Abstract
Purpose
Individual variations in response of C-reactive protein (CRP) to acute strenuous exercise are less well known. The purpose of this study was to investigate the relationship between running economy and systemic inflammation following a marathon.
Materials and methods
Sixteen college recreational runners participated in this study. To measure maximal oxygen uptake and running economy, the treadmill running test was performed 1–2 weeks before the marathon race. Running economy was defined as oxygen cost (mL/kg/km) at submaximal running. CRP and muscle damage markers (creatine kinase and lactate dehydrogenase) were measured before and 1, 2, and 3 days after the race.
Results
All subjects completed the race in 4 hours 7 minutes 43 seconds±44 minute 29 seconds [mean±SD]. The marathon running significantly increased CRP and muscle damage markers. The levels of inflammation and muscle damage peaked after 1 day and remained high throughout the 3-day recovery period compared to that before the race. Spearman correlation analysis showed that the change in CRP level was significantly positively correlated with oxygen cost (r=0.619, P=0.011) but not maximal oxygen uptake. There was no significant relationship in responses between muscle damage markers and CRP.
Conclusion
These findings suggest that running economy is related to postmarathon race CRP response. Further study to clarify the cause of the relationship and clinical significance of transient increase in CRP is necessary.
Keywords: inflammation, oxygen consumption, C-reactive protein, muscle damage
Introduction
Running is one of the most popular forms of aerobic exercise. It is well known that habitual moderate aerobic exercise is effective in improving cardiovascular fitness.1 However, acute strenuous running, such as marathon running, can create acute physiological impacts.2–6 Biomarkers of muscle damage and inflammation have been measured as indicators of exercise stress.7 Kobayashi et al4 investigated creatine kinase (CK) and lactate dehydrogenase (LDH) to quantify muscle damage before and after a marathon race. In this report, post-race CK and LDH were significantly increased in comparison with pre-race value. C-reactive protein (CRP) is one of the markers of systemic inflammation following acute exercise. Kim et al2 investigated the CRP before and for 6 days after a marathon run and reported that it peaked 1 day after the race, returning to the prerace level on day 4. The conclusion from these studies was that a marathon run has a negative impact on markers of muscle damage and systemic inflammation.
Although the overall effects of marathon running on muscle damage and systemic inflammation have been investigated, individualization of factors that influence the magnitude of these responses has been less studied. It had been reported that differences in race time explained 32% of variability in changes in CRP after a marathon run.5 In addition, the most recent study indicated that high physical fitness was related to the attenuation of CRP elevation after an endurance cycling race.8 In this report, however, race time was used as a measure of physical fitness and no objective aerobic fitness markers were assessed. It is well known that endurance race performance is closely related to maximal oxygen uptake (VO2max) and running economy, which is defined as steady-state oxygen cost (O2 cost).9 In particular, the running economy is closely related to energy expenditure during the marathon race since the runner generates energy based on the oxygen consumption.10 In addition, one of the major energy sources is glycogen, as demonstrated by a significant reduction in glycogen concentration after a marathon run.11 Pedersen12 suggests that an acute increase in plasma IL-6, which stimulates CRP release via the hepatocyte, is dependent on muscle glycogen stores. Transcription of IL-6 is faster with low muscle glycogen content compared to high muscle glycogen content.12 Thus, it is reasonable to assume that CRP elevation post-race in runners with excellent running economies (ie, lower O2 cost) is minor because the energy expenditure is smaller. However, to our knowledge, the relationship between running economy and CRP response has not been taken into consideration to a significant extent thus far.
Kampus et al13 reported that acute elevation of the CRP after strenuous exercise may affect small artery elasticity, which is an important cardiovascular factor. Indeed, acute inflammation plays an important role in the development of atrial fibrosis and the progression to atrial fibrillation.14 Moreover, using two experimental groups consisting of a cherry juice (CJ) group and a placebo (PL) group, Dimitriou et al15 found that the CRP increase at 24 and 48 hours post-marathon was lower in the CJ group compared with the PL group. In the previous study, interestingly, no upper respiratory tract symptoms were reported in the CJ group, whereas 50% of runners in the PL group reported these symptoms after the race. Collectively, these previous studies indicate that excessive systemic inflammation after a marathon run is detrimental to health-related physiological functions. It is therefore important to understand factors related to individual variation in CRP response.
