Abstract
The first surgical modalities to reduce aqueous humor production by damaging the ciliary body date back to the early twentieth century. Until recently, however, cyclodestructive procedures (e.g., cyclocryotherapy and transscleral diode laser photocoagulation) have been reserved as last option procedures in refractory glaucoma patients with poor visual potential. Emerging technologic innovation has led to the development of promising, safer and less destructive techniques, such as micropulse diode cyclophotocoagulation, endoscopic cyclophotocoagulation and ultrasound cyclodestruction. Consequently, an emerging paradigm shift is under way with the selection of these surgical options in eyes with less severe glaucoma and good visual potential. Although existing evidence has not, as yet, adequately defined the role and value of these procedures, their emergence is a welcome expansion of available options for patients with moderate-to-severe glaucoma. This article reviews the pertinent evidence on both established and evolving cyclodestructive techniques and describes their growing role in the management of glaucoma.
Keywords: Cyclocryotherapy, Cyclodestruction, Cyclophotocoagulation, Diode laser, Endoscopic photocoagulation, High-intensity focused ultrasound, Micropulse diode, Ophthalmology, Refractory glaucoma, Ultrasound cyclodestruction
Introduction
Attempts to reduce aqueous humor synthesis by means of ciliary body surgery or cyclodestruction are by no means novel. Since the early twentieth century, diathermy [1, 2] or surgical excision of the ciliary body (“cyclectomy”) [3] and penetrating cyclodiathermy [4] have been employed in eyes with refractory glaucoma and poor prognosis. These early treatment modalities were technically demanding and typically had a poor safety profile. Later advents in technology led to the introduction of techniques with a more satisfactory safety profile such as cyclocryotherapy, various types of Nd:YAG and diode cyclophotocoagulation and recently ultrasound cyclodestruction.
This review presents selected evidence on the technical aspects and efficacy of currently established and emerging cyclodestructive modalities. It also highlights the evolving role and range of indications of these techniques in eyes with less severe glaucoma and better prognosis.
Methods
A literature search was performed in PubMed using the following keywords: cyclocryotherapy, cyclophotocoagulation, transscleral cyclophotocoagulation, endoscopic cyclophotocoagulation, cyclodiode, ultrasound cyclodestruction and cyclodestruction. No language filter was used. Publications that appeared until 15 August 2018 were considered. The reference lists of the documents identified with the literature search were reviewed, and additional publications were retrieved if deemed relevant to this review. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Overview
Mechanism of Action
Cyclocryotherapy refers to the trans-scleral application of a cryo-probe over the ciliary processes with the aim of ablating sufficient ciliary tissue so that aqueous humor inflow (and hence IOP) is reduced to clinically acceptable levels [5]. Technical details of the procedure are described in the following sections. Typically, rapid freezing to temperatures around − 70 °C results in the formation of intracellular micro-crystals that eventually leads to cellular destruction. Cryoablation also leads to small-vessel obliteration and necrosis of the ciliary body in addition to the destruction of ciliary epithelial cells. The resultant tissue ischemia is thought to be an additional mechanism leading to the reduction of aqueous humor synthesis. An undesirable collateral effect of cyclocryotherapy, however, may be the damage inflicted upon the neighboring trabecular meshwork due to the extension of the cryoablated area. This trabecular outflow damage may lead to the procedure losing its effect over time, especially since ciliary epithelium regeneration can partly restore aqueous synthesis. Another, mostly desirable, collateral effect of cyclocryotherapy is the reduction of corneal sensitivity due to the damage of corneal nerves; this may allow some patients with painful eyes to experience less pain, despite the IOP remaining high [6].
Cyclophotocoagulation (CPC) refers to the application of laser energy for the destruction of ciliary tissue. To date, both trans-scleral and endoscopic approaches have been successfully utilized. For example, Nd:YAG and diode lasers have been widely used trans-sclerally for the last several years, whereas the endoscopic diode laser was recently developed. A detailed discussion on the use of these modalities is provided below. The mechanism of action of CPC is thought to entail the coagulative necrosis of the secretory ciliary apparatus following the absorption of laser energy by the pigmented ciliary epithelium. Disruptive tissue damage (micro-explosions often audible as “pops”) and tissue ischemia due to the dissipated energy are supplementary mechanisms in laser cyclodestruction.
The newer techniques of CPC such as endoscopic (ECP) and micropulse transscleral cyclophotocoagulation seem to exert less damage on the ciliary epithelium and surrounding structures. In the former, the endoscopic approach allows direct application and titration of the laser energy to the target ciliary epithelium and maintains the basic cellular architecture while still reducing aqueous formation. The micropulse CPC approach delivers the laser energy in short bursts with rest intervals, allowing for a tissue effect while minimizing collateral energy absorption and damage.
Cycloablation by means of ultrasound has also been investigated for several years, but recent advances may lead to a more widespread clinical use. With this method, the operating frequency of the piezoelectric transducer induces coagulative necrosis of the ciliary processes with reportedly very little collateral damage in adjacent tissues.
The clinical effect seen in cyclodestructive procedures is mainly exerted through reduction of the rate of aqueous humor formation. However, evidence indicates that transscleral CPC may also increase ciliary body and scleral permeability to aqueous humor, thereby enhancing the uveoscleral outflow pathway [7, 8].
Histopathology Data on Different Techniques
Most cyclodestructive modalities lead to extensive damage and necrosis to the ciliary epithelia and vasculature as well as adjacent structures. The effects of cyclocryotherapy were characterized by Quigley in rabbit and human eyes [9]. In that study, the ciliary body, non-pigmented ciliary epithelium and pigmented ciliary epithelium were separated from the stroma, which exhibited vascular congestion, edema and hemorrhage. Destructive changes were not selective and were seen in the pars plana, pars plicata, trabecular meshwork, Schlemm’s canal and iris stroma. Necrosis of the ciliary epithelia and stroma, vessel atrophy and damage to adjacent tissues were also observed in other studies after cyclocryotherapy [10, 11]. Diode laser CPC also leads to necrotic changes in the ciliary epithelia, stroma and vasculature and eventually focal atrophy of ciliary processes with fibrosis of the ciliary epithelia and stroma [12]. Similar changes with coagulative necrosis of the pigmented and non-pigmented ciliary epithelium and stroma with vascular congestion and thrombosis were also seen in a human eye after contact Nd:YAG laser CPC [13, 14].
In contrast, histologic evaluation of autopsy eyes comparing transscleral diode laser CPC and ECP has provided evidence for a much more targeted effect after ECP [15]. In a recent study in enucleated human and monkey eyes, selective changes in the secretory epithelium were observed, with pigment loss and a decrease in patent small capillaries (Francis et al., submitted for publication). Minimal disruption of the ciliary body stroma and adjacent tissues of the iris, cornea, sclera or pars plana was detected. These histopathology findings may also explain differences in observed complications with each treatment modality.
Indications
Traditionally, cyclodestructive procedures are employed when filtration surgery, or the aqueous tube shunts bear high risk of failure, have already failed or are not feasible for other reasons, especially in eyes with already significantly compromised visual function [16]. These procedures have been increasingly used as alternatives to drainage devices because of the comparatively favorable safety profile in advanced refractory glaucomas [16, 17]. However, it is worth noting that there is enormous variation worldwide in the frequency and range of indications for these procedures depending on various factors such as resources, surgeon preference, patient profile, etc., [18–21]. The Ophthalmic Technology Assessment of the American Academy of Ophthalmology has suggested a framework of indications for CPC (Table 1) [22]. This framework of indications does not apply to ECP, since the latter is an intraocular procedure with particular requirements (sterile conditions, sophisticated instrumentation) and different complications (e.g., intraocular hemorrhage, endophthalmitis, etc.).
Table 1.
Indications for cyclodestructive procedures according to the Ophthalmic Technology Assessment of the American Academy of Ophthalmology [22]
| Indications |
|---|
| Eyes in which trabeculectomy and/or drainage devices have a high risk of failure or have already failed |
| Eyes with silicone oil following retinal detachment surgery |
| Eyes with uncontrolled intraocular pressure and minimal useful vision |
| Painful eyes with no (or minimal) useful vision |
| Eyes with no visual potential and very high intraocular pressure despite treatment in which corneal complications are expected because of the high pressure |
| Patients with poor general medical condition who cannot undergo incisional surgery |
| Patients who refuse to undergo incisional surgery |
| Emergency situations (e.g., acute onset of neovascular glaucoma) |
A detailed account of the available research for each particular technique in the following sections of this review suggests a paradigm shift in recent years: these procedures are increasingly used earlier in the course of the disease, for a wider range of indications and in eyes with good visual function or potential. Nonetheless, there is currently insufficient high-quality controlled evidence to evaluate the relative safety and effectiveness of cyclodestructive techniques for the management of non-refractory glaucoma [23].
Perioperative Considerations
All cyclodestructive procedures are considered painful and require adequate anesthesia. Based on the individual patient profile and surgeon preference, the range of options for analgesia includes general, retrobulbar, peribulbar, sub-Tenon’s or subconjunctival anesthesia. Supplementary sedation can also be considered. For trans-scleral diode laser CPC (DLCPC) in particular, subconjunctival or sub-Tenon’s injections carry the risk of subconjunctival hematomas that may impede delivery of photon energy to the ciliary body and lead to conjunctival burns. Postoperative pain is usually not severe and can be controlled with a short course of systemic non-opioid analgesics.
Although systemic medications that predispose to bleeding such as anticoagulants or antiplatelets are not usually discontinued prior to cyclodestructive procedures, perioperative or postoperative intraocular, or subconjunctival hemorrhages are not uncommon in patients treated with these drugs. Antiglaucoma drops should be taken as usual and tapered off in the days or weeks following treatment according to postoperative IOP. Oral carbonic anhydrase inhibitors can be continued until the first postoperative visit and discontinued at that time.
All cyclodestructive techniques induce mild-to-moderate intraocular inflammation. Consequently, a typical postoperative regimen includes topical steroids (e.g., dexamethasone in doses that range from hourly to four times daily) and non-steroid antiinflammatory drugs (e.g., diclofenac three times daily), with the addition of cycloplegics (usually atropine twice daily) if needed. Routine use of topical antibiotics is not indicated after transscleral delivery, but are routinely used after ECP.
Techniques and Evidence on Efficacy
Cyclocryotherapy
The cryotherapy unit is composed of a digital or analog control module that regulates gas flow (and hence temperature) from a nitric oxide or carbon dioxide reservoir so that the probe tip freezes. The cryoprobe tip typically has a 2.5-mm diameter and is capable of reaching temperatures between − 60 °C and − 80 °C. Such freezing in turn produces temperatures around − 10 °C at the target tissue. The anterior edge of the cryoprobe tip is placed at a distance of approximately 1.5 mm from the limbus; firm pressure is applied, and the cryoprobe is allowed to reach − 60 °C to − 80 °C for 60 s. The probe is allowed to thaw so that it can be detached from the ocular tissues, and the process is repeated five to seven times over an arc of 180°. To prevent the risk of significant discomfort, pain and inflammation, treating the 3 and 9 o’clock positions should be avoided so that damage to the long posterior vessels and nerves is minimized. At least 4 weeks are necessary before the final hypotensive effect can be judged. Retreatment may be performed if the initial result is unsatisfactory. Although there is no consensus, some experts prefer to retreat in an arch of 180° that includes 90° of a previously treated area.
This technique is not very commonly used nowadays and has been largely replaced by DLCPC because the latter is associated with a lower risk of hypotony and phthisis [24]. However, a recent comparative study between cyclocryotherapy and DLCPC performed as primary surgical procedures in open-angle glaucoma patients suggested that both techniques offered a similar, moderate IOP-lowering effect and a generally safe profile [25].
Transscleral CPC
Contact and Non-Contact Nd-YAG Laser CPC
The Nd-YAG laser (1064 nm) is used in the non-Q switched free-running thermal mode. In the non-contact method, typical energy settings are 4–8 J for a duration of 0.02 s for 8–10 applications per quadrant. Treatment is usually performed over an arc of 270°–360°. With the aiming beam of the laser focused on the conjunctiva, the Nd:YAG laser beam should be defocused so as to offset the focal point 3.6 mm into the eye. The use of a contact lens offers the advantage of immobilizing the globe, keeping the eyelids open and blanching the conjunctiva so that energy is more efficiently delivered to the ciliary body.
In the contact method, an eyelid speculum is used and the patient lies supine. The tip of the fiberoptic probe is centered 1.5–2.0 mm behind the limbus, pressure is exerted to stabilize the globe and blanche conjunctival vessels, and 8–10 pulses per quadrant are delivered (5–8 W with duration 0.7–2 s) until 270°–360° is treated.
