Abstract
Objective
Raynaud’s phenomenon consists of vasospastic disease of the digital arteries after exposure to cold or stress. It causes an important reduction in the patient’s quality of life when severe. The available treatments do not always offer favorable results.
Methods
A 3-year retrospective study was presented. A total of 15 patients with severe Raynaud’s phenomenon who required infiltration with botulinum toxin type A participated in the study. In the first and follow-up visits (30 min, 7 days, 3 months, 6 months, and annual), the overall response by the patient was assessed as was the reduction in the number of weekly episodes of Raynaud’s phenomenon, improvement in pain by means of the Visual Analogue Scale, and resolution of ulcers and necrosis as efficacy variables.
Results
A total of 15 patients were included in the study. After 30 min of infiltration, the immediate results showed a very good perception of response in four patients. After 1 month of treatment, eight patients had obtained and maintained a very good response, persisting throughout the study. A statistically significant reduction in pain was obtained, as well as the number of weekly episodes of Raynaud’s phenomenon. Of the seven patients with basal ulcers, five were completely healed at 3 months. Of the patients, 64.3% showed an overall satisfaction level of >8 at the end of treatment. No serious adverse events were observed.
Conclusion
Botulinum toxin is a useful treatment for severe Raynaud’s phenomenon that is generally well tolerated. Its mechanism of action is not based exclusively on vasodilation. Further studies are necessary to define the ideal patient for this treatment, the most appropriate method of administration, and the number of units and frequency of the infiltrations.
Keywords: Raynaud’s phenomenon, botulinum toxin, botulinum toxin type A
Introduction
Raynaud’s phenomenon is a vasospastic disorder of the digital arteries after exposure to cold or stress, accompanied by pain and ulcers (1–3), consequently altering the patient’s quality of life. Described by Maurice Raynaud in 1862, a primary form can be distinguished, as well as a form that is secondary or associated with systemic diseases (3).
In addition to general measures, such as patients avoiding tobacco and protecting themselves from the cold, drugs may be required in the treatment, and there is no current standardized therapeutic plan (3). Calcium antagonists are used as first-line treatment as they reduce the number and severity of weekly episodes (4). Other drugs, such as angiotensin II receptor blockers (5), phosphodiesterase inhibitors (6), prostaglandin analogs (7), and endothelin receptor antagonists, have been used in refractory cases (8, 9). Surgical treatment involving sympathectomy or arterial reconstruction may be necessary in certain patients who have incapacitating pain and ulcers with torpid evolution, but these techniques are not exempt from comorbidities and may not always offer satisfactory results (10).
Botulinum toxin, a polypeptide produced by the Clostridium botulinum bacteria, is an acetylcholine release inhibitor in the peripheral nerve endings of the motor plate and sweat glands. The protein is formed by a light chain of 50 kDa and a heavy chain of 100 kDa linked by a disulfide bridge. The light chain is bound to synaptosomal-associated protein 25 of acetylcholine vesicles, to avoid its transport and exocytosis (11). It is used to treat blepharospasm, facial spasm, cervical dystonia, facial wrinkles, axillary hyperhidrosis, and anal fissures, among others. However, its use in severe Raynaud’s phenomenon is a recent therapeutic possibility (12).
Methods
We performed a 3-year retrospective study involving, according to the inclusion and exclusion criteria shown in Table 1, patients with severe Raynaud’s phenomenon referred for dermatology consultations between December 2013 and March 2017 and who required treatment with botulinum toxin type A (Botox®; Allergan, Westport, Ireland). This treatment was given in the Dermatology Unit at Hospital Universitario Príncipe de Asturias de Alcalá de Henares (Madrid) and always administered by the same qualified medical staff.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Severe, primary, or secondary Raynaud’s phenomenon (intense and incapacitating pain) | Active infection at the injection site |
| Presence of ulcers and/or necrosis | Hypersensitivity to botulinum toxin or drug delivery vehicle |
| Distal capillary filling of >8 s | Earlier thoracic sympathectomy |
| Refractory reaction to conventional medical treatments (calcium antagonists) | Pregnant or breastfeeding |
| Absence of obstructive disease confirmed by Doppler echocardiography |
The Pharmacy Committee and Clinical Research Ethics Committee approved the study protocol. Written informed consent was obtained from all the patients to participate in the study.
