D., Bonfield C., Anderson J.W., editors. Dietary Fiber. Chemistry, Physiology, and Health Effects. 1st ed. Springer; New York, NY, USA: 1990. pp. 317–337. [Google Scholar]
- 10.Schönfeld P., Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016;57:943–954. doi: 10.1194/jlr.R067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beermann C., Jelinek J., Reinecker T., Hauenschild A., Boehm G., Klör H.U. Short term effects of dietary medium-chain fatty acids and n-3 long-chain polyunsaturated fatty acids on the fat metabolism of healthy volunteers. Lipids Health Dis. 2003;2:10. doi: 10.1186/1476-511X-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braverman N., Eichler F. Peroxisomal Disorders and Neurological Disease. In: Squire L.R., editor. Encyclopedia of Neuroscience. Volume 7. Academic Press; Oxford, UK: 2009. pp. 579–588. [Google Scholar]
- 13.Sassa T., Kihara A. Metabolism of very long-chain fatty acids: Genes and pathophysiology. Biomol. Ther. (Seoul) 2014;22:83–92. doi: 10.4062/biomolther.2014.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poulos A., Johnson D.W., Beckman K., White I.G., Easton C. Occurrence of unusual molecular species of sphingomyelin containing 28-34-carbon polyenoic fatty acids in ram spermatozoa. Biochem. J. 1987;248:961–964. doi: 10.1042/bj2480961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy S.J., Ferrante A., Poulos A., Robinson B.S., Johnson D.W., Murray A.W. Effect of exogenous fatty acids with greater than 22 carbon atoms (very long chain fatty acids) on superoxide production by human neutrophils. J. Immunol. 1994;153:1754–1761. [PubMed] [Google Scholar]
- 16.Hardy S.J., Ferrante A., Robinson B.S., Johnson D.W., Poulos A., Clark K.J., Murray A.W. In vitro activation of rat brain protein kinase C by polyenoic very-long-chain fatty acids. J. Neurochem. 1994;62:1546–1551. doi: 10.1046/j.1471-4159.1994.62041546.x. [DOI] [PubMed] [Google Scholar]
- 17.Singh M. Essential fatty acids, DHA and human brain. Indian J. Pediatr. 2005;72:239–242. doi: 10.1007/BF02859265. [DOI] [PubMed] [Google Scholar]
- 18.Arts M.T., Ackman R.G., Holub B.J. “Essential fatty acids” in aquatic ecosystems: A crucial link between diet and human health and evolution. Can. J. Fish. Aquat. Sci. 2001;58:122–137. doi: 10.1139/f00-224. [DOI] [Google Scholar]
- 19.Osborne T.B., Mendel L.B. Growth on diets poor in true fats. J. Biol. Chem. 1920;45:145–152. [Google Scholar]
- 20.Burr G.O., Burr M.M. A new deficiency disease produced by the rigid exclusion of fat from the diet. J. Biol. Chem. 1929;82:345–367. doi: 10.1111/j.1753-4887.1973.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 21.Holman R.T. Essential fatty acids. Nutr. Rev. 1958;16:33–35. doi: 10.1111/j.1753-4887.1958.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 22.Das U. Essential Fatty Acids—Biochemistry, Physiology and Clinical Significance. In: Das U., editor. Molecular Basis of Health and Disease. 1st ed. Springer; Dordrecht, The Netherlands: 2011. pp. 101–151. [Google Scholar]
- 23.Semba R.D. Essential Fatty Acids and Visual Development in Infants. In: Semba R.D., editor. Nutrition & Health: Handbook of Nutrition and Ophtalmology. 1st ed. Humana Press; Totowa, NJ, USA: 2007. pp. 415–441. [Google Scholar]
- 24.van Goor S.A., Dijck-Brouwer D.A.J., Muskiet F.A.J. Mother–Child Long Chain Polyunsaturated Fatty Acid Relationships: Implications for Diet and Behavior. In: Preedy V.R., Watson R.R., Martin C.R., editors. Handbook of Behavior, Food and Nutrition. 1st ed. Volume 2. Springer; New York, NY, USA: 2011. pp. 1139–1156. [Google Scholar]
- 25.Parrish C.C. Essential fatty acids in aquatic food webs. In: Arts M.T., Brett M.T., Kainz M., editors. Lipids in Aquatic Ecosystems. 1st ed. Springer; New York, NY, USA: 2009. pp. 309–326. [Google Scholar]
- 26.Nakamura M.T., Nara T.Y. Essential fatty acid synthesis and its regulation in mammals. Prostaglandins Leukot. Essent. Fat. Acids. 2003;68:145–150. doi: 10.1016/S0952-3278(02)00264-8. [DOI] [PubMed] [Google Scholar]
- 27.Gurr M.I., Harwood J.L. Lipid Biochemistry. An Introduction. 4th ed. Chapman and Hall; London, UK: 1991. p. 406. [Google Scholar]
- 28.Cunnane S.C., Likhodii S.S. 13C NMR spectroscopy and gas chromatograph–combustion–isotope ratio mass spectrometry: Complementary applications in monitoring the metabolism of 13C-labelled polyunsaturated fatty acids. Can. J. Physiol. Pharmacol. 1996;74:761–768. doi: 10.1139/y96-071. [DOI] [PubMed] [Google Scholar]
- 29.Dunstan G.A., Volkman J.K., Jeffrey S.W., Barrett S.M. Biochemical composition of microalgae from green algal classes Chlorophyceae and Prasinophyceae. 2. Lipid classes and fatty acids. J. Exp. Mar. Biol. Ecol. 1992;16:115–134. doi: 10.1016/0022-0981(92)90193-E. [DOI] [Google Scholar]
- 30.Viso C.A., Marty J.-C. Fatty acids from 28 marine microalgae. Phytochemistry. 1993;34:1521–1533. doi: 10.1016/S0031-9422(00)90839-2. [DOI] [Google Scholar]
- 31.Nanton D.A., Castell J.D. The effects of dietary fatty acids on the fatty acid composition of the harpacticoid copepod, Tisbe sp., for use as a live food for marine fish larvae. Aquaculture. 1998;163:251–261. doi: 10.1016/S0044-8486(98)00236-1. [DOI] [Google Scholar]
- 32.Cunnane S.C. The Canadian Society for Nutritional Sciences 1995 Young Scientist Award Lecture. Recent studies on the synthesis, β-oxidation and deficiency of linoleate and α-linolenate: Are essential fatty acids more aptly named indispensable or conditionally dispensable fatty acids? Can. J. Physiol. Pharmacol. 1996;74:629–639. doi: 10.1139/y96-089. [DOI] [PubMed] [Google Scholar]
- 33.Cunnane S.C. The conditional nature of the dietary need for a polyunsturates: A proposal to reclassify ‘essential fatty acids’ as ‘conditionally-indispensable’ or ‘conditionally-dispensable’ fatty acids. Br. J. Nutr. 2000;84:803–812. doi: 10.1017/S0007114500002415. [DOI] [PubMed] [Google Scholar]
- 34.Cunnane S.C. Problems with essential fatty acids: Time for a new paradigm? Prog. Lipid Res. 2003;42:544–568. doi: 10.1016/S0163-7827(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 35.Algarra M., Sánchez C., Esteves da Silva J.C.G., Jiménez-Jiménez J. Fatty acid and cholesterol content of Manchego type cheese prepared with incorporated avocado oil. Int. J. Food Prop. 2012;15:796–808. doi: 10.1080/10942912.2010.503358. [DOI] [Google Scholar]
- 36.Clément M., Tremblay J., Lange M., Thibodeau J., Belhumeur P. Purification and identification of bovine cheese whey fatty acids exhibiting in vitro antifungal activity. J. Dairy Sci. 2008;91:2535–2544. doi: 10.3168/jds.2007-0806. [DOI] [PubMed] [Google Scholar]
- 37.Moussa T.A., Almaghrabi O.A. Fatty acid constituents of Peganum harmala plant using gas chromatography-mass spectroscopy. Saudi J. Biol. Sci. 2016;23:397–403. doi: 10.1016/j.sjbs.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antony B., Fujii T., Moto K., Matsumoto S., Fukuzawa M., Nakano R., Tatsuki S., Ishikawa Y. Pheromone-gland-specific fatty-acyl reductase in the adzuki bean borer, Ostrinia scapulalis (Lepidoptera: Crambidae) Insect Biochem. Mol. Biol. 2009;39:90–95. doi: 10.1016/j.ibmb.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Bhuiyan M.N.I., Begum J., Sultana M. Chemical composition of leaf and seed essential oil of Coriandrum sativum L. from Bangladesh. Bangladesh J. Pharmacol. 2009;4:150–153. doi: 10.3329/bjp.v4i2.2800. [DOI] [Google Scholar]