A suboptimal hypotensive effect might be observed after a primary treatment with either the contact or the non-contact technique [26]. Because the ultimate efficacy of the primary intervention can be safely judged after 4 weeks, the decision for a retreatment should be deferred until then.
Currently, the Nd:YAG CPC techniques have largely been replaced by DLCPC as the latter is easier to perform, has equivalent efficacy and has a better safety profile [27–29].
Semiconductor Diode Laser Cyclophotocoagulation
The semiconductor diode laser incorporates two light-emitting diodes with a combined wavelength of 810 nm and can be deployed in a non-contact fashion (attached to a slitlamp) or on a contact probe.
In the non-contact mode, the device is set at a power of 1200–1500 mW, spot-size of 100–400 μm and duration of 1 s. It is usually recommended that 30–40 equally spaced applications be placed 1 mm behind the limbus over 270°–360°. In the contact mode, the specially designed G-Probe (IRIS Medical Instruments, Inc., Mountain View, CA, USA) is used to deliver energy from a semiconductor diode 810 nm laser in evenly spaced areas at a distance of 1.5 mm behind the limbus over a variable length of arc. The design and dimensions of the G-Probe contact plate are such that they enable the clinician to place the probe at the correct distance from the limbus. In addition, it facilitates the delivery of the laser energy in an axis parallel to the visual axis so that the area of treatment is directed toward the ciliary body and the risk of collateral tissue damage is minimized. Typical settings are 1300–2000 mW and duration of 1.5–3.5 s. However, a wide range of settings has been reported in the literature: energy levels of 1250–2600 mW and pulse duration of 1.5–4.0 s for 15–40 applications over 180°–360°. In practice, in any given patient, the energy should be titrated so that the power is set just below the level at which audible “pops” are produced. Treating the 3 and 9 o’clock positions should be avoided to minimize the risk of damage to the long posterior vessels and nerves. The position of the ciliary body can vary around the quadrants and can be found at a very posterior location in highly myopic or buphthalmic eyes. Thus, localization of the ciliary body is very important and can easily be done with trans-illumination through the pupil or trans-sclerally. To facilitate the identification of the ciliary body during the procedure, a G-probe with a built-in fiberoptic element designed to offer trans-illumination was recently introduced (Iridex G-Probe™ Illuminate). Peer-reviewed data on the usefulness of this probe are currently unavailable. In addition to the conventional energy delivery mode with the G-Probe where high laser energy is emitted in a continuous fashion, a micropulse delivery mode has recently been developed. A similar DLCPC platform of 810 nm is used with a novel contact probe for the emission of laser energy in a series of repetitive short pulses (“on” periods) separated by rest periods (“off” periods). In contrast to the operative technique used with the conventional G-probe where energy is delivered following firm pressure in distinct locations around the limbus, with micropulse DLCPC, a novel probe is used in a slow, continuous back-and-forth sliding motion (“painting”) along two arcs surrounding the limbus (9:30–2:30 and 3:30–8:30, avoiding the 3 o’clock and 9 o’clock positions). Settings described in published studies were 2 W delivered over 100 s for a 360° treatment area; this translates to 62,500 micropulses with the 0.5 s “on”/1.1 s “off” cycle (duty cycle: 31.3%). The total energy delivered to an eye with such settings would be 62.6 J [30, 31]. However, the authors of the current review believe that exposures of 90 s per 180 degrees for a total of 180 s may be appropriate for most patients. In theory, micropulse DLCPC photocoagulates the pigmented ciliary epithelium while allowing adjacent tissues to cool during the “off” cycle. Thus, the temperature in collateral tissue remains below the coagulation threshold and damage is minimized. Unlike continuous mode DLCPC, micropulse DLCPC does not induce clinically evident tissue disruption and micro-explosions (audible “pops”) during treatment. Therefore, it may be difficult to titrate therapy. Because micropulse DLCPC delivers a smaller amount of energy per session, treatment with this mode may be less painful compared with continuous DLCPC [32, 33].
Efficacy of DLCPC
In a prospective trial with uncontrolled open-angle or secondary glaucoma patients, Kosoko et al. [34] enrolled 27 eyes of 27 patients who were treated with DLCPC and were followed up for a median of 19 months. Baseline IOP was significantly lowered from 36.4 ± 12.4 mmHg to 20.3 ± 8.7 mmHg (44% reduction) at the last available visit. At 2 years post-laser, 62% of patients had > 20% IOP reduction from baseline with or without medication, while 52% had achieved both > 20% IOP reduction from baseline and IOP < 22 mmHg with or without medication. In a large retrospective study that included 210 eyes of 195 refractory glaucoma patients aged 1 to 89 years (mean: 51 years), Bloom et al. [35] reported that the mean pretreatment IOP (34.1 ± 10.6 mmHg) was significantly reduced after an average follow-up of 10 months (20.1 ± 9.3 mmHg) with a mean of 1.75 treatments per eye. The mean number of anti-glaucoma medications was reduced from 2.3 to 1.7. At the last follow-up visit, 71% of patients had unchanged or better visual acuity compared with baseline. In a retrospective study that included 47 eyes of 43 patients with refractory glaucoma (36 eyes had POAG), Hauber et al. [36] examined the effectiveness of a single session of DLCPC with predetermined laser parameters (power: 2 W, exposure time: 2 s, average number of laser spots: 25.6). The average total energy ± SD delivered to the ciliary body was 102.5 ± 16.2 J. The average pretreatment IOP was significantly reduced from 29.4 ± 10.6 mmHg to 16.3 ± 4.2 mmHg (P < 0.005, 44.5% reduction) at 3 to 6 months and 16.2 ± 1.3 mmHg at 1 year (P < 0.005, 44.8% reduction). At the 1-year visit, 17 of the 18 eyes (94.4%) that were not lost to follow-up satisfied the definition of success (IOP < 21 mmHg with or without medications). At the 3–6-month postoperative visit, patients had lost an average of 0.5 ± 1.1 lines of best corrected visual acuity. At the same postoperative visits, the average number of medications was insignificantly reduced from 2.8 to 2.3. The authors combined their data with those from previous studies and found a direct linear correlation (r = 0.91) between the percentage of patients achieving success (IOP < 21 or 22 mmHg) and the total energy delivered to the ciliary body. A notable difference between this and previous studies was that Hauber et al. used a total energy of 102.5 ± 16.2 J compared with an average of 59.5 J in the other studies reviewed.
The efficacy of DLCPC in various types of refractory pediatric glaucoma has also been investigated. Kirwan et al. [37] reported on data from a retrospective chart review that included 77 eyes of 61 patients with a mean age of 7.4 years. Sixty-four percent of eyes had undergone at least one previous operation for glaucoma, and 60% were aphakic. The mean pretreatment IOP was 32.0 ± 6.4 mmHg. Twelve months following a single DLCPC session, 37% of eyes maintained a clinically useful response (IOP < 22 mmHg or at least 30% reduction). After a repeat DLCPC treatment, 72% had a clinically useful IOP reduction for at least 1 year. Compared with phakic eyes, aphakic eyes had more sustained IOP reduction. Visual acuity estimation was possible for 53 of 61 eyes. In 4 of these 53 eyes (8%), visual acuity decreased by at least one “level of vision” after DLCPC. All eyes with visual deterioration had pretreatment acuity of 1/10 or worse. Retinal detachment possibly related to the treatment was observed in one phakic and two aphakic eyes with end-stage glaucoma. The authors observed that success in their sample was worse than that reported in adult patients possibly because in younger eyes ciliary body function recovers faster because of tissue regeneration. They concluded that despite its short-lived efficacy, this modality might have a role as an adjunct to surgery or in selected patients for whom surgery is not possible.
Since patients with refractory glaucoma that are not good candidates for, or have already failed, a trabeculectomy are usually offered CPC or tube surgery, Malik et al. [38] performed a retrospective study to compare the efficacy and safety profiles with the two approaches. Data from patients with open-angle or secondary refractory glaucoma who underwent CPC (n = 28, mean follow-up: 51 months) or double-plate Molteno tube implantation (n = 26, mean follow-up: 24.5 months) were analyzed. Mean preoperative IOP was 39 ± 16 mmHg for the DLCPC group and 37 ± 12 mmHg for the tube group (P = 0.61). The final IOP was 17 ± 12 mmHg for the diode group and 17 ± 9 mmHg for the tube group (P = 0.98). Surgical success (defined as IOP > 5 mmHg and < 22 mmHg without medication) was noted in 11% of diode eyes and 46% of tube eyes, while qualified success (defined as IOP > 5 mmHg and < 22 mmHg with medication) was noted in 64% of DLCPC eyes and 81% of tube eyes. Retreatment for the DLCPC group was needed in 17 eyes (61%) with 2 eyes requiring seven treatment sessions. The mean number of antiglaucoma medications was similar at the last visit (P = 0.36). Forty-six percent of DLCPC procedures were complication-free versus 31% of tube surgeries. However, phthisis was observed in two eyes in the DLCPC group. The authors of this retrospective study acknowledge that the two groups may have been somewhat unbalanced, because more cases with neovascular glaucoma were included in the DLCPC group. Since eyes with neovascular glaucoma carry a worse prognosis, the results may be favorably biased toward the tube group. A survival analysis following the exclusion of cases with neovascular glaucoma showed that both DLCPC and tube patients fared equally. The much longer follow-up period available for the DLCPC group (51 months compared with 24.5 months for the tube group) may have also affected the observed survival of the two procedures.
In another study, Schaefer et al. [39] reported on the efficacy of CPC as a secondary procedure in eyes with a failed glaucoma tube compared with a second tube implantation. The authors performed a retrospective chart review of 47 eyes of 43 adult and pediatric patients who had received either a double-plate Molteno or a Baerveldt 350-mm2 tube. CPC was performed in 32 eyes (DLCPC: n = 23, Nd:YAG laser: n = 9), and a second tube was inserted in 15 eyes (double-plate Molteno: n = 4, Baerveldt 350 mm2: n = 11). The IOPs after the primary tube insertion and before the secondary procedure in the CPC and the secondary tube insertion groups were similar (22.4 mmHg and 22.6 mmHg, respectively; P = 0.918). Medication use, visual acuity, age and diagnosis were also similar between groups. Excluding eyes that required further intervention in the first postoperative months following the secondary procedure, the mean IOP at 12 months for the CPC (n = 25) and the tube groups (n = 14) were 16.0 ± 6.1 mmHg (27% reduction) and 15.3 ± 6.5 mmHg (33% reduction), respectively. At the last follow-up, the IOP for the CPC (mean: 63 months, n = 28) and the tube groups (mean: 132 months, n = 14) were 15.9 ± 6.6 mmHg (27% reduction) and 14.8 ± 6.8 mmHg (30.5% reduction), respectively. There was also a significant decrease in the logMAR from 1.20 ± 1.0 to 1.66 ± 1.20 (P = 0.006) in the CPC group and from 1.0 ± 0.9 to 1.67 ± 1.14 (P = 0.040) in the tube group. Survival analysis indicated that secondary tube implantation had a high probability of success (IOP < 18 mmHg) initially, but a rather high likelihood of late failure (usually after 6 years). Eyes treated with CPC tended to fail earlier, often within the first post-laser year, but had relatively few late failures. Interestingly, the average follow-up for the CPC group (just over 5 years) was markedly reduced compared with that of the tube group (11 years). Consequently, this difference in length of follow-up might have affected the reported survival of the two procedures.
In a recent retrospective review, Levinson et al. [40] compared the efficacy of sequential glaucoma drainage device implantation (GDD group, n = 32) versus DLCPC (CPC group, n = 21) after failure of a primary drainage device. In the GDD group, any of four different glaucoma tubes could have been used. Success was defined as the absence of all of the following: IOP < 6 mmHg or > 21 mmHg at two consecutive visits after the initial 3-month postoperative period, reoperation for glaucoma and loss of light perception. The follow-up for the GDD and the CPC groups were 37.9 months and 46.3 months, respectively. Before the secondary procedure, eyes in the CPC group had worse IOP (33.2 vs. 27.8 mmHg, P = 0.027) and visual acuity (LogMAR 1.40 vs. 0.95, P = 0.041), reflecting the institution’s practice to use DLCPC as a treatment of last resort in refractory glaucoma. The IOP decreased from baseline by 56.3% for the CPC and 40.7% for the GDD groups, respectively (P = 0.017), while the 5-year probability of success was 58.0% for CPC and 65.3% for sequential GDD (P = 0.868). The number of medications needed postoperatively was significantly reduced compared with the preoperative number, and this reduction was comparable in both groups. Although the power of the study was inadequate for the statistical analysis of postoperative complications, eyes in the CPC group had fewer serious adverse events. In a similar retrospective study, Wang et al. [41] recently examined the efficacy and safety of a second glaucoma drainage device (n = 35) versus transscleral CPC (n = 40) following failure of a previous drainage device. The authors found that although both groups achieved significant IOP reduction, eyes implanted with a second drainage device had longer mean survival time (45.0 months) than eyes treated with CPC (26.5 months), but they suffered significantly more postoperative complications (60% versus 20% of eyes) [41].
