Abstract
Purpose
Cardiovascular magnetic resonance (CMR) perfusion has been established as a useful imaging modality for the detection of coronary artery disease (CAD). However, there are several limitations when applying standard, ECG-gated stress/rest perfusion CMR to patients with atrial fibrillation (AF). In this study we investigate an approach with no ECG gating and a rapid rest/stress perfusion protocol to determine its accuracy for detection of CAD in patients with AF.
Methods
26 patients with AF underwent a rapid rest/regadenoson stress CMR perfusion imaging protocol, and all patients had X-ray coronary angiography. An ungated radial myocardial perfusion sequence was used. Imaging protocol included: rest perfusion image acquisition, followed nearly immediately by administration of regadenoson to induce hyperemia, 60 second wait, and stress image acquisition. CMR perfusion images were interpreted by three blinded readers as normal or abnormal. Diagnostic accuracy was evaluated by comparison to X-ray angiography.
Results
21 of the CMR rest/stress perfusion scans were negative, and 5 were positive by angiography criteria. Majority results of the ungated datasets from all of the readers showed a sensitivity, specificity and accuracy of 80%, 100% and 96%, respectively, for detection of CAD.
Conclusions
An ungated, rapid rest/stress regadenoson perfusion CMR protocol appears to be useful for the diagnosis of obstructive CAD in patients with AF.
Keywords: Atrial fibrillation, ECG gating, ungated cardiac MR, myocardial perfusion imaging, cardiac perfusion
Background
Coronary artery disease (CAD) remains a common cause of morbidity and mortality [1]. Due to advancements in technology, perfusion cardiac magnetic resonance imaging (CMR) has emerged as a modality with superior accuracy to single positron emission computed tomography (SPECT)[2–4]. Perfusion CMR has several advantages over SPECT, including high spatial and temporal resolution and lack of exposure to radiation[5]. In addition, CMR can provide comprehensive assessment of ventricular function and myocardial infarction. However, perfusion CMR is traditionally limited by both the need for ECG gating, which is challenging in patients with an irregular cardiac rhythm[6], and by long acquisition times. In particular, non-invasive imaging for assessment of CAD can be challenging in patients with atrial fibrillation (AF)[7]. Although multiple studies have shown sensitivity and specificity of perfusion CMR for obstructive CAD greater than 80%,[2–4, 8, 9] diagnostic accuracy of this modality has received little study in patients with AF. Greulich et al. recently reported a diagnostic accuracy of 70% in 64 patients with AF using a standard ECG-gated breath-hold perfusion CMR protocol[10]. This suggests that diagnostic accuracy of conventional perfusion CMR may be reduced in patients with AF.
Standard ECG-gated CMR imaging protocols rely on repetitive timing of a cardiac cycle to obtain image information synchronized to the same phase of the cardiac cycle over multiple heartbeats to eliminate cardiac motion, which is problematic in arrhythmias with variable R-R intervals such as AF. In addition, magnetic field gradients and magneto-hydrodynamic effects make ECG-gating challenging in the MR environment[11]. Ungated perfusion acquisitions have previously been described by our group and others[12–14]. These acquisition methods run continuously without any ECG gating, and thus are unaffected by poor gating. This continuous, rapid acquisition allows for collection of multiple images in each cardiac cycle, resulting in increased temporal resolution relative to standard gated acquisitions. Our group previously reported favorable initial results in 8 subjects using adenosine stress and an ungated acquisition, with a sensitivity of 92% and specificity of 92%[13].
In addition to ECG-gating challenges, perfusion CMR is limited by long acquisition times. In most perfusion protocols, stress imaging is performed prior to rest imaging because it has the advantage of obtaining stress perfusion while the myocardium is contrast naïve, possibly increasing sensitivity and ensuring that there are no late enhancement effects related to myocardial infarct during this critical portion of the study. With regadenoson, stress first can be more challenging since flow and heartrate can remain elevated longer than with adenosine. Even with aminophylline to reverse the effects, mixed results are reported as to whether perfusion does[15] or does not[16] return to baseline after 15 minutes. To address these issues we utilize a rest-first protocol, where rest perfusion imaging is performed and immediately followed by administration of regadenoson for stress perfusion imaging. Additionally, by performing the rest portion first, we allow for both perfusion sequences to be obtained in rapid succession, minimizing overall imaging time. In this work, we aim to evaluate the diagnostic accuracy of a rapid, rest-first perfusion CMR protocol using a unique ungated pulse sequence for detection of CAD in patients with AF.
