Abstract
Objective
Pulmonary hypertension (PH) may occur in Takayasu arteritis (TA), mostly due to pulmonary arteritis, but also due to left heart disease and/or chronic thromboembolism (CTE). Using transthoracic echocardiography (TTE), we investigated the frequency of PH caused by pulmonary arteritis.
Methods
This cross-sectional study included 70 patients with TA fulfilling the 1990 ACR criteria, 68 healthy controls, and 67 patients with systemic sclerosis (SSc) fulfilling the 1980 ACR criteria representing the disease control group. Patients with severe left heart disease or CTE were excluded. The ESC-ERS guideline definition was considered for diagnosis of PH.
Results
The mean systolic pulmonary artery pressure (SPAP) values in TA, SSc, and healthy control groups were 20.93±6.06, 31.57±12.75, and 18.88±5.39 mmHg, respectively. While the SPAP values were similar between TA and healthy groups, the SPAP values in the SSc group were significantly higher than in other groups. Based on conventional and/or magnetic resonance angiography findings, pulmonary arteritis was present in 4 out of 70 TA patients; however, PH was not detected in any patients with TA, including those with pulmonary arteritis.
Conclusion
The TTE findings suggested that the frequency of PH was not increased in TA. However, a low frequency of pulmonary arteritis in our series might have affected our results.
Keywords: Pulmonary arteritis, pulmonary hypertension, Takayasu arteritis
Introduction
Takayasu arteritis (TA) is a systemic vasculitis affecting large and medium-sized arteries with unknown etiopathogenesis. It causes granulomatous inflammation of the vessel wall. TA generally has an insidious onset, and it predominantly affects young females, especially during the second or third decades of life (1, 2). Although aortic arch and its primary branches, ascending aorta, thoracic descending aorta, and abdominal aorta are mainly involved, visceral arteries originating from abdominal aorta, as well as pulmonary and extremity arteries, may also be affected in TA. The frequency of pulmonary artery involvement, that is, pulmonary arteritis in TA, has been reported to range between 6.9% and 86% (2–4).
Pulmonary hypertension (PH) and pulmonary arterial hypertension (PAH) are not same pathologies. PH is a pathological condition that may occur in a variety of clinical situations, including PAH. Among many other causes, PAH represents the most prominent type of PH. Other than PAH, various pathologies including lung diseases, chronic hypoxia, and chronic thromboembolic diseases may also cause occurrence of precapillary subgroup of PH. PAH refers specifically to a pulmonary hypertensive state limited predominantly to the arterial component of the pulmonary vasculature. In other words, PAH is a term used to describe various clinical progressive conditions that cause increased pulmonary artery resistance, which leads to right heart failure and death, and it has many different etiologies, including connective tissue diseases (5–8). The prognosis of PAH is poor, and it has nearly a 15% mortality within 1 year (9).
In different case series, the frequency of PH in TA has been reported to range between 12% and 15% (2, 10–12). In the course of TA, PH may develop not only due to pulmonary arteritis, classified as PAH, but also due to presence of left heart disease and aortic regurgitation (post-capillary PH). In addition, chronic thromboembolic pulmonary hypertension may also occur in TA (5). Since PAH carries a poor prognosis, early diagnosis is essential; however, clinical signs of PAH are nonspecific. Transthoracic echocardiography (TTE) should be performed initially when PAH is suspected. TTE may be useful to evaluate systolic pulmonary arterial pressure (SPAP), based on the peak velocity of the jet of tricuspid regurgitation (13, 14).
In literature, the reported incidence of PH in patients with TA includes patients with left heart disease, which may overestimate the exact incidence of PH due to pulmonary arteritis. Therefore, we aimed to investigate PH in TA patients without left heart disease, by using TTE. In addition to healthy controls, patients with systemic sclerosis (SSc) were also included as a disease control group.