Recently, Rodriguez-Garcia et al. [42] presented data on the efficacy and safety of DLCPC in patients with refractory glaucoma following penetrating keratoplasty. In this case series of 16 eyes of 15 patients, the mean pre-laser IOP (31.5 mmHg) was significantly reduced after the first laser application (17.5 mmHg, 44.4% reduction). During the follow-up (mean: 29.2 months), five eyes (31%) needed a second DLCPC session and one of these eyes needed a third session. The authors report that despite the low preoperative visual acuity of the patients, 81% of them experienced an improvement in visual acuity. Two serious complications were observed: phthisis bulbi in one eye and endothelial allograft rejection in another.
The efficacy and safety of DLCPC and cyclocryotherapy as primary surgical procedures in poorly controlled eyes with open-angle glaucoma were examined in a recent study [25]. The authors reviewed the records of 184 eyes of 112 patients treated with a single session of DLCPC (n = 133) or cyclocryotherapy (n = 51). After a mean follow-up time of 5.5 months, the preoperative IOP in the DLCPC group fell from 16.13 ± 2.50 mmHg to 14.58 ± 2.40 mmHg; in the cyclocryotherapy group, the preoperative IOP fell from 17.50 ± 3.42 mmHg to 15.17 ± 2.82 mmHg. The mean IOP reduction with DLCPC (1.55 mmHg) was not statistically different from that observed with cyclocryotherapy (2.33 mmHg, P = 0.08). The mean number of medications was reduced from 2.9 to 2.1 and from 3.4 to 2.4 for the DLCPC and cyclocryotherapy groups, respectively. The mean IOP was reduced by at least 20% without an increase in medications or with a decrease of at least one medication in 45% and 70% of patients treated with DLCPC and cyclocryotherapy, respectively. Loss of best-corrected visual acuity of at least two lines was seen in 10.5% and 9.8% of patients in the DLCPC and cyclocryotherapy groups, respectively.
Using micropulse DLCPC, Tan et al. [31] reported on the efficacy of this modality in 40 eyes of 38 patients with various types of refractory glaucomas. In this prospective case series, the mean pre-laser IOP was reduced from 39.3 ± 12.6 mmHg to 26.2 ± 14.3 mmHg at 18 months post-laser. After a mean of 1.3 sessions, the overall success (defined as IOP of < 21 mmHg or IOP reduction ≥ 30% with or without topical medication) was 80% at last follow-up. The mean number of medications was reduced from 2.1 before treatment to 1.3 at last follow-up. In this cohort with poor pre-laser visual acuity (light perception to Snellen 6/60), visual acuity improved in 10% and remained constant in 90% of eyes. Recently, the comparative efficacy of micropulse versus continuous DLCPC was examined in a randomized trial with a follow-up of 18 months [30]. One eye from each of 48 patients with refractory end-stage glaucoma was randomized to either treatment. The mean baseline IOP was similar in the two groups (micropulse DLCPC: 36.5 mmHg; continuous DLCPC: 35.0 mmHg; P = 0.50) and was significantly reduced by the same percentage at 18 months (45%, P = 0.70). At the end of follow-up, success (defined as IOP between 6 and 21 mmHg and at least 30% reduction with or without anti-glaucoma medications) was noted in 52% of eyes treated with micropulse DLCPC and 30% of eyes treated with continuous DLCPC (P = 0.13). There was no significant difference in retreatment rates or number of IOP-lowering medications in the two groups. Ocular pain assessed with the verbal analog scale was not significantly different in the two groups. The authors found that complications were more common in eyes treated with continuous DLCPC (P = 0.01). However, despite randomization, 50% of eyes treated with continuous DLCPC had neovascular glaucoma (n = 12) versus 29% of those treated with micropulse DLCPC (n = 7). Notably, all cases with prolonged hypotony (n = 5) were treated with continuous DLCPC, and four of them had neovascular glaucoma.
Finally, Vernon et al. [43] investigated the long-term results of DLCPC for refractory glaucoma. They retrospectively evaluated data from 42 eyes with a minimum follow-up of 36 months after DLCPC. Treatment was performed under a standard protocol without titrating the number of burns (except for six eyes, where local anatomy dictated otherwise), energy or pulse duration, and the treatment session could be repeated if needed. Mean follow-up for this cohort was 66 months and results supported good hypotensive results, with a 50.3% drop in IOP and 88% of eyes achieving an IOP < 22 mmHg. In 60% of eyes, DLCPC was repeated (up to 6 times). Twenty-seven eyes experienced visual loss, 11 were stable and 4 improved their acuity at final follow-up.
Transpupillary CPC
This option is limited to eyes in which a sufficient number of ciliary processes can be viewed on gonioscopy (e.g., in cases with extensive anterior synechiae that displace the iris anteriorly, in aniridia or an unusually large peripheral iridectomy). An argon laser and Goldmann gonioscopy lens can be used for the coagulation of as many ciliary processes as possible. Typical settings of 125-μm spot size, 700–740 mW of energy and 0.3–0.5 s of pulse duration are used. The energy and duration of pulses are adjusted so that the ciliary processes are seen to shrink and change color to whitish-gray or just before minute disruptions (“pops”) are seen and heard.
Ultrasound Cyclodestruction
Ultrasound has been used for cyclodestruction since the 1980s [20, 44]. Transscleral cyclodestruction by means of ultrasound has an important advantage over laser energy; because scleral tissue has significant light-scattering properties, a large amount of laser energy may be needed for clinically meaningful ciliary body ablation. This excessive laser energy may produce significant damage to collateral tissues such as conjunctiva, sclera and crystalline lens [20, 44].
An early commercially available device (Therapeutic Ultrasound System; Sonocare, Inc., Ridgewood, NJ) utilized high-intensity focused ultrasound (HIFU), but was not widely adopted because of its bulky construction, demanding operation and safety profile. The interest in ultrasound technology for cyclodestructive procedures was recently revived with the development of the EyeOP1 device (EyeTechCare, Rillieux-la-Pape, France) [45–47]. This instrument is composed of a control panel used for the adjustment of parameters and a concave probe designed to be positioned over the full circumference of a patient’s anterior sclera. When placed in this fashion, the six miniature piezoelectric elements of the concave probe are placed over the patient’s ciliary body. The stability of the probe on the eye is maintained with a vacuum pump exerting negative pressure through a suction ring. To accommodate for differences in globe size, three models of the device with different ring sizes (11, 12 and 13 mm) are available. Following ultrasound biomicroscopy or optical coherence tomography imaging of the anterior segment or simple measurement of white-to-white distance and axial length, the device with the appropriate ring size is chosen so that the focal zones of the delivered ultrasound energy closely match the patient’s ciliary body. Depending on surgeon preferences, some or all six of the high-frequency (21 MHz) piezoelectric transducers are activated sequentially. The emitted energy induces coagulative necrosis of the underlying ciliary processes with reportedly no collateral tissue damage and little inflammation. Ultrasound absorption does not depend on the individual pigmentation, and as such energy deposition is much better controlled. Ultrasound can be used to heat and treat a well-defined and adjustable tissue volume at any depth or location. Further, ultrasound cyclocoagulation involves a much slower temperature rise than trans-scleral cyclophotocoagulation, thus eliminating the risks of tissue explosion. Additionally, temperature variability is much more tightly controlled with ultrasound cyclocoagulation (Figs. 1, 2, 3). In the recently developed second-generation probe, the active transducer area is broader compared with the first-generation probe (4 mm2 vs. 2.5 mm2, respectively). The six transducers are located equidistantly on the circumference of the ring and oriented so that they create a focal zone. The focal volume of each transducer is characterized by axial length of about 1 mm, transverse focal width of about 0.1 mm and lateral focal width of about 3.5 mm. Because the heat generated in the area of the focal volume propagates circumferentially, the heated volume is proportional to the time of exposure [48]. In principle, compared with the low operating frequency of the earlier Sonocare™ device (4.6 MHz), the higher operating frequency of the newer EyeOP1 device (21 MHz) allows for a steeper transition between the treated and untreated zones, thus minimizing the risk of collateral damage in adjacent tissues [49]. An animal study by Aptel et al. found circumferentially distributed coagulation of the ciliary processes and ciliary body that apparently explains the reduced aqueous production [50]. These authors treated 34 rabbit eyes with the ring-shaped probe used in the ultrasound cyclocoagulation technique and reported that the epithelium of the ciliary processes was degenerated or necrotic and sloughed off; months later, examinations confirmed the involution of the ciliary processes. Further, a vascular corrosion cast determined the focal interruption of the ciliary body microvasculature and the existence of a sustained fluid space between the sclera, ciliary body and choroid. These findings confirm that the uveoscleral pathway also plays a role in the mechanism of action of the ultrasound technique [50]. Recent in vivo clinical evidence in human eyes with refractory glaucoma has shown that the newly developed HIFU technique with the ultrasonic circular cyclocoagulator induces anatomical alterations at the sclera and conjunctiva that suggests augmented trans-scleral aqueous humor percolation [51]. Specifically, the authors used anterior segment optical coherence tomography and in vivo confocal microscopy and observed that the area of intrascleral hyporeflective spaces and the mean density and area of conjunctival microcysts increased after treatment with HIFU [51]. These findings suggest that in addition to the reduction of aqueous humor inflow due to cyclodestruction, increased outflow may be responsible for the observed ocular hypotensive effect with this modality.
Fig. 1.
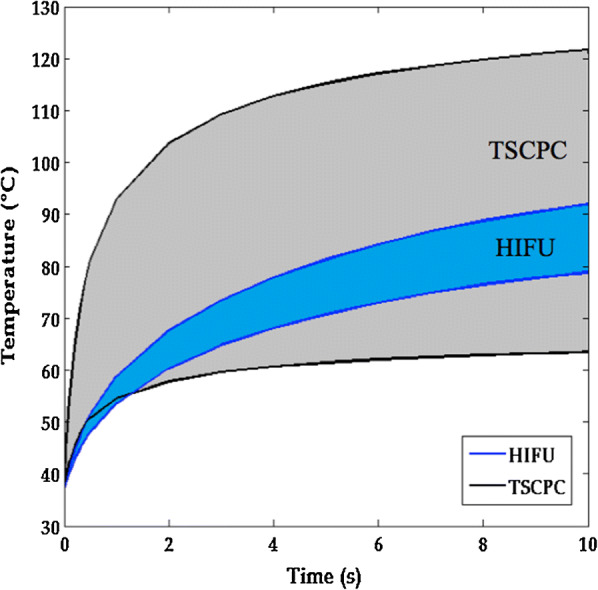
Thermal modelization for ultrasound cyclocoagulation high-intensity focused ultrasound (HIFU) and trans-scleral cyclophotocoagulation (TSCPC). The temperature evolution curves show that thermal variability is smaller with HIFU. Permission to use this figure was granted by EyeTechCare SA
Fig. 2.
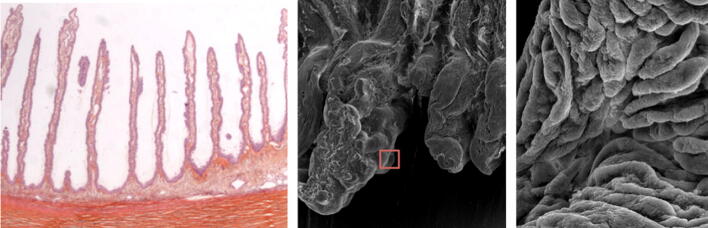
Histologic preparation (left) and scanning electron microscopy images (middle and right) of an untreated area of rabbit ciliary processes covered by epithelial cells, which serve to secrete aqueous humor. Red square in the middle photograph indicates the magnified area shown in the right photograph. Permission to use this figure was granted by EyeTechCare SA
Fig. 3.
