Abstract
Mesenchymal Stem Cells (MSCs) possess important characteristics that could be exploited in therapeutic strategies for Type 1 Diabetes (T1D) and for certain complications of Type 2 Diabetes (T2D). MSCs can inhibit autoimmune, alloimmune and inflammatory processes. Moreover, they can promote the function of endogenous and transplanted pancreatic islets. Furthermore, they can stimulate angiogenesis. MSC functions are largely mediated by their secretome, which includes growth factors, exosomes, and other extracellular vesicles. MSCs have shown a good safety profile in clinical trials. MSC-derived exosomes are emerging as an alternative to the transplantation of live MSCs. MSCs harvested from different anatomical locations (e.g. bone marrow, umbilical cord, placenta, adipose tissue, and pancreas) have shown differences in gene expression profiles and function. Data from clinical trials suggest that umbilical cord-derived MSCs could be superior to bone marrow-derived MSCs for the treatment of T1D. Autologous MSCs from diabetic patients may present abnormal functions. BM-MSCs from T1D patients exhibit gene expression differences that may impact in vivo function. BM-MSCs from T2D patients seem to be significantly impaired due to the T2D diabetic milieu. In this review, we highlight how the harvesting site and donor derivation can affect the efficacy of MSC-based treatments for T1D and T2D.
Keywords: Islet transplantation, type 1 diabetes, beta cell replacement, cell therapy, xenotransplantation, stem cells
Cell-based strategies for diabetes
Glucose metabolism and glycemia are controlled by the secretion of insulin from pancreatic islet beta cells. Beta cells can be lost, can be impaired, or can become impaired due to very different mechanisms. The lack or insufficiency of their insulin release function leads to a group of diseases with characteristic pathological features: abnormal metabolism of carbohydrates and elevated levels of glucose in the blood and urine1.
Type 1 diabetes (T1D) is a multifactorial chronic disorder that is characterized by the autoimmune destruction of insulin-producing pancreatic beta cells: the disease becomes clinically overt when the vast majority of beta cell lose function or are lost2–4. To date, there is no definitive cure for this disease and life-long exogenous insulin replacement is required5.
Differently, Type 2 diabetes (T2D) is characterized by insulin resistance, hyperglycemia and eventually dysfunction of the insulin-producing cells, and it is mainly caused by diet and lifestyle choices6.
Transplantation of cadaveric pancreas or pancreatic islets can correct diabetes-restoring normo-glycemia in T1D patients7–11. Unfortunately, the low number of organs available for transplantation and the need for immunosuppression (often characterized by serious side effects12) limit these transplantation strategies13. The identification of an inexhaustible source of transplantable insulin producing beta cells is an important hurdle that still needs to be overcome13, but the recent activation of clinical trials testing the safety of embryonic stem cell-derived pancreatic progenitor cells represents an important milestone in that direction (NCT 02239354, ClinicalTrials.gov). Beta cell replacement would be extremely beneficial for T1D patients, and beta cell supplementation could be beneficial for a subset of T2D patients. Nevertheless, patients with T1D and T2D would benefit from strategies that modulate immunity and inflammation, that protect or sustain beta cells, that improve islet transplantation and that stimulate angiogenesis. During the last decade, a cell population has catalyzed significant interest and has been tested in a number of clinical trials for diabetes: Mesenchymal stem cells (MSCs)14. MSC possess important characteristics that could be exploited in cell-based strategies for T1D and for complications of T2D. These cells showed a good safety profile in initial clinical trials for T1D and T2D. In order to maximize their therapeutic efficacy, important considerations related to the harvesting site and to donor derivation need to be made.
