Abstract
Background
Lung cancer (LC) is the most prevalent malignancy worldwide, and non-small-cell LC (NSCLC) cell is associated with high mortality. As a member of the second generation of anaplastic lymphoma kinase (ALK) suppressors, ceritinib has considerable therapeutic effects for ALK and c-ros oncogene 1 (ROS1)-positive NSCLC cell. Nevertheless, patients inevitably develop resistance to the drug. Our research focused on the exploration of whether afatinib was able to counteract ceritinib resistance (CR) in NSCLC cells with positive ALK or ROS1.
Materials and methods
Acquired CR cell sublines (HCC78R and H1299R) were induced by stepwise escalation of ceritinib exposure. MTT assay was used to validate cell proliferation. Fluorescence assay was performed for apoptosis analysis. Quantitative real-time PCR and Western blot assays were used to assess the alterations of signaling pathway-related mRNA and proteins, respectively.
Results
We found that prolonged treatment of HCC78 and H1299 with ceritinib brought about 10 times weaker ceritinib sensitivity (CS) in comparison with parent cells. Additionally, the results showed that afatinib efficiently promoted CS, which was evidenced as reduced proliferation and cell death promotion, in NSCLC cells, irrespective of their previous sensitivity or resistance to ceritinib. Moreover, afatinib decreased neuregulin-1 (NRG1) signaling stimulation in CR as well as CS cells. Furthermore, supplementing NRG1 in H1299 and HCC78 cells triggered CR, which was attenuated by afatinib.
Conclusion
These results demonstrated that afatinib overcame CR in NSCLC cells with positive ALK or ROS1 by inhibiting the NRG1 signaling pathway, which might be a promising therapeutic approach.
Keywords: afatinib, ceritinib, NRG1, lung cancer, ALK/ROS1
Introduction
Lung cancer (LC) is the most prevalent malignancy worldwide; 80% of the associated mortality is attributable to non-small-cell LC (NSCLC) cell.1 Chromosomal changes or somatic mutations leading to the stimulation of protein kinase are responsible for cancer generation. Anaplastic lymphoma kinase (ALK) was the first identified fusion oncokinase, held accountable for 4%–6% of pulmonary adenocarcinomas.2 ALK rearrangements have been reported in various cancers such as NSCLC, providing a novel target for treating a proportion of NSCLCs.3 c-ros oncogene 1 (ROS1) is a member of the receptor tyrosine kinase family, which generates fusions and defines additional active oncogenic driver mutations with regard to NSCLC.4,5 Recent studies have proved that almost 1.4% of NSCLCs display rearrangements in ROS1. The discovery of selective and promising suppressors of ALK as well as ROS1 kinase has promoted research utilizing these agents as an innovative therapeutic approach to treat LC with positive ROS1 and ALK.
As the earliest second-generation ALK/ROS1 suppressor, ceritinib (LDK378) received US Food and Drug Administration approval in 2014 and is currently used in NSCLC therapy for patients resistant to crizotinib.6–8 Although ceritinib is a remarkable drug, ceritinib resistance (CR) can develop. Recent research on ceritinib has reported that 60% of patients developed CR, while their cancers displayed no obvious mutations resistant to ALK.9,10 These findings indicate the possible existence of pathways besides those targeted by second-generation ALK suppressors.
Previous studies have demonstrated the existence of an autocrine loop between HER3 and its ligand NRG3 in prostate cancer, ovarian tumor, and melanoma.11,12 Research has suggested that NRG1–EGFR axis participates in the regulation of resistance to second-generation ALK suppressors such as ceritinib.1 Consequently, the neuregulin-1 (NRG1) pathway serves as a promising target to treat NSCLC with positive ALK.
Meanwhile, afatinib, also called BIBW2992, suppresses HER2 as well as EGFR irreversibly and has been approved for the treatment of NSCLC with mutated EGFR in several countries. In two randomized Phase III trials, afatinib displayed a remarkable promotion of survival without progression in comparison to standardized doublet chemotherapy for NSCLC with mutated EGFR.13,14
Therefore, we aimed to explore whether afatinib was able to recover CS in CR cells via suppression of the NRG1 axis.