A total of 15 patients were included in the study. Table 2 shows the demographic and clinical characteristics of the patients.
Table 2.
Demografic and clinical characteristics of the patients
| Patient ID | Sex | Age (year) | Smoker or ex-smoker | Raynaud’s phenomenon type | Previous treatments | Ulcers and/or necrosis | Infiltrated toxin units |
|---|---|---|---|---|---|---|---|
| 1 | F | 52 | No | Secondary LSS | Iloprost, nifedipine | Yes | L: 48 R: 48 |
| 2 | F | 41 | Yes | Secondary LSS | Nifedipine | No | L: 40 R: 40 |
| 3 | F | 51 | No | Secondary LSS | Nifedipine | Yes | L: 52 R: 48 |
| 4 | F | 35 | No | Secondary LSS | Nifedipine, bosentan, iloprost, pentoxifylline | Yes | L: 36 R: 40 |
| 5 | F | 41 | No | Secondary MCTD | Nifedipine | Yes | L: 32 R: 64 |
| 6 | F | 37 | No | Primary | Pentoxifylline, nifedipine | No | L: 52 R: 44 |
| 7 | F | 48 | Ex-smoker (10 years) | Secondary LSS | Nifedipine | No | L: 34 R: 36 |
| 8 | F | 46 | NA | Secondary DSS | Amlodipine | No | L: 40 R: 44 |
| 9 | F | 71 | NA | Secondary LSS | Diltiazem | No | L: 44 R: 56 |
| 10 | F | 52 | Ex-smoker (1 year) | Secondary LSS | Nifedipine | No | L: 34 R: 34 |
| 11 | F | 54 | NA | Primary | Nifedipine | No | L: 44 R: 46 |
| 12 | F | 41 | Ex-smoker (10 years) | Secondary DSS | Pentoxifylline, amlodipine | Yes | L: 50 R: 50 |
| 13 | F | 44 | NA | Secondary MCTD | Pentoxifylline, nifedipine | No | L: 46 R: 50 |
| 14 | F | 43 | NA | Secondary MCTD | Nifedipine | No | L: 40 R: 60 |
| 15 | M | 69 | NA | Secondary MCTD | Nifedipine | No | L: 50 R: 50 |
DSS: diffuse systemic scleroderma; F: female; ID: identification; L: left; LSS: limited systemic scleroderma; MCTD: mixed connective tissue disease; M: male; NA: not available; R: right
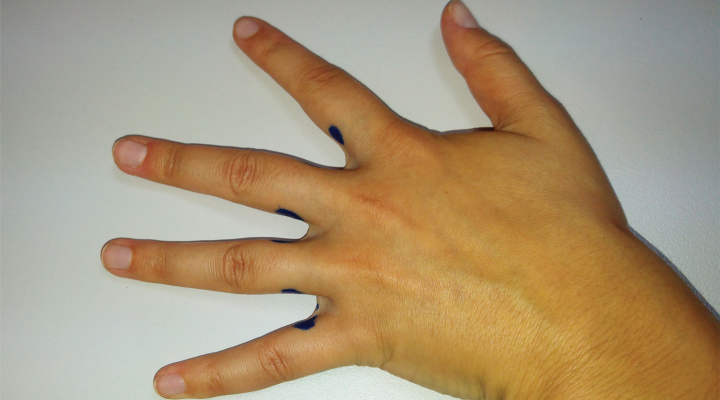
The infiltration protocol comprises the following steps (Figure 1): reconstitution of a vial containing 100 botulinum toxin units type A in 5 mL of saline serum to 0.9% (dilution: 20 IU/mL), marking of the infiltration sites, at the base of the lateral aspects of all the fingers, except the first as this is the least frequently affected and the one that is most at risk of suffering muscle weakness as a side effect, and injection of 4 and 8 IU of toxin per site using a 30 G needle, depending on the severity.
Figure 1.
Infiltration sites at the base of the lateral aspects of all the fingers, except the first
For the first four patients, there was an interval of 1 week before infiltration into the second hand, to evaluate the possible side effects. As the drug was tolerated well, it was decided that for the remaining cases, the drug would be infiltrated into both hands on the same day.
