Abstract
Objective
Approximately 10%–20% of patients with familial Mediterranean fever (FMF) show an inadequate response to colchicine. In our cohort study, patients with FMF with or without amyloidosis and with an inadequate response to colchicine were treated with anakinra or canakinumab.
Methods
Clinical and laboratory parameters, Mediterranean fever (MEFV) mutations, and patient-reported outcomes were analyzed in 31 patients treated with anakinra or canakinumab.
Results
In a cohort of 250 adult patients with FMF, 31 patients were treated with anakinra (n=29) or canakinumab (n=2). The median Pras FMF severity score was 8 (range, 5–14) and correlated with the presence of high-penetrance MEFV mutations (p.Met-694-Val or p.Met-680-Ile). The FMF severity score was 11 in patients with two high-penetrance MEFV mutations (68%), 9 in those with a single high-penetrance MEFV mutation (19%), and 7.5 in those without high-penetrance MEFV mutations (13%, p=0.2). FMF-related amyloid A amyloidosis was diagnosed in 12 (39%) patients. Anakinra was used daily in 20 patients, thrice a week in 7, and upon demand during attacks in 2. Two patients were treated with canakinumab. IL-1-blocking treatment showed a rapid (2±3 days) and persistent suppression of FMF symptoms and inflammatory parameters. The frequency of FMF attacks was significantly reduced (p<0.003). Both patient- and physician-reported FMF activity significantly improved (p<0.0001).
Conclusion
IL-1-blocking therapy was well tolerated over a median period of 2 years and reduced the frequency of FMF attacks in patients with colchicine-resistant FMF.
Keywords: Familial Mediterranean fever, colchicin, amyloidosis, IL1
Introduction
Familial Mediterranean fever (FMF) is an autoinflammatory disorder that is more prevalent in individuals with an ancestry from the Mediterranean basin (1). FMF is characterized by recurrent attacks of fever with serosal and synovial inflammation lasting for 1272 h (2). Patients with FMF are at risk to develop amyloid A (AA) amyloidosis, which predominantly affects renal functions in patients with FMF (2, 3). Majority of patients with FMF have two homozygous or compound heterozygous mutations in the Mediterranean fever (MEFV) gene, which encodes the pyrin protein (4, 5). The standard of care for FMF is colchicine, and it has been considered a safe and effective prophylaxis in FMF attacks and in reducing the risk of amyloidosis (6–9). Although colchicine prophylaxis is cost effective compared to the biological treatment, it is not approved for FMF in all European countries. Nevertheless, the current EULAR recommendations for the management of FMF recently confirmed colchicine as an anchor for treatment (9). Despite the efficacy of colchicine, some manifestations, such as arthritis, are less responsive to colchicine. Furthermore, approximately 5%10% of patients are considered non-responders to colchicine (colchicine-resistant FMF) or cannot tolerate colchicine (colchicine-intolerant FMF) of up to 2.0 mg per day (10, 11). These patients were considered inadequate responders to colchicine. Unfortunately, there is no generally accepted definition of colchicine resistance in the literature. EULAR defines colchicine resistance as having one or more attacks per month in compliant patients who had been receiving the maximally tolerated colchicine dose for at least 6 months (9). EULAR also stated that patients with FMF and AA amyloidosis should receive an intensified treatment with a maximal tolerable colchicine dose and biological treatment as required (9).
A case series and two trials have suggested that I L1-blocking therapy is efficient in preventing FMF attacks and progression of FMF-associated amyloidosis (9, 11–21). The objective of our study was to investigate the safety and efficacy of IL-1-blocking therapy in colchicine-resistant FMF and in patients with FMF and AA amyloidosis.
Methods
FMF diagnosis, severity, and genetic analyses
The University Hospital of Heidelberg serves as a referral center for autoinflammatory diseases and amyloidosis. FMF diagnosis was established according the Tel Hashomer criteria (22). Genetic tests for MEFV mutations were conducted in all patients. In cases with less than two MEFV variants, a complete analysis of MEFV exons 1–10 was performed. The FMF severity score was determined according to method used by Pras et al. (23). The FMF severity score comprised age at FMF onset, frequency of attacks, presence of arthritis, erysipelas-like erythema, amyloidosis, and the required dose of colchicine prophylaxis that is necessary to control FMF symptoms. The FMF severity score indicates mild (score, 1–5), moderate (score, 6–9), and severe (score, 10–19) FMF activity.