Materials and methods
Cell culture and reagents
NSCLC cells H1299 and HCC78 with positive ALK or ROS1 were bought from the American Type Culture Collection (ATCC) (Manassas, VA, USA). DMEM supplemented with 10% FBS (Thermo Fisher Scientific, Waltham, MA, USA), 100 µg/mL streptomycin, and 100 U/mL penicillin was used for culturing under 5% CO2 at 37°C. Afatinib and ceritinib were bought from Selleckchem (Houston, TX, USA). NRG1 was bought from R&D Systems, Inc. (Minneapolis, MN, USA). Cells were preserved at −20°C.
Preparation of CR cells
CR cells (HCC78R and H1299R) were cultivated by increasing supplementation of the agents (1 µM) for 3 days. Cells resistant to agents underwent expansion and recovered growth rates similar to those of parent cells. Subsequently, cells that survived received supplement of agents for 3 additional days. This procedure was repeated until growth rates with 1 µM ceritinib were similar to those without ceritinib.
Quantitative real-time PCR (qRT-PCR)
Separation of total RNA was carried out using the Mini RNA Isolation II Kit (Zymo Research, Orange, CA, USA). Total RNA (1 µg) was used to produce cDNA using the Super-Script II reverse transcriptase (Thermo Fisher Scientific). Primary denaturation of qRT-PCR was conducted as follows: 2 minutes at 94°C, 30 seconds at 94°C, 30 seconds at 58°C, and 1 minute at 72°C. After 35 amplification cycles, extra extension reactions were conducted for 10 minutes at 72°C.
MTT assay
Plates with 96 wells were used to culture 2,000 cells in 100 µL of media. Every well received an additional 10 µL of MTT agent (Sigma-Aldrich Co., St Louis, MO, USA) at 5 mg/mL concentration. The media were drained after 4 hours. To promote the lysis of formazan crystals, 150 µL of DMSO was added to every well. The absorbance was recorded at 490 nm using a Thermo Fisher Spectrophotometer 1510 (Molecular Devices LLC, Sunnyvale, CA, USA). The survival rate was calculated as a ratio of the absorbance of supplemented cells to that of the control groups. This process was repeated thrice.
Apoptosis assay
Subsequently, cells that received supplementation underwent 10 minute staining with Hoechst 33342 and were observed using UV/488 dual stimulation under an inverted fluorescence microscope (DM16000B, Leica Microsystems, Wetzlar, Germany). Fluorescence was evaluated against the emission of Hoechst 33342 dye at 460 nm. Cell death manifested as fragmented nuclei. No <200 cells were assessed from five randomly selected fields.
Western blot (WB)
Cells were obtained and underwent washing with PBS. In order to obtain total protein to carry out WB, lysis buffer with protease suppressors was used. Proteins, totally 20 µg from the lysates, were placed on a SDS-PAGE and underwent electrophoresis. Nitrocellulose membrane was utilized in order to transfer the protein blots. Blocking solution including PBS, 5% skimmed milk, and 0.1% Tween-20 was supplemented for 1 hour at room temperature. Subsequently, overnight incubation of primary antibodies (anti-EGFR, anti-Actin, as well as anti-p-EGFR; Abcam, Cambridge, MA, USA) was done at 4°C. Tris-buffered saline containing 0.05% Tween-20, used for membrane bathing, was incubated for 90 minutes with secondary antibodies at room temperature. Enhanced chemiluminescence (ECL; Pierce, Rockford, IL, USA) was used to observe protein bands.
Statistical analysis
The outcome is presented in the form of mean ± SD. One-way ANOVA or Student’s t-test was utilized to evaluate the difference. SPSS 17.0 (SPSS Inc., Chicago, IL, USA) was adopted to carry out analyses. Results of a two-tailed test were regarded as significant at P<0.05.
