Abstract
The clinical syndrome of heart failure (HF) can be described as the reduced capacity of the heart to deliver blood throughout the body. To compensate for inadequate tissue perfusion, the renin–angiotensin aldosterone system (RAAS) and the sympathetic nervous system (SNS) become activated, resulting in increased blood pressure, heart rate, and blood volume. Consequent activation of the natriuretic peptide system (NPS) typically balances these effects; however, the NPS is unable to sustain compensation for excessive neurohormonal activation over time. Until recently, mortality benefits have been provided to patients with HF only by therapies that target the RAAS and SNS, including angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), mineralocorticoid receptor antagonists, and beta-blockers. Sacubitril/valsartan, the first-in-class angiotensin receptor/neprilysin inhibitor (ARNI), targets both the NPS and RAAS to further improve clinical outcomes. This review discusses the focused management of patients with HF with reduced ejection fraction (HFrEF) and suggests changes to current management paradigms. From this assessment, the evidence supports favoring sacubitril/valsartan over ACEIs or ARBs, and this therapy should be used in conjunction with beta-blockers to further decrease morbidity and mortality in patients with HFrEF.
Key Points
| Therapies targeting the renin–angiotensin aldosterone system (RAAS) and the sympathetic nervous system reduce mortality risk for patients with heart failure with reduced ejection fraction. |
| Angiotensin receptor/neprilysin inhibitor (ARNI) therapy targets both the natriuretic peptide system and RAAS to further reduce the risk for mortality. |
| Guidelines recommend switching appropriate patients from angiotensin-converting enzyme inhibitors/angiotensin receptor blockers treatment to ARNI therapy. |
Introduction
With the advancements of medical therapy in treating infections, hypertension, diabetes mellitus, cancers, and acute myocardial infarction, life expectancy has been steadily increasing [1]. However, along with this increase in longevity comes an associated increased risk for an individual to develop other chronic conditions, particularly chronic heart failure (HF) [2]. In 2012, HF affected more than 5 million Americans; by 2030, approximately 8.5 million adults may have HF in the United States [3, 4]. Thus, physicians can expect to see an increased number of patients who have or are at risk for chronic HF.
HF is a complex clinical syndrome that is traditionally considered to be caused by the heart not pumping effectively, thereby reducing its capacity to deliver blood throughout the body to achieve appropriate levels of tissue perfusion and resulting in dyspnea, edema, and fatigue [5, 6]. Cardiac dysfunction caused by HF can be systolic, as a result of depressed myocardial contractility, or diastolic, as a result of reduced ventricular relaxation and filling. Both types of dysfunction can contribute to the development of HF and can coexist in many patients [5, 7]. However, for disease management purposes, HF that is predominantly systolic is classified as HF with reduced ejection fraction (HFrEF) [defined as left ventricular ejection fraction (LVEF) ≤ 40%], and predominantly diastolic HF is classified as HF with preserved ejection fraction (HFpEF) (defined as LVEF ≥ 50%). Patients with LVEF between 41 and 49% are classified as having borderline HFpEF. Guideline-directed medical therapy (GDMT) differs based on this classification [5]. In this review, the recommended management of outpatients with New York Heart Association (NYHA) class II–IV HFrEF and inpatients with HFrEF recovering from an acute episode will be discussed and graded using the Strength of Recommendation Taxonomy (SORT) [8], with a focus on recent treatment advances that may alter current management paradigms.
Compensatory Mechanisms in Heart Failure
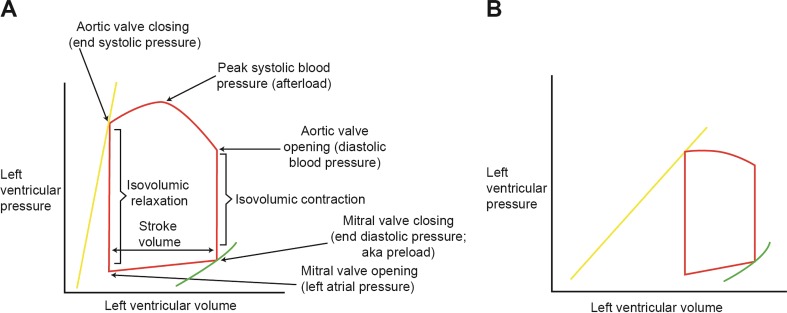
The key biomechanical principle governing the function of the heart is the relationship between the degree of pressure present in the heart, which is related to myocardial contractility, and the volume of blood circulated throughout the body (Fig. 1) [7].
Fig. 1.
