Abstract
Background
The association between metabolic syndrome (MS) and bladder cancer (BC) was not fully investigated, and most primary studies and pooled analyses were only focused on certain specific components.
Objective
To further investigate this issue and obtain more precise findings, we conducted this updated evidence synthesis of published studies, which involved not only MS components but also the MS in its entirety.
Materials and methods
We searched the PubMed, EMBASE, and Web of Science databases for observational studies on the association between BC susceptibility and/or mortality, and MS and its components. We extracted data from included studies, evaluated heterogeneity, and performed meta-analytic quantitative syntheses.
Results
A total of 95 studies with 97,795,299 subjects were included in the present study. According to the results, MS significantly increased the risk of BC (risk ratio [RR]=1.11, 95% CI=1.00–1.23); diabetes significantly increased the risk of BC (RR=1.29, 95% CI=1.19–1.39) and associated with poor survival (RR=1.24, 95% CI=1.08–1.43). Excessive body weight was associated with increased susceptibility (RR=1.07, 95% CI=1.02–1.12), recurrence (RR=1.46, 95% CI=1.18–1.81), and mortality (RR=1.17, 95% CI=1.00–1.37). As indicated by cumulative meta-analysis, sample size was inadequate for the association between BC susceptibility and MS, the association between BC recurrence and excessive body weight, and the association between BC survival and diabetes. The sample size of the meta-analysis was enough to reach a stable pooled effect for other associations.
Conclusion
Diabetes and excessive body weight as components of MS are associated with increased susceptibility and poor prognosis of BC. Uncertainty remains concerning the impact of overall MS, hypertension, and dyslipidemia on BC susceptibility and prognosis, for which further investigations are needed.
Keywords: metabolic syndrome, bladder cancer, diabetes, excessive body weight, susceptibility, prognosis, meta-analysis, cumulative meta-analysis
Introduction
Metabolic syndrome (MS) is defined by a collection of biochemical and physiologic abnormalities associated with the development of cardiovascular disease (CVD) and type 2 diabetes. Abdominal obesity, atherogenic dyslipidemia, high blood pressure, and insulin resistance are major components of MS.1 According to the US National Health and Nutrition Examination Survey data, the overall MS prevalence among adults was 21.8% during 1988–1994, and it had increased up to 34.7% by the end of 2012. The prevalence of MS also increases with age, from 18.3% among those aged 20–39 years to 46.7% among those aged 60 years or more. Almost half of the US population will be diagnosed of MS throughout their life as the population-aging continues.2,3 The MS was previously identified as a strong contributor to cardiovascular morbidity and mortality; in addition, it has also been recognized as a potential etiologic factor for the development and progression of multiple types of cancers.4 Bladder cancer (BC) is the most common malignancy that affects the urinary tract. Evidence from epidemiologic investigations, clinical studies, and pooled analyses suggests that the MS may increase the risk, recurrence, and mortality of BC. However, most primary studies and pooled analyses only focused on certain specific MS components, such as diabetes and excessive body weight, but not the overall MS; as a result, the impact of MS on the carcinogenesis and prognosis of BC patients remains unclear. On the other hand, the stability of pooled effects (robustness to inclusion of additional studies), which could be investigated using cumulative meta-analysis, was left unexamined in previous meta-analysis. Therefore, in order to further investigate this issue and obtain more precise findings, an updated summarization of published studies, which integrates pooled analysis and stability examination and involves not only MS components but also the MS in its entirety, needs to be performed. To this end, we conducted the present comprehensive evidence synthesis incorporating pooled analysis and cumulative meta-analysis, to summarize all published studies to date, concerning the association between MS, its components, and BC in terms of susceptibility and prognosis.
Materials and methods
Literature search strategy
To identify observational studies on the association between BC risk and prognosis, we searched literature databases including the PubMed, EMBASE, and Web of Science. All possible combinations of the following search terms were used for the search: “bladder cancer”, “urothelial carcinoma”, “metabolic syndrome”, “diabetes”, “overweight”, “obesity”, “hypertension”, “dyslipidemia”, “risk”, “susceptibility”, “survival”, “recurrence”, and “prognosis”. The time limit for the search was between the establishment of the database and 31 March 2018. No language limits were applied to the search. The references of previous meta-analyses on similar topics were also screened for potential relevant studies.
Study selection and data extraction
Eligible studies were selected according to the predefined selection criteria. Included studies should be observational studies and provided data on the association between BC susceptibility and/or survival, and MS or any of its known components. Two authors independently reviewed the literature search results and selected studies for inclusion. Any discrepancies were resolved by discussion with a third author.
The following data were extracted from the included studies: surname of the first author, year of publication, country of origin, research design, sample size, exposures examined (MS and/or its components), outcomes analyzed (susceptibility and/or prognosis), and data of effect sizes measuring the association between exposures and outcomes. Two authors independently conducted data extraction, and any discrepancies were resolved by discussion or consulting with a third author.
Statistical analysis
Quantitative evidence syntheses using a fixed effects or random effects meta-analytic model were performed. When significant heterogeneity among studies was detected, a fixed effects model was used, otherwise a random effects model was used. Leave-one-out sensitivity analysis was performed to identify influential studies for a given meta-analysis. Subgroup analysis by gender was performed concerning the association between MS and BC susceptibility, and subgroup analysis by degree of excessive body weight (overweight vs obesity) was performed for the association between excessive body weight and BC risk, recurrence, and mortality. Z-test was performed to examine if the difference between pooled effects across subgroups was significant. Cumulative meta-analyses by chronologic order were performed to examine if a stable pooled effect was reached in meta-analysis. Publication bias was investigated by funnel graphs and Egger’s test, and trim-and-fill analysis was performed when significant publication bias was detected.
Results
Basic characteristics of included studies
The primary literature search identified 884 publications. After comprehensive screening according to the selection criteria, a total of 95 studies were included in the present evidence synthesis. Among the included studies, 74 were cohort studies, 16 were case–control studies, and five were cross-sectional studies. The basic characteristics of the included studies are shown in Table S1.
Association between MS and BC susceptibility and prognosis
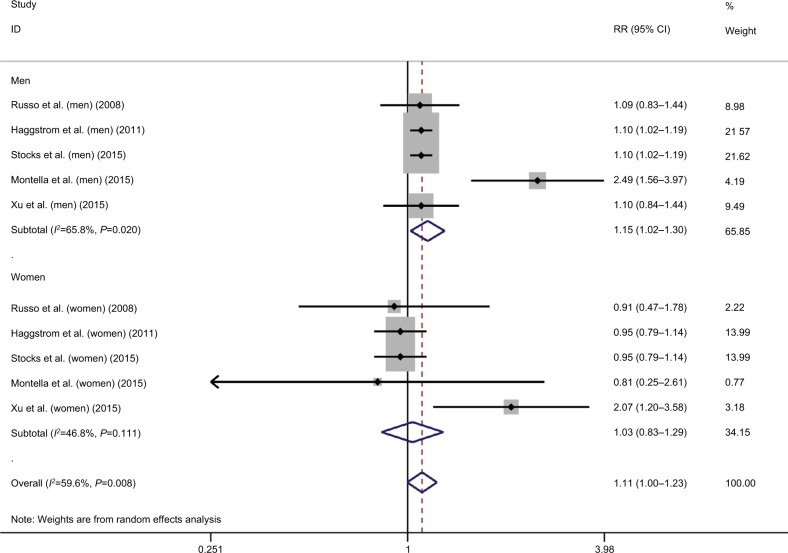
As to the correlation between overall MS and susceptibility of BC, a total of five studies were included, among which significant heterogeneity was detected (I2=59.6, P=0.008).5–9 Based on the results of pooled analysis, MS significantly increased the risk of BC (risk ratio [RR]=1.11, 95% CI=1.00–1.23; Figure 1). Sensitivity analysis showed that the study by Montella et al was influential and was a potential source of observed heterogeneity.8 According to the subgroup analysis by gender, MS significantly increased the incidence of BC in males (RR=1.15, 95% CI=1.02–1.30; Figure 1), but not in females (RR=1.03, 95% CI=0.83–1.29; Figure 1). However, the observed subgroup difference was not statistically significant (Z=0.88, P=0.38).
