Abstract
Objective
Matching-adjusted indirect comparison was used to assess the comparative effectiveness of secukinumab 150 mg and adalimumab 40 mg in biologic-naïve patients with ankylosing spondylitis (AS) for up to 1 year.
Methods
Pooled individual patient data from the secukinumab arms of MEASURE 1 (NCT01358175) and MEASURE 2 (NCT01649375) trials (n=197) were matched against the ATLAS (NCT00085644) adalimumab population (n=208). Logistic regression analysis was used to determined weights to match for age, sex, Bath AS Functional Index, C-reactive protein levels, and previous tumor necrosis factor inhibitor therapy. Recalculated Assessment of SpondyloArthritis International Society (ASAS) 20 and 40 responses at weeks 8, 12, 16, 24, and 52 from MEASURE 1/2 (effective sample size=120) were compared with those of ATLAS. Anchored (placebo-adjusted) comparisons were possible until week 12, and unanchored (non-placebo-adjusted) comparisons were necessary thereafter.
Results
For placebo-anchored ASAS 20 and 40 comparisons up to week 12, there were no differences between secukinumab and adalimumab. For unanchored comparisons at week 16, ASAS 20 was higher for secukinumab [odds ratio 1.60 (95% confidence interval, 1.01–2.54); p=0.047]; at week 24, ASAS 20 and 40 were higher for secukinumab [1.76 (1.11–2.79); p=0.017 and 1.79 (1.14–2.82); p=0.012, respectively]; and at week 52, ASAS 40 was higher for secukinumab [1.54 (1.06–2.23); p=0.023] than for adalimumab.
Conclusion
There were no differences observed in placebo-adjusted ASAS 20 and 40 responses up to 12 weeks between secukinumab- and adalimumab-treated patients with ankylosing spondylitis. After week 12, secukinumab demonstrated signs of greater improvement in non-placebo-adjusted ASAS 20 and 40 responses than adalimumab.
Keywords: Adalimumab, comparative effectiveness, matching-adjusted indirect comparison, ankylosing spondylitis, secukinumab
Introduction
Patients with ankylosing spondylitis (AS) receive biologic disease-modifying antirheumatic drugs (bDMARDs) if disease activity remains high, despite therapy with nonsteroidal anti-inflammatory drugs, according to the recommendations from the Assessment of SpondyloArthritis International Society (ASAS)/European League Against Rheumatism (EULAR), and the American College of Rheumatology (1, 2). Until recently, bDMARD choice was limited only to tumor necrosis factor inhibitors (TNFis); however, now patients also have the option of using the fully human interleukin 17A inhibitor secukinumab, which has also been recommended by ASAS/EULAR (2–4).
There are no controlled head-to-head superiority trials (randomized controlled trials, RCTs) to inform the choice of bDMARD in AS. To date, one open-label TNFi-intraclass trial of infliximab versus etanercept has been reported (5), but none have compared different mechanisms of action. Indirect comparisons using results from separate RCTs can provide estimates of comparative efficacy. Standard indirect comparisons, such as network meta-analyses, are feasible when there is a common comparator arm between RCTs, but are subject to bias from differences in trial design, including heterogeneity in patient populations due to differences in patient inclusion and exclusion criteria (6, 7).
Matching-adjusted indirect comparison (MAIC) is an analytical method for an indirect comparison that uses a form of propensity score weighting of individual patient data (IPD) to match patients from one trial with those from another for baseline characteristics, especially those that may influence treatment response (8, 9). By reweighting patient data, MAIC targets comparison of treatment efficacy in the matched population, reducing the impact of heterogeneity between study populations. This technique has been increasingly used in the area of spondyloarthritides, as well as other diseases (8–12), and is an accepted technique used by agencies, such as the UK’s National Institute for Health and Care Excellence (NICE) in their decision-making processes (13–16).
Our study follows the NICE guidelines on MAIC methodology [Decision Support Unit (DSU) Technical Support Document (TSD) 18] (14, 17) and provides evidence for comparative effectiveness of medium-term (≤1 year) biologic therapy for biologic-naïve patients with active AS. We compared the TNFi adalimumab with secukinumab, using common primary and secondary outcome measures from the pivotal phase 3 RCTs.
Methods
MAIC
Identification of source data by systematic literature review
A systematic literature review (conducted: September 2014; updated: September 2015) identified three relevant clinical trials for use in this MAIC: MEASURE 1 (18), MEASURE 2 (18), and ATLAS (19, 20). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart is shown in Supplementary Figure 1 and eligibility criteria are shown in Supplementary Table 1. Details of excluded and included trials are shown in Supplementary Table 2, whereas study designs are shown in Supplementary Figure 2.
Selection of baseline characteristics for matching
Selection of matching variables complied with NICE DSU TSD 18 (14, 17), following (1) advice from clinical experts in the treatment of AS, (2) a review of the clinical literature, and (3) statistical analyses of prognostic variables and effect modifiers using logistic regression analysis (Supplementary Table 3) (14, 17), as described previously (9).
The principal analysis matched for previous use of TNFi therapy, age, sex, mean Bath Ankylosing Spondylitis Functional Index (BASFI) score, and mean C-reactive protein (CRP) level (Supplementary Table 4). A sensitivity analysis additionally included mean Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score as a baseline matching variable.
Matching and adjustment of IPD to published aggregate data
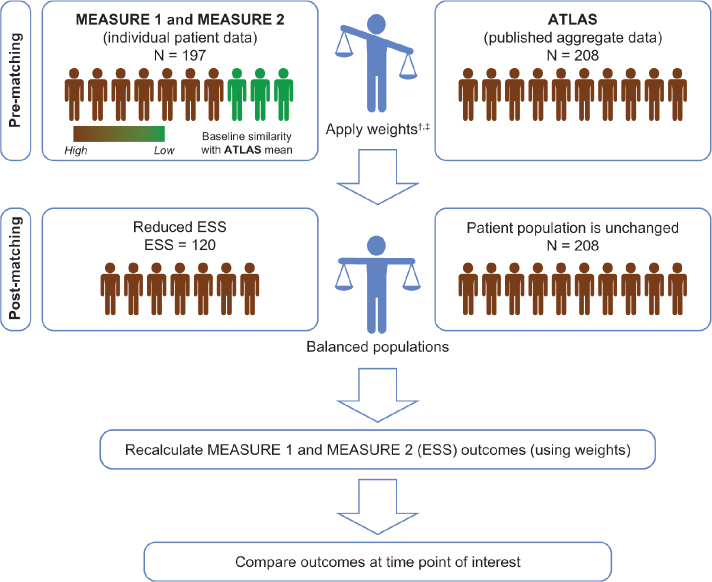
Three MAIC analyses against ATLAS were developed: pooled MEASURE 1/2, MEASURE 1 individually, and MEASURE 2 individually. The pooled MEASURE 1/2 analysis is presented in our study because it has the largest effective sample size (ESS). Patients receiving secukinumab in each MEASURE study were matched to patients in ATLAS; results are therefore targeted to a population similar to ATLAS (Figure 1). For post-matching, the MEASURE secukinumab 150 mg population was compared with the adalimumab arm of ATLAS because 150 mg is the licensed secukinumab dose in AS (4, 21). The methodology for matching and adjustment was based on Signorovitch et al. (12) and subsequent studies (11, 15, 30) and is in line with NICE DSU TSD 18 (11, 14, 15, 17, 22). Regression results were used to weight patients in the MEASURE trials, so that each patient’s weighting corresponded to their relative propensity to match with ATLAS for those variables considered treatment effect modifiers (age, sex, BASFI, CRP, and prior TNFi exposure).
Figure 1.
Matching-adjusted indirect comparison using pooled MEASURE 1 and MEASURE 2 data as an example
*An MAIC was similarly performed for MEASURE 2 only, in which the pooled secukinumab 75 mg and 150 mg arms were matched; however, outcome data are shown only for secukinumab 150 mg
†Weights were derived by logistic regression. The choice of matching parameters was made by consensus of all authors (including both clinical and methodological experts). Patients were matched for key characteristics known or expected to influence clinical outcomes in individuals with active AS
‡Pooled placebo arms of MEASURE 1 and 2 were also matched to the placebo arm of ATLAS
AS: ankylosing spondylitis, ESS: effective sample size, MAIC: matching-adjusted indirect comparison
Comparison of outcomes using weighted patient data
The weights were used to recalculate outcomes for each patient. Outcomes were then aggregated and used to estimate the comparative effectiveness of secukinumab and adalimumab (15).
Analyses
Missing data
In the ATLAS trial, missing data for all binary outcomes (ASAS 20, ASAS 40, and ≥50% improvement in BASDAI score) were imputed using non-responder imputation (NRI) up to week 24. After week 24, last observation carried forward (LOCF) data were reported for patients who had switched to adalimumab weekly, used early escape therapy, or switched from placebo (19, 20). LOCF was used for all continuous outcomes. In both MEASURE studies, missing data for binary outcomes were imputed by NRI at all time points (18). Between-group differences in continuous variables were evaluated using a mixed-model repeated-measures approach. To compensate for these differences and to match the ATLAS design for missing data as closely as possible (19, 20), week 52 data from both MEASURE studies were recalculated using LOCF and included placebo switchers to the 150 mg dose. Missing continuous outcomes from both MEASURE studies were calculated to match ATLAS using LOCF. If ATLAS data were reported in graphs only, specific software (DigitizeIt, Braunschweig, Germany) was used for data extraction.
Placebo-adjusted and non-placebo-adjusted outcome comparisons
At weeks 8 and 12, anchored (placebo-adjusted) comparisons were possible. Placebo-adjusted comparisons were not possible after week 12 because patients randomized to placebo could receive active treatment from week 16 in MEASURE 1/2 and from week 12 in ATLAS (Supplementary Figure 2). Therefore, after week 12, outcomes from the adalimumab arm of ATLAS were directly compared with outcomes from the adjusted and recalculated pooled secukinumab 150 mg arms of MEASURE 1/2. This unanchored (non-placebo-adjusted) MAIC methodology is recommended by NICE in cases when anchored comparisons are not possible (14, 17, 22–24).
Pairwise comparisons
For the ASAS 20, ASAS 40, ASAS 5/6, and ASAS partial remission (PR) outcomes, odds ratios (ORs) were estimated along with their corresponding 95% confidence intervals (CIs) and p values (two-sided). Relative likelihoods of response (RRs) were also calculated for all ASAS responses (Supplementary Table 5). For placebo-adjusted comparisons, ORs and corresponding standard errors were calculated using the Bucher method (7). For non-placebo-adjusted comparisons, standard errors for OR values were estimated based on the information provided by a 2×2 contingency table that shows outcomes in the adalimumab arm of the ATLAS trial and outcomes in the recalculated pooled secukinumab 150 mg arm of MEASURE 1/2.
Continuous outcome scores
Comparisons for continuous outcomes were made at week 12 (placebo-adjusted) and weeks 24 and 52 (non-placebo-adjusted). For placebo-adjusted comparisons, differences in mean scores between adalimumab and placebo and secukinumab and placebo, respectively, were calculated, along with 95% CIs and p values based on a normal approximation. For non-placebo-adjusted comparisons, the mean change scores of patients in the adalimumab arm of the ATLAS trial were compared with the mean change scores of patients in the reweighted secukinumab arms of the MEASURE 1/2 population. Normal approximations were used to calculate 95% CIs; however, complete week 52 data were not reported from ATLAS, and it was therefore not possible to calculate p values.
For all ASAS and continuous outcome scores, the commonly used threshold of p<0.05 was considered the threshold for statistical significance. In acknowledgment of the recent American Statistical Association (ASA) guidelines (25, 26), ASAS data were also interpreted using a more modern definition of statistical evidence (27, 28), as described previously (9).
Results
Principal analysis
Matching baseline characteristics
Figure 1 illustrates the MAIC matching process. Supplementary Table 4 shows the baseline characteristics of patients in MEASURE 1/2 in the pooled secukinumab 150 mg (n=197) and placebo arms (n=196) before and after matching to the ATLAS adalimumab 40 mg (n=208) and placebo arms (n=107). Before matching, there was heterogeneity between the trial populations. In addition to demographic characteristics, an important difference between populations was that 31.0% of patients randomized to secukinumab were TNFi-inadequate responders (TNFi-IR; 69.0% were TNFi-naïve), whereas all patients receiving adalimumab were TNFi-naïve. After matching, all patients receiving secukinumab were TNFi-naïve. Achieving homogeneity between the two populations reduced the sample size the ESS of pooled MEASURE 1/2 after matching was 120 for secukinumab 150 mg and 120 for placebo.
ASAS response rates
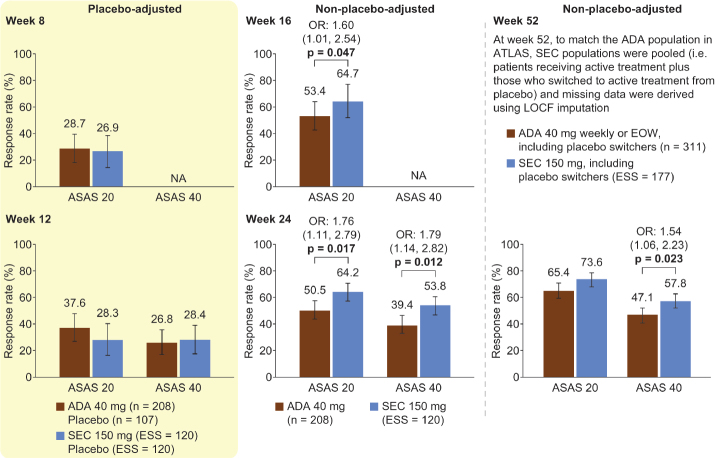
Figure 2, Table 1, and Supplementary Figure 3 show the ASAS response rates in the principal analysis. At weeks 8 and 12, patients receiving adalimumab had numerically higher placebo-adjusted ASAS 20, 40, 5/6, and PR response rates than those receiving secukinumab; however, there were no significant differences (p>0.05). Compared with adalimumab, at week 16, the ASAS 20 response rate was significantly higher for patients receiving secukinumab [OR 1.60 (95% CI, 1.01–2.54); p=0.047]; at week 24, ASAS 20 and ASAS 40 response rates were significantly higher for secukinumab [OR 1.76 (95% CI, 1.11–2.79); p=0.017 and OR 1.79 (95% CI, 1.14–2.82); p=0.012]; and at week 52, the ASAS 40 response rate was significantly higher for secukinumab [OR 1.54 (95% CI, 1.06–2.23); p=0.023]. Data analysis using RR instead of OR resulted in similar observations (Supplementary Table 5). Using an additional interpretation of data acknowledging the recent ASA guidelines yielded similar observations (Supplementary Table 6).
Figure 2.
Principal analysis results for ASAS 20 and ASAS 40
All p values (shown when significant, p<0.05) were derived from OR values. Error bars and figures in brackets show 95% confidence intervals. Numbers above each bar are the absolute mean response rate (ATLAS) and the mean response rate after reweighting (MEASURE 1/2)
ADA: adalimumab, ASAS 20/40: 20%/40% improvement in the Assessment of SpondyloArthritis International Society response criteria, EOW: every other week, ESS: effective sample size, LOCF: last observation carried forward, NA: not available, OR: odds ratio, SEC: secukinumab
Table 1.