Methods
Study Participants
26 patients with a history of AF (age 69 ± 12 years, 15 males and 11 females) who were either being referred to X-ray coronary angiography or who had recently undergone clinically indicated X-ray coronary angiography without intervention within 30 days were included in this prospective study from January 2013 to November 2015. Exclusion criteria were contraindications to regadenoson stress agent (e.g. atrioventricular block, reversible airway disease), contraindication to gadolinium based contrast agent (allergy or GFR < 30 ml/min per 1.73 m2), the presence of pacemakers or defibrillators, inability to lie flat for the study, pregnancy, and claustrophobia. Baseline clinical characteristics of the study population are summarized in Table 1. Participants were instructed to avoid caffeine 12 hours prior to CMR imaging. Written consent was obtained from all participants. The University of Utah Institutional Review Board approved the study.
Table 1.
| Number | Percent | |
|---|---|---|
|
|
||
| Female Gender | 11 | 42 |
|
|
||
| Hypertension | 16 | 62 |
|
|
||
| Diabetes | 7 | 27 |
|
|
||
| CKD | 1 | 4 |
|
|
||
| Hyperlipidemia | 19 | 73 |
|
|
||
| PVD | 3 | 12 |
|
|
||
| Stroke | 1 | 4 |
|
|
||
| Prior MI | 3 | 12 |
|
|
||
| CAD | 11 | 42 |
|
|
||
| Heart failure | 12 | 46 |
|
|
||
| Valvular disease | 5 | 19 |
|
|
||
| Family Hx CAD | 13 | 52 |
|
|
||
| Tobacco use | 2 | 8 |
|
|
||
| Prior PCI | 4 | 15 |
|
|
||
| Prior CABG | 2 | 8 |
|
|
||
| Prior ablation | 4 | 15 |
|
|
||
CKD = chronic kidney disease; PVD = peripheral vascular disease; CAD = coronary artery disease; PCI = percutaneous coronary intervention; Hx = history; CABG = coronary artery bypass grafting
CMR Imaging Protocol and Image Reconstruction
A unique ungated saturation recovery radial turboFLASH sequence was used on a 3T MRI scanner (Verio, Siemens Healthcare, Erlangen, Germany). The sequence did not use any information from the ECG. Instead the sequence obtained images rapidly (~50ms) and consecutively, with a short break after each set of 5 slices to perform another saturation pulse and 40 ms delay. TR/TE=2.2/1.2 ms, 26cm field of view and 144 points in each readout (oversampled to 288 acquisition points) were used. Acquired spatial resolution was ~1.8×1.8×8mm, although the reconstructed resolution can vary spatially[17]. Five slices were acquired after a single saturation pulse with a 40 ms delay. For each slice, twenty radial k-space lines were acquired with golden ratio based angular spacing[18]. Each image was acquired during free breathing in 42–53 ms. The set of a saturation pulse plus five slices was repeatedly acquired, approximately four times per second, with no ECG gating. Hence, each slice was acquired at multiple phases of the cardiac cycle, and these phases varied each beat. This approach provides a “real-time” like set of images of a beating heart during gadolinium uptake and washout. We have described this type of ungated sequence in detail in previous works [13, 19].
Instead of a standard stress/rest sequence, we performed a rest/stress protocol in the following order: rest image acquisition (0.05 mmol/kg gadoteridol), administration of 0.4 mg regadenoson intravenously into a peripheral vein to induce pharmacological hyperemia, ~60 second wait to allow for peak effect [20, 21], and then stress image acquisition (0.075 mmol/kg gadoteridol) (Fig. 1). To overcome the potential issue of peri-infarct ischemia with a rest-first sequence, we allowed very little time between rest and stress perfusion scans. In order to minimize the risk of signal saturation during the stress scan, a lower dose of contrast agent was used in the rest portion of the study. Time for the entire perfusion CMR protocol was recorded for each patient. The patient was instructed to breathe shallowly during both rest and stress imaging. Ungated perfusion images were then reconstructed using our previously described, iterative compressed sensing method that includes parallel imaging and spatial and temporal total variation constraints[13, 22]. Reconstructions consisted of five short axis slices, evenly spaced through the left ventricle, at both rest and stress. Standard cine and late gadolinium enhanced (LGE) images were obtained in all patients, but were not made available to the readers for analysis. Therefore the diagnostic accuracy results and imaging time reported reflect those of the perfusion rest and stress perfusion sequences only.