Figure 1.
Meta-analysis on the association between bladder cancer susceptibility and metabolic syndrome.
Abbreviation: RR, risk ratio.
Only one study that investigated the association between MS and BC mortality was found, in which a strong positive correlation between MS and BC mortality was reported (RR=4.03, 95% CI=2.03–8.01).10
According to the results of cumulative meta-analysis by year of publication, stable pooled effects were not yet reached in both overall meta-analysis and subgroup analysis by gender, regarding the association between MS and BC susceptibility (Figure S1).
Association between diabetes and BC susceptibility and prognosis
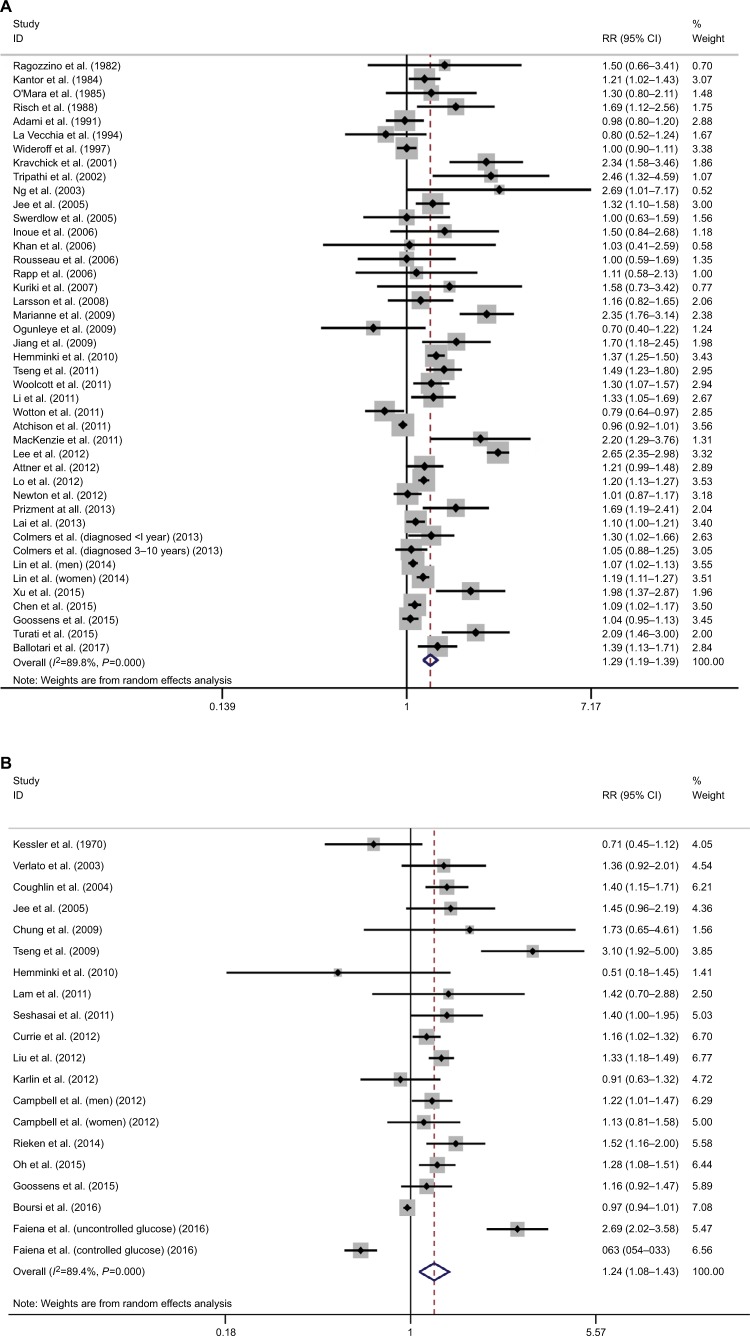
In terms of the correlation between diabetes and risk of BC, a total of 41 studies were included, among which significant heterogeneity was detected (I2=89.8%; P<0.001).11–51 Based on the results of pooled analysis, diabetes significantly increased the risk of BC (RR=1.29, 95% CI=1.19–1.39; Figure 2A). Sensitivity analysis revealed that the study by Lee et al was influential, which was a potential source of observed heterogeneity.39
Figure 2.
Meta-analysis on the association between bladder cancer susceptibility (A), mortality (B), and diabetes.
Abbreviation: RR, risk ratio.
In terms of the correlation between diabetes and mortality of BC patients, a total of 18 studies were included.21,32,49,52–67 Significant heterogeneity was detected (I2=89.4%; P<0.001). The results of pooled analysis showed that diabetes was significantly associated with poor survival in BC patients (RR=1.24, 95% CI=1.08–1.43; Figure 2B). Sensitivity analysis revealed that the studies by Faiena et al66 and Tseng et al55 were influential, as potential sources of observed heterogeneity.
According to the results of cumulative meta-analysis by year of publication, a stable pooled effect was reached in meta-analysis regarding the association between diabetes and BC susceptibility (Figure S2A), but not the association between diabetes and BC mortality (Figure S2B).