- 40.Abad J.L., Camps F., Fabriàs G. Stereospecificity of the (Z)-9 desaturase that converts (E)-11-tetradecenoic acid into (Z,E)-9,11-tetradecadienoic acid in the biosynthesis of Spodoptera littoralis sex pheromone. Insect Biochem. Mol. Biol. 2001;31:799–803. doi: 10.1016/S0965-1748(00)00185-5. [DOI] [PubMed] [Google Scholar]
- 41.Frolov A.V., Pankov S.L. The reproduction strategy of oyster Ostrea edulis L. from the biochemical point of view. Comp. Biochem. Physiol. B. 1992;103:161–182. doi: 10.1016/0305-0491(92)90428-T. [DOI] [Google Scholar]
- 42.Aziz A., Larher F. Osmotic stress induced changes in lipid composition and peroxidation in leaf discs of Brassica napus L. J. Plant Physiol. 1998;153:754–762. doi: 10.1016/S0176-1617(98)80231-9. [DOI] [Google Scholar]
- 43.Rodriguez S., Hao G., Liu W., Pina B., Rooney A.P., Camps F., Roelofs W.L., Fabrias G. Expression and evolution of Δ9 and Δ11 desaturase genes in the moth Spodoptera littoralis. Insect Biochem. Mol. Biol. 2004;34:1315–1328. doi: 10.1016/j.ibmb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Keusgen M., Curtis J.M., Ayer S.W. The use of nicotinates and sulfoquinovosyl monoacylglycerols in the analysis of monounsaturated n-3 fatty acids by mass spectrometry. Lipids. 1996;31:231–238. doi: 10.1007/BF02522625. [DOI] [PubMed] [Google Scholar]
- 45.Bragagnolo N., Rodriguez-Amaya D.B. Simultaneous determination of total lipid, cholesterol and fatty acids in meat and backfat of suckling and adult pigs. Food Chem. 2002;79:255–260. doi: 10.1016/S0308-8146(02)00136-X. [DOI] [Google Scholar]
- 46.Leheska J.M., Thompson L.D., Howe J.C., Hentges E., Boyce J., Brooks J.C., Shriver B., Hoover L., Miller M.F. Effects of conventional and grass-feeding systems on the nutrient composition of beef. J. Anim. Sci. 2008;86:3575–3585. doi: 10.2527/jas.2007-0565. [DOI] [PubMed] [Google Scholar]
- 47.Couvreur S., Hurtaud C., Lopez C., Delaby L., Peyraud J.L. The linear relationship between the proportion of fresh grass in the cow diet, milk fatty acid composition, and butter properties. J. Dairy Sci. 2006;89:1956–1969. doi: 10.3168/jds.S0022-0302(06)72263-9. [DOI] [PubMed] [Google Scholar]
- 48.Bicalho B., David F., Rumplel K., Kindt E., Sandra P. Creating a fatty acid methyl ester database for lipid profiling in a single drop of human blood using high resolution capillary gas chromatography and mass spectrometry. J. Chromatogr. A. 2008;1211:120–128. doi: 10.1016/j.chroma.2008.09.066. [DOI] [PubMed] [Google Scholar]
- 49.Li J., Fan Y., Zhang Z., Yu H., An Y., Kramer J.K., Deng Z. Evaluating the trans fatty acid, CLA, PUFA and erucic acid diversity in human milk from five regions in China. Lipids. 2009;44:257–271. doi: 10.1007/s11745-009-3282-x. [DOI] [PubMed] [Google Scholar]
- 50.Mosley E.E., Wright A.L., McGuire M.K., McGuire M.A. trans Fatty acids in milk produced by women in the United States. Am. J. Clin. Nutr. 2005;82:1292–1297. doi: 10.1093/ajcn/82.6.1292. [DOI] [PubMed] [Google Scholar]
- 51.Wang H.L., Zhao C.H., Millar J.G., Cardé R.T., Löfstedt C. Biosynthesis of unusual moth pheromone components involves two different pathways in the navel orangeworm, Amyelois transitella. J. Chem. Ecol. 2010;36:535–547. doi: 10.1007/s10886-010-9777-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hădărugă D.I., Birău Mitroi C.L., Gruia A.T., Păunescu V., Bandur G.N., Hădărugă N.G. Moisture evaluation of β-cyclodextrin/fish oils complexes by thermal analyses: A data review on common barbel (Barbus barbus L.), Pontic shad (Alosa immaculata Bennett), European wels catfish (Silurus glanis L.), and common bleak (Alburnus alburnus L.) living in Danube river. Food Chem. 2017;236:49–58. doi: 10.1016/j.foodchem.2017.03.093. [DOI] [PubMed] [Google Scholar]
- 53.Nasirullah, Werner G., Seher A. Fatty acid composition of lipids from edible parts and seeds of vegetables. Eur. J. Lipid Sci. Technol. 1984;86:264–268. doi: 10.1002/lipi.19840860702. [DOI] [Google Scholar]
- 54.Jamieson G.R., Reid E.H. The leaf lipids of some conifer species. Phytochemistry. 1972;11:269–275. doi: 10.1016/S0031-9422(00)90002-5. [DOI] [Google Scholar]
- 55.Bohannon M.B., Kleiman R. Unsaturated C18 α-hydroxy acids in Salvia nilotica. Lipids. 1975;10:703–706. doi: 10.1007/BF02532764. [DOI] [Google Scholar]
- 56.Amin S., Mir S.R., Kohli K., Ali B., Ali M. A study of the chemical composition of black cumin oil and its effect on penetration enhancement from transdermal formulations. Nat. Prod. Res. 2010;24:1151–1157. doi: 10.1080/14786410902940909. [DOI] [PubMed] [Google Scholar]
- 57.Chisholm M.J., Hopkins C.Y. Fatty acids of filbert oil and Nasturtium seed oil. Can. J. Chem. 1953;31:1131–1137. doi: 10.1139/v53-146. [DOI] [Google Scholar]
- 58.Memon N.N., Talpur F.N., Bhanger M.I., Balouch A. Changes in fatty acid composition in muscle of three farmed carp fish species (Labeo rohita, Cirrhinus mrigala, Catla catla) raised under the same conditions. Food Chem. 2011;126:405–410. doi: 10.1016/j.foodchem.2010.10.107. [DOI] [Google Scholar]
- 59.Shirai N., Higuchi T., Suzuki H. Analysis of lipid classes and the fatty acid composition of the salted fish roe food products, Ikura, Tarako, Tobiko and Kazunoko. Food Chem. 2006;94:61–67. doi: 10.1016/j.foodchem.2004.10.050. [DOI] [Google Scholar]
- 60.Pickova J., Kiessling A., Pettersson A., Dutta P.C. Comparison of fatty acid composition and astaxanthin content in healthy and by M74 affected salmon eggs from three Swedish river stocks. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998;120:265–271. doi: 10.1016/S0305-0491(98)10016-0. [DOI] [Google Scholar]
- 61.Bandarra N.M., Marçalo A., Cordeiro A.R., Pousão-Ferreira P. Sardine (Sardina pilchardus) lipid composition: Does it change after one year in captivity? Food Chem. 2018;244:408–413. doi: 10.1016/j.foodchem.2017.09.147. [DOI] [PubMed] [Google Scholar]
- 62.Ackman R.G., Epstein S., Eaton C.A. Differences in the fatty acid compositions of blubber fats from northwestern Atlantic finwhales (Balaenoptera physalus) and harp seals (Pagophilus groenlandica) Comp. Biochem. Physiol. B. 1971;40:683–697. doi: 10.1016/0305-0491(71)90143-X. [DOI] [PubMed] [Google Scholar]
- 63.Mu H., Jin J., Xie D., Zou X., Wang X., Wang X., Jin Q. Combined urea complexation and argentated silica gel column chromatography for concentration and separation of PUFAs from tuna oil: Based on improved DPA level. J. Am. Oil Chem. Soc. 2016;93:1157–1167. doi: 10.1007/s11746-016-2842-5. [DOI] [Google Scholar]
- 64.Saito H., Aono H. Characteristics of lipid and fatty acid of marine gastropod Turbo cornutus: High levels of arachidonic and n-3 docosapentaenoic acid. Food Chem. 2014;145:135–144. doi: 10.1016/j.foodchem.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 65.Jin F., Nieman D.C., Sha W., Xie G., Qiu Y., Jia W. Supplementation of milled chia seeds increases plasma ALA and EPA in postmenopausal women. Plant Foods Hum. Nutr. 2012;67:105–110. doi: 10.1007/s11130-012-0286-0. [DOI] [PubMed] [Google Scholar]