Fig. 1.
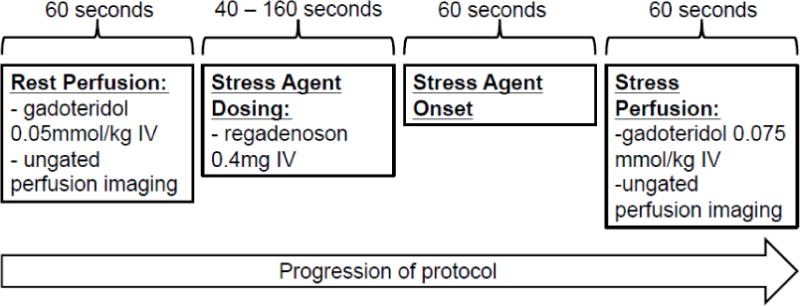
The protocol for rapid regadenoson ungated CMR perfusion imaging is shown. Rest perfusion imaging was completed and stress agent dosing was administered immediately with stress perfusion imaging. Average scan time for the entire perfusion protocol was 4 minutes and 40 seconds ± 60 seconds.
Image Analysis
Ungated CMR perfusion images were interpreted by 3 experienced readers as normal or abnormal, without viewing cine or late gadolinium enhancement (LGE) images. Readers were blinded to angiography results and all other clinical data. Images were presented to the readers in a random order. A 16-segment AHA model was used to report perfusion defects by visual assessment of short axis slices by comparison of rest and stress images. Presence of a perfusion defect in a single segment was considered positive. Fleiss’ kappa statistic was computed to assess interobserver agreement. All readers scored images for quality on a scale of 1 (lowest) to 5 (highest).
X-Ray Coronary Angiography
All patients underwent clinically indicated X-ray coronary angiography performed and analyzed visually by interventional cardiologists who were blinded to the perfusion CMR findings. Obstructive CAD was defined as luminal narrowing with ≥50% stenosis in 2 orthogonal planes present in the left main coronary artery, or ≥70% stenosis in 2 orthogonal planes present in ≥1 of the three main coronary arteries or in a major side branch of ≥2 mm diameter. In patients with a history of coronary artery bypass grafting, ≥70% stenosis in the grafts or non-grafted vessels was defined as obstructive CAD. Fractional flow reserve (FFR) was performed at the clinical discretion of the operator. If performed, FFR < 0.8 was considered obstructive CAD. Perfusion CMR was performed either prior to X-ray coronary angiography or within 30 days after X-ray coronary angiography if there was no intervention.
Statistical analysis
STATA 14 (StataCorp, College Station, Texas) was used for statistical analyses. Results for the diagnostic accuracy of perfusion CMR and its confidence intervals were calculated both by using the majority diagnosis of 3 readers (diagnosis of at least 2 of 3 readers) and by pooling each diagnosis from all three readers. Unpaired t-tests were used to compare image quality scores between patients in AF versus sinus rhythm at the time of the scan.