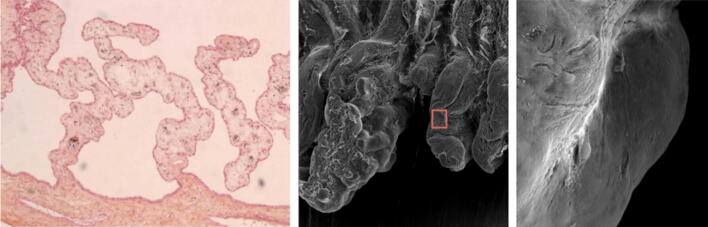
Histologic preparation (left) and scanning electron microscopy images (middle and right) following ultrasound cyclocoagulation. Red square in middle photograph indicates the magnified area shown in the right photograph. The epithelial cell layer is removed but the stromal base is preserved with no explosion of ciliary processes. Permission to use this figure was granted by EyeTechCare SA
Efficacy of EyeOP1
In an animal study designed to examine the safety of the new HIFU method, 18 healthy adult New Zealand white rabbits underwent treatment with an EyeOP1 prototype adapted for the rabbit eye [46]. Six eyes of six animals were treated with all six piezoelectric transducers activated, six were treated with five transducers and six with only four transducers. Nearly 1 month after the procedure, all treated eyes had significantly lower IOP compared with the pre-treatment levels but the reduction was larger in eyes treated with five or six transducers. Histologic examination revealed segmental-to-annular damage of the ciliary processes caused primarily by coagulation necrosis. Inflammation was minimal, and the sclera and crystalline lens appeared intact.
The first clinical trial with the EyeOP1 platform enrolled 12 patients with advanced refractory glaucoma who were followed for a mean period of 6.5 ± 4.3 months [45]. The patients’ diagnoses were: POAG (n = 5), neovascular glaucoma (n = 3), congenital glaucoma (n = 2), chronic angle-closure glaucoma (n = 1) and iridocorneal endothelial syndrome (n = 1). The mean preoperative IOP (37.9 ± 10.7 mmHg) was significantly reduced at the 6th month visit (26.3 ± 5.1 mmHg; P < 0.01). At the final visit, the cohort’s mean IOP reduction was 33.9%, and surgical success (IOP > 5 mmHg and IOP reduction > 20%) was achieved in 10 of 12 patients (83.3%). No major intra- or postoperative complications were observed, and the mean visual acuity remained unchanged. The most frequent adverse events included superficial punctate keratitis (n = 3) and superficial corneal ulceration (n = 1) in patients with previous keratopathy (e.g., corneal edema due to congenital glaucoma or iridocorneal endothelial syndrome). All cases responded well to conservative treatment.
The early promising results from animal studies [46] and human eyes with advanced refractory glaucoma [45] led to a dose-escalation clinical trial. Denis et al. [52] recently reported results of a 12-month prospective multicenter study with 52 patients with POAG (n = 36) or secondary glaucoma (n = 16) who were treated with the EyeOP1 device. The transducer pulse duration was set at 4 s (group 1, n = 24) or 6 s (group 2, n = 28). Patients in group 1 had an IOP decrease of 32%, from 29.7 ± 6.7 mmHg with 3.5 glaucoma medications preoperatively to 20.1 ± 6.7 mmHg with 3.2 medications at 12 months postoperatively. Patients in group 2 had an IOP decrease of 36% from 29.0 ± 7.4 mmHg with 3.3 medications preoperatively to 18.5 ± 6.6 mmHg with 3.5 medications at 12 months postoperatively. Five patients from group 1 and three from group 2 underwent the procedure twice during the study. Surgical success (IOP reduction > 20% and IOP > 5 mmHg) was achieved in 57.1% of patients in group 1 and 48% of patients in group 2 (P = 0.56). The cohort’s mean visual acuity remained statistically unchanged. Common adverse events were minor and included conjunctival hyperemia (n = 25), superficial punctate keratitis (n = 17) and transient anterior chamber inflammation (n = 13). One patient presented with transient postoperative hypotony (4 mmHg) and choroidal detachment that resolved with conservative treatment within a month. Twelve patients required additional surgical interventions (trabeculectomy, diode cyclocoagulation or valve implantation) 6 to 12 months after the HIFU procedure due to insufficient IOP control.
In another recent two-center study, 28 patients with refractory POAG were treated with 6-s pulse duration and followed for a mean period of 9.3 ± 3.1 months [48]. The mean preoperative IOP decreased by 26%, from 29.0 ± 7.2 mmHg to 21.6 ± 9.4 mmHg at the final visit (P < 0.05). Complete success (IOP reduction > 20% and IOP > 5 mmHg without medication or other intervention) was obtained in 50% of eyes at final follow-up, and qualified success (IOP reduction > 20% and IOP > 5 mmHg with possible re-intervention) was obtained in 68% of eyes at last follow-up. Regarding safety and adverse events, although the mean visual acuity of the cohort remained statistically unchanged (logMAR 0.84 ± 1.09 preoperatively vs. 1.09 ± 1.20 postoperatively; P = 0.42), four patients (15.4%) lost three or more lines. In addition, one patient presented 1 week postoperatively with a large choroidal detachment that resolved within 2 months.
In a prospective trial, Melamed et al. [53] recently evaluated the safety and efficacy of HIFU in 20 eyes of 20 patients with refractory glaucoma. The authors used 6 s duration pulses. The mean preoperative IOP was significantly lowered by 38% from 36.4 ± 5.7 mmHg to 22.5 ± 10.3 mmHg at 12 months (P < 0.01). No major complications were observed. Surgical success (defined as IOP reduction from baseline ≥ 20% and IOP > 5 mmHg) was achieved in 13 of 20 eyes (65%) and 4 eyes had to be retreated. Visual acuity deteriorated by 2 or more lines in 2 eyes (10%), remained unchanged in 14 eyes (70%) and improved in 4 eyes (20%).
Another prospective trial evaluated the efficacy of HIFU in 30 eyes of 30 patients with refractory glaucoma [54]. The investigators included patients with open-angle, chronic-angle closure and neovascular glaucoma who were followed for 6 months. They also examined the efficacy of the procedure using different duration of ultrasound activation (4, 6 and 8 s). Overall, the mean preoperative IOP (30.1 ± 10.5 mmHg) was significantly reduced after 6 months (20.2 ± 6.2 mmHg; P < 0.001). The mean number of daily topical medications and acetazolamide tablets used preoperatively (2.7 ± 0.9 and 0.8 ± 0.5, respectively) were also significantly reduced (2.0 ± 1.0 and 0.3 ± 0.5, respectively) after 6 months (both P < 0.01). The mean percentage IOP reduction was highest in eyes with chronic-angle closure glaucoma (37.8%), followed by those with neovascular glaucoma (26.2%) and open-angle glaucoma (20.0%). Patients treated with 8-s HIFU duration had significantly greater IOP reduction (− 16.2 ± 8.3 mmHg) compared with patients treated with 6-s (− 8.8 ± 6.6 mmHg) or 4-s (− 3.7 ± 6.5 mmHg) duration. No major complications were recorded except for a case with temporarily fixed dilated pupil.
After having substantiated the safety of HIFU in patients with very advanced disease and limited prognosis, use of this modality in less advanced cases was also investigated [45, 46, 52]. In a recent prospective multicenter trial, Aptel et al. [55] evaluated the efficacy and safety of HIFU in 30 eyes of 30 patients with moderate or severe open-angle glaucoma (mean MD: − 12.6 ± 12.0 dB) who had not undergone previous cyclodestructive procedures or filtering surgery. The authors used pulses of 6 s duration and followed the patients for up to 12 months. The mean preoperative IOP (28.2 ± 7.2 mmHg with an average of 3.6 hypotensive medications) was significantly reduced by 30% at 12 months (19.6 ± 7.9 mmHg with an average of 3.1 hypotensive medications) after a mean of 1.1 procedures. Qualified success (defined as IOP reduction ≥ 20% and IOP > 5 mmHg with possible re-intervention and without antiglaucoma medication) was achieved in 63% of eyes, and complete success (defined as IOP reduction ≥ 20%, IOP between 5 to 21 mmHg with possible re-intervention and without antiglaucoma medication) was achieved in 46.7% of eyes. Although the mean visual acuity of the group remained statistically unchanged (P = 0.38), six patients (20%) lost more than three lines. The authors noted cataract progression in four of these six patients that already had cataract preoperatively, sustained superficial keratitis in one patient and progression of advanced glaucoma in another patient. The efficacy of HIFU in this study is comparable to previous studies by the same group that included advanced cases with refractory glaucoma [48, 52], but inferior to their initial pilot study [45]. According to the authors, a plausible explanation could be that 25% of patients recruited in the initial pilot study had already been treated with other cyclodestructive methods and thus may have responded more favorably to additional cyclodestruction by HIFU.
Much of the published evidence on ultrasound cyclocoagulation was produced with the EyeOP1 device and the early, first-generation probe. In its current state of development, the device is equipped with a second-generation probe featuring a broader transducer area, more precise transducer calibration and improved suction, ultrasound coupling and ergonomics. A recent meta-analysis on 251 patients (160 male, 91 female) included 141 patients who had undergone the procedure with the first-generation probe and 110 with the second-generation probe [56]. Of the seven studies, four involved solely refractory glaucoma patients (n = 111), two involved non-refractory glaucoma patients (n = 120), and one involved a combination of refractory and non-refractory glaucoma patients (n = 20). The mean patient age was 63 ± 13 years. The overwhelming majority of patients (n = 211, 84%) were diagnosed with POAG, and the remainder (n = 40, 16%) had secondary glaucoma. Of the 110 patients who underwent the procedure with the second-generation probe, 90 (82%) were of Indian descent. The average IOP reduction for the first-generation probes across all indications was 34.3% at day 360 and 35.3% at day 180 with the second-generation probes. The number of mean medications dropped from 3.8 at baseline to 3.3 at month 12 across all indications in the first-generation probes. At month 6, the mean medication use increased slightly from 1.0 to 1.2 in the second-generation probes. The success rate, defined as IOP reduction of at least 20% compared with baseline with no medication added, was 54% for the first-generation probe and 64% in the second-generation probe. The meta-analysis found consistent safety results between the first- and second-generation probes. Conjunctival hyperemia was observed in 173 patients (69%), but was attributed to the placement of the suction cone and was frequently pre-existing because of the long-term topical medication use. Superficial punctate keratitis (24%) and anterior chamber reaction (21%) were also less pronounced with the second-generation than with the first-generation probe. On the other hand, scleral marks were more pronounced with the second-generation probe (20%) than with the first-generation probe (3%). Chemosis and loss of visual acuity were more pronounced with the first-generation than with the second-generation probe (5% vs. 0% and 4% vs. 0%, respectively). There were 20 patients (8%) with pre-existing corneal edema due to already compromised corneas. A major source of error that may adversely affect the efficacy of the procedure is the choice of probe diameter [56]. Should the procedure prove unsuccessful in a surgery-naïve patient, filtration surgery is recommended before laser photocoagulation [56].
Newer studies presented after the aforementioned meta-analysis continue to add to the literature on the safety and efficacy of this procedure. In one multicenter study conducted in university glaucoma centers, 52 eyes of 50 patients with refractory POAG underwent ultrasound cyclodestruction [57]. The mean IOP was significantly decreased from 24.3 ± 7.0 mmHg at baseline to 16.8 ± 7.6 mmHg (30.8%) by the last follow-up after month 6. There were no major intraoperative complications, and the entire procedure lasted less than 5 min.
The device is CE marked for use in patients with refractory and non-refractory glaucoma. The recommended candidate profile includes cases with POAG, pigmentary and pseudoexfoliative glaucoma, patients with previously failed filtration surgery or those with an elevated risk for surgical failure and patients poorly controlled after maximally tolerated glaucoma therapy with IOP between 21 mmHg and 35 mmHg.
Endoscopic Cyclophotocoagulation
The ECP device is composed of a console that contains the semiconductor diode laser generator (810 nm) and the settings panel, a video display and a 20-gauge fiberoptic endoscope through which the image, laser and light guides pass (Beaver Visitech International and Endo Optiks, Little Silver, NJ, USA) [21]. The surgeon performs the procedure while viewing the monitor rather than looking through the operating microscope, which involves a learning curve for the anterior segment surgeon (Fig. 4). ECP is performed through a limbal approach or through the pars plana. The former can be utilized in phakic, pseudophakic or aphakic eyes through a clear corneal or scleral tunnel 1.5–2.0-mm incision. Viscoelastic is injected under the iris and over the ciliary processes to widen the ciliary sulcus to facilitate visualization and minimize the risk of thermal burn to the adjacent iris. In phakic eyes, viscoelastic injection under the iris also pushes the crystalline lens backwards so that the likelihood of lens damage and cataract formation is decreased. At the end of the procedure, viscoelastic is removed to avoid postoperative IOP spikes. The limbal approach can be combined with either phacoemulsification in eyes with cataract or anterior vitrectomy in eyes with aphakia. The pars plana approach can be utilized in eyes with aphakia or pseudophakia, but the presence of the crystalline lens precludes this option. After the anterior vitreous is removed with a vitrector, the laser endoscope is introduced through the pars plana incision, and the ciliary processes are photocoagulated under endoscopic view.