Screening and diagnosis of AA amyloidosis
All patients were screened for clinical and laboratory signs of amyloidosis, such as proteinuria, edema, reduced creatinine clearance, and gastrointestinal or cardiac involvement. In patients with suspected amyloidosis due to, for example, proteinuria, periumbilical fat aspiration biopsy and biopsy of the involved organs (mainly the kidneys) were performed. Tissue staining with Congo red and immunohistochemistry were used to confirm AA amyloidosis and to exclude other forms of amyloidosis, particularly light chain amyloidosis. In patients with no clinical or laboratory signs of amyloidosis, no routine biopsy of periumbilical fat or rectal biopsy was performed.
Definition of colchicine-resistant FMF and FMF treatment in AA amyloidosis
The EULAR recommendations for the management of FMF (9) define colchicine resistance as having one or more attacks per month in compliant patients who had been receiving the maximally tolerated colchicine dose for at least 6 months (9). EULAR also states that patients with FMF and AA amyloidosis should receive an intensified treatment with a maximal tolerable colchicine dose and biological treatment as required (9). Both indications suggest FMF treatment with a maximal tolerable colchicine dose and supplemented with a biological treatment (9).
Study patients and study treatment
All FMF patients were aged at least 18 years and were eligible for providing informed consent. The maximum dose of colchicine was 2.0 mg per day. Patients were not encouraged to take >2.0 mg of colchicine to avoid toxic adverse events. Patients with an inadequate response to colchicines, which was defined as three or more FMF attacks per year, or those with persistently elevated C-reactive protein (CRP) and serum amyloid A (SAA) serum levels were identified in patients with FMF and biopsy-proven AA amyloidosis. Between 2013 and 2017, a combination of colchicine and IL-1-blocking therapy (anakinra 100 mg subcutaneous [s.c.] daily) was provided to this consecutive cohort of patients to reduce FMF attacks. Two patients were on dialysis and received anakinra thrice a week to compensate the reduced renal clearance of the drug. Two patients did not tolerate anakinra injections and were switched to canakinumab 150 mg s.c. every 8 weeks.
Clinical characteristics, laboratory parameters, and response to treatment were recorded. All patients continued the colchicine treatment. The response to treatment was evaluated using patients’ global and physicians’ global assessment of disease severity on a visual analog scale (VAS) with a range of 0 to 100. High VAS scores indicate severe disease activity. Laboratory parameters (CRP, SAA, creatinine, and proteinuria) were determined during each visit. The efficacy of anti-IL-1-treatment was quantified as ≥50% reduction of FMF attack frequency, patient and physician global assessment, and reduction of CRP and SAA levels, which has been used previously as the modified FMF50 score (24, 25).
After 3 months without FMF attacks and with normal range acute phase reactants, patients were offered to reduce anakinra to thrice a month or less to maintain remission. The follow-up period was ≥6 months. Patients with FMF attacks under less than daily anakinra were supported to use anakinra daily again.
All patients agreed upon informed consent to participate in this study, which was approved by our local ethics committee (S-103/2013).
Statistical analysis
Unless otherwise stated, values were calculated as means and ranges (minimum–maximum) or percentage. Study parameters were not normally distributed. Therefore, the Wilcoxon’s signed rank test was used to compare repeated measurements, and p values of <0.05 were considered statistically significant. All statistical analyses were conducted using the Statistical Package for Social Sciences (SPSS) software version 25 (IBM Crop.; Armonk, NY, USA).
A power analysis for CRP, SAA, and FMF improvement revealed that if an alpha of 0.05 and power of 0.8 were acceptable, then the required minimal sample size is 24 patients.
Results
Thirty-one patients with FMF (12.4%, 14 females and 17 males) and an inadequate response to colchicine were identified in a cohort of 261 patients with FMF. Twenty-eight (90%) patients were of the Turkish-Armenian ancestry, one from Germany, one from Azerbaijan, and one from the Czech Republic. Genetic analyses revealed 58 MEFV variants in 31 patients with FMF. High-penetrance MEFV variants of p.Met-694-Val or p.Met-680-Ile were detected in 48 of 58 MEFV variants (83%). FMF-related symptoms were reported at baseline (Table 1). The median FMF severity score was 8 (range, 5–14).
Table 1.