Pressure–volume graphs illustrating the key biomechanical principle governing the function of the heart. In these illustrations, the green curves represent the end diastolic pressure–volume relationship, which determines diastolic function. The yellow lines represent the end systolic pressure–volume relationship, which determines the degree of systolic function. The slopes of the yellow lines are known as the end systolic slopes and the angle of the slopes indicates the contractile function of the heart. a Pressure–volume loop representing a normal heart. b Pressure–volume loop in a heart with systolic dysfunction. In a heart with systolic dysfunction, the slope of the yellow line is less steep, indicating depressed left ventricular (LV) contractility. The impaired contractility shifts the whole loop to the right because incomplete LV emptying leads to a higher remaining volume at end systole. Altogether, the stroke volume is diminished (represented by a shorter and narrower loop with reduced total area) as a result of systolic dysfunction
With the diminished stroke volume in systolic dysfunction, there is reduced blood circulation and poor tissue perfusion [7]. The body responds to inadequate perfusion by activating various compensatory neurohormonal mechanisms that are identical to those activated in response to hemorrhage [7]. Blood pressure, heart rate, and blood volume are increased through activation of the renin–angiotensin aldosterone system (RAAS) and the sympathetic nervous system (SNS) [9, 10]. In the context of normal cardiac physiology, the RAAS and SNS are balanced by the natriuretic peptide system (NPS) to maintain homeostasis [7]. The volume overload associated with excessive neurohormonal activation, which also triggers upregulation of the antidiuretic hormone vasopressin, results in myocardial stretch. Subsequently, this stretching triggers the release of natriuretic peptides to induce vasodilation, natriuresis, and diuresis [7, 10]. As HF progresses, the effects of the NPS become blunted through several proposed mechanisms, including reduced production of the biologically active forms of atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP), increased degradation of natriuretic peptides by neprilysin, increased receptor-mediated clearance of natriuretic peptides in circulation, and desensitization or reduced expression of natriuretic peptide receptors in target organs [11]. As a result, the NPS is further stimulated, but eventually, the NPS becomes unable to compensate for the overactivation of the RAAS and SNS [11]. The increased blood volume and pressure that results from neurohormonal activation forces fluid from blood vessels, resulting in congestive symptoms [7]. In addition, the chronic hemodynamic stress from neurohormonal activation further strains the failing heart, stimulating cardiac remodeling [7]. Cardiac remodeling initially increases ventricular contractility and volume, but eventually the increased wall thickness and resultant fibrosis from prolonged neurohormonal activation become detrimental, further limiting cardiac function [7]. Altogether, neurohormonal activation and subsequent cardiac remodeling propagates a vicious cycle that results in a cardiac crisis [7].
Treating Chronic Heart Failure with Reduced Ejection Fraction
Advances in treatment have led to improvements in survival for many patients with HF [5, 12]. Until recently, reductions in mortality have been provided only by therapies that target the RAAS and SNS, initially demonstrated with the angiotensin-converting enzyme inhibitor (ACEI) enalapril [10, 13]. The pivotal trials demonstrating the benefits with beta-blockers, mineralocorticoid receptor antagonists (MRAs), and angiotensin receptor blockers (ARBs) soon followed [5, 14–16]. In addition to reducing the risk of mortality, these therapies offer other benefits for patients with HFrEF, including antihypertensive effects [5, 17] and reduced ventricular remodeling [18–20]. Despite the availability of effective treatments, the average 5-year mortality for patients with HF remains at 24.4% for those who are 60 years old and 54.4% for patients aged 80 years [4], indicating the need for additional options. Research has demonstrated that targeting only the increased activation of the RAAS and SNS eventually leads to upregulation of other compensatory escape pathways [9, 10]. Increased understanding of some of the endogenous escape pathways and mechanisms that the body uses to try to compensate for increased activation of the RAAS and SNS has yielded additional targets for therapeutic intervention. For example, preservation of endogenous peptides such as natriuretic peptides, bradykinin, and adrenomedullin could be beneficial by counterbalancing neurohormonal activity [21].
Particular attention has been given to the NPS as a therapeutic target [21]. Binding of ANP and BNP to natriuretic peptide receptor A stimulates an intracellular signaling pathway that leads to activation of protein kinase G, resulting in vasorelaxation, natriuresis, and diuresis. Other effects of ANP and BNP signaling include RAAS inhibition, enhancement of myocardial relaxation, reduced cardiovascular remodeling, apoptosis, hypertrophy, and fibrosis [21]. A third natriuretic peptide released primarily from endothelial cells (and not found in high levels in the blood) is C-type natriuretic peptide (CNP), which acts as a vasodilator [21]. These peptides are cleared through natriuretic-peptide-receptor-mediated clearance and enzymatic degradation by neprilysin [21]. Neprilysin also degrades the vasodilator peptides, adrenomedullin and bradykinin, as well as the vasoconstrictors, angiotensin I, angiotensin II, and endothelin-1 [21]. These actions to degrade regulators of opposing mechanisms of vasodilation and vasoconstriction essentially neutralize each other, and as a result, monotherapy with a neprilysin inhibitor has little effect on HF. Additionally, the increase in RAAS activation stimulated by neprilysin inhibitor-mediated upregulation of angiotensin II could actually worsen HF, and the breakdown of bradykinin could increase risk of angioedema.
Development of Sacubitril/Valsartan
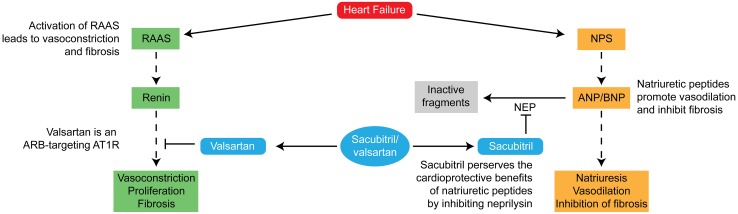
Recognition of the need for a therapeutic agent that could target both the RAAS and the NPS without increasing risk for angioedema led researchers to test the combination of a neprilysin inhibitor with an ARB. The first such combination angiotensin receptor/neprilysin inhibitor (ARNI) to be developed is sacubitril/valsartan (Fig. 2) [21].
Fig. 2.
Mechanism of action of sacubitril/valsartan [21]. The inhibition of neprilysin works synergistically with the inhibition of angiotensin receptors by valsartan. ANP/BNP atrial natriuretic peptide/brain natriuretic peptide, ARB angiotensin receptor blocker, AT1R angiotensin II type 1 receptor, NEP neprilysin, NPS natriuretic peptide system, RAAS renin–angiotensin aldosterone system.