For the efficacy variables, we recorded the patient’s overall assessment of response (no response, mild response, moderate response, and very good response), as well as the reduction in the number of Raynaud episodes per week, improvement in pain (quantified using the Visual Analogue Scale (VAS) with scores between 0 and 10), and any resolution of ulcers and necrosis.
Data were recorded in both the first visit and during follow-up, with patient evaluation at 30 min, 7 days, 1 month, 3 months, 6 months, and a year from the infiltration. In addition, the patients’ overall level of satisfaction was registered at the end of the data collection period with scores between 0 (very unsatisfied) and 10 (totally satisfied).
Statistical analysis was performed using the PASW Statistics 18. The non-parametric Wilcoxon test was used to analyze the quantitative variable of the number of weekly Raynaud’s phenomenon episodes and the qualitative variable of ordinal pain measured using VAS. The significance level was set at alpha ≤0.05.
The patients’ assessment of response and resolution of ulcers and necrosis were expressed descriptively.
Results
The present study included a total of 15 (14 women and 1 man) patients. The average age of the patients was 46 (35–71) years. The follow-up period was 3 years for six patients, 2 years for four patients, and 1 year for four patients, with at least two winter periods after treatment for all the patients.
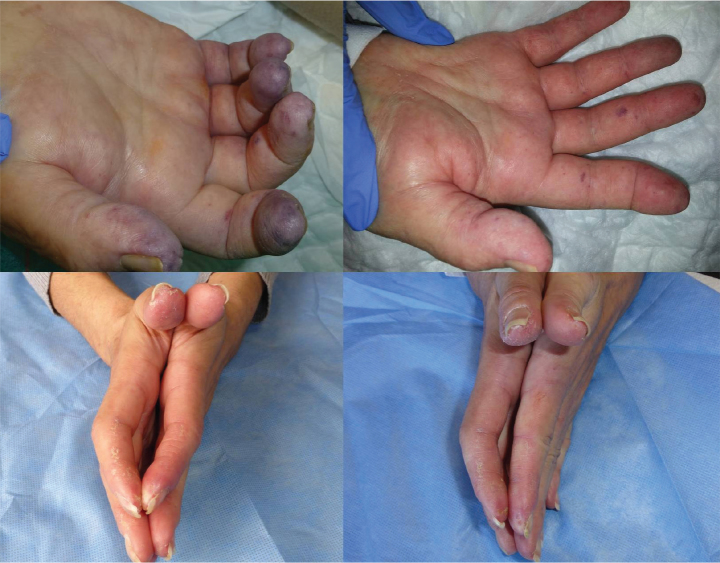
The immediate post-infiltration results are exemplified by some of the patients as well as the associated clinical images. After 30 min of infiltration with the toxin, five patients showed no change, three had mild response, two had moderate response, and four had very good response. One patient presented with purpuric coloration in all the fingers, together with a necrotic injury on the fifth finger of the left hand (Figure 2), accompanied by intense pain. After 6 h of treatment, there was a significant improvement in color, changing from a purpuric to a pinkish tone (Figure 2). In their initial visit, two patients presented with a significant limitation in the flexion and extension of the second finger in both hands (Figure 2). Half an hour after infiltration, they had full movement, accompanied by a total absence of pain and improved color (Figure 2). Four patients had a very painful ulcer in the pad of the second finger on the right hand. After infiltration, they reported that the pain had disappeared, and there was a subjective increase in temperature.
Figure 2.
One patient presented with purpuric coloration in all the fingers, together with a necrotic injury on the fifth finger of the left hand (up, left). After 6 h of treatment, there was a significant improvement in color (up, right). Two patients presented with a significant limitation in the flexion and extension of the second finger in both hands (down, left). Half an hour after infiltration of the left hand, the fingers had full movement, accompanied by a total absence of pain and improved color (down, right)
One patient died due to peritonitis. Owing to this reason, we will now refer to 14 patients.