Odds ratios for secukinumab 150 mg and adalimumab 40 mg at weeks 8, 12, 16, 24, and 52
| Principal analysis (SEC vs. ADA) | Sensitivity analysis (SEC vs. ADA) | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| ASAS 20 | ASAS 40 | ASAS 5/6 | ASAS PR | ASAS 20 | ASAS 40 | ASAS 5/6 | ASAS PR |
| Week 8 (placebo-adjusted) | |||||||
| 0.91 (0.44–1.89); p=0.795 | NR | NR | NR | 0.99 (0.47–2.06); p=0.971 | NR | NR | NR |
| Week 12 (placebo-adjusted) | |||||||
| 0.60 (0.28–1.28); p=0.185 | 0.93 (0.39–2.21); p=0.867 | 0.50 (0.22–1.18); p=0.115 | 0.97 (0.23–4.04); p=0.961 | 0.67 (0.31–1.44); p=0.304 | 0.96 (0.40–2.30); p=0.927 | 0.52 (0.22–1.21); p=0.129 | 0.97 (0.23–4.08); p=0.972 |
| Week 16 (non-placebo-adjusted) | |||||||
| 1.60 (1.01–2.54); p=0.047 | NR | NR | NR | 1.70 (1.06–2.73); p=0.028 | NR | NR | NR |
| Week 24 (non-placebo-adjusted) | |||||||
| 1.76 (1.11–2.79); p=0.017 | 1.79 (1.14–2.82); p=0.012 | 1.51 (0.96–2.38); p=0.072 | 1.34 (0.80–2.25); p=0.265 | 1.87 (1.17–3.01); p=0.009 | 1.85 (1.17–2.94); p=0.009 | 1.55 (0.98–2.46); p=0.060 | 1.37 (0.81–2.31); p=0.239 |
| Week 52 (non-placebo-adjusted) | |||||||
| 1.48 (0.98–2.22); p=0.062 | 1.54 (1.06–2.23); p=0.023 | 1.42 (0.97–2.07); p=0.072 | 0.71 (0.47–1.08); p=0.110 | 1.53 (1.00–2.34); p=0.048 | 1.52 (1.04–2.23); p=0.031 | 1.40 (0.95–2.07); p=0.089 | 0.72 (0.47–1.11); p=0.137 |
Data are OR (95% CI). Data in italics indicate statistically significant differences (p<0.05) in OR. At week 52, SEC populations were pooled (i.e., patients receiving active treatment plus those switching to active treatment from placebo), and missing data were derived using LOCF imputation to match the ADA patient population in ATLAS
ADA: adalimumab, ASAS: Assessment of SpondyloArthritis International Society, ASAS 20/40: 20%/40% improvement in the ASAS response criteria, ASAS 5/6: 20% improvement in any five of the six domains in the ASAS response criteria, ASAS PR: ASAS partial remission (a value of <2 on a 0–10 scale in each of the four domains of the ASAS response criteria), CI: confidence interval, LOCF: last observation carried forward, NR: not reported, OR: odds ratio, SEC: secukinumab
ASAS 20 response rates in patients receiving placebo were 29.0% (ATLAS; aggregate data) and 32.3% (MEASURE 1/2; after matching) at week 8 and 20.6% (ATLAS; aggregate data) and 33.7% (MEASURE 1/2; after matching) at week 12; ASAS 40 response rates were 13.1% (ATLAS; aggregate data) and 16.7% (MEASURE 1/2; after matching) at week 12. Given that ASAS 40 is a more stringent outcome than ASAS 20, the near equivalence of the ASAS 40 placebo response between ATLAS and recalculated rates from MEASURE 1/2 suggests a good match. Supplementary Table 7 shows placebo responses from principal and sensitivity analyses.
Continuous outcome
Table 2 shows the principal analysis comparison of the ASAS core set and additional key continuous outcomes primarily suggested by the Outcome Measures in Rheumatology group (29, 30). At week 12 (placebo-adjusted), adalimumab was associated with a significantly greater change from baseline in Patient Global Assessment (PtGA; −45.6 vs. −15.4; p<0.001), BASFI (−2.8 vs. −1.0; p<0.001), BASDAI inflammation (−2.6 vs. −1.1; p=0.024), and Bath Ankylosing Spondylitis Metrology Index (BASMI; −0.6 vs. −0.3; p=0.039) scores compared with secukinumab. At week 24, secukinumab showed significantly greater improvements than adalimumab in total back pain (−34.9 vs. −27.7; p=0.004), nocturnal pain (−34.1 vs. −27.3; p=0.011), tender joint count (−2.7 vs. −0.9; p=0.001), and swollen joint count (−1.1 vs. −0.4; p=0.010). Adalimumab was associated with significantly greater improvements than secukinumab in BASFI (−3.8 vs. −2.2; p<0.001) and BASDAI inflammation (−4.3 vs. −2.9; p<0.001) scores at week 24.
Table 2.
Principal analysis of continuous outcome comparisons
| Placebo-adjusted change from baseline (95% CI)* | Change from baseline (95% CI)* | Change from baseline (95% CI)† | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Week 12 | Week 24 | Week 52 | ||||
|
|
|
|
||||
| ADA 40 mg | SEC 150 mg | ADA 40 mg | SEC 150 mg | ADA 40 mg | SEC 150 mg | |
| ASAS core set | ||||||
| PtGA, 0–100 mm VAS | −45.6 (−60.9 to −30.3); p<0.001 | −15.4 (−20.5 to −10.4) | −37.8 (−47.6 to −28.0); p=0.503 | −34.2 (−38.0 to −30.5) | −31.0 (NR) | −34.5 (−37.5 to −31.5) |
| Total back pain, 0–100 mm VAS | −18.9 (−24.8 to −13.0); p=0.664 | −17.2 (−22.1 to −12.3) | −27.7 (−31.2 to −24.2) | −34.9 (−38.3 to −31.5); p=0.004 | −31.0 (NR) | −35.0 (−37.9 to −32.1) |
| Nocturnal pain, 0–100 mm VAS | −18.0 (−24.0 to −12.0); p=0.688 | −16.4 (−21.4 to −11.4) | −27.3 (−31.0 to −23.6) | −34.1 (−37.7 to −30.4); p=0.011 | NR | −37.3 (−40.3 to −34.3) |
| BASFI, 0–100 mm VAS | −2.8 (−3.7 to −1.8); p<0.001 | −1.0 (−1.4 to −0.6) | −3.8 (−4.4 to −3.2); p<0.001 | −2.2 (−2.5 to −2.0) | −2.1 (NR) | −2.3 (−2.6 to −2.1) |
| BASDAI inflammation‡ | −2.6 (−3.8 to −1.5); p=0.024 | −1.1 (−1.7 to −0.6) | −4.3 (−5.0 to −3.6); p<0.001 | −2.9 (−3.3 to −2.4) | −3.6 (NR) | −3.1 (−3.4 to −2.8) |
| BASMI total score | −0.6 (−0.9 to −0.3); p=0.039 | −0.3 (−0.4 to −0.1) | −0.6 (−0.8 to −0.4); p=0.729 | −0.6 (−0.7 to −0.4) | −0.7 (NR) | −0.5 (−0.7 to −0.4) |
| CRP, mg/dL | −1.2 (−1.5 to −0.9); p=0.589 | −1.1 (−1.5 to −0.7) | −1.3 (−1.5 to −1.1); p=0.780 | −1.2 (−1.6 to −0.9) | −1.2 (NR) | −1.3 (−1.6 to −1.0) |
| Additional key outcomes | ||||||
| ASQoL, 0–18 | −2.2 (−3.2 to −1.2); p=0.756 | −2.0 (−3.0 to −0.9) | −3.6 (−5.0 to −2.2) | −4.2 (−5.0 to −3.3); p=0.478 | −3.9 (NR) | −4.6 (−5.2 to −4.0) |
| Tender joint count, 44 counts | −0.5 (−1.9 to 0.9) | NA | −0.9 (−1.7 to −0.1) | −2.7 (−3.4 to −2.0); p=0.001 | −1.5 (NR) | −2.5 (−3.2 to −1.7) |
| Swollen joint count, 44 counts | 0.1 (−0.6 to 0.8) | NA | −0.4 (−0.8 to 0.0) | −1.1 (−1.5 to −0.7); p=0.010 | −0.2 (NR) | −0.7 (−1.1 to −0.2) |
| BASDAI, 0–100 mm VAS | −1.8 (−2.4 to −1.2); p=0.104 | −1.2 (−1.6 to −0.8) | −2.6 (−3.0 to −2.2) | −2.9 (−3.2 to −2.6); p=0.267 | −3.0 (NR) | −2.9 (−3.2 to −2.6) |
| BASDAI fatigue§, 0–100 mm VAS | −1.5 (−2.2 to −0.8); p=0.470 | −1.2 (−1.6 to −0.7) | −2.4 (−2.8 to −2.0) | −2.9 (−3.2 to −2.5); p=0.085 | NR | −2.9 (−3.2 to −2.6) |
95% CI, ranges for ADA are not reported and were calculated for the purpose of the comparison
Complete week 52 data were not available from ATLAS, and it was therefore not possible to calculate p values
The BASDAI inflammation score was calculated as the mean of scores for questions 5 and 6 of the BASDAI
This score is based on the fatigue item of the BASDAI
Data in italics indicate statistically significant evidence supporting SEC superiority over ADA (p value shown). Bold text indicates statistically significant evidence supporting ADA superiority over SEC (p value shown)
ADA: adalimumab, ASAS: Assessment of SpondyloArthritis, ASQoL: Ankylosing Spondylitis Quality of Life questionnaire, BASDAI: Bath Ankylosing Spondylitis Disease Activity Index, BASFI: Bath Ankylosing Spondylitis Functional Index, BASMI: Bath Ankylosing Spondylitis Metrology Index, CI: confidence interval, CRP: C-reactive protein, NA: not available, NR: not reported, PtGA: Patient Global Assessment, SEC: secukinumab, VAS: Visual Analog Scale.
Sensitivity analyses
Matching baseline characteristics
The sensitivity analysis matched for previous use of TNFi therapy, age, sex, BASFI score, and CRP level, as in the principal analysis, and additionally included BASDAI score as a baseline matching variable. The ESS in MEASURE 1/2 after matching was 114 for secukinumab 150 mg and 117 for placebo (Supplementary Table 4).
ASAS response rates
Results were broadly consistent with the principal analysis (Table 1), except for the observation of a significantly higher ASAS 20 response for secukinumab than that for adalimumab at week 52 [OR 1.53 (95% CI, 1.00–2.34); p=0.048]. Supplementary Table 6 shows an additional interpretation of data acknowledging the recent ASA guidelines.
Continuous outcome scores
The sensitivity analysis demonstrated significant improvements in total back pain, nocturnal pain, BASDAI fatigue, and tender and swollen joint counts for secukinumab compared with adalimumab, and in PtGA, BASFI, BASMI, and BASDAI inflammation scores for adalimumab compared with secukinumab (Supplementary Table 8).
Individual MEASURE 1 and MEASURE 2 MAIC study analyses versus ATLAS
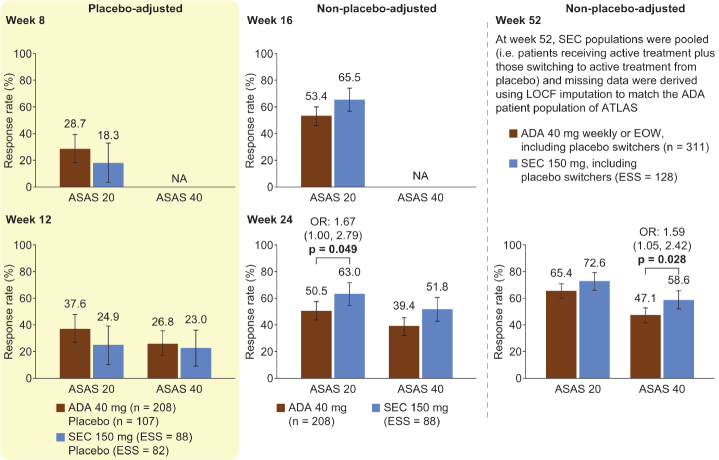
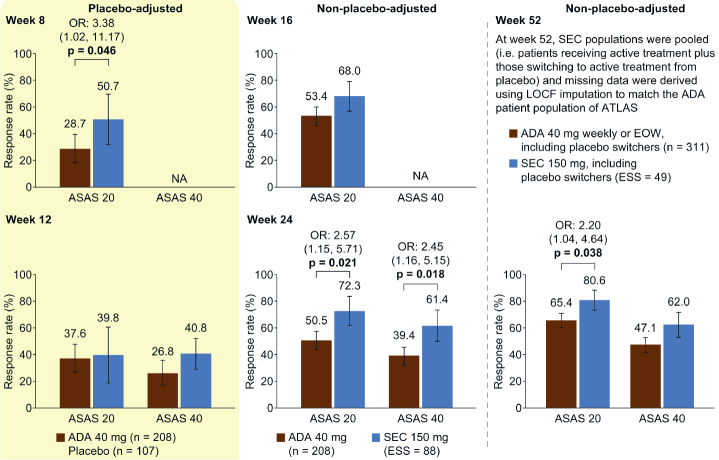
As shown in Figures 3 and 4, separately matching individual MEASURE 1 (secukinumab 150 mg ESS=88 and placebo ESS=82) or MEASURE 2 (secukinumab 150 mg ESS=34 and placebo ESS=34) data gave similar results to the pooled MEASURE 1/2 analysis. In the MEASURE 2 analysis, in which patients received the licensed dosing with subcutaneous loading, we identified additional evidence for a significantly higher ASAS 20 response with secukinumab than that with adalimumab at week 8 [OR 3.38 (95% CI, 1.02–11.17); p=0.046] and week 52 [OR 2.20 (95% CI, 1.04–4.64); p=0.038]. In contrast to the pooled MEASURE 1/2 analysis, differences in week 16 ASAS 20 and week 52 ASAS 40 responses were not significant; however, they were numerically higher for secukinumab than for adalimumab. In MEASURE 2, week 24 and week 52 ASAS 5/6 responses were significantly higher for secukinumab than for adalimumab [OR 2.84 (95% CI, 1.30–6.21); p=0.009 and OR 2.30 (95% CI, 1.17–4.52); p=0.016], which was not seen in the pooled MEASURE 1/2 analysis (data not shown).
Figure 3.
MEASURE 1 MAIC analysis. ASAS 20 and ASAS 40 response rates placebo-adjusted at weeks 8 and 12 and non-placebo-adjusted at weeks 16, 24, and 52.
All p values (shown when significant, p<0.05) were derived from OR values. Error bars and figures in brackets show 95% confidence intervals. Numbers above each bar are the absolute mean response rate (ATLAS) and the mean response rate after reweighting (MEASURE 1)
ADA: adalimumab, ASAS 20/40: 20%/40% improvement in the Assessment of Spondyloarthritis International Society response criteria, EOW: every other week, ESS: effective sample size, LOCF: last observation carried forward, MAIC: matching-adjusted indirect comparison, NA: not available, OR: odds ratio, SEC: secukinumab
Figure 4.
MEASURE 2 MAIC analysis. ASAS 20 and ASAS 40 response rates placebo-adjusted at weeks 8 and 12 and non-placebo-adjusted at weeks 16, 24, and 52.