Fig. 4.
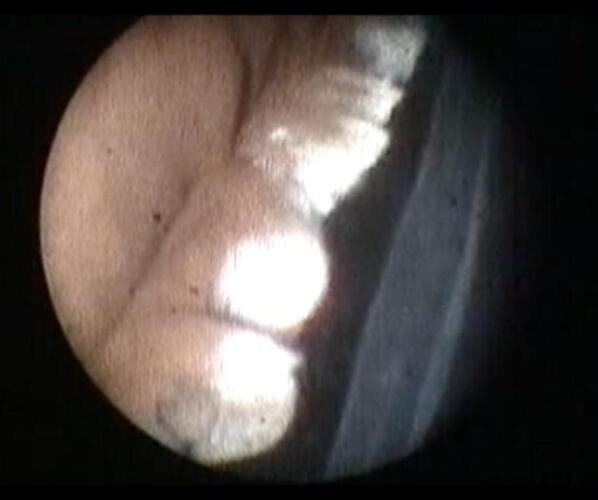
View during endoscopic cyclophotocoagulation. Treated ciliary process has shrunk and appears gray (upper part of image). From the archive of Dr. Brian A. Francis
Efficacy of Endoscopic Cyclophotocoagulation
More than 2 decades ago, Uram [58] reported results of diode laser endoscopic photocoagulation of the ciliary processes through the pars plana for the management of ten patients with intractable neovascular glaucoma and IOP ranging from 36 mmHg to 62 mmHg. After a mean follow-up of almost 9 months, nine patients had IOP < 21 mmHg, three of them with medication. Hypotony was observed in two eyes with preexisting chronic retinal detachment. In an early paper, Uram also published favorable results of the combined endoscopic cyclophotocoagulation and phacoemulsification with intraocular lens implantation in ten eyes with uncontrolled open-angle glaucoma and cataract [59]. With a mean follow-up of 19 months, the mean preoperative IOP was reduced from 31.4 mmHg to 13.5 mmHg (57% reduction), and half of the patients were well controlled without medications.
Chen et al. [60] reported results on the use of ECP in 68 eyes of 68 patients with refractory open-angle, congenital or secondary glaucoma, most of whom had a history of at least one failed glaucoma surgery. Twelve of these eyes underwent concurrent cataract extraction. With a follow-up of 13 months, the authors noted a significant reduction in mean preoperative IOP from 27.7 ± 10.3 mmHg to 17.0 ± 6.7 mmHg (34%, P < 0.0001) and a significant reduction in glaucoma medications from 3.0 ± 1.3 to 2.0 ± 1.3 (33%, P < 0.0001). At the last follow-up visit, 90% of eyes achieved IOP ≤ 21 mmHg with or without medications, and 74% had ≥ 20% IOP reduction with or without medications. Best-corrected visual acuity remained stable or improved in 94% of eyes, but 6% of eyes lost more than two lines of Snellen visual acuity. No serious complications such as phthisis, sympathetic ophthalmia, endophthalmitis or retinal detachment were noted.
In a prospective randomized trial with 58 eyes of 58 patients with glaucoma, Gayton et al. [61] compared the efficacy of combined phacoemulsification and trabeculectomy (n = 29, adjunctive use of mitomycin C in 14 eyes) versus combined phacoemulsification and ECP (n = 29). Eligible patients had to have one of the following: IOP ≥ 30 mmHg, progressive cupping or visual field loss. At the final available visit (> 6 months of follow-up), cases treated with phacoemulsification and trabeculectomy had an IOP reduction of 31.9% from baseline (24.6 ± 6.2 mmHg), while cases treated with phacoemulsification and ECP had an IOP reduction of 28.8% from baseline (24.8 ± 8.6 mmHg). In the phacoemulsification/trabeculectomy group, IOP control (defined as IOP < 19 mmHg without visual field progression or increase in cup/disc ratio) was achieved in 40% of patients without medications and 52% with medications. The respective percentages for the phacoemulsification/ECP group were 30% and 65%. Three patients in the former group (10%) and four patients in the latter group (14%) required further surgery and were considered failures.
Lima et al. [62] prospectively compared the efficacy of ECP and Ahmed valve implantation in pseudophakic eyes with refractory glaucoma. Using a quasi-randomized allocation method, they included 68 eyes of 68 patients who were followed for a mean of 21.3 ± 6.4 months (ECP group, n = 34) and 19.8 ± 8.3 months (Ahmed group, n = 34) (P = 0.4). The mean preoperative IOP was similar for the two groups (ECP: 41.6 ± 3.4 mmHg; Ahmed: 41.3 ± 3.0 mmHg, P = 0.5). At 24 months, the mean postoperative IOP in the two groups was not significantly different (ECP: 14.1 ± 7.2; Ahmed: 14.7 ± 6.4 mmHg, P = 0.7). Both groups used the same number of medications preoperatively (ECP: 3.0 ± 1.3; Ahmed: 3.5 ± 1.0, P = 0.7) and postoperatively (ECP: 2.0 ± 1.2; Ahmed: 2.5 ± 1.3, P = 0.3). After 24 months, success (defined as IOP between 6 and 21 mmHg with or without topical anti-hypertensive medications) was achieved in 73.5% and 70.6% of patients in the ECP and Ahmed groups, respectively (P = 0.7). Patients treated with ECP, however, had fewer complications. Moreover, patients in the Ahmed group had a greater incidence of visual acuity loss (37.5% vs. 16%, P = 0.001). The authors hypothesized that the ocular hypertensive phase observed after valve implantation may have resulted in visual decline in these eyes with advanced disease.
In a prospective trial, Francis et al. [63] examined the effectiveness of 360-degree ECP in 25 eyes of 25 patients with various types of refractory glaucoma that had undergone insertion of one or more aqueous shunts. After 12 months, the mean IOP dropped from 24.0 mmHg to 15.4 mmHg (30.8%), and the mean number of medications decreased from 3.2 to 1.5 (P < 0.001). Success (defined as IOP reduction ≥ 3 mmHg or a reduction in medications in the medication intolerant group and IOP < 21 mmHg) was 88% at 12 months (n = 18) and remained at that level until the end of the follow-up period of 2 years (n = 11, P < 0.01). No serious complications were observed.
Recently, Murakami et al. [64] retrospectively compared the efficacy of ECP versus the implantation of a second Baerveldt 350 or 250 drainage device in patients with an existing failed (but still functioning) Baerveldt 350 device. The sample consisted of patients with different types of refractory glaucoma excluding neovascular glaucoma. Twenty-five patients underwent 330–360-degree ECP, and 48 had either a Baerveldt 250 (n = 26) or a Baerveldt 350 (n = 22) device implanted. Failure was defined as continued uncontrolled IOP > 21 mmHg or IOP < 5 mmHg on two consecutive visits after 1 month, IOP reduction < 20% from preoperative baseline, need for additional glaucoma surgery or loss of light perception. The baseline IOP of the ECP (24.0 mmHg) and the Baerveldt group (23.5 mmHg) was similar (P = 0.85) and was significantly reduced in all postoperative visits up to the 24 months visit. There was no statistically significant difference in IOP at the 6-month (14.9 mmHg vs. 15.2 mmHg, P = 0.98), 12-month (15.4 mmHg vs. 14.2 mmHg, P = 0.61) or 24-month (18.1 mmHg vs. 14.6 mmHg, P = 0.14) visit for the ECP and the Baerveldt groups, respectively. Similarly, there was no significant difference in the number of medications used pre- or postoperatively at any visit for the two groups. There was no statistically significant difference in the cumulative probability of success up to the last follow-up at 24 months. The occurrence of complications was not significantly different in the two groups (P > 0.05).
An enhancement of the original technique was described by Tan et al. [32] in an effort to augment efficacy. In a retrospective, non-comparative case series, the authors examined the efficacy of ECP combined with pars plana laser ablation (“ECP-Plus”) in 53 eyes of 53 patients with various types of refractory glaucoma that remained uncontrolled (IOP > 21 mmHg) after at least one glaucoma surgery and maximally tolerated medical treatment. This modification of the standard ECP procedure was performed through a pars plana approach following pars plana vitrectomy. According to the authors, pars plana ablation may offer a more profound hypotensive effect by destroying any extension of the ciliary epithelium from the pars plicata, by limiting the blood flow to the ciliary body and by enhancing uveoscleral outflow. The mean preoperative IOP was reduced from baseline (27.9 ± 7.5 mmHg) at 12 months (10.7 ± 5.2 mmHg, 63% reduction) and 18–24 months (9.4 ± 5.8 mmHg, 66% reduction) (all P < 0.001). The mean number of medications used at the respective time points was 3.5 ± 1.2, 0.7 ± 1.0 and 0.7 ± 1.1 (all P < 0.001). Complications after the 6th postoperative month were seen in 16% of patients and included hypotony (8%), choroidal detachment (8%), cystoid macular edema without hypotony (6%) and corneal graft failure (2%).
ECP has also been tried in pediatric glaucoma patients with modest efficacy [65–69]. In one of the more recent reports, Carter et al. [65] retrospectively studied 34 eyes of 25 patients with a mean age at first treatment of 4.2 years with aphakic or pseudophakic glaucoma who were followed for a mean of 44.4 months. An average of 0.4 glaucoma procedures per eye had been performed before the initial ECP, with 82% of eyes receiving ECP as an initial procedure. The mean pretreatment IOP was 32.6 mmHg and was significantly reduced to 22.9 mmHg after a mean number of 1.5 ECP treatments per eye. The mean number of pre- and postoperative medications remained unchanged (1.2). Overall, 53% of patients achieved success (defined as IOP < 24 mmHg and IOP reduction > 15% with or without medications). Contrary to these results, a retrospective chart review study by Kraus et al. [70] found that ECP is an effective option in pediatric glaucoma cases. The authors compared data from 52 eyes of 43 patients who underwent ECP and 72 eyes of 56 patients who underwent transcleral CPC. The average number of ECP (3.24) and transcleral CPC (2.29) procedures was not statistically different. The respective treatments were performed as either a primary or secondary procedure. The IOP reduction after ECP was statistically similar to that after transcleral CPC (33.2% vs. 28.6%, respectively). Statistically similar rates of success (defined as IOP < 21 mmHg with or without medications) were achieved in cases treated with ECP (62%) and transcleral CPC (67.6%). Finally, a recent meta-analysis that included six studies with pediatric and adult cases with refractory glaucoma did not find any difference in efficacy or occurrence of complications between ECP (n = 204) and non-ECP procedures (n = 225) [71].
With increasing use and more data on safety of the technique, the use of ECP expanded beyond complex and refractory cases, and physicians started offering this option to patients with well-controlled glaucoma, typically in the setting of cataract extraction [72]. In a study with 626 eyes treated with concurrent phacoemulsification and ECP and a comparable cohort of 81 eyes treated with phacoemulsification alone, the authors found that after a mean follow-up of 3.2 years, IOP in the phaco/ECP group decreased from 19.1 mmHg to 15.7 mmHg and increased from 18.2 mmHg to 18.9 mmHg in the phaco group [73]. The number of antiglaucoma medications decreased from 1.53 preoperatively to 0.65 at the end of follow-up in the phaco/ECP group but remained unchanged (1.2) in the phaco group. There were no serious complications in either group.
The safety profile of the procedure was also documented in a study involving 5824 eyes and a follow-up of 5.2 years, with cystoid macular edema occurring in 0.7%, vision loss of more than two lines seen in 1% and other serious complications seen in less than 1% [74].
Kahook et al. [75] used a retrospective chart review to examine the effectiveness of ECP through one or two clear cornea incisions in patients undergoing phacoemulsification. The rationale of their investigation was to assess whether treatment of the complete arc of ciliary processes (360°, through two side ports) would be preferable to a less extensive treatment (240°–300°, through a single side port). Patients were grouped in a single-incision (n = 15) or a two-incision group (n = 25) and followed for up to 6 months. The respective preoperative pressures in the groups were similar (23.6 ± 3.9 vs. 24.5 ± 9.0, P = 0.72). No serious complications were observed in either group. The two-incision group achieved significantly lower IOP values at all postoperative measurement time points and a significantly greater reduction in glaucoma medication (2.6 ± 0.7 to 0.5 ± 0.6) compared with the single-incision group (2.5 ± 0.7 to 1.9 ± 0.9) (P < 0.001).