Adapted with permission from J Am Coll Cardiol 2015;65(10):1029–41. Copyright 2015: American College of Cardiology Foundation
A pharmacodynamic study in patients with HFrEF showed that, 21 days after receiving sacubitril/valsartan, plasma concentrations of the negative heart function modulators aldosterone and endothelin-1 were significantly decreased [22]. In addition, concentrations of N-terminal pro B-type natriuretic peptide (NT-proBNP) (the byproduct of cleavage of pre-proBNP) were significantly decreased at all time points assessed (7 and 21 days after sacubitril/valsartan treatment; all p < 0.05). These results suggest that the ARNI inhibits both the RAAS and neprilysin.
Efficacy of HF Treatments
In 2015, sacubitril/valsartan was approved in Europe for the treatment of adults with chronic HFrEF and in the United States to reduce the risk of cardiovascular death and hospitalization for HF in patients with chronic HFrEF (NYHA class II–IV) [23, 24]. These approvals occurred following the results of the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial (NCT01035255), which compared sacubitril/valsartan with enalapril, an ACEI recognized as a standard-of-care medication for HFrEF [23–27]. The trial enrolled 8442 patients with stable HFrEF (EF ≤ 40%) who were on GDMT, which included ACEIs, ARBs, beta-blockers, and/or MRAs [26]. Patients received either enalapril 10 mg twice daily or sacubitril/valsartan 97/103 mg twice daily (randomly assigned in a 1:1 ratio) in addition to concomitant GDMT.
Compared with enalapril, sacubitril/valsartan showed a 20% reduction [hazard ratio (HR) 0.80; 95% confidence interval (CI) 0.73–0.87; p < 0.001] in the primary endpoint of composite cardiovascular death or first hospitalization for HF [26]. Both drugs were reasonably well tolerated, although significantly fewer patients in the sacubitril/valsartan group versus the enalapril group had to discontinue treatment because of an adverse event (10.7 vs. 12.3%; p = 0.03) or renal impairment (0.7 vs. 1.4%; p = 0.002) [26].
The potential of ARNI therapy to improve outcomes may support recommendations for its broad adoption into clinical practice. Using the number needed to treat calculated for HF therapies in previous clinical trials, it has been estimated that if ARNI therapy was initiated in all eligible patients in the United States, 28,484 deaths per year could be prevented or postponed [28]. The 2016 American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Failure Society of America (HFSA) focused update of the 2013 American College of Cardiology Foundation (ACCF)/AHA guideline for the management of HF included the new findings from PARADIGM-HF, adding the ARNIs as a treatment option in the class I recommendation for RAAS inhibition for patients with HFrEF, and also recommended switching from ACEI/ARB to ARNI therapy for eligible patients to further reduce morbidity and mortality [29]. The guidelines also recommend that only one ACEI/ARB/ARNI agent should be used in the treatment of HF [5, 29, 30], because combined RAAS inhibition causes excessive bradykinin accumulation, which increases the risk for angioedema [21].
Now that sacubitril/valsartan has been on the market for several years, real-world data related to patient characteristics and clinical outcomes are emerging. Retrospective analyses of medical records have shown that patients receiving sacubitril/valsartan in real-world settings typically had more severe disease than patients who were included in PARADIGM-HF [31]. Beneficial outcomes in the real world are similar to those observed in PARADIGM-HF, including improvements in NYHA functional class, decreases in NT-proBNP, reduced requirements for diuretic therapy, and improvements in exercise tolerance; sacubitril/valsartan is generally safe and well tolerated in patients with chronic symptomatic HFrEF [32–35]. In the future, data from HF registries may be particularly useful sources of information for more acute gauging of the real-world effectiveness and safety of sacubitril/valsartan. Specifically, the PAtient RegisTry Assessing Effectiveness and Safety of HEart Failure treatmeNt with LCZ696 acrOss CaNada (PARTHENON) (NCT02957409) will provide observational, naturalistic data on rates of all-cause hospitalization and all-cause mortality in relation to NT-proBNP levels in approximately 1000 patients with HFrEF who initiate treatment with sacubitril/valsartan. Additionally, results from the ongoing Prospective Comparison of ARNI with ARB Global Outcomes in HF with Preserved Ejection Fraction (PARAGON-HF) trial (NCT01920711) may become available in the next year. PARAGON-HF is a large-scale, randomized, controlled, event-driven study comparing morbidity and mortality outcomes in patients with HFpEF receiving treatment with sacubitril/valsartan compared with those receiving valsartan treatment alone [36]. If the results of PARAGON-HF show meaningful benefits with sacubitril/valsartan, HFpEF treatment would have a significant advancement.
Sacubitril/Valsartan Use in Clinical Practice
Effective change in clinical practice typically requires thorough education of the clinicians who manage patients with HF. This shift entails clinicians being comfortable with not only identifying which patients are candidates for treatment with ARNIs, but also understanding the proper use of these medications.
The approved target dose of sacubitril/valsartan is 97/103 mg twice per day. Treatment should be initiated at a starting dose of sacubitril/valsartan 49/51 mg twice per day for those patients already on an ACEI or ARB at ≥ 50% of the target dose. Sacubitril/valsartan uptitration should be performed after 2–4 weeks to the target dose of 97/103 mg, as tolerated by the patient [23]. To initiate therapy with sacubitril/valsartan in patients who are not currently on an ACEI or ARB or who were previously taking low doses of these agents, the US prescribing information recommends starting at a lower dose of 24/26 mg twice daily and uptitrating every 2–4 weeks to the target dose [23]. A starting dose of 24/26 mg twice daily is also recommended for patients with severe renal impairment (i.e., estimated glomerular filtration rate < 30 mL/min/1.73 m2) or moderate hepatic impairment (Child–Pugh class score B), with uptitration every 2–4 weeks, as tolerated [23].