- 66.Madkour H.M.F., Ghareeb M.A., Abdel-Aziz M.S., Khalaf O.M., Saad A.M., El-Ziaty A.K., Abdel-Mogib M. Gas chromatography-mass spectrometry analysis, antimicrobial, anticancer and antioxidant activities of n-hexane and methylene chloride extracts of Senna italica. J. Appl. Pharm. Sci. 2017;7:23–32. doi: 10.7324/japs.2017.70604. [DOI] [Google Scholar]
- 67.Wolff R.L., Pédrono F., Marpeau A.M. Fokienia hodginsii seed oil, another source of all-cis 5,9,12,15-18:4 (coniferonic) acid. J. Am. Oil Chem. Soc. 1999;76:535–536. doi: 10.1007/s11746-999-0037-z. [DOI] [Google Scholar]
- 68.Souza A.L., Martinez F.P., Ferreira S.B., Kaiser C.R. A complete evaluation of thermal and oxidative stability of chia oil. The richest natural source of a-linolenic acid. J. Therm. Anal. Calorim. 2017;130:1307–1315. doi: 10.1007/s10973-017-6106-x. [DOI] [Google Scholar]
- 69.Gómez-Cortés P., Tyburczy C., Brenna J.T., Juárez M., de la Fuente M.A. Characterization of cis-9 trans-11 trans-15 C18:3 in milk fat by GC and covalent adduct chemical ionization tandem MS. J. Lipid Res. 2009;50:2412–2420. doi: 10.1194/jlr.M800662-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang J., Zhou C., Yuan G., Li D. Effects of geographical origin on the conjugated linolenic acid of Trichosanthes kirilowii Maxim seed oil. J. Am. Oil Chem. Soc. 2012;89:401–407. doi: 10.1007/s11746-011-1928-3. [DOI] [Google Scholar]
- 71.Bjelková M., Nôžková J., Fatrcová-Šramková K., Tejklová E. Comparison of linseed (Linum usitatissimum L.) genotypes with respect to the content of polyunsaturated fatty acids. Chem. Pap. 2012;66:972–976. doi: 10.2478/s11696-012-0209-4. [DOI] [Google Scholar]
- 72.Pérez-Gálveza A., Garrido-Fernándeza J., Mínguez-Mosqueraa M.I., Lozano-Ruizb M., Montero-de-Espinosab V. Fatty acid composition of two new pepper varieties (Capsicum annuum L. cv. Jaranda and Jariza). Effect of drying process and nutritional aspects. J. Am. Oil Chem. Soc. 1999;76:205–208. doi: 10.1007/s11746-999-0219-8. [DOI] [Google Scholar]
- 73.Goren A.C., Kilic T., Dirmenci T., Bilsel G. Chemotaxonomic evaluation of Turkish species of Salvia: Fatty acid compositions of seed oils. Biochem. Syst. Ecol. 2006;34:160–164. doi: 10.1016/j.bse.2005.09.002. [DOI] [Google Scholar]
- 74.Giannelos P.N., Zannikos F., Stournas S., Lois E., Anastopoulos G. Tobacco seed oil as an alternative diesel fuel: Physical and chemical properties. Ind. Crop. Prod. 2002;16:1–9. doi: 10.1016/S0926-6690(02)00002-X. [DOI] [Google Scholar]
- 75.Alves S.P., Bessa R.J.B. Identification of cis-12, cis-15 octadecadienoic acid and other minor polyenoic fatty acids in ruminant fat. Eur. J. Lipid Sci. Technol. 2007;109:879–883. doi: 10.1002/ejlt.200700035. [DOI] [Google Scholar]
- 76.Wolff R.L., Lavialle O., Pédrono F., Pasquier E., Destaillats F., Marpeau A.M., Angers P., Aitzetmüller K. Abietoid seed fatty acid compositions—A review of the genera Abies, Cedrus, Hesperopeuce, Keteleeria, Pseudolarix, and Tsuga and preliminary inferences on the taxonomy of Pinaceae. Lipids. 2002;37:17–26. doi: 10.1007/s11745-002-0859-5. [DOI] [PubMed] [Google Scholar]
- 77.Mongrand S., Badoc A., Patouille B., Lacomblez C., Chavent M., Cassagne C., Bessoule J.J. Taxonomy of Gymnospermae: Multivariate analyses of leaf fatty acid composition. Phytochemistry. 2001;58:101–115. doi: 10.1016/S0031-9422(01)00139-X. [DOI] [PubMed] [Google Scholar]
- 78.Kuhnt K., Degen C., Jaudszus A., Jahreis G. Searching for health beneficial n-3 and n-6 fatty acids in plant seeds. Eur. J. Lipid Sci. Technol. 2012;114:153–160. doi: 10.1002/ejlt.201100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guil-Guerrero J.L., López-Martínez J.C., Gómez-Mercado F., Campra-Madrid P. Gamma-linolenic and stearidonic acids from Moroccan Boraginaceae. Eur. J. Lipid Sci. Technol. 2006;108:43–47. doi: 10.1002/ejlt.200500251. [DOI] [Google Scholar]
- 80.Khalid M.N., Shameel M. Studies on the phycochemistry and biological activity of Spirogyra rhizoides (Chlorophycota) Pak. J. Bot. 2012;44:1815–1820. [Google Scholar]
- 81.Gunstone F.D., Subbarao R. New tropical seed oils. Part I. Conjugated trienoic and tetraenoic acids and their oxo derivatives in the seed oils of Chrysobalanus icaco and Parinarium laurinum. Chem. Phys. Lipids. 1967;1:349–359. doi: 10.1016/0009-3084(67)90012-6. [DOI] [Google Scholar]
- 82.Heintz M., Gregoire J., Lefort D. Chromatographie en phase gazeuse et lipochemie, 11. Contribution a l’etude de la composition en acides gras des huiles de Sideroxylon argania, Balanites aegyptiaca et Parinarium macrophyllum. Oleagineux. 1965;20:603–608. [Google Scholar]
- 83.Werdelmann B.W., Schmid R.D. The biotechnology of fats—A challenge and an opportunity. J. Lipid Sci. Technol. 1982;84:436–443. doi: 10.1002/lipi.19820841104. [DOI] [Google Scholar]
- 84.Kaufmann H.P., Sud R.K. Die Papier-Chromatographie auf dem Fettgebiet XLI: Uber die Fettsaure-Zusammensetzung einiger Konjuenole. Eur. J. Lipid Sci. Technol. 1960;62:160–164. doi: 10.1002/lipi.19600620302. [DOI] [Google Scholar]
- 85.Spitzer V., Tomberg W., Zucolotto M. Identification of α-parinaric acid in the seed oil of Sebastiana brasiliensis Sprengel (Euphorbiaceae) J. Am. Oil Chem. Soc. 1996;73:569–573. doi: 10.1007/BF02518109. [DOI] [Google Scholar]
- 86.Tulloch A.P. 13C nuclear magnetic resonance spectroscopic analysis of seed oils containing conjugated unsaturated acids. Lipids. 1982;17:544–550. doi: 10.1007/BF02535382. [DOI] [Google Scholar]
- 87.Riley J.P. The seed fat of Parinarium laurinum. Part I. Component acids of the seed fat. J. Chem. Soc. 1950:12–18. doi: 10.1039/jr9500000012. [DOI] [Google Scholar]
- 88.Schroeder F., Holland J.F., Vagelos P.R. Use of β-parinaric acid, a novel fluorimetric probe, to determine characteristic temperatures of membranes and membrane lipids from cultured animal cells. J. Biol. Chem. 1976;251:6739–6746. [PubMed] [Google Scholar]
- 89.Kaufmann H.P., Keller M.C. Konjugiert-ungesattigte Verbindungen in der Fettchemie VI: Uber das Vorkommen von Parinarsaure in den Samenfetten der Balsaminaceen. Eur. J. Lipid Sci. Technol. 1950;52:389–398. doi: 10.1002/lipi.19500520702. [DOI] [Google Scholar]
- 90.Shibahara A., Yamamoto K., Shinkai K., Nakayama T., Kajimoto G. cis-9, cis-15-Octadecadienoic acid: A novel fatty acid found in higher plants. Biochim. Biophys. Acta. 1993;1170:245–252. doi: 10.1016/0005-2760(93)90006-U. [DOI] [PubMed] [Google Scholar]
- 91.Galla N.R., Pamidighantam P.R., Satyanarayana A. Chemical, amino acid and fatty acid composition of Sterculia urens L. seed. Food Hydrocoll. 2012;28:320–324. doi: 10.1016/j.foodhyd.2012.01.003. [DOI] [Google Scholar]
- 92.Hoffmann G., Meijboom P.W. Identification of 11,15-octadecadienoic acid from beef and mutton tallow. J. Am. Oil Chem. Soc. 1969;46:620–622. doi: 10.1007/BF02544981. [DOI] [Google Scholar]
- 93.Akinoso R., Suleiman A. Heat treatment effects on extraction of roselle (Hibiscus sabdariffa L.) seed oil. Eur. J. Lipid Sci. Technol. 2011;113:1527–1532. doi: 10.1002/ejlt.201100067. [DOI] [Google Scholar]
