Abstract
Purpose
The aim of this study was to investigate the clinical significance of the combined fibrinogen and neutrophil–lymphocyte ratio (F-NLR) in patients with resectable colorectal cancer (CRC).
Patients and methods
We retrospectively recruited 693 patients with stage I–III CRC following curative surgery. Cutoff values of the preoperative fibrinogen and neutrophil–lymphocyte ratio (NLR) were determined with the receiver operating characteristic analysis. Patients were divided into three groups based on the F-NLR value and were further divided into the chemotherapy and nonchemotherapy groups. The overall survival (OS) and disease-free survival (DFS) were evaluated with the Kaplan–Meier survival method, the log-rank test, univariate and multivariate Cox proportional hazards models, and subgroup analyses.
Results
The Kaplan–Meier survival curves revealed that the 5-year OS rates in the F-NLR 0, 1, and 2 groups were 78.4%, 52%, 42.6%, respectively (P<0.001), and the 5-year DFS rates were 54.9%, 43.9%, 26.7%, respectively (P<0.001). Multivariate analyses revealed that the F-NLR score was an independent prognostic factor for both the OS (P=0.035) and the DFS (P=0.001). In addition, subgroup analyses based on the histological type showed that an elevated F-NLR score was significantly associated with worse OS (P=0.001) and DFS (P<0.001) in patients with colorectal adenocarcinoma. Furthermore, DFS in the F-NLR 0–1 group was significantly shortened after the administration of chemotherapy (P=0.005); however, patients with a relatively higher F-NLR score showed slight OS benefit from adjuvant chemotherapy (P=0.144).
Conclusion
The F-NLR score, as a novel inflammation-based grading index, was a potential predictor for the prognosis and responses to chemotherapy in patients with resectable CRC.
Keywords: inflammation, fibrinogen, neutrophil-lymphocyte ratio, colorectal cancer, prognosis
Introduction
Colorectal cancer (CRC) is one of the most aggressive malignancies among gastrointestinal tract cancers worldwide.1,2 Surgery is the mainstay treatment method for patients with early stage CRCs, and chemotherapy is recommended for patients with stage III and high-risk stage II CRCs.3,4 Despite the dramatic development in surgical techniques and adjuvant therapies, the 5-year survival rate remains poor.5,6 Thus, it is necessary and important to search for sensitive biomarkers to evaluate the prognosis and responses to chemotherapy before starting treatments.
Over the past several decades, cancer-related systemic inflammation has been proved to be crucial in the progression and prognosis of several cancers.7–9 Recently, several inflammation-based biomarkers, including the neutrophil–lymphocyte ratio (NLR),10–12 platelet–lymphocyte ratio (PLR),13,14 and fibrinogen,15–17 were reported as prognostic factors in several types of malignancies. The Glasgow Prognostic Score (GPS), defined as the combination of serum C-reactive protein (CRP) and albumin, has been demonstrated to have predictive value in various malignancies, including CRC.18–21 The systemic immune inflammation index (SII), which was calculated with peripheral lymphocyte, neutrophil, and platelet counts, was a powerful prognostic factor for hepatocellular carcinoma and CRC.22,23
Recent studies have emphasized that the F-NLR score, which was the combination of the fibrinogen and NLR, was associated with malignant behaviors and clinical outcomes of various carcinomas, such as non-small cell lung cancer,24 esophageal cancer,25 and gastric cancer.26 However, to date, the prognostic value of F-NLR for CRC patients has not been investigated and whether F-NLR has effects on prognosis and chemotherapeutic efficacy needs to be investigated.
In the present study, we investigated the prognostic and predictive value of F-NLR in CRC patients who underwent curative resection.
Patients and methods
Patients
We retrospectively recruited 693 CRC patients who underwent radical surgery at the Shandong Provincial Hospital Affiliated to Shandong University between March 2000 and July 2016. The inclusion criteria were as follows: 1) patients with pathologically diagnosed primary adenocarcinoma or mucinous adenocarcinoma; 2) patients who underwent complete resection without positive margins; 3) patients with stage I–III CRCs; and 4) patients with intact data of preoperative peripheral blood counts, follow-up information (more than 2 months), and medical record. The exclusion criteria were as follows: 1) patients with hematological disorders, active infectious diseases, or autoimmune diseases; 2) patients who received preoperative treatment (chemotherapy or radiotherapy); 3) patients who received anti-inflammatory or immunosuppressive treatment; 4) patients with more than one primary carcinoma; 5) patients with a history of venous thrombosis or blood transfusion within the past 3 months; and 6) patients who underwent emergency surgeries due to obstruction or enterobrosis.
Data collection
Clinical parameters were obtained from the medical records: gender, age, differentiation, histological type, T stage, N stage, TNM stage, morphology, primary tumor location, tumor size, venous invasion, perineural invasion, tumor deposits, and chemotherapy treatment. Patients were divided into three groups according to the primary tumor location: right colon cancer (RCC, cecum to transverse), left colon cancer (LCC, splenic flexure to rectosigmoid), and rectal cancer (RECC, rectum). The TNM stage was assessed with the seventh edition of the American Joint Committee on Cancer staging manual.27
F-NLR evaluation
The results of preoperative laboratory examinations including the levels of fibrinogen, lymphocytes, and neutrophils were extracted to evaluate the F-NLR score. The NLR was defined as the neutrophil count divided by the lymphocyte count. Using cancer-specific death as the endpoint, the receiver operating characteristic (ROC) analysis was performed to obtain the optimal cutoff value with the highest Youden index. The cutoff values were 2.34 for NLR and 2.97 g/L for fibrinogen (sensitivity and specificity: 54.7% and 62.7% for NLR, 82.3% and 40% for fibrinogen, respectively; Figure 1). The areas under the concentration–time curve (AUC) were 0.597 and 0.639, respectively. The F-NLR score was calculated as follows: patients with an elevated fibrinogen (>2.97 g/L) and an increased NLR (>2.34) were assigned a score of 2, those with only one of the two abnormalities were classified as a score of 1, and those with neither of the two abnormalities were assigned a score of 0.