Methods
This is a cross-sectional study. Seventy-seven patients with TA fulfilling the 1990 American College of Rheumatology (ACR) criteria were initially enrolled in this study (15). TTE was performed in all participants. Patients with severe left-sided heart disease and/or thromboembolic pulmonary disease that could cause pulmonary hypertension were excluded. Therefore, 7 patients (3 with severe mitral valve regurgitation, 2 with severe mitral valve stenosis, and 2 with dilated cardiomyopathy) were excluded, and remaining 70 patients with TA (mean age 42.1±10.4 years; F/M: 63/7) were included. Clinical data of those patients were collected retrospectively from patient charts. Conventional angiographies were available for all those patients, and conventional angiographic findings were grouped according to the classification defined at the International Conference on TA in 1994. According to this classification, Type I involves the branches of aortic arch; Type IIa involves both ascending aorta, aortic arch, and branches of aortic arch; Type IIb involves thoracic descending aorta with the involvement of Type IIa; Type III involves thoracic descending aorta, abdominal aorta, and/or renal arteries; Type IV involves abdominal aorta and/or renal arteries; and Type V is the combination of Type IIb and Type IV (16).
The presence or absence of disease activity was assessed using the Kerr criteria (17). Computed tomography angiography (CTA) and/or magnetic resonance angiography (MRA) findings obtained during the follow-up were also noted. All the patients were questioned for the presence of breathlessness, fatigue, weakness, angina, and syncope, which are nonspecific symptoms of PAH. A through physical examination was also made. The patients were investigated especially for the presence of left parasternal lift, pansystolic murmur of tricuspid regurgitation, and diastolic murmur of pulmonary insufficiency.
Sixty-eight age- and gender-matched healthy subjects constituted the healthy control group (39.5±9.5 years; F/M: 61/7). Since SSc is known to have the highest prevalence of PAH among various autoimmune disorders, 67 patients with SSc (56.7±11.6 years; F/M: 59/8; diffuse/limited 41/26), fulfilling the 1980 ACR classification criteria were also included, serving as a disease control group (18). The study protocol was approved by the Human Research Ethics Committee of Ege University School of Medicine, and informed consent was obtained from all subjects.
Transthoracic echocardiography (ACUSON CV70, Siemens, Germany) was performed by the same cardiologist in all participants. SPAP was calculated for each individual, based on the peak velocity of the jet of tricuspid regurgitation, using the simplified Bernoulli equation (19). This equation allowed for estimation of SPAP, using the right atrial pressure (19).
Arbitrary criteria defined in the European Society of Cardiology (ESC) and European Respiratory Society (ERS) guidelines were used for echocardiographic diagnosis of PH. These criteria were based on tricuspid regurgitation peak velocity and Doppler-calculated PA systolic pressure at rest (assuming a normal right atrial pressure of 5 mmHg) (5, 13, 14):
Echocardiographic diagnosis of PH is unlikely if tricuspid regurgitation velocity (TRV) is <2.8 m/s (SPAP<36 mmHg), and there is no additional echocardiographic signs suggestive of PH, as described in the 2015 ESC-ERS Guidelines for PH.
Echocardiographic diagnosis of PH is possible if TRV<2.8 m/s (SPAP<36 mmHg), but additional echocardiographic PH signs are present, or TRV is 2.9–3.4 m/s (SPAP 37–50 mmHg) with/without additional echocardiographic PH signs.
Echocardiographic diagnosis of PH is likely if TRV>3.4 m/s (SPAP>50 mmHg), with/without additional echocardiographic PH signs.
Patients with SPAP>40 mmHg with compatible echocardiographic PH signs were planned to undergo right heart catherization.
The Statistical Package for Social Sciences 15.0 (SPSS Inc.; Chicago, IL, USA) was used for statistical analysis. The continuous variables were described as the mean±standard deviation (mean±SD). The categorical variables were presented as numbers and percentages. For the comparison of continuous variables, an one-way analysis of variance test was used. For post-hoc analysis, the Bonferroni correction test was also used. A p-value <0.05 was accepted as statistically significant.
Results
The demographic data, disease duration, types of angiographic involvement, and disease activity state (according to the Kerr criteria) of the patients with TA are presented in Table 1. Only six out of 70 TA patients were active (8.6%) according to the Kerr Criteria. With respect to angiographic classification, Type V (31/70; 44.3%) and Type I (27/70; 38.6%) were the most frequent types of involvement. Based on conventional and/or magnetic resonance angiography findings, pulmonary arteritis was present in 4 out of 70 TA patients (5.7%) (Table 1).