All p values (shown when significant, p<0.05) were derived from OR values. Error bars and figures in brackets show 95% confidence intervals. Numbers above each bar are the absolute mean response rate (ATLAS) and the mean response rate after reweighting (MEASURE 2)
ADA: adalimumab, ASAS 20/40: 20%/40% improvement in the Assessment of SpondyloArthritis International Society response criteria, EOW: every other week, ESS: effective sample size, LOCF: last observation carried forward, MAIC: matching-adjusted indirect comparison, NA: not available, OR: odds ratio, SEC: secukinumab
Discussion
This MAIC demonstrated no significant differences in placebo-adjusted ASAS 20 and 40 responses up to 12 weeks between secukinumab- and adalimumab-treated patients with AS who were matched for treatment effect modifiers (age, sex, BASFI, CRP, and prior TNFi exposure). Our data suggest that patients with AS treated with secukinumab may be more likely to experience ASAS 20 responses than those treated with adalimumab after week 12. In the pooled MEASURE 1/2 analysis, the strongest statistical evidence supporting higher ASAS responses (ASAS 20 and 40) in patients treated with secukinumab was observed at weeks 24 and 52, suggesting that secukinumab might provide further treatment responses with prolonged therapy.
Taken together, our analyses provide evidence that TNFi-naïve patients with active AS receiving secukinumab 150 mg achieve similar short-term (weeks 8 and 12, placebo-adjusted) and medium- to long-term (weeks 16, 24, and 52, non-placebo-adjusted) reductions in signs and symptoms to those receiving adalimumab 40 mg.
Patient-reported outcomes are central to understanding how any therapeutic agent impacts a patient’s ability to function and to perform daily activities. At week 24, we observed that the majority of significantly improved disease activity scores (i.e., total back pain, nocturnal back pain, tender joint count, and swollen joint count) were reported by patients treated with secukinumab. Additionally, we observed several improved week 12 scores (e.g., PtGA, BASFI, BASDAI inflammation, and BASMI) with adalimumab relative to secukinumab. These findings could reflect differences in patient populations, multiplicity, or real differences in outcome domains relative to axial versus peripheral inflammation.
Our study has certain potential limitations, both inherent to the methodology and specific to our analysis. Despite matching observed patient variables considered treatment effect modifiers at baseline, unobserved variables or variables reported by only one study cannot be controlled via MAIC. We also present non-placebo-adjusted comparisons that, while providing a transparent way to compare long-term data, are conducted in a non-randomized fashion at time points when patients are aware of their biologic treatment. We also used pooled IPD from MEASURE 1 and MEASURE 2 to allow an increased ESS, although there were differences in the design of these two trials, including the intravenous loading regimen used in MEASURE 1 (secukinumab 10 mg/kg body weight or placebo sham infusion) versus subcutaneous used in MEASURE 2 (secukinumab 75 mg or 150 mg or placebo subcutaneous doses). However, we consider this acceptable because the MEASURE 1 and 2 data sets were also interrogated individually by MAIC, free from composite mean data bias, and showed results similar to the pooled analysis. Of note, it was only possible to adjust for characteristics that were reported and defined in the same way across the included RCTs. Methods to quantify the remaining unexplained heterogeneity (e.g., associated with study design, conduct, or patient eligibility) in MAICs are an important area of future research. Moreover, the MAIC analysis was restricted to TNFi-naïve patients. Differences between outcomes tended to be small in this MAIC with sometimes marginal statistical significance. Finally, another limitation of this MAIC is that we did not compare safety outcomes between treatments.
There has been one previous MAIC between secukinumab and adalimumab, which compared ASAS 20 and ASAS 40 responses at week 12 (ATLAS) with those at week 16 (MEASURE 1/2) and showed similar efficacy between the two agents, even though the biologic-naïve cohort of ATLAS was compared with a mixed population of biologic-naïve and biologic-experienced patients from MEASURE 1/2 (10). Based on our regression model, TNFi-IR patients were less likely to achieve ASAS 20 responses with secukinumab in MEASURE 1/2 than those who were TNFi-naïve [OR 0.545 (95% CI, 0.33–0.92); p=0.022]. A similar relationship has been shown between ASAS 40 responses to adalimumab in TNFi-naïve and TNFi-IR patients (31). Thus, not matching for TNFi use is expected to lead to a strong potential for bias in favor of adalimumab. Despite this bias, the previous MAIC showed no significant difference between secukinumab and adalimumab at weeks 12 and 16. In contrast, in the current MAIC, we used data that were matched for prior TNFi exposure.
In conclusion, the current MAIC of patients with active AS in the MEASURE 1/2 RCTs receiving secukinumab 150 mg who were matched for treatment effect modifiers to the ATLAS RCT population receiving adalimumab demonstrates comparable placebo-adjusted ASAS 20 and 40 responses up to 12 weeks but suggests a higher probability of achieving both medium- and long-term ASAS-defined responses (ASAS 20 and ASAS 40) in those receiving secukinumab. The authors are not aware of a planned or ongoing head-to-head RCT powered for superiority of AS sign and symptom improvement of secukinumab over adalimumab. However, a currently recruiting head-to-head RCT, SURPASS (NCT03259074), is powered for superiority of secukinumab over the adalimumab biosimilar GP2017 regarding the primary end-point of no radiographic structural progression of the spine (32). This will allow the direct comparison of secukinumab with an adalimumab biosimilar for an important clinical end-point.
Supplementary Materials
Supplementary Table 1.
Eligibility criteria (PICOS) for systematic literature review
| Criteria | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population |
|
|
| Intervention |
|
|
| Comparators |
|
|
| Outcomes (note that references were only limited by outcomes at full-text review stage)* |
Efficacy
|
|
| Study design |
|
|
| Language |
|
|
| Date |
|
|
In line with the original review, studies were screened for outcomes only during the full-text review; at the title/abstract screening stage, any outcomes will be permitted
In line with the original review, systematic reviews, meta-analyses, and pooled analyses will be included at the title/abstract screening stage and used for identification of any additional primary studies not identified through the database searches, but will be excluded during the full-text review
AE: adverse event; AS: ankylosing spondylitis; ASAS: Assessment of SpondyloArthritis International Society; ASAS 20/40/70: 20%/40%/70% improvement in the ASAS response criteria; ASAS 5/6: 20% improvement in any five of the six domains in the ASAS response criteria; ASDAS: Ankylosing Spondylitis Disease Activity Score; ASQoL: Ankylosing Spondylitis Quality of Life; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; BASFI: Bath Ankylosing Spondylitis Functional Index; BASMI: Bath Ankylosing Spondylitis Metrology Index; DMARD: disease-modifying anti-rheumatic drug; EQ-5D: 5-dimensions EuroQol Questionnaire; FACIT: Functional Assessment of Chronic Illness Therapy; HAQ: Health Assessment Questionnaire; MASES: Maastricht Ankylosing Spondylitis Enthesitis Score; mSASSS: modified Stoke Ankylosing Spondylitis Spinal Score; NSAID: non-steroidal anti-inflammatory drug; PICOS: population, intervention, comparators, outcomes, and study design; SF-36: 36-item Short-Form Health Survey; TNFi: tumor necrosis factor inhibitor
Supplementary Table 2.
Trials identified by the SLR and reasons for inclusion or exclusion in the MAIC
| Trial acronym | Intervention | Comparator | Population reference | Primary study Secondary references | Study included in MAIC analysis? | |
|---|---|---|---|---|---|---|
| A2209 trial | Two doses of intravenous secukinumab 10 mg/kg given 3 weeks apart | Placebo | 30 patients aged 18–65 years with moderate to severe AS were randomly assigned to treatment | Baeten et al. (33) 2013 | NA | No - treatment regimen is unlicensed use of secukinumab |
| ATLAS | Adalimumab 40 mg Q2W | Placebo | 315 patients aged ≥18 years with definite active AS were randomly assigned to treatment | van der Heijde et al. (19) 2006 | van der Heijde et al. (34) 2015 Sieper et al. (35) 2012 Maksymowych et al. (36) 2010 van der Heijde et al. (37) 2009 van der Heijde et al. (20) 2009 Revicki et al. (38) 2008 van der Heijde et al. (39) 2008 Davis et al. (40) 2007 Sieper et al. (41) 2014 van der Heijde et al. (42) 2008 |
Yes |
| Hu et al. 2012 | Adalimumab 40 mg Q2W | Placebo | 46 patients who had been treated unsuccessfully (non-responsive or lack of tolerance) with ≥1 NSAID | Hu et al. (43) 2012 | NA | No - ASAS outcome not measured |
| Huang et al. 2014 | Adalimumab 40 mg Q2W | Placebo | 334 patients with inadequate response or intolerance to previous treatment | Huang et al. (44) 2014 | NA | No - mono-ethnic study population (Chinese) |
| MEASURE 1 | Secukinumab 75 mg or 150 mg Q4W | Placebo | 371 patients aged ≥18 years with moderate to severe active AS were randomly assigned to treatment | Baeten et al. (45) 2014 | Deodhar et al. (46) 2014 Baeten et al. (47) 2015 Baraliakos et al. (48) 2015 Deodhar et al. (49) 2015 Baeten et al. (50) 2015 Baraliakos et al. (51) 2015 Wei et al. (52) 2015 |
Yes |
| MEASURE 2 | Secukinumab 75 mg or 150 mg Q4W | Placebo | 219 patients aged ≥18 years with moderate to severe active AS were randomly assigned to treatment | Sieper et al. (53) 2014 | Baeten et al. (50) 2015 Braun et al. (54) 2015 Braun et al. (55) 2015 Deodhar et al. (49) 2015 Deodhar et al. (56) 2015 Sieper et al. (57) 2015 Sieper et al. (58) 2015 |
Yes |
| M03-606 Canadian AS Study | Adalimumab 40 mg Q2W | Placebo | 82 patients with active AS who had inadequate response or were intolerant to >1 NSAID, and had no previous exposure to TNFi therapy | Lambert et al. (59) 2007 |
Sieper et al. (41) 2014 Van der Heijde et al. (60) 2009 |
No - the study did not report any end-points of interest |
Studies excluded from the MAIC analysis are shown in bold
AS: ankylosing spondylitis, ASAS: Assessment of SpondyloArthritis International Society, MAIC: matching-adjusted indirect comparison, NA: not applicable, NSAID: non-steroidal anti-inflammatory drug, Q2W: every 2 weeks, Q4W: every 4 weeks, SLR: systematic literature review, TNFi: tumor necrosis factor inhibitor
Supplementary Table 3.
Logistic regression model for ASAS 20 and ASAS 40 (pooled MEASURE 1/2) responses at week 52
| OR (95% CI), p | ||
|---|---|---|
|
|
||
| Variable | ASAS 20 | ASAS 40 |
| Age (estimated OR for a 10-unit change) | 0.89 (0.74–1.08) p=0.237 | 0.82 (0.69–0.99) p=0.035 |
| Weight (estimated OR for a 5-unit change) | 0.93 (0.86–1.00) p=0.048 | 0.91 (0.85–0.98) p=0.018 |
| Sex (female vs. male) | 1.00 (0.59–1.68) p=0.997 | 0.87 (0.53–1.41) p=0.557 |
| Smoking history at baseline (yes vs. no) | 0.92 (0.57–1.49) p=0.724 | 0.88 (0.56–1.39) p=0.584 |
| TNFi status (inadequate responders vs. naïve) | 0.55 (0.33–0.92) p=0.022 | 0.53 (0.32–0.88) p=0.014 |
| Methotrexate use at baseline (yes vs. no) | 0.74 (0.37–1.45) p=0.376 | 0.98 (0.51–1.87) p=0.940 |
| Corticosteroid use at baseline (yes vs. no) | 0.40 (0.19–0.85) p=0.018 | 0.74 (0.36–1.55) p=0.428 |
| BASDAI total score at baseline | 1.27 (1.08–1.49) p=0.005 | 1.18 (1.01–1.37) p=0.036 |
| BASFI score at baseline | 1.06 (0.95–1.18) p=0.326 | 1.05 (0.95–1.17) p=0.347 |
| Human leukocyte antigen B27 at baseline (positive vs. negative) | 1.56 (0.84–2.91) p=0.157 | 1.20 (0.66–2.18) p=0.545 |
| CRP at baseline | 1.02 (1.01–1.04) p=0.007 | 1.02 (1.01–1.04) p<0.001 |
| AS duration at baseline | 1.00 (0.97–1.02) p=0.707 | 0.98 (0.95–1.00) p=0.087 |
Italic format indicates statistically significant results with a p value of <0.05. Standard error estimates (95% CI) and p values are from a logistic regression model with baseline factors including age, weight, sex, smoking history, TNFi status, methotrexate use, corticosteroid use, NSAID use, BASDAI total score, BASFI score, human leukocyte antigen B27, C-reactive protein, and disease duration. The model includes n=192 observations. Missing responders are considered as non-responders
AS: ankylosing spondylitis, ASAS 20/40: 20%/40% improvement in the Assessment of SpondyloArthritis International Society response criteria, BASDAI: Bath Ankylosing Spondylitis Disease Activity Index, BASFI: Bath Ankylosing Spondylitis Functional Index, CI: confidence interval, CRP: C-reactive protein, NSAID: non-steroidal anti-inflammatory drug, OR: odds ratio, TNFi: tumor necrosis factor inhibitor
Supplementary Table 4.