- 94.Huang J.J., Cheung P.C. Enhancement of polyunsaturated fatty acids and total carotenoid production in microalgae by ultraviolet band A (UVA, 365 nm) radiation. J. Agric. Food Chem. 2011;59:4629–4636. doi: 10.1021/jf200910p. [DOI] [PubMed] [Google Scholar]
- 95.Kahraman A. Nutritional value and foliar fertilization in soybean. J. Elem. 2017;22:55–66. doi: 10.5601/jelem.2016.21.1.1106. [DOI] [Google Scholar]
- 96.He Z., Zhu H., Li W., Zeng M., Wua S., Chen S., Qin F., Chen J. Chemical components of cold pressed kernel oils from different Torreya grandis cultivars. Food Chem. 2016;209:196–202. doi: 10.1016/j.foodchem.2016.04.053. [DOI] [PubMed] [Google Scholar]
- 97.Kobelnik M., Fontanari G.G., Ribeiro C.A., Crespi M.S. Evaluation of thermal behavior and chromatographic characterization of oil extracted from seed of Pittosporum undulatum. J. Therm. Anal. Calorim. 2017;131:371–378. doi: 10.1007/s10973-017-6763-9. [DOI] [Google Scholar]
- 98.Kemertelidze E.P., Dalakishvili T.M., Bitadze M.A., Khatiashvili N.S. Lipids of Phellodendron lavalei seeds. Chem. Nat. Compd. 2000;36:272–275. doi: 10.1007/BF02238333. [DOI] [Google Scholar]
- 99.Cheikh-Rouhou S., Hentai B., Besbes S., Blecker C., Deroanne C., Attia H. Chemical composition and lipid fraction characteristics of aleppo pine (Pinus halepensis Mill.) seeds cultivated in Tunisia. Food Sci. Technol. Int. 2006;15:407–415. doi: 10.1177/1082013206069910. [DOI] [Google Scholar]
- 100.Aitzetmüller K., Vosmann K. Cyclopropenoic fatty acids in gymnosperms: The seed oil of Welwitschia. J. Am. Oil Chem. Soc. 1998;75:1761–1765. doi: 10.1007/s11746-998-0329-8. [DOI] [Google Scholar]
- 101.Pintea A., Dulf F.V., Bunea A., Matea C., Andrei S. Comparative analysis of lipophilic compounds in eggs of organically raised ISA Brown and Araucana hens. Chem. Pap. 2012;66:955–963. doi: 10.2478/s11696-012-0219-2. [DOI] [Google Scholar]
- 102.Samel N., Järving I., Lõhmus M., Lopp A., Kobzar G., Sadovskaya V., Välimäe T., Lille U. Identification and biological activity of a novel natural prostaglandin, 5,6-dihydro-prostaglandin E3. Prostaglandins. 1987;33:137–146. doi: 10.1016/0090-6980(87)90311-X. [DOI] [PubMed] [Google Scholar]
- 103.Moffat C.F., McGill A.S. Variability of the composition of fish oils: Significance for the diet. Proc. Nutr. Soc. 1993;52:441–456. doi: 10.1079/PNS19930085. [DOI] [PubMed] [Google Scholar]
- 104.Ikeda I., Oka T., Koba K., Sugano M., Lie Ken Jie M.S. 5c,11c,14c-eicosatrienoic acid and 5c,11c,14c,17c-eicosatetraenoic acid of Biota orientalis seed oil affect lipid metabolism in the rat. Lipids. 1992;27:500–504. doi: 10.1007/BF02536130. [DOI] [PubMed] [Google Scholar]
- 105.Kim G.-W., Itabashi Y. Non-methylene-interrupted fatty acids with Δ5 unsaturation in Sargassum species. J. Oleo Sci. 2012;61:311–319. doi: 10.5650/jos.61.311. [DOI] [PubMed] [Google Scholar]
- 106.Smith C.R., Jr., Kleiman R., Wolff I.A. Caltha palustris L. seed oil. A source of four fatty acids with cis-5-unsaturation. Lipids. 1968;3:37–42. doi: 10.1007/BF02530966. [DOI] [PubMed] [Google Scholar]
- 107.Kang J.-Y., Chun B.-S., Lee M.-C., Choi J.-S., Choi I.S., Hong Y.-K. Anti-inflammatory activity and chemical composition of essential oil extracted with supercritical CO2 from the brown seaweed Undaria pinnatifida. J. Essent. Oil Bear. Plants. 2016;19:46–51. doi: 10.1080/0972060X.2014.989181. [DOI] [Google Scholar]
- 108.Carballeira N.M., Cartagena M., Tasdemir D. Fatty acid composition of Turkish Rhododendron species. J. Am. Oil Chem. Soc. 2008;85:605–611. doi: 10.1007/s11746-008-1233-y. [DOI] [Google Scholar]
- 109.Mayzaud P., Ackman R.G. The 6,9,12,15,18-heneicosapentaenoic acid of seal oil. Lipids. 1978;13:24–28. doi: 10.1007/BF02533362. [DOI] [PubMed] [Google Scholar]
- 110.Solomon G., Aman D., Bachheti R.K. Fatty acids, metal composition, nutritional value and physicochemical parameters of Lepidium sativium seed oil collected from Ethiopia. Int. Food Res. J. 2016;23:827–831. [Google Scholar]
- 111.Hoffman W.H., Zuckerman A., Grace N.H. Canadian erucic acid oils. VIII. Component fatty acids of the oil from weed seed screenings, largely charlock. J. Am. Oil Chem. Soc. 1951;28:522–524. doi: 10.1007/BF02645834. [DOI] [Google Scholar]
- 112.Kallio H., Lehtinen T., Laakso P., Tahvonen R. Fatty acids of a salami-type sausage made of Baltic herring fillets, pork and lard. Zeitschrift für Lebensmitteluntersuchung und-Forschung A. 1998;207:276–280. doi: 10.1007/s002170050333. [DOI] [Google Scholar]
- 113.Narsing Rao G., Prabhakara Rao P.G., Satyanarayana A. Chemical, fatty acid, volatile oil composition and antioxidant activity of shade dried neem (Azadirachta indica L.) flower powder. Int. Food Res. J. 2014;21:807–813. [Google Scholar]
- 114.Zengin H., Vural N., Çelik V.K. Comparison of changes in fatty acid composition of starved and fed rainbow trout, (Oncorhynchus mykiss) larvae. Turk. J. Fish. Aquat. Sci. 2013;13:397–405. doi: 10.4194/1303-2712-v13_3_02. [DOI] [Google Scholar]
- 115.Varljen J., Baticic L., Sincic-Modric G., Varljen N., Kapovic M. Liver and muscle tissue fatty acid composition of the lipid fractions of Diplodus vulgaris from the north Adriatic Sea, Croatia. J. Food Lipids. 2005;12:286–298. doi: 10.1111/j.1745-4522.2005.00024.x. [DOI] [Google Scholar]
- 116.Flakemore A.R., Malau-Aduli B.S., Nichols P.D., Malau-Aduli A.E.O. Degummed crude canola oil, sire breed and gender effects on intramuscular long-chain omega-3 fatty acid properties of raw and cooked lamb meat. J. Anim. Sci. Technol. 2017;59:17. doi: 10.1186/s40781-017-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Johns R.B., Nichols P.D., Perry G.J. Fatty acid composition of ten marine algae from Australian waters. Phytochemistry. 1979;18:799–802. doi: 10.1016/0031-9422(79)80018-7. [DOI] [Google Scholar]
- 118.Linko R.R., Karinkanta H. Fatty acids of long chain length in baltic herring lipids. J. Am. Oil Chem. Soc. 1970;47:42–46. doi: 10.1007/BF02541455. [DOI] [Google Scholar]
- 119.Litchfield C., Tyszkiewicz J., Marcantonio E.E., Note G. 15,18,21,24-triacontatetraenoic and 15,18,21,24,27-triacontapentaenoic acids: New C30 fatty acids from the marine sponge Cliona celata. Lipids. 1979;14:619–622. doi: 10.1007/BF02533446. [DOI] [Google Scholar]
- 120.Carballeira N.M., Reyes E.D. Novel very long chain fatty acids from the sponge Petrosia pellasarca. J. Nat. Prod. 1990;53:836–840. doi: 10.1021/np50070a010. [DOI] [Google Scholar]
- 121.Zhang H.Y., Yamakawa Y., Matsuya Y., Toyooka N., Tohda C., Awale S., Li F., Kadota S., Tezuka Y. Synthesis of long-chain fatty acid derivatives as a novel anti-Alzheimer’s agent. Bioorg. Med. Chem. Lett. 2014;24:604–608. doi: 10.1016/j.bmcl.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 122.Poulos A., Sharp P., Johnson D., Easton C. The occurrence of polyenoic very long chain fatty acids with greater than 32 carbon atoms in molecular species of phosphatidylcholine in normal and peroxisome-deficient (Zellweger’s syndrome) brain. Biochem. J. 1988;253:645–650. doi: 10.1042/bj2530645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yae E., Yahara S., El-Aasr M., Ikeda T., Yoshimitsu H., Masuoka C., Ono M., Hide I., Nakata Y., Nohara T. Studies on the constituents of whole plants of Youngia japonica. Chem. Pharm. Bull. 2009;57:719–723. doi: 10.1248/cpb.57.719. [DOI] [PubMed] [Google Scholar]