The purpose of the present study was to investigate the relationship between running economy and systemic inflammation following a marathon race. We hypothesized that running economy is related to post-race CRP response.
Materials and methods
Subjects
Sixteen recreational runners (13 males and 3 females, aged 24.1±3 years, height 170±7 cm, and weight 61.6±10.2 kg [mean±SD]) participated in this study. The subjects were recruited through advertisement and word of mouth. All the subjects were free of injury and disease. All subjects had taken part in regular running training for the race. Training distance was 24±14 km/week and the longest training session was 33±19 km during the 3 months before the race.
Before participating, all subjects signed an informed consent, while ethical approval for the present study was granted by the Research Ethics Committee of the University of Tsukuba. This study was conducted according to the Declaration of Helsinki.
Experimental design
The present study was conducted before and after the 37th Tsukuba Marathon held in Ibaraki, Japan. This race was held on November 26, 2017 and was officially recognized by the Japan Association of Athletics Federations and a flat-road race course. At the start of the race, the temperature was 7.1°C, the relative humidity 64%, and wind speed 2.6 m/s.
To measure the aerobic fitness, a treadmill running test was performed 1–2 weeks before the race. Blood sampling was conducted 4 days before (PRE) and 1 (POST1), 2 (POST2), and 3 (POST3) days after the marathon race. The dependent variables measured were serum CK and serum LDH as muscle damage markers and serum CRP as indicator of systemic inflammation.
Treadmill running test
The treadmill running test was performed on a motorized treadmill (ORK-7000; Ohtake-Root Kogyo Co, Iwate, Japan) set at 1% gradient according to previous studies.9,16 This test consisted of a running economy test and a maximal incremental test. First, the subjects underwent a 5-minute running economy test at submaximal intensity that was set individually by the treadmill velocity at 85% of the velocity corresponding with the target velocity of the marathon race. Following a 5-minute recovery period after the running economy test, the maximal incremental test was performed. This test was initiated with the velocity at 8.4 km/h and was increased by 0.6 km/h per minute until volitional exhaustion.
Expired gas analysis was performed on a breath-by-breath basis using the computerized standard open circuit technique by the metabolic gas analyzer (AE-310s; Minato Medical Science, Osaka, Japan). The data were analyzed by obtaining mean values at 20-second intervals. Before the test, the metabolic system was calibrated using known gas concentrations and 2-L syringe according to the manufacturer’s instructions. Heart rate (HR) was measured using a surface electrode chest strap (Polar H7 and M400; Polar Electro, Kempele, Finland).
The O2 cost was calculated based on the last 1-minute VO2 during the running economy test. VO2max was defined as the attainment of at least two of the following four criteria: 1) a leveling-off VO2 despite an increase in the velocity; 2) peak respiratory exchange ratio (RER) ≥1.10, 3) peak HR ≥95% of the age-predicted HRmax, and 4) ratings of perceived exertion at the end of the test ≥19. All subjects consumed a light meal at least 3 hours before the treadmill running test and during the 3 hours prior to the test, and water intake was allowed as needed.
Blood sampling
After overnight fasting, the blood sample (~6 mL per sample) was drawn from an antecubital vein using a butterfly needle (Terumo, Tokyo, Japan) in a sitting position between 7:00 and 10:00 am. The collected blood was rapidly handed to the Tsukuba i-Laboratory LLP. After centrifugation at 3,000 rpm for 8 minutes, the serum was tested with an automatic clinical chemistry analyzer (LABOSPECT 008; Hitachi, Tokyo, Japan). CK and LDH were quantified by the Japan Society of Clinical Chemistry standardization method. The CRP was quantified using a latex coagulating nephelometry. The detection limit of CRP was 0.03 mg/dL. Where activity was undetectable, the sample was assigned a value equivalent to the detection limit. The reference values were as follows: CK, 63–257 U/L (male), 42–150 U/L (female); LDH, 106–211 U/L, and CRP <0.2 mg/dL.