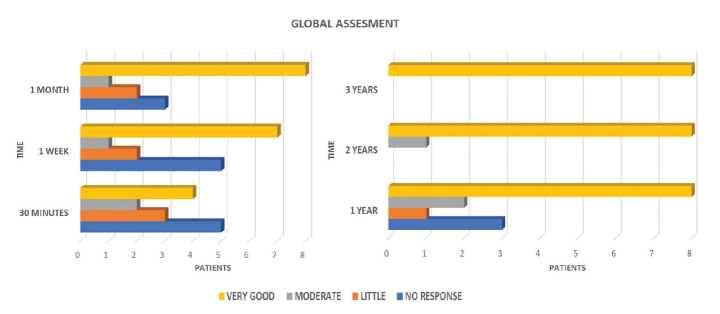
At the 7-day visit, seven patients showed a very good response. After 1 month of treatment, eight patients had a very good response, one had a moderate response, two had a mild response, and three showed no response (Figure 3). Eight patients with a very good response maintained this with an annual dose, with the exception of two patients who required no further infiltrations, until 3 years of follow-up. Regarding the remaining six patients, three of them presented with moderate or mild response, and only one continued the treatment (annual infiltration) up to 2 years; the other three did not show a response, so treatment and monitoring were discontinued (Figure 3). Some patients experienced a significant improvement in mobility (reduced stiffness and numbness).
Figure 3.
Patient evaluation at 30 min, 7 days, 1 month, 3 months, 6 months, and a year from the infiltration. Eight patients with a very good response maintained this with an annual dose, with the exception of two patients who required no further infiltrations
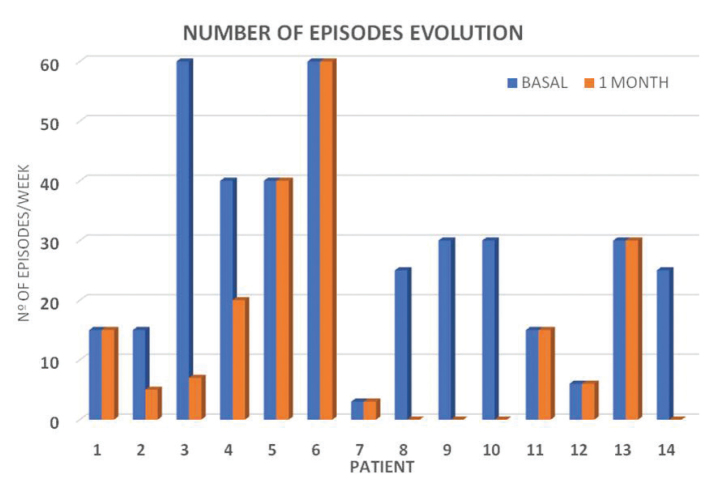
The average number of episodes per week of Raynaud’s phenomenon recorded at the first visit was 30 (range 3–60), and the pain assessment was 8 out of 10 (range 2–10). The number of episodes of Raynaud’s phenomenon significantly decreased in the first month post-treatment, with an average of 14 (range 0–60, p<0.009) (Figure 4). The pain intensity decreased to 2.7 (range 0–8, p<0.005), making this variable the earliest and most striking response (Figure 5). Only five patients continued to experience a reduction in pain in the 3- and 6-month visits. For the others, both the number of episodes and pain remained stable with respect to the data recorded 1 month after treatment, coinciding with summer.
Figure 4.
Number of episodes of Raynaud’s phenomenon significantly decreased in the first month post-treatment
Figure 5.
The pain intensity significantly decreased in the first month of treatment, making this variable the earliest and most striking response
With regard to ulcers, of the seven patients with basal ulceration, five were completely healed at 3 months, with no subsequent worsening, and two remained unchanged.
Of the patients, 63% showed a satisfaction level of >8 at the end of treatment.
We documented no adverse systemic effects in our patients. Four patients reported a temporary decrease in strength lasting a few weeks, and two had pain at the injection site that resolved spontaneously after 2–3 days.
Discussion
Raynaud’s phenomenon is triggered by stimuli, such as cold and stress, leading to an imbalance between the constriction and expansion of the small digital arteries. This leads to the appearance of pain, stiffness, numbness, a sensation of cold, and ulceration, with a consequent alteration in the quality of life of patients. Conventional treatments do not always control this process well, making it necessary to look for alternative therapies.
In 2004, Sycha et al. (13) published a pilot study involving two patients with severe Raynaud’s phenomenon who responded excellently to infiltration with botulinum toxin. Similar results have also been observed in previous studies (13–19).