Association between excessive body weight and BC susceptibility and prognosis
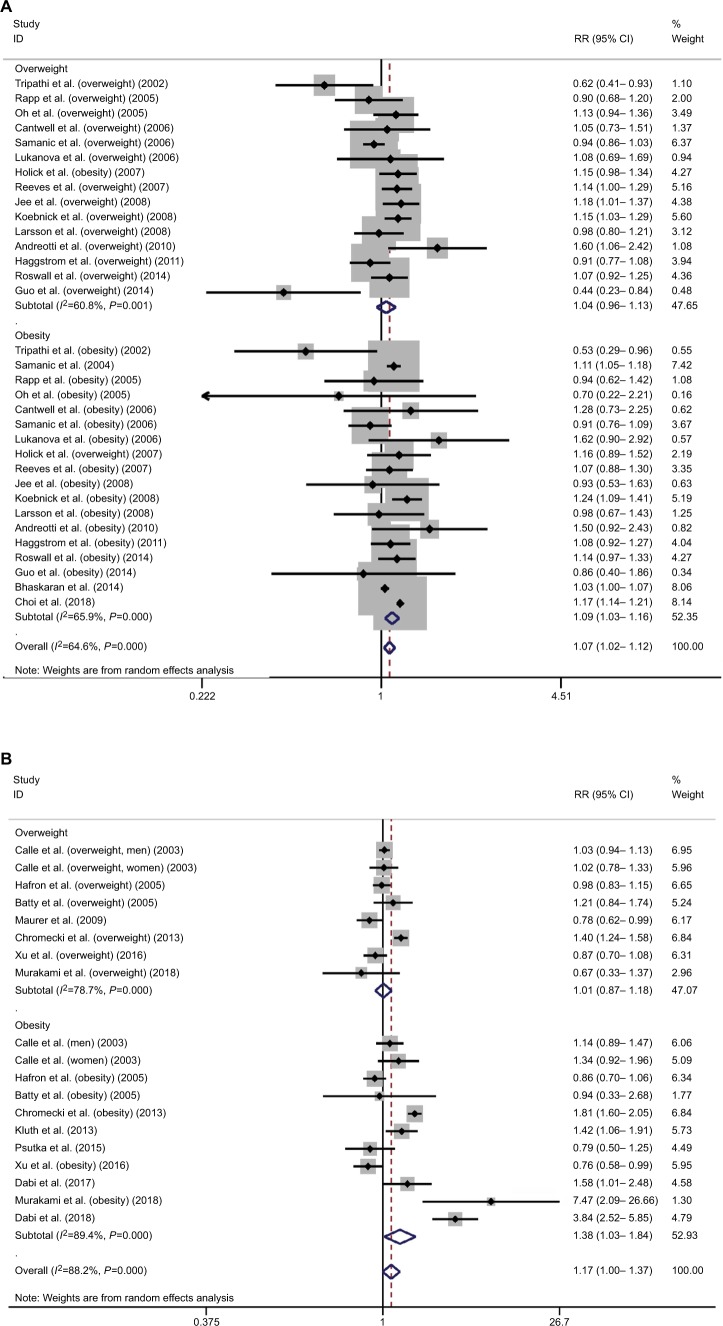
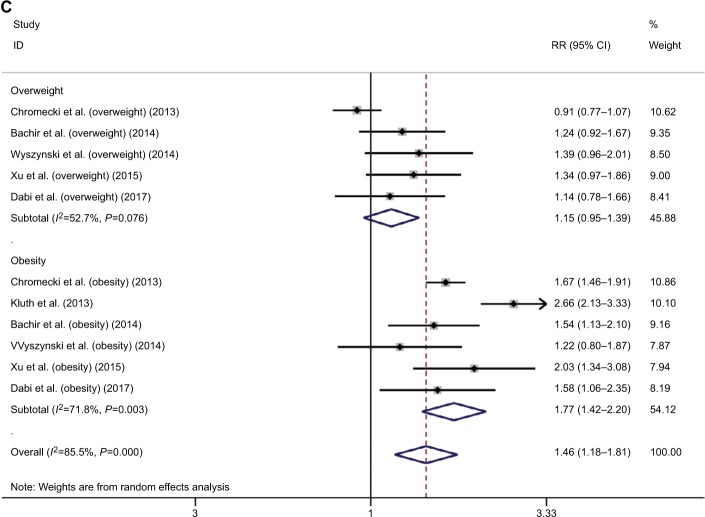
In terms of the association between excessive body weight and risk of BC, a total of 18 studies were included in the overall meta-analysis of both overweight and obesity, among which significant heterogeneity was detected (s=64.6, P<0.001).6,19,28,68–82 Excessive body weight was significantly associated with increased risk of BC (RR=1.07, 95% CI=1.02–1.12; Figure 3A). Sensitivity analysis revealed that the studies by Samanic et al72, Koebnick et al77, and Häggström et al6 were influential and were potential sources of observed heterogeneity. The overall meta-analysis investigating the association between excessive body weight and BC recurrence indicated increased risk of recurrence in BC patients with excessive body weight (RR=1.46, 95% CI=1.18–1.81; Figure 3B). Significant heterogeneity was detected (I2=85.5, P<0.001) among the included 11 studies.83–93 Sensitivity analysis revealed that the study by Kluth et al was influential, a potential source of observed heterogeneity.90 The overall meta-analysis investigating association between excessive body weight and BC survival indicated unfavorable survival of BC patients with excessive body weight (RR=1.17, 95% CI=1.00–1.37; Figure 3C). Significant heterogeneity was detected (I2=88.2, P<0.001) among the included six studies.87,90,92,94–96 Sensitivity analysis revealed that the studies by Chromecki et al and Dabi et al were influential and were potential sources of observed heterogeneity.87,92
Figure 3.
Meta-analysis on the association between bladder cancer susceptibility (A), recurrence (B), mortality (C), and excessive body weight.
Abbreviation: RR, risk ratio.
According to subgroup analysis by the degree of excessive body weight, overweight did not have a significant impact on the susceptibility of BC (RR=1.04, 95% CI=0.96–1.13), but obesity was significantly associated with increased risk of BC (RR=1.09, 95% CI=1.03–1.16). Similar results were revealed in subgroup analysis for prognosis. Overweight did not have a significant impact on recurrence and mortality of BC (recurrence: RR=1.15, 95% CI=0.95–1.39; mortality: RR=1.01, 95% CI=0.87–1.18), but obesity was associated with an increased recurrence risk and poor survival in BC patients (recurrence: RR=1.46, 95% CI=1.18–1.81; mortality: RR=1.38, 95% CI=1.03–1.84). However, the observed subgroup differences were all statistically nonsignificant (susceptibility: Z=0.93, P=0.35; recurrence: Z=1.64, P=0.10; mortality: Z=1.88, P=0.06). Significant heterogeneity was detected in each subgroup analysis (Figure 3A–C).
According to the results of cumulative analysis by chronologic order, a stable pooled effect was reached in meta-analysis regarding the association between excessive body weight and BC susceptibility (Figure S3A) and subgroup analysis by severity (Figure S3B). In contrast, instability was detected for recurrence (Figure S3C, D). An almost stable pooled effect was reached in meta-analysis for mortality (Figure S3E, F).