Statistical analysis
Data are expressed as means with SD. Friedman rank sums test and post hoc Wilcoxon-signed rank test were used for comparing between days. The relationships between selected variables were determined using Spearman correlation analysis. Because significant relationships were observed in each blood marker from POST1 to POST3, the POST1 values were taken as the representative value for the magnitude of damage. The Spearman correlation analysis was conducted based on delta difference between PRE and POST1. The statistical analyses were performed with SPSS Statistics 24 (IBM Japan, Tokyo, Japan).
Results
All subjects completed the race. The finishing time was 4 h 7 min 43 s±44 min 29 s (range: 2 h 27 min 16 s to 5 h 0 min 11 s). The average velocity during the race was 10.6±2.4 km/h.
Aerobic fitness
The subjects had the following characteristics: VO2max of 57.4±7.1 mL/kg/min (range: 47.9–75.5 mL/kg/min), O2 cost of 225.1±21.3 mL/kg/km (range: 193.6–264.8 mL/kg/km). In the running economy test, the RER of all subjects was <1.0.
Changes in markers of muscle damage and inflammation
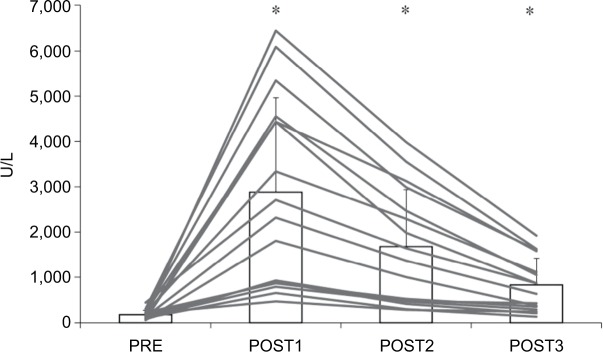
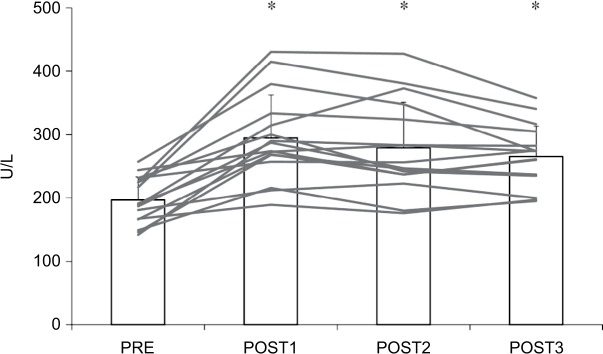
Figures 1 and 2 show the changes in muscle damage markers before and after the marathon race. There were significant effects for each subsequent day following the race, and post hoc analyses showed that the peak was achieved on POST1 and remained high throughout the 3-day recovery period compared to PRE.
Figure 1.
Serum creatine kinase before and after a marathon run (n=16).
Notes: *P<0.05 – significant difference compared to PRE. Gray lines indicate individual subject results and bar graph indicates the group mean with SD values.
Abbreviations: PRE, blood sampling done 4 days before the marathon race; POST1, blood sampling done 1 day after the marathon race; POST2, blood sampling done 1 day after the marathon race; POST3, blood sampling done 3 days after the marathon race.
Figure 2.
Serum lactate dehydrogenase before and after a marathon run (n=16).
Notes: *P<0.05 – significant difference compared to PRE. Gray lines indicate individual subject results and bar graph indicates the group mean with SD values.