Figure 1.
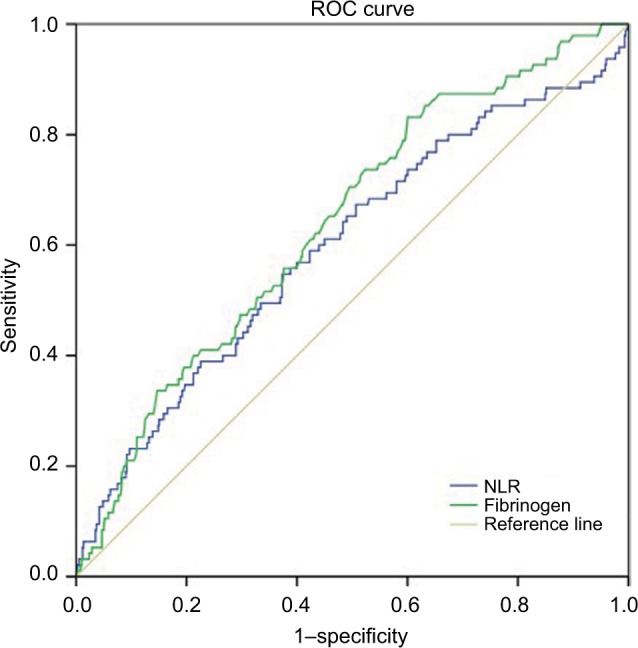
ROC curves to assess the predictive value of plasma fibrinogen and NLR.
Notes: The cutoff values were 2.34 for NLR and 2.97 g/L for fibrinogen (sensitivity and specificity: 54.7% and 62.7% for NLR, 82.3% and 40% for fibrinogen, respectively).
Abbreviations: NLR, neutrophil–lymphocyte ratio; F-NLR, combined fibrinogen and NLR; ROC, receiver operating characteristic.
Follow-up
The overall survival (OS) and disease-free survival (DFS) were chosen as primary endpoints. The OS was defined as the interval between the date of surgery and death. The DFS was defined as the time between the date of surgery and the time of first recurrence or metastasis or the end of life. The median duration of follow-up was 21.69 months (range: 2–202 months).
Statistical analyses
All data were analyzed with SPSS software version 22.0 (IBM Corporation, Armonk, NY, USA). The AUC was obtained with the ROC analysis. The optimal cutoff values for NLR and fibrinogen were calculated with the Youden index. The chi-squared test or the Fisher’s exact test was performed to compare the relationship among F-NLR and other variables. The Kaplan–Meier method, the log-rank test, univariate and multivariate analyses, and subgroup analyses were performed to compare the survival outcomes. Variables with a P-value of <0.1 in the univariate analysis were included in the multivariate Cox proportional hazards regression model. A two-sided P<0.05 was considered statistically significant.
Ethics statement
The present study was approved by the medical ethics committee of Shandong Provincial Hospital Affiliated to Shan-dong University. Written informed consent was obtained from all patients involved.
Results
Baseline characteristics of CRC patients
A total of 693 CRC patients (64.2% male and 35.8% female) were enrolled in this study; 58.6% of the patients were younger than 60 years; 41.1% had small tumors <4 cm in size; and 79.9% received adjuvant chemotherapy. Patients with TNM stages I, II, and III accounted for 9.2%, 39.8%, and 50.9%, respectively. Of all the patients, 108 (15.6%) tumors were located in the right colon, 156 (22.5%) tumors in the left colon, and the remaining 429 (61.9%) tumors were located in the rectum. Patients with the histological type of adenocarcinoma and mucinous adenocarcinoma accounted for 84.3% and 15.7%, respectively.
Patients were categorized into three groups based on the F-NLR score, of whom 181 (26.1%) patients had an F-NLR score of 0, 295 (42.6%) patients had an F-NLR score of 1, and 217 (31.3%) patients had an F-NLR score of 2. Significant differences were observed among the F-NLR 0, 1, and 2 groups in terms of age (P=0.001), histological type (P=0.043), T stage (P=0.034), TNM stage (P=0.021), morphology (P=0.026), primary tumor location (P=0.015), and tumor size (P=0.016; Table 1).
Table 1.