Association between hypertension, dyslipidemia, and BC susceptibility and prognosis
A quantitative evidence synthesis regarding the association between hypertension and BC risk or mortality cannot be performed due to limited number of relevant studies. Stocks’ study revealed that every 10 mmHg blood pressure increment brought significant increase in BC susceptibility and mortality in males (susceptibility: RR=1.12, 95% CI=1.04–1.21; mortality: RR=1.26, 95% CI=1.05–1.51); in contrast, similar effects were not observed among females (susceptibility: RR=0.95, 95% CI=0.81–1.11; mortality: RR=1.14, 95% CI=0.82–1.59).97 Batty’s study reported no significant association between increased systolic or diastolic blood pressure BC (high systolic blood pressure: RR=1.06, 95% CI=0.96–1.18; high diastolic blood pressure: RR=1.08, 95% CI=0.92–1.27).98 In Tai’s study, investigators reported a nonsignificant association between hypertension and BC recurrence (HR=1.3, 95% CI=0.88–1.93).99
Concerning the association between dyslipidemia and BC, only two studies were identified in our literature search, in which inconsistent findings were reported. A retrospective study with 2,070 Chinese participants revealed a non-significant correlation between hypertriglyceridemia and BC susceptibility (adjusted OR=1.3, 95% CI=0.88–1.93).9 However, another study by Stocks et al reported a significant association between hypertriglyceridemia and both BC susceptibility (HR=1.18, 95% CI=1.06–1.32) and mortality (HR=1.72, 95% CI=1.39–2.12).7
Publication bias
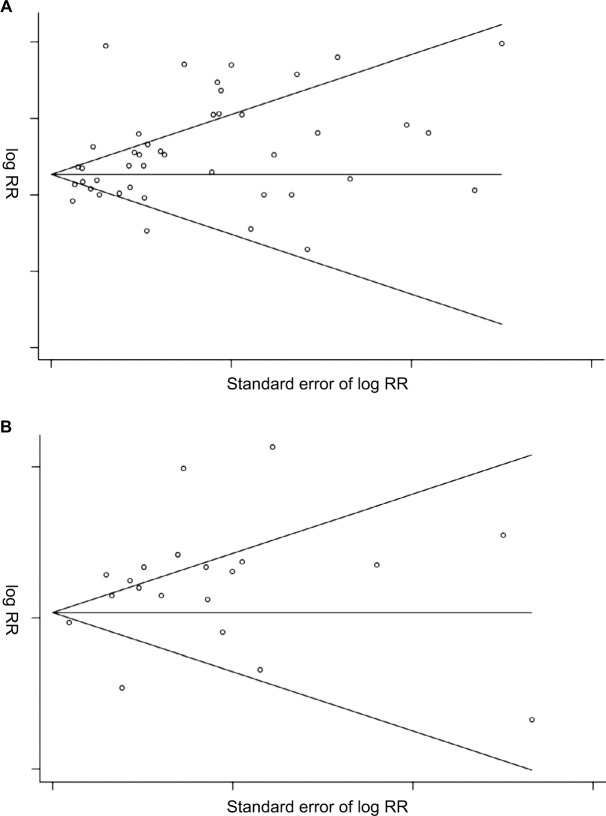
No significant publication bias was detected except for the meta-analyses regarding the association between diabetes and BC susceptibility (P=0.015; Figure 4A) and mortality (P=0.036; Figure 4B). The trim-and-fill adjusted pooled RR and 95% CI was 1.28 (1.19–1.38) and 1.11 (0.97–1.28), respectively.
Figure 4.
Funnel plot for the meta-analyses regarding the association between bladder cancer susceptibility (A), mortality (B), and diabetes.
Abbreviation: RR, risk ratio.
Discussion
The prevalence of MS continues to increase worldwide. According to the US National Cholesterol Education Program’s Adult Treatment Panel III report, MS has been identified as a multiplex risk factor for CVD that deserves more clinical attention.1 On the other hand, we should also spare attention on the probable association between MS and BC, which is a common and costly malignancy.100 In this comprehensive evidence synthesis of 95 studies involving 97,795,299 participants, we confirmed that MS and its components did confer contributing effects on the development and mortality of BC. In a Northern Italy prospective cohort of 407,157 subjects in the Reggio Emilia diabetes registry project, diabetes was associated with increases in the incidence of all cancers, including BC.101 Our pooled analysis also reported a positive association between diabetes and BC. Our findings indicated that diabetes was the strongest single risk factor among the components of MS, which significantly increased the risk of BC and overall mortality. Our findings were similar to those reported in Fang et al’s meta-analysis and identified a stronger association compared with Xu et al’s meta-analysis.102,103 The underlying mechanisms between this association have been proposed. First, insulin may play a role as growth factor by exerting mitosis-promoting effects. Insulin binds and activates the insulin-like growth factor-1 (IGF-1) receptor and triggers the downstream pathways having potent mitogenic and transforming activity.104 Increased insulin and IGF-1 in patients with MS may contribute to cancer progression and facilitate the growth of tumors by binding to the overexpressed insulin receptor in many cancers.105 In addition, an association between insulin resistance and aberrant level of proinflammatory cytokine tumor necrosis factor a, which may induce development and progression of many tumors, has been reported.106,107 This could explain the increased cancer risk in adults with type 2 diabetes.108,109 Second, diabetes was associated with mitochondrial malfunction, which will lead to insufficient DNA repair. Moreover, mitochondria malfunction will increase the production of ROS, raising oxidative stress.110 Third, diabetes, especially under conditions of poor metabolic control, causes a permanent proinflammatory state. This will consume intracellular antioxidant capacity, predisposing susceptible cells to carcinogenesis and cancer progression.111 This could explain why uncontrolled diabetes was significantly associated with higher overall mortality, as reported in both Tai and Faiena’s studies.66,99 On the other hand, it has been suggested that antidiabetic drugs, such as metformin, may influence cancer risk among diabetic patients.108
Obesity has been previously reported to be associated with a higher incidence and mortality of cancer.112,113 In the present meta-analysis, we observed a positive correlation between obesity and BC risk, BC survival, and recurrence; in contrast, overweight was not significantly associated with BC risk, BC survival, or recurrence. Overall, it seems that there was a positive correlation between body weight and BC risk, jBC survival or recurrence, with a possible dose–response effect. Similar to our findings, previous meta-analyses had revealed that each 1 kg/m2 increase in body mass index (BMI) was related to a 1.3% increase in risk of BC recurrence and each 5 kg/m2 increase in BMI was related to a 4.2% increase in BC incidence.114,115 A meta-analysis by Qin et al also confirmed that obesity was associated with an increased risk for BC, which was consistent with our results.116 However, a meta-analysis by Lin et al reported that obesity was not significantly associated with BC overall survival, which was partially different from our observations.115 The mechanism for the potential effects of excessive body weight on the BC risk and outcome has not been fully clarified. A frequently proposed hypothesis is that excessive body weight may cause metabolic and hormone changes, including hyperinsulinemia, leptin level elevation, and adiponectin reduction, which may promote carcinogenesis and progression of certain cancers.117,118 Moreover, obesity is generally accompanied by unrestrained diet and physical inactivity, and previous studies have proved that excessive calorie intake and physical inactivity may influence cancer development and progression.113
Unlike obesity and diabetes, the role of hypertension and hyperlipidemia in BC development and progression has not been well investigated. Existing studies concerning hypertension and BC have not reached an unified conclusion. In Stocks et al’s study, blood pressure increment was significantly associated with BC incidence and mortality in men, whereas other studies did not find a significant association between hypertension and BC. Interestingly, even Stocks et al did not find the same correlation in women.97–99 A link between hypertension and cancer may be mediated via proliferative abnormalities in vascular smooth muscle cells. However, it needs more proof to clarify the correlation between hypertension and BC, and the underlying mechanism as well.119 A case–control study in China revealed that hypertriglyceridemia was significantly associated with BC risk, while there was no positive correlation between low high-density lipoprotein-cholesterol and BC risk.120 The association between hypertriglyceridemia and BC also needs further investigations.