Other studies with a retrospective design also investigated the efficacy of ECP. Yip et al. [76] conducted a retrospective case review of 29 eyes from 29 Asian patients with various types of open-angle, closed-angle and secondary glaucomas who were followed up for a mean of 15.9 ± 8.9 months. Twenty of their patients underwent combined phaco/ECP. The mean pretreatment IOP (21.8 ± 6.6 mmHg) was significantly reduced at 18 months (16.2 ± 4.1 mmHg, n = 17) (P = 0.02). The mean number of medications was also significantly reduced (2.0 ± 1.0 vs. 0.9 ± 0.9, P = 0.04) at 18 months. At 24 months, however (n = 10), the differences were not statistically significant for IOP (21.8 ± 6.6 vs. 17.9 ± 4.9 mmHg, P = 0.18) and medications (2.0 ± 1.0 vs. 1.2 ± 0.8, P = 0.13). Overall, 48.3% of patients satisfied the definition of success used by the authors (IOP reduction > 20% without an increase in number of medications). As these results are poorer than the ones reported in Caucasians (e.g., 74% success reported by Chen et al. [60] using the same definition), the authors speculated that the procedure might be less effective in Asian eyes.
Lima et al. [62] retrospectively studied 368 eyes from 243 patients with POAG and cataract treated with combined phaco/ECP and found that IOP was significantly lowered from 23.07 mmHg preoperatively to 12.14 mmHg (47.4% reduction) while the mean number of medications was reduced from 1.44 ± 0.97 to 0.37 ± 0.74 after 2 years.
Lindfield et al. [77] conducted a retrospective chart review of 56 cases with cataract and mild open- or narrow-angle glaucoma that were treated with phaco/ECP. The preoperative IOP (21.5 mmHg) was significantly reduced (14.4 mmHg) at the last follow-up at 24 months (P < 0.001). However, the mean number of medications (1.97) remained unchanged up to the 24-month visit (2.07).
In another retrospective uncontrolled case series with 63 eyes of 59 patients with various types of glaucoma and a follow-up of 12 months, Clement et al. [78] reported that combined phaco/ECP reduced the mean preoperative IOP from 21.13 ± 6.21 mmHg to 16.09 ± 5.27 mmHg (23.9%, P < 0.01) while the mean number of medications was reduced from 2.71 ± 1.06 to 1.47 ± 1.30 (P < 0.01). Additionally, the authors observed that IOP reduction had a positive correlation with preoperative IOP levels (r = 0.63) and age (r = 0.55).
In a recent prospective non-randomized case-control study, Francis et al. [79] compared the effect of concurrent phacoemulsification and ECP (study group, n = 80) and phacoemulsification alone (control group, n = 80) in patients with controlled open-angle glaucoma and cataract. In the study group, the preoperative IOP significantly decreased from 18.1 ± 3.0 mmHg to 16.0 ± 3.3 mmHg after 2 years of follow-up. The number of medications in this group was decreased from 1.5 ± 0.8 preoperatively to 0.4 ± 0.7 after 2 years of follow-up. With a similar follow-up period, the respective IOP values in the control group were 18.1 ± 3.0 mmHg and 17.3 ± 3.2 mmHg, whereas the number of medications decreased from 2.4 ± 1.0 to 2.0 ± 1.0. The reduction in the IOP and number of medications was statistically significant in both groups (P < 0.05 for all comparisons). However, patients in the study group had significantly lower IOP (16.0 ± 3.3 vs. 17.3 ± 3.2 mmHg, P = 0.01) with significantly fewer medications (0.4 ± 0.7 vs. 2.0 ± 1.0, P < 0.001) at 2 years. Visual acuity outcomes and complication rates were similar in the two groups.
Siegel et al. [80] in a recent retrospective study with 36 months of follow-up compared the efficacy of combined phaco and ECP in 261 eyes and phacoemulsification alone in 52 eyes with open-angle or chronic angle-closure glaucoma. The IOP in the phacoemulsification/ECP group decreased from 17.2 ± 4.8 mmHg preoperatively to 14.7 ± 3.1 mmHg at the end of follow-up (P < 0.001). The respective values for the phaco group were 17.7 ± 4.4 mmHg and 15.5 ± 3.6 mmHg (P < 0.001). Although the IOP of the groups at the last follow-up visit (14.7 mmHg vs. 15.5 mmHg) was not significantly different (P = 0.34), eyes in the phaco/ECP group relied on significantly fewer medications (0.2 vs. 1.2, P < 0.001). The authors defined full success as IOP reduction > 20% with a decrease of at least one antiglaucoma drug and qualified success as IOP no higher than baseline with a decrease of at least one antiglaucoma medication. Using these definitions, significantly more eyes in the phacoemulsification/ECP group achieved full success (61.4% vs. 23.3%, P < 0.001) and qualified success (72.6% vs. 23.3%, P < 0.001).
A recent retrospective study examined the efficacy of combined phacoemulsification and ECP in 91 eyes of 73 patients with various types of glaucoma who were followed up for 12 months. Almost half of the eyes (43 of 91) had already undergone at least one glaucoma laser or incisional procedure [81]. Failure was defined as < 20% IOP reduction from baseline or IOP ≥ 21 mmHg or ≤ 5 mmHg on two consecutive visits or additional glaucoma surgery performed within 12 months after the procedure. The mean baseline IOP was reduced from 16.65 mmHg to 13.88 mmHg at 12 months (P < 0.0001), and the mean number of medications was reduced from 1.88 at baseline to 1.48 at 12 months (P < 0.001). The criterion of success was satisfied in 49.7% of patients at 12 months. The authors also noted that the only patient characteristic associated with success was higher baseline IOP.
There is evidence [75, 77–80, 82] to support that concurrent phacoemulsification and ECP can lower the IOP and allow a reduction of the total number of antiglaucoma medications. However, there is little research to suggest that IOP levels in the low teens can be achieved with this approach [81]. Consequently, the place of the combined phacoemulsification/ECP procedure in the management of advanced glaucoma remains unclear. To investigate if phaco/ECP can indeed achieve such low pressures, Morales et al. [83] conducted a retrospective chart review of 104 eyes from 104 patients with cataract and advanced glaucoma of various types who underwent phaco/ECP and were followed-up for a mean of 17.3 months. Because the sample consisted of patients with advanced glaucoma, the authors used two different stringent definitions of complete success: IOP < 15 mmHg or IOP reduction > 30% without medications. By analogy, qualified success was defined as: IOP < 15 mmHg or IOP reduction > 30% with medications. The mean IOP decreased from 17 ± 1.4 mmHg preoperatively to 14.7 ± 1.3 mmHg at the last follow-up visit. Using the “< 15 mmHg” criterion, complete and qualified success was observed for 12% and 72% of patients, respectively. Using the “> 30% IOP reduction” criterion, the respective percentages were nearly identical (14% and 71%, respectively).
The role of phaco/ECP in the treatment of plateau iris syndrome was also investigated recently [84]. In a prospective case series that included 12 eyes of 6 patients, the authors used UBM to evaluate several anterior chamber parameters before and 3–6 months following the procedure. The rationale of the study was that shrinkage of the large, anteriorly rotated ciliary processes with ECP (“endoscopic cycloplasty”) could diminish forward displacement of the peripheral iris, thus widening the anterior chamber angle. Several UBM parameters indicated that the combination of cycloplasty and phacoemulsification favorably affects the angle anatomy, though the IOP was not significantly reduced after 6 months (15.1 ± 4.7 mmHg vs. 15.6 ± 5.4 mmHg), and the mean number of medications only showed a tendency to decrease (1.6 ± 1.4 vs. 0.9 ± 1.4, P = 0.07). On the other hand, Hollander et al. [85] recently reported that the combination of phacoemulsification and ECP not only widens the anterior chamber angle, but also leads to significant IOP reduction in patients with plateau iris syndrome. In this small series with nine eyes of six patients followed for a mean period of approximately 6 years, the mean baseline IOP was significantly reduced from 25.2 ± 10.9 mmHg to 17.1 ± 5.3 mmHg (P < 0.05). The mean number of medications was also significantly reduced from 3.4 ± 1.0 preoperatively to 1.9 ± 1.5 at last visit (P < 0.001).
Recently, Vila-Arteaga et al. [86] described an ab-interno CPC technique that does not require expensive endoscopic instrumentation and is suited for the treatment of pseudophakic and aphakic eyes. Instead, the “gonioprism-assisted diode CPC” technique requires a surgical gonioprism, an ophthalmic operating microscope, iris hooks and an 810-nm laser diode probe similar to the one used in endolaser retinal photocoagulation. To perform this treatment, four iris hooks are placed 45 degrees apart from one another to expose the ciliary processes over the nasal side. Next, the laser probe is introduced in the anterior chamber through a temporal clear corneal incision. The ciliary processes are lasered under gonioscopic view until whitened and shrunk. The same process is repeated in the temporal side with the iris hooks placed temporally and the probe entered nasally. Published results on the efficacy and safety of this procedure are currently unavailable.
Finally, with the increasing popularity of different minimally invasive glaucoma surgeries, ECP has been performed as a combined procedure with phacoemulsification and iStent® (Glaukos Corp., Laguna Hills, CA, USA) implantation and referred to as the ‘ICE’ procedure [87]. Further evidence on the safety and efficacy of this procedure is currently lacking.
Conclusions
Cyclodestructive procedures such as cryotherapy or transscleral diode laser have been used for several decades in the management of patients with advanced refractory glaucoma who have already undergone, or are poor candidates for, filtering surgery. In recent years, based on cumulated evidence on the safety and efficacy of these techniques, the range of indications for cyclodestructive procedures may be expanding so that patients with less severe disease and better overall prognosis are treated with such modalities. The role of cyclodestruction in glaucoma management will most likely continue to evolve because of the introduction of novel technologies such as endoscopic diode cyclophotocoagulation, circular high-frequency cyclocoagulation and micropulse transscleral diode cyclophotocoagulation. Early evidence with these recently introduced technologies suggests they have a clinically acceptable efficacy and favorable safety profile. Confirmation of these first promising data with larger studies with adequate follow-up is needed before these modalities are more widely adopted in clinical practice. Further investigations on mechanisms mediating the physiologic responses in cyclodestruction [51] or novel experimental approaches [88] may prove fruitful directions for future research.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Medical writing, editorial and other assistance
No writing, editorial or other assistance was used in the preparation of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosures
Anna I. Dastiridou has nothing to disclose. Andreas Katsanos has received honoraria and congress expenses by Allergan, Novartis, Laboratoires Théa and Vianex. Philippe Denis has received lecture fees from Alcon, Allergan, EyeTechCare, Novartis, Santen and Laboratoires Théa. Brian A. Francis has received research support from BVI Endo Optiks and Iridex. Dimitrios G. Mikropoulos has received honoraria from Allergan, Novartis and Vianex. Miguel A. Teus has received lecture fees from Alcon, Allergan, Johnson & Johnson, Novartis; consultancy fees from Alcon, Allergan, Novartis; study & travel support from Alcon, Novartis and Johnson & Johnson. Anastasios-Georgios Konstas has received honoraria from Allergan, Bayer Healthcare, Mundipharma, Santen and Vianex; research funding from Allergan, Novartis and Santen.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
To view enhanced digital features for this article go to 10.6084/m9.figshare.7291136.
Anna I. Dastiridou and Andreas Katsanos have contributed equally and should both be considered first authors.