Comparison of demographic and clinicopathological features among patients with different F-NLRs
| Features | F-NLR
|
All (N=693) | P-value* | ||
|---|---|---|---|---|---|
| 0 (n=181) | 1 (n=295) | 2 (n=217) | |||
|
| |||||
| Gender | 0.523 | ||||
| Male | 114 (63%) | 185 (62.7%) | 146 (67.3%) | 445 (64.2%) | |
| Female | 67 (37%) | 110 (37.3%) | 71 (32.7%) | 248 (35.8%) | |
| Age (years) | 0.001 | ||||
| ≤60 | 127 (70.2%) | 154 (52.2%) | 125 (57.6%) | 406 (58.6%) | |
| >60 | 54 (29.8%) | 141 (47.8%) | 92 (42.4%) | 287 (41.4%) | |
| Differentiation | 0.052 | ||||
| Well | 12 (6.6%) | 22 (7.5%) | 11 (5.1%) | 45 (6.5%) | |
| Moderate | 130 (71.8%) | 211 (71.5%) | 137 (63.1%) | 478 (69%) | |
| Poor | 31 (17.1%) | 39 (13.2%) | 45 (20.7%) | 115 (16.6%) | |
| Unknown | 8 (4.4%) | 23 (7.8%) | 24 (11.1%) | 55 (7.9%) | |
| Histological type | 0.043 | ||||
| Adenocarcinoma | 163 (90.1%) | 244 (82.7%) | 177 (81.6%) | 584 (84.3%) | |
| Mucinous type | 18 (9.9%) | 51 (17.3%) | 40 (18.4%) | 109 (15.7%) | |
| T stage | 0.034 | ||||
| 1–2 | 31 (17.1%) | 42 (14.2%) | 16 (7.4%) | 89 (12.8%) | |
| 3 | 49 (27.1%) | 84 (28.5%) | 58 (26.7%) | 191 (27.6%) | |
| 4 | 101 (55.8%) | 169 (57.3%) | 143 (65.9%) | 413 (59.6%) | |
| N stage | 0.652 | ||||
| 0 | 98 (54.1%) | 139 (47.1%) | 104 (47.9%) | 341 (49.2%) | |
| 1 | 45 (24.9%) | 86 (29.2%) | 63 (29%) | 194 (28%) | |
| 2 | 38 (21%) | 70 (23.7%) | 50 (23%) | 158 (22.8%) | |
| TNM stage | 0.021 | ||||
| 1 | 27 (14.9%) | 25 (8.5%) | 12 (5.5%) | 64 (9.2%) | |
| 2 | 71 (39.2%) | 113 (38.3%) | 92 (42.4%) | 276 (39.8%) | |
| 3 | 83 (45.9%) | 157 (53.2%) | 113 (52.1%) | 353 (50.9%) | |
| Morphology | 0.026 | ||||
| Expansive | 27 (14.9%) | 64 (21.7%) | 25 (11.5%) | 116 (16.7%) | |
| Infiltrative | 3 (1.7%) | 11 (3.7%) | 7 (3.2%) | 21 (3%) | |
| Ulcerative | 149 (82.3%) | 217 (73.6%) | 179 (82.5%) | 545 (78.6%) | |
| Complex | 2 (1.1%) | 3 (1%) | 6 (2.8%) | 11 (1.6%) | |
| Location | 0.015 | ||||
| RCC | 17 (9.4%) | 51 (17.3%) | 40 (18.4%) | 108 (15.6%) | |
| LCC | 37 (20.4%) | 61 (20.7%) | 58 (26.7%) | 156 (22.5%) | |
| RECC | 127 (70.2%) | 183 (62%) | 119 (54.8%) | 429 (61.9%) | |
| Tumor size | 0.016 | ||||
| ≤4 cm | 90 (49.7%) | 123 (41.7%) | 72 (33.2%) | 285 (41.1%) | |
| >4 cm | 86 (47.5%) | 158 (53.6%) | 132 (60.8%) | 376 (54.3%) | |
| Unknown | 5 (2.8%) | 14 (4.7%) | 13 (6%) | 32 (4.6%) | |
| Venous invasion | 0.693 | ||||
| Positive | 9 (5%) | 10 (3.4%) | 9 (4.1%) | 28 (4%) | |
| Negative | 172 (95%) | 285 (96.6%) | 208 (95.9%) | 665 (96%) | |
| Perineural invasion | 0.595 | ||||
| Positive | 5 (2.8%) | 10 (3.4%) | 10 (4.6%) | 25 (3.6%) | |
| Negative | 176 (97.2%) | 285 (96.6%) | 207 (95.4%) | 668 (96.4%) | |
| Tumor deposits | 0.295 | ||||
| Present | 5 (2.8%) | 17 (5.8%) | 9 (4.1%) | 31 (4.5%) | |
| Absent | 176 (97.2%) | 278 (94.2%) | 208 (95.9%) | 662 (95.5%) | |
| Chemotherapy | 0.768 | ||||
| Yes | 143 (79%) | 234 (79.3%) | 177 (81.6%) | 554 (79.9%) | |
| No | 38 (21%) | 61 (20.7%) | 40 (18.4%) | 139 (20.1%) | |
Notes:
P-values were calculated by the chi-squared test or the Fisher’s exact test. P-value for significance was <0.05.
Abbreviations: LCC, left colon cancer; NLR, neutrophil–lymphocyte ratio; F-NLR, combined fibrinogen and NLR; RCC, right colon cancer; RECC, rectal cancer.
Univariate and multivariate analyses for OS
The age (HR=0.600; 95% CI=0.407–0.884; P=0.010), histological type (HR=0.574; 95% CI=0.351–0.938; P=0.027), T stage (P=0.007), N stage (P<0.001), venous invasion (HR=2.810; 95% CI=1.416–5.578; P=0.003), perineural invasion (HR=4.065; 95% CI=1.946–8.491; P<0.001), tumor deposits (HR=2.579; 95% CI=1.296–5.133; P=0.007), and F-NLR (P=0.001) were significantly associated with OS in the univariate analysis. Multivariate analysis after controlling for these cofounders revealed that age (HR=0.538; 95% CI=0.357–0.811; P=0.003), N stage (P<0.001), and F-NLR (P=0.035) were independent prognostic factors for OS (Table 2).