In our meta-analysis, we found overall that MS significantly increased the risk and mortality of BC, but this effect was only observed among male subjects. This finding is consistent with those of Esposito et al’s and Cantiello et al’s meta-analyses, which also indicated that MS was associated with higher risk of BC in men.4,121 However, Cantiello et al’s work reported a nonsignificant association between MS and BC prognosis, and this was different from our observation.121
For the first time we investigated whether the sample size was large enough to support conclusions with confidence. We need additional samples (primary studies) for the association between BC susceptibility and MS, the association between BC recurrence and excessive body weight, and the association between BC survival and diabetes. The sample size was adequate to reach a stable pooled effect regarding other associations. To the best of our knowledge, the present study is the most recent evidence synthesis connecting BC and MS, which included nearly 100 studies with nearly 100,000,000 participants, examined the overall MS and individual components, evaluated both susceptibility and prognosis, and explored adequacy of sample size by cumulative meta-analysis; however, our work also has certain limitations. First, significant heterogeneity was detected for most of the pooled analysis. In addition to the effect by influential studies identified by sensitivity analysis, the variation in race, sample size, and study design could also be the potential sources of the observed heterogeneity. Second, the publication bias was detected for certain analyses, and we performed trim- and-fill adjustment as complementary information.
Conclusions
Certain components of MS, that is, diabetes and excessive body weight, are associated with increased susceptibility and poor prognosis of BC. Uncertainty remains concerning the impact of overall MS, hypertension, and dyslipidemia on BC susceptibility and prognosis, for which further investigations are needed.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Grundy SM, Brewer HB, Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among us adults: findings from the third national health and nutrition examination survey. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 3.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313(19):1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 4.Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35(11):2402–2411. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo A, Autelitano M, Bisanti L. Metabolic syndrome and cancer risk. Eur J Cancer. 2008;44(2):293–297. doi: 10.1016/j.ejca.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Häggström C, Stocks T, Rapp K, et al. Metabolic syndrome and risk of bladder cancer: prospective cohort study in the metabolic syndrome and cancer project (Me-Can) Int J Cancer. 2011;128(8):1890–1898. doi: 10.1002/ijc.25521. [DOI] [PubMed] [Google Scholar]
- 7.Stocks T, Bjørge T, Ulmer H, et al. Metabolic risk score and cancer risk: pooled analysis of seven cohorts. Int J Epidemiol. 2015;44(4):1353–1363. doi: 10.1093/ije/dyv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montella M, di Maso M, Crispo A, et al. Metabolic syndrome and the risk of urothelial carcinoma of the bladder: a case-control study. BMC Cancer. 2015;15:720. doi: 10.1186/s12885-015-1769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu S, Zhang GM, Guan FJ, et al. The association between metabolic syndrome and the risk of urothelial carcinoma of the bladder: a case-control study in China. World J Surg Oncol. 2015;13(1):236. doi: 10.1186/s12957-015-0631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sha N, Xu H, Chen T, et al. The evaluation of the association between the metabolic syndrome and tumor grade and stage of bladder cancer in a Chinese population. Onco Targets Ther. 2016;9:1175–1179. doi: 10.2147/OTT.S102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ragozzino M, Melton LJ, Chu CP, Palumbo PJ. Subsequent cancer risk in the incidence cohort of Rochester, Minnesota, residents with diabetes mellitus. J Chronic Dis. 1982;35(1):13–19. doi: 10.1016/0021-9681(82)90025-x. [DOI] [PubMed] [Google Scholar]
- 12.Kantor AF, Hartge P, Hoover RN, Narayana AS, Sullivan JW, Fraumeni JF. Urinary tract infection and risk of bladder cancer. Am J Epidemiol. 1984;119(4):510–515. doi: 10.1093/oxfordjournals.aje.a113768. [DOI] [PubMed] [Google Scholar]
- 13.O’Mara BA, Byers T, Schoenfeld E. Diabetes mellitus and cancer risk: a multisite case-control study. J Chronic Dis. 1985;38(5):435–441. doi: 10.1016/0021-9681(85)90139-0. [DOI] [PubMed] [Google Scholar]
- 14.Risch HA, Burch JD, Miller AB, Hill GB, Steele R, Howe GR. Dietary factors and the incidence of cancer of the urinary bladder. Am J Epidemiol. 1988;127(6):1179–1191. doi: 10.1093/oxfordjournals.aje.a114911. [DOI] [PubMed] [Google Scholar]
- 15.Adami HO, Mclaughlin J, Ekbom A, et al. Cancer risk in patients with diabetes mellitus. Cancer Causes Control. 1991;2(5):307–314. doi: 10.1007/BF00051670. [DOI] [PubMed] [Google Scholar]
- 16.La Vecchia C, Negri E, Franceschi S, D’Avanzo B, Boyle P. A case-control study of diabetes mellitus and cancer risk. Br J Cancer. 1994;70(5):950–953. doi: 10.1038/bjc.1994.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wideroff L, Gridley G, Mellemkjaer L, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89(18):1360–1365. doi: 10.1093/jnci/89.18.1360. [DOI] [PubMed] [Google Scholar]
- 18.Kravchick S, Gal R, Cytron S, et al. Increased incidence of diabetes mellitus in the patients with transitional cell carcinoma of urinary bladder. Pathol Oncol Res. 2001;7(1):56–59. doi: 10.1007/BF03032606. [DOI] [PubMed] [Google Scholar]
- 19.Tripathi A, Folsom AR, Anderson KE, Iowa Women’s Health Study Risk factors for urinary bladder carcinoma in postmenopausal women. The Iowa Women’s Health Study. Cancer. 2002;95(11):2316–2323. doi: 10.1002/cncr.10975. [DOI] [PubMed] [Google Scholar]
- 20.Ng Y, Husain I, Waterfall N. Diabetes mellitus and bladder cancer--an epidemiological relationship? Pathol Oncol Res. 2003;9(1):30–31. doi: 10.1007/BF03033711. [DOI] [PubMed] [Google Scholar]
- 21.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293(2):194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 22.Swerdlow AJ, Laing SP, Qiao Z, et al. Cancer incidence and mortality in patients with insulin-treated diabetes: a UK cohort study. Br J Cancer. 2005;92(11):2070–2075. doi: 10.1038/sj.bjc.6602611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med. 2006;166(17):1871–1877. doi: 10.1001/archinte.166.17.1871. [DOI] [PubMed] [Google Scholar]
- 24.Khan M, Mori M, Fujino Y, et al. Site-specific cancer risk due to diabetes mellitus history: evidence from the Japan Collaborative Cohort (JACC) Study. Asian Pac J Cancer Prev. 2006;7(2):253–259. [PubMed] [Google Scholar]
- 25.Rousseau MC, Parent ME, Pollak MN, Siemiatycki J. Diabetes mellitus and cancer risk in a population-based case-control study among men from Montreal, Canada. Int J Cancer. 2006;118(8):2105–2109. doi: 10.1002/ijc.21600. [DOI] [PubMed] [Google Scholar]