Abbreviations: PRE, blood sampling done 4 days before the marathon race; POST1, blood sampling done 1 day after the marathon race; POST2, blood sampling done 1 day after the marathon race; POST3, blood sampling done 3 days after the marathon race.
Figure 3 shows the change in CRP before and after a marathon run. The CRP level peaked on POST1 and remained high throughout the 3-day recovery period compared to PRE. All subjects had at least one above reference value at 3 days after the race.
Figure 3.
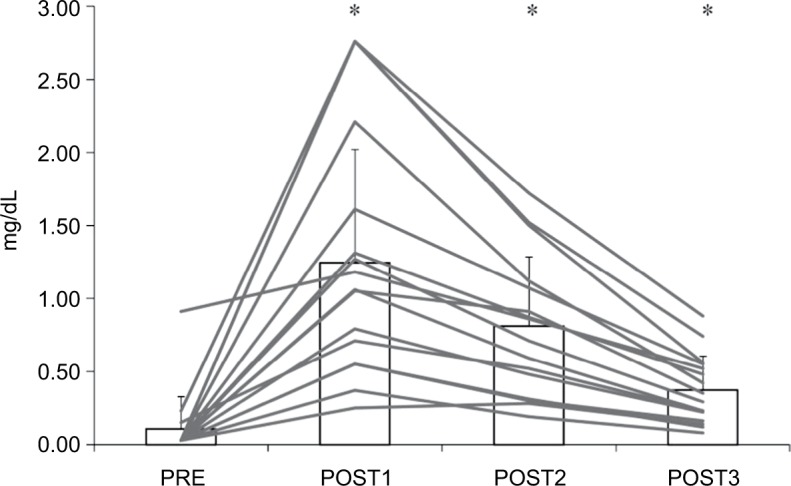
Serum C-reactive protein before and after a marathon run (n=16).
Notes: *P<0.05 – significant difference compared to PRE. Gray lines indicate individual subject results and bar graph indicates the group mean with SD values.
Abbreviations: PRE, blood sampling done 4 days before the marathon race; POST1, blood sampling done 1 day after the marathon race; POST2, blood sampling done 1 day after the marathon race; POST3, blood sampling done 3 days after the marathon race.
Relationship between the variables
The coefficient of variation at POST1 was 73.2% for CK, 58.3% for LDH, and 71.3% for CRP, showing remarkable individual variations. Table 1 shows the relationships between the variables. VO2max was not related to CK, LDH, and CRP at POST1. There was a significant positive correlation in O2 cost with CRP elevation at POST1 (r=0.619, P=0.011) (Figure 4). O2 cost was not related to CK and LDH. When excluding female subjects, the significant relationship was unchanged (shown in parentheses).
Table 1.
Spearman correlation analysis of aerobic fitness, race time, and blood sampling 1 day after a marathon run
| Variables | VO2max | O2 cost | Race time | CK | LDH | CRP |
|---|---|---|---|---|---|---|
|
| ||||||
| VO2max | – | |||||
| O2 cost | 0.096 (−0.135) | – | ||||
| Race time | −0.609* (−0.746*) | 0.368 (0.407) | – | |||
| CK | −0.147 (−0.281) | 0.209 (0.291) | 0.374 (0.456) | – | ||
| LDH | −0.218 (−0.253) | 0.091 (0.214) | 0.450 (0.555*) | 0.844* (0.791*) | – | |
| CRP | −0.066 (−0.450) | 0.619* (0.567*) | 0.647* (0.725*) | 0.427 (0.399) | 0.255 (0.278) | – |
Notes:
P<0.05, significant correlation. Parentheses indicate excluding females.
Abbreviations: VO2max, maximal oxygen uptake; O2 cost, oxygen cost; CK, creatine kinase; LDH, lactate dehydrogenase; CRP, C-reactive protein.
Figure 4.