Mesenchymal stem cells
Mesenchymal Stem Cells (MSCs) were first described in the 1970s by Friedenstein et al15 who isolated a population of cells from mouse bone marrow (BM) and showed these had the ability to form colonies. About twenty years later Caplan16 defined the corresponding terminology, and approximately ten years later MSCs were identified in human adult BM17,18. MSCs are characterized by the adherence to plastic in culture, expression of a set of surface markers in the absence of lineage-specific marker expression, and potential to differentiate into multiple mesodermal lineages (osteoblasts, adipocytes, and chondroblasts)19,20. MSCs are potent immunomodulators, exerting suppressive functions on immune effector cells and orchestrating the action of other regulatory cells21–29. MSCs are able to migrate to sites of inflammation and to regulate the traffic of different hematopoietic cells30. Moreover, MSCs have been shown to promote repair and regeneration of endogenous and transplanted islets14,31,32. Furthermore, they have shown a good safety profile in clinical trials, including a very limited risk of tumor formation32–34.
The functional capacity of MSCs, together with their responsiveness to inflamed or damaged microenvironments, have made them an attractive potential agent for many regenerative, anti-inflammatory, and auto-immune applications for a wide range of disorders35. MSC-based therapies for T1D are mostly focused on alleviating hyperglycemia by inhibiting autoimmunity, stimulating pancreatic beta cell regeneration and function. MSC-based therapies for T2D are more focused on the treatment of co-morbidities36. The main therapeutic effect of MSCs seems to derive from their release of cytokines and soluble factors – molecules determine immunosuppressive, anti-inflammatory, pro-angiogenic and pro-regenerative changes (Figure 1)37. Alleviation of hyperglycemia seems to be the net result of a dampening of the immune responses, along with a stimulation of the survival and of the proliferation of pancreatic progenitors/beta cells38. MSC transdifferentiation towards insulin producing beta cells is not considered a major therapeutic mechanism.
Figure 1.
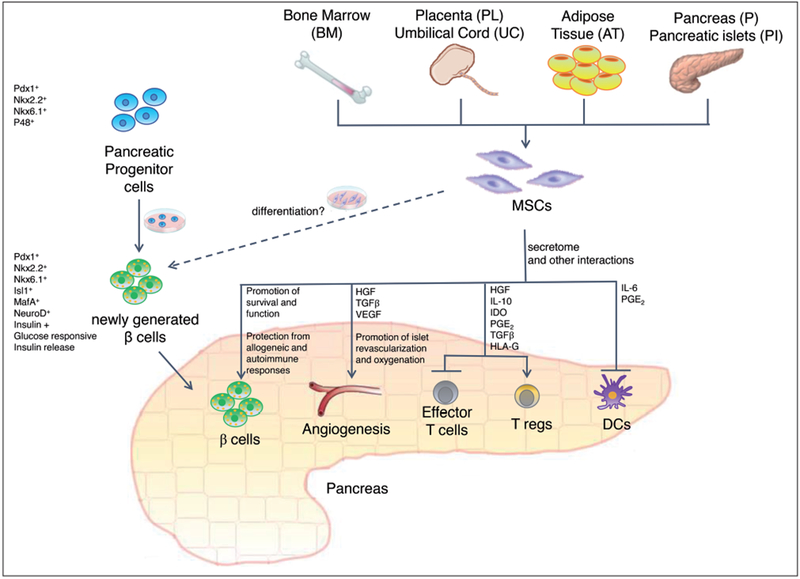
The effect of MSCs is largely mediated by their secretome. MSCs can promote the survival and function of islet beta cells. Moreover, they can stimulate islet revascularization and oxygenation. Furthermore, MSCs can protect islet beta cells from allogeneic and autoimmune responses. MSCs exert multiple immune-modulatory functions, including an inhibition of Effector T cell functions and of DC differentiation, and a stimulation of T reg functions. MSCs could be employed in therapeutic strategies for T1D and T2D: they could be utilized to protect and sustain endogenous or transplanted islet beta cells. Moreover, MSCs could be employed to stimulate angiogenesis and inhibit inflammation in complications of T1D and T2D.