Table 2.
Univariate and multivariate Cox analyses of OS in CRC patients
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Gender (male/female) | 1.043 (0.699–1.557) | 0.873 | ||
| Age (≤60/>60) (years) | 0.600 (0.407–0.884) | 0.010 | 0.538 (0.357–0.811) | 0.003 |
| Differentiation | 0.112 | |||
| Well/poor | 0.662 (0.283–1.551) | |||
| Moderate/poor | 0.718 (0.441–1.167) | |||
| Histological subtype | 0.027 | 0.215 | ||
| Adenocarcinoma/mucinous type | 0.574 (0.351–0.938) | 0.727 (0.439–1.203) | ||
| T stage | 0.007 | 0.052 | ||
| 1–2/4 | 0.054 (0.008–0.391) | 0.085 (0.012–0.620) | ||
| 3/4 | 0.724 (0.465–1.127) | 0.937 (0.588–1.493) | ||
| N stage | <0.001 | <0.001 | ||
| 0/2 | 0.283 (0.174–0.458) | 0.319 (0.192–0.531) | ||
| 1/2 | 0.663 (0.420–1.047) | 0.563 (0.346–0.915) | ||
| Primary tumor location | 0.319 | |||
| RCC/RECC | 1.379 (0.838–2.268) | |||
| LCC/RECC | 0.891 (0.546–1.452) | |||
| Morphology | 0.292 | |||
| Infiltrative/expansive | 1.686 (0.663–4.289) | |||
| Ulcerative/expansive | 0.830 (0.490–1.408) | |||
| Tumor size (≤4/>4 cm) | 0.988 (0.649–1.504) | 0.955 | ||
| Venous invasion | 0.003 | 0.179 | ||
| Positive/negative | 2.810 (1.416–5.578) | 1.696 (0.785–3.665) | ||
| Perineural invasion | <0.001 | 0.072 | ||
| Positive/negative | 4.065 (1.946–8.491) | 2.176 (0.933–5.075) | ||
| Tumor deposit (present/absent) | 2.579 (1.296–5.133) | 0.007 | 1.580 (0.767–3.258) | 0.215 |
| Chemotherapy (yes/no) | 0.913 (0.574–1.454) | 0.702 | ||
| F-NLR | 0.001 | 0.035 | ||
| 0/2 | 0.281 (0.143–0.551) | 0.399 (0.198–0.803) | ||
| 1/2 | 0.648 (0.428–0.980) | 0.813 (0.528–1.250) | ||
Abbreviations: CRC, colorectal cancer; LCC, left colon cancer; NLR, neutrophil–lymphocyte ratio; F-NLR, combined fibrinogen and NLR; OS, overall survival; RCC, right colon cancer; RECC, rectal cancer.
Univariate and multivariate analyses for DFS
T stage (P=0.031), N stage (P<0.001), primary tumor location (P=0.042), morphology (P=0.002), venous invasion (HR=2.160; 95% CI=1.254–3.719; P=0.005), perineural invasion (HR=4.561; 95% CI=2.784–7.473; P<0.001), tumor deposits (HR=3.019; 95% CI=1.920–4.747; P<0.001), chemotherapy (HR=1.811; 95% CI=1.236–2.654; P=0.002), and F-NLR (P<0.001) were significantly associated with DFS in the univariate analysis. Multivariate analysis after controlling for these variables revealed that N stage (P<0.001), primary tumor location (P=0.013), perineural invasion (HR=2.557; 95% CI=1.424–4.590; P=0.002), tumor deposits (HR=2.194; 95% CI=1.331–3.619; P=0.002), and F-NLR (P=0.001) were independent prognostic factors for DFS (Table 3).
Table 3.
Univariate and multivariate Cox analyses of DFS in CRC patients
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Gender (male/female) | 1.209 (0.909–1.607) | 0.192 | ||
| Age (≤60/>60) (years) | 0.930 (0.710–1.219) | 0.599 | ||
| Differentiation | 0.216 | |||
| Well/poor | 0.541 (0.286–1.023) | |||
| Moderate/poor | 0.759 (0.542–1.063) | |||
| Histological subtype | 0.338 | |||
| Adenocarcinoma/mucinous type | 0.839 (0.586–1.202) | |||
| T stage | 0.031 | 0.778 | ||
| 1–2/4 | 0.534 (0.327–0.873) | 0.878 (0.517–1.492) | ||
| 3/4 | 0.817 (0.597–1.118) | 1.067 (0.763–1.491) | ||
| N stage | <0.001 | <0.001 | ||
| 0/2 | 0.370 (0.266–0.514) | 0.453 (0.318–0.646) | ||
| 1/2 | 0.762 (0.550–1.056) | 0.674 (0.480–0.947) | ||
| Primary tumor location | 0.042 | 0.013 | ||
| RCC/RECC | 1.153 (0.813–1.637) | 1.350 (0.927–1.965) | ||
| LCC/RECC | 0.674 (0.470–0.967) | 0.686 (0.471–1.000) | ||
| Morphology | 0.002 | 0.372 | ||
| Infiltrative/expansive | 3.118 (1.614–6.025) | 1.412 (0.689–2.896) | ||
| Ulcerative/expansive | 1.196 (0.800–1.787) | 1.005 (0.657–1.535) | ||
| Tumor size (≤4/>4 cm) | 0.920 (0.692–1.223) | 0.565 | ||
| Venous invasion | 0.005 | 0.511 | ||
| Positive/negative | 2.160 (1.254–3.719) | 1.225 (0.668–2.247) | ||
| Perineural invasion | <0.001 | 0.002 | ||
| Positive/negative | 4.561 (2.784–7.473) | 2.557 (1.424–4.590) | ||
| Tumor deposit (present/absent) | 3.019 (1.920–4.747) | <0.001 | 2.194 (1.331–3.619) | 0.002 |
| Chemotherapy (yes/no) | 1.811 (1.236–2.654) | 0.002 | 1.379 (0.921–2.064) | 0.119 |
| F-NLR | <0.001 | 0.001 | ||
| 0/2 | 0.417 (0.281–0.619) | 0.474 (0.317–0.708) | ||
| 1/2 | 0.659 (0.491–0.884) | 0.684 (0.505–0.926) | ||
Abbreviations: CRC, colorectal cancer; DFS, disease-free survival; LCC, left colon cancer; NLR, neutrophil–lymphocyte ratio; F-NLR, combined fibrinogen and NLR; RCC, right colon cancer; RECC, rectal cancer.