In a study of 11 patients, Van Beek et al. (14) found an improvement in pain in the first 24–48 h, as well as a lower frequency of vasospasm episodes, which was maintained throughout 9.6 months of follow-up. The ulcers present in nine patients healed. The reported pain decreased from 9–10 to 0–2 (14).
In 2009, Neumeister et al. (16) published a study of 19 patients who were administered an infiltration of 50 to 100 IU of botulinum toxin type A per hand, reporting an 84% reduction in patient pain and improved flow in the Doppler study 30 min after the infiltration in 10 of the 14 patients treated, as well as ulcer healing in 60 days in all the patients. However, in a recent study, Bello et al. (20) could not demonstrate improvement in flow in the Doppler study after treatment with botulinum toxin in patients with Raynaud’s phenomenon secondary to systemic scleroderma (20).
In their prospective study published in 2011, Serri et al. (21) found that in 18 patients with scleroderma and Raynaud’s phenomenon, there is an improvement in pain measured using the QuickDASH scale, enhanced O2 partial pressure, and complete healing of ulcers (21).
Motegi et al. (11), in a prospective study of 10 Japanese patients with scleroderma and Raynaud’s phenomenon, reported a decrease in the frequency of episodes and improved color, duration, and pain, as well as resolution of digital ulcers. In addition, they used thermography to measure changes in finger temperature after immersion in cold water (11).
In our study, 30 min after infiltration of the toxin, six patients already showed a response, and four of these were very good. This immediate response has been observed by other authors, with particular significance when it comes to a reduction in pain (13, 14, 16, 21, 22). On the other hand, the reduction in the number of weekly vasospasm episodes is significant 1 month after treatment, decreasing from an average of 30 to an average of 14 (p<0.009) (Figure 1). The average pain intensity decreased from 8 to 2.7 (p<0.005), making this variable the earliest and most striking response (Figure 2). In addition, five patients continued to improve beyond the first month, reaching the maximum response at 6 months. For the others, after 6 months, both the number of episodes and pain remained stable with respect to the data recorded 1 month after treatment.
It is known that cold and stress produce a norepinephrine-mediated stimulus of the adrenergic receptor in the pericytes and vascular smooth muscle cells, causing vasoconstriction. In patients with systemic scleroderma, the response of the alpha-adrenergic receptors is increased in the digital arteries, suggesting that norepinephrine intervenes in the genesis of Raynaud’s phenomenon (1, 2). On the other hand, norepinephrine, substance P, glutamate, and calcitonin gene-related peptide increase in the peripheral nerves of the affected skin, inducing severe pain and paresthesia in the fingers (22–26).
The reduced number of Raynaud’s phenomenon episodes, its decreased duration, and the improved pain, which may be immediate, are reported in several studies and were observed in the present study, as mentioned previously. The exact mechanism of action is unknown, but it seems that it does not only cause vasodilation due to the paralysis of the acetylcholine-mediated arterial muscles (27), as this mechanism does not explain the rapid response experienced by some patients. The blocking of norepinephrine release and the inhibition of alpha-adrenergic receptor expression in the vascular walls reduce vasoconstriction and pain (12).
On the other hand, in animal models, it has been demonstrated that botulinum toxin inhibits these pain-mediating neurotransmitters in the nociceptive sensory neurons, reducing the peripheral stimulus toward the spinal cord and its progression toward the cerebral cortex. To this, the blocking of ectopic sodium channels that are overexpressed and aberrant in ischemic and chronic pain processes must be added (16, 25). In line with previously published studies, in our study, the variable showing the best response was pain, with immediate improvement in five patients.
The improved mobility observed in some patients is in agreement with the reduced stiffness and numbness documented by Sycha et al. (13) it is likely that the reduced pain caused by the aforementioned inhibition of the neurotransmitters plays an important role in this process.
Similarly, the response of ulcers to the treatment, as well as the 4- to 8-week healing period, also agrees with published studies (12, 14–17, 21). The study by Motegi et al. (28) supports the resolution of ulcers with the demonstration of increased blood flow through angiography and dermoscopy tests.