- 26.Rapp K, Schroeder J, Klenk J, et al. Fasting blood glucose and cancer risk in a cohort of more than 140,000 adults in Austria. Diabetologia. 2006;49(5):945–952. doi: 10.1007/s00125-006-0207-6. [DOI] [PubMed] [Google Scholar]
- 27.Kuriki K, Hirose K, Tajima K. Diabetes and cancer risk for all and specific sites among Japanese men and women. Eur J Cancer Prev. 2007;16(1):83–89. doi: 10.1097/01.cej.0000228404.37858.40. [DOI] [PubMed] [Google Scholar]
- 28.Larsson SC, Andersson SO, Johansson JE, Wolk A. Diabetes mellitus, body size and bladder cancer risk in a prospective study of Swedish men. Eur J Cancer. 2008;44(17):2655–2660. doi: 10.1016/j.ejca.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Ulcickas Yood M, Oliveria SA, Campbell UB, Koro CE. Incidence of cancer in a population-based cohort of patients with type 2. Diabetes. 2009;3:12–16. [Google Scholar]
- 30.Ogunleye AA, Ogston SA, Morris AD, Evans JM. A cohort study of the risk of cancer associated with type 2 diabetes. Br J Cancer. 2009;101(7):1199–1201. doi: 10.1038/sj.bjc.6605240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang X, Castelao JE, Groshen S, et al. Urinary tract infections and reduced risk of bladder cancer in Los Angeles. Br J Cancer. 2009;100(5):834–839. doi: 10.1038/sj.bjc.6604889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hemminki K, Li X, Sundquist J, Sundquist K. Risk of cancer following hospitalization for type 2 diabetes. Oncologist. 2010;15(6):548–555. doi: 10.1634/theoncologist.2009-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tseng CH. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia. 2011;54(8):2009–2015. doi: 10.1007/s00125-011-2171-z. [DOI] [PubMed] [Google Scholar]
- 34.Woolcott CG, Maskarinec G, Haiman CA, Henderson BE, Kolonel LN. Diabetes and urothelial cancer risk: the Multiethnic Cohort study. Cancer Epidemiol. 2011;35(6):551–554. doi: 10.1016/j.canep.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li C, Balluz LS, Ford ES, Okoro CA, Tsai J, Zhao G. Association between diagnosed diabetes and self-reported cancer among U.S. adults: findings from the 2009 Behavioral Risk Factor Surveillance System. Diabetes Care. 2011;34(6):1365–1368. doi: 10.2337/dc11-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wotton CJ, Yeates DG, Goldacre MJ. Cancer in patients admitted to hospital with diabetes mellitus aged 30 years and over: record linkage studies. Diabetologia. 2011;54(3):527–534. doi: 10.1007/s00125-010-1987-2. [DOI] [PubMed] [Google Scholar]
- 37.Atchison EA, Gridley G, Carreon JD, Leitzmann MF, Mcglynn KA. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer. 2011;128(3):635–643. doi: 10.1002/ijc.25362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackenzie T, Zens MS, Ferrara A, Schned A, Karagas MR. Diabetes and risk of bladder cancer: evidence from a case-control study in New England. Cancer. 2011;117(7):1552–1556. doi: 10.1002/cncr.25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee MY, Lin KD, Hsiao PJ, Shin SJ. The association of diabetes mellitus with liver, colon, lung, and prostate cancer is independent of hypertension, hyperlipidemia, and gout in Taiwanese patients. Metabolism. 2012;61(2):242–249. doi: 10.1016/j.metabol.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 40.Attner B, Landin-Olsson M, Lithman T, Noreen D, Olsson H. Cancer among patients with diabetes, obesity and abnormal blood lipids: a population-based register study in Sweden. Cancer Causes Control. 2012;23(5):769–777. doi: 10.1007/s10552-012-9946-5. [DOI] [PubMed] [Google Scholar]
- 41.Lo SF, Chang SN, Muo CH, et al. Modest increase in risk of specific types of cancer types in type 2 diabetes mellitus patients. Int J Cancer. 2013;132(1):182–188. doi: 10.1002/ijc.27597. [DOI] [PubMed] [Google Scholar]
- 42.Newton CC, Gapstur SM, Campbell PT, Jacobs EJ. Type 2 diabetes mellitus, insulin-use and risk of bladder cancer in a large cohort study. Int J Cancer. 2013;132(9):2186–2191. doi: 10.1002/ijc.27878. [DOI] [PubMed] [Google Scholar]
- 43.Prizment AE, Anderson KE, Yuan JM, Folsom AR. Diabetes and risk of bladder cancer among postmenopausal women in the Iowa Women’s Health Study. Cancer Causes Control. 2013;24(3):603–608. doi: 10.1007/s10552-012-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai GY, Park Y, Hartge P, Hollenbeck AR, Freedman ND. The association between self-reported diabetes and cancer incidence in the NIH-AARP Diet and Health Study. J Clin Endocrinol Metab. 2013;98(3):E497–E502. doi: 10.1210/jc.2012-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colmers IN, Majumdar SR, Yasui Y, Bowker SL, Marra CA, Johnson JA. Detection bias and overestimation of bladder cancer risk in type 2 diabetes: a matched cohort study. Diabetes Care. 2013;36(10):3070–3075. doi: 10.2337/dc13-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin CC, Chiang JH, Li CI, et al. Cancer risks among patients with type 2 diabetes: a 10-year follow-up study of a nationwide population-based cohort in Taiwan. BMC Cancer. 2014;14:381. doi: 10.1186/1471-2407-14-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu HL, Fang H, Xu WH, et al. Cancer incidence in patients with type 2 diabetes mellitus: a population-based cohort study in Shanghai. BMC Cancer. 2015;15:852. doi: 10.1186/s12885-015-1887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen HF, Chen SW, Chang YH, Li CY, Cy L. Risk of malignant neoplasms of kidney and bladder in a cohort study of the diabetic population in Taiwan with age, sex, and geographic area stratifications. Medicine. 2015;94(38):e1494. doi: 10.1097/MD.0000000000001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goossens ME, Zeegers MP, Bazelier MT, de Bruin ML, Buntinx F, de Vries F. Risk of bladder cancer in patients with diabetes: a retrospective cohort study. BMJ Open. 2015;5(6):e7470. doi: 10.1136/bmjopen-2014-007470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turati F, Polesel J, di Maso M, et al. Diabetes mellitus and the risk of bladder cancer: an Italian case–control study. Br J Cancer. 2015;113(1):127–130. doi: 10.1038/bjc.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ballotari P, Vicentini M, Manicardi V, et al. Diabetes and risk of cancer incidence: results from a population-based cohort study in northern Italy. BMC Cancer. 2017;17(1):703. doi: 10.1186/s12885-017-3696-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kessler II. Cancer mortality among diabetics. J Natl Cancer Inst. 1970;44(3):673–686. [PubMed] [Google Scholar]
- 53.Verlato G, Zoppini G, Bonora E, Muggeo M. Mortality from site-specific malignancies in type 2 diabetic patients from Verona. Diabetes Care. 2003;26(4):1047–1051. doi: 10.2337/diacare.26.4.1047. [DOI] [PubMed] [Google Scholar]
- 54.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159(12):1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 55.Chung H, Chen S, Li M. POD-11.08: Diabetes and risk of death from cancer of the prostate, kidney, and urinary bladder. Urology. 2009;74(4):S36–S37. [Google Scholar]