FMF symptoms at baseline
| FMF symptoms | Number of Patients n=31 (%) |
|---|---|
| Abdominal pain | 28 (90) |
| Fever | 25 (81) |
| Myalgia | 8 (26) |
| Arthralgia | 17 (55) |
| Chest Pain | 16 (52) |
| Arthritis | 7 (23) |
| Amyloidosis | 12 (39) |
Patients carrying 2, 1, and none of the high-penetrance MEFV mutations (p.Met-694-Val or p.Met-680-Ile) had a severity score of 11.0 (n=21), 9.0 (n=6), and 7.5 (n=4; p=0.2; Table 2), respectively. AA amyloidosis was diagnosed in 12 (39%) patients with a median FMF severity score of 10.0 (severe FMF activity). The 19 patients without amyloidosis had an FMF severity score of 7.0 (moderate FMF activity, p=0.3).
Table 2.
Genotype-phenotype correlation
| High penetrance MEFV mutations | 2 | 1 | 0 |
|---|---|---|---|
| Number of patients (%) | n=21 (68%) | n=6 (19%) | n=4 (13%) |
| Age at onset (years, median (range)) | 8 (1–50) | 9 (2–21) | 41 (8–61) |
| Age at baseline (years) | 36 (19–54) | 44 (36–51) | 38 (21–64) |
| Maximum CRP (mg/l, median (range)) | 143 (17–330) | 153 (48–350) | 90 (76–220) |
| Amyloidosis (%) | n=9 (43%) | n=3 (50%) | 0 |
| FMF activity score (range 1–14) | 11 (5–14) | 9 (5–12) | 7.5 (5–10) |
| Attacks per year with colchicines | 12 (4–24) | 12 (5–24) | 7 (2–18) |
| Colchicine dose (mg/d) | 2 (0.5–3) | 1.5 (1–2) | 1.5 (0.5–2) |
| Anakinra daily (n=20) | 17 (81%) | 1 (17%) | 2 (50%) |
| Anakinra less than daily (n=9) | 3 (14%) | 4 (66%) | 2 (50%) |
| Canakinumab (n=2) | 1 (5%) | 1 (17%) | 0 |
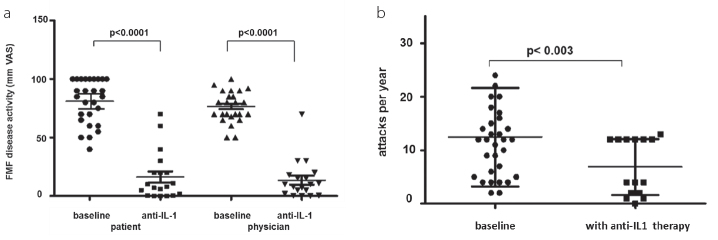
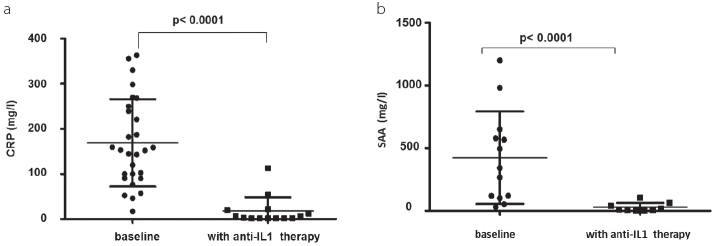
Twenty-nine patients were treated with a combination of colchicine (1.5±0.7 mg per day) and anakinra (94%). Two (6%) patients reported non-tolerable side effects to anakinra with protracted severe painful skin swelling at the site of anakinra injections. These two patients received colchicine and canakinumab 150 mg s.c. every 8 weeks because of poor compliance with anakinra injections. Both patients did not have any FMF attack or subclinically elevated CRP during treatment with canakinumab. Anakinra was used once daily in 18 patients and twice daily in one patient. Seven patients used anakinra thrice a week, and two used it only during FMF attacks. All patients with IL-1-blocking therapy showed a rapid improvement of the global health and a persistent suppression of FMF attacks (Figure 1a, b) and inflammatory parameters (Figure 2a, b).
Figure 1. a, b.
Patient and physician assessment of FMF disease activity at baseline and after IL-1 blocking therapy (a); frequency of FMF attacks at baseline and after IL-1 blocking therapy (b)
Figure 2. a, b.