Baseline characteristics before and after matching
| Before matching | MEASURE 1/2 after matching | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| ATLAS | MEASURE 1/2 | Principal analysis | Sensitivity analysis | |||||
| Baseline characteristics | ADA 40 mg n=208 |
Placebo n=107 |
SEC 150 mg n=197 |
Placebo n=196 |
SEC 150 mg ESS=120 |
Placebo ESS=120 |
SEC 150 mg ESS=114 |
Placebo ESS=117 |
| Demographic characteristics used for matching | ||||||||
| Age, years, mean (SD) | 41.7 (11.7) | 43.4 (11.3) | 40.8 (11.9) p=0.4433 |
43.3 (12.7) | 41.7 (9.8) p=1.0000 |
43.4 (9.7) | 41.7 (9.8) p=1.0000 |
43.4 (12.2) |
| Female, n (%) | 51 (24.5) | 28 (26.2) | 67 (34.0) p=0.0356 |
55 (28.1) | (24.5)* p=0.9969 |
(26.2)* | (24.5)* p=0.9969 |
(26.2)* |
| Disease characteristics used for matching | ||||||||
| BASDAI, mean† (SD) | 6.3 (1.7) | 6.3 (1.7) | 6.4 (1.5) p=0.5314 |
6.6 (1.4) | 6.1 (1.2)† p=0.2570 |
6.5 (1.2)† | 6.3 (1.2) p=1.0000 |
6.3 (1.3) |
| BASFI, mean (SD) | 5.2 (2.2) | 5.6 (2.2) | 5.9 (2.2) p=0.0015 |
5.9 (2.0) | 5.2 (1.8) p=1.0000 |
5.6 (1.7) | 5.2 (1.8) p=1.0000 |
5.6 (1.7) |
| CRP, mg/dL, mean (SD) | 1.8 (2.2) | 2.2 (2.9) | 2.0 (3.5) p=0.4891 |
1.6 (2.1) | 1.8 (2.3) p=1.0000 |
2.2 (2.2) | 1.8 (2.3) p=1.0000 |
2.2 (2.2) |
| Previous therapy used for matching‡ | ||||||||
| TNFi-naïve, n (%) | 208 (100) | 107 (100) | 136 (69.0) p<0.0001 |
134 (68.4) | (100)* p=1.0000 |
(100)* | (100)* p=1.0000 |
(100)* |
| Characteristics not used for matching¶ | ||||||||
| BASDAI fatigue item§, mean (SD) | 6.5 (2.0) | 6.7 (1.9) | 6.8 (1.6) | 6.9 (1.7) | 6.5 (1.3) | 6.7 (1.4) | 6.6 (1.2) | 6.5 (1.5) |
| BASMI total score, mean (SD) | 3.8 (2.2) | 4.2 (2.1) | 3.8 (1.8) | 4.0 (1.6) | 3.7 (1.5) | 4.1 (1.3) | 3.7 (1.4) | 4.1 (1.3) |
| Tender joint count, mean (SD) | 5.1 (7.4) | 5.6 (6.8) | 5.5 (7.6) | 5.9 (8.2) | 4.3 (5.7) | 5.3 (5.9) | 4.4 (5.7) | 5.1 (5.7) |
| Swollen joint count, mean (SD) | 1.5 (3.3) | 1.4 (2.8) | 1.9 (3.7) | 2.2 (4.6) | 1.6 (2.8) | 2.0 (3.6) | 1.6 (2.7) | 1.9 (3.4) |
| SF-36 PCS, mean (SD) | 32.9 (8.0) | 31.8 (8.0) | 35.9 (6.8) | 36.3 (6.3) | 37.8 (5.7) | 37.0 (5.2) | 37.6 (5.5) | 37.0 (5.1) |
| SF-36 MCS, mean (SD) | 43.4 (12.0) | 44.4 (12.0) | 39.9 (10.6) | 39.5 (10.4) | 41.6 (8.4) | 40.2 (8.2) | 41.3 (8.4) | 40.6 (8.1) |
| PtGA, mean (SD) | 6.3 (2.2) | 6.5 (2.0) | 6.5 (1.9) | 6.8 (1.8) | 6.2 (1.5) | 6.5 (1.5) | 6.4 (1.5) | 6.4 (1.5) |
| Total back pain, mean (SD) | 6.4 (2.1) | 6.7 (2.2) | 6.5 (1.8) | 6.8 (1.7) | 6.2 (1.5) | 6.7 (1.4) | 6.4 (1.4) | 6.6 (1.4) |
| Nocturnal pain, mean (SD) | 6.1 (2.4) | 6.5 (2.4) | 6.3 (1.9) | 6.5 (2.0) | 6.0 (1.5) | 6.6 (1.4) | 6.2 (1.4) | 6.5 (1.4) |
| ASQoL, mean (SD) | 10.2 (4.0) | 10.6 (4.0) | 11.3 (4.5) | 11.6 (4.2) | 10.3 (3.9) | 11.3 (3.7) | 10.4 (3.9) | 11.2 (3.7) |
| Corticosteroid use, mean (%) | 25 (12.0) | 6 (5.6) | 22 (11.2) | 23 (11.7) | (12.5)* | (14.0)* | (11.2)* | (14.5)* |
Integer population (n) values not available owing to calculation of pooled SEC ESS using the equation:
BASDAI scores were not included in the matching for the principal analysis, but were included in the sensitivity analysis
TNFi-experienced patients in MEASURE 1 and MEASURE 2 are TNFi-inadequate responders
This score is based on a question on fatigue from the BASDAI
Duration of AS was also identified as a relevant factor, but this could not be matched because it was defined as time from diagnosis in MEASURE 1/2 and time from symptom onset in ATLAS
All p values were calculated for secukinumab vs. adalimumab
ADA: adalimumab, ASQoL: Ankylosing Spondylitis Quality of Life, BASDAI: Bath Ankylosing Spondylitis Disease Activity Index, BASFI: Bath Ankylosing Spondylitis Functional Index, BASMI: Bath Ankylosing Spondylitis Metrology Index, CRP: C-reactive protein, ESS: effective sample size, MCS: Mental Component Summary, PCS: Physical Component Summary, PtGA: patient global assessment, SD: standard deviation, SEC: secukinumab, SF-36: 36-item Short-Form Health Survey, TNFi: tumor necrosis factor inhibitor
Supplementary Table 5.
Relative likelihoods of response for secukinumab 150 mg and adalimumab 40 mg at weeks 8, 12, 16, 24, and 52
| Principal analysis (SEC vs. ADA) | Sensitivity analysis (SEC vs. ADA) | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| ASAS 20 | ASAS 40 | ASAS 5/6 | ASAS PR | ASAS 20 | ASAS 40 | ASAS 5/6 | ASAS PR |
| Week 8 (placebo-adjusted) | |||||||
| 0.92 (0.59–1.43) p=0.707 | NR | NR | NR | 0.96 (0.62–1.49) p=0.851 | NR | NR | NR |
| Week 12 (placebo-adjusted) | |||||||
| 0.65 (0.40–1.06) p=0.084 | 0.88 (0.45–1.75) p=0.725 | 0.57 (0.30–1.08) p=0.084 | 0.91 (0.24–3.46) p=0.893 | 0.69 (0.42–1.12) p=0.130 | 0.90 (0.46–1.79) p=0.767 | 0.57 (0.30–1.09) p=0.088 | 0.93 (0.24–3.53) p=0.914 |
| Week 16 (non-placebo-adjusted) | |||||||
| 1.21 (1.01–1.46) p=0.040 | NR | NR | NR | 1.24 (1.03–1.49) p=0.022 | NR | NR | NR |
| Week 24 (non-placebo-adjusted) | |||||||
| 1.27 (1.05–1.54) p=0.013 | 1.37 (1.08–1.73) p=0.010 | 1.23 (0.99–1.54) p=0.065 | 1.25 (0.85–1.84) p=0.261 | 1.30 (1.08–1.57) p=0.006 | 1.39 (1.09–1.76) p=0.007 | 1.25 (1.00–1.56) p=0.053 | 1.27 (0.86–1.87) p=0.235 |
| Week 52 (non-placebo-adjusted) | |||||||
| 1.13 (1.00–1.27) p=0.053 | 1.23 (1.03–1.46) p=0.020 | 1.15 (0.99–1.34) p=0.065 | 0.78 (0.58–1.06) p=0.116 | 1.14 (1.01–1.28) p=0.038 | 1.22 (1.02–1.46) p=0.026 | 1.15 (0.98–1.34) p=0.080 | 0.79 (0.58–1.08) p=0.144 |
Data are RR (95% CI). All p values (shown in italics when significant, i.e., p<0.05) were derived from RR values. At week 52, SEC populations were pooled (i.e., patients receiving active treatment plus those switching to active treatment from placebo) and missing data were derived using LOCF imputation to match the ADA patient population in ATLAS
ADA: adalimumab, ASAS 20/40: 20%/40% improvement in Assessment of SpondyloArthritis International Society response criteria, ASAS 5/6: 20% improvement in any five of the six domains in the Assessment of SpondyloArthritis International Society response criteria, ASAS PR: Assessment of SpondyloArthritis International Society partial remission, CI: confidence interval, LOCF: last observation carried forward, NR: not reported, RR: relative likelihood of response, SEC: secukinumab
Supplementary Table 6.
Odds ratios for secukinumab 150 mg and adalimumab 40 mg using a possible statistical interpretation in acknowledgment of the American Statistical Association guidance
| Principal analysis (SEC vs. ADA) | Sensitivity analysis (SEC vs. ADA) | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| ASAS 20 | ASAS 40 | ASAS 5/6 | ASAS PR | ASAS 20 | ASAS 40 | ASAS 5/6 | ASAS PR |
| Week 8 (placebo-adjusted) | |||||||
| 0.91 (0.44–1.89) p=0.795 | NR | NR | NR | 0.99 (0.47–2.06) p=0.971 | NR | NR | NR |
| Week 12 (placebo-adjusted) | |||||||
| 0.60 (0.28–1.28) p=0.185 | 0.93 (0.39–2.21) p=0.867 | 0.50 (0.22–1.18) p=0.115 | 0.97 (0.23–4.04) p=0.961 | 0.67 (0.31–1.44) p=0.304 | 0.96 (0.40–2.30) p=0.927 | 0.52 (0.22–1.21) p=0.129 | 0.97 (0.23–4.08) p=0.972 |
| Week 16 (non-placebo-adjusted) | |||||||
| 1.60 (1.01–2.54) p=0.047 | NR | NR | NR | 1.70 (1.06–2.73) p=0.028 | NR | NR | NR |
| Week 24 (non-placebo-adjusted) | |||||||
| 1.76 (1.11–2.79) p=0.017 | 1.79 (1.14–2.82) p=0.012 | 1.51 (0.96–2.38) p=0.072 | 1.34 (0.80–2.25) p=0.265 | 1.87 (1.17–3.01) p=0.009 | 1.85 (1.17–2.94) p=0.009 | 1.55 (0.98–2.46) p=0.060 | 1.37 (0.81–2.31) p=0.239 |
| Week 52 (non-placebo-adjusted) | |||||||
| 1.48 (0.98–2.22) p=0.062 | 1.54 (1.06–2.23) p=0.023 | 1.42 (0.97–2.07) p=0.072 | 0.71 (0.47–1.08) p=0.110 | 1.53 (1.00–2.34) p=0.048 | 1.52 (1.04–2.23) p=0.031 | 1.40 (0.95–2.07) p=0.089 | 0.72 (0.47–1.11) p=0.137 |
Data are OR (95% CI). We avoid strict thresholds when interpreting statistical p values, as per the American Statistical Association definition (7 March 2016) (25), but loosely interpret p values (two-sided) between 0.1 and 0.001 as increasing evidence (weak through moderate to strong) and p values (two-sided) below 0.001 as strong evidence (27). All p values (two-sided) are shown. Italic format indicates differences with p values between 0.1 and 0.001. At week 52, SEC populations were pooled (i.e., patients receiving active treatment plus those switching to active treatment from placebo) and missing data were derived using LOCF imputation to match the ADA patient population in ATLAS
ADA: adalimumab, ASAS 20/40: 20%/40% improvement in the Assessment of SpondyloArthritis International Society response criteria, ASAS 5/6: 20% improvement in any five of the six domains in the Assessment of SpondyloArthritis International Society response criteria, ASAS PR: Assessment of SpondyloArthritis International Society partial remission, CI: confidence interval, LOCF: last observation carried forward, NR: not reported, OR: odds ratio, SEC: secukinumab
Supplementary Table 7.
Principal analysis placebo arm responses across selected outcomes
| Principal analysis | Sensitivity analysis | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Week 8 | Week 12 | Week 8 | Week 12 | |||||
|
|
|
|
|
|||||
| Outcome | ATLAS | MEASURE 1/2 | ATLAS | MEASURE 1/2 | ATLAS | MEASURE 1/2 | ATLAS | MEASURE 1/2 |
| ASAS 20, % patients | 29.0 | 32.3 | 20.6 | 33.7 | 29.0 | 38.6 | 20.6 | 36.0 |
| ASAS 40, % patients | NA | NA | 13.1 | 16.7 | NA | NA | 13.1 | 22.2 |
| BASDAI 50, % patients | NA | NA | 15.9 | 17.6 | NA | NA | 15.9 | 21.1 |
| ASAS 5/6, % patients | NA | NA | 13.1 | 23.2 | NA | NA | 13.1 | 26.2 |
| ASAS PR, % patients | NA | NA | 3.7 | 5.2 | NA | NA | 3.7 | 6.1 |
| PtGA, 0–100 mm VAS | NA | NA | 6.5 | −13.2 | NA | NA | 6.5 | −14.5 |
| BASFI*, 0–100 mm VAS | NA | NA | −0.80 | −0.87 | NA | NA | −0.80 | −0.96 |
| CRP*, mg/dL | NA | NA | −0.10 | −0.26 | NA | NA | −0.10 | −0.20 |
Mean change from baseline
ASAS 20/40: 20%/40% improvement in the Assessment of SpondyloArthritis International Society response criteria, ASAS 5/6: 20% improvement in any five of the six domains in the Assessment of SpondyloArthritis International Society response criteria, ASAS PR: Assessment of SpondyloArthritis International Society partial remission, BASDAI 50: 50% improvement in the Bath Ankylosing Spondylitis Disease Activity Index, BASFI: Bath Ankylosing Spondylitis Functional Index, CRP: C-reactive protein, NA: not available, PtGA: patient global assessment, VAS: visual analog scale
Supplementary Table 8.
Sensitivity analysis continuous outcome comparisons
| Placebo-adjusted change from baseline* (95% CI) | Change from baseline* (95% CI) | Change from baseline† (95% CI) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Week 12 | Week 24 | Week 52 | ||||
|
|
|
|
||||
| ADA 40 mg | SEC 150 mg | ADA 40 mg | SEC150 mg | ADA 40 mg | SEC 150 mg | |
| ASAS core set PtGA, 0–100 mm VAS |
−45.6 (−60.9 to −30.3) p<0.001 |
−16.9 (−22.0 to −11.8) | −37.8 (−47.6 to −28.0) p=0.675 |
−35.6 (−39.3 to −1.9) | −31.0 (NR) | −34.8 (−37.8 to −31.8) |
| Total back pain, 0–100 mm VAS | −18.9 (−24.8 to −13.0) p=0.941 |
−18.6 (−23.5 to −3.7) | −27.7 (−31.2 to −24.2) | −36.0 (−39.4 to −32.7) p<0.001 |
−31.0 (NR) | −34.9 (−37.9 to −32.0) |
| Nocturnal back pain, 0–100 mm VAS | −18.0 (−24.0 to −12.0) p=0.918 |
−17.6 (−22.6 to −12.6) | −27.3 (−31.0 to −23.6) | −34.9 (−38.5 to −31.3) p=0.004 |
NR | −37.1 (−40.1 to −34.1) |
| BASFI, 0–100 mm VAS |
−2.8 (−3.7 to −1.8) p<0.001 |
−1.0 (−1.4 to −0.7) |
−3.8 (−4.4 to −3.2) p<0.001 |
−2.3 (−2.5 to −2.0) | −2.1 (NR) | −2.3 (−2.6 to −2.1) |
| BASDAI inflammation‡ | NA | NA |
−4.3 (−5.0 to −3.6) p=0.004 |
−3.1 (−3.5 to −2.6) | −3.6 (NR) | −3.2 (−3.5 to −2.9) |
| BASMI total score |
−0.6 (−0.9 to −0.3) p=0.044 |
−0.3 (−0.4 to −0.1) | −0.6 (−0.8 to −0.4) p=0.798 |
−0.6 (−0.7 to −0.5) | −0.7 (NR) | −0.5 (−0.6 to −0.4) |
| CRP, mg/dL | −1.2 (−1.5 to −0.9) p=0.637 |
−1.1 (−1.5 to −0.7) | −1.3 (−1.5 to −1.1) p=0.814 |
−1.3 (−1.6 to −0.9) | −1.2 (NR) | −1.3 (−1.6 to −1.0) |
| Additional key outcomes ASQoL, 0–18 | NA | NA | −3.6 (−5.0 to −2.2) | −4.3 (−5.1 to −3.4) p=0.409 |
−3.9 (NR) | −4.6 (−5.2 to −4.0) |
| Tender joint count, 44 counts | −0.5 (−1.9 to 0.9) | NA | −0.9 (−1.7 to −0.1) | −2.7 (−3.5 to −2.0) p<0.001 |
−1.5 (NR) | −2.4 (−3.1 to −1.7) |
| Swollen joint count, 44 counts | 0.1 (−0.6 to 0.8) | NA | −0.4 (−0.8 to 0.0) | −1.1 (−1.5 to −0.7) p=0.013 |
−0.2 (NR) | −0.6 (−1.1 to −0.2) |
| BASDAI, 0–100 mm VAS | −1.8 (−2.4 to −1.2) p=0.372 |
−1.5 (−1.9 to −1.1) | −2.6 (−3.0 to −2.2) | −3.1 (−3.3 to −2.8) p=0.059 |
−3.0 (NR) | −2.9 (−3.2 to −2.7) |
| BASDAI fatigue§, 0–100 mm VAS | −1.5 (−2.2 to −0.8) p=0.691 |
−1.3 (−1.8 to −0.9) | −2.4 (−2.8 to −2.0) | −3.0 (−3.3 to −2.6) p=0.031 |
NR | −2.9 (−3.2 to −2.6) |
95% CI, ranges for ADA are not reported and were calculated for the purpose of the comparison
Complete week 52 data were not available from ATLAS, and it was not possible to calculate p values
The BASDAI inflammation score was calculated as the mean of scores for questions 5 and 6 of the BASDAI
This score is based on the fatigue item of the BASDAI
Italic format indicates statistically significant evidence supporting SEC superiority over ADA (p value shown). Bold format indicates statistically significant evidence supporting ADA superiority over SEC (p value shown)
ADA: adalimumab, ASAS: Assessment of SpondyloArthritis International Society, ASQoL: Ankylosing Spondylitis Quality of Life questionnaire, BASDAI: Bath Ankylosing Spondylitis Disease Activity Index, BASFI: Bath Ankylosing Spondylitis Functional Index, BASMI: Bath Ankylosing Spondylitis Metrology Index, CI: confidence interval, CRP: C-reactive protein, NA: not available, NR: not reported, PtGA: patient global assessment, SEC: secukinumab, VAS: visual analog scale
PRISMA diagram
A systematic literature review (conducted: September 2014; updated: September 2015) was used to identify relevant clinical evidence of secukinumab and biologic comparators in the treatment of adult patients with AS. Twenty-three trials were judged suitable for inclusion according to the eligibility criteria (Supplementary Table 1); 16 RCTs included neither secukinumab nor adalimumab and were excluded; a further four trials were excluded based on the research question, leaving three [MEASURE 1 (18), MEASURE 2 (18), and ATLAS (19, 20)] for use in the MAIC. Details of the four excluded and three included trials are provided in Supplementary Table 2; study designs are summarized in Supplementary Figure 2. Outcomes selected for comparison were in line with ASAS and Outcome Measures in Rheumatology (OMERACT) recommendations (29, 30).