- 124.Olias J.M., Rios J.J., Valle M., Zamora R., Sanz L.C., Axelrod B. Fatty acid hydroperoxide lyase in germinating soybean seedlings. J. Agric. Food Chem. 1990;38:624–630. doi: 10.1021/jf00093a009. [DOI] [Google Scholar]
- 125.Matsui K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006;9:274–280. doi: 10.1016/j.pbi.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 126.Tarchevsky I.A., Karimova F.G., Grechkin A.N., Moukhametchina N.U. Influence of (9Z)-12-hydroxy-9-dodecenoic acid and methyl jasmonate on plant protein phosphorylation. Biochem. Soc. Trans. 2000;28:870–871. doi: 10.1042/bst0280870. [DOI] [PubMed] [Google Scholar]
- 127.Grechkin A.N. Hydroperoxide lyase and divinyl ether synthase. Prostaglandins Other Lipid Mediat. 2002;68–69:457–470. doi: 10.1016/S0090-6980(02)00048-5. [DOI] [PubMed] [Google Scholar]
- 128.Kallenbach M., Gilardoni P.A., Allmann S., Baldwin I.T., Bonaventure G. C12 derivatives of the hydroperoxide lyase pathway are produced by product recycling through lipoxygenase-2 in Nicotiana attenuata leaves. New Phytol. 2011;191:1054–1068. doi: 10.1111/j.1469-8137.2011.03767.x. [DOI] [PubMed] [Google Scholar]
- 129.Schreier P., Lorenz G. Separation, partial purification and characterization of a fatty acid hydroperoxide cleaving enzyme from apple and tomato fruits. Z. Naturforsch. C. 1982;37:165–173. doi: 10.1515/znc-1982-3-405. [DOI] [Google Scholar]
- 130.Kleiman R., Spencer G.F., Earle F.R., Nieshlag H.J., Barclay A.S. Tetra-acid triglycerides containing a new hydroxy eicosadienoyl moiety in Lesquerella auriculata seed oil. Lipids. 1972;7:660–665. doi: 10.1007/BF02533073. [DOI] [Google Scholar]
- 131.Jenderek M.M., Dierig D.A., Isbell T.A. Fatty-acid profile of Lesquerella germplasm in the National Plant Germplasm System collection. Ind. Crop. Prod. 2009;29:154–164. doi: 10.1016/j.indcrop.2008.04.019. [DOI] [Google Scholar]
- 132.Reed D.W., Taylor D.C., Covello P.S. Metabolism of hydroxy fatty acids in developing seeds in the genera Lesquerella (Brassicaceae) and Linum (Linaceae) Plant Physiol. 1997;114:63–68. doi: 10.1104/pp.114.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rodé-Gowal H., Abbott S., Meyer D., Röller H., Dahm K.H. Propionate as a precursor of Juvenile Hormone in the Cecropia moth. Z. Naturforsch. C. 1975;30:392–397. doi: 10.1515/znc-1975-5-615. [DOI] [Google Scholar]
- 134.Richter C.K., Bowen K.J., Mozaffarian D., Kris-Etherton P.M., Skulas-Ray A.C. Total long-chain n-3 fatty acid intake and food sources in the United States compared to recommended intakes: NHANES 2003–2008. Lipids. 2017;52:917–927. doi: 10.1007/s11745-017-4297-3. [DOI] [PubMed] [Google Scholar]
- 135.Mori T.A. Marine OMEGA-3 fatty acids in the prevention of cardiovascular disease. Fitoterapia. 2017;123:51–58. doi: 10.1016/j.fitote.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 136.Fialkow J. Omega-3 fatty acid formulations in cardiovascular disease: Dietary supplements are not substitutes for prescription products. Am. J. Cardiovasc. Drugs. 2016;16:229–239. doi: 10.1007/s40256-016-0170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Monroig Ó., Tocher D.R., Navarro J.C. Biosynthesis of polyunsaturated fatty acids in marine invertebrates: Recent advances in molecular mechanisms. Mar. Drugs. 2013;11:3998–4018. doi: 10.3390/md11103998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Puri R., Mahajan M., Sahajpal N.S., Singh H., Singh H., Jain S.K. Self-nanoemulsifying drug delivery system of docosahexanoic acid: Development, in vitro, in vivo characterization. Drug Dev. Ind. Pharm. 2016;42:1032–1041. doi: 10.3109/03639045.2015.1107089. [DOI] [PubMed] [Google Scholar]
- 139.Ezeagua I.E., Petzkeb K.J., Langeb E., Metges C.C. Fat content and fatty acid composition of oils extracted from selected wild-gathered tropical plant seeds from Nigeria. J. Am. Oil Chem. Soc. 1998;75:1031–1035. doi: 10.1007/s11746-998-0282-6. [DOI] [Google Scholar]
- 140.Yang N., Sampathkumar K., Loo S.C.J. Recent advances in complementary and replacement therapy with nutraceuticals in combating gastrointestinal illnesses. Clin. Nutr. 2017;36:968–979. doi: 10.1016/j.clnu.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 141.Abedi E., Sahari M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014;2:443–463. doi: 10.1002/fsn3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Martin J., Stapleton R.D. Parenteral and Enteral Nutrition with Omega-3 Fatty Acids. In: Rajendram R., Preedy V.R., Patel V.B., editors. Diet and Nutrition in Critical Care. 1st ed. Volume 2. Springer; New York, NY, USA: 2015. pp. 1695–1710. [Google Scholar]
- 143.Couëdelo L., Amara S., Lecomte M., Meugnier E., Monteil J., Fonseca L., Pineau G., Cansell M., Carrière F., Michalski M.C., et al. Impact of various emulsifiers on ALA bioavailability and chylomicron synthesis through changes in gastrointestinal lipolysis. Food Funct. 2015;6:1726–1735. doi: 10.1039/C5FO00070J. [DOI] [PubMed] [Google Scholar]
- 144.Osipova E.V., Lantsova N.V., Chechetkin I.R., Mukhitova F.K., Hamberg M., Grechkin A.N. Hexadecanoid pathway in plants: Lipoxygenase dioxygenation of (7Z,10Z,13Z)-hexadecatrienoic acid. Biochemistry. 2010;75:708–716. doi: 10.1134/S0006297910060052. [DOI] [PubMed] [Google Scholar]
- 145.Belury M.A. Conjugated dienoic linoleate: A polyunsaturated fatty acid with unique chemoprotective properties. Nutr. Rev. 1995;53:83–89. doi: 10.1111/j.1753-4887.1995.tb01525.x. [DOI] [PubMed] [Google Scholar]
- 146.Cahoon E.B., Carlson T.J., Ripp K.G., Schweiger B.J., Cook G.A., Hall S.E., Kinney A.J. Biosynthetic origin of conjugated double bonds: Production of fatty acid components of high-value drying oils in transgenic soybean embryos. Proc. Natl. Acad. Sci. USA. 1999;96:12935–12940. doi: 10.1073/pnas.96.22.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tecoma E.S., Sklar L.A., Simoni R.D., Hudson B.S. Conjugated polyene fatty acids as fluorescent probes: Biosynthetic incorporation of parinaric acid by Escherichia coli and studies of phase transitions. Biochemistry. 1977;16:829–835. doi: 10.1021/bi00624a003. [DOI] [PubMed] [Google Scholar]
- 148.Haldar S., Jena N., Croce C.M. Inactivation of Bcl-2 by phosphorylation. Proc. Natl. Acad. Sci. USA. 1995;92:4507–4511. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Soares J.R., Dinis T.C., Cunha A.P., Almeida L.M. Antioxidant activities of some extracts of Thymus zygis. Free Radic. Res. 1997;26:469–478. doi: 10.3109/10715769709084484. [DOI] [PubMed] [Google Scholar]
- 150.Drummen G.P., Op den Kamp J.A., Post J.A. Validation of the peroxidative indicators, cis-parinaric acid and parinaroyl-phospholipids, in a model system and cultured cardiac myocytes. Biochim. Biophys. Acta. 1999;1436:370–382. doi: 10.1016/S0005-2760(98)00142-8. [DOI] [PubMed] [Google Scholar]