Results
Extended ceritinib supplemented to LC cells brought about CR
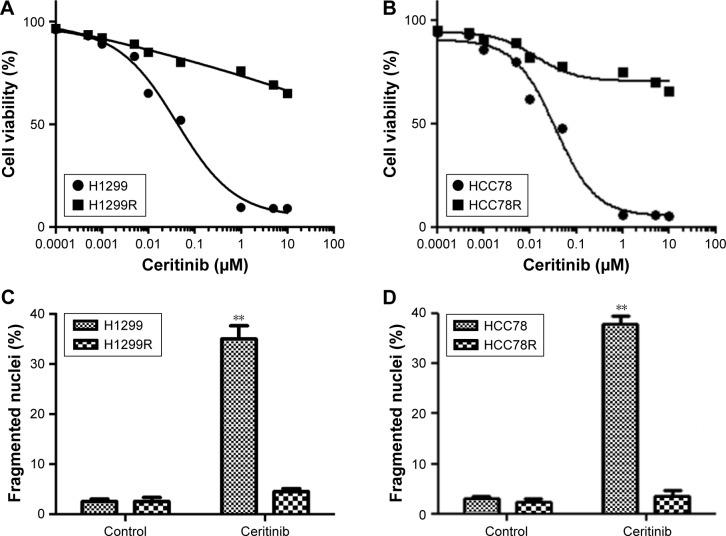
Repeated exposure to prolonged ceritinib addition was used to generate adaptive H1299 and HCC78 cells. Cells developed CR and were designated H1299R and HCC78R subsequent to intermittent ceritinib supplements (1 µM) for 3 days. The two kinds of cells were cultured with ceritinib (1 µM) to maintain resistance. CS in HCC78R and H1299R cells was lower by a factor of 10 times than that of the parent cells (Figure 1A and B). Furthermore, ceritinib supplementation triggered cell death in parent cells, a reaction thoroughly eliminated in not only HCC78R cells but also in H1299R cells (Figure 1C and D).
Figure 1.
Extended therapy of human LC cells with ceritinib induced CR.
Notes: (A) H1299R (generated by ceritinib supplement to H1299 cells) and (B) HCC78R (generated by ceritinib supplement to HCC78 cells), as well as corresponding parent cells, received the supplement of ceritinib for 4 days. MTT assay was used to evaluate the survival. (C) H1299R and (D) HCC78R, as well as corresponding parent cells, received the supplement of 500 nM ceritinib for 2 days. Calculation of fragmented as well as condensed nuclei was used to evaluate cell death. Data are presented as mean ± SD from three independent experiments. **P<0.01, ceritinib vs control.
Abbreviations: CR, ceritinib resistance; LC, lung cancer.
Afatinib efficiently promoted CS in both ceritinib-resistant and ceritinib-sensitive NSCLC cells
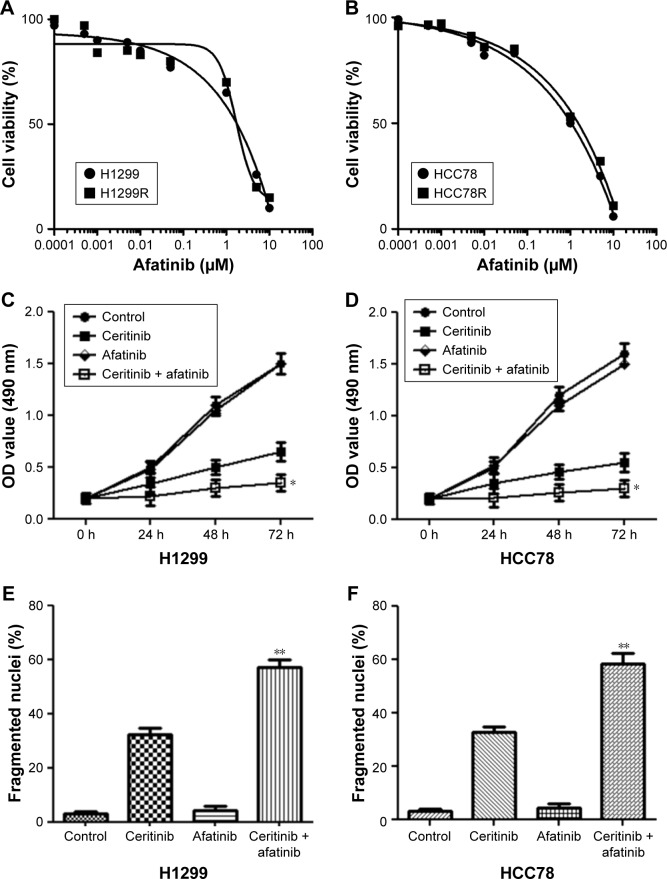
In order to explore whether afatinib was able to promote the suppressive effect of ceritinib on the proliferation of CS HCC78 and H1299 cells, the effect of afatinib on cell viability at different concentrations in both two ceritinib-resistant cells and their parental cells was detected using the MTT assay first. The results showed that afatinib at concentration <0.5 µM only gently impacted cell viability in both CR and parental cells. In contrast, high concentration of afatinib significantly suppressed the cell survival (Figure 2A and B). Supplement of ceritinib noticeably decelerated the growth of HCC87 and H1299 cells (Figure 2C and D). These cells supplemented with afatinib (200 nM) experienced CS (Figure 2C and D). Furthermore, cell death triggered by ceritinib in both parent cells was remarkably promoted by treatment with both afatinib and ceritinib together (Figure 2E and F).