MAIC: matching-adjusted indirect comparison, PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
MEASURE 1, MEASURE 2, and ATLAS trial designs
MEASURE 1 and 2 were phase 3 RCTs (18) in which patients who were refractory to NSAID therapy as defined by ASAS recommendations were recruited. Patients were also eligible if they had previously inadequately responded to ≥3 months of treatment with an approved dose of one TNFi (primary or secondary lack of efficacy); these accounted for 27.0% and 39.7% of the respective patient populations. Patients receiving placebo who did not achieve an ASAS20 response by week 16 were re-randomized 1:1 to secukinumab 75 mg or 150 mg; those who achieved responses were re-randomized 1:1 at week 24. In MEASURE 1, secukinumab was administered as an intravenous loading dose (10 mg/kg body weight) at weeks 0, 2, and 4, then subcutaneously (75 mg or 150 mg) every 4 weeks starting from week 8; in MEASURE 2, secukinumab was given subcutaneously (75 mg or 150 mg) at weeks 0, 1, 2, and 3, and every 4 weeks starting from week 4. ATLAS was a phase 3 RCT that recruited NSAID-refractory patients (19, 20); patients were ineligible if they had received any TNFi therapy. Adalimumab was given subcutaneously (40 mg) every other week. Patients receiving placebo without ASAS 20 responses at week 12, 16, or 20 were eligible for “early escape” to receive open-label treatment with adalimumab; all patients were eligible from week 24, with an option to uptitrate to weekly adalimumab (19, 20). Across trials, the primary end-point was the proportion of ASAS 20 responders - at week 16 for MEASURE 1 and 2, and at week 12 for ATLAS. The predominant difference between ATLAS and MEASURE trials was that the latter included patients for whom a previous TNFi treatment had failed, while participants in the ATLAS trial were TNFi-naïve.
ASAS 20: at least a 20% improvement in Assessment of SpondyloArthritis International Society response criteria, EOW: every other week, MAIC: matching-adjusted indirect comparison, M1: MEASURE 1, M2: MEASURE 2, R: randomization.
*Patients classified as “responders” (achieved ≥ASAS 20 response) at week 16. †Patients classified as “non-responders” (did not achieve ASAS 20 response) at week 16. ‡Patients who did not achieve an ASAS 20 response were eligible. §Patients who did not achieve an ASAS 20 response after ≥12 weeks of open-label treatment with adalimumab 40 mg EOW were eligible for adalimumab 40 mg weekly.
¶296 patients completed the double-blind phase and were eligible to enter the open-label extension. Post-week 24 efficacy data used in our MAIC analysis used published LOCF data of ATLAS patients who received at least one dose of adalimumab (n=311, of whom 239 received adalimumab EOW and 72 received adalimumab weekly). Placebo-adjusted comparisons were possible only up to week 12 because patients in ATLAS who did not achieve an ASAS 20 response were eligible for early escape (open-label adalimumab) from week 12 to week 24 (the cumulative number of patients entering early escape from each arm is indicated in red numerals; patients who withdrew from the study are not shown). Reference numbering in this figure caption is from the main paper
Principal analysis results for ASAS 20 showing placebo and active treatment responses
In the MEASURE 1 and MEASURE 2 trials, patients receiving placebo who did not achieve an ASAS 20 response by week 16 were re-randomized 1:1 to secukinumab 75 mg or 150 mg; those who did achieve this response were re-randomized 1:1 at week 24. In the ATLAS trials, patients receiving placebo who did not achieve an ASAS 20 response at week 12, 16, or 20 were eligible for the “early escape” option of open-label treatment with adalimumab; all patients were eligible for this from week 24, with an option to uptitrate to weekly adalimumab
ADA: adalimumab, ASAS 20: 20% improvement in Assessment of SpondyloArthritis International Society response criteria, ESS: effective sample size, i.v.: intravenous, LOCF: last observation carried forward, M1: MEASURE 1, M2: MEASURE 2, PBO: placebo, s.c.: subcutaneous, SEC: secukinumab
Supplementary References
- 33.Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, van der Heijde D, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382:1705–13. doi: 10.1016/S0140-6736(13)61134-4. https://doi.org/10.1016/S0140-6736(13)61134-4 [DOI] [PubMed] [Google Scholar]
- 34.van der Heijde D, Breban M, Halter D, DiVittorio G, Bratt J, Cantini F, et al. Maintenance of improvement in spinal mobility, physical function and quality of life in patients with ankylosing spondylitis after 5 years in a clinical trial of adalimumab. Rheumatology (Oxford) 2015;54:1210–9. doi: 10.1093/rheumatology/keu438. https://doi.org/10.1093/rheumatology/keu438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sieper J, van der Heijde D, Dougados M, Brown LS, Lavie F, Pangan AL. Early response to adalimumab predicts long-term remission through 5 years of treatment in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71:700–6. doi: 10.1136/annrheumdis-2011-200358. https://doi.org/10.1136/annrheumdis-2011-200358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maksymowych WP, Gooch KL, Wong RL, Kupper H, van der Heijde D. Impact of age, sex, physical function, health-related quality of life, and treatment with adalimumab on work status and work productivity of patients with ankylosing spondylitis. J Rheumatol. 2010;37:385–92. doi: 10.3899/jrheum.090242. https://doi.org/10.3899/jrheum.090242 [DOI] [PubMed] [Google Scholar]
- 37.van der Heijde DM, Revicki DA, Gooch KL, Wong RL, Kupper H, Harnam N, et al. Physical function, disease activity, and health-related quality-of-life outcomes after 3 years of adalimumab treatment in patients with ankylosing spondylitis. Arthritis Res Ther. 2009;11:R124. doi: 10.1186/ar2790. https://doi.org/10.1186/ar2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Revicki DA, Luo MP, Wordsworth P, Wong RL, Chen N, Davis JC., Jr Adalimumab reduces pain, fatigue, and stiffness in patients with ankylosing spondylitis: results from the adalimumab trial evaluating long-term safety and efficacy for ankylosing spondylitis (ATLAS) J Rheumatol. 2008;35:1346–53. [PubMed] [Google Scholar]
- 39.van der Heijde D, Pangan AL, Schiff MH, Braun J, Borofsky M, Torre J, et al. Adalimumab effectively reduces the signs and symptoms of active ankylosing spondylitis in patients with total spinal ankylosis. Ann Rheum Dis. 2008;67:1218–21. doi: 10.1136/ard.2007.082529. https://doi.org/10.1136/ard.2007.082529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis JC, Jr, Revicki D, van der Heijde DM, Rentz AM, Wong RL, Kupper H, et al. Health-related quality of life outcomes in patients with active ankylosing spondylitis treated with adalimumab: results from a randomized controlled study. Arthritis Rheum. 2007;57:1050–7. doi: 10.1002/art.22887. https://doi.org/10.1002/art.22887 [DOI] [PubMed] [Google Scholar]
- 41.Sieper J, van der Heijde D, Vorathai NA, Anderson JK. Comparison of baseline extra-articular manifestations, comorbidities, and long-term safety in patients treated with adalimumab for ankylosing spondylitis and non-radiographic axial spondyloarthritis. Arthritis Rheumatol. 2014;66(Suppl 10):S242–3. [Google Scholar]
- 42.van der Heijde D, Dijkmans B, Schiff M, Kivitz A, de Vlam K, Wordsworth P. Maintenance of reduction in disease activity and partial remission in patients with ankylosing spondylitis (AS) - 3 year results from the adalimumab (Humira) trial evaluating long-term efficacy and safety in AS (ATLAS) Arthritis Rheum. 2008:S579. [Google Scholar]
- 43.Hu Z, Xu M, Li Q, Lin Z, Liao Z, Cao S, et al. Adalimumab significantly reduces inflammation and serum DKK-1 level but increases fatty deposition in lumbar spine in active ankylosing spondylitis. Int J Rheum Dis. 2012;15:358–65. doi: 10.1111/j.1756-185X.2012.01734.x. https://doi.org/10.1111/j.1756-185X.2012.01734.x [DOI] [PubMed] [Google Scholar]
- 44.Huang F, Gu J, Zhu P, Bao C, Xu J, Xu H, et al. Efficacy and safety of adalimumab in Chinese adults with active ankylosing spondylitis: results of a randomised, controlled trial. Ann Rheum Dis. 2014;73:587–94. doi: 10.1136/annrheumdis-2012-202533. https://doi.org/10.1136/annrheumdis-2012-202533 [DOI] [PubMed] [Google Scholar]
- 45.Baeten D, Braun J, Baraliakos X, et al. Secukinumab, a monoclonal antibody to interleukin-17A, significantly improves signs and symptoms of active ankylosing spondylitis: results of a 52-week phase 3 randomized placebo-controlled trial with intravenous loading and subcutaneous maintenance dosing. Arthritis Rheumatol. 2014;66(Suppl 10) [Google Scholar]
- 46.Deodhar A, Baeten D, Braun J, Baraliakos X, Sieper J, Dougados M. Secukinumab, a monoclonal antibody to interleukin-17A, significantly improves physical function and quality of life in subjects with active ankylosing spondylitis: results of a phase 3 randomized, placebo-controlled trial with intravenous loading and subcutaneous maintenance dosing. Arthritis Rheumatol. 2014;66(Suppl 10):S233–4. [Google Scholar]
- 47.Baeten D, Braun J, Sieper J, Dougados M, Deodhar A, Baraliakos X, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis: 2-year efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2015;67(Suppl 10) [Google Scholar]
- 48.Baraliokos X, Deodhar A, Braun J, Baeten D, Dougados M, Sieper J, et al. Effect of interleukin-17A inhibition on spinal radiographic changes through 2 years in patients with active ankylosing spondylitis: results of a phase 3 study with secukinumab. Arthritis Rheumatol. 2015;67 [Google Scholar]
- 49.Deodhar A, Baeten D, Sieper, Porter B, Widmer A, Richards H. Safety and tolerability of secukinumab in patients with active ankylosing spondylitis: pooled safety analysis of two phase 3, randomized, controlled trials. American College of Rheumatology 2015 Congress; 2015, Nov 6–11; San Francisco, CA. Abstract 2887. [Google Scholar]
- 50.Baeten D, Blanco R, Geusens P, Sieper J, Jui-Cheng T, Martin R, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis in anti-TNF-naïve patients and those previously exposed to anti-TNF therapy: 52-week results from two randomized, double-blind, placebo-controlled phase 3 trials. Arthritis Rheumatol. 2015;67(Suppl 10) [Google Scholar]
- 51.Baraliakos X, Braun J, Sieper J, Baeten DL, Readie A, Ligozio G, et al. Secukinumab reduces sacroiliac joint and spinal inflammation in patients with ankylosing spondylitis: MRI data from a phase 3 randomized, double-blind, placebo-controlled study (MEASURE 1) Ann Rheum Dis. 2015;74(Suppl 2):281. https://doi.org/10.1136/annrheumdis-2015-eular.3008 [Google Scholar]
- 52.Wei JC-C, Baeten DL, Geusens P, Porter B, Martin R, Richards H. Intravenous loading and subcutaneous maintenance with secukinumab provides sustained improvement in multiple measures of disease activity in subjects with active ankylosing spondylitis: 52-week data from the randomized, double-blind, placebo-controlled, phase 3 MEASURE 1 study. Ann Rheum Dis. 2015;74(Suppl 2):1146–7. https://doi.org/10.1136/annrheumdis-2015-eular.3066 [Google Scholar]
- 53.Sieper J, Braun J, Baraliakos X, Baeten D, Dougados M, Emery P. Secukinumab, a monoclonal antibody to interleukin-17A, significantly improves signs and symptoms of active ankylosing spondylitis: results of a phase 3, randomized, placebo-controlled trial with subcutaneous loading and maintenance dosing. American College of Rheumatology 2014 Congress; 2014, Nov 14–19; Boston, MA. Poster 536. [Google Scholar]
- 54.Braun J, Deodhar A, Sieper J, Dougados M, Porter B, Andersson M, et al. Secukinumab significantly improves signs and symptoms of active ankylosing spondylitis: 52-week results from a randomized, double-blind, placebo-controlled phase 3 trial with subcutaneous loading and maintenance dosing. Arthritis Rheumatol. 2015;67(Suppl 10) [Google Scholar]
- 55.Braun J, Sieper J, Aelion J, Emery P, Deodhar A, Porter B, et al. Secukinumab improves multiple parameters of disease activity in subjects with active ankylosing spondylitis through 52 weeks of subcutaneous therapy: data from the phase 3 MEASURE 2 study. Ann Rheum Dis. 2015;74(Suppl 2):1147. https://doi.org/10.1136/annrheumdis-2015-eular.3031 [Google Scholar]
- 56.Deodhar A, Sieper J, Emery P, Porter B, Andersson M, Richards H. Secukinumab significantly improves physical function, quality of life, and work productivity through 52 weeks in subjects with active ankylosing spondylitis in the phase 3 MEASURE 2 study. Ann Rheum Dis. 2015;74(Suppl 2):1144. https://doi.org/10.1136/annrheumdis-2015-eular.3392 [Google Scholar]
- 57.Sieper J, Braun J, Baraliakos X, Baeten DL, Dougados M, Emery P, et al. Secukinumab efficacy in anti-TNF-naive patients and patients previously exposed to anti-TNF therapy: results of a randomized, double-blind, placebo-controlled phase 3 study (MEASURE 2) in active ankylosing spondylitis. Ann Rheum Dis. 2015;74(Suppl 2):272. https://doi.org/10.1136/annrheumdis-2015-eular.3089 [Google Scholar]
- 58.Sieper J, Braun J, Baraliakos X, Baeten DL, Dougados M, Emery P, et al. Secukinumab significantly improves signs and symptoms of active ankylosing spondylitis: 52-week data from MEASURE 2, a randomized, double-blind, placebo-controlled phase 3 trial with subcutaneous loading and maintenance dosing. Ann Rheum Dis. 2015;74(Suppl 2):132–3. https://doi.org/10.1136/annrheumdis-2015-eular.2787 [Google Scholar]
- 59.Lambert RG, Salonen D, Rahman P, Inman RD, Wong RL, Einstein SG, et al. Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2007;56:4005–14. doi: 10.1002/art.23044. [DOI] [PubMed] [Google Scholar]
- 60.van der Heijde D, Salonen D, Weissman BN, Landewe R, Maksymowych WP, Kupper H, et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther. 2009;11:R127. doi: 10.1186/ar2794. https://doi.org/10.1186/ar2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
Acknowledgements
The authors would like to acknowledge Christian Eichinger and Martin Dalziel of Oxford PharmaGenesis, Oxford, UK, who provided medical writing support during the development of this manuscript.