The pathways determining anti-inflammatory and immunosuppressive effects have not been completely elucidated, but direct contact with effector cells, the production of soluble mediators and the activation of regulatory cell subtypes, all may contribute to the MSC effect21–29,39,40. MSCs can inhibit dendritic cells differentiation and maturation, suppress the proliferation of CD4+ and CD8+ T cells, impair the cytotoxic activity of cytotoxic lymphocytes, induce and expand T regulatory cells, and can balance T helper subsets41,42. Thanks to the interaction of MSC receptors with ligands indicating inflamed environments, MSCs selectively home in inflamed tissues and promote tissue repair and regeneration37. MSCs secrete several molecules (such as IL-6, IL-8, TGF-beta, TIMP-2, VEGF, HGF), which can stimulate tissue repair and act as chemo-attractants, recruiting macrophages and endothelial cells at the site of injury or inflammation43,44. MSCs also appear to have angiogenic and trophic potential that improve, in a co-transplant setting, the ability of pancreatic islets to survive the first few days after transplantation13.
In fact, several models of islet transplantation showed positive effects of MSCs in promoting engraftment and increasing survival and function of beta cells45. MSCs from recipient rats mediated such an effect when co-transplanted with allogeneic islets, resulting in long term survival and sustained normoglycemia46. The effect of MSCs in this model could be due either to an anti-inflammatory effect or an immunomodulatory effect, or to a combination of both.
The positive effect observed in this study was paralleled by increased neoangiogenesis at the implant site, a key observation that highlights the multiple mechanisms of action of MSCs46,47.
In stringent models of transplantation in fully allogeneic recipients, the co-administration of MSCs with islets led to highly significant prolongation of graft survival in rodent models48,49. In nonhuman primates, allogeneic MSCs significantly enhanced engraftment and function of co-transplanted islets50. In a subset of animals, additional infusions of MSCs resulted in reversal of rejection episodes and prolongation of islet function50.
Several clinical trials are currently testing MSCs transplantation in patients with T1D and T2D14,32,51. So far, MSC transplantation has showed a good safety profile with a very limited risk of tumor formation32.
An open label pilot trial52 enrolled T1D patients with recent onset of diabetes. Twenty patients were randomized to the group receiving transplantation of autologous bone marrow-derived MSCs (BM-MSCs), or to the control group which only received insulin therapy. The treatment was found to be safe; moreover, at one year after MSCs infusion, most of the patients treated with MSC transplantation showed the preservation of stimulated C-peptide secretion, a key marker of the insulin release from residual beta cells, whereas the control patients showed a decline in C-peptide levels. Patients in both the treated and control groups continued to require insulin therapy and there were no statistically significant differences in insulin requirements and glycated hemoglobin levels between the two groups.
Ongoing clinical trials are also testing the safety and efficacy of MSCs transplantation in patients with T2D14,31 in order to treat common complications of diabetes such as ulcers, limb ischemia, and nephropathy, and to improve metabolic control31,53,54. Treatment with MSCs was reported to be safe, it appeared effective in facilitating wound closure of diabetic foot ulcers55 and in inducing T reg cells in T2D patients56. Allogeneic placenta (PL)-derived MSCs were transplanted in 10 patients with T2D and the infusion was reported to be safe. The patients experienced a reduction in daily insulin requirement, showed a better control of blood glucose fluctuations, and experienced improvements in quality of life57. Interestingly, a recent meta-analysis on clinical reports highlighted that the tissue source of the MSCs impacts the outcome of the cell therapy58.
Harvesting site
An important open issue is represented by the site of MSCs harvest (Figure 1). Historically, as it was mentioned before, the bone marrow has been long investigated as a source of stem cells, and therefore also the studies concerning MSCs in the context of diabetes were conducted on BM-MSCs. However, the clinical applicability of BM-MSCs is limited due to the relatively invasive procedure required for sample collection as well as the marked reduction in cell number, proliferation, and differentiation capacity with the age of the donor76. Thus, various different tissues have been studied as alternatives sources of MSCs.
It is now accepted that MSCs can be harvested from multiple anatomical locations, and it has been widely assumed that MSCs derived from different sources are largely equivalent, at least in terms of surface marker expression and differentiation potential. On the other hand, evidence suggests differences in term of marker/gene expression profiles; these differences may have a profound impact on MSCs function13,77,78 and clinical efficacy58.