The technique used on our patients, involving infiltration at the base of the fingers, is one of two proposals by Fregene et al. (15) These show no significant differences in efficacy, although there is a greater tendency to weakness in the intrinsic musculature, the more proximal the injection (palmar or wrist) (15). In addition, the infiltration on the lateral aspect of the base of the fingers was chosen because it is similar to the one used for the digital nerve block anesthesia of the fingers, targeting the neurovascular plexus.
Patient satisfaction with the treatment is high, despite this technique requiring periodic infiltrations, which are very well tolerated in practically all cases.
We have not been able to establish a clear pattern associated with a better response. Some patients demonstrate an excellent response that is maintained over time, whereas others show no improvements. Regarding doses of 100–200 IU of botulinum toxin, they could be insufficient, as suggested by Motegi et al. (29) in a study in which a greater response is observed using a dose of 1000–2000 IU. Owing to these reasons, we consider multicentric, prospective, controlled studies to be necessary in order to establish the ideal candidate patient for the treatment, the most adequate doses, and the best injection pattern. A possible explanation for the variability in response is that certain patients may be immunologically resistant to botulinum toxin (3).
In conclusion, the present study establishes botulinum toxin as a safe, accessible, and effective therapeutic alternative for the treatment of severe Raynaud’s phenomenon, allowing non-responders to conventional treatments to maintain a good quality of life, through annual infiltrations prior to the winter period.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the HUPA Ethical Committee (Decision Date: 06/2013; Decision No.CF 06/2013-DER).
Informed Consent: Written informed consent was obtained from all the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.M., I.P., C.I., L.R.; Design - S.M., I.P., C.I., L.R., N.V., C.G.; Supervision - S.M., I.P., C.I., L.R., L.T.; Resources - S.M., I.P., C.I., L.R., L.T.; Materials - S.M., I.P., C.I., L.R., C.G., A.C.; Data Collection and/or Processing - S.M., I.P., C.I., L.R., N.V., C.G.; Analysis and/or Interpretation - S.M., I.P., C.I., L.R., N.V., A.G.Z.; Literature Search - S.M., I.P., C.I., L.R., N.V., A.G.Z., A.C., C.G.; Writing Manuscript - S.M., A.G.Z.; Critical Review - S.M., I.P., C.I., L.R., A.G.Z., A.C., L.T.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Herrick AL. The pathogenesis, diagnosis and treatment of Raynaud phenomenon. Nat Rev Rheumatol. 2012;8:469–79. doi: 10.1038/nrrheum.2012.96. https://doi.org/10.1038/nrrheum.2012.96 [DOI] [PubMed] [Google Scholar]
- 2.Wigley FM. Clinical practice Raynaud’s phenomenon. N Engl J Med. 2002;347:1001–8. doi: 10.1056/NEJMcp013013. https://doi.org/10.1056/NEJMcp013013 [DOI] [PubMed] [Google Scholar]
- 3.Neumeister MW. The role of botulinum toxin in vasospactic disorders of the hand. Hand Clin. 2015;31:23–37. doi: 10.1016/j.hcl.2014.09.003. https://doi.org/10.1016/j.hcl.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 4.Thompson AE, Pope JE. Calcium channel blockers for primay Raynaud’s phenomenon: a meta-analysis. Rheumatology (Oxford) 2005;44:145–50. doi: 10.1093/rheumatology/keh390. https://doi.org/10.1093/rheumatology/keh390 [DOI] [PubMed] [Google Scholar]