Footnotes
Ethics Committee Approval: This study was of non-interventional nature and did not include primary data collection (i.e. was based on published secondary data only). Therefore, ethic committee or institutional review board approval was not required. Data used were taken from published randomized controlled trials, which were conducted according to the principles of the Declaration of Helsinki and with informed consent from participants.
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - W.P.M., P.N., V.S., S.M.J., B.P., K.K.G.; Design - W.P.M., S.M.J, K.K.G, M.H., C.K., H.T.; Supervision - W.P.M., S.M.J., K.K.G., B.P.; Resources - S.M.J.; Data Collection and/or Processing - M.H., C.K., H.T.; Analysis and/or Interpretation - W.P.M., V.S., P.N., Y.Y., H.T., M.H., C.K., B.P., K.K.G., S.M.J.; Literature Search - S.M.J., K.K.G., B.P., H.T.; Writing Manuscript - W.P.M., V.S., P.N., Y.Y., H.T., M.H., C.K., B.P., K.K.G., S.M.J.; Critical Reviews - W.P.M., V.S., P.N., Y.Y., H.T., M.H., C.K., B.P., K.K.G., S.M.J.
You can reach the supplemental tables, figures, and references of this article at https://doi.org/10.5152/eurjrheum.2018.18162
Conflict of Interest: W.P.M. has received research grants from AbbVie, Novartis, and Pfizer, and consultancy fees from AbbVie, Boehringer, Eli Lilly, Galapagos, Janssen, Merck, Novartis, Pfizer, and UCB. V.S. has received consultancy fees from AbbVie, Amgen, AstraZeneca, Bayer, Biogen Idec, BMS, Boehringer Ingelheim, Celltrion, Crescendo, Genentech/Roche, GlaxoSmithKline, Janssen, Eli Lilly, Merck, Novartis, Pfizer, Regeneron, Samsung, Sandoz, Sanofi, and UCB. P.N. has received funding from AbbVie, BMS, Celgene, Janssen, Eli Lilly, Novartis, Pfizer, Roche, Sanofi, and UCB for research and consultancy, and as a speaker. Y.Y. has received research grants from BMS, Celgene, and Genentech, and consultancy fees from BMS, Celgene, and Novartis. H.T. has received consultancy fees from Eli Lilly, F. Hoffmann-La Roche AG, Novartis Pharma AG, and Pfizer. M.H. is a paid employee of ICON plc (formerly Mapi Group). C.K. was a paid employee of ICON plc (formerly Mapi Group), at the time of this study. The Mapi Group received funding from Novartis Pharma AG for this study. K.K.G. and B.P. are paid employees of Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA. S.M.J. is a paid employee of Novartis Pharma AG, Basel, Switzerland. K.K.G., B.P., and S.M.J. own Novartis stock.
Financial Disclosure: Medical writing support was provided by Christian Eichinger and Martin Dalziel of Oxford PharmaGenesis, Oxford, UK, and was funded by Novartis Pharma AG, Basel, Switzerland. MAPI Group performed the MAIC and associated report, funded by Novartis Pharma AG. RTI Health Solutions (Research Triangle Park, NC, USA) performed the original systematic literature review and was funded by Novartis Pharma AG. RTI Health Solutions also conducted the regression analysis of baseline parameters in MEASURE 1/2. The updated literature review was performed by Costello Medical Consulting Ltd (Cambridge, UK) who received funds from Novartis Pharma AG.
References
- 1.Ward MM, Deodhar A, Akl EA, Lui A, Ermann J, Gensler LS, et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2015;68:282–98. doi: 10.1002/art.39298. https://doi.org/10.1002/art.39298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Heijde D, Ramiro S, Landewe R, Baraliakos X, Van den Bosch F, Sepriano A, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017 doi: 10.1136/annrheumdis-2016-210770. https://doi.org/10.1136/annrheumdis-2016-210770 [DOI] [PubMed] [Google Scholar]
- 3.European Medicines Agency. Cosentyx EPAR Product Information. 2015. Available from: URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000603/WC500044686.pdf.
- 4.Food and Drug Administration (FDA) US Prescribing Information COSENTYX® (secukinumab) injection, for subcutaneous use. 2015. Available from: URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125504s001s002lbl.pdf.
- 5.Giardina AR, Ferrante A, Ciccia F, Impastato R, Miceli MC, Principato A, et al. A 2-year comparative open label randomized study of efficacy and safety of etanercept and infliximab in patients with ankylosing spondylitis. Rheumatol Int. 2010;30:1437–40. doi: 10.1007/s00296-009-1157-3. https://doi.org/10.1007/s00296-009-1157-3 [DOI] [PubMed] [Google Scholar]
- 6.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33:607–17. doi: 10.1177/0272989X12458724. https://doi.org/10.1177/0272989X12455847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–91. doi: 10.1016/s0895-4356(97)00049-8. https://doi.org/10.1016/S0895-4356(97)00049-8 [DOI] [PubMed] [Google Scholar]
- 8.Deodhar A. Mirror, mirror, on the wall, which is the most effective biologic of all? J Rheumatol. 2018;45:449–50. doi: 10.3899/jrheum.171279. https://doi.org/10.3899/jrheum.171279 [DOI] [PubMed] [Google Scholar]
- 9.Nash P, McInnes IB, Mease PJ, Thom H, Hunger M, Karabis A, et al. Secukinumab versus adalimumab for psoriatic arthritis: comparative effectiveness up to 48 weeks using a matching-adjusted indirect comparison. Rheumatol Ther. 2018 doi: 10.1007/s40744-018-0106-6. https://doi.org/10.1007/s40744-018-0106-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Betts KA, Mittal M, Song J, Skup M, Joshi A. Relative efficacy of adalimumab versus secukinumab in active ankylosing spondylitis: a matching-adjusted indirect comparison. Ann Rheum Dis. 2016;75:98–9. https://doi.org/10.1136/annrheumdis-2016-eular.2754 [Google Scholar]
- 11.Kirson NY, Rao S, Birnbaum HG, Kantor E, Wei RS, Cifaldi M. Matching-adjusted indirect comparison of adalimumab vs etanercept and infliximab for the treatment of psoriatic arthritis. J Med Econ. 2013;16:479–89. doi: 10.3111/13696998.2013.768530. https://doi.org/10.3111/13696998.2013.768530 [DOI] [PubMed] [Google Scholar]
- 12.Signorovitch JE, Wu EQ, Yu AP, Gerrits CM, Kantor E, Bao Y, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics. 2010;28:935–45. doi: 10.2165/11538370-000000000-00000. https://doi.org/10.2165/11538370-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 13.Thom H, Jugl S, Palaka E, Jawla S. Matching adjusted indirect comparisons to assess comparative effectiveness of therapies: usage in scientific literature and health technology appraisals (ID 4483). International Society for Pharmacoeconomics and Outcomes Research: 21st Annual International Meeting; 2016; Washington DC, USA. [Google Scholar]
- 14.Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. NICE DSU Technical Support Document 18: methods for population-adjusted indirect comparisons in submission to NICE. 2016. Available from: URL: http://nicedsu.org.uk/technical-support-documents/population-adjusted-indirect-comparisons-maic-and-stc/
- 15.Signorovitch JE, Sikirica V, Erder MH, Xie J, Lu M, Hodgkins PS, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15:940–7. doi: 10.1016/j.jval.2012.05.004. https://doi.org/10.1016/j.jval.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 16.Thom H, Jugl S, Nikoglou E, Jawla S. Matching-adjusted indirect comparisons to assess comparative effectiveness: a systematic review of application in scientific literature and health technology appraisals. Value Health. 2016;19:100–1. [Google Scholar]
- 17.Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2017 doi: 10.1177/0272989X17725740. 272989X17725740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373:2534–48. doi: 10.1056/NEJMoa1505066. https://doi.org/10.1056/NEJMoa1505066 [DOI] [PubMed] [Google Scholar]
- 19.van der Heijde D, Kivitz A, Schiff MH, Sieper J, Dijkmans BA, Braun J, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006;54:2136–46. doi: 10.1002/art.21913. https://doi.org/10.1002/art.21913 [DOI] [PubMed] [Google Scholar]
- 20.van der Heijde D, Schiff MH, Sieper J, Kivitz AJ, Wong RL, Kupper H, et al. Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long-term results from the ATLAS trial. Ann Rheum Dis. 2009;68:922–9. doi: 10.1136/ard.2007.087270. https://doi.org/10.1136/ard.2007.087270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.European Medicines Agency (EMA) Summary of product characteristics. Cosentyx 150 mg powder for solution for injection. 2015. Available from: URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003729/WC500183129.pdf.
- 22.Signorovitch J, Swallow E, Kantor E, Wang X, Klimovsky J, Haas T, et al. Everolimus and sunitinib for advanced pancreatic neuroendocrine tumors: a matching-adjusted indirect comparison. Exp Hematol Oncol. 2013;2:32. doi: 10.1186/2162-3619-2-32. https://doi.org/10.1186/2162-3619-2-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swallow E, Song J, Yuan Y, Kalsekar A, Kelley C, Mu F, et al. Daclatasvir + sofosbuvir versus standard of care for hepatitis C genotype 3: a matching-adjusted indirect comparison. J Comp Eff Res. 2016;5:129–39. doi: 10.2217/cer.15.49. https://doi.org/10.2217/cer.15.69 [DOI] [PubMed] [Google Scholar]
- 24.Dearden L, Majer I, Heeg B, Liwing J, Sandstrom K, Diels J. Comparison of mean overall survival (OS) and radiographic progression free survival (RPFS) based on matching adjusted indirect comparison of abiraterone acetate and enzalutamide for the treatment of castration-resistant prostate cancer in chemotherapy naive patients. Value Health. 2014;17:A616. doi: 10.1016/j.jval.2014.08.2170. https://doi.org/10.1016/j.jval.2014.08.2170 [DOI] [PubMed] [Google Scholar]
- 25.Wasserstein RL, Lazar NA. The ASA’s statement on p-values: context, process, and purpose. Am Stat. 2016;70:129–33. https://doi.org/10.1080/00031305.2016.1154108 [Google Scholar]
- 26.Best AM, 3rd, Greenberg BL, Glick M. From tea tasting to t test: a p value ain’t what you think it is. J Am Dent Assoc. 2016;147:527–9. doi: 10.1016/j.adaj.2016.05.004. https://doi.org/10.1016/j.adaj.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 27.Sterne JA, Davey Smith G. Sifting the evidence-what’s wrong with significance tests? BMJ. 2001;322:226–31. doi: 10.1136/bmj.322.7280.226. https://doi.org/10.1136/bmj.322.7280.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodman S, Greenland S. Why most published research findings are false: problems in the analysis. PLoS Med. 2007;4:e168. doi: 10.1371/journal.pmed.0040168. https://doi.org/10.1371/journal.pmed.0040168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Heijde D, van der Linden S, Bellamy N, Calin A, Dougados M, Khan MA. Which domains should be included in a core set for endpoints in ankylosing spondylitis? Introduction to the ankylosing spondylitis module of OMERACT IV. J Rheumatol. 1999;26:945–7. [PubMed] [Google Scholar]
- 30.van der Heijde D, Calin A, Dougados M, Khan MA, van der Linden S, Bellamy N. Selection of instruments in the core set for DC-ART, SMARD, physical therapy, and clinical record keeping in ankylosing spondylitis. Progress report of the ASAS Working Group. Assessments in Ankylosing Spondylitis. J Rheumatol. 1999;26:951–4. [PubMed] [Google Scholar]
- 31.Rudwaleit M, Claudepierre P, Wordsworth P, Cortina EL, Sieper J, Kron M, et al. Effectiveness, safety, and predictors of good clinical response in 1250 patients treated with adalimumab for active ankylosing spondylitis. J Rheumatol. 2009;36:801–8. doi: 10.3899/jrheum.081048. https://doi.org/10.3899/jrheum.081048 [DOI] [PubMed] [Google Scholar]
- 32.Clinicaltrials.gov. NCT03259074, Effect of secukinumab on radiographic progression in ankylosing spondylitis as compared to GP2017 (adalimumab biosimilar) (SURPASS) Available from: URL: https://clinicaltrials.gov/ct2/show/NCT03259074.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Eligibility criteria (PICOS) for systematic literature review
| Criteria | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population |
|
|
| Intervention |
|
|
| Comparators |
|
|
| Outcomes (note that references were only limited by outcomes at full-text review stage)* |
Efficacy
|
|
| Study design |
|
|
| Language |
|
|
| Date |
|
|
In line with the original review, studies were screened for outcomes only during the full-text review; at the title/abstract screening stage, any outcomes will be permitted
In line with the original review, systematic reviews, meta-analyses, and pooled analyses will be included at the title/abstract screening stage and used for identification of any additional primary studies not identified through the database searches, but will be excluded during the full-text review
AE: adverse event; AS: ankylosing spondylitis; ASAS: Assessment of SpondyloArthritis International Society; ASAS 20/40/70: 20%/40%/70% improvement in the ASAS response criteria; ASAS 5/6: 20% improvement in any five of the six domains in the ASAS response criteria; ASDAS: Ankylosing Spondylitis Disease Activity Score; ASQoL: Ankylosing Spondylitis Quality of Life; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; BASFI: Bath Ankylosing Spondylitis Functional Index; BASMI: Bath Ankylosing Spondylitis Metrology Index; DMARD: disease-modifying anti-rheumatic drug; EQ-5D: 5-dimensions EuroQol Questionnaire; FACIT: Functional Assessment of Chronic Illness Therapy; HAQ: Health Assessment Questionnaire; MASES: Maastricht Ankylosing Spondylitis Enthesitis Score; mSASSS: modified Stoke Ankylosing Spondylitis Spinal Score; NSAID: non-steroidal anti-inflammatory drug; PICOS: population, intervention, comparators, outcomes, and study design; SF-36: 36-item Short-Form Health Survey; TNFi: tumor necrosis factor inhibitor
Supplementary Table 2.