Results
Clinical characteristics of patients enrolled in the study are shown in Table 1. All of the patients underwent clinically indicated X-ray coronary angiography. 21 (81%) were negative and 5 (19%) were positive by angiography criteria as described above. 15 patients were in AF during perfusion CMR imaging. Average scan time for the rest/stress perfusion protocol was 4.7±1.0 minutes. Using the majority of 3 readers, 4 studies were read as positive, and 22 were read as negative. Sensitivity, specificity, and accuracy for obstructive CAD were 80% (CI 30 – 99%), 100% (CI 81 – 100%), and 96% (CI 78 – 100%), respectively. Using the majority result, there was one false negative out of the 22 studies read as negative. There were no false positives. Using the pooled results, there were 4 total false negative reads out of 63 negative reads. Sensitivity, specificity, and accuracy were 73% (CI 45% – 91%), 94% (CI 84% – 98%), and 88% (CI 77% – 94%), respectively (Table 2). There were 4 false positive reads out of 15 total positive reads. Fleiss’ kappa was 0.82, suggesting good interobserver agreement. Figure 2 demonstrates a perfusion defect detected on CMR along with the corresponding vessel involvement on the coronary angiogram. Out of 5 patients who were diagnosed to have significant CAD by angiography, 2 patients had right coronary artery involvement, 1 patient had left anterior descending artery involvement, and 2 patients had three-vessel disease. Figure 3 shows example stress perfusion images from each positive case. Pooled image quality scores were not significantly different in patients with normal sinus rhythm vs. those in AF at the time of the scan (3.2±0.4 vs. 3.2±0.4, p = 0.74). Accuracy was 93% in patients in AF during the scan and 100% in patients in normal sinus rhythm during the scan using the majority results (p = 0.40).
Table 2.
| Majority Results (CI) | Pooled Results (CI) | |
|---|---|---|
|
|
||
| Sensitivity | 80% (30 – 99%) | 73% (45 – 91%) |
|
|
||
| Specificity | 100% (81 – 100%) | 94% (84 – 98%) |
|
|
||
| Accuracy | 96% (78 – 100%) | 88% (77 – 94%) |
|
|
||
CI = 95% confidence interval
Fig. 2.
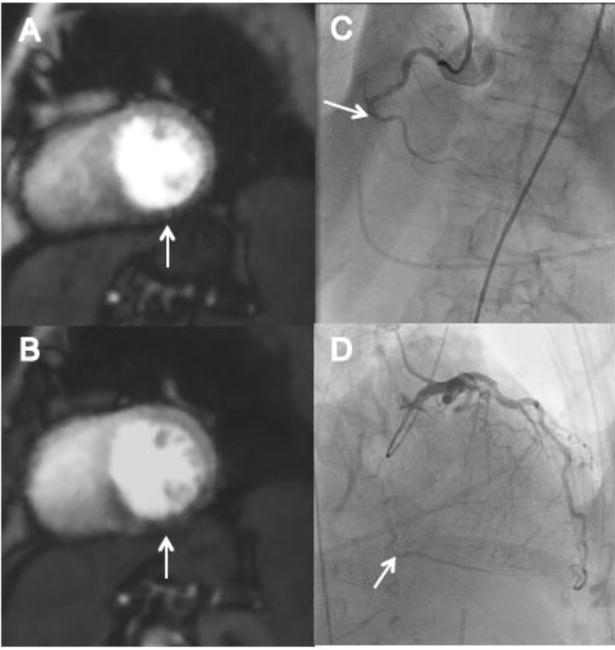
Rest and stress perfusion images showing inferior wall defect with corresponding coronary X-ray angiography of an example patient are shown. The patient is a 74 year old female with a history of hypertension who presented with syncope and chest pain, found to have onset of atrial fibrillation in the setting of a non-ST segment elevation acute myocardial infarction. (A) Rest perfusion image demonstrating mid inferior wall defect. (B) Stress perfusion image demonstrating mid inferior wall defect with septal wall extension. (C) Coronary X-ray angiography demonstrating chronic total occlusion of the mid right coronary artery. (D) Coronary X-ray angiography demonstrating distal right coronary artery filling via collaterals.
Fig. 3.