Table 1.
Characteristics of patients with Takayasu arteritis
| Age, years | 42.1±10.4 |
|---|---|
| Female/male | 63/7 |
| Disease duration, years | 7.0±6.8, (0.1–29) |
| ESR, mm/h | 23.9±22.2, (3–140) |
| CRP, mg/dL | 2.2±3.8, (0.1–22) |
| Active disease, Kerr criteria | 6/70 (8.6%) |
| Angiographic classification | |
| Type I | 27/70 (38.6%) |
| Type Ila | 2/70 (2.9%) |
| Type Ilb | 3/70 (4.3%) |
| Type III | 0/70 (0%) |
| Type IV | 7/70 (10%) |
| Type V | 31/70 (44.3%) |
| Pulmonary artery involvement | 4/70 (5.7%) |
CRP: C-reactive protein; ESR: erythrocyte sedimentation rate
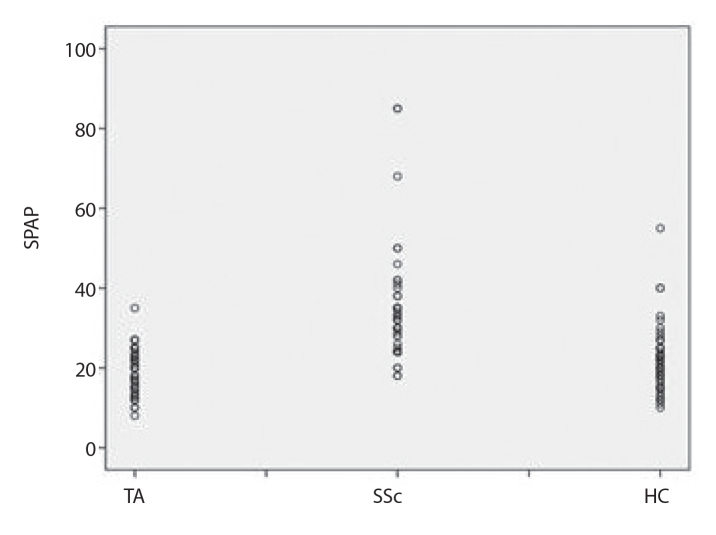
The mean SPAP values in TA, SSc, and healthy control groups were 20.9±6.1, 31.6±12.8, and 18.9±5.4 mmHg, respectively (Figure 1). The SPAP values in the SSc group were significantly higher than in other two groups (p<0.001), and 5 out of 67 SSc patients (7.4%) fulfilled possible echocardiographic diagnosis of PH. On the other hand, there was no significant difference between TA and healthy groups (p=0.17), and none of the patients with TA, including those 6 cases with pulmonary arteritis, fulfilled possible echocardiographic diagnosis of PH. In 69 out of 70 TA patients, SPAP was equal to or less than 36 mmHg. There was only a single TA patient with a SPAP value of 40 mmHg, but TRV was within the normal range without any additional echocardiographic findings denoting PH. Moreover, she had no clinical symptoms or signs of PH. Therefore, like the rest of the TA group, this patient also did not fulfill the possible echocardiographic diagnosis of PH based on the ESC-ERS guideline definition (5). The mean SPAP values of the 7 TA patients, excluded from the study due to secondary cardiac problems were 37.1±14.53 mmHg, which was significantly higher than the rest of the TA patients included in the study (p<0.001). In patients with TA, there was no significant correlation between disease activity based on the Kerr criteria and SPAP values. None of the patients with TA had symptoms and/or clinical findings suggesting the presence of PH. The SPAP values and other available echocardiographic findings of the study groups are presented in Table 2.
Figure 1.
The mean SPAP values
Table 2.