In recent years, multiple alternative sources of MSCs have shown a great potential, including umbilical cord (UC), umbilical cord blood (UCB), and adipose tissue (AT). Cells derived from UC and UCB are easily bankable and offer the theoretical advantage of youth6. The advantages of using MSCs from birth-associated tissues have been highlighted by Hass et al79; the use of parts of the neonatal placenta and umbilical cord/Wharton’s jelly is not invasive and raises no ethical concerns, MSCs from these tissues possess increased proliferative capacity in vitro, especially under hypoxic conditions, in comparison to certain MSCs populations obtained from adult tissues.
Another MSCs source that currently commands great attention is the adipose tissue (AT), which can be readily collected and processed for autologous use. AT-MSCs have been found to have proliferative ability and differentiation potential comparable to those of BM-MSCs80. Therefore, adipose tissue offers important advantages when compared to bone marrow, given its availability and ease of collection.
It is now evident that MSCs from these tissues and from BM are morphologically and immunophenotypically similar, but not identical81. UCB-derived MSCs form the fewest colonies and show the highest proliferative capacity, whereas AT-MSCs form the greatest number of colonies, and BM-MSCs have the lowest proliferative capacity. MSCs from AT and UCB82 may gain more popularity because of the versatility of the tissue sources and because of their great potential for a wide range of clinical applications.
Jeon et al83 isolated MSCs from the placenta and adipose tissue, and showed significant molecular differences in the properties of the MSCs according to their cellular source. The cytoskeleton proteins were abundantly expressed in BM-MSCs and in AT-MSCs, while the oxidative stress proteins and apoptosis proteins were abundantly expressed in PL-MSCs. Therefore, authors suggest that PL-MSCs may be more appropriate for treatments that aim to increase therapeutic ability.
In the context of diabetes, the source of the MSCs is considered important. Pancreas and pancreatic islet-derived MSCs (first isolated in 200184) could be considered a better option than other commonly used MSCs85. Pancreatic-islet derived MSCs may have the peculiar ability to enter the pancreatic endocrine differentiation path, although the level of transcriptional and functional maturation is still far from that expected of true beta cells13. The increasing interest in pancreas-derived MSCs is due to their potential use for the modulation of immune function, stimulation of angiogenesis, and potentiation of islet endocrine functions; moreover, these cells may differentiate into beta like cells with a yield superior to that of MSCs from different sources, without the need of additional genetic engineering – but this differentiation potential is still debated85–89. As stated, the tissue source of the transplanted MSCs seems to impact the outcome of the therapy in the clinical setting: importantly, UC-MSCs appeared to be superior to BM-MSCs in improving C-peptide levels in T1D patients58. The main characteristics of MSC harvested from different sources are summarized in Table 1.
Table 1.
Main characteristics of MSC harvested from different sources.
| MSC Source | Main characteristics |
|---|---|
| BM | Long investigated. Invasive procedure for sample collection. Yield may be limited in aging individuals. |
| AT | Morphologically and immunophenotypically similar to BM-MSC. Proliferative ability and differentiation potential similar to BM-MSC. Easy accessible, highly available, easily bankable, no invasive procedure for sample collection. Important advantages for autologous applications. |
| UC, UCB | Morphologically and immunophenotypically similar to BM-MSC. Increased proliferative capacity. Increased expression of oxidative stress proteins. Easy bankable, no invasive procedure for sample collection. |
| PL | Morphologically and immunophenotypically similar to BM-MSC. Increased proliferative capacity. Increased expression of oxidative stress proteins. Easy bankable, no invasive procedure for sample collection. |
| P, PI | Features and differentiation capacity in line with those of MSC from other sources; potential for the stimulation of islet-specific functions, potentially easier differentiation into beta cells. |
Abbreviations: BM, bone marrow; AT, adipose tissue; UC, umbilical cord (including Wharton’s Jelly); UCB, umbilical cord blood; PL, placenta; P, Pancreas; PI, Pancreatic Islets.