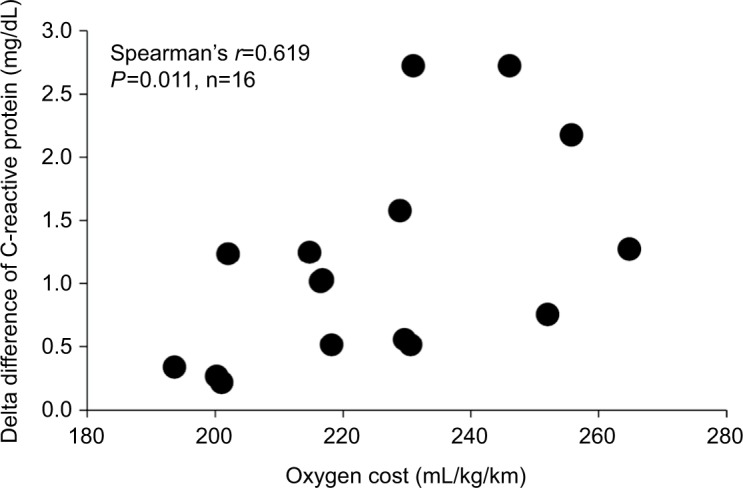
Relationship between oxygen cost and delta difference of C-reactive protein (n=16).
Notes: Delta difference is calculated between PRE and POST1.
Abbreviations: PRE, blood sampling done 4 days before the marathon race; POST1, blood sampling done 1 day after the marathon race.
A significant relationship was observed between CK and LDH. However, CRP was not related to CK and LDH. There was a significant positive correlation between race time (min) and CRP elevation at POST1 (r=0.647, P=0.007) and a significant negative correlation to VO2max (r=– 0.609, P=0.012).
Discussion
The purpose of the present study was to investigate the relationship between running economy and systemic inflammation after a marathon race. The most important finding was that O2 cost is related to post-race CRP response in college recreational runners. This finding suggests that systemic inflammation is minor in runners with superior running economies.
VO2max and running economy were recognized as the main physiological factors determining marathon running performance. Of the two factors, VO2max had the larger impact in heterogeneous performance runners,9 supported by the present result showing the relationship between aerobic fitness and race time (Table 1). Several studies have investi gated the relationship between VO2max and CRP response after acute exercise.3,17 Kim et al3 investigated the relationship between VO2max and CRP response immediately after a marathon race and reported no relationship between them (r=−0.03). A previous study by Plaisance et al17 showed that response of systemic inflammation after acute aerobic exercise was no different between individuals of at least average peak oxygen uptake levels. In the present study, although the value of VO2max is heterogeneous, all subjects achieved a value greater than the reference value for a population of the same age and sex.18 Thus, higher VO2max does not appear to be related to superior resistance to systemic inflammation after a marathon race in young recreational runners.
Unlike other inflammatory markers, CRP levels peak 24 hours after a marathon race. Thus, since we could not conduct blood sampling immediately after the race, CRP was selected to assess the systemic inflammation. A significant positive relationship was observed between O2 cost and CRP response after a marathon race, suggesting that systemic inflammation in runners with superior running economies was minor. This is an important finding because acute excessive systemic inflammation has detrimental effects on health-related physiological functions as discussed in the introduction. The detailed mechanism is unknown but it may be that energy expenditure is different during a marathon race. Since the runners generate energy based on the amount of oxygen consumed during submaximal running, O2 cost can estimate energy expenditure (1 L of O2 haŝ5 kcal). Assuming that the running economy was similar among the participants during the race, the difference in energy expenditure was calculated to reach 37% in the present subjects. Therefore, it is possible that differences in energy expenditure affected the extent of systemic inflammation following the race.
It is known that causes of increased CRP levels include repeated hypoxic stress.19,20 Guven et al19 reported that the increased CRP levels were observed in subjects with obstructive sleep apnea and were related to oxygen desaturation. A high O2 cost means that oxygen consumption by the muscles is high. Therefore, the significant relationship between O2 cost and CRP response after a marathon race is likely in part due to oxygen desaturation during the race. However, to our knowledge, no study has compared O2 cost and CRP response. Furthermore, oxygen saturation is closely affected by relative intensity (ie, %VO2max).21 In the present study, we did not measure HR or oxygen saturation during the race. Thus, there is a need to further investigate the cause of the significant relationship found between O2 cost and CRP response.