F-NLR as a prognostic factor in patients with different histological types
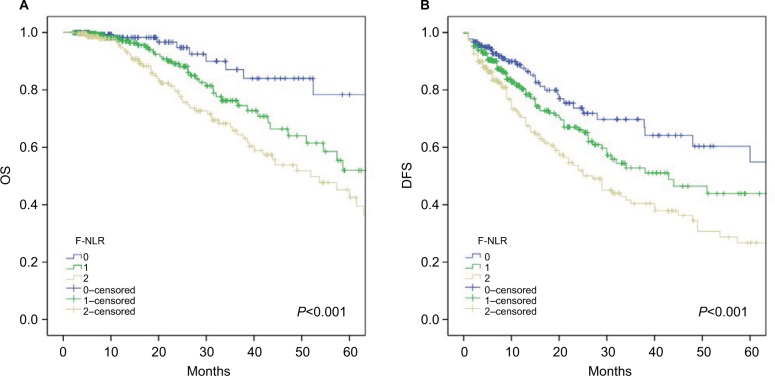
The Kaplan–Meier survival curves revealed that the 5-year OS rates in the F-NLR 0, 1, and 2 groups differed significantly and were 78.4%, 52%, and 42.6%, respectively (P<0.001; Figure 2A), and the 5-year DFS rates were 54.9%, 43.9%, and 26.7%, respectively (P<0.001; Figure 2B).
Figure 2.
Kaplan–Meier survival curves according to the F-NLR score for (A) OS and (B) DFS.
Abbreviations: DFS, disease-free survival; NLR, neutrophil–lymphocyte ratio; F-NLR, combined fibrinogen and NLR; OS, overall survival.
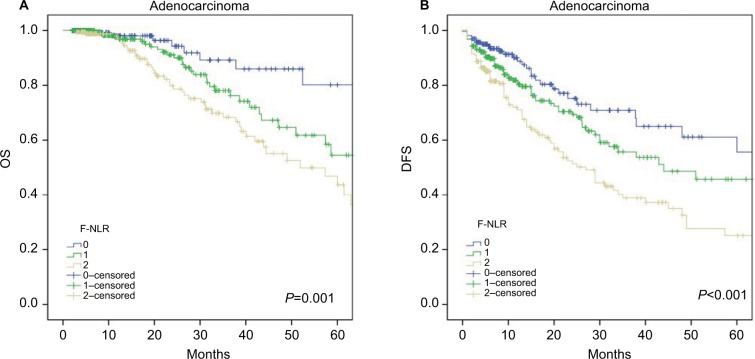
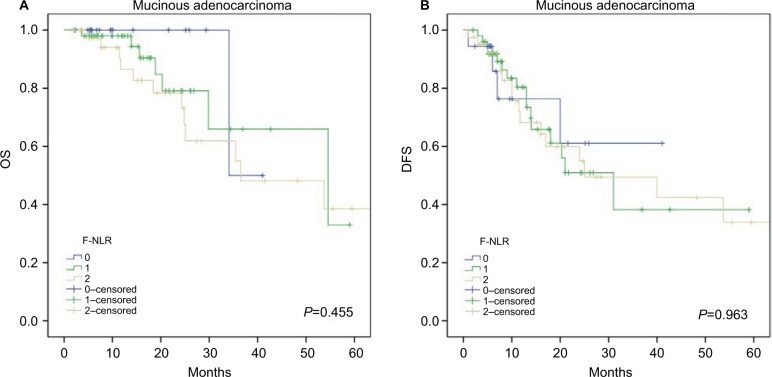
Further subgroup analyses were performed to investigate the prognostic value of F-NLR in CRC patients with different histological types. The results showed that F-NLR was a prognostic factor for OS (P=0.001; Figure 3A) and DFS (P<0.001; Figure 3B) in patients with colorectal adenocarcinoma, whereas no differences in OS (P=0.455; Figure 4A) and DFS (P=0.963; Figure 4B) were observed for patients with mucinous adenocarcinoma.
Figure 3.
Kaplan–Meier survival curves in patients with adenocarcinoma according to the F-NLR score for (A) OS and (B) DFS.
Abbreviations: DFS, disease-free survival; NLR, neutrophil–lymphocyte ratio; F-NLR, combined fibrinogen and NLR; OS, overall survival.
Figure 4.
Kaplan–Meier survival curves in patients with mucinous adenocarcinoma according to the F-NLR score for (A) OS and (B) DFS.