Figure 2.
Afatinib promoted CS in CS LC cells.
Notes: (A) H1299R and (B) HCC78R, as well as corresponding parent cells, received supplement of afatinib for 4 days. MTT assay was used to evaluate the survival. (C) H1299 and (D) HCC78 cells received the supplement of 500 nM ceritinib, 200 nM afatinib, or both at specific time points. MTT assay was used to evaluate the survival. (E) H1299 and (F) HCC78 cells received the supplement of 500 nM ceritinib, 200 nM afatinib, or both for 2 days. Cell death was evaluated by the calculation of fragmented as well as condensed nuclei. Data are presented as mean ± SD from three independent experiments. *P<0.01 and **P<0.01, ceritinib + afatinib vs ceritinib.
Abbreviations: CS, ceritinib sensitivity; LC, lung cancer.
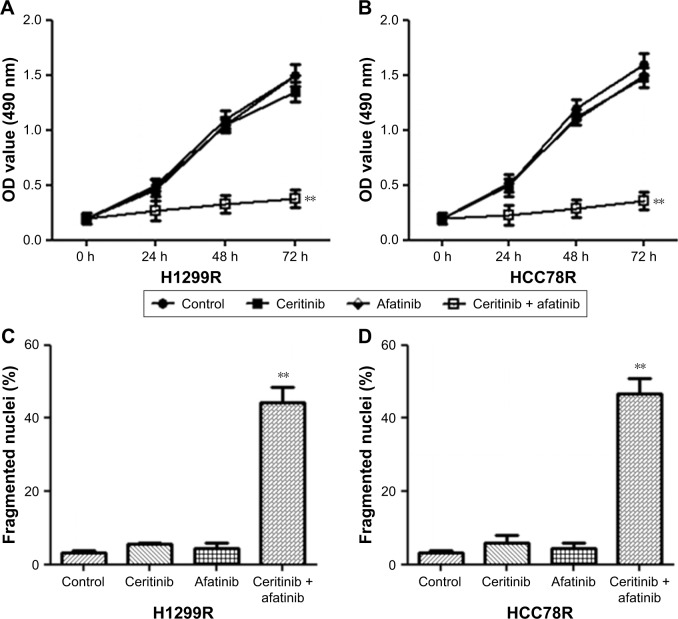
Subsequently, our research investigated whether afatinib was able to attenuate CR in LC cells. Findings from MTT assays suggested that HCC78R and H1299R cells displayed stronger CR than did parent cells. However, afatinib addition noticeably promoted the CS of both resistant cell types (Figure 3A and B). Despite the fact that afatinib failed to promote cell death in these two resistant classes of cells, the combination of afatinib and ceritinib triggered cell death iñ60% of HCC78R and H1299R cells (Figure 3C and D).
Figure 3.
Afatinib promoted CS in CR LC cells.
Notes: (A) H1299R and (B) HCC78R cells received the supplement of 500 nM ceritinib, 200 nM afatinib, or both at specific time points. MTT assay was used to evaluate survival. (C) H1299R and (D) HCC78R cells received the supplement of 500 nM ceritinib, 200 nM afatinib, or both for 2 days. Cell death was evaluated by the calculation of fragmented as well as condensed nuclei. Data are presented as mean ± SD from three independent experiments. **P<0.01, ceritinib + afatinib vs ceritinib.
Abbreviations: CS, ceritinib sensitivity; CR, ceritinib resistance; LC, lung cancer.