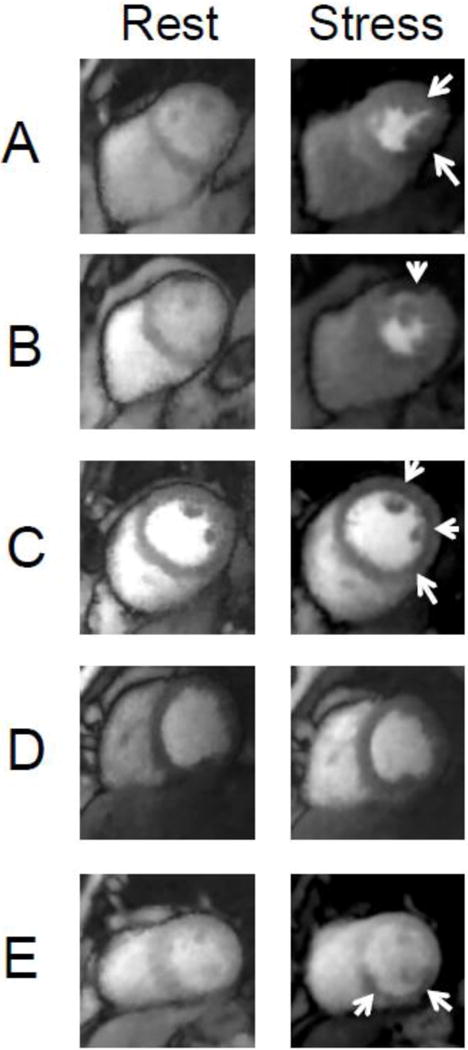
Example ungated rest and stress perfusion images for each patient with obstructive CAD by X-ray angiography are shown. (A) A patient with a total occlusion of the posterior descending artery with an inferior wall stress perfusion defect. (B) A patient with three-vessel obstructive CAD with a matched anterior perfusion defect. (C) A patient with three-vessel obstructive CAD with a diffuse subendocardial perfusion defect. (D) A patient with obstructive lesions of the mid left anterior descending artery and first diagonal branch without associated perfusion defect (false negative study). (E) A patient with multiple obstructive lesions in the right coronary artery with a stress perfusion defect in the inferior wall and inferior septum.
Discussion
While there are many patients with AF in need of noninvasive assessment of CAD, current techniques have significant limitations[7]. We propose a rapid, ungated perfusion CMR imaging protocol using regadenoson, and perform a preliminary analysis of its accuracy and feasibility. The overall diagnostic accuracy of 96% compares favorably with that in previously published CMR perfusion imaging studies in patients without arrhythmia[2–4, 8]. Greulich et al. recently report results in 64 patients with AF using a standard stress-first, ECG-gated perfusion CMR protocol[10]. They report a sensitivity of 71%, specificity of 69%, and accuracy of 70%. In addition, the readers in the current study do not view any cine or LGE images during image analysis. This is in contrast to the Greulich study and others[2–4, 10] where cine and LGE images are analyzed with the perfusion CMR. Thus, the data presented here does not account for the further diagnostic benefit that cine and LGE images may provide. This may suggest that an ungated approach could improve accuracy in this population. However, it would be valuable to perform a direct comparison between a gated and ungated approach, and to perform a larger multi-center study.
Our results are consistent with our previously published preliminary study using an adenosine ungated stress-first protocol[13], which also showed high diagnostic accuracy. In addition to having a significantly larger number of patients, in this study we use a rest-first protocol. In stress-first protocols, the myocardium is contrast naïve during the stress portion of the study. This, in theory, maximizes contrast-to-noise ratio, and thus, the sensitivity for detection of a perfusion defect during this critical portion of the study. However, this also requires that regadenoson be given prior to rest imaging, and this may result in lingering hyperemia even after administration of aminophylline and a standard 15 minute rest period[16]. A rest-first approach avoids any residual hyperemia during rest imaging. As well, it may be possible that late enhancement of infarcted myocardium after the rest contrast dose could mask a perfusion defect on the stress imaging. Therefore, we perform the stress acquisition immediately after the rest acquisition, before late enhancement effects occur. This has the additional advantage of reducing overall study time. The results of our study suggest that the rapid rest-first protocol employed in this study does not significantly reduce diagnostic accuracy.
Conventional ECG-gated perfusion CMR protocols have a number of inherent challenges. It is often difficult to choose the number of slices to acquire in each heartbeat since the heart rate typically increases significantly with stress. Even a slight change in heart rate can lead to data acquisition every other beat, which is only 50% efficient even when there is perfect ECG triggering (no missed or extra triggers from the waveform). The ungated approach addresses these issues. The number of slices is fixed prior to data acquisition, and increasing the number of slices leads to a consistent, predictable change in temporal resolution. Data acquisition is inherently more efficient as compared to ECG-gated protocols. As the acquisition is continuous for each block of 5 slices, maximal information is acquired during the brief first-pass of the contrast agent. In this study, we obtained 5 slices through the myocardium every 250 ms, significantly higher temporal resolution than a single frame of each slice per cardiac cycle that would be achieved with a gated-acquisition.