Maximum CRP at baseline and after IL-1-blocking therapy (a); maximum SAA at baseline and after IL-1-blocking therapy (b)
The number of FMF attacks was significantly reduced by anti-IL-1 treatment (Figure 1b; p<0.003). Both patient-reported (VAS 75±23 mm) and physician-reported (VAS 70±12 mm) FMF severity significantly improved (Figure 1a; p<0.0001). Consistent with the clinical improvement, the maximum serum CRP and SAA levels were also significantly decreased (Figure 2, p<0.0001). The modified FMF50 score showed an improvement in 28 of 31 patients (90%, Table 3).
Table 3.
FMF 50 response to IL-1-blocking therapy
| 50% reduction of | Number of Patients (%) total n=31 |
|---|---|
| frequency of FMF attacks | 23 (74) |
| frequency of joint attacks | 4 (13) |
| CRP or normalization of CRP | 30 (97) |
| patient assessment of FMF disease activity | 30 (97) |
| physician assessment of FMF disease activity | 31 (100) |
Among the 12 patients with amyloidosis, three (25%) presented with nephrotic syndrome, five with chronic kidney disease, two with end-stage renal disease, and two were in the fifth and seventh year after renal transplantation. The 24-h urine protein excretion was 5.0±3.5 g (median±SD) at baseline and decreased to 0.4±1.0 g with anakinra at the last visit after 24±18 months. Patients with AA amyloidosis had baseline creatinine serum levels of 2.4±0.6 mg/dL, which decreased in four patients to 1.6±0.6 mg/dL and remained stable at 2.1±0.4 mg/dL in eight.
Patients with AA amyloidosis reported high FMF disease activity at baseline (VAS 70±21 mm) that improved with IL-1-blocking therapy (VAS 10±20 mm; p=0.001). The physician-reported FMF disease activity was comparably high (VAS 80±16 mm) and significantly improved (VAS 15±20 mm; p=0.0009) after 24 months (±18 mm) of IL-1-blocking therapy.
At the end of this trial, anakinra was used once daily in 19 patients (Table 2). One patient used anakinra twice daily, seven used it thrice a week, and two used it only during attacks. The presence of two high-penetrance MEFV variants (n=20) required daily anakinra injections in 17 (75%) patients to maintain a good control of inflammation, whereas in the absence of two high-penetrance MEFV mutations (n=9), less than daily anakinra injections were sufficient in six patients (67%, p=0.001, Table 2).
Patients in this cohort were treated with IL-1-blocking therapy and followed over 24±12 months. No secondary loss of efficacy was observed. Patients report mild infections of the upper respiratory tract (16%) and local reactions to anakinra (25%). No severe adverse events were noted during 58 patient-years with IL-1-blocking therapy.
Discussion
We reported about the open-label treatment of 31 patients with colchicine-resistant FMF with a combination of colchicine and IL-1-blocking therapy. Our report is consistent with previous reports, which indicated a good safety and efficacy of IL-1-blocking therapy in patients with FMF (11–21, 25–29). None of the 12 patients with systemic AA progressed to organ failure, and 19 patients without AA showed significant improvement of global health, inflammatory parameters, and modified FMF50 score. This study substantiates previous anecdotal reports and small case series showing safety and efficacy of IL-1-blocking therapy in patients with FMF with and without amyloidosis.
Twenty-nine patients were treated with anakinra and two with canakinumab. Both drugs were safe and well tolerated by our patients. Local reactions at the anakinra injection site were reported from 25% of the patients, which is rather low compared with the 30%–70% of injection site reactions that has been observed in previous trials (27). However, the majority of injection site reactions were transient and tolerable. To increase the adherence to anakinra injections, the potential for injection site reactions and their management should be discussed with the patient prior to the initiation of therapy. Some patients used anakinra every second or third day during the initial 6 weeks to desensitize against injection site reactions in addition to the general anakinra application advice (27). Two patients with a poor compliance for daily injections were treated with canakinumab with good comparable results. Mild infections of the upper respiratory tract were reported by 16% of the patients, and none of them discontinued anakinra. One patient reported a severe gastrointestinal infection that remitted after hospitalization. Systemic infections were not observed, and none of the patients discontinued therapy because of infections or injection site reactions.
A direct comparison of the quality of life in patients treated with anakinra and canakinumab might reveal a general preference of canakinumab by the patient. Although medical costs of canakinumab are much higher than those of anakinra, canakinumab can be used as a second-line treatment for patients with compromised compliance or side effects to anakinra injections. In addition, results of a clinical trial of canakinumab for patients with colchicine-resistant FMF were recently published (28).