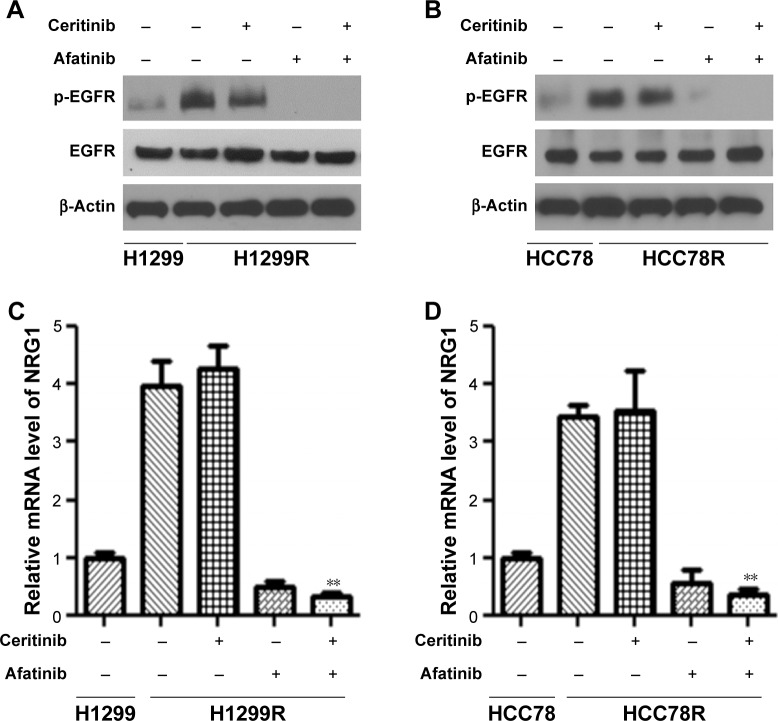
Afatinib decreased NRG1 pathway stimulation in not only CS cells but also in CR cells
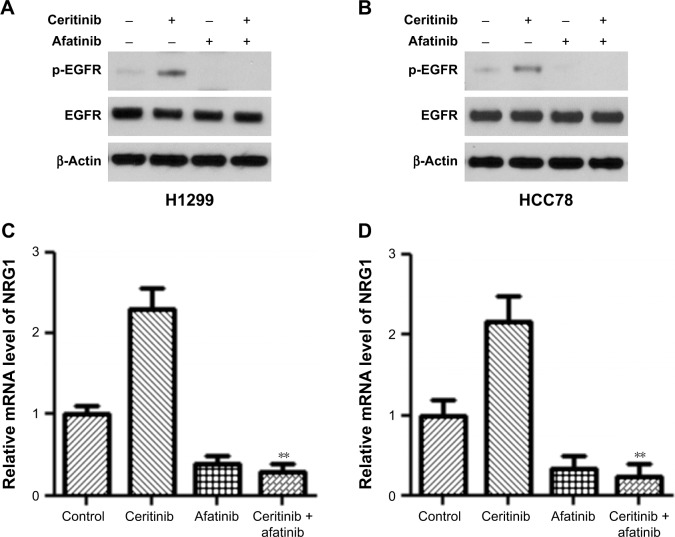
In order to illuminate how afatinib counteracted acquired CR, our research investigated the effect of afatinib on stimulation of the NRG1–EGFR pathway, which was demonstrated to participate in CR.13 First, WB was done for both CS HCC78 and H1299 cells. The results showed that afatinib reduced the phosphorylation of EGFR with or without ceritinib, while ceritinib alone promoted its phosphorylation (Figure 4A and B). Reverse transcriptase-PCR (RT-PCR) revealed that transcription of EGF-like ligand NRG1 was elevated subsequent to ceritinib addition. Afatinib remarkably reduced NRG1 transcription with or without ceritinib (Figure 4C and D).
Figure 4.
Afatinib decreased NRG1 pathway stimulation in CS LC cells.
Notes: (A) H1299 and (B) HCC78 cells, as well as corresponding parent cells, received the supplement of 500 nM ceritinib, 200 nM afatinib, or both for 2 days. WB was used to evaluate the expression of certain proteins. (C–D) RT-PCR was used to assess NRG1 transcription. Data are presented as mean ± SD from three independent experiments. **P<0.01, ceritinib + afatinib vs ceritinib.
Abbreviations: CS, ceritinib sensitivity; LC, lung cancer; WB, Western blot.
Taking into consideration the stimulation of NRG1 pathway in parent cells as well as CR cells, phosphorylation of EGFR and transcription of NRG1 were remarkably elevated in HCC78R and H1299R cells (Figure 5A–D). In conformity with ceritinib sensitivity (CS) cells, afatinib remarkably reduced the promoted phosphorylation of EGFR and transcription of NRG1 with or without ceritinib owing to the long-term supplementation of ceritinib (Figure 5A–D).
Figure 5.
Afatinib decreased NRG1 pathway stimulation in CR LC cells.
Notes: (A) H1299R and (B) HCC78R cells, as well as corresponding parent cells, received the supplement of 500 nM ceritinib, 200 nM afatinib, or both for 2 days. WB was used to evaluate the expression of certain proteins. (C–D) NRG1 transcription was assessed by RT-PCR. Data are presented as mean ± SD from three independent experiments. **P<0.01, ceritinib + afatinib vs ceritinib.