- 5.Matucci-Cerinic M, Denton CP, Frust DE, Mayes MD, Hsu VM, Carpentier P, et al. Bosentan treatment of digital ulcers related to systemic sclerosis. Results from RAPIDS-2 randomised, double-blind placebo-controlled trial. Ann Rheum Dis. 2011;70:32–8. doi: 10.1136/ard.2010.130658. https://doi.org/10.1136/ard.2010.130658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wigley FM, Wise RA, Seibold JR, McCloskey DA, Kujala G, Medsger TA, Jr, et al. Intravenous iloprost infusion in patients with Raynaud phenomenon secondary to systemic sclerosis. A multicentric placebo-controlled, double-blind study. Ann Intern Med. 1994;120:199–206. doi: 10.7326/0003-4819-120-3-199402010-00004. https://doi.org/10.7326/0003-4819-120-3-199402010-00004 [DOI] [PubMed] [Google Scholar]
- 7.Dziadzio M, Denton CP, Smith R, Howell K, Blann A, Bowers E, et al. Losartan therapy for Raynaud phenomenon and scleroderma. Arthitis Rheum. 1999;42:2646–55. doi: 10.1002/1529-0131(199912)42:12<2646::AID-ANR21>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen VA, Eisendle K, Gruber I, Hughe B, Reider D, Reider N. Effect of the dual endothelin receptor antagonist bosentan on Raynaud’s phenomenon secondary to systemic sclerosis: a double-blind prospective, randomized, placebo controlled pilot study. Rheumatology (Oxford) 2010;49:583–7. doi: 10.1093/rheumatology/kep413. https://doi.org/10.1093/rheumatology/kep413 [DOI] [PubMed] [Google Scholar]
- 9.Merkel PA, Herlyn K, Martin RW, Anderson JJ, Mayes MD, Bell P, et al. Measuring disease activity and functional status in patients with scleroderma and Raynaud’s phenomenon. Arthritis Rheum. 2002;46:2410–20. doi: 10.1002/art.10486. https://doi.org/10.1002/art.10486 [DOI] [PubMed] [Google Scholar]
- 10.Kostis SV, Chung KC. A systematic review of the outcomes of digital sympathectomy for treatment of chronic digital ischemia. J Rheumatol. 2003;30:1788–92. [PubMed] [Google Scholar]
- 11.Motegi S, Yamada K, Toki S, Uchiyama A, Kubota Y, Nakamura T, et al. Beneficial effect of botulinum toxin A on Raynaud’s phenomenon in Japanese patients with systemic sclerosis: A prospective, case series study. J Dermatol. 2016;43:56–62. doi: 10.1111/1346-8138.13030. https://doi.org/10.1111/1346-8138.13030 [DOI] [PubMed] [Google Scholar]
- 12.Setler P. Therapeutic use of botulinum toxins: back-ground and history. Clin J Pain. 2002;18(Suppl):S119–24. doi: 10.1097/00002508-200211001-00002. https://doi.org/10.1097/00002508-200211001-00002 [DOI] [PubMed] [Google Scholar]
- 13.Sycha T, Graninger M, Auff E, Shinider P. Botulinum toxin in the treatment of Raynaud phenomenon: A pilot study. Eur J Clin Invest. 2004;34:312–3. doi: 10.1111/j.1365-2362.2004.01324.x. https://doi.org/10.1111/j.1365-2362.2004.01324.x [DOI] [PubMed] [Google Scholar]
- 14.Van Beek AL, Lim PK, Gear AJ, Pritzker MR. Management of vasospastic disorders with botulinum toxin. Plast Reconstr Surg. 2007;119:217–26. doi: 10.1097/01.prs.0000244860.00674.57. https://doi.org/10.1097/01.prs.0000244860.00674.57 [DOI] [PubMed] [Google Scholar]
- 15.Fregene A, Ditmars D, Siddiqui A. Botulinum toxin type A: A treatment option for digital ischemia in patients with Raynaud’s phenomenon. Hand Surg Am. 2009;34:446–52. doi: 10.1016/j.jhsa.2008.11.026. https://doi.org/10.1016/j.jhsa.2008.11.026 [DOI] [PubMed] [Google Scholar]
- 16.Neumeister MW, Chambers CB, Herron MS, Webb K, Wietfeldt I, Gillespie JN, et al. Botox therapy for ischemic digits. Plast Reconstr Surg. 2009;124:191–201. doi: 10.1097/PRS.0b013e3181a80576. https://doi.org/10.1097/PRS.0b013e3181a80576 [DOI] [PubMed] [Google Scholar]
- 17.Neumeister MW. Botulinum toxin type A in the treatment of Raynaud’s phenomenon. J Hand Surg Am. 2010;35:2085–92. doi: 10.1016/j.jhsa.2010.09.019. https://doi.org/10.1016/j.jhsa.2010.09.019 [DOI] [PubMed] [Google Scholar]