- 151.Steenbergen R.H., Drummen G.P., Op den Kamp J.A., Post J.A. The use of cis-parinaric acid to measure lipid peroxidation in cardiomyocytes during ischemia and reperfusion. Biochim. Biophys. Acta. 1997;1330:127–137. doi: 10.1016/S0005-2736(97)00144-2. [DOI] [PubMed] [Google Scholar]
- 152.Hedley D., Chow S. Flow cytometric measurement of lipid peroxidation in vital cells using parinaric acid. Cytometry. 1992;13:686–692. doi: 10.1002/cyto.990130704. [DOI] [PubMed] [Google Scholar]
- 153.Kuypers F.A., van den Berg J.J., Schalkwijk C., Roelofsen B., Op den Kamp J.A. Parinaric acid as a sensitive fluorescent probe for the determination of lipid peroxidation. Biochim. Biophys. Acta. 1987;921:266–274. doi: 10.1016/0005-2760(87)90027-0. [DOI] [PubMed] [Google Scholar]
- 154.Zsila F., Bikádi Z. trans-Parinaric acid as a versatile spectroscopic label to study ligand binding properties of bovine β-lactoglobulin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005;62:666–672. doi: 10.1016/j.saa.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 155.Terzaghi W.B., Fork D.C., Berry J.A., Field C.B. Low and high temperature limits to PSII: A survey using trans-parinaric acid, delayed light emission, and Fo chlorophyll fluorescence. Plant Physiol. 1989;91:1494–1500. doi: 10.1104/pp.91.4.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Polidori P., Vincenzetti S., Pucciarelli S., Polzonetti V. CLAs in Animal Source Foods: Healthy Benefits for Consumers. In: Mérillon J.M., Ramawat K., editors. Bioactive Molecules in Food. Reference Series in Phytochemistry. Springer; Cham, Switzerland: 2018. pp. 1–32. [Google Scholar]
- 157.Warnaar F. Deca-2,4,6-trienoic acid, a new conjugated fatty acid, isolated from the latex of Euphorbia pulcherrima Willd. Lipids. 1977;12:707–710. doi: 10.1007/BF02570899. [DOI] [Google Scholar]
- 158.Sharp P., Poulos A., Fellenberg A., Johnson D. Structure and lipid distribution of polyenoic very-long-chain fatty acids in the brain of peroxisome-deficient patients (Zellweger syndrome) Biochem. J. 1987;248:61–67. doi: 10.1042/bj2480061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sharp P., Johnson D., Poulos A. Molecular species of phosphatidylcholine containing very long chain fatty acids in human brain: Enrichment in X-linked adrenoleukodystrophy brain and diseases of peroxisome biogenesis brain. J. Neurochem. 1991;56:30–37. doi: 10.1111/j.1471-4159.1991.tb02558.x. [DOI] [PubMed] [Google Scholar]
- 160.Bridges R.B., Coniglio J.G. The biosynthesis of Δ9,12,15,18-tetracosatetraenoic and of Δ6,9,12,15,18-tetracosapentaenoic acids by rat testes. J. Biol. Chem. 1970;245:46–49. [PubMed] [Google Scholar]
- 161.Grogan W.M., Huth E.G. Biosynthesis of long-chain polyenoic acids from arachidonic acid in cultures of enriched spermatocytes and spermatids from mouse testis. Lipids. 1983;18:275–284. doi: 10.1007/BF02534702. [DOI] [PubMed] [Google Scholar]
- 162.Rosenthal M.D., Hill J.R. Human vascular endothelial cells synthesize and release 24- and 26-carbon polyunsaturated fatty acids. Biochim. Biophys. Acta. 1984;795:171–178. doi: 10.1016/0005-2760(84)90063-8. [DOI] [PubMed] [Google Scholar]
- 163.Salywon A.M., Dierig D.A., Rebman J.P., de Rodríguez D.J. Evaluation of new Lesquerella and Physaria (Brassicaceae) oilseed germplasm. Am. J. Bot. 2005;92:53–62. doi: 10.3732/ajb.92.1.53. [DOI] [PubMed] [Google Scholar]
- 164.Smith C.R., Wilson T.L., Bates R.B., Scholfield C.R. Densipolic acid: A unique hydroxydienoid acid from Lesquerella densipila seed oil. J. Org. Chem. 1962;27:3112–3117. doi: 10.1021/jo01056a031. [DOI] [Google Scholar]
- 165.Engeseth N., Stymne S. Desaturation of oxygenated fatty acids in Lesquerella and other oil seeds. Planta. 1996;198:238–245. doi: 10.1007/BF00206249. [DOI] [Google Scholar]
- 166.Hayes D.G., Kleiman R., Phillips B.S. The triglyceride composition, structure, and presence of estolides in the oils of Lesquerella and related species. J. Am. Oil Chem. Soc. 1995;72:559–569. doi: 10.1007/BF02638857. [DOI] [Google Scholar]
- 167.Schuchardt J.P., Hahn A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids. 2013;89:1–8. doi: 10.1016/j.plefa.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 168.Maki K.C., Johns C., Harris W.S., Puder M., Freedman S.D., Thorsteinsson T., Daak A., Rabinowicz A.L., Sancilio F.D. Bioequivalence Demonstration for Ω-3 Acid Ethyl Ester Formulations: Rationale for Modification of Current Guidance. Clin. Ther. 2017;39:652–658. doi: 10.1016/j.clinthera.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 169.Köhler A., Sarkkinen E., Tapola N., Niskanen T., Bruheim I. Bioavailability of fatty acids from krill oil, krill meal and fish oil in healthy subjects—A randomized, single-dose, cross-over trial. Lipids Health Dis. 2015;14 doi: 10.1186/s12944-015-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cicero A.F.G., Colletti A. Krill oil: Evidence of a new source of polyunsaturated fatty acids with high bioavailability. Clin. Lipidol. 2015;10:1–4. doi: 10.2217/clp.14.67. [DOI] [Google Scholar]
- 171.Castro-Gómez M.P., Holgado F., Rodríguez-Alcalá L.M., Montero O., Fontecha J. Comprehensive Study of the Lipid Classes of Krill Oil by Fractionation and Identification of Triacylglycerols, Diacylglycerols, and Phospholipid Molecular Species by Using UPLC/QToF-MS. Food Anal. Methods. 2015;8:2568–2580. doi: 10.1007/s12161-015-0150-6. [DOI] [Google Scholar]
- 172.Davidson M.H., Johnson J., Rooney M.W., Kyle M.L., Kling D.F. A novel omega-3 free fatty acid formulation has dramatically improved bioavailability during a low-fat diet compared with omega-3-acid ethyl esters: The ECLIPSE (Epanova® compared to Lovaza® in a pharmacokinetic single-dose evaluation) study. J. Clin. Lipidol. 2012;6:573–584. doi: 10.1016/j.jacl.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 173.Dyerberg J., Madsen P., Møller J.M., Aardestrup I., Schmidt E.B. Bioavailability of marine n−3 fatty acid formulations. Prostaglandins Leukot. Essent. Fat. Acids. 2010;83:137–141. doi: 10.1016/j.plefa.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 174.Bandarra N.M., Lopes P.A., Martins S.V., Ferreira J., Alfaia C.M., Rolo E.A., Correia J.J., Pinto R.M., Ramos-Bueno R.P., Batista I., et al. Docosahexaenoic acid at the sn-2 position of structured triacylglycerols improved n-3 polyunsaturated fatty acid assimilation in tissues of hamsters. Nutr. Res. 2016;36:452–463. doi: 10.1016/j.nutres.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 175.Miura K., Hughes M.C., Ungerer J.P., Smith D.D., Green A.C. Absolute versus relative measures of plasma fatty acids and health outcomes: Example of phospholipid omega-3 and omega-6 fatty acids and all-cause mortality in women. Eur. J. Nutr. 2018;52:713–722. doi: 10.1007/s00394-016-1358-y. [DOI] [PubMed] [Google Scholar]
- 176.Fekete K., Marosvölgyi T., Jakobik V., Decsi T. Methods of assessment of n-3 long-chain polyunsaturated fatty acid status in humans: A systematic review. Am. J. Clin. Nutr. 2009;89:2070S–2084S. doi: 10.3945/ajcn.2009.27230I. [DOI] [PubMed] [Google Scholar]