Abbreviations: CR, ceritinib resistance; LC, lung cancer; WB, Western blot.
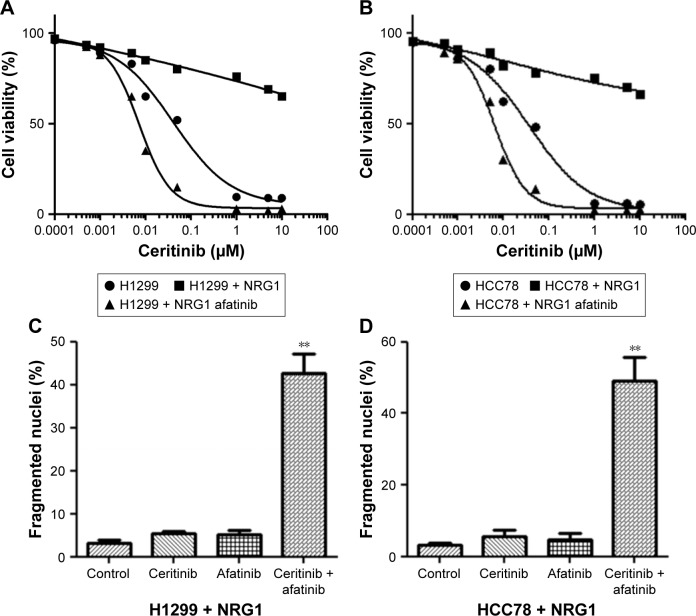
Afatinib counteracted CR triggered by NRG1 in human LC cells
Forty-eight-hour culture in medium containing NRG1 reduced the CS of HCC78 and H1299 cells, as revealed by the MTT assay, but afatinib supplement recovered CS (Figure 6A and B). Furthermore, the use of ceritinib and afatinib together remarkably triggered cell death in HCC78 and H1299 cells cultivated in media containing NRG1 (Figure 6C and D).
Figure 6.
Afatinib counteracted CR triggered by NRG1 in LC cells.
Notes: (A) H1299 and (B) HCC78 cells received the supplement of 100 ng/mL NRG1 or 100 ng/mL NGRI and 200 nM afatinib and subsequently underwent incubation with ceritinib for 4 days. MTT assay was used to evaluate the survival. (C) H1299 and (D) HCC78 cells received the supplement of 100 ng/mL of NRG1 and subsequently underwent incubation with 500 nM ceritinib, 200 nM afatinib, or both for 2 days. Cell death was evaluated by the calculation of fragmented as well as condensed nuclei. Data are presented as mean ± SD from three independent experiments. **P<0.01, ceritinib + afatinib vs ceritinib.
Abbreviations: CR, ceritinib resistance; LC, lung cancer.
Discussion
This study demonstrated that afatinib can promote CS in CS cells and counteract CR in CR cells with the help of ceritinib. The counteracting influence of afatinib on CR was because of its capability to decrease NRG1 stimulation. Additionally, we established that the combination of both ceritinib and afatinib counteracted CR in NSCLC patients with positive ROS1 or ALK.
Rearrangement of ALK usually leads to remarkable reactions to the first generation of ALK suppressors including crizotinib. However, drug resistance occurs unavoidably.15–21 Apart from progress in the second generation of ALK suppressors, adding secondary agents with crizotinib could serve as an innovative strategy to counteract CR. Recent studies have explored the effect of the combined application of crizotinib and pemetrexed or some targeted reagents like an Hsp90 suppressor in terminal NSCLC with positive ALK.22 Since resistance to the second generation of ALK suppressors including ceritinib exists,23 investigation of the promising joint therapy could suggest innovative approaches to treat NSCLC with positive ALK or ROS1 instead of relying on the successful preparation of ALK suppressors.
As a member of the second generation of pan-EGFR tyrosine kinase inhibitor, which has received FDA approval, afatinib irreversibly binds to HER4, HER2, and EGFR1,24,25 leading to persistent suppression in comparison with the first generation of TKI suppressors, such as erlotinib and gefitinib.26,27 Previous research has demonstrated that afatinib remarkably suppresses the proliferation of tumors, which excessively express either wild-type HER2 and/or EGFR1 or EGFR1 with L858R/T790M dual mutations.26,27 Furthermore, it has been proved by in vitro studies in hypo-pharyngeal cell line FaDu that afatinib suppresses growth and promotes sensitivity to radiotherapy.24,25 In our study, afatinib supplementation promoted CS in HCC78 and H1299 cells. Furthermore, in terms of HCC78R and H1299R cells, the addition of afatinib counteracted resistance. Inhibited growth as well as promoted cell death could serve as the mechanism of the combination of two agents, which was evidenced via MTT and fragmented nuclei calculation.