Trials identified by the SLR and reasons for inclusion or exclusion in the MAIC
| Trial acronym | Intervention | Comparator | Population reference | Primary study Secondary references | Study included in MAIC analysis? | |
|---|---|---|---|---|---|---|
| A2209 trial | Two doses of intravenous secukinumab 10 mg/kg given 3 weeks apart | Placebo | 30 patients aged 18–65 years with moderate to severe AS were randomly assigned to treatment | Baeten et al. (33) 2013 | NA | No - treatment regimen is unlicensed use of secukinumab |
| ATLAS | Adalimumab 40 mg Q2W | Placebo | 315 patients aged ≥18 years with definite active AS were randomly assigned to treatment | van der Heijde et al. (19) 2006 | van der Heijde et al. (34) 2015 Sieper et al. (35) 2012 Maksymowych et al. (36) 2010 van der Heijde et al. (37) 2009 van der Heijde et al. (20) 2009 Revicki et al. (38) 2008 van der Heijde et al. (39) 2008 Davis et al. (40) 2007 Sieper et al. (41) 2014 van der Heijde et al. (42) 2008 |
Yes |
| Hu et al. 2012 | Adalimumab 40 mg Q2W | Placebo | 46 patients who had been treated unsuccessfully (non-responsive or lack of tolerance) with ≥1 NSAID | Hu et al. (43) 2012 | NA | No - ASAS outcome not measured |
| Huang et al. 2014 | Adalimumab 40 mg Q2W | Placebo | 334 patients with inadequate response or intolerance to previous treatment | Huang et al. (44) 2014 | NA | No - mono-ethnic study population (Chinese) |
| MEASURE 1 | Secukinumab 75 mg or 150 mg Q4W | Placebo | 371 patients aged ≥18 years with moderate to severe active AS were randomly assigned to treatment | Baeten et al. (45) 2014 | Deodhar et al. (46) 2014 Baeten et al. (47) 2015 Baraliakos et al. (48) 2015 Deodhar et al. (49) 2015 Baeten et al. (50) 2015 Baraliakos et al. (51) 2015 Wei et al. (52) 2015 |
Yes |
| MEASURE 2 | Secukinumab 75 mg or 150 mg Q4W | Placebo | 219 patients aged ≥18 years with moderate to severe active AS were randomly assigned to treatment | Sieper et al. (53) 2014 | Baeten et al. (50) 2015 Braun et al. (54) 2015 Braun et al. (55) 2015 Deodhar et al. (49) 2015 Deodhar et al. (56) 2015 Sieper et al. (57) 2015 Sieper et al. (58) 2015 |
Yes |
| M03-606 Canadian AS Study | Adalimumab 40 mg Q2W | Placebo | 82 patients with active AS who had inadequate response or were intolerant to >1 NSAID, and had no previous exposure to TNFi therapy | Lambert et al. (59) 2007 |
Sieper et al. (41) 2014 Van der Heijde et al. (60) 2009 |
No - the study did not report any end-points of interest |
Studies excluded from the MAIC analysis are shown in bold
AS: ankylosing spondylitis, ASAS: Assessment of SpondyloArthritis International Society, MAIC: matching-adjusted indirect comparison, NA: not applicable, NSAID: non-steroidal anti-inflammatory drug, Q2W: every 2 weeks, Q4W: every 4 weeks, SLR: systematic literature review, TNFi: tumor necrosis factor inhibitor
Supplementary Table 3.
Logistic regression model for ASAS 20 and ASAS 40 (pooled MEASURE 1/2) responses at week 52
| OR (95% CI), p | ||
|---|---|---|
|
|
||
| Variable | ASAS 20 | ASAS 40 |
| Age (estimated OR for a 10-unit change) | 0.89 (0.74–1.08) p=0.237 | 0.82 (0.69–0.99) p=0.035 |
| Weight (estimated OR for a 5-unit change) | 0.93 (0.86–1.00) p=0.048 | 0.91 (0.85–0.98) p=0.018 |
| Sex (female vs. male) | 1.00 (0.59–1.68) p=0.997 | 0.87 (0.53–1.41) p=0.557 |
| Smoking history at baseline (yes vs. no) | 0.92 (0.57–1.49) p=0.724 | 0.88 (0.56–1.39) p=0.584 |
| TNFi status (inadequate responders vs. naïve) | 0.55 (0.33–0.92) p=0.022 | 0.53 (0.32–0.88) p=0.014 |
| Methotrexate use at baseline (yes vs. no) | 0.74 (0.37–1.45) p=0.376 | 0.98 (0.51–1.87) p=0.940 |
| Corticosteroid use at baseline (yes vs. no) | 0.40 (0.19–0.85) p=0.018 | 0.74 (0.36–1.55) p=0.428 |
| BASDAI total score at baseline | 1.27 (1.08–1.49) p=0.005 | 1.18 (1.01–1.37) p=0.036 |
| BASFI score at baseline | 1.06 (0.95–1.18) p=0.326 | 1.05 (0.95–1.17) p=0.347 |
| Human leukocyte antigen B27 at baseline (positive vs. negative) | 1.56 (0.84–2.91) p=0.157 | 1.20 (0.66–2.18) p=0.545 |
| CRP at baseline | 1.02 (1.01–1.04) p=0.007 | 1.02 (1.01–1.04) p<0.001 |
| AS duration at baseline | 1.00 (0.97–1.02) p=0.707 | 0.98 (0.95–1.00) p=0.087 |
Italic format indicates statistically significant results with a p value of <0.05. Standard error estimates (95% CI) and p values are from a logistic regression model with baseline factors including age, weight, sex, smoking history, TNFi status, methotrexate use, corticosteroid use, NSAID use, BASDAI total score, BASFI score, human leukocyte antigen B27, C-reactive protein, and disease duration. The model includes n=192 observations. Missing responders are considered as non-responders
AS: ankylosing spondylitis, ASAS 20/40: 20%/40% improvement in the Assessment of SpondyloArthritis International Society response criteria, BASDAI: Bath Ankylosing Spondylitis Disease Activity Index, BASFI: Bath Ankylosing Spondylitis Functional Index, CI: confidence interval, CRP: C-reactive protein, NSAID: non-steroidal anti-inflammatory drug, OR: odds ratio, TNFi: tumor necrosis factor inhibitor
Supplementary Table 4.
Baseline characteristics before and after matching
| Before matching | MEASURE 1/2 after matching | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| ATLAS | MEASURE 1/2 | Principal analysis | Sensitivity analysis | |||||
| Baseline characteristics | ADA 40 mg n=208 |
Placebo n=107 |
SEC 150 mg n=197 |
Placebo n=196 |
SEC 150 mg ESS=120 |
Placebo ESS=120 |
SEC 150 mg ESS=114 |
Placebo ESS=117 |
| Demographic characteristics used for matching | ||||||||
| Age, years, mean (SD) | 41.7 (11.7) | 43.4 (11.3) | 40.8 (11.9) p=0.4433 |
43.3 (12.7) | 41.7 (9.8) p=1.0000 |
43.4 (9.7) | 41.7 (9.8) p=1.0000 |
43.4 (12.2) |
| Female, n (%) | 51 (24.5) | 28 (26.2) | 67 (34.0) p=0.0356 |
55 (28.1) | (24.5)* p=0.9969 |
(26.2)* | (24.5)* p=0.9969 |
(26.2)* |
| Disease characteristics used for matching | ||||||||
| BASDAI, mean† (SD) | 6.3 (1.7) | 6.3 (1.7) | 6.4 (1.5) p=0.5314 |
6.6 (1.4) | 6.1 (1.2)† p=0.2570 |
6.5 (1.2)† | 6.3 (1.2) p=1.0000 |
6.3 (1.3) |
| BASFI, mean (SD) | 5.2 (2.2) | 5.6 (2.2) | 5.9 (2.2) p=0.0015 |
5.9 (2.0) | 5.2 (1.8) p=1.0000 |
5.6 (1.7) | 5.2 (1.8) p=1.0000 |
5.6 (1.7) |
| CRP, mg/dL, mean (SD) | 1.8 (2.2) | 2.2 (2.9) | 2.0 (3.5) p=0.4891 |
1.6 (2.1) | 1.8 (2.3) p=1.0000 |
2.2 (2.2) | 1.8 (2.3) p=1.0000 |
2.2 (2.2) |
| Previous therapy used for matching‡ | ||||||||
| TNFi-naïve, n (%) | 208 (100) | 107 (100) | 136 (69.0) p<0.0001 |
134 (68.4) | (100)* p=1.0000 |
(100)* | (100)* p=1.0000 |
(100)* |
| Characteristics not used for matching¶ | ||||||||
| BASDAI fatigue item§, mean (SD) | 6.5 (2.0) | 6.7 (1.9) | 6.8 (1.6) | 6.9 (1.7) | 6.5 (1.3) | 6.7 (1.4) | 6.6 (1.2) | 6.5 (1.5) |
| BASMI total score, mean (SD) | 3.8 (2.2) | 4.2 (2.1) | 3.8 (1.8) | 4.0 (1.6) | 3.7 (1.5) | 4.1 (1.3) | 3.7 (1.4) | 4.1 (1.3) |
| Tender joint count, mean (SD) | 5.1 (7.4) | 5.6 (6.8) | 5.5 (7.6) | 5.9 (8.2) | 4.3 (5.7) | 5.3 (5.9) | 4.4 (5.7) | 5.1 (5.7) |
| Swollen joint count, mean (SD) | 1.5 (3.3) | 1.4 (2.8) | 1.9 (3.7) | 2.2 (4.6) | 1.6 (2.8) | 2.0 (3.6) | 1.6 (2.7) | 1.9 (3.4) |
| SF-36 PCS, mean (SD) | 32.9 (8.0) | 31.8 (8.0) | 35.9 (6.8) | 36.3 (6.3) | 37.8 (5.7) | 37.0 (5.2) | 37.6 (5.5) | 37.0 (5.1) |
| SF-36 MCS, mean (SD) | 43.4 (12.0) | 44.4 (12.0) | 39.9 (10.6) | 39.5 (10.4) | 41.6 (8.4) | 40.2 (8.2) | 41.3 (8.4) | 40.6 (8.1) |
| PtGA, mean (SD) | 6.3 (2.2) | 6.5 (2.0) | 6.5 (1.9) | 6.8 (1.8) | 6.2 (1.5) | 6.5 (1.5) | 6.4 (1.5) | 6.4 (1.5) |
| Total back pain, mean (SD) | 6.4 (2.1) | 6.7 (2.2) | 6.5 (1.8) | 6.8 (1.7) | 6.2 (1.5) | 6.7 (1.4) | 6.4 (1.4) | 6.6 (1.4) |
| Nocturnal pain, mean (SD) | 6.1 (2.4) | 6.5 (2.4) | 6.3 (1.9) | 6.5 (2.0) | 6.0 (1.5) | 6.6 (1.4) | 6.2 (1.4) | 6.5 (1.4) |
| ASQoL, mean (SD) | 10.2 (4.0) | 10.6 (4.0) | 11.3 (4.5) | 11.6 (4.2) | 10.3 (3.9) | 11.3 (3.7) | 10.4 (3.9) | 11.2 (3.7) |
| Corticosteroid use, mean (%) | 25 (12.0) | 6 (5.6) | 22 (11.2) | 23 (11.7) | (12.5)* | (14.0)* | (11.2)* | (14.5)* |
Integer population (n) values not available owing to calculation of pooled SEC ESS using the equation:
BASDAI scores were not included in the matching for the principal analysis, but were included in the sensitivity analysis
TNFi-experienced patients in MEASURE 1 and MEASURE 2 are TNFi-inadequate responders
This score is based on a question on fatigue from the BASDAI
Duration of AS was also identified as a relevant factor, but this could not be matched because it was defined as time from diagnosis in MEASURE 1/2 and time from symptom onset in ATLAS
All p values were calculated for secukinumab vs. adalimumab
ADA: adalimumab, ASQoL: Ankylosing Spondylitis Quality of Life, BASDAI: Bath Ankylosing Spondylitis Disease Activity Index, BASFI: Bath Ankylosing Spondylitis Functional Index, BASMI: Bath Ankylosing Spondylitis Metrology Index, CRP: C-reactive protein, ESS: effective sample size, MCS: Mental Component Summary, PCS: Physical Component Summary, PtGA: patient global assessment, SD: standard deviation, SEC: secukinumab, SF-36: 36-item Short-Form Health Survey, TNFi: tumor necrosis factor inhibitor
Supplementary Table 5.
Relative likelihoods of response for secukinumab 150 mg and adalimumab 40 mg at weeks 8, 12, 16, 24, and 52
| Principal analysis (SEC vs. ADA) | Sensitivity analysis (SEC vs. ADA) | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| ASAS 20 | ASAS 40 | ASAS 5/6 | ASAS PR | ASAS 20 | ASAS 40 | ASAS 5/6 | ASAS PR |
| Week 8 (placebo-adjusted) | |||||||
| 0.92 (0.59–1.43) p=0.707 | NR | NR | NR | 0.96 (0.62–1.49) p=0.851 | NR | NR | NR |
| Week 12 (placebo-adjusted) | |||||||
| 0.65 (0.40–1.06) p=0.084 | 0.88 (0.45–1.75) p=0.725 | 0.57 (0.30–1.08) p=0.084 | 0.91 (0.24–3.46) p=0.893 | 0.69 (0.42–1.12) p=0.130 | 0.90 (0.46–1.79) p=0.767 | 0.57 (0.30–1.09) p=0.088 | 0.93 (0.24–3.53) p=0.914 |
| Week 16 (non-placebo-adjusted) | |||||||
| 1.21 (1.01–1.46) p=0.040 | NR | NR | NR | 1.24 (1.03–1.49) p=0.022 | NR | NR | NR |
| Week 24 (non-placebo-adjusted) | |||||||
| 1.27 (1.05–1.54) p=0.013 | 1.37 (1.08–1.73) p=0.010 | 1.23 (0.99–1.54) p=0.065 | 1.25 (0.85–1.84) p=0.261 | 1.30 (1.08–1.57) p=0.006 | 1.39 (1.09–1.76) p=0.007 | 1.25 (1.00–1.56) p=0.053 | 1.27 (0.86–1.87) p=0.235 |
| Week 52 (non-placebo-adjusted) | |||||||
| 1.13 (1.00–1.27) p=0.053 | 1.23 (1.03–1.46) p=0.020 | 1.15 (0.99–1.34) p=0.065 | 0.78 (0.58–1.06) p=0.116 | 1.14 (1.01–1.28) p=0.038 | 1.22 (1.02–1.46) p=0.026 | 1.15 (0.98–1.34) p=0.080 | 0.79 (0.58–1.08) p=0.144 |
Data are RR (95% CI). All p values (shown in italics when significant, i.e., p<0.05) were derived from RR values. At week 52, SEC populations were pooled (i.e., patients receiving active treatment plus those switching to active treatment from placebo) and missing data were derived using LOCF imputation to match the ADA patient population in ATLAS
ADA: adalimumab, ASAS 20/40: 20%/40% improvement in Assessment of SpondyloArthritis International Society response criteria, ASAS 5/6: 20% improvement in any five of the six domains in the Assessment of SpondyloArthritis International Society response criteria, ASAS PR: Assessment of SpondyloArthritis International Society partial remission, CI: confidence interval, LOCF: last observation carried forward, NR: not reported, RR: relative likelihood of response, SEC: secukinumab
Supplementary Table 6.