A significant positive relationship was observed between race time and CRP response, showing that longer race time increases the CRP response. This finding is in agreement with the result of several previous studies for marathon running5 or an endurance cycling race.8 In the present study, interestingly, even though no significant relationship was observed between O2 cost and race time, the O2 cost was significantly related to CRP response. This result indicates that running economy may be related to systemic inflammation after marathon running, independently of race time.
Several previous studies indicated a similar response of both CRP and CK after acute exercise.22,23 For example, a previous investigation in an Ironman triathlon showed a significant relationship between responses of CK and CRP 1 day after the race.23 In contrast, no significant relationships were observed between muscle damage markers and CRP response in the present study. In a well-controlled laboratory study, Croisier et al24 measured serum myoglobin as a marker of muscle damage, as well as IL-6 after two damaging eccentric exercise sessions, separated by a period of 3 weeks during which the subjects underwent five training sessions. After training, serum myoglobin was significantly lower than that measured before training, suggesting that these training sessions conferred a protective effect against muscle damage. However, the response of IL-6 was no different before and after training. Based on this result, they concluded that there was no causal relationship between exercise-induced muscle damage and post-exercise elevation in IL-6. Taking all these findings into account, the influence of muscle damage on systemic inflammation after marathon running appears to be minor.
A significant relationship was observed between race time and LDH response when excluding females, suggesting longer race time induces more severe muscle damage. It is well known that exercise-induced muscle damage is attenuated in well-trained subjects by the repeated bout effect.25 In brief, a fast runner is well trained and has a strong protective effect against muscle damage. Indeed, a previous study showed that muscle damage was more severe in the novice runner group (average race velocity: 9.5 km/h) compared to an experienced runner group (average race speed 13.8 km/h) after a 56-km ultramarathon race.26 Therefore, the significant relationship between race time and LDH may be caused by the differences in protective effects against muscle damage in the individual runners.
In the present study, there were significant differences in markers of muscle damage and inflammation between PRE and POST3. Moreover, all subjects had at least one above reference value at 3 days after the race. These results indicate that, in college recreational runners, a 3-day recovery period does not seem to be long enough for recovery following a marathon race. Retraining in a state of insufficient recovery could lead to further increase in CK after a marathon.27 Meanwhile, it has been reported that physiological capacity recovered within 7 days of a marathon race.16 Therefore, we encourage recreational unners to take a passive rest for at least 3 days after the race, but recommend that they can resume training 1 week later.
The present study was a field study, and thus we acknowledge several limitations. The first limitation is that evaluation of running economy was based on the result of the treadmill running test and not on measuring muscle glycogen stores. Therefore, the causal relationship between running economy and glycogen stores after the race is unknown. Second, we did not control food intake during the experimental period. Third, the present study included both males and females. However, the significant relationship between O2 cost and CRP response was unchanged when excluding females. Finally, the present study has a cross-sectional design and relatively small sample size. Further studies should be conducted to clarify causal relationships between running economy and CRP response after marathon running.
Conclusion
The present study confirmed the hypothesis that O2 cost is related to postmarathon race CRP response. This finding suggests that systemic inflammation is minor in the runners with superior running economies. Further study to clarify the cause of the relationship and clinical significance of transient increase in CRP is necessary.