Sacubitril/valsartan is contraindicated in patients with concurrent ACEI use and in patients with a history of angioedema [23]. The risk of angioedema is comparable with ACEIs and with sacubitril, and as such, combining these agents poses a safety risk [37]. Consequently, for those patients who are already on ACEI therapy, a 36-h washout period is required to decrease the risk for angioedema [23]. In patients being treated with an ARB, no washout period is necessary because the risk of angioedema is typically low with these agents [37], and the regular dose initiation and uptitration schedule can be followed [23].
The same precautions/contraindications that are associated with ACEIs and ARBs apply to ARNIs; however, ARNIs decrease intravascular volume, which may result in an increased risk of symptomatic hypotension [21, 26]. When using any medication that can lower blood pressure, patients’ cardiac performance should be monitored based on mean arterial pressure [(2 × diastolic + systolic blood pressure)/3]. To maintain adequate total body perfusion, mean arterial pressure should be maintained at ≥ 65 mmHg; if it falls below this threshold, uptitration of sacubitril/valsartan should be stopped. If symptomatic hypotension occurs in a patient who is on a combination therapy of sacubitril/valsartan and a diuretic, the diuretic dose should be decreased prior to decreasing the sacubitril/valsartan dosage or discontinuing therapy [23]. If necessary, the diuretic dose should either be decreased by 50% or discontinued completely, depending on the current dose of diuretic and the individual patient’s known response to diuretics (personal observation). Close follow-up should be provided to ensure that the clinical responses to such changes are not detrimental. A phone call to follow-up should be performed within 24 hours, and an office visit should be conducted within one week. In addition, treatment with sacubitril/valsartan results in an increase in BNP because the degradation of this protein is inhibited [23]. Thus, caution should be taken when interpreting increased BNP concentration with ARNI therapy [30]. Alternatively, NT-proBNP levels can be monitored because it is not a substrate for neprilysin and, therefore, will more accurately reflect HF clinical status [38]. In the following sections, we describe two clinical settings—outpatient and inpatient (post-acute episode)—and discuss how ARNI use may be applicable.
Prescribing Sacubitril/Valsartan for Outpatients
In the PARADIGM-HF study, patients enrolled exhibited medical histories that may have been related to their disease, including prior hospitalization for HF (63%), myocardial infarction (43%), and/or atrial fibrillation/flutter (37%), in addition to risk factors for HF such as hypertension (71%) and diabetes mellitus (35%) [39]. Baseline LVEF varied widely in the study, from 5 to 42% [40]. Thus, the trial assessed the use of ARNI therapy on a diverse outpatient HFrEF population, with the primary goal of preventing occurrences of acute episodes and decreasing mortality. Notably, a post hoc analysis showed that baseline LVEF did not significantly impact clinical outcomes [40]. However, prescribers should review a number of factors when considering optimal outpatient GDMT for chronic symptomatic HFrEF. The first is to ensure inhibition of the SNS and the RAAS with combined beta-blocker, ACEI/ARB/ARNI, and MRA therapy [strength of recommendation (SOR) A; level of evidence (LOE) 1] [5, 29, 30, 41].
After patients have demonstrated tolerability to concurrent therapy with a RAAS antagonist and beta-blocker at low doses, each agent prescribed should be uptitrated to the target or highest tolerated dose (SOR A; LOE 1), with beta-blockers uptitrated until the target heart rate is achieved [42–44]. In a post hoc analysis of data from PARADIGM-HF, patients treated with sacubitril/valsartan experienced lower risk for mortality compared with enalapril, both at target doses (HR 0.79; 95% CI 0.71–0.88) and at suboptimal doses between 50 and 100% of the target dose (HR 0.79; 95% CI 0.67–0.92); however, patients treated at target doses experienced greater reductions for the risk for mortality, irrespective of therapy [44]. Uptitration should be performed in small increments, dictated by orthostatic vital signs and physical examination findings (SOR C; LOE 3) [5]. For example, if the resting heart rate is not considered to be at goal (i.e., < 70 beats per minute), then the beta-blocker should be uptitrated first [5].
Once the goal resting heart rate is achieved, then RAAS antagonist therapy should be uptitrated (SOR C; LOE 3). In contrast, if beta-blocker uptitration to achieve the target heart rate results in symptomatic hypotension, thereby limiting the initiation or uptitration of a RAAS antagonist, then it is reasonable to switch from a nonselective beta-blocker such as carvedilol, which may increase risk of vasodilation as a result of alpha-1 blockade, to a beta-1-selective beta-blocker (e.g., metoprolol succinate or bisoprolol) [45]. Blood pressure should be reduced to the lowest tolerable level for each patient (SOR C; LOE 3), which may limit the maximum dosage of therapy [5, 30]. To guide uptitration, clinicians may consider assessing mean arterial pressure—which contributes to perfusion pressure and systemic vascular resistance—rather than systolic blood pressure alone [46]. As a point of clarification, when substitution for or initiation of an ARNI is being considered, patients do not need to be on the maximal recommended dose of GDMT, but rather on the highest doses of GDMT that they are able to tolerate. These doses can be far below the recommended doses in the US prescribing information [23]. One can consider an ARNI to be a “super ACEI” or “super ARB” and address its use accordingly, as one would use an ACEI or ARB.