- 56.Tseng CH, Chong CK, Tseng CP, Chan TT. Age-related risk of mortality from bladder cancer in diabetic patients: a 12-year follow-up of a national cohort in Taiwan. Ann Med. 2009;41(5):371–379. doi: 10.1080/07853890902729778. [DOI] [PubMed] [Google Scholar]
- 57.Lam EK, Batty GD, Huxley RR, et al. Associations of diabetes mellitus with site-specific cancer mortality in the Asia-Pacific region. Ann Oncol. 2011;22(3):730–738. doi: 10.1093/annonc/mdq405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35(2):299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu X, Ji J, Sundquist K, Sundquist J, Hemminki K. The impact of type 2 diabetes mellitus on cancer-specific survival: a follow-up study in Sweden. Cancer. 2012;118(5):1353–1361. doi: 10.1002/cncr.26420. [DOI] [PubMed] [Google Scholar]
- 61.Karlin NJ, Dueck AC, Cook CB. Cancer with diabetes: prevalence, metabolic control, and survival in an academic oncology practice. Endocr Pract. 2012;18(6):898–905. doi: 10.4158/EP12128.OR. [DOI] [PubMed] [Google Scholar]
- 62.Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care. 2012;35(9):1835–1844. doi: 10.2337/dc12-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rieken M, Xylinas E, Kluth L, et al. Effect of diabetes mellitus and metformin use on oncologic outcomes of patients treated with radical cystectomy for urothelial carcinoma. Urol Oncol. 2014;32(1):49.e7–14. doi: 10.1016/j.urolonc.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 64.Oh JJ, Kang MY, Jo JK, et al. Association between diabetes mellitus and oncological outcomes in bladder cancer patients undergoing radical cystectomy. Int J Urol. 2015;22(12):1112–1117. doi: 10.1111/iju.12901. [DOI] [PubMed] [Google Scholar]
- 65.Boursi B, Giantonio BJ, Lewis JD, Haynes K, Mamtani R, Yang YX. Serum glucose and hemoglobin A1C levels at cancer diagnosis and disease outcome. Eur J Cancer. 2016;59:90–98. doi: 10.1016/j.ejca.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Faiena I, Dombrovskiy VY, Sultan RC, Salmasi AH, Singer EA, Weiss RE. Effect of uncontrolled diabetes on outcomes after cystectomy in patients with bladder cancer: a population-based study. Clin Genitourin Cancer. 2016;14(5):e509–e514. doi: 10.1016/j.clgc.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 67.Hwang EC, Kim YJ, Hwang IS, et al. Impact of diabetes mellitus on recurrence and progression in patients with non-muscle invasive bladder carcinoma: a retrospective cohort study. Int J Urol. 2011;18(11):769–776. doi: 10.1111/j.1442-2042.2011.02845.x. [DOI] [PubMed] [Google Scholar]
- 68.Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni JF. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15(1):35–44. doi: 10.1023/B:CACO.0000016573.79453.ba. [DOI] [PubMed] [Google Scholar]
- 69.Rapp K, Schroeder J, Klenk J, et al. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer. 2005;93(9):1062–1067. doi: 10.1038/sj.bjc.6602819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005;23(21):4742–4754. doi: 10.1200/JCO.2005.11.726. [DOI] [PubMed] [Google Scholar]
- 71.Cantwell MM, Lacey JV, Schairer C, Schatzkin A, Michaud DS. Reproductive factors, exogenous hormone use and bladder cancer risk in a prospective study. Int J Cancer. 2006;119(10):2398–2401. doi: 10.1002/ijc.22175. [DOI] [PubMed] [Google Scholar]
- 72.Samanic C, Chow WH, Gridley G, Jarvholm B, Fraumeni JF. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control. 2006;17(7):901–909. doi: 10.1007/s10552-006-0023-9. [DOI] [PubMed] [Google Scholar]
- 73.Lukanova A, Björ O, Kaaks R, et al. Body mass index and cancer: results from the Northern Sweden Health and Disease Cohort. Int J Cancer. 2006;118(2):458–466. doi: 10.1002/ijc.21354. [DOI] [PubMed] [Google Scholar]
- 74.Holick CN, Giovannucci EL, Stampfer MJ, Michaud DS. Prospective study of body mass index, height, physical activity and incidence of bladder cancer in US men and women. Int J Cancer. 2007;120(1):140–146. doi: 10.1002/ijc.22142. [DOI] [PubMed] [Google Scholar]
- 75.Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335(7630):1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jee SH, Yun JE, Park EJ, et al. Body mass index and cancer risk in Korean men and women. Int J Cancer. 2008;123(8):1892–1896. doi: 10.1002/ijc.23719. [DOI] [PubMed] [Google Scholar]
- 77.Koebnick C, Michaud D, Moore SC, et al. Body mass index, physical activity, and bladder cancer in a large prospective study. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1214–1221. doi: 10.1158/1055-9965.EPI-08-0026. [DOI] [PubMed] [Google Scholar]
- 78.Andreotti G, Hou L, Beane Freeman LE, et al. Body mass index, agricultural pesticide use, and cancer incidence in the Agricultural Health Study cohort. Cancer Causes Control. 2010;21(11):1759–1775. doi: 10.1007/s10552-010-9603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roswall N, Freisling H, Bueno-de-Mesquita HB, et al. Anthropometric measures and bladder cancer risk: a prospective study in the EPIC cohort. Int J Cancer. 2014;135(12):2918–2929. doi: 10.1002/ijc.28936. [DOI] [PubMed] [Google Scholar]
- 80.Guo L, Li N, Wang G, et al. Body mass index and cancer incidence: a prospective cohort study in northern China. Zhonghua Liu Xing Bing Xue Za Zhi. 2014;35(3):231–236. [PubMed] [Google Scholar]
- 81.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384(9945):755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choi JB, Lee EJ, Han KD, Hong SH, Ha US. Estimating the impact of body mass index on bladder cancer risk: stratification by smoking status. Sci Rep. 2018;8(1):947. doi: 10.1038/s41598-018-19531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 84.Hafron J, Mitra N, Dalbagni G, Bochner B, Herr H, Donat SM. Does body mass index affect survival of patients undergoing radical or partial cystectomy for bladder cancer? J Urol. 2005;173(5):1513–1517. doi: 10.1097/01.ju.0000154352.54965.14. [DOI] [PubMed] [Google Scholar]
- 85.Batty GD, Shipley MJ, Jarrett RJ, Breeze E, Marmot MG, Smith GD. Obesity and overweight in relation to organ-specific cancer mortality in London (UK): findings from the original Whitehall study. Int J Obes. 2005;29(10):1267–1274. doi: 10.1038/sj.ijo.0803020. [DOI] [PubMed] [Google Scholar]
- 86.Maurer T, Maurer J, Retz M, et al. Influence of body mass index on operability, morbidity and disease outcome following radical cystectomy. Urol Int. 2009;82(4):432–439. doi: 10.1159/000218533. [DOI] [PubMed] [Google Scholar]
- 87.Chromecki TF, Cha EK, Fajkovic H, et al. Obesity is associated with worse oncological outcomes in patients treated with radical cystectomy. BJU Int. 2013;111(2):249–255. doi: 10.1111/j.1464-410X.2012.11322.x. [DOI] [PubMed] [Google Scholar]