References
- 1.Dunphy EB, Albaugh CH. Cyclodiathermy: an operation for the treatment of glaucoma. Trans Am Ophthalmol Soc. 1941;39:193–213. [PMC free article] [PubMed] [Google Scholar]
- 2.Shahan W, Post L. Thermophore studies in glaucoma. Am J Ophthalmol. 1921;4:109–18. 10.1016/S0002-9394(21)90829-8 [DOI] [Google Scholar]
- 3.Verhoeff F. Cyclectomy: a new operation for glaucoma. Arch Ophthalmol. 1924;53:228–9. [Google Scholar]
- 4.Vogt A. Cyclodiathermypuncture in cases of glaucoma. Br J Ophthalmol. 1940;24:288–97. 10.1136/bjo.24.6.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bietti G. Surgical intervention on the ciliary body; new trends for the relief of glaucoma. J Am Med Assoc. 1950;142:889–97. 10.1001/jama.1950.02910300027006 [DOI] [PubMed] [Google Scholar]
- 6.Benson MT, Nelson ME. Cyclocryotherapy: a review of cases over a 10-year period. Br J Ophthalmol. 1990;74:103–5. 10.1136/bjo.74.2.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu GJ, Mizukawa A, Okisaka S. Mechanism of intraocular pressure decrease after contact transscleral continuous-wave Nd:YAG laser cyclophotocoagulation. Ophthalmic Res. 1994;26:65–79. 10.1159/000267395 [DOI] [PubMed] [Google Scholar]
- 8.Schubert HD, Agarwala A, Arbizo V. Changes in aqueous outflow after in vitro neodymium: yttrium aluminum garnet laser cyclophotocoagulation. Invest Ophthalmol Vis Sci. 1990;31:1834–8. [PubMed] [Google Scholar]
- 9.Quigley HA. Histological and physiological studies of cyclocryotherapy in primate and human eyes. Am J Ophthalmol. 1976;82:722–32. 10.1016/0002-9394(76)90009-X [DOI] [PubMed] [Google Scholar]
- 10.Ferry AP, King MH, Richards DW. Histopathologic observations on human eyes following neodymium: YAG laser cyclophotocoagulation for glaucoma. Trans Am Ophthalmol Soc. 1995;93:315–31 (discussion 332–336). [PMC free article] [PubMed] [Google Scholar]
- 11.Smith RS, Boyle E, Rudt LA. Cyclocryotherapy: a light and electron microscopic study. Arch Ophthalmol Chic Ill. 1960;1977(95):285–8. [DOI] [PubMed] [Google Scholar]
- 12.Schuman JS, Jacobson JJ, Puliafito CA, Noecker RJ, Reidy WT. Experimental use of semiconductor diode laser in contact transscleral cyclophotocoagulation in rabbits. Arch Ophthalmol Chic Ill. 1960;1990(108):1152–7. [DOI] [PubMed] [Google Scholar]
- 13.Brancato R, Trabucchi G, Verdi M, Carassa RG, Gobbi PG. Diode and Nd:YAG laser contact transscleral cyclophotocoagulation in a human eye: a comparative histopathologic study of the lesions produced using a new fiber optic probe. Ophthalmic Surg. 1994;25:607–11. [PubMed] [Google Scholar]
- 14.Brancato R, Leoni G, Trabucchi G, Cappellini A. Histopathology of continuous wave neodymium: yttrium aluminum garnet and diode laser contact transscleral lesions in rabbit ciliary body. A comparative study. Invest Ophthalmol Vis Sci. 1991;32:1586–92. [PubMed] [Google Scholar]
- 15.Pantcheva MB, Kahook MY, Schuman JS, Noecker RJ. Comparison of acute structural and histopathological changes in human autopsy eyes after endoscopic cyclophotocoagulation and trans-scleral cyclophotocoagulation. Br J Ophthalmol. 2007;91:248–52. 10.1136/bjo.2006.103580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Glaucoma Society. Terminology and guidelines for glaucoma. 4th ed. Savona: PubliComm; 2014. [Google Scholar]
- 17.Topouzis F, Yu F, Coleman AL. Factors associated with elevated rates of adverse outcomes after cyclodestructive procedures versus drainage device procedures. Ophthalmology. 1998;105:2276–81. 10.1016/S0161-6420(98)91229-5 [DOI] [PubMed] [Google Scholar]
- 18.Kramp K, Vick H-P, Guthoff R. Transscleral diode laser contact cyclophotocoagulation in the treatment of different glaucomas, also as primary surgery. Graefes Arch Clin Exp Ophthalmol. 2002;240:698–703. 10.1007/s00417-002-0508-5 [DOI] [PubMed] [Google Scholar]
- 19.Holló G, Katsanos A, Konstas AG. Management of exfoliative glaucoma: challenges and solutions. Clin Ophthalmol. 2015;9:907–19. 10.2147/OPTH.S77570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bloom P, Negi A, Kersey T, Crawley L. Cyclodestructive techniques. In: Shaarawy T, Sherwood M, Hitchings R, Crowston J, editors. Glaucoma. 2nd ed. London, UK: Elsevier; 2015. p. 1150–9. [Google Scholar]
- 21.Endophotocoagulation Berke S. In: Shaarawy T, Sherwood M, Hitchings R, Crowston J, editors. Glaucoma. 2nd ed. London, UK: Elsevier; 2015. p. 1160–6. [Google Scholar]
- 22.Pastor SA, Singh K, Lee DA, Juzych MS, Lin SC, Netland PA, et al. Cyclophotocoagulation: a report by the American Academy of Ophthalmology. Ophthalmology. 2001;108:2130–8. 10.1016/S0161-6420(01)00889-2 [DOI] [PubMed] [Google Scholar]
- 23.Michelessi M, Bicket AK, Lindsley K. Cyclodestructive procedures for non-refractory glaucoma. Cochrane Database Syst Rev. 2018;4:CD009313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldenberg-Cohen N, Bahar I, Ostashinski M, Lusky M, Weinberger D, Gaton DD. Cyclocryotherapy versus transscleral diode laser cyclophotocoagulation for uncontrolled intraocular pressure. Ophthalmic Surg Lasers Imaging. 2005;36:272–9. 10.3928/1542-8877-20050701-04 [DOI] [PubMed] [Google Scholar]
- 25.Gorsler I, Thieme H, Meltendorf C. Cyclophotocoagulation and cyclocryocoagulation as primary surgical procedures for open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2015;253:2273–7. 10.1007/s00417-015-3159-z [DOI] [PubMed] [Google Scholar]
- 26.Agarwal HC, Gupta V, Sihota R. Evaluation of contact versus non-contact diode laser cyclophotocoagulation for refractory glaucomas using similar energy settings. Clin Exp Ophthalmol. 2004;32:33–8. 10.1046/j.1442-9071.2004.00754.x [DOI] [PubMed] [Google Scholar]
- 27.Oguri A, Sogano S, Yamamoto T, Kitazawa Y. Incidence of elevation of intraocular pressure over time and associated factors in normal-tension glaucoma. J Glaucoma. 1998;7:117–20. 10.1097/00061198-199804000-00009 [DOI] [PubMed] [Google Scholar]
- 28.Youn J, Cox TA, Herndon LW, Allingham RR, Shields MB. A clinical comparison of transscleral cyclophotocoagulation with neodymium: YAG and semiconductor diode lasers. Am J Ophthalmol. 1998;126:640–7. 10.1016/S0002-9394(98)00228-1 [DOI] [PubMed] [Google Scholar]
- 29.Lin P, Wollstein G, Glavas IP, Schuman JS. Contact transscleral neodymium:yttrium-aluminum-garnet laser cyclophotocoagulation Long-term outcome. Ophthalmology. 2004;111:2137–43. 10.1016/j.ophtha.2004.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aquino MCD, Barton K, Tan AMWT, Sng C, Li X, Loon SC, et al. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: a randomized exploratory study. Clin Exp Ophthalmol. 2015;43:40–6. 10.1111/ceo.12360 [DOI] [PubMed] [Google Scholar]
- 31.Tan AM, Chockalingam M, Aquino MC, Lim ZI-L, See JL-S, Chew PT. Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin Exp Ophthalmol. 2010;38:266–72. [DOI] [PubMed] [Google Scholar]
- 32.Tan JCH, Francis BA, Noecker R, Uram M, Dustin L, Chopra V. Endoscopic cyclophotocoagulation and pars plana ablation (ECP-plus) to treat refractory glaucoma. J Glaucoma. 2016;25:e117–22. 10.1097/IJG.0000000000000278 [DOI] [PubMed] [Google Scholar]
- 33.Friberg TR, Venkatesh S. Alteration of pulse configuration affects the pain response during diode laser photocoagulation. Lasers Surg Med. 1995;16:380–3. 10.1002/lsm.1900160409 [DOI] [PubMed] [Google Scholar]
- 34.Kosoko O, Gaasterland DE, Pollack IP, Enger CL. Long-term outcome of initial ciliary ablation with contact diode laser transscleral cyclophotocoagulation for severe glaucoma. The diode laser ciliary ablation study group. Ophthalmology. 1996;103:1294–302. 10.1016/S0161-6420(96)30508-3 [DOI] [PubMed] [Google Scholar]
- 35.Bloom PA, Tsai JC, Sharma K, Miller MH, Rice NS, Hitchings RA, et al. “Cyclodiode”. Trans-scleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. Ophthalmology. 1997;104:1508–19 (discussion 1519–1520). 10.1016/S0161-6420(97)30109-2 [DOI] [PubMed] [Google Scholar]
- 36.Hauber FA, Scherer WJ. Influence of total energy delivery on success rate after contact diode laser transscleral cyclophotocoagulation: a retrospective case review and meta-analysis. J Glaucoma. 2002;11:329–33. 10.1097/00061198-200208000-00009 [DOI] [PubMed] [Google Scholar]
- 37.Kirwan JF, Shah P, Khaw PT. Diode laser cyclophotocoagulation: role in the management of refractory pediatric glaucomas. Ophthalmology. 2002;109:316–23. 10.1016/S0161-6420(01)00898-3 [DOI] [PubMed] [Google Scholar]
- 38.Malik R, Ellingham RB, Suleman H, Morgan WH. Refractory glaucoma–tube or diode? Clin Exp Ophthalmol. 2006;34:771–7. 10.1111/j.1442-9071.2006.01339.x [DOI] [PubMed] [Google Scholar]
- 39.Schaefer JL, Levine MA, Martorana G, Koenigsman H, Smith MF, Sherwood MB. Failed glaucoma drainage implant: long-term outcomes of a second glaucoma drainage device versus cyclophotocoagulation. Br J Ophthalmol. 2015;99:1718–24. 10.1136/bjophthalmol-2015-306725 [DOI] [PubMed] [Google Scholar]
- 40.Levinson JD, Giangiacomo AL, Beck AD, Pruett PB, Superak HM, Lynn MJ, et al. A comparison of sequential glaucoma drainage device implantation versus cyclophotocoagulation following failure of a primary drainage device. J Glaucoma. 2017;26:311–4. 10.1097/IJG.0000000000000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang MY, Patel K, Blieden LS, Chuang AZ, Baker LA, Bell NP, et al. Comparison of efficacy and complications of cyclophotocoagulation and second glaucoma drainage device after initial glaucoma drainage device failure. J Glaucoma. 2017;26:1010–8. 10.1097/IJG.0000000000000766 [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez-García A, González-González LA, Carlos Alvarez-Guzmán J. Trans-scleral diode laser cyclophotocoagulation for refractory glaucoma after high-risk penetrating keratoplasty. Int Ophthalmol. 2016;36:373–83. 10.1007/s10792-015-0130-2 [DOI] [PubMed] [Google Scholar]
- 43.Vernon SA, Koppens JM, Menon GJ, Negi AK. Diode laser cycloablation in adult glaucoma: long-term results of a standard protocol and review of current literature. Clin Exp Ophthalmol. 2006;34:411–20. 10.1111/j.1442-9071.2006.01241.x [DOI] [PubMed] [Google Scholar]
- 44.Aptel F, Denis P. Ultrasonic circular cyclocoagulation. In: Samples J, Ahmed I, editors. Surg innov glaucoma. New York: Springer; 2014. [Google Scholar]
- 45.Aptel F, Charrel T, Lafon C, Romano F, Chapelon J-Y, Blumen-Ohana E, et al. Miniaturized high-intensity focused ultrasound device in patients with glaucoma: a clinical pilot study. Invest Ophthalmol Vis Sci. 2011;52:8747–53. 10.1167/iovs.11-8137 [DOI] [PubMed] [Google Scholar]
- 46.Aptel F, Charrel T, Palazzi X, Chapelon J-Y, Denis P, Lafon C. Histologic effects of a new device for high-intensity focused ultrasound cyclocoagulation. Invest Ophthalmol Vis Sci. 2010;51:5092–8. 10.1167/iovs.09-5135 [DOI] [PubMed] [Google Scholar]