Demographic features and echocardiographic findings of study groups
| TA | SSc | HC | |
|---|---|---|---|
| Age, years | 42.1±10.4 | 56.7±11.6 | 39.5±9.5 |
| Female/male | 63/7 | 59/8 | 61/7 |
| mSPAP | 20.9±6.1 | 31.6±12.8 | 18.9±5.4 |
| SPAP, mmHg | |||
| <36 | 69/70 (98.6%) | 48/67 (71.7%) | 67/68 (98.5%) |
| 37–49 | 1/70 (1.4%) | 14/67 (20.9%) | 1/68 (1.5%) |
| >50 | 0/70 (0%) | 5/67 (7.5%) | 0/68 (0%) |
| Possible echocardiographic PH | 0/70 (0%) | 5/67 (7.5%) | 0/68 (0%) |
| Interventricular septum mm | 9±1.5 (5–13) | 11±1.7 (8–14.5) | 9±1.5 (6–12) |
| Posterior wall, mm | 9±1.5 (5–12) | 10±1.4 (6–14) | 8±1.3 (5–11) |
| LV end-systolic diameter, mm | 25±5 (15–45) | 25±4 (18–35) | 25±3 (17–33) |
| LV end-diastolic diameter, mm | 44±5 (32–64) | 42±4 (32–51) | 44±4 (35–52) |
| RA volume, mL | 31±11 (12–57) | NA | 32±12 (8–78) |
| RV end-diastolic volume, mL | 20±9 (6–57) | NA | 23±10 (6–54) |
| RV end-systolic volume, mL | 7±4 (2–22) | NA | 9±5 (2–25) |
| PA diameter, mm | 20±3 (15–27) | 22±3 (18–28) | 19±3 (13–27) |
| TAPSE, mm | 23±4 (15–33) | 18±5 (12–18) | 24±3 (17–32) |
| PA acceleration time, msec | 121±20 (70–160) | 122±30 (50–170) | 122±20 (80–192) |
| Tricuspid regurgitation velocity, msec | 1.9±0.4 (1.3–3) | 2.5±0.3 (2–3) | 1.9±0.4 (0.9–2.7) |
| Velocity of the tricuspid annular systolic motion, RV S′ | 17±4 (9–33) | 11±4 (8–18) | 16±4 (9–26) |
| LV diastolic dysfunction | 9/70 (12.9%) | 30/63 (47.6%) | 11/68 (16.2%) |
HC: healthy controls; LV: left ventricle; NA: not available; PH: pulmonary hypertension; RA: right atrium; RV: right ventricle; SPAP: systolic pulmonary artery pressure; SSc: systemic sclerosis; TA: Takayasu arteritis; TAPSE: tricuspid annular plane systolic excursion; PA: pulmonary artery
Discussion
Unlike previous studies in the literature, in the present cross-sectional and controlled study, which reflects the experience of a single center in Turkey, we could not find an increased frequency of PH in TA. The mean SPAP values in 70 patients with TA were not significantly higher than in healthy controls. Based on clinical findings and TTE results, none of our TA patients, including those four patients with pulmonary arteritis, fulfilled the criteria of possible PH as defined in the ESC-ERS guidelines (5, 13, 14). We believe that the low frequency of pulmonary arteritis (6%) in our series, as well as exclusion of TA patients with severe left heart disease and/or chronic thromboembolic diseases, that is, other causes of PH in TA, may explain our findings.
The frequency of pulmonary arteritis in TA has been reported to range from 6.9% to 86% in various series (10–12). Interestingly the lowest figures came from the largest published Turkish multicenter series of TA patients, which reported pulmonary artery involvement in only 17 out of 247 patients (6.9%) (2). In other words, frequency of pulmonary arteritis in Turkish patients with TA seems to be low, which may possibly be explained by genetic and ethnic differences. Therefore, very low frequency of pulmonary arteritis (6%) in our series of TA patients is not a surprise.
On the other hand, in literature, the frequency of PH in patients with TA has been reported to range from 12% to 15% (2, 10–12). Based on echocardiographic evaluation, PH was detected in 12% of 248 cases in the largest Turkish series (2), and 15% of 160 cases in Korean (11) series of TA patients. However, unlike our study, the presence of left heart disease and other etiologies that may cause PH had not been excluded in both of these series. The mean SPAP values of the excluded 7 TA patients in the present study was higher than in the 70 TA patients included, and this finding could explain the lower rate of PH found in this study. Based on the data from previous studies and patient series, the prevalence of PH in TA, applied exclusion criteria, the frequency of pulmonary artery involvement, and applied diagnostic tools are summarized in Table 3 (2, 11, 20–24).
Table 3.