Patients with chronic symptomatic NYHA class II or III HFrEF already receiving an ACEI or ARB should also be switched to an ARNI (SOR A; LOE 1), in line with the 2016 and subsequent 2017 ACC/AHA/HFSA focused guideline updates [26, 29, 30]. Patients with NYHA class IV HFrEF were underrepresented in the PARADIGM-HF trial [60 (< 1%) of 8442 subjects were enrolled with NYHA class IV HFrEF] [26]. In European market authorizations, this lack of clinical experience is cited as a reason to exercise caution when using sacubitril/valsartan in patients with NYHA class IV HFrEF [24]. However, in the United States, sacubitril/valsartan is indicated for the treatment of patients with chronic NYHA class II–IV HFrEF [23], and results suggest that treatment with sacubitril/valsartan, compared with enalapril, may reduce the risk of cardiovascular death in this patient population [26]. Thus, it is reasonable for clinicians to consider initiating sacubitril/valsartan therapy in patients with NYHA class IV HFrEF. One additional important consideration is the potential for drug interactions, mainly those that involve the additive pharmacodynamic effects of hypotension and hyperkalemia between guideline-recommended therapies that suppress angiotensin stimulation [5, 30]. Because RAAS inhibitors are known to inhibit renal potassium excretion, patients with HFrEF treated with ACEI/ARB/ARNI are at increased risk for hyperkalemia, especially if they have comorbid kidney disease. If hyperkalemia develops in such patients, outpatient therapy with the potassium binder patiromer is a reasonable treatment option to reduce serum potassium levels [47].
Consideration should also be given to improving the overall cardiac health of the patient. Through proper and complete management of a patient’s hypertension and HF, negative long-term effects associated with HFrEF (including cardiac remodeling) may be avoided and contractility may be improved. Although it has not yet been established whether sacubitril/valsartan can prevent or reverse cardiac remodeling, the Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodeling During Sacubitril/Valsartan Therapy for Heart Failure (PROVE-HF) (NCT02887183) is underway to determine whether the benefits of this drug extend from improved clinical outcomes and into measurable changes in cardiac health [48]. Additional potential benefits include the cessation or reversal of damage to other organs caused by hypoperfusion.
Prescribing Sacubitril/Valsartan for Inpatients After an Acute Episode
Acute episodes of worsening HF may be attributed to either a lack of treatment in patients previously undiagnosed with HF or inadequate treatment of HF in patients with a confirmed diagnosis. For patients who are not responding as expected to GDMT, consultation with an HF cardiologist is advisable. Inadequate responses could include no significant diuresis as defined by a urine output of < 100 mL/h while on intravenous loop diuretics, worsening renal function with diuretic therapy, hypotension with use of GDMT at low doses, or persistent signs and symptoms of HF (e.g., dyspnea/edema/tachycardia) despite appropriate GDMT.
Following resolution of an acute episode of HF, a patient must receive appropriate medications as part of the discharge planning (SOR B; LOE 2) [49, 50], which should include assessment of the risk for future episodes, and evaluation of a patient’s ability to adhere to therapy (SOR C; LOE 3) [5]. It may be prudent to initiate therapies prior to discharge to ensure tolerability, particularly with delayed follow-up care (SOR B; LOE 2) [51, 52]. The Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) trial (NCT00344513) observed that initiation of carvedilol at discharge in patients hospitalized for acute HF (AHF) was associated with a reduced risk for mortality at 60–90 days (HR 0.46; p = 0.0006) and mortality or rehospitalization [odds ratio (OR) 0.71; p = 0.0175] compared with patients who were not initiated on beta-blocker therapy prior to discharge [51]. In an analysis of 16,052 patients hospitalized with HF from the Get With The Guidelines®-Heart Failure registry, patients who discontinued or did not start ACEI/ARB therapy at the time of hospital discharge had significantly higher 1-year mortality (all p ≤ 0.001) and higher 30-day hospital readmission rates (all p < 0.05) compared with patients who continued or initiated treatment [52]. Furthermore, medication reconciliation and counseling at discharge improves transitions of care [5, 53].
Multidisciplinary HF clinic-based interventions should be performed to improve patient outcomes (SOR A; LOE 1) [54]. It is useful for patients to receive financial and social evaluation and counseling prior to discharge; having a lower socioeconomic status has been associated with increased risk for poor clinical outcomes and adherence in patients with HF [55, 56]. In addition, referrals to healthcare professionals for outpatient care should be planned. HF specialist or cardiologist referral may improve adherence to GDMT and ensure the timely initiation of invasive cardiac therapies when indicated [57]. Similarly, referrals to dietitians and pharmacists for further education after discharge may improve adherence to interventions [58, 59]. Moreover, multidisciplinary care improves outcomes in patients with HF, and hospitalization presents an opportunity to coordinate care [6, 57].
While pending discharge following stabilization of AHF, careful thought must be given to the chronic HF treatments prescribed, as this regimen is the first line of defense against rehospitalization. Although the PARADIGM-HF study population did not include inpatients, the occurrence of hospitalizations during the study allowed for some insight into the use of sacubitril/valsartan in this population. In particular, it is worth noting that patients receiving sacubitril/valsartan experienced a reduced risk for HF readmission within 30 days (OR 0.62; 95% CI 0.45–0.87; p = 0.006) and 60 days (OR 0.68; 95% CI 0.50–0.92; p = 0.01) compared with those patients receiving enalapril [60]. In addition, an analysis of Medicare data observed that patients with a discharge order for ACEI therapy had higher prescription-fill rates within 30 days of discharge (HR 10.93; 95% CI 5.28–22.61) [61]; thus, discharge orders for ARNI therapy may similarly improve adherence [62]. Also, cost, insurance coverage and/or requirement for preauthorization, and tolerability for the medication are adherence factors. However, in real-world practice, these issues can usually be managed with relative ease. In many cases, inpatient hospital management teams can provide patients with a manufacturer’s voucher for a free 30-day prescription trial of ARNI therapy prior to discharge. During this first month of therapy, the team schedules a follow-up visit at an outpatient HF clinic, where tolerance of the ARNI is evaluated, with particular attention paid to weight gain, hypotension (defined as mean arterial pressure < 65 mmHg), orthostatic symptoms, and signs of decompensated HF. If tolerance to therapy is demonstrated, a prescription for continued therapy will be issued. Once the prescription is received by the patient’s pharmacy, a prior authorization form is usually generated automatically. Thus, prior authorization is in place after tolerability is confirmed and before the patient needs to get the first prescription refill at the pharmacy. Although the cost of sacubitril/valsartan is high, data from PARADIGM-HF indicate that benefits for patients can be tremendous, and the financial costs must be weighed relative to benefits. The relative risk reduction of death with sacubitril/valsartan is comparable to that with an implantable cardioverter defibrillator, which can cost tens of thousands of dollars to implant and replace [63]. In this author’s opinion, physicians have a responsibility to offer ARNI therapy to those patients who have clear indications for its use, because data from PARADIGM-HF were so compelling.