- 47.Charrel T, Aptel F, Birer A, Chavrier F, Romano F, Chapelon J-Y, et al. Development of a miniaturized HIFU device for glaucoma treatment with conformal coagulation of the ciliary bodies. Ultrasound Med Biol. 2011;37:742–54. 10.1016/j.ultrasmedbio.2011.01.017 [DOI] [PubMed] [Google Scholar]
- 48.Aptel F, Dupuy C, Rouland J-F. Treatment of refractory open-angle glaucoma using ultrasonic circular cyclocoagulation: a prospective case series. Curr Med Res Opin. 2014;30:1599–605. 10.1185/03007995.2014.910509 [DOI] [PubMed] [Google Scholar]
- 49.Lizzi FL, Driller J, Ostromogilsky M. Thermal model for ultrasonic treatment of glaucoma. Ultrasound Med Biol. 1984;10:289–98. 10.1016/0301-5629(84)90163-7 [DOI] [PubMed] [Google Scholar]
- 50.Aptel F, Béglé A, Razavi A, Romano F, Charrel T, Chapelon J-Y, et al. Short- and long-term effects on the ciliary body and the aqueous outflow pathways of high-intensity focused ultrasound cyclocoagulation. Ultrasound Med Biol. 2014;40:2096–106. 10.1016/j.ultrasmedbio.2014.04.017 [DOI] [PubMed] [Google Scholar]
- 51.Mastropasqua R, Agnifili L, Fasanella V, Toto L, Brescia L, Di Staso S, et al. Uveo-scleral outflow pathways after ultrasonic cyclocoagulation in refractory glaucoma: an anterior segment optical coherence tomography and in vivo confocal study. Br J Ophthalmol. 2016;100:1668–75. 10.1136/bjophthalmol-2015-308069 [DOI] [PubMed] [Google Scholar]
- 52.Denis P, Aptel F, Rouland J-F, Nordmann J-P, Lachkar Y, Renard J-P, et al. Cyclocoagulation of the ciliary bodies by high-intensity focused ultrasound: a 12-month multicenter study. Invest Ophthalmol Vis Sci. 2015;56:1089–96. 10.1167/iovs.14-14973 [DOI] [PubMed] [Google Scholar]
- 53.Melamed S, Goldenfeld M, Cotlear D, Skaat A, Moroz I. High-intensity focused ultrasound treatment in refractory glaucoma patients: results at 1 year of prospective clinical study. Eur J Ophthalmol. 2015;25:483–9. 10.5301/ejo.5000620 [DOI] [PubMed] [Google Scholar]
- 54.Giannaccare G, Vagge A, Gizzi C, Bagnis A, Sebastiani S, Del Noce C, et al. High-intensity focused ultrasound treatment in patients with refractory glaucoma. Graefes Arch Clin Exp Ophthalmol. 2017;255:599–605. 10.1007/s00417-016-3563-z [DOI] [PubMed] [Google Scholar]
- 55.Aptel F, Denis P, Rouland J-F, Renard J-P, Bron A. Multicenter clinical trial of high-intensity focused ultrasound treatment in glaucoma patients without previous filtering surgery. Acta Ophthalmol (Copenh). 2016;94:e268–77. 10.1111/aos.12913 [DOI] [PubMed] [Google Scholar]
- 56.Denis P. Clinical research of ultrasound ciliary plasty and implications for clinical practice. Eur Ophthalmic Rev. 2016;10:108–12. 10.17925/EOR.2016.10.02.108 [DOI] [Google Scholar]
- 57.Aptel F, Rouland J, Stalmans I, Denis F. Ultrasonic circular cyclo coagulation in patients with primary open-angle glaucoma with a second generation probe: Results of a multicenter clinical trial. Prague, Czech Republic: Eur Glaucoma Soc Meet; 2016. [Google Scholar]
- 58.Uram M. Ophthalmic laser microendoscope ciliary process ablation in the management of neovascular glaucoma. Ophthalmology. 1992;99:1823–8. 10.1016/S0161-6420(92)31718-X [DOI] [PubMed] [Google Scholar]
- 59.Uram M. Combined phacoemulsification, endoscopic ciliary process photocoagulation, and intraocular lens implantation in glaucoma management. Ophthalmic Surg. 1995;26:346–52. [PubMed] [Google Scholar]
- 60.Chen J, Cohn RA, Lin SC, Cortes AE, Alvarado JA. Endoscopic photocoagulation of the ciliary body for treatment of refractory glaucomas. Am J Ophthalmol. 1997;124:787–96. 10.1016/S0002-9394(14)71696-4 [DOI] [PubMed] [Google Scholar]
- 61.Gayton JL, Van Der Karr M, Sanders V. Combined cataract and glaucoma surgery: trabeculectomy versus endoscopic laser cycloablation. J Cataract Refract Surg. 1999;25:1214–9. 10.1016/S0886-3350(99)00141-8 [DOI] [PubMed] [Google Scholar]
- 62.Lima FE, Magacho L, Carvalho DM, Susanna R, Avila MP. A prospective, comparative study between endoscopic cyclophotocoagulation and the Ahmed drainage implant in refractory glaucoma. J Glaucoma. 2004;13:233–7. 10.1097/00061198-200406000-00011 [DOI] [PubMed] [Google Scholar]
- 63.Francis BA, Kawji AS, Vo NT, Dustin L, Chopra V. Endoscopic cyclophotocoagulation (ECP) in the management of uncontrolled glaucoma with prior aqueous tube shunt. J Glaucoma. 2011;20:523–7. [DOI] [PubMed] [Google Scholar]
- 64.Murakami Y, Akil H, Chahal J, Dustin L, Tan J, Chopra V, et al. Endoscopic cyclophotocoagulation versus second glaucoma drainage device after prior aqueous tube shunt surgery. Clin Exp Ophthalmol. 2017;45:241–6. 10.1111/ceo.12828 [DOI] [PubMed] [Google Scholar]
- 65.Carter BC, Plager DA, Neely DE, Sprunger DT, Sondhi N, Roberts GJ. Endoscopic diode laser cyclophotocoagulation in the management of aphakic and pseudophakic glaucoma in children. J AAPOS. 2007;11:34–40. 10.1016/j.jaapos.2006.08.015 [DOI] [PubMed] [Google Scholar]
- 66.Wallace DK, Plager DA, Snyder SK, Raiesdana A, Helveston EM, Ellis FD. Surgical results of secondary glaucomas in childhood. Ophthalmology. 1998;105:101–11. 10.1016/S0161-6420(98)91519-6 [DOI] [PubMed] [Google Scholar]
- 67.Plager DA, Neely DE. Intermediate-term results of endoscopic diode laser cyclophotocoagulation for pediatric glaucoma. J AAPOS. 1999;3:131–7. 10.1016/S1091-8531(99)70057-1 [DOI] [PubMed] [Google Scholar]
- 68.Neely DE, Plager DA. Endocyclophotocoagulation for management of difficult pediatric glaucomas. J AAPOS. 2001;5:221–9. 10.1067/mpa.2001.116868 [DOI] [PubMed] [Google Scholar]
- 69.Al-Haddad CE, Freedman SF. Endoscopic laser cyclophotocoagulation in pediatric glaucoma with corneal opacities. J AAPOS. 2007;11:23–8. 10.1016/j.jaapos.2006.08.005 [DOI] [PubMed] [Google Scholar]
- 70.Kraus CL, Tychsen L, Lueder GT, Culican SM. Comparison of the effectiveness and safety of transscleral cyclophotocoagulation and endoscopic cyclophotocoagulation in pediatric glaucoma. J Pediatr Ophthalmol Strabismus. 2014;51:120–7. 10.3928/01913913-20140211-01 [DOI] [PubMed] [Google Scholar]
- 71.Yang Y, Zhong J, Dun Z, Liu X, Yu M. Comparison of efficacy between endoscopic cyclophotocoagulation and alternative surgeries in refractory glaucoma: a meta-analysis. Medicine (Baltimore). 2015;94:e1651. 10.1097/MD.0000000000001651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berke S, Cohen A, Sturm R, Caronia R, Nelson D. Endoscopic cyclophotocoagulation (ECP) and phacoemulsification in the treatment of medically controlled open angle glaucoma. Annual Meeting of the American Glaucoma Society 2000.
- 73.Berke S, Sturm R, Caronia R. Phacoemulsification combined with endoscopic cyclophotocoagulation (ECP) in the management of cataract and medically controlled glaucoma: a large, long-term study. Las Vegas: Annual Meeting of the American Academy of Ophthalmology; 2006. [Google Scholar]
- 74.Noecker R. Complications of endoscopic cyclophotocoagulation: ECP Collaborative Study Group. The ASCRS Symposium on Cataract, IOL and Refractive Surgery, San Diego, CA, USA; 2007.
- 75.Kahook MY, Lathrop KL, Noecker RJ. One-site versus two-site endoscopic cyclophotocoagulation. J Glaucoma. 2007;16:527–30. 10.1097/IJG.0b013e3180575215 [DOI] [PubMed] [Google Scholar]
- 76.Yip LW, Yong SO, Earnest A, Ji J, Lim BA. Endoscopic cyclophotocoagulation for the treatment of glaucoma: an Asian experience. Clin Exp Ophthalmol. 2009;37:692–7. 10.1111/j.1442-9071.2009.02120.x [DOI] [PubMed] [Google Scholar]
- 77.Lindfield D, Ritchie RW, Griffiths MF. “Phaco-ECP”: combined endoscopic cyclophotocoagulation and cataract surgery to augment medical control of glaucoma. BMJ Open. 2012;2:e000578. 10.1136/bmjopen-2011-000578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clement CI, Kampougeris G, Ahmed F, Cordeiro MF, Bloom PA. Combining phacoemulsification with endoscopic cyclophotocoagulation to manage cataract and glaucoma. Clin Exp Ophthalmol. 2013;41:546–51. 10.1111/ceo.12051 [DOI] [PubMed] [Google Scholar]
- 79.Francis BA, Berke SJ, Dustin L, Noecker R. Endoscopic cyclophotocoagulation combined with phacoemulsification versus phacoemulsification alone in medically controlled glaucoma. J Cataract Refract Surg. 2014;40:1313–21. 10.1016/j.jcrs.2014.06.021 [DOI] [PubMed] [Google Scholar]
- 80.Siegel MJ, Boling WS, Faridi OS, Gupta CK, Kim C, Boling RC, et al. Combined endoscopic cyclophotocoagulation and phacoemulsification versus phacoemulsification alone in the treatment of mild to moderate glaucoma. Clin Exp Ophthalmol. 2015;43:531–9. 10.1111/ceo.12510 [DOI] [PubMed] [Google Scholar]
- 81.Roberts SJ, Mulvahill M, SooHoo JR, Pantcheva MB, Kahook MY, Seibold LK. Efficacy of combined cataract extraction and endoscopic cyclophotocoagulation for the reduction of intraocular pressure and medication burden. Int J Ophthalmol. 2016;9:693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lima FEL, de Carvalho DM. Avila MP de [Phacoemulsification and endoscopic cyclophotocoagulation as primary surgical procedure in coexisting cataract and glaucoma]. Arq Bras Oftalmol. 2010;73:419–22. 10.1590/S0004-27492010000500006 [DOI] [PubMed] [Google Scholar]
- 83.Morales J, Al Qahtani M, Khandekar R, Al Shahwan S, Al Odhayb S, Al Mobarak F, et al. Intraocular pressure following phacoemulsification and endoscopic cyclophotocoagulation for advanced glaucoma: 1-year outcomes. J Glaucoma. 2015;24:e157–62. 10.1097/IJG.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 84.Francis BA, Pouw A, Jenkins D, Babic K, Vakili G, Tan J, et al. Endoscopic cycloplasty (ECPL) and lens extraction in the treatment of severe plateau iris syndrome. J Glaucoma. 2016;25:e128–33. 10.1097/IJG.0000000000000156 [DOI] [PubMed] [Google Scholar]
- 85.Hollander DA, Pennesi ME, Alvarado JA. Management of plateau iris syndrome with cataract extraction and endoscopic cyclophotocoagulation. Exp Eye Res. 2017;158:190–4. 10.1016/j.exer.2016.07.018 [DOI] [PubMed] [Google Scholar]
- 86.Vila-Arteaga J, Stirbu O, Suriano MM, Vila-Mascarell E. A new technique for diode laser cyclophotocoagulation. J Glaucoma. 2014;23:35–6. 10.1097/IJG.0b013e31826981b1 [DOI] [PubMed] [Google Scholar]
- 87.Radcliffe N, Parekh P, Noecker R, Sarkisian S, Chapman K. ICE surgical technique outcomes: MIGS implantation of trabecular bypass stent, cataract extraction and endoscopic cyclophtocoagulation. Washington: Annual Meeting of the American Glaucoma Society; 2014. [Google Scholar]
- 88.Charisis SK, Detorakis ET, Vitanova VS, Panteleontidis VA, Kounis GA, Tsilimbaris MK. Contact transcleral photodynamic cyclo-suppression in human eyes: a feasibility study. Can J Ophthalmol. 2011;46:196–8. 10.3129/i10-109 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.