Autologous or allogenic
Another important matter of debate is whether autologous or allogenic MSCs are more suitable for therapeutic strategies in T1D and T2D. Under pathological conditions, MSCs can become functionally compromised. Autologous MSCs may present abnormal functions due to the autoimmune process in T1D, or due to the diabetic microenvironment in both T1D and T2D. The main characteristics of autologous BMMSCs isolated from T1D and T2D patients are summarized in Table 2 Allogeneic MSCs may be recognized and may be rejected by the competent immune system of the recipient90, may transmit donor-derived infections or diseases52.
Table 2.
Main characteristics of autologous BM-MSCs isolated from T1D and T2D patients.
| Autologous T1D BM-MSCs | Autologous T2D BM-MSCs |
|---|---|
| No differences compared to healthy BM-MSCs in term of morphology, immune-suppressive activity, and migration capacity | No differences compared to healthy BM-MSCs in term of phenotype, morphology, and multilineage differentiation potential |
| Differential expression of genes related to cytokines, immunomodulation, and wound-healing potential | Decreased potency, these cells appear to be terminally differentiated |
| Dysfunctional secretome composition, affecting pro-angiogenic functions | |
| Several oxidative stress-dependent dysfunctions |
Studies addressing potential abnormalities in MSCs derived from patients with autoimmune or inflammatory disorders are scarce and somewhat contradictory. To date, available evidence is still not strong enough to support a recommendation, and more studies should be performed in order to fully establish advantages and weaknesses of autologous or allogeneic MSCs.
Thus, studies that investigate characteristics of autologous MSCs isolated from both T1D and T2D patients are essential to improve the knowledge of the effect that the host environment has on stem cell function, and therefore to guide future clinical applications.
Autologous T1D-MSCs
Recent studies analyzing functions of T1D BM-MSCs demonstrated that T1D and healthy BM-MSCs exhibit no differences in term of morphology, immune-suppressive activity, and migration capacity91–93. However, some studies revealed differential expression of genes related to cytokines, immunomodulation, and wound-healing potential, which would be important to investigate further.
A study by Yaochite et al93 evaluated the in vitro properties and the in vivo therapeutic efficacy of BM-MSCs isolated from newly diagnosed (6 weeks, corresponding to early stages after clinically overt disease) T1D patients. T1D BM-MSCs showed morphology, immunophenotypic profile, and adipocyte differentiation capacity comparable to healthy MSCs. MSCs in inflammatory environments develop immunosuppressive functions by molecules of acute phase inflammation, especially tumor necrosis factor alpha (TNFα) and interferon gamma (IFN-γ), or toll-like receptor (TLR) ligands94. In the study by Yaochite et al93, microarray analysis was performed and no significant differences were observed in the expression of immunomodulatory genes (PDL1, NOS2, IL10, PTGES, TGFB1, PDL2, HLAG, and TGS6) and licensing-related genes (IFNGR2, TNFR1, IFNGR1, TNFR2, TLR4, and TLR3). However, the HGF gene was significantly downregulated in T1D BM-MSCs93.
When administered to diabetic mice, both T1D-MSCs and healthy donor-derived MSCs showed equal contribution to improving β-cell mass, increasing insulin production and glucose tolerance93. Therefore it seems that T1D-MSCs do not present functional abnormalities93.
Accordingly, Dong et al95 reported that MSCs isolated from diabetic rats decreased blood glucose levels and prevented body weight loss when transplanted into diabetic animals, suggesting that diabetes does not influence MSCs properties and supporting the use of autologous MSCs in the treatment of T1D patients.
On the contrary, Fiorina et al96 supported the hypothesis that transplantation of MSCs derived from nondiabetic donors, rather than autologous MSCs, would be the best option for the treatment of T1D; in fact, they reported that MSCs isolated from non-obese diabetic (NOD) mice were unable to delay the onset of diabetes when administered to pre-diabetic NOD mice and did not reverse hyperglycemia with already established diabetes.