Prevalence of pulmonary hypertension in patients with Takayasu arteritis in various series
| Series | PH Prevalence | PA | Definition | Exclusion Criteria |
|---|---|---|---|---|
| Robles et al. (20) | 7/44 (15.9%) | 7 PA | Aortogram | No |
| Kerr et al. (21) | 4/4 (100%) | 4 PA with thromboembolic disease | Pulmonary angiogram | No |
| Soto et al. (22) | 10/76 (13.0%) | 5 PA | CA ECHO |
No |
| Bicakci et al. (2) | 22/184 (12.0%) | 17 PA | ECHO | No |
| Toledano et al. (23) | 19/47 (42.2%) | 47 PA 6 with poststenotic dilatation, 1 with thrombosis | CTA/MRA | No |
| Lee et al. (11) | 24/160 (15.0%) | 27 PA | CA/CTA/MRA ECHO |
No |
| Yang et al. (24) | 88/566 (17.8%) | 83 PA 32 with occlusion | CTA/MRA ECHO pulmonary angiogram |
No |
| Kalfa et al. | 0/70 (0%) | 4 PA | ECHO | Severe left-sided HD Thromboembolic pulmonary disease 3 severe MVR 2 severe MVS 2 dilated CMP |
PH: pulmonary hypertension; PA: pulmonary artery involvement; ECHO: echocardiography; CA: conventional angiography; CTA: computer tomography angiography; MRA: magnetic resonance angiography; HD: heart disease; MVR: mitral valve regurgitation; MVS: mitral valve stenosis; CMP: cardiomyopathy
On the other hand, in the Korean study, it was suggested that high disease activity played an important role for the cardiovascular manifestations of TA, including PH (11). However, in the present study, we could not show a relationship between active disease and SPAP values, although the number of patients with active disease was low in our series.
In TA, pulmonary arteritis may be rarely isolated, but it is generally associated with involvement of the aorta and large thoracic vessels in many cases (4, 25–31). Diagnosis of pulmonary arteritis may be performed by showing stenosis, narrowing, occlusion, and irregularity of pulmonary arteries using the CTA or MRA, or by showing abnormal uptake on pulmonary perfusion scan. Pulmonary arteritis may also be detected by autopsy. Autopsy series of TA patients reported the frequency of pulmonary arterial involvement to range between 20% and 56% (12). In general, TA patients with PH due to pulmonary arteritis have worse prognosis and higher mortality rates (23).
Toledano et al. (23) reported two cases of TA having pulmonary involvement and reviewed the literature, finding out 45 more such cases. The analysis of all those 47 cases showed that most of patients were female (89.1%), and the mean age was 34.6 years (range, 11–66 years). Interestingly, only 14 (31.8%) had isolated pulmonary vasculitis and no involvement of the aorta. Upper-lobe branches of the right-sided pulmonary arteries were found to be most frequently involved. Among those 47 cases, PH was found in 19 (42.2%) patients (23).
Recently, Wang et al. (10) reported that patients with TA were at increased risk for PH, based on the retrospective analysis of 48 patients with TA having PH. Among those 48 patients, 36 (75.0%) cases had pulmonary arteritis, and 12 (25.0%) had evidence of left heart disease, which was a secondary cause of PH. A high frequency of pulmonary arteritis and inclusion of secondary cardiac causes of PH were notable in this study. Moreover, their series consisted of hospitalized TA patients, representing more severe disease compared to our series. They also showed that serum levels of big endothelin 1 were correlated with SPAP values, confirming the positive association between pulmonary arteritis and PH in their series (10).
Since the clinical signs of PH are not specific, and the primary involvement of the aorta may mask the symptoms, the clinical diagnosis of PH may be delayed (32). It is well known that right heart catheterization (RHC) is the gold standard for diagnosing PH. However, RHC is a non-invasive method; therefore, TTE should be initially performed when PH is suspected. TTE is helpful to evaluate SPAP, based on the peak velocity of the jet of tricuspid regurgitation (13, 14). Various parameters obtained by TTE were shown to have good correlations with right heart hemodynamics. So, TTE is widely used for screening PH, as in the present study. In the present study, had we found high SPAP values in our patients with TA, we should have performed RHC to confirm the final diagnosis of PH. However, since SPAP values as detected by TTE were already within the normal limits, there was no indication for RHC.