The guidelines do not specify that ARNI therapy should not be used in an inpatient setting [29, 30]; therefore, the initiation of sacubitril/valsartan prior to discharge for stabilized patients with acute HF exacerbation may be considered. Two trials will evaluate the safety and efficacy of sacubitril/valsartan initiated prior to hospital discharge after stabilization for AHF: Comparison of Sacubitril/Valsartan Versus Enalapril on Effect on NT-proBNP in Patients Stabilized From an AHF Episode (PIONEER-HF) (NCT02554890) and Comparison of Pre- and Post-Discharge Initiation of LCZ696 Therapy in HFrEF Patients After an Acute Decompensation Event (TRANSITION) (NCT02661217) [62].
After discharge, it is important to follow up with patients to assess adherence, monitor clinical status, and optimize GDMT (SOR A; LOE 1) [5, 64]. It is reasonable to schedule a follow-up visit within 7–14 days post-discharge and an early telephone call within 3 days of hospital discharge to improve transitions of care [5].
Conclusions
To adequately reduce the mortality and morbidity associated with chronic HF, it is important to treat both mechanisms of disease associated with it, i.e., the increased activation of the RAAS and the adrenergic system. Sacubitril/valsartan is designed to target both systems, and clinical data support its use for reducing mortality in patients with HFrEF.
In the largest trial to date for HFrEF patients (PARADIGM-HF), sacubitril/valsartan was shown to be superior over enalapril in improving both mortality and morbidity [26], which led to the current guideline recommendation to initiate ARNI therapy in all appropriate patients with RAAS-antagonist–naïve HFrEF and also to substitute an ARNI for an ACEI/ARB in patients with NYHA class II–III HFrEF [30]. For appropriate patients with HFrEF, sacubitril/valsartan therapy to target both the RAAS and NPS should, therefore, be the drug of choice over ACEIs and ARBs—in combination with beta-blocker therapy for the SNS—to decrease morbidity and mortality.
Acknowledgements
Medical writing assistance was provided by Marcel Kuttab, PharmD, of Oxford PharmaGenesis Inc., which was funded by Novartis Pharmaceuticals Corporation.
Funding
Medical writing assistance for this manuscript was provided by Oxford PharmaGenesis Inc., Newtown, Pennsylvania, USA, which was funded by Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA. The author was fully responsible for all content and editorial decisions and received no financial support or other form of compensation related to the development of this manuscript.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Conflict of interest
Dr. Liu has received a consultant fee from Novartis.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
References
- 1.Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65:1–122. [PubMed] [Google Scholar]
- 2.Huffman MD, Berry JD, Ning H, Dyer AR, Garside DB, Cai X, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol. 2013;61:1510–1517. doi: 10.1016/j.jacc.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e319. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 6.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 7.Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012;21:365–371. doi: 10.1016/j.carpath.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Ebell MH, Siwek J, Weiss BD, Woolf SH, Susman J, Ewigman B, et al. Strength of Recommendation Taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. J Am Board Fam Pract. 2004;17:59–67. doi: 10.3122/jabfm.17.1.59. [DOI] [PubMed] [Google Scholar]
- 9.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;113:739–753. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reed BN, Street SE, Jensen BC. Time and technology will tell: the pathophysiologic basis of neurohormonal modulation in heart failure. Heart Fail Clin. 2014;10:543–557. doi: 10.1016/j.hfc.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Díez J. Chronic heart failure as a state of reduced effectiveness of the natriuretic peptide system: implications for therapy. Eur J Heart Fail. 2017;19:167–176. doi: 10.1002/ejhf.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loh JC, Creaser J, Rourke DA, Livingston N, Harrison TK, Vandenbogaart E, et al. Temporal trends in treatment and outcomes for advanced heart failure with reduced ejection fraction from 1993–2010: findings from a university referral center. Circ Heart Fail. 2013;6:411–419. doi: 10.1161/CIRCHEARTFAILURE.112.000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The CONSENSUS Trial Study Group Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 14.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 15.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 16.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 17.Baker DW. Prevention of heart failure. J Card Fail. 2002;8:333–346. doi: 10.1054/jcaf.2002.0805333. [DOI] [PubMed] [Google Scholar]
- 18.Konstam MA, Rousseau MF, Kronenberg MW, Udelson JE, Melin J, Stewart D, et al. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dysfunction in patients with heart failure. SOLVD Investigators. Circulation. 1992;86:431–438. doi: 10.1161/01.CIR.86.2.431. [DOI] [PubMed] [Google Scholar]
- 19.Devereux RB, Dahlöf B, Gerdts E, Boman K, Nieminen MS, Papademetriou V, et al. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation. 2004;110:1456–1462. doi: 10.1161/01.CIR.0000141573.44737.5A. [DOI] [PubMed] [Google Scholar]
- 20.Wong M, Staszewsky L, Latini R, Barlera S, Volpi A, Chiang YT, et al. Valsartan benefits left ventricular structure and function in heart failure: Val-HeFT echocardiographic study. J Am Coll Cardiol. 2002;40:970–975. doi: 10.1016/S0735-1097(02)02063-6. [DOI] [PubMed] [Google Scholar]
- 21.Braunwald E. The path to an angiotensin receptor antagonist-neprilysin inhibitor in the treatment of heart failure. J Am Coll Cardiol. 2015;65:1029–1041. doi: 10.1016/j.jacc.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 22.Kobalava Z, Kotovskaya Y, Averkov O, Pavlikova E, Moiseev V, Albrecht D, et al. Pharmacodynamic and pharmacokinetic profiles of sacubitril/valsartan (LCZ696) in patients with heart failure and reduced ejection fraction. Cardiovasc Ther. 2016;34:191–198. doi: 10.1111/1755-5922.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Entresto (sacubitril and valsartan) [prescribing information]. East Hanover: Novartis Pharmaceuticals Corporation; 2017.