Ungated CMR acquisitions for both cine[11, 23, 24] and perfusion[12–14] imaging have been described. These acquisitions also allow for retrospective “self-gating” where either the k-space rays or the images themselves are used to bin each into the correct phase of the cardiac cycle. Ungated CMR perfusion acquisition leads to visualization of myocardial perfusion during all phases of the cardiac cycle, which could affect diagnostic accuracy. However, Motwani et al. demonstrated similar diagnostic accuracy using systolic or diastolic datasets, although they acquired data of only a single slice[25], suggesting that phase of the cardiac cycle does not affect diagnostic accuracy. Guttman et al. demonstrated a single slice perfusion study with data sharing using the “real-time” acquisition that provided wall motion and first pass perfusion simultaneously[26], another potential advantage of an ungated approach.
In this study, we use highly accelerated acquisitions with advanced reconstruction methods and apply the “real-time” concept to multi-slice myocardial perfusion imaging. We use a radial acquisition which has inherently high spatial resolution in all directions and is robust to motion. Likely as a result, we do not perceive dark rim artifacts, which often complicate the interpretation of perfusion CMR, in any of the cases in this study. In Cartesian acquisitions, the lower spatial resolution of the phase encoding direction contributes to dark rim artifact[27]. Others have shown reduced dark rim artifact by smoothing (apodizing) the images along with a radial acquisition [28]. The smoothing step was not used here but the reconstruction includes temporal and spatial total variation regularization that may contribute to seeing less dark rim artifact. The 2D ungated sequence here uses a saturation recovery pulse because it is a well tested and universally accepted method for perfusion studies. Performing ungated steady-state acquisitions with a 3D readout without the saturation pulse, which is not required in an ungated approach, may be the subject of future work [12].
Given that this was a study designed to determine diagnostic accuracy, X-ray coronary angiography was used as the reference as it remains the gold standard for diagnosis of obstructive CAD. Other perfusion imaging techniques such as SPECT, echocardiography, computed tomography, or positron emission tomography were not used in comparison, although future studies to directly compare the diagnostic accuracy to these techniques would be useful to determine the most accurate noninvasive perfusion imaging method. FFR was used only when the operator thought it would be useful to clarify significance of an observed stenosis. FFR was performed in 2 of 26 patients and was negative in both. FFR has been shown to be a better predictor of ischemia and benefit from revascularization than X-ray angiography alone[29]. A number of recent studies of perfusion CMR have used invasive FFR as a reference standard with good results.[9, 30] A meta-analysis by Jiang et al. reports a sensitivity and specificity of 88% each when FFR is used as a reference standard. Future studies of the rapid ungated approach could possibly be better validated using FFR as the reference standard in all patients, even in patients without stenoses that appear significant by visual assessment.
The prevalence of obstructive CAD was lower than expected, which is a limitation of this work, where 5 of 26 patients had obstructive CAD. This may reflect the referral of lower-risk AF patients for coronary angiography, as non-invasive alternatives are less reliable in this population. Given this limitation, the sensitivity for CAD has a wide confidence interval in this study. Larger studies in populations with a higher prevalence of obstructive CAD are necessary to further validate the sensitivity of this novel approach to detect CAD in patients with AF.
Conclusions
Common challenges in perfusion CMR protocols include lingering hyperemia after administration of regadenoson when using a stress-first protocol, long total imaging time, and ECG-gating in patients with atrial fibrillation. The unique, ungated myocardial perfusion sequence evaluated in this study, with a rapid rest-first regadenoson stress perfusion CMR protocol, shows good diagnostic accuracy in patients with AF, while addressing these concerns and reducing overall imaging time.
Supplementary Material
Acknowledgments
We would like to thank Ashlee Rooks, who coordinated the study, the three readers for the study, and the MRI technologists who performed the image acquisition. We would also like to thank each of the patients who participated.