Recently, an expert consensus defined the most appropriate criteria for referring patients with SSc for RHC when PH was suspected (33). These criteria may be summarized as the presence of at least one of the clinical signs or echocardiographic findings or abnormal DLCO findings suggesting PH. If these criteria are also applied for TA, performing RHC seemed to be not justifiable and/or ethical for our patients with TA.
The present study has certainly some limitations. The major limitation was the small sample size of TA patients. Despite being a cross-sectional study, most of the clinical and angiographic data were evaluated retrospectively. Therefore, we admit that some of the data might have not been complete. Another limitation was a very low percentage of active patients (only 6 of 70). If our series had consisted of patients with a more severe and active disease, the frequency of pulmonary arteritis might have been higher, thereby causing occurrence of a higher PH frequency. It was suggested that a high disease activity played an important role for the cardiovascular manifestations of TA, including PH.
Another limitation was a higher mean age of the control group of patients with SSc. Given that the frequency of ventricular diastolic dysfunction that may affect SPAP values tend to amplify with an increasing age, a higher mean age of patients with SSc might have contributed to higher SPAP values detected in those patients, compared to the TA group with a lower mean age.
In conclusion, using TTE and the ESC-ERS guidelines, none of our TA patients, including those four with pulmonary artery involvement fulfilled the criteria of possible PH. Moreover, the mean SPAP values in the TA group were not significantly higher than in healthy controls. A low frequency of pulmonary arteritis in our TA series, as well as a low frequency of patients with active disease at the time of enrollment might have affected our findings. To illuminate whether the prevalence of PH is increased or not in TA, larger screening studies are warranted.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ege University School of Medicine Human Research Ethics Committee.
Informed Consent: Written informed consent was obtained from all the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - K.A., M.K.; Design - K.A., M.K., G.K.; Supervision - K.A., M.K., F.Ö., G.K., N.A., S.A.; Materials - M.K., O.M.; Data Collection and/or Processing - M.K., O.M., H.E., Ö.S.G., G.K.; Literature Search - M.K., O.M.; Writing Manuscript - M.K.; Critical Review - K.A., M.K., G.K.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Lupi-Herrera E, Sanchez-Torres G, Marcushamer J, Mispireta J, Horwitz S, Vela JE. Takayasu’s arteritis. Clinical study of 107 cases. Am Heart J. 2007;93:94–103. doi: 10.1016/s0002-8703(77)80178-6. https://doi.org/10.1016/S0002-8703(77)80178-6 [DOI] [PubMed] [Google Scholar]
- 2.Bicakcigil M, Aksu K, Kamali S, Ozbalkan Z, Ates A, Karadag O, et al. Takayasu’s arteritis in Turkey-clinical and angiographic features of 248 patients. Clin Exp Rheumatol. 2009;27:59–64. [PubMed] [Google Scholar]
- 3.Sharma S, Kamalakar T, Rajani M, Talwar KK, Shrivastava S. The incidence and patterns of pulmonary artery involvement in Takayasu’s arteritis. Clin Radiol. 1990;42:177–81. doi: 10.1016/s0009-9260(05)81929-4. https://doi.org/10.1016/S0009-9260(05)81929-4 [DOI] [PubMed] [Google Scholar]
- 4.Lupi E, Sanchez G, Horwitz S, Gutierrez E. Pulmonary artery involvement in Takayasu’s arteritis. Chest. 1995;67:69–74. doi: 10.1378/chest.67.1.69. [DOI] [PubMed] [Google Scholar]