Odds ratios for secukinumab 150 mg and adalimumab 40 mg using a possible statistical interpretation in acknowledgment of the American Statistical Association guidance
| Principal analysis (SEC vs. ADA) | Sensitivity analysis (SEC vs. ADA) | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| ASAS 20 | ASAS 40 | ASAS 5/6 | ASAS PR | ASAS 20 | ASAS 40 | ASAS 5/6 | ASAS PR |
| Week 8 (placebo-adjusted) | |||||||
| 0.91 (0.44–1.89) p=0.795 | NR | NR | NR | 0.99 (0.47–2.06) p=0.971 | NR | NR | NR |
| Week 12 (placebo-adjusted) | |||||||
| 0.60 (0.28–1.28) p=0.185 | 0.93 (0.39–2.21) p=0.867 | 0.50 (0.22–1.18) p=0.115 | 0.97 (0.23–4.04) p=0.961 | 0.67 (0.31–1.44) p=0.304 | 0.96 (0.40–2.30) p=0.927 | 0.52 (0.22–1.21) p=0.129 | 0.97 (0.23–4.08) p=0.972 |
| Week 16 (non-placebo-adjusted) | |||||||
| 1.60 (1.01–2.54) p=0.047 | NR | NR | NR | 1.70 (1.06–2.73) p=0.028 | NR | NR | NR |
| Week 24 (non-placebo-adjusted) | |||||||
| 1.76 (1.11–2.79) p=0.017 | 1.79 (1.14–2.82) p=0.012 | 1.51 (0.96–2.38) p=0.072 | 1.34 (0.80–2.25) p=0.265 | 1.87 (1.17–3.01) p=0.009 | 1.85 (1.17–2.94) p=0.009 | 1.55 (0.98–2.46) p=0.060 | 1.37 (0.81–2.31) p=0.239 |
| Week 52 (non-placebo-adjusted) | |||||||
| 1.48 (0.98–2.22) p=0.062 | 1.54 (1.06–2.23) p=0.023 | 1.42 (0.97–2.07) p=0.072 | 0.71 (0.47–1.08) p=0.110 | 1.53 (1.00–2.34) p=0.048 | 1.52 (1.04–2.23) p=0.031 | 1.40 (0.95–2.07) p=0.089 | 0.72 (0.47–1.11) p=0.137 |
Data are OR (95% CI). We avoid strict thresholds when interpreting statistical p values, as per the American Statistical Association definition (7 March 2016) (25), but loosely interpret p values (two-sided) between 0.1 and 0.001 as increasing evidence (weak through moderate to strong) and p values (two-sided) below 0.001 as strong evidence (27). All p values (two-sided) are shown. Italic format indicates differences with p values between 0.1 and 0.001. At week 52, SEC populations were pooled (i.e., patients receiving active treatment plus those switching to active treatment from placebo) and missing data were derived using LOCF imputation to match the ADA patient population in ATLAS
ADA: adalimumab, ASAS 20/40: 20%/40% improvement in the Assessment of SpondyloArthritis International Society response criteria, ASAS 5/6: 20% improvement in any five of the six domains in the Assessment of SpondyloArthritis International Society response criteria, ASAS PR: Assessment of SpondyloArthritis International Society partial remission, CI: confidence interval, LOCF: last observation carried forward, NR: not reported, OR: odds ratio, SEC: secukinumab
Supplementary Table 7.
Principal analysis placebo arm responses across selected outcomes
| Principal analysis | Sensitivity analysis | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Week 8 | Week 12 | Week 8 | Week 12 | |||||
|
|
|
|
|
|||||
| Outcome | ATLAS | MEASURE 1/2 | ATLAS | MEASURE 1/2 | ATLAS | MEASURE 1/2 | ATLAS | MEASURE 1/2 |
| ASAS 20, % patients | 29.0 | 32.3 | 20.6 | 33.7 | 29.0 | 38.6 | 20.6 | 36.0 |
| ASAS 40, % patients | NA | NA | 13.1 | 16.7 | NA | NA | 13.1 | 22.2 |
| BASDAI 50, % patients | NA | NA | 15.9 | 17.6 | NA | NA | 15.9 | 21.1 |
| ASAS 5/6, % patients | NA | NA | 13.1 | 23.2 | NA | NA | 13.1 | 26.2 |
| ASAS PR, % patients | NA | NA | 3.7 | 5.2 | NA | NA | 3.7 | 6.1 |
| PtGA, 0–100 mm VAS | NA | NA | 6.5 | −13.2 | NA | NA | 6.5 | −14.5 |
| BASFI*, 0–100 mm VAS | NA | NA | −0.80 | −0.87 | NA | NA | −0.80 | −0.96 |
| CRP*, mg/dL | NA | NA | −0.10 | −0.26 | NA | NA | −0.10 | −0.20 |
Mean change from baseline
ASAS 20/40: 20%/40% improvement in the Assessment of SpondyloArthritis International Society response criteria, ASAS 5/6: 20% improvement in any five of the six domains in the Assessment of SpondyloArthritis International Society response criteria, ASAS PR: Assessment of SpondyloArthritis International Society partial remission, BASDAI 50: 50% improvement in the Bath Ankylosing Spondylitis Disease Activity Index, BASFI: Bath Ankylosing Spondylitis Functional Index, CRP: C-reactive protein, NA: not available, PtGA: patient global assessment, VAS: visual analog scale
Supplementary Table 8.
Sensitivity analysis continuous outcome comparisons
| Placebo-adjusted change from baseline* (95% CI) | Change from baseline* (95% CI) | Change from baseline† (95% CI) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Week 12 | Week 24 | Week 52 | ||||
|
|
|
|
||||
| ADA 40 mg | SEC 150 mg | ADA 40 mg | SEC150 mg | ADA 40 mg | SEC 150 mg | |
| ASAS core set PtGA, 0–100 mm VAS |
−45.6 (−60.9 to −30.3) p<0.001 |
−16.9 (−22.0 to −11.8) | −37.8 (−47.6 to −28.0) p=0.675 |
−35.6 (−39.3 to −1.9) | −31.0 (NR) | −34.8 (−37.8 to −31.8) |
| Total back pain, 0–100 mm VAS | −18.9 (−24.8 to −13.0) p=0.941 |
−18.6 (−23.5 to −3.7) | −27.7 (−31.2 to −24.2) | −36.0 (−39.4 to −32.7) p<0.001 |
−31.0 (NR) | −34.9 (−37.9 to −32.0) |
| Nocturnal back pain, 0–100 mm VAS | −18.0 (−24.0 to −12.0) p=0.918 |
−17.6 (−22.6 to −12.6) | −27.3 (−31.0 to −23.6) | −34.9 (−38.5 to −31.3) p=0.004 |
NR | −37.1 (−40.1 to −34.1) |
| BASFI, 0–100 mm VAS |
−2.8 (−3.7 to −1.8) p<0.001 |
−1.0 (−1.4 to −0.7) |
−3.8 (−4.4 to −3.2) p<0.001 |
−2.3 (−2.5 to −2.0) | −2.1 (NR) | −2.3 (−2.6 to −2.1) |
| BASDAI inflammation‡ | NA | NA |
−4.3 (−5.0 to −3.6) p=0.004 |
−3.1 (−3.5 to −2.6) | −3.6 (NR) | −3.2 (−3.5 to −2.9) |
| BASMI total score |
−0.6 (−0.9 to −0.3) p=0.044 |
−0.3 (−0.4 to −0.1) | −0.6 (−0.8 to −0.4) p=0.798 |
−0.6 (−0.7 to −0.5) | −0.7 (NR) | −0.5 (−0.6 to −0.4) |
| CRP, mg/dL | −1.2 (−1.5 to −0.9) p=0.637 |
−1.1 (−1.5 to −0.7) | −1.3 (−1.5 to −1.1) p=0.814 |
−1.3 (−1.6 to −0.9) | −1.2 (NR) | −1.3 (−1.6 to −1.0) |
| Additional key outcomes ASQoL, 0–18 | NA | NA | −3.6 (−5.0 to −2.2) | −4.3 (−5.1 to −3.4) p=0.409 |
−3.9 (NR) | −4.6 (−5.2 to −4.0) |
| Tender joint count, 44 counts | −0.5 (−1.9 to 0.9) | NA | −0.9 (−1.7 to −0.1) | −2.7 (−3.5 to −2.0) p<0.001 |
−1.5 (NR) | −2.4 (−3.1 to −1.7) |
| Swollen joint count, 44 counts | 0.1 (−0.6 to 0.8) | NA | −0.4 (−0.8 to 0.0) | −1.1 (−1.5 to −0.7) p=0.013 |
−0.2 (NR) | −0.6 (−1.1 to −0.2) |
| BASDAI, 0–100 mm VAS | −1.8 (−2.4 to −1.2) p=0.372 |
−1.5 (−1.9 to −1.1) | −2.6 (−3.0 to −2.2) | −3.1 (−3.3 to −2.8) p=0.059 |
−3.0 (NR) | −2.9 (−3.2 to −2.7) |
| BASDAI fatigue§, 0–100 mm VAS | −1.5 (−2.2 to −0.8) p=0.691 |
−1.3 (−1.8 to −0.9) | −2.4 (−2.8 to −2.0) | −3.0 (−3.3 to −2.6) p=0.031 |
NR | −2.9 (−3.2 to −2.6) |
95% CI, ranges for ADA are not reported and were calculated for the purpose of the comparison
Complete week 52 data were not available from ATLAS, and it was not possible to calculate p values
The BASDAI inflammation score was calculated as the mean of scores for questions 5 and 6 of the BASDAI
This score is based on the fatigue item of the BASDAI
Italic format indicates statistically significant evidence supporting SEC superiority over ADA (p value shown). Bold format indicates statistically significant evidence supporting ADA superiority over SEC (p value shown)
ADA: adalimumab, ASAS: Assessment of SpondyloArthritis International Society, ASQoL: Ankylosing Spondylitis Quality of Life questionnaire, BASDAI: Bath Ankylosing Spondylitis Disease Activity Index, BASFI: Bath Ankylosing Spondylitis Functional Index, BASMI: Bath Ankylosing Spondylitis Metrology Index, CI: confidence interval, CRP: C-reactive protein, NA: not available, NR: not reported, PtGA: patient global assessment, SEC: secukinumab, VAS: visual analog scale
PRISMA diagram
A systematic literature review (conducted: September 2014; updated: September 2015) was used to identify relevant clinical evidence of secukinumab and biologic comparators in the treatment of adult patients with AS. Twenty-three trials were judged suitable for inclusion according to the eligibility criteria (Supplementary Table 1); 16 RCTs included neither secukinumab nor adalimumab and were excluded; a further four trials were excluded based on the research question, leaving three [MEASURE 1 (18), MEASURE 2 (18), and ATLAS (19, 20)] for use in the MAIC. Details of the four excluded and three included trials are provided in Supplementary Table 2; study designs are summarized in Supplementary Figure 2. Outcomes selected for comparison were in line with ASAS and Outcome Measures in Rheumatology (OMERACT) recommendations (29, 30).
MAIC: matching-adjusted indirect comparison, PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
MEASURE 1, MEASURE 2, and ATLAS trial designs
MEASURE 1 and 2 were phase 3 RCTs (18) in which patients who were refractory to NSAID therapy as defined by ASAS recommendations were recruited. Patients were also eligible if they had previously inadequately responded to ≥3 months of treatment with an approved dose of one TNFi (primary or secondary lack of efficacy); these accounted for 27.0% and 39.7% of the respective patient populations. Patients receiving placebo who did not achieve an ASAS20 response by week 16 were re-randomized 1:1 to secukinumab 75 mg or 150 mg; those who achieved responses were re-randomized 1:1 at week 24. In MEASURE 1, secukinumab was administered as an intravenous loading dose (10 mg/kg body weight) at weeks 0, 2, and 4, then subcutaneously (75 mg or 150 mg) every 4 weeks starting from week 8; in MEASURE 2, secukinumab was given subcutaneously (75 mg or 150 mg) at weeks 0, 1, 2, and 3, and every 4 weeks starting from week 4. ATLAS was a phase 3 RCT that recruited NSAID-refractory patients (19, 20); patients were ineligible if they had received any TNFi therapy. Adalimumab was given subcutaneously (40 mg) every other week. Patients receiving placebo without ASAS 20 responses at week 12, 16, or 20 were eligible for “early escape” to receive open-label treatment with adalimumab; all patients were eligible from week 24, with an option to uptitrate to weekly adalimumab (19, 20). Across trials, the primary end-point was the proportion of ASAS 20 responders - at week 16 for MEASURE 1 and 2, and at week 12 for ATLAS. The predominant difference between ATLAS and MEASURE trials was that the latter included patients for whom a previous TNFi treatment had failed, while participants in the ATLAS trial were TNFi-naïve.
ASAS 20: at least a 20% improvement in Assessment of SpondyloArthritis International Society response criteria, EOW: every other week, MAIC: matching-adjusted indirect comparison, M1: MEASURE 1, M2: MEASURE 2, R: randomization.
*Patients classified as “responders” (achieved ≥ASAS 20 response) at week 16. †Patients classified as “non-responders” (did not achieve ASAS 20 response) at week 16. ‡Patients who did not achieve an ASAS 20 response were eligible. §Patients who did not achieve an ASAS 20 response after ≥12 weeks of open-label treatment with adalimumab 40 mg EOW were eligible for adalimumab 40 mg weekly.
¶296 patients completed the double-blind phase and were eligible to enter the open-label extension. Post-week 24 efficacy data used in our MAIC analysis used published LOCF data of ATLAS patients who received at least one dose of adalimumab (n=311, of whom 239 received adalimumab EOW and 72 received adalimumab weekly). Placebo-adjusted comparisons were possible only up to week 12 because patients in ATLAS who did not achieve an ASAS 20 response were eligible for early escape (open-label adalimumab) from week 12 to week 24 (the cumulative number of patients entering early escape from each arm is indicated in red numerals; patients who withdrew from the study are not shown). Reference numbering in this figure caption is from the main paper
Principal analysis results for ASAS 20 showing placebo and active treatment responses
In the MEASURE 1 and MEASURE 2 trials, patients receiving placebo who did not achieve an ASAS 20 response by week 16 were re-randomized 1:1 to secukinumab 75 mg or 150 mg; those who did achieve this response were re-randomized 1:1 at week 24. In the ATLAS trials, patients receiving placebo who did not achieve an ASAS 20 response at week 12, 16, or 20 were eligible for the “early escape” option of open-label treatment with adalimumab; all patients were eligible for this from week 24, with an option to uptitrate to weekly adalimumab
ADA: adalimumab, ASAS 20: 20% improvement in Assessment of SpondyloArthritis International Society response criteria, ESS: effective sample size, i.v.: intravenous, LOCF: last observation carried forward, M1: MEASURE 1, M2: MEASURE 2, PBO: placebo, s.c.: subcutaneous, SEC: secukinumab