- 88.Xu X, Zhou L, Miao R, et al. Association of cancer mortality with postdiagnosis overweight and obesity using body mass index. Oncotarget. 2016;7(4):5023–5029. doi: 10.18632/oncotarget.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murakami Y, Matsumoto K, Ikeda M, et al. Impact of body mass index on the oncological outcomes of patients with upper and lower urinary tract cancers treated with radical surgery: a multi-institutional retrospective study. Asia Pac J Clin Oncol. 2018;14(4):310–317. doi: 10.1111/ajco.12848. [DOI] [PubMed] [Google Scholar]
- 90.Kluth LA, Xylinas E, Crivelli JJ, et al. Obesity is associated with worse outcomes in patients with T1 high grade urothelial carcinoma of the bladder. J Urol. 2013;190(2):480–486. doi: 10.1016/j.juro.2013.01.089. [DOI] [PubMed] [Google Scholar]
- 91.Psutka SP, Boorjian SA, Moynagh MR, et al. Mortality after radical cystectomy: impact of obesity versus adiposity after adjusting for skeletal muscle wasting. J Urol. 2015;193(5):1507–1513. doi: 10.1016/j.juro.2014.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dabi Y, Rouscoff Y, Anract J, et al. Impact of body mass index on the oncological outcomes of patients treated with radical cystectomy for muscle-invasive bladder cancer. World J Urol. 2017;35(2):229–235. doi: 10.1007/s00345-016-1852-0. [DOI] [PubMed] [Google Scholar]
- 93.Dabi Y, El Mrini M, Duquesnes I, et al. Impact of body mass index on the oncological outcomes of patients treated with radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol. 2018;36(1):65–71. doi: 10.1007/s00345-017-2095-4. [DOI] [PubMed] [Google Scholar]
- 94.Bachir BG, Aprikian AG, Izawa JI, et al. Effect of body mass index on the outcomes of patients with upper and lower urinary tract cancers treated by radical surgery: results from a Canadian multicenter collaboration. Urol Oncol. 2014;32(4):441–448. doi: 10.1016/j.urolonc.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 95.Wyszynski A, Tanyos SA, Rees JR, et al. Body mass and smoking are modifiable risk factors for recurrent bladder cancer. Cancer. 2014;120(3):408–414. doi: 10.1002/cncr.28394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu T, Zhu Z, Wang X, et al. Impact of body mass on recurrence and progression in Chinese patients with Ta, T1 urothelial bladder cancer. Int Urol Nephrol. 2015;47(7):1135–1141. doi: 10.1007/s11255-015-1013-1. [DOI] [PubMed] [Google Scholar]
- 97.Stocks T, van Hemelrijck M, Manjer J, et al. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension. 2012;59(4):802–810. doi: 10.1161/HYPERTENSIONAHA.111.189258. [DOI] [PubMed] [Google Scholar]
- 98.Batty GD, Shipley MJ, Marmot MG, Davey Smith G, Davey SG, White-hall Study Blood pressure and site-specific cancer mortality: evidence from the original Whitehall study. Br J Cancer. 2003;89(7):1243–1247. doi: 10.1038/sj.bjc.6601255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tai YS, Chen CH, Huang CY, et al. Diabetes mellitus with poor glycemic control increases bladder cancer recurrence risk in patients with upper urinary tract urothelial carcinoma. Diabetes Metab Res Rev. 2015;31(3):307–314. doi: 10.1002/dmrr.2614. [DOI] [PubMed] [Google Scholar]
- 100.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21(18):1315–1330. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 101.Ballotari P, Vicentini M, Manicardi V, et al. Diabetes and risk of cancer incidence: results from a population-based cohort study in northern Italy. BMC Cancer. 2017;17(1):703. doi: 10.1186/s12885-017-3696-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fang H, Yao B, Yan Y, et al. Diabetes mellitus increases the risk of bladder cancer: an updated meta-analysis of observational studies. Diabetes Technol Ther. 2013;15(11):914–922. doi: 10.1089/dia.2013.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu Y, Huo R, Chen X, Yu X. Diabetes mellitus and the risk of bladder cancer: a PRISMA-compliant meta-analysis of cohort studies. Medicine. 2017;96(46):e8588. doi: 10.1097/MD.0000000000008588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc. 2001;60(1):91–106. doi: 10.1079/pns200070. [DOI] [PubMed] [Google Scholar]
- 105.Frasca F, Pandini G, Scalia P, et al. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 1999;19(5):3278–3288. doi: 10.1128/mcb.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280(5):E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 107.Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-alpha as a tumour promoter. Eur J Cancer. 2006;42(6):745–750. doi: 10.1016/j.ejca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 108.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16(4):1103–1123. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 109.Vigneri R. Diabetes: diabetes therapy and cancer risk. Nat Rev Endocrinol. 2009;5(12):651–652. doi: 10.1038/nrendo.2009.219. [DOI] [PubMed] [Google Scholar]
- 110.Cebioglu M, Schild HH, Golubnitschaja O. Diabetes mellitus as a risk factor for cancer: stress or viral etiology? Infect Disord Drug Targets. 2008;8(2):76–87. doi: 10.2174/187152608784746501. [DOI] [PubMed] [Google Scholar]
- 111.Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121(11):2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 112.Adami HO, Trichopoulos D. Obesity and mortality from cancer. N Engl J Med. 2003;348(17):1623–1624. doi: 10.1056/NEJMp030029. [DOI] [PubMed] [Google Scholar]
- 113.Vigneri P, Frasca F, Sciacca L, Frittitta L, Vigneri R. Obesity and cancer. Nutr Metab Cardiovasc Dis. 2006;16(1):1–7. doi: 10.1016/j.numecd.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 114.Sun JW, Zhao LG, Yang Y, Ma X, Wang YY, Xiang YB. Obesity and risk of bladder cancer: a dose-response meta-analysis of 15 cohort studies. PLoS One. 2015;10(3):e119313. doi: 10.1371/journal.pone.0119313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin Y, Wang Y, Wu Q, et al. Association between obesity and bladder cancer recurrence: a meta-analysis. Clin Chim Acta. 2018;480:41–46. doi: 10.1016/j.cca.2018.01.039. [DOI] [PubMed] [Google Scholar]
- 116.Qin Q, Xu X, Wang X, Zheng XY. Obesity and risk of bladder cancer: a meta-analysis of cohort studies. Asian Pac J Cancer Prev. 2013;14(5):3117–3121. doi: 10.7314/apjcp.2013.14.5.3117. [DOI] [PubMed] [Google Scholar]
- 117.Cleary MP, Grossmann ME. Minireview: obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150(6):2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002;94(22):1704–1711. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- 119.Lindgren A, Pukkala E, Nissinen A, Tuomilehto J. Blood pressure, smoking, and the incidence of lung cancer in hypertensive men in North Karelia, Finland. Am J Epidemiol. 2003;158(5):442–447. doi: 10.1093/aje/kwg179. [DOI] [PubMed] [Google Scholar]
- 120.Xu S, Zhang GM, Guan FJ, et al. The association between metabolic syndrome and the risk of urothelial carcinoma of the bladder: a case-control study in China. World J Surg Oncol. 2015;13:236. doi: 10.1186/s12957-015-0631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cantiello F, Cicione A, Salonia A, et al. Association between metabolic syndrome, obesity, diabetes mellitus and oncological outcomes of bladder cancer: a systematic review. Int J Urol. 2015;22(1):22–32. doi: 10.1111/iju.12644. [DOI] [PubMed] [Google Scholar]