Acknowledgments
This work was supported by Grant-in-Aid for JSPS Research Fellow (No. 17J01363). The authors would like to thank Dr N Mukai for assistance in blood sample collection. We would like to thank Editage (www.editage.jp) for English language editing. Part of this study was presented in Oral Presentation format at 23rd Annual Congress of the European College of Sport Science, Dublin, Ireland, 4–7 July 2018 (Session-ID: OP-PM80).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Plaisance EP, Grandjean PW. Physical activity and high-sensitivity C-reactive protein. Sports Med. 2006;36(5):443–458. doi: 10.2165/00007256-200636050-00006. [DOI] [PubMed] [Google Scholar]
- 2.Kim HJ, Lee YH, Kim CK. Changes in serum cartilage oligomeric matrix protein (COMP), plasma CPK and plasma hs-CRP in relation to running distance in a marathon (42.195 km) and an ultra-marathon (200 km) race. Eur J Appl Physiol. 2009;105(5):765–770. doi: 10.1007/s00421-008-0961-x. [DOI] [PubMed] [Google Scholar]
- 3.Kim YJ, Ahn JK, Shin KA, Kim CH, Lee YH, Park KM. Correlation of cardiac markers and biomarkers with blood pressure of middle-aged marathon runners. J Clin Hypertens. 2015;17(11):868–873. doi: 10.1111/jch.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi Y, Takeuchi T, Hosoi T, Yoshizaki H, Loeppky JA. Effect of a marathon run on serum lipoproteins, creatine kinase, and lactate dehydrogenase in recreational runners. Res Q Exerc Sport. 2005;76(4):450–455. doi: 10.1080/02701367.2005.10599318. [DOI] [PubMed] [Google Scholar]
- 5.Mündermann A, Geurts J, Hügle T, et al. Marathon performance but not BMI affects post-marathon pro-inflammatory and cartilage biomarkers. J Sports Sci. 2017;35(7):711–718. doi: 10.1080/02640414.2016.1184301. [DOI] [PubMed] [Google Scholar]
- 6.Wilhelm M, Zueger T, De Marchi S, et al. Inflammation and atrial remodeling after a mountain marathon. Scand J Med Sci Sports. 2014;24(3):519–525. doi: 10.1111/sms.12030. [DOI] [PubMed] [Google Scholar]
- 7.Bessa AL, Oliveira VN, Agostini GG, et al. Exercise intensity and recovery: biomarkers of injury, inflammation, and oxidative Stress. J Strength Cond Res. 2016;30(2):311–319. doi: 10.1519/JSC.0b013e31828f1ee9. [DOI] [PubMed] [Google Scholar]
- 8.Kleiven Ø, Bjørkavoll-Bergseth M, Melberg T, et al. High physical fitness is associated with reduction in basal- and exercise-induced inflammation. Scand J Med Sci Sports. 2018;28(1):172–179. doi: 10.1111/sms.12878. [DOI] [PubMed] [Google Scholar]
- 9.Takayama F, Aoyagi A, Nabekura Y. Relationship between classic physiological variables and running performance in recreational runners. Int J Sport Health Sci. 2018;16:33–40. [Google Scholar]
- 10.Rapoport BI. Metabolic factors limiting performance in marathon runners. PLoS Comput Biol. 2010;6(10):e1000960. doi: 10.1371/journal.pcbi.1000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asp S, Daugaard JR, Rohde T, Adamo K, Graham T. Muscle glycogen accumulation after a marathon: roles of fiber type and pro- and macro-glycogen. J Appl Physiol. 1999;86(2):474–478. doi: 10.1152/jappl.1999.86.2.474. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen BK. Muscular interleukin-6 and its role as an energy sensor. Med Sci Sports Exerc. 2012;44(3):392–396. doi: 10.1249/MSS.0b013e31822f94ac. [DOI] [PubMed] [Google Scholar]
- 13.Kampus P, Kals J, Unt E, et al. Association between arterial elasticity, C-reactive protein and maximal oxygen consumption in well-trained cadets during three days extreme physical load: a pilot study. Physiol Meas. 2008;29(4):429–437. doi: 10.1088/0967-3334/29/4/001. [DOI] [PubMed] [Google Scholar]