Studies have demonstrated the beneficial role of MSCs on in vivo and in vitro induction/proliferation of Treg cells97,98, but neither the study conducted by Yaochite et al93, nor the study by Fiorina et al96 observed significant modifications. Opposite results were reported by Madec et al99. Yaochite93 suggested that their analyses were performed 35 days after MSCs administration, which may represent too long a period of time to detect alterations in Treg cell frequency. Therefore, on the one hand further experiments should be performed earlier after cell transplantation, and on the other hand the beneficial effects promoted by administration MSCs are not related to late or long-standing expansion of Treg cells93.
Another recent study by Davies et al91 investigated whether BM-MSCs from T1D patients offer a therapeutic cell source equivalent to healthy donors BM-MSCs. Differences in gene expression were observed between healthy and late-stage T1D donors in relation to cytokine secretion, immunomodulatory activity, and wound-healing potential - suggesting a state of disease memory in these cells. Long-term exposure to the diabetic environment has been suggested to induce disease memory in BM-MSCs100. Despite differential gene expression, T1D-MSCs did not demonstrate a significant difference from healthy controls in immunosuppressive activity, migratory capacity, or hemocompatibility. Therefore, the authors concluded that MSCs from T1D donors are phenotypically and functionally similar to healthy control MSCs indicating their suitability for use in autologous cell therapy91.
In another recent study by de Lima et al92, BM-MSCs from newly-diagnosed T1D patients (within 6 weeks from diagnosis) were compared with those from healthy individuals for morphological characteristics, immunophenotypical characteristics, differentiation potential, and gene expression profile. T1D-MSCs and control MSCs showed similar morphology, immunophenotype, and multipotent differentiation, as reported by others, but T1D-MSCs showed an increased migratory capacity. Importantly, T1D-MSCs showed abnormalities in mRNA expression, including a downregulation of the immunomodulatory molecules VC AM-1, CXCL12, CCL2, CCL24, CXCL5, of the pro-regenerative molecule HGF, of the stemness-related EGFR and FGFR, along with the activation of sympathetic nervous system and JAK STAT signaling92. This gene expression profile suggests that human T1D-MSCs may have impairments in their interactions with immune/hematopoietic cell populations and in their ability to suppress immune effector functions. In accordance with what Davies et al91 had found, the study by de Lima et al92 also confirmed the down-modulation of HGF in T1D-MSCs. HGF is associated with angiogenesis and cell survival101,102, it can stimulate kidney and liver regeneration. Moreover, HGF is believed to be a protective factor for pancreatic β cells, and consequently its downregulation may indicate a decreased potential for the stimulation of pancreatic islet regeneration. Additionally, EGFR and FGFR were also found downregulated in T1D-MSCs: these receptors regulate stemness, inhibit senescence, are essential for cell growth, tissue repair, and homeostasis103,104; a downregulation of EGFR signaling may determine the downregulation of HGF103,105,106. This study analyzed MSCs after in vitro culture; therefore, the abnormalities found could be influenced by culture conditions beyond the exposure to the altered diabetic bone marrow milieu. Further functional experiments will be required in order to better elucidate how these gene expression alterations may affect therapeutic efficacy of autologous MSCs in T1D patients92.
Autologous T2D-MSCs
The studies focused on autologous T2D BM-MSCs suggest that long-term exposure to the disease-related inflammatory and hyperglycemic environment affect their functions.
Shin and Peterson107 examined the influence of T2D on the therapeutic potential of endogenous BM-MSCs, showing that the diabetic mice had BM-MSCs occurring in lower numbers, with impaired proliferation and survival in vitro.
The study conducted in 2009 by Phadnis et al108 investigated the characteristics of BM-MSCs derived from T2D patients. As it was described by the articles cited in this review about T1D91–93, also T2D-MSCs appear similar to healthy MSCs in phenotype, morphology, and multilineage differentiation potential. However, the diabetic environment seems to have an impact on MSCs: C-peptide and insulin transcripts can be detected in T2D-MSCs108. Kojima and colleagues had previously observed that hyperglycemia, with or without established diabetes, activates insulin gene transcription and proinsulin production in multiple extrapancreatic and extrathymic tissues109.