- 18.Jenkins SN, Neyman KM, Veledar E, Chen SC. A pilot evaluating the efficacy of botulinum toxin A in the treatment of Raynaud phenomenon. J Am Acad Dermatol. 2013;69:834–5. doi: 10.1016/j.jaad.2013.06.029. https://doi.org/10.1016/j.jaad.2013.06.029 [DOI] [PubMed] [Google Scholar]
- 19.Iorio ML, Masden DL, Higgins JP. Botulinum toxin A treatment of Raynaud’s phenomenon: A review. Semin Arthritis Rheum. 2012;41:599–603. doi: 10.1016/j.semarthrit.2011.07.006. https://doi.org/10.1016/j.semarthrit.2011.07.006 [DOI] [PubMed] [Google Scholar]
- 20.Bello RJ, Cooney CM, Melamed E, Follmar K, Yenokyan G, Leatherman G, et al. The Therapeutic Efficacy of Botulinum Toxin in Treating Scleroderma-Associated Raynaud’s Phenomenon: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Arthritis Rheumatol. 2017;69:1661–9. doi: 10.1002/art.40123. https://doi.org/10.1002/art.40123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serri J, Legre R, Veit V, Guardia C, Gay AM. Intérêt de la toxine botulinique de type A dans le traitement des syndromes de Raynaud sévères secondaires à la sclérodermie systémique. Ann Chir Plast Esthet. 2013;58:658–62. doi: 10.1016/j.anplas.2011.11.001. https://doi.org/10.1016/j.anplas.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 22.Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology. 2005;26:785–93. doi: 10.1016/j.neuro.2005.01.017. https://doi.org/10.1016/j.neuro.2005.01.017 [DOI] [PubMed] [Google Scholar]
- 23.Welch MJ, Purkiss JR, Foster KA. Sensitivity of embryonic rat dorsal root ganglia neurons to clostridium botulinum neurotoxins. Toxicon. 2000;38:245–58. doi: 10.1016/s0041-0101(99)00153-1. https://doi.org/10.1016/S0041-0101(99)00153-1 [DOI] [PubMed] [Google Scholar]
- 24.Lawrence GW, Foran P, Dolly JO. Insights into a basis for incomplete inhibition by botulinum toxin A of Ca2+ evoked exocitosis from a permmeabilised chromaffin cells. Toxicology. 2002;181:249–53. doi: 10.1016/s0300-483x(02)00453-5. https://doi.org/10.1016/S0300-483X(02)00453-5 [DOI] [PubMed] [Google Scholar]
- 25.Durham PL, Cady R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: Implications for migraine therapy. Headache. 2004;44:35–42. doi: 10.1111/j.1526-4610.2004.04007.x. https://doi.org/10.1111/j.1526-4610.2004.04007.x [DOI] [PubMed] [Google Scholar]
- 26.Cui M, Khanijou S, Rubino J, Aoki KR. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain. 2004;107:125–33. doi: 10.1016/j.pain.2003.10.008. https://doi.org/10.1016/j.pain.2003.10.008 [DOI] [PubMed] [Google Scholar]
- 27.Stone AV, Koman AL, Callahan MF, Eckman DM, Smith BP, Plate JF, et al. The effect of botulinum neurotoxin-A on blood flow in rats: A potencial mechanism for treatment of Raynaud phenomenon. J Hand Surg. 2012;37:795–802. doi: 10.1016/j.jhsa.2012.01.021. https://doi.org/10.1016/j.jhsa.2012.01.021 [DOI] [PubMed] [Google Scholar]
- 28.Motegi SI, Uehara A, Yamada K, Sekiguchi A, Fujiwara C, Toki S, et al. Efficacy of Botulinum Toxin B Injection for Raynaud’s Phenomenon and Digital Ulcers in Patients with Systemic Sclerosis. Acta Derm Venereol. 2017;97:843–50. doi: 10.2340/00015555-2665. https://doi.org/10.2340/00015555-2665 [DOI] [PubMed] [Google Scholar]
- 29.Motegi SI, Sekiguchi A, Saito S, Ishibuchi H, Kishi C, Yasuda M, et al. Successful treatment of Raynaud’s phenomenon and digital ulcers in systemic sclerosis patients with botulinum toxin B injection: Assessment of peripheral vascular disorder by angiography and dermoscopic image of nail fold capillary. J Dermatol. 2018;45:349–52. doi: 10.1111/1346-8138.14140. https://doi.org/10.1111/1346-8138.14140 [DOI] [PubMed] [Google Scholar]