A limitation of our study was the observational design and heterogeneity of patients with FMF with and without AA. However, our results are perfectly consistent with other reports of a good safety and efficacy of IL-1-blocking agents for the treatment of colchicine-resistant FMF.
After the patients attained clinical remission, nine reduced anakinra to less than daily injections to maintain remission. It seemed that in these patients, the biological effect of anakinra lasted longer than the pharmacological effects (29). Patients with FMF with two high-penetrance MEFV mutations are more likely to require daily anakinra to maintain their remission. This finding is consistent with a previous report of a correlation between high-penetrance MEFV mutations and the increased production of IL-1-beta (30). It is also consistent with the concept of a gene-dose effect on the clinical phenotype as recently discussed (31).
The predominance of high-penetrance MEFV mutations in our German cohort is consistent with previous reports on patients with FMF with the Turkish-Armenian ancestry. A recent report from south Italy has described an endemic area for a mild-to-moderate FMF variant with predominant E148Q and R761H MEFV mutations (32), which were not observed in our cohort.
The treatment of colchicine-resistant FMF with anakinra and canakinumab was safe and efficient in patients with and without AA amyloidosis.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Heildelberg University (S-103/2013).
Informed Consent: Written informed consent was obtained from all the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - N.B., B.M.K.; Design - N.B., B.M.K.; Supervision - N.B., H.M.L.; Resources - N.B., H.M.L.; Data Collection and/or Processing - N.B., B.M.K.; Analysis and/or Interpretation - N.B., B.M.K., H.M.L.; Literature Search - N.B., B.M.K.; Writing Manuscript - B.M.K.; Critical Review - N.B., H.M.L.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Ben-Chetrit E, Touitou I. Familial Mediterranean fever in the world. Arthritis Rheum. 2009;61:1447–53. doi: 10.1002/art.24458. https://doi.org/10.1002/art.24458 [DOI] [PubMed] [Google Scholar]
- 2.Sohar E, Gafni J, Pras M, Heller H. Familial Mediterranean fever. A survey of 470 cases and review of the literature. Am J Med. 1967;43:227–53. doi: 10.1016/0002-9343(67)90167-2. https://doi.org/10.1016/0002-9343(67)90167-2 [DOI] [PubMed] [Google Scholar]
- 3.Tunca M, Akar S, Onen F, Ozdogan H, Kasapcopur O, Yalcinkaya F, et al. Turkish FMF Study Group. Familial Mediterranean fever (FMF) in Turkey: results of a nationwide multicenter study. Medicine. 2005;84:1–11. doi: 10.1097/01.md.0000152370.84628.0c. https://doi.org/10.1097/01.md.0000152370.84628.0c [DOI] [PubMed] [Google Scholar]
- 4.French FMF Consortium. A candidate gene for familial Mediterranean fever. Nat Genet. 1997;17:25–31. doi: 10.1038/ng0997-25. https://doi.org/10.1038/ng0997-25 [DOI] [PubMed] [Google Scholar]
- 5.The International FMF consortium. Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell. 1997;90:797–807. doi: 10.1016/s0092-8674(00)80539-5. https://doi.org/10.1016/S0092-8674(00)80539-5 [DOI] [PubMed] [Google Scholar]
- 6.Goldfinger SE. Colchicine for familial Mediterranean fever. N Engl J Med. 1972;287:1302. doi: 10.1056/NEJM197212212872514. https://doi.org/10.1056/NEJM197212212872514 [DOI] [PubMed] [Google Scholar]
- 7.Zemer D, Revach M, Pras M, Modan B, Schor S, Sohar E, et al. A controlled trial of colchicine in preventing attacks of familial Mediterranean fever. N Engl J Med. 1974;291:932–4. doi: 10.1056/NEJM197410312911803. https://doi.org/10.1056/NEJM197410312911803 [DOI] [PubMed] [Google Scholar]