- 5.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. https://doi.org/10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 6.Peacock AJ, Murphy NF, Mcmurray JJV, Caballero L, Stewart S. An epidemiological study of pulmonary arterial hypertension. Eur Respir J. 2007;30:104–9. doi: 10.1183/09031936.00092306. https://doi.org/10.1183/09031936.00092306 [DOI] [PubMed] [Google Scholar]
- 7.Hachulla E, Gressin V, Guillevin L, Carpentier P, Diot E, Sibilia J, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: A French nationwide prospective multicenter study. Arthritis Rheum. 2005;52:3792–800. doi: 10.1002/art.21433. https://doi.org/10.1002/art.21433 [DOI] [PubMed] [Google Scholar]
- 8.Kayikçioğlu M, Kültürsay H. Seven years of experience in patients with pulmonary arterial hypertension in Ege University Hospital: diagnostic approach of a single center. Anadolu Kardiyol Derg. 2008;8:279–85. [PubMed] [Google Scholar]
- 9.Thenappan T, Shah SJ, Rich S, Gomberg-Maitland M. A USA-based registry for pulmonary arterial hypertension: 1982–2006. Eur Respir J. 2007;30:1103–10. doi: 10.1183/09031936.00042107. https://doi.org/10.1183/09031936.00042107 [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Dang A, Chen B, Lv N, Liu Q. Takayasu Arteritis-associated Pulmonary Hypertension. J Rheumatol. 2015;42:495–503. doi: 10.3899/jrheum.140436. https://doi.org/10.3899/jrheum.140436 [DOI] [PubMed] [Google Scholar]
- 11.Lee GY, Jang SY, Ko SM, Kim EK, Lee SH, Han H, et al. Cardiovascular manifestations of Takayasu arteritis and their relationship to the disease activity: Analysis of 204 Korean patients at a single center. Int J Cardiol. 2012;159:14–20. doi: 10.1016/j.ijcard.2011.01.094. https://doi.org/10.1016/j.ijcard.2011.01.094 [DOI] [PubMed] [Google Scholar]
- 12.Sharma BK, Jain S, Radotra BD. An autopsy study of Takayasu arteritis in India. Int J Cardiol. 1998;1:85–91. doi: 10.1016/s0167-5273(98)00155-7. https://doi.org/10.1016/S0167-5273(98)00155-7 [DOI] [PubMed] [Google Scholar]
- 13.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. https://doi.org/10.1093/eurheartj/ehp297 [DOI] [PubMed] [Google Scholar]
- 14.Mclaughlin V, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. CCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension A Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association Developed in Collaboration with the American Collage of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. JACC. 2009;53:1573–619. doi: 10.1016/j.jacc.2009.01.004. https://doi.org/10.1016/j.jacc.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 15.Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33:1129–34. doi: 10.1002/art.1780330811. https://doi.org/10.1002/art.1780330811 [DOI] [PubMed] [Google Scholar]
- 16.Hata A, Noda M, Moriwaki R, Numano F. Angiographic findings of Takayasu arteritis: new classification. Int J Cardiol. 1996;54:155–63. doi: 10.1016/s0167-5273(96)02813-6. https://doi.org/10.1016/S0167-5273(96)02813-6 [DOI] [PubMed] [Google Scholar]
- 17.Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, et al. Takayasu arteritis. Ann Intern Med. 1994;120:919–29. doi: 10.7326/0003-4819-120-11-199406010-00004. https://doi.org/10.7326/0003-4819-120-11-199406010-00004 [DOI] [PubMed] [Google Scholar]
- 18.Subcommittee for Scleroderma Criteria of the American Rheumatism Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of scleroderma. Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. https://doi.org/10.1002/art.1780230510 [DOI] [PubMed] [Google Scholar]
- 19.Hatle L, Angelsen BA, Tromsdal A. Non-invasive estimation of pulmonary artery systolic pressure with Doppler ultrasound. Br Heart J. 1981;45:157–65. doi: 10.1136/hrt.45.2.157. https://doi.org/10.1136/hrt.45.2.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robles M, Reyes PA. Takayasu’s atreritis in Mexico: a clinical review of 44 consecutive cases. Clin Exp Rheumatol. 1994;12:381–8. [PubMed] [Google Scholar]