- 177.Browning L.M., Walker C.G., Mander A.P., West A.L., Madden J., Gambell J.M., Young S., Wang L., Jebb S.A., Calder P.C. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am. J. Clin. Nutr. 2012;96:748–758. doi: 10.3945/ajcn.112.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Köhler A., Heinrich J., von Schacky C. Bioavailability of dietary omega-3 fatty acids added to a variety of sausages in healthy individuals. Nutrients. 2017;9:629. doi: 10.3390/nu9060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tero-Vescan A., Vancea S., Hutanu A., Borka-Balas R., Dobreanu M. Concordance and controversy in determining the omega-3 index in plasma and red blood cells membrane. Farmacia. 2015;63:504–509. [Google Scholar]
- 180.Gurzell E.A., Wiesinger J.A., Morkam C., Hemmrich S., Harris W.S., Fenton J.I. Is the omega-3 index a valid marker of intestinal membrane phospholipid EPA+DHA content? Prostaglandins Leukot. Essent. Fat. Acids. 2014;91:87–96. doi: 10.1016/j.plefa.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sanguansri L., Augustin M.A., Lockett T.J., Abeywardena M.Y., Royle P.J., Mano M.T., Patten G.S. Bioequivalence of n-3 fatty acids from microencapsulated fish oil formulations in human subjects. Br. J. Nutr. 2015;113:822–831. doi: 10.1017/S000711451400436X. [DOI] [PubMed] [Google Scholar]
- 182.Cook C.M., Hallaråker H., Sæbø P.C., Innis S.M., Kelley K.M., Sanoshy K.D., Berger A., Maki K.C. Bioavailability of long chain omega-3 polyunsaturated fatty acids from phospholipid-rich herring roe oil in men and women with mildly elevated triacylglycerols. Prostaglandins Leukot. Essent. Fat. Acids. 2016;111:17–24. doi: 10.1016/j.plefa.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 183.Offman E., Davidson M., Abu-Rashid M., Chai P., Nilsson C. Systemic bioavailability and dose proportionality of omega-3 administered in free fatty acid form compared with ethyl ester form: Results of a phase 1 study in healthy volunteers. Eur. J. Drug Metab. Pharmacokinet. 2017;42:815–825. doi: 10.1007/s13318-016-0398-2. [DOI] [PubMed] [Google Scholar]
- 184.Yurko-Mauro K., Kralovec J., Bailey-Hall E., Smeberg V., Stark J.G., Salem N., Jr. Similar eicosapentaenoic acid and docosahexaenoic acid plasma levels achieved with fish oil or krill oil in a randomized double-blind four-week bioavailability study. Lipids Health Dis. 2015;14:99. doi: 10.1186/s12944-015-0109-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wood K.E., Mantzioris E., Gibson R.A., Ramsden C.E., Muhlhausler B.S. The effect of modifying dietary LA and ALA intakes on omega-3 long chain polyunsaturated fatty acid (n-3 LCPUFA) status in human adults: A systematic review and commentary. Prostaglandins Leukot. Essent. Fat. Acids. 2015;95:47–55. doi: 10.1016/j.plefa.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nordøy A., Barstad L., Connor W.E., Hatcher L. Absorption of the n-3 eicosapentaenoic and docosahexaenoic acids as ethyl esters and triglycerides by humans. Am. J. Clin. Nutr. 1991;53:1185–1190. doi: 10.1093/ajcn/53.5.1185. [DOI] [PubMed] [Google Scholar]
- 187.Schuchardt J.P., Schneider I., Meyer H., Neubronner J., von Schacky C., Hahn A. Incorporation of EPA and DHA into plasma phospholipids in response to different omega-3 fatty acid formulations—A comparative bioavailability study of fish oil vs. krill oil. Lipids Health Dis. 2011;10:145. doi: 10.1186/1476-511X-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ramprasath V.R., Eyal I., Zchut S., Shafat I., Jones P.J. Supplementation of krill oil with high phospholipid content increases sum of EPA and DHA in erythrocytes compared with low phospholipid krill oil. Lipids Health Dis. 2015;14:142. doi: 10.1186/s12944-015-0142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ghasemifard S., Hermon K., Turchini G.M., Sinclair A.J. Metabolic fate (absorption, β-oxidation and deposition) of long-chain n-3 fatty acids is affected by sex and by the oil source (krill oil or fish oil) in the rat. Br. J. Nutr. 2015;114:684–692. doi: 10.1017/S0007114515002457. [DOI] [PubMed] [Google Scholar]
- 190.Freund Levi Y., Vedin I., Cederholm T., Basun H., Faxén Irving G., Eriksdotter M., Hjorth E., Schultzberg M., Vessby B., Wahlund L.O., et al. Transfer of omega-3 fatty acids across the blood-brain barrier after dietary supplementation with a docosahexaenoic acid-rich omega-3 fatty acid preparation in patients with Alzheimer’s disease: The OmegAD study. J. Intern. Med. 2014;275:428–436. doi: 10.1111/joim.12166. [DOI] [PubMed] [Google Scholar]
- 191.Hachem M., Géloën A., Van A.L., Foumaux B., Fenart L., Gosselet F., Da Silva P., Breton G., Lagarde M., Picq M., et al. Efficient docosahexaenoic acid uptake by the brain from a structured phospholipid. Mol. Neurobiol. 2016;53:3205–3215. doi: 10.1007/s12035-015-9228-9. [DOI] [PubMed] [Google Scholar]
- 192.Kalder P.C., Jensen G.L., Koletzko B.V., Singer P., Wanten G.J.A. Lipid emulsions in parenteral nutrition of intensive care patients: Current thinking and future directions. Intensive Care Med. 2010;36:735–749. doi: 10.1007/s00134-009-1744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vlaardingerbroek H., Vermeulen M.J., Carnielli V.P., Vaz F.M., van den Akker C.H., van Goudoever J.B. Growth and fatty acid profiles of VLBW infants receiving a multicomponent lipid emulsion from birth. J. Pediatr. Gastroenterol. Nutr. 2014;58:417–427. doi: 10.1097/MPG.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 194.Kyrana E., Dhawan A. Omega-3 fatty acid-rich parenteral nutrition: Is it a double-edged sword? J. Pediatr. Gastroenterol. Nutr. 2015;61:469–471. doi: 10.1097/MPG.0000000000000863. [DOI] [PubMed] [Google Scholar]
- 195.Al-Taan O., Stephenson J.A., Spencer L., Pollard C., West A.L., Calder P.C., Metcalfe M., Dennison A.R. Changes in plasma and erythrocyte omega-6 and omega-3 fatty acids in response to intravenous supply of omega-3 fatty acids in patients with hepatic colorectal metastases. Lipids Health Dis. 2013;12:64. doi: 10.1186/1476-511X-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Qin Y., Nyheim H., Haram E.M., Moritz J.M., Hustvedt S.O. A novel self-micro-emulsifying delivery system (SMEDS) formulation significantly improves the fasting absorption of EPA and DHA from a single dose of an omega-3 ethyl ester concentrate. Lipids Health Dis. 2017;16:204. doi: 10.1186/s12944-017-0589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.U.S. Department of Agriculture, Agricultural Research Service . What We Eat in America, NHANES 2013–2014. Food Surveys Research Group; Beltsville, MD, USA: 2016. [(accessed on 20 January 2018)]. Breakfast: Percentages of Selected Nutrients Contributed by Food and Beverages Consumed at Breakfast, by Gender and Age, in the United States, 2013–2014. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1314/Table_13_BRK_GEN_13.pdf. [Google Scholar]
- 198.Orishadipe A.T., Ibekwe N.N., Adesomoju A.A., Okogun J.I. Chemical composition and antimicrobial activity of the seed oil of Entandrophragma angolense (Welw) C.DC. Afr. J. Pure Appl. Chem. 2012;6:184–187. doi: 10.5897/AJPAC12.028. [DOI] [Google Scholar]