Funding
We would like to acknowledge funding sources Astellas Pharma and the National Heart, Lung, and Blood Institute of the National Institutes of Health R01HL113224. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding entities.
Abbreviations
- CMR
cardiovascular magnetic resonance
- CAD
coronary artery disease
- AF
atrial fibrillation
- SPECT
single positron emission computed tomography
- MRI
magnetic resonance imaging
- LGE
late gadolinium enhancement
- FFR
fractional flow reserve
Footnotes
Trial Registrations: ClinicalTrials.gov Identifier: NCT01710254, Date of registration: October 2, 2012.
Declarations
Ethics approval and consent to participate
The University of Utah Institutional Review Board approved the study. Written consent for participation was obtained from all participants.
Consent for publication
Written consent for publication was obtained from all study participants.
Availability of data and material
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
EB, IH, GA, and LC made significant contributions to the data acquisition, analysis and interpretation, made significant contributions to drafting the manuscript, and read and approved the final manuscript. PS and DL made significant contributions to data acquisition, and read and approved the final manuscript. AS and LJ made significant contributions to the image interpretation design and read and approved the final manuscript. CM made significant contributions to the conception and design of the study, analysis and interpretation, and read and approved the final manuscript. BW and ED made significant contributions to the conception and design of the study, data acquisition, analysis and interpretation, and critically reviewed and approved the final manuscript. All authors agreed to be accountable for the accuracy and integrity of the work.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379:453–460. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwitter J, Wacker CM, van Rossum AC, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29:480–489. doi: 10.1093/eurheartj/ehm617. [DOI] [PubMed] [Google Scholar]
- 4.Schwitter J, Wacker CM, Wilke N, et al. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J. 2013;34:775–781. doi: 10.1093/eurheartj/ehs022. [DOI] [PubMed] [Google Scholar]
- 5.Lanza GA, Buffon A, Sestito A, et al. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol. 2008;51:466–472. doi: 10.1016/j.jacc.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 6.Ishida N, Sakuma H, Motoyasu M, et al. Noninfarcted myocardium: correlation between dynamic first-pass contrast-enhanced myocardial MR imaging and quantitative coronary angiography. Radiology. 2003;229:209–216. doi: 10.1148/radiol.2291021118. [DOI] [PubMed] [Google Scholar]
- 7.Smit MD, Tio RA, Slart RH, Zijlstra F, Van Gelder IC. Myocardial perfusion imaging does not adequately assess the risk of coronary artery disease in patients with atrial fibrillation. Europace. 2010;12:643–648. doi: 10.1093/europace/eup404. [DOI] [PubMed] [Google Scholar]
- 8.Hamon M, Fau G, Née G, Ehtisham J, Morello R. Meta-analysis of the diagnostic performance of stress perfusion cardiovascular magnetic resonance for detection of coronary artery disease. J Cardiovasc Magn Reson. 2010;12:29. doi: 10.1186/1532-429X-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang B, Cai W, Lv X, Liu H. Diagnostic Performance and Clinical Utility of Myocardial Perfusion MRI for Coronary Artery Disease with Fractional Flow Reserve as the Standard Reference: A meta-analysis. Heart Lung Circ. 2016 doi: 10.1016/j.hlc.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Greulich S, Steubing H, Birkmeier S, et al. Impact of arrhythmia on diagnostic performance of adenosine stress CMR in patients with suspected or known coronary artery disease. J Cardiovasc Magn Reson. 2015;17:94. doi: 10.1186/s12968-015-0195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson AC, White RD, Laub G, McVeigh ER, Li D, Simonetti OP. Self-gated cardiac cine MRI. Magn Reson Med. 2004;51:93–102. doi: 10.1002/mrm.10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiBella EV, Chen L, Schabel MC, Adluru G, McGann CJ. Myocardial perfusion acquisition without magnetization preparation or gating. Magn Reson Med. 2012;67:609–613. doi: 10.1002/mrm.23318. [DOI] [PubMed] [Google Scholar]