- 21.Kerr KM, Auger WR, Fedullo PF, Channick RH, Yi ES, Moser KM. Large Vessel Pulmonary Arteritis Mimicking Chronic Thromboembolic Disease. Am J Respir Crit Care Med. 1995;152:367–73. doi: 10.1164/ajrccm.152.1.7599847. https://doi.org/10.1164/ajrccm.152.1.7599847 [DOI] [PubMed] [Google Scholar]
- 22.Soto ME, Espinola-Zavaleta N, Ramirez-Quito O, Reyes PA. Echocardiograpy. 2006;23:353–60. doi: 10.1111/j.1540-8175.2006.00238.x. https://doi.org/10.1111/j.1540-8175.2006.00238.x [DOI] [PubMed] [Google Scholar]
- 23.Toledano K, Guralnik L, Lorber A, Ofer A, Yigla M, Rozin A, et al. Pulmonary Arteries Involvement in Takayasu’s Arteritis: Two Cases and Literature Review. Sem Arthritis Rheum. 2011;41:461–70. doi: 10.1016/j.semarthrit.2011.06.001. https://doi.org/10.1016/j.semarthrit.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Zhang H, Jiang X, Zou Y, Qin F, Song L, et al. Clinical Manifestations and Longterm Outcome for Patients with Takayasu Arteritis in China. J Rheumatol. 2014;41:2439–46. doi: 10.3899/jrheum.140664. https://doi.org/10.3899/jrheum.140664 [DOI] [PubMed] [Google Scholar]
- 25.Lie JT. Isolated pulmonary Takayasu arteritis: clinicopathologic characteristics. Mod Pathol. 1996;9:469–74. [PubMed] [Google Scholar]
- 26.Yamazaki I, Ichikawa Y, Ishii M, Hamada T, Kajiwara H. Surgical case of isolated pulmonary Takayasu’s arteritis. Circ J. 2005;69:500–2. doi: 10.1253/circj.69.500. https://doi.org/10.1253/circj.69.500 [DOI] [PubMed] [Google Scholar]
- 27.Cassling RJ, Lois JF, Gomes AS. Unusual pulmonary angiographic findings in suspected pulmonary embolism. Am J Roentgenol. 1985;145:995–9. doi: 10.2214/ajr.145.5.995. https://doi.org/10.2214/ajr.145.5.995 [DOI] [PubMed] [Google Scholar]
- 28.Fukuda Y, Shirai K, Takamiya Y, Nathan M, Mito T, Yamagi D, et al. Isolated pulmonary arterial stenosis caused by Takayasu’s arteritis in an elderly male. J Cardiol. 2008;51:196–200. doi: 10.1016/j.jjcc.2007.12.003. https://doi.org/10.1016/j.jjcc.2007.12.003 [DOI] [PubMed] [Google Scholar]
- 29.Haas A, Stiehm ER. Takayasu’s arteritis presenting as pulmonary hypertension. Am J Dis Child. 1986;140:372–4. doi: 10.1001/archpedi.1986.02140180106036. https://doi.org/10.1001/archpedi.1986.02140180106036 [DOI] [PubMed] [Google Scholar]
- 30.Şentürk T, Kaderli AA, Karabacak S, Yeşilbursa D, Serdar OA. Pulmonary artery hypertension as an initial manifestation of Takayasu’s arteritis: A case report. Respir Med CME. 2010;6:211–3. https://doi.org/10.1016/j.rmedc.2009.11.006 [Google Scholar]
- 31.Elsassera S, Solèra M, Bolligera CT, Jager K, Steiger U, Perruchoud AP. Takayasu Disease with Predominant Pulmonary Involvement. Respiration. 2000;67:213–5. doi: 10.1159/000029490. https://doi.org/10.1159/000029490 [DOI] [PubMed] [Google Scholar]
- 32.Karadag B, Kilic H, Duman D, Ongen Z, Vural VA, Yazici H. Takayasu disease with prominent pulmonary artery involvement: confusion with pulmonary disease leading to delayed diagnosis. Mod Rheumatol. 2008;18:507–10. doi: 10.1007/s10165-008-0081-9. https://doi.org/10.3109/s10165-008-0081-9 [DOI] [PubMed] [Google Scholar]
- 33.Avouac J, Huscher D, Furst DE, Opitz CF, Distler O, Allanore Y. EPOSS GROUP: Expert consensus for performing right heart catheterisation for suspected pulmonary arterial hypertension in systemic sclerosis: a Delphi consensus study with cluster analysis. Ann Rheum Dis. 2014;73:191–7. doi: 10.1136/annrheumdis-2012-202567. https://doi.org/10.1136/annrheumdis-2012-202567 [DOI] [PubMed] [Google Scholar]