Abbreviations: DFS, disease-free survival; NLR, neutrophil–lymphocyte ratio; F-NLR, combined fibrinogen and NLR; OS, overall survival.
Univariate and multivariate analyses showed that the F-NLR score was an independent prognostic factor for both the OS (P=0.035) and DFS (P=0.001). In addition, a higher F-NLR score was significantly associated with worse prognosis (Tables 2 and 3).
F-NLR as a predictive factor for the responses to chemotherapy in CRC patients
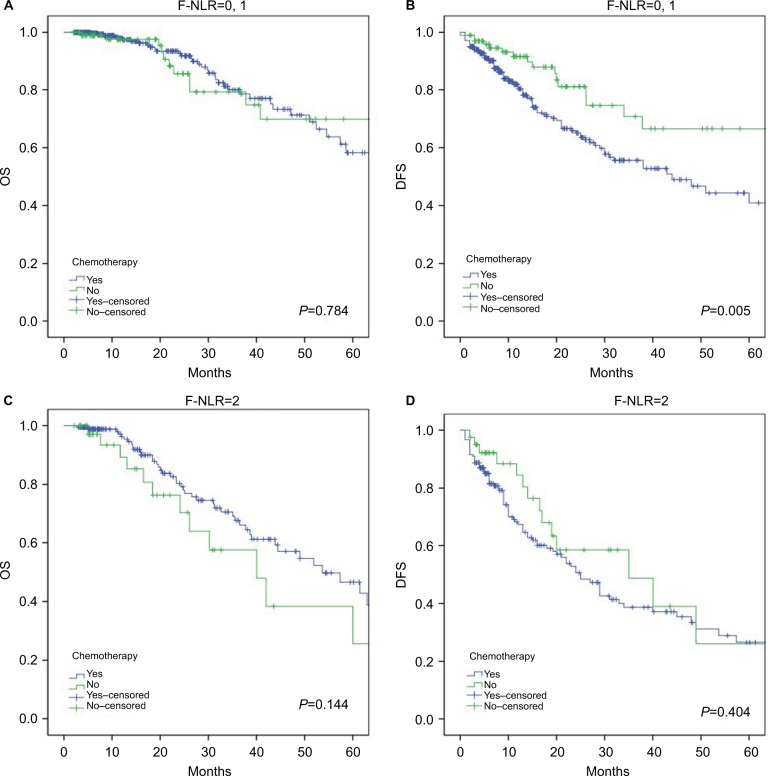
We further divided the patients into a chemotherapy group and a nonchemotherapy group based on the treatment of chemotherapy. The Kaplan–Meier survival curves revealed that the DFS of the F-NLR 0 and 1 groups could be shortened significantly after the administration of chemotherapy (Figure 5B; P<0.001), whereas the F-NLR 2 group patients did not show an DFS harm from the administration of chemotherapy (Figure 5D). The Kaplan–Meier survival curves showed that chemotherapy had no effects on the OS in the F-NLR 0 and 1 groups (Figure 5A); however, patients with chemotherapy may show slightly better survival in the F-NLR 2 group, although the P-value is above 0.05 (Figure 5C).
Figure 5.
Kaplan–Meier survival curves for patients who received chemotherapy or did not receive chemotherapy.
Notes: (A) OS in patients with the F-NLR scores of 0 and 1. (B) DFS in patients with the F-NLR scores of 0 and 1. (C) OS in patients with the F-NLR score of 2. (D) DFS in patients with the F-NLR score of 2.
Abbreviations: DFS, disease-free survival; NLR, neutrophil–lymphocyte ratio; F-NLR, combined fibrinogen and NLR; OS, overall survival.
Discussion
Cancer-related inflammation encompassed not only the tumor-derived but also the host-derived inflammatory cytokines, chemokines, proinflammatory mediators, and immune cells, which were correlated with the initiation, progression, and development of malignancies.28–31 Several inflammatory-based markers have been recognized to be associated with poor clinical outcomes and have the ability to predict the prognosis in various malignancies, including CRC. For example, NLR,32 PLR,33 GPS,34 and lymphocyte–monocyte ratio (LMR)35,36 were risk factors for poor clinical outcomes in patients with CRC.
Neutrophils were demonstrated to promote tumor growth, invasion, and metastasis via the intrinsic pathway through secreting inflammatory mediators and the extrinsic pathway through altering the tumor microenvironment.37,38 Lymphocytes suppress the tumor proliferation and metastasis by inducing the cytotoxic cell death and producing inhibitive cytokines.39,40 Thus, lymphopenia may result in an insufficient immunological response to malignancies, thus facilitating tumor progression and leading to poor prognosis.41,42 Recent studies have emphasized that the elevated plasma fibrinogen plays an important role in malignant behaviors of various tumors43,44 through impeding the elimination of cancer cells mediated by cytotoxic cells or natural killer cells.45 Therefore, the F-NLR score, as an integrated index based on the plasma fibrinogen, peripheral neutrophil, and lymphocyte counts, reflects the alterations in the cancer microenvironment and the preoperative inflammatory responses of hosts to tumors. We hypothesized that F-NLR could favor cancer initiation, progression, and metastasis.
The F-NLR score has been demonstrated to be a predictive marker for the prognosis in patients with advanced esophageal cancer,25 gastric cancer,26 and resectable non-small-cell lung cancer.24 However, there have been no researches regarding the prognostic and predictive value of F-NLR in patients with resectable CRC. Thus, in the present study, we for the first time evaluated the prognostic value of F-NLR in patients with resectable CRC.