- 13.Harrison A, Adluru G, Damal K, et al. Rapid ungated myocardial perfusion cardiovascular magnetic resonance: preliminary diagnostic accuracy. J Cardiovasc Magn Reson. 2013;15:26. doi: 10.1186/1532-429X-15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen D, Sharif B, Bi X, et al. Quantification of myocardial blood flow using non-electrocardiogram-triggered MRI with three-slice coverage. Magn Reson Med. 2016;75:2112–2120. doi: 10.1002/mrm.25787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dandekar VK, Bauml MA, Ertel AW, Dickens C, Gonzalez RC, Farzaneh-Far A. Assessment of global myocardial perfusion reserve using cardiovascular magnetic resonance of coronary sinus flow at 3 Tesla. J Cardiovasc Magn Reson. 2014;16:24. doi: 10.1186/1532-429X-16-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhave NM, Freed BH, Yodwut C, et al. Considerations when measuring myocardial perfusion reserve by cardiovascular magnetic resonance using regadenoson. J Cardiovasc Magn Reson. 2012;14:89. doi: 10.1186/1532-429X-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wech T, Stab D, Budich JC, et al. Resolution evaluation of MR images reconstructed by iterative thresholding algorithms for compressed sensing. Med Phys. 2012;39:4328–4338. doi: 10.1118/1.4728223. [DOI] [PubMed] [Google Scholar]
- 18.Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans Med Imaging. 2007;26:68–76. doi: 10.1109/TMI.2006.885337. [DOI] [PubMed] [Google Scholar]
- 19.Likhite D, Adluru G, Hu N, McGann C, DiBella E. Quantification of myocardial perfusion with self-gated cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2015;17:14. doi: 10.1186/s12968-015-0109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Booker OJ, Bandettini P, Kellman P, et al. Time resolved measure of coronary sinus flow following regadenoson administration. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2011;13:O74. [Google Scholar]
- 21.DiBella EV, Fluckiger JU, Chen L, et al. The effect of obesity on regadenoson-induced myocardial hyperemia: a quantitative magnetic resonance imaging study. The international journal of cardiovascular imaging. 2012;28:1435–1444. doi: 10.1007/s10554-011-9949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adluru G, McGann C, Speier P, Kholmovski EG, Shaaban A, Dibella EV. Acquisition and reconstruction of undersampled radial data for myocardial perfusion magnetic resonance imaging. J Magn Reson Imaging. 2009;29:466–473. doi: 10.1002/jmri.21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowe ME, Larson AC, Zhang Q, et al. Automated rectilinear self-gated cardiac cine imaging. Magn Reson Med. 2004;52:782–788. doi: 10.1002/mrm.20212. [DOI] [PubMed] [Google Scholar]
- 24.Larson AC, Kellman P, Arai A, et al. Preliminary investigation of respiratory self-gating for free-breathing segmented cine MRI. Magn Reson Med. 2005;53:159–168. doi: 10.1002/mrm.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motwani M, Fairbairn TA, Larghat A, et al. Systolic versus diastolic acquisition in myocardial perfusion MR imaging. Radiology. 2012;262:816–823. doi: 10.1148/radiol.11111549. [DOI] [PubMed] [Google Scholar]
- 26.Guttman MA, Dick AJ, Raman VK, Arai AE, Lederman RJ, McVeigh ER. Imaging of myocardial infarction for diagnosis and intervention using real-time interactive MRI without ECG-gating or breath-holding. Magn Reson Med. 2004;52:354–361. doi: 10.1002/mrm.20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Bella EV, Parker DL, Sinusas AJ. On the dark rim artifact in dynamic contrast-enhanced MRI myocardial perfusion studies. Magn Reson Med. 2005;54:1295–1299. doi: 10.1002/mrm.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharif B, Dharmakumar R, LaBounty T, et al. Towards elimination of the dark-rim artifact in first-pass myocardial perfusion MRI: removing Gibbs ringing effects using optimized radial imaging. Magn Reson Med. 2014;72:124–136. doi: 10.1002/mrm.24913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 30.Li M, Zhou T, Yang LF, Peng ZH, Ding J, Sun G. Diagnostic accuracy of myocardial magnetic resonance perfusion to diagnose ischemic stenosis with fractional flow reserve as reference: systematic review and meta-analysis. JACC Cardiovasc Imaging. 2014;7:1098–1105. doi: 10.1016/j.jcmg.2014.07.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