- 8.Dinarello CA, Wolff SM, Goldfinger SE, Dale DC, Alling DW. Colchicine therapy for familial mediterranean fever. A double-blind trial. N Engl J Med. 1974;291:934–7. doi: 10.1056/NEJM197410312911804. https://doi.org/10.1056/NEJM197410312911804 [DOI] [PubMed] [Google Scholar]
- 9.Ozen S, Demirkaya E, Erer B, Livneh A, Ben-Chetrit E, Giancane G, et al. EULAR recommendations for the management of familial Mediterranean fever. Ann Rheum Dis. 2016;75:644–51. doi: 10.1136/annrheumdis-2015-208690. https://doi.org/10.1136/annrheumdis-2015-208690 [DOI] [PubMed] [Google Scholar]
- 10.Ben-Chetrit E, Levy M. Familial Mediterranean fever. Lancet. 1998;351:659–64. doi: 10.1016/S0140-6736(97)09408-7. https://doi.org/10.1016/S0140-6736(97)09408-7 [DOI] [PubMed] [Google Scholar]
- 11.Ozen S, Kone-Paut I, Gül A. Colchicine resistance and intolerance in familial mediterranean fever: Definition, causes, and alternative treatments. Semin Arthritis Rheum. 2017;47:115–20. doi: 10.1016/j.semarthrit.2017.03.006. https://doi.org/10.1016/j.semarthrit.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 12.Akar S, Cetin P, Kalyoncu U, Karadag O, Sari I, Cınar M, et al. Nationwide Experience with Off-Label Use of Interleukin-1 Targeting Treatment in Familial Mediterranean Fever Patients. Arthritis Care Res. 2017;70:1090–4. doi: 10.1002/acr.23446. https://doi.org/10.1002/acr.23446 [DOI] [PubMed] [Google Scholar]
- 13.Mitroulis I, Papadopoulos VP, Konstantinidis T, Ritis K. Anakinra suppresses familial Mediterranean fever crises in a colchicine-resistant patient. Neth J Med. 2008;66:489–91. [PubMed] [Google Scholar]
- 14.Moser C, Pohl G, Haslinger I, Knapp S, Rowczenio D, Russel T, et al. Successful treatment of familial Mediterranean fever with anakinra and outcome after renal transplantation. Nephrol Dial Transplant. 2009;24:676–8. doi: 10.1093/ndt/gfn646. https://doi.org/10.1093/ndt/gfn646 [DOI] [PubMed] [Google Scholar]
- 15.Ozen S, Bilginer Y, Aktay Ayaz N, Calguneri M. Anti-interleukin 1 treatment for patients with familial Mediterranean fever resistant to colchicine. J Rheumatol. 2011;38:516–8. doi: 10.3899/jrheum.100718. https://doi.org/10.3899/jrheum.100718 [DOI] [PubMed] [Google Scholar]
- 16.Meinzer U, Quartier P, Alexandra JF, Hentgen V, Retornaz F, Kone-Paut I. Interleukin-1 targeting drugs in familial Mediterranean fever: a case series and a review of the literature. Semin Arthritis Rheum. 2011;41:265–71. doi: 10.1016/j.semarthrit.2010.11.003. https://doi.org/10.1016/j.semarthrit.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 17.Stankovic Stojanovic K, Delmas Y, Torres PU, Peltier J, Pelle G, Jeru I. Dramatic beneficial effect of interleukin-1 inhibitor treatment in patients with familial Mediterranean fever complicated with amyloidosis and renal failure. Nephrol Dial Transplant. 2012;27:1898–901. doi: 10.1093/ndt/gfr528. https://doi.org/10.1093/ndt/gfr528 [DOI] [PubMed] [Google Scholar]
- 18.Alpay N, Sumnu A, Calıskan Y, Yazıcı H, Turkmen A, Gul A. Efficacy of anakinra treatment in a patient with colchicine-resistant familial Mediterranean fever. Rheumatol Int. 2012;32:3277–9. doi: 10.1007/s00296-010-1474-6. https://doi.org/10.1007/s00296-010-1474-6 [DOI] [PubMed] [Google Scholar]
- 19.Eroglu FK, Besbas N, Topaloglu R, Ozen S. Treatment of colchicine-resistant Familial Mediterranean fever in children and adolescents. Rheumatol Int. 2015;35:1733–7. doi: 10.1007/s00296-015-3293-2. https://doi.org/10.1007/s00296-015-3293-2 [DOI] [PubMed] [Google Scholar]
- 20.Basaran O, Uncu N, Celikel BA, Taktak A, Gur G, Cakar N. Interleukin-1 targeting treatment in familial Mediterranean fever: an experience of pediatric patients. Mod Rheumatol. 2015;25:621–4. doi: 10.3109/14397595.2014.987437. https://doi.org/10.3109/14397595.2014.987437 [DOI] [PubMed] [Google Scholar]