Throwing light upon how afatinib counteracts CR is crucial to the generation of innovative reagents to treat ALK/ROS1 NSCLC. Currently, NRG1–EGFR axis is related to CR. NRG1 concentration seems to be linked to the risk of human tumors.28 Generally, elevated levels of EGFR are linked to increased mortality.29,30 With studies available on specific suppressors of NRG1, indications that malignant cells with NRG1 expression could respond to EGFR suppressors and EGFR TKIs are able to decrease EGFR expression and downstream agents.31 One recent study also revealed that gene fusions involving the ERBB ligand gene, NRG1, might lead to the expression and presentation of the EGF-like domain of NRG1 on the cell surface, which binds to ERBB3 in an autocrine and juxtacrine manner, thus inducing the formation of ERBB2–ERBB3 heterodimers and subsequent activation of the PI3K–AKT and MAPK signaling pathways.32 We discovered that afatinib supplement decreased the stimulation of NRG1 pathway in both parent cells and CR cells, which was expected. Furthermore, afatinib counteracted resistance triggered by NRG1 in both sets of parent cells. This indicates that afatinib counteracts CR via the suppression of NRG1 pathway.
Conclusion
Our study elucidated that NRG1–HER3–EGFR participates in the regulation of CR in ALK/ROS1 NSCLC. Afatinib is able to counteract CR in ALK/ROS1 NSCLC cells by the inhibition of NRG1 axis, indicating a promising innovative approach to overcome CR in ALK/ROS1 NSCLC.
Acknowledgments
This work was supported by National Key Research and Development Program of China (grant number 2016YFC0905501 and 2016YFC0905500) and the National Natural Science Foundation of China (grant number 81772484).
Footnotes
Author contributions
HC conceived the study and designed the experiments. HC, QZ, YZ, BJ, and BZ contributed to data extraction, performed the analysis, and interpreted the results. CW wrote the first draft. HC, QY, YZ, BJ, and BZ contributed to the critical revision of the article. All authors contributed toward data analysis, drafting and critically revising the paper, gave approval of the final version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 4.Bergethon K, Shaw AT, Ou SH, Shi O, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18(3):378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 6.Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4(6):662–673. doi: 10.1158/2159-8290.CD-13-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen L, Ji HF. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370(26):2537. doi: 10.1056/NEJMc1404894. [DOI] [PubMed] [Google Scholar]
- 8.Vansteenkiste JF. Ceritinib for treatment of ALK-rearranged advanced non-small-cell lung cancer. Future Oncol. 2014;10(12):1925–1939. doi: 10.2217/fon.14.94. [DOI] [PubMed] [Google Scholar]
- 9.Toyokawa G, Inamasu E, Shimamatsu S, et al. Identification of a Novel ALK G1123S Mutation in a Patient with ALK-rearranged Non–small-cell Lung Cancer Exhibiting Resistance to Ceritinib. Journal of Thoracic Oncology. 2015;10(7):e55–e57. doi: 10.1097/JTO.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 10.Kodityal S, Elvin JA, Squillace R, et al. A novel acquired ALK F1245C mutation confers resistance to crizotinib in ALK-positive NSCLC but is sensitive to ceritinib. Lung Cancer. 2016;92:19–21. doi: 10.1016/j.lungcan.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Buac K, Xu M, Cronin J, Weeraratna AT, Hewitt SM, Pavan WJ. NRG1/ERBB3 signaling in melanocyte development and melanoma: inhibition of differentiation and promotion of proliferation. Pigment Cell Melanoma Res. 2009;22(6):773–784. doi: 10.1111/j.1755-148X.2009.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong X, Fernandez-Salas E, Li E, Wang S. Elucidation of Resistance Mechanisms to Second-Generation ALK Inhibitors Alectinib and Ceritinib in Non-Small Cell Lung Cancer Cells. Neoplasia. 2016;18(3):162–171. doi: 10.1016/j.neo.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 14.Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 15.Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18(5):1472–1482. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Akbay E, Mikse O, et al. Co-clinical trials demonstrate superiority of crizotinib to chemotherapy in ALK-rearranged non-small cell lung cancer and predict strategies to overcome resistance. Clin Cancer Res. 2014;20(5):1204–1211. doi: 10.1158/1078-0432.CCR-13-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song A, Kim TM, Kim DW, et al. Molecular Changes Associated with Acquired Resistance to Crizotinib in ROS1-Rearranged Non-Small Cell Lung Cancer. Clin Cancer Res. 2015;21(10):2379–2387. doi: 10.1158/1078-0432.CCR-14-1350. [DOI] [PubMed] [Google Scholar]