- 14.Swanson DR. Atrial fibrillation in athletes: implicit literature-based connections suggest that overtraining and subsequent inflammation may be a contributory mechanism. Med Hypotheses. 2006;66(6):1085–1092. doi: 10.1016/j.mehy.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Dimitriou L, Hill JA, Jehnali A, et al. Influence of a montmorency cherry juice blend on indices of exercise-induced stress and upper respiratory tract symptoms following marathon running: a pilot investigation. J Int Soc Sports Nutr. 2015;12:22. doi: 10.1186/s12970-015-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takayama F, Aoyagi A, Shimazu W, Nabekura Y. Effects of marathon running on aerobic fitness and performance in recreational runners one week after a race. J Sports Med. 2017;2017:1–6. doi: 10.1155/2017/9402386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plaisance EP, Taylor JK, Alhassan S, Abebe A, Mestek ML, Grandjean PW. Cardiovascular fitness and vascular inflammatory markers after acute aerobic exercise. Int J Sport Nutr Exerc Metab. 2007;17(2):152–162. doi: 10.1123/ijsnem.17.2.152. [DOI] [PubMed] [Google Scholar]
- 18.Ministry of Health Labour and Welfare of Japan [homepage on the Internet] Physical activity reference for health promotion. 2013. [Accessed July 29, 2018]. Available from: https://www.mhlw.go.jp/stf/houdou/2r9852000002xple-att/2r9852000002xpqt.pdf.
- 19.Guven SF, Turkkani MH, Ciftci B, Ciftci TU, Erdogan Y. The relationship between high-sensitivity C-reactive protein levels and the severity of obstructive sleep apnea. Sleep Breath. 2012;16(1):217–221. doi: 10.1007/s11325-011-0492-2. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann G, Tschöp M, Fischer R, et al. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine. 2000;12(3):246–252. doi: 10.1006/cyto.1999.0533. [DOI] [PubMed] [Google Scholar]
- 21.Peltonen JE, Leppävuori AP, Kyrö KP, Mäkelä P, Rusko HK. Arterial haemoglobin oxygen saturation is affected by F(I)O2 at submaximal running velocities in elite athletes. Scand J Med Sci Sports. 1999;9(5):265–271. doi: 10.1111/j.1600-0838.1999.tb00244.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim HJ, Lee YH, Kim CK. Biomarkers of muscle and cartilage damage and inflammation during a 200 km run. Eur J Appl Physiol. 2007;99(4):443–447. doi: 10.1007/s00421-006-0362-y. [DOI] [PubMed] [Google Scholar]
- 23.Neubauer O, König D, Wagner KH. Recovery after an Ironman triathlon: sustained inflammatory responses and muscular stress. Eur J Appl Physiol. 2008;104(3):417–426. doi: 10.1007/s00421-008-0787-6. [DOI] [PubMed] [Google Scholar]
- 24.Croisier JL, Camus G, Venneman I, et al. Effects of training on exercise-induced muscle damage and interleukin 6 production. Muscle Nerve. 1999;22(2):208–212. doi: 10.1002/(sici)1097-4598(199902)22:2<208::aid-mus8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 25.Mchugh MP, Connolly DA, Eston RG, Gleim GW. Exercise-induced muscle damage and potential mechanisms for the repeated bout effect. Sports Med. 1999;27(3):157–170. doi: 10.2165/00007256-199927030-00002. [DOI] [PubMed] [Google Scholar]
- 26.Noakes TD, Carter JW. The responses of plasma biochemical parameters to a 56-km race in novice and experienced ultra-marathon runners. Eur J Appl Physiol Occup Physiol. 1982;49(2):179–186. doi: 10.1007/BF02334066. [DOI] [PubMed] [Google Scholar]
- 27.Maresh CM, Allison TG, Noble BJ, Drash A, Kraemer WJ. Substrate and hormone responses to exercise following a marathon run. Int J Sports Med. 1989;10(2):101–106. doi: 10.1055/s-2007-1024883. [DOI] [PubMed] [Google Scholar]