In the present study, interesting associations between the F-NLR score and clinicopathological characteristics were observed. F-NLR was associated with more advanced T stage, larger tumor size, more perineural invasion, and more mucinous adenocarcinoma, supporting the abovementioned hypothesis that the elevated F-NLR might favor tumor proliferation, invasion, and metastasis. Univariate and multivariate analyses revealed that both the F-NLR score and the N stage were independent risk factors in resectable CRC patients. In addition, subgroup analyses based on the histological type revealed that the elevated F-NLR score was correlated with poor OS and DFS in patients with colorectal adenocarcinoma. Furthermore, patients with the F-NLR score of 0 and 1 were harmed by chemotherapy, whereas patients with the elevated F-NLR score lost the DFS harm and might have slightly better OS from the administration of chemotherapy. As we all know, chemotherapy was a double-edged sword and not all patients with malignancies could benefit from chemotherapy. As chemotherapy did harm not only cancer cells but also normal cells and immune cells, patients with lower stage of F-NLR may be harmed by chemotherapy.
To date, the TNM staging system is the gold standard for predicting the prognosis and the treatment selection for various types of cancers. However, as the TNM staging only reflects the pathological behaviors of resected tumors after surgery, preoperative survival prediction and decision making for further treatment seemed difficult. Our findings demonstrated that the preoperative F-NLR was a novel clinical biomarker for resectable CRC patients and had a powerful prognostic value. Thus, as a simple, convenient, cheap, easily acquired parameter in clinical practice, F-NLR may serve as a complementary to the TNM staging system to identify high-risk patients among patients with the same TNM stage.
To the best of our knowledge, this was the first report investigating the prognostic value of F-NLR in patients with resectable CRC. However, there were a few limitations associated with the present study. First of all, as a retrospective study, selection bias may not be avoided and there may have some mistakes in the data collection. Second, as a single-institution study, the number of patients enrolled was relatively small and the follow-up duration was not that long. Third, only stage I–III CRC patients who received curative resection were enrolled, and thus the results are not applicable for patients with advanced unresectable CRCs. Thus, a larger-scale, prospective, multicenter investigation is required to further validate the findings of the present study.
Conclusion
This is the first study to demonstrate the prognostic value of the preoperative F-NLR score in patients with resectable CRC. Patients with an elevated preoperative F-NLR had higher risks of mortality, recurrence, or metastasis for adenocarcinomas, suggesting doctors to perform more careful surgeries and conduct more rigorous follow-up for these patients.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no. 81472685 and 81600469), the Major Special Plan of Science and Technology of Shandong Province (no. 2015ZDXX0802A01), the Science and Technology Development Projects of Shandong Province (no. 2016GSF201126), and the Natural Science Foundation of Shandong Province (no. ZR2016HB06 and ZR2017MH035).
Footnotes
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Jonker DJ, Spithoff K, Maroun J. Adjuvant systemic chemotherapy for stage II and III colon cancer after complete resection: an updated practice guideline. Clin Oncol. 2011;23(5):314–322. doi: 10.1016/j.clon.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Verdecchia A, Francisci S, Brenner H, et al. Recent cancer survival in Europe: a 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8(9):784–796. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]
- 6.Lin J, Qiu M, Xu R, Dobs AS. Comparison of survival and clinicopathologic features in colorectal cancer among African American, Caucasian, and Chinese patients treated in the United States: Results from the surveillance epidemiology and end results (SEER) database. Oncotarget. 2015;6(32):33935–33943. doi: 10.18632/oncotarget.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diakos CI, Charles KA, Mcmillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 8.Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12(10):584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Jia H, Yu W, et al. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer. 2016;139(1):220–231. doi: 10.1002/ijc.30071. [DOI] [PubMed] [Google Scholar]
- 11.Cho IR, Park JC, Park CH, et al. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer. 2014;17(4):703–710. doi: 10.1007/s10120-013-0330-2. [DOI] [PubMed] [Google Scholar]
- 12.Mcnamara MG, Templeton AJ, Maganti M, et al. Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancer. Eur J Cancer. 2014;50(9):1581–1589. doi: 10.1016/j.ejca.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Zou ZY, Liu HL, Ning N, Li SY, Du XH, Li R. Clinical significance of pre-operative neutrophil lymphocyte ratio and platelet lymphocyte ratio as prognostic factors for patients with colorectal cancer. Oncol Lett. 2016;11(3):2241–2248. doi: 10.3892/ol.2016.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.You J, Zhu GQ, Xie L, et al. Preoperative platelet to lymphocyte ratio is a valuable prognostic biomarker in patients with colorectal cancer. Oncotarget. 2016;7(18):25516–25527. doi: 10.18632/oncotarget.8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Z, Wen H, Bi R, Duan Y, Yang W, Wu X. Thrombocytosis and hyperfibrinogenemia are predictive factors of clinical outcomes in high-grade serous ovarian cancer patients. BMC Cancer. 2016;16:43. doi: 10.1186/s12885-016-2070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka N, Kikuchi E, Matsumoto K, et al. Prognostic value of plasma fibrinogen levels in patients with localized upper tract urothelial carcinoma. BJU Int. 2013;111(6):857–864. doi: 10.1111/j.1464-410X.2012.11353.x. [DOI] [PubMed] [Google Scholar]