- 21.Cetin P, Sari I, Sozeri B, Cam O, Birlik M, Akkoc N. Efficacy of interleukin-1 targeting treatments in patients with familial Mediterranean fever. Inflammation. 2015;38:27–31. doi: 10.1007/s10753-014-0004-1. https://doi.org/10.1007/s10753-014-0004-1 [DOI] [PubMed] [Google Scholar]
- 22.Livneh A, Langevitz P, Zemer D, Zaks N, Kees S, Lidar T, et al. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum. 1997;40:1879–85. doi: 10.1002/art.1780401023. https://doi.org/10.1002/art.1780401023 [DOI] [PubMed] [Google Scholar]
- 23.Pras E, Livneh A, Balow JE, Jr, Pras E, Kastner DL, Pras M, et al. Clinical differences between North African and Iraqi Jews with familial Mediterranean fever. Am J Med Genet. 1998;75:216–9. doi: 10.1002/(sici)1096-8628(19980113)75:2<216::aid-ajmg20>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 24.Ozen S, Demirkaya E, Duzova A, Erdogan O, Erken E, Gul A, et al. FMF50: a score for assessing outcome in familial Mediterranean fever. Ann Rheum Dis. 2014;73:897–901. doi: 10.1136/annrheumdis-2013-204719. https://doi.org/10.1136/annrheumdis-2013-204719 [DOI] [PubMed] [Google Scholar]
- 25.Ben-Zvi I, Kukuy O, Giat E, Pras E, Feld O, Kivity S, et al. Anakinra for Colchicine-Resistant Familial Mediterranean Fever: A Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheumatol. 2017;69:854–62. doi: 10.1002/art.39995. https://doi.org/10.1002/art.39995 [DOI] [PubMed] [Google Scholar]
- 26.Kucuksahin O, Yildizgoren MT, Ilgen U, Ates A, Kinikli G, Turgay M, et al. Anti-interleukin-1 treatment in 26 patients with refractory familial mediterranean fever. Mod Rheumatol. 2017;27:350–5. doi: 10.1080/14397595.2016.1194510. https://doi.org/10.1080/14397595.2016.1194510 [DOI] [PubMed] [Google Scholar]
- 27.Kaiser C, Knight A, Nordström D, Pettersson T, Fransson J, Florin-Robertsson E, et al. Injection-site reactions upon Kineret (anakinra) administration: experiences and explanations. Rheumatol Int. 2012;32:295–9. doi: 10.1007/s00296-011-2096-3. https://doi.org/10.1007/s00296-011-2096-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Benedetti F, Gattorno M, Anton J, Ben-Chetrit E, Frenkel J, Hoffman HM, et al. Canakinumab for the Treatment of Autoinflammatory Recurrent Fever Syndromes. N Engl J Med. 2018;378:1908–19. doi: 10.1056/NEJMoa1706314. https://doi.org/10.1056/NEJMoa1706314 [DOI] [PubMed] [Google Scholar]
- 29.Granowitz EV, Porat R, Mier JW, Pribble JP, Stiles DM, Bloedow DC, et al. Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin-1 receptor antagonist in healthy humans. Cytokine. 1992;4:353–60. doi: 10.1016/1043-4666(92)90078-6. https://doi.org/10.1016/1043-4666(92)90078-6 [DOI] [PubMed] [Google Scholar]
- 30.Omenetti A, Carta S, Delfino L, Martini A, Gattorno M, Rubartelli A. Increased NLRP3-dependent interleukin 1β secretion in patients with familial Mediterranean fever: correlation with MEFV genotype. Ann Rheum Dis. 2014;73:462–9. doi: 10.1136/annrheumdis-2012-202774. https://doi.org/10.1136/annrheumdis-2012-202774 [DOI] [PubMed] [Google Scholar]
- 31.Kallinich T, Orak B, Wittkowski H. Role of genetics in familial Mediterranean fever. Review in German. Z Rheumatol. 2017;76:303–12. doi: 10.1007/s00393-017-0265-9. https://doi.org/10.1007/s00393-017-0265-9 [DOI] [PubMed] [Google Scholar]
- 32.Bonfrate L, Scaccianoce G, Palasciano G, Ben-Chetrit E, Portincasa P. A novel cluster of patients with Familial Mediterranean Fever (FMF) in southern Italy. Eur J Clin Invest. 2017;47:622–9. doi: 10.1111/eci.12783. https://doi.org/10.1111/eci.12783 [DOI] [PubMed] [Google Scholar]