- 24.Entresto [summary of product characteristics]. Nuremberg: Novartis Pharma GmbH; 2015.
- 25.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF) Eur J Heart Fail. 2013;15:1062–1073. doi: 10.1093/eurjhf/hft052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 27.McMurray JJ. Neprilysin inhibition to treat heart failure: a tale of science, serendipity, and second chances. Eur J Heart Fail. 2015;17:242–247. doi: 10.1002/ejhf.250. [DOI] [PubMed] [Google Scholar]
- 28.Fonarow GC, Hernandez AF, Solomon SD, Yancy CW. Potential mortality reduction with optimal implementation of angiotensin receptor neprilysin inhibitor therapy in heart failure. JAMA Cardiol. 2016;1:714–717. doi: 10.1001/jamacardio.2016.1724. [DOI] [PubMed] [Google Scholar]
- 29.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68:1476–1488. doi: 10.1016/j.jacc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 31.Martens P, Beliën H, Dupont M, Mullens W. Insights into implementation of sacubitril/valsartan into clinical practice. ESC Heart Fail. 2018 doi: 10.1002/ehf2.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wachter R, Viriato D, Klebs S, Grunow SS, Schindler M, Engelhard J, et al. Early insights into the characteristics and evolution of clinical parameters in a cohort of patients prescribed sacubitril/valsartan in Germany. Postgrad Med. 2018;130:308–316. doi: 10.1080/00325481.2018.1442090. [DOI] [PubMed] [Google Scholar]
- 33.Pogge EK, Davis LE. Evaluating the safety and tolerability of sacubitril/valsartan for HFrEF managed within a pharmacist clinic. Am J Cardiovasc Drugs. 2018;18:143–151. doi: 10.1007/s40256-018-0264-5. [DOI] [PubMed] [Google Scholar]
- 34.Hormann SM, Davis LE, Pogge EK. The diuretic potential of sacubitril/valsartan: a tale of 2 patients. J Cardiovasc Nurs. 2018;33:104–110. doi: 10.1097/JCN.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 35.Kałużna-Oleksy M, Kolasa J, Migaj J, Pawlak A, Lelonek M, Nessler J, et al. Initial clinical experience with the first drug (sacubitril/valsartan) in a new class - angiotensin receptor neprilysin inhibitors in patients with heart failure with reduced left ventricular ejection fraction in Poland. Kardiol Pol. 2018;76:381–387. doi: 10.5603/KP.a2017.0230. [DOI] [PubMed] [Google Scholar]
- 36.Solomon SD, Rizkala AR, Gong J, Wang W, Anand IS, Ge J, et al. Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON-HF trial. JACC Heart Fail. 2017;5:471–482. doi: 10.1016/j.jchf.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Owens RE, Oliphant CS. Angioedema spotlight: a closer examination of sacubitril/valsartan safety results. J Am Board Fam Med. 2017;30:556–557. doi: 10.3122/jabfm.2017.04.170111. [DOI] [PubMed] [Google Scholar]
- 38.Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, et al. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation. 2017;135:e1054–e1091. doi: 10.1161/CIR.0000000000000490. [DOI] [PubMed] [Google Scholar]
- 39.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz M, Rizkala AR, et al. Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF) Eur J Heart Fail. 2014;16:817–825. doi: 10.1002/ejhf.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solomon SD, Claggett B, Desai AS, Packer M, Zile M, Swedberg K, et al. Influence of ejection fraction on outcomes and efficacy of sacubitril/valsartan (LCZ696) in heart failure with reduced ejection fraction: the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) Trial. Circ Heart Fail. 2016;9:e002744. doi: 10.1161/CIRCHEARTFAILURE.115.002744. [DOI] [PubMed] [Google Scholar]
- 41.Burnett H, Earley A, Voors AA, Senni M, McMurray JJ, Deschaseaux C, et al. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction: a network meta-analysis. Circ Heart Fail. 2017;10:e003529. doi: 10.1161/circheartfailure.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150:784–794. doi: 10.7326/0003-4819-150-11-200906020-00006. [DOI] [PubMed] [Google Scholar]
- 43.Khan MS, Fonarow GC, Ahmed A, Greene SJ, Vaduganathan M, Khan H, et al. Dose of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers and outcomes in heart failure: a meta-analysis. Circ Heart Fail. 2017;10:e003956. doi: 10.1161/circheartfailure.117. [DOI] [PubMed] [Google Scholar]
- 44.Vardeny O, Claggett B, Packer M, Zile MR, Rouleau J, Swedberg K, et al. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016;18:1228–1234. doi: 10.1002/ejhf.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taniguchi T, Ohtani T, Mizote I, Kanzaki M, Ichibori Y, Minamiguchi H, et al. Switching from carvedilol to bisoprolol ameliorates adverse effects in heart failure patients with dizziness or hypotension. J Cardiol. 2013;61:417–422. doi: 10.1016/j.jjcc.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Kosmala W, Marwick TH, Stanton T, Abhayaratna WP, Stowasser M, Sharman JE. Guiding hypertension management using central blood pressure: effect of medication withdrawal on left ventricular function. Am J Hypertens. 2016;29:319–325. doi: 10.1093/ajh/hpv108. [DOI] [PubMed] [Google Scholar]
- 47.Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211–221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 48.ClinicalTrials.gov. Effects of sacubitril/valsartan therapy on biomarkers, myocardial remodeling and outcomes (PROVE-HF). https://clinicaltrials.gov/ct2/show/NCT02887183. Accessed 7 Sep 2017.