- 18.Casaluce F, Sgambato A, Sacco PC, et al. Resistance to Crizotinib in Advanced Non-Small Cell Lung Cancer (NSCLC) with ALK Rearrangement: Mechanisms, Treatment Strategies and New Targeted Therapies. Curr Clin Pharmacol. 2016;11(2):77–87. doi: 10.2174/1574884711666160502124134. [DOI] [PubMed] [Google Scholar]
- 19.Cuyàs E, Pérez-Sánchez A, Micol V, Menendez JA, Bosch-Barrera J. STAT3-targeted treatment with silibinin overcomes the acquired resistance to crizotinib in ALK-rearranged lung cancer. Cell Cycle. 2016;15(24):3413–3418. doi: 10.1080/15384101.2016.1245249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong E, Huang H. Crizotinib in ROS1 rearranged non-small cell lung cancer (NSCLC), from response to resistance. BMJ Case Rep. 2016;2016:bcr2016217322. doi: 10.1136/bcr-2016-217322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Jiang T, Li X, et al. Clinical features of Bim deletion polymorphism and its relation with crizotinib primary resistance in Chinese patients with ALK/ROS1 fusion-positive non-small cell lung cancer. Cancer. 2017;123(15):2927–2935. doi: 10.1002/cncr.30677. [DOI] [PubMed] [Google Scholar]
- 22.Iacono D, Chiari R, Metro G, et al. Future options for ALK-positive non-small cell lung cancer. Lung Cancer. 2015;87(3):211–219. doi: 10.1016/j.lungcan.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Toyokawa G, Inamasu E, Shimamatsu S, et al. Identification of a Novel ALK G1123S Mutation in a Patient with ALK-rearranged Non-small-cell Lung Cancer Exhibiting Resistance to Ceritinib. J Thorac Oncol. 2015;10(7):e55–e57. doi: 10.1097/JTO.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 24.Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27(34):4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther. 2012;343(2):342–350. doi: 10.1124/jpet.112.197756. [DOI] [PubMed] [Google Scholar]
- 26.Ferrarotto R, Gold KA. Afatinib in the treatment of head and neck squamous cell carcinoma. Expert Opin Investig Drugs. 2014;23(1):135–143. doi: 10.1517/13543784.2014.858696. [DOI] [PubMed] [Google Scholar]
- 27.Modjtahedi H, Cho BC, Michel MC, Solca F. A comprehensive review of the preclinical efficacy profile of the ErbB family blocker afatinib in cancer. Naunyn Schmiedebergs Arch Pharmacol. 2014;387(6):505–521. doi: 10.1007/s00210-014-0967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheng Q, Liu X, Fleming E, et al. An activated ErbB3/NRG1 autocrine loop supports in vivo proliferation in ovarian cancer cells. Cancer Cell. 2010;17(3):298–310. doi: 10.1016/j.ccr.2009.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen HJ, Yan HH, Yang JJ, et al. Disease flare after EGFR tyrosine kinase inhibitor cessation predicts poor survival in patients with non-small cell lung cancer. Pathol Oncol Res. 2013;19(4):833–838. doi: 10.1007/s12253-013-9651-z. [DOI] [PubMed] [Google Scholar]
- 30.Traynor AM, Weigel TL, Oettel KR, et al. Nuclear EGFR protein expression predicts poor survival in early stage non-small cell lung cancer. Lung Cancer. 2013;81(1):138–141. doi: 10.1016/j.lungcan.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakaoku T, Tsuta K, Ichikawa H, et al. Druggable oncogene fusions in invasive mucinous lung adenocarcinoma. Clin Cancer Res. 2014;20(12):3087–3093. doi: 10.1158/1078-0432.CCR-14-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez-Cuesta L, Thomas RK. Molecular Pathways: Targeting NRG1 Fusions in Lung Cancer. Clin Cancer Res. 2015;21(9):1989–1994. doi: 10.1158/1078-0432.CCR-14-0854. [DOI] [PubMed] [Google Scholar]