- 17.Luo Y, Kim HS, Kim M, Lee M, Song YS. Elevated plasma fibrinogen levels and prognosis of epithelial ovarian cancer: a cohort study and meta-analysis. J Gynecol Oncol. 2017;28(3):e36. doi: 10.3802/jgo.2017.28.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mcmillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–540. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, He X, Pan J, Chen S, Wang L. Prognostic role of Glasgow prognostic score in patients with colorectal cancer: evidence from population studies. Sci Rep. 2017;7(1):6144. doi: 10.1038/s41598-017-06577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forrest LM, Mcmillan DC, Mcardle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89(6):1028–1030. doi: 10.1038/sj.bjc.6601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang CX, Wang SY, Chen SQ, Yang SL, Wan L, Xiong B. Association between pretreatment Glasgow prognostic score and gastric cancer survival and clinicopathological features: a meta-analysis. Onco Targets Ther. 2016;9:3883–3891. doi: 10.2147/OTT.S103996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23(34):6261–6272. doi: 10.3748/wjg.v23.i34.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Zhao J, Zhang M, Han L, Wang M, Xingde L. The combination of plasma fibrinogen and neutrophil lymphocyte ratio (F-NLR) is a predictive factor in patients with resectable non small cell lung cancer. J Cell Physiol. 2018;233(5):4216–4224. doi: 10.1002/jcp.26239. [DOI] [PubMed] [Google Scholar]
- 25.Kijima T, Arigami T, Uchikado Y, et al. Combined fibrinogen and neutrophil-lymphocyte ratio as a prognostic marker of advanced esophageal squamous cell carcinoma. Cancer Sci. 2017;108(2):193–199. doi: 10.1111/cas.13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arigami T, Uenosono Y, Ishigami S, et al. A novel scoring system based on fibrinogen and the neutrophil-lymphocyte ratio as a predictor of chemotherapy response and prognosis in patients with advanced gastric cancer. Oncology. 2016;90(4):186–192. doi: 10.1159/000444494. [DOI] [PubMed] [Google Scholar]
- 27.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 28.West NR, Mccuaig S, Franchini F, Powrie F. Emerging cytokine networks in colorectal cancer. Nat Rev Immunol. 2015;15(10):615–629. doi: 10.1038/nri3896. [DOI] [PubMed] [Google Scholar]
- 29.Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. 2015;125(9):3347–3355. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 31.Balkwill FR. The chemokine system and cancer. J Pathol. 2012;226(2):148–157. doi: 10.1002/path.3029. [DOI] [PubMed] [Google Scholar]
- 32.Pine JK, Morris E, Hutchins GG, et al. Systemic neutrophil-to-lymphocyte ratio in colorectal cancer: the relationship to patient survival, tumour biology and local lymphocytic response to tumour. Br J Cancer. 2015;113(2):204–211. doi: 10.1038/bjc.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ying HQ, Deng QW, He BS, et al. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31(12):305. doi: 10.1007/s12032-014-0305-0. [DOI] [PubMed] [Google Scholar]
- 34.Lin MS, Huang JX, Yu H. Prognostic significance of Glasgow prognostic score in patients with stage II colorectal cancer. Int J Clin Exp Med. 2015;8(10):19138–19143. [PMC free article] [PubMed] [Google Scholar]
- 35.Shibutani M, Maeda K, Nagahara H, Iseki Y, Ikeya T, Hirakawa K. Prognostic significance of the preoperative lymphocyte-to-monocyte ratio in patients with colorectal cancer. Oncol Lett. 2017;13(2):1000–1006. doi: 10.3892/ol.2016.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibutani M, Maeda K, Nagahara H, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with metastatic colorectal cancer. World J Gastroenterol. 2015;21(34):9966–9973. doi: 10.3748/wjg.v21.i34.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moses K, Brandau S. Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol. 2016;28(2):187–196. doi: 10.1016/j.smim.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Felix K, Gaida MM. Neutrophil-derived proteases in the microenvironment of pancreatic cancer-active players in tumor progression. Int J Biol Sci. 2016;12(3):302–313. doi: 10.7150/ijbs.14996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138(6):2101–2114. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 40.Lin EY, Pollard JW. Role of infiltrated leucocytes in tumour growth and spread. Br J Cancer. 2004;90(11):2053–2058. doi: 10.1038/sj.bjc.6601705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann TK, Dworacki G, Tsukihiro T, et al. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8(8):2553–2562. [PubMed] [Google Scholar]
- 42.Cézé N, Thibault G, Goujon G, et al. Pre-treatment lymphopenia as a prognostic biomarker in colorectal cancer patients receiving chemotherapy. Cancer Chemother Pharmacol. 2011;68(5):1305–1313. doi: 10.1007/s00280-011-1610-3. [DOI] [PubMed] [Google Scholar]
- 43.Qiu J, Yu Y, Fu Y, Ye F, Xie X, Lu W. Preoperative plasma fibrinogen, platelet count and prognosis in epithelial ovarian cancer. J Obstet Gynaecol Res. 2012;38(4):651–657. doi: 10.1111/j.1447-0756.2011.01780.x. [DOI] [PubMed] [Google Scholar]
- 44.Lu K, Zhu Y, Sheng L, Liu L, Shen L, Wei Q. Serum fibrinogen level predicts the therapeutic response and prognosis in patients with locally advanced rectal cancer. Hepatogastroenterology. 2011;58(110–111):1507–1510. doi: 10.5754/hge11133. [DOI] [PubMed] [Google Scholar]
- 45.Palumbo JS, Talmage KE, Massari JV, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105(1):178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]