- 199.Deol P., Fahrmann J., Yang J., Evans J.R., Rizo A., Grapov D., Salemi M., Wanichthanarak K., Fiehn O., Phinney B., et al. Omega-6 and omega-3 oxylipins are implicated in soybean oil-induced obesity in mice. Sci. Rep. 2017;7:12488. doi: 10.1038/s41598-017-12624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cavazos-Garduño A., Flores A.A.O., Serrano-Niño J.C., Martínez-Sanchez C.E., Beristain C.I., García H.S. Preparation of betulinic acid nanoemulsions stabilized by x-3 enriched phosphatidylcholine. Ultrason. Sonochem. 2015;24:204–213. doi: 10.1016/j.ultsonch.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 201.Lopez-Toledano M.A., Thorsteinsson T., Daak A., Maki K.C., Johns C., Rabinowicz A.L., Sancilio F.D. A Novel ω-3 acid ethyl ester formulation incorporating Advanced Lipid Technologies™ (ALT®) improves docosahexaenoic acid and eicosapentaenoic acid bioavailability compared with Lovaza®. Clin. Ther. 2017;39:581–591. doi: 10.1016/j.clinthera.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 202.Gumus C.E., Decker E.A., McClements D.J. Formation and stability of ω-3 oil emulsion-based delivery systems using plant proteins as emulsifiers: Lentil, pea, and faba bean proteins. Food Biophys. 2017;12:186–197. doi: 10.1007/s11483-017-9475-6. [DOI] [Google Scholar]
- 203.Lane K.E., Li W., Smith C.J., Derbyshire E.J. The development of vegetarian omega-3 oil in water nanoemulsions suitable for integration into functional food products. J. Funct. Foods. 2016;23:306–314. doi: 10.1016/j.jff.2016.02.043. [DOI] [Google Scholar]
- 204.Walker R., Decker E.A., McClements D.J. Development of food-grade nanoemulsions and emulsions for delivery of omega-3 fatty acids: Opportunities and obstacles in the food industry. Food Funct. 2015;6:41–54. doi: 10.1039/C4FO00723A. [DOI] [PubMed] [Google Scholar]
- 205.Hinriksdottir H.H., Jonsdottir V.L., Sveinsdottir K., Martinsdottir E., Ramel A. Bioavailability of long-chain n-3 fatty acids from enriched meals and from microencapsulated powder. Eur. J. Clin. Nutr. 2015;69:344–348. doi: 10.1038/ejcn.2014.250. [DOI] [PubMed] [Google Scholar]
- 206.Ottestad I., Nordvi B., Vogt G., Holck M., Halvorsen B., Brønner K.W., Retterstøl K., Holven K.B., Nilsson A., Ulven S.M. Bioavailability of n-3 fatty acids from n-3-enriched foods and fish oil with different oxidative quality in healthy human subjects: A randomised single-meal cross-over study. J. Nutr. Sci. 2016;5:e43. doi: 10.1017/jns.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Garaiova I., Guschina I.A., Plummer S.F., Tang J., Wang D., Plummer N.T. A randomised cross-over trial in healthy adults indicating improved absorption of omega-3 fatty acids by pre-emulsification. Nutr. J. 2007;6:4. doi: 10.1186/1475-2891-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Raatz S.K., Redmon J.B., Wimmergren N., Donadio J.V., Bibus D.M. Enhanced absorption of n-3 fatty acids from emulsified compared with encapsulated fish oil. J. Am. Diet. Assoc. 2009;109:1076–1081. doi: 10.1016/j.jada.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Taha A.Y., Cheon Y., Faurot K.F., Macintosh B., Majchrzak-Hong S.F., Mann J.D., Hibbeln J.R., Ringel A., Ramsden C.E. Dietary omega-6 fatty acid lowering increases bioavailability of omega-3 polyunsaturated fatty acids in human plasma lipid pools. Prostaglandins Leukot. Essent. Fat. Acids. 2014;90:151–157. doi: 10.1016/j.plefa.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Arsic A., Prekajski N., Vucic V., Tepsic J., Popovic T., Vrvic M., Glibetic M. Milk in human nutrition: Comparison of fatty acid profiles. Acta Vet.-Beograd. 2009;59:569–578. doi: 10.2298/avb0906569a. [DOI] [Google Scholar]
- 211.Gerster H. Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int. J. Vitam. Nutr. Res. 1998;68:159–173. [PubMed] [Google Scholar]
- 212.Simopoulos A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002;56:365–379. doi: 10.1016/S0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 213.Russo G.L. Dietary n − 6 and n − 3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009;77:937–946. doi: 10.1016/j.bcp.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 214.Berger M.E., Smesny S., Kim S.-W., Davey C.G., Rice S., Sarnyai Z., Schlögelhofer M., Schäfer M.R., Berk M., McGorry P.D., et al. Omega-6 to omega-3 polyunsaturated fatty acid ratio and subsequent mood disorders in young people with at-risk mental states: A 7-year longitudinal study. Transl. Psychiatry. 2017;7:e1220. doi: 10.1038/tp.2017.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Simopoulos A.P. Evolutionary aspects of diet: The omega-6/omega-3 ratio and the brain. Mol. Neurobiol. 2011;44:203–215. doi: 10.1007/s12035-010-8162-0. [DOI] [PubMed] [Google Scholar]
- 216.Cordain L., Eaton S.B., Sebastian A., Mann N., Lindeberg S., Watkins B.A., O’Keefe J.H., Brand-Miller J. Origins and evolution of the western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 217.Yehuda S. Omega-6/omega-3 ratio and brain-related functions. In: Simopoulos A.P., Cleland L.G., editors. Omega-6/Omega-3 Essential Fatty Acid Ratio: The Scientific Evidence. Volume 92. Karger; Basel, Switzerland: 2003. pp. 37–56. [Google Scholar]
- 218.Wainwright P.E., Huang Y.S., Bulman-Fleming B., Dalby D., Mills D.E., Redden P.R., McCutcheon D. The effects of dietary n-3/n-6 ratio on brain development in the mouse: A dose response study with long-chain n-3 fatty acids. Lipids. 1999;27:98–103. doi: 10.1007/BF02535807. [DOI] [PubMed] [Google Scholar]
- 219.Bartram H.P., Gostner A., Scheppach W., Reddy B.S., Rao C.V., Dusel G., Richter F., Richter A., Kasper H. Effects of fish oil on rectal cell proliferation, mucosal fatty acids, and prostaglandin E2 release in healthy subjects. Gastroenterology. 1993;105:1317–1322. doi: 10.1016/0016-5085(93)90135-Y. [DOI] [PubMed] [Google Scholar]
- 220.Sheppard K.W., Cheatham C.L. Omega-6/omega-3 fatty acid intake of children and older adults in the U.S.: Dietary intake in comparison to current dietary recommendations and the Healthy Eating Index. Lipids Health Dis. 2018;17:43. doi: 10.1186/s12944-018-0693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Eratte D., Wang B., Dowling K., Barrow C.J., Adhikari B.P. Complex coacervation with whey protein isolate and gum arabic for the microencapsulation of omega-3 rich tuna oil. Food Funct. 2014;5:2743–2750. doi: 10.1039/C4FO00296B. [DOI] [PubMed] [Google Scholar]
- 222.Us-Medina U., Ruiz-Ruiz J.C., Quintana-Owen P., Segura-Campos M.R. Salvia hispanica mucilage-alginate properties and performance as an encapsulation matrix for chia seed oil. J. Food Process. Preserv. 2017;41:e13270. doi: 10.1111/jfpp.13270. [DOI] [Google Scholar]
- 223.Staprãns I., Rapp J.H., Pan X.M., Kim K.Y., Feingold K.R. Oxidized lipids in the diet are a source of oxidized lipid in chylomicrons of human serum. Arterioscler. Thromb. 1994;14:1900–1905. doi: 10.1161/01.ATV.14.12.1900. [DOI] [PubMed] [Google Scholar]
- 224.Naruszewicz M., Woźny E., Mirkiewicz E., Nowicka G., Szostak W.B. The effect of thermally oxidized soya bean oil on metabolism of chylomicrons. Increased uptake and degradation of oxidized chylomicrons in cultured mouse macrophages. Atherosclerosis. 1987;66:45–53. doi: 10.1016/0021-9150(87)90178-X. [DOI] [PubMed] [Google Scholar]