- 49.Gislason GH, Rasmussen JN, Abildstrom SZ, Schramm TK, Hansen ML, Buch P, et al. Persistent use of evidence-based pharmacotherapy in heart failure is associated with improved outcomes. Circulation. 2007;116:737–744. doi: 10.1161/CIRCULATIONAHA.106.669101. [DOI] [PubMed] [Google Scholar]
- 50.Lappé JM, Muhlestein JB, Lappé DL, Badger RS, Bair TL, Brockman R, et al. Improvements in 1-year cardiovascular clinical outcomes associated with a hospital-based discharge medication program. Ann Intern Med. 2004;141:446–453. doi: 10.7326/0003-4819-141-6-200409210-00010. [DOI] [PubMed] [Google Scholar]
- 51.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, et al. Carvedilol use at discharge in patients hospitalized for heart failure is associated with improved survival: an analysis from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Am Heart J. 2007;153:82.e1–11. doi: 10.1016/j.ahj.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 52.Gilstrap LG, Fonarow GC, Desai AS, Liang L, Matsouaka R, DeVore AD, et al. Initiation, continuation, or withdrawal of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and outcomes in patients hospitalized with heart failure with reduced ejection fraction. J Am Heart Assoc. 2017;6:e004675. doi: 10.1161/jaha.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leguelinel-Blache G, Dubois F, Bouvet S, Roux-Marson C, Arnaud F, Castelli C, et al. Improving patient’s primary medication adherence: the value of pharmaceutical counseling. Medicine (Baltimore) 2015;94:e1805. doi: 10.1097/MD.0000000000001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eisenberg JM. Center for Clinical Decisions and Communications Science. Transitional care interventions to prevent readmissions for people with heart failure. 2015 Oct 16. In: Comparative effectiveness review summary guides for clinicians [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2007. https://www.ncbi.nlm.nih.gov/books/NBK327018/. Accessed 7 Dec 2017.
- 55.Eapen ZJ, McCoy LA, Fonarow GC, Yancy CW, Miranda ML, Peterson ED, et al. Utility of socioeconomic status in predicting 30-day outcomes after heart failure hospitalization. Circ Heart Fail. 2015;8:473–480. doi: 10.1161/CIRCHEARTFAILURE.114.001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osborn CY, Kripalani S, Goggins KM, Wallston KA. Financial strain is associated with medication nonadherence and worse self-rated health among cardiovascular patients. J Health Care Poor Underserved. 2017;28:499–513. doi: 10.1353/hpu.2017.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cowie MR, Lopatin YM, Saldarriaga C, Fonseca C, Sim D, Magana JA, et al. The optimize heart failure care program: initial lessons from global implementation. Int J Cardiol. 2017;236:340–344. doi: 10.1016/j.ijcard.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 58.Al-Sutari MM, Ahmad MM. Effect of educational program on self-care behaviors and health outcome among patients with heart failure: an experimental study. Int J Evid Based Healthc. 2017;15:178–185. doi: 10.1097/XEB.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 59.Murray MD, Young J, Hoke S, Tu W, Weiner M, Morrow D, et al. Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Ann Intern Med. 2007;146:714–725. doi: 10.7326/0003-4819-146-10-200705150-00005. [DOI] [PubMed] [Google Scholar]
- 60.Desai AS, Claggett BL, Packer M, Zile MR, Rouleau JL, Swedberg K, et al. Influence of sacubitril/valsartan (LCZ696) on 30-day readmission after heart failure hospitalization. J Am Coll Cardiol. 2016;68:241–248. doi: 10.1016/j.jacc.2016.04.047. [DOI] [PubMed] [Google Scholar]
- 61.Butler J, Arbogast PG, Daugherty J, Jain MK, Ray WA, Griffin MR. Outpatient utilization of angiotensin-converting enzyme inhibitors among heart failure patients after hospital discharge. J Am Coll Cardiol. 2004;43:2036–2043. doi: 10.1016/j.jacc.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 62.Riddell E, Vader JM. Potential expanded indications for neprilysin inhibitors. Curr Heart Fail Rep. 2017;14:134–145. doi: 10.1007/s11897-017-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med. 2005;353:1471–1480. doi: 10.1056/NEJMsa051989. [DOI] [PubMed] [Google Scholar]
- 64.Driscoll A, Meagher S, Kennedy R, Hay M, Banerji J, Campbell D, et al. What is the impact of systems of care for heart failure on patients diagnosed with heart failure: a systematic review. BMC Cardiovasc Disord. 2016;16:195. doi: 10.1186/s12872-016-0371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.