However, unlike in β-cells, MSCs from T2D exclusively produced proinsulin and very little mature insulin, and did not contribute significantly to insulin production in vivo108. Although high glucose concentration induces proinsulin transcription, it also stimulates the secretion of cytokines such as interleukin1, which cause β-cell apoptosis in vitro and in vivo110. Kojima et al109 hypothesized that these cells may mediate the ill effects of hyperglycemia, and may contribute to chronic diabetic complications such as diabetic neuropathy.
Although the amount of proinsulin produced by the BM cells exposed to hyperglycemia in vivo was extremely small, the appearance of proinsulin-producing cells outside the pancreas may represent the body’s attempt to reverse hyperglycemia108. Thus, chronic exposure to hyperglycemia may be important for the decreased potential of these BM-MSCs, precluding them for autologous stem cell therapy in T2D patients. In fact, these cells appear to be terminally differentiated, therefore leading to a loss of stemness and failure of further propagation108.
Furthermore, the persistent hyperglycemic milieu in T2D is also associated with several pathological complications, mostly related with compromised vascularization and/or aberrant angiogenesis111. By releasing growth factors and cytokines such as IGF-1, BM-MSCs stimulate endothelial cell migration112, inhibit endothelial apoptosis, stimulate angiogenesis, promote neovascularization and tissue regeneration112–114. The influence of T2D on the secretome and pro-angiogenic functions of BM-MSCs deserves thorough investigations. Ribot et al111 analyzed the impact of T2D on BM-MSCs secretome and functions, hypothesizing that in the diabetic milieu these could have different composition and properties. The results obtained provided the evidence that short-term T2D alters the BM-MSC secretome composition and promotes angiogenic capabilities111.
Angiogenesis-related genes are differentially expressed in BM-MSCs from diabetic fatty rats (ZDF, a T2D model) when compared with lean animals (control). In particular, several pro-angiogenic genes were found to be overexpressed, while anti-angiogenic genes were downregulated. The up-regulated genes included IGF-1 and TIE1, which are critical regulators of angiogenesis115, MCP-1 and IL-6, homing factors for BM-MSCs and endothelial cells/endothelial progenitor cells116,117, and IL-6 and TNFa, critical mediators of the inflammatory process. Moreover, proteomic analysis of the T2D BM-MSC secretome showed decreased levels of ab-crystallin, a chaperone for VEGF-A, and increased levels of LTBP1 and LTBP2, regulators of TGF-b availability118, as well as of OSTP and FMOD, which are components of the extracellular matrix and might be involved in the paracrine action of T2D BM-MSCs on endothelial cells119–122. In addition, the proteomic analysis of T2D BM-MSC demonstrated a specific secretory phenotype of extracellular matrix remodeling and glucose metabolism, showing overexpressed proteins involved in extracellular matrix homeostasis and remodeling-related molecules123; in contrast, proteins involved in the metabolism of glucose (such as ALDOA, LDHA, KPYM, G6P, PTMA, OAS2, ALD1, and IBP2) were secreted at lower levels.
Functional impairment of T2D MSCs is evident from preclinical and clinical studies that have been performed to determine their efficacy in the treatment of peripheral arterial disease (PAD). PAD is frequently associated with diabetes, hypertension, atherosclerosis, and aging - all of which could damage the regenerative function of stem cells and progenitor cells124–129. Yan et al130 have shown that experimental T2D causes hyperinsulinemia-induced oxidant stress in murine MSCs, a stress that restricts their multipotency and impairs their capacity to promote neovascularization.
The same authors observed that MSCs harvested from T2D mice show several dysfunctions deriving from oxidative stress131. Rather than increasing postischemic neovascularization and limb blood flow, injection of MSCs from T2D mice impaired blood flow recovery. Should human MSCs display similar oxidative stress-induced impairment of function, these findings recommend a therapeutic approach aimed maximizing the potential of MSC transplantation, particularly in the increasingly common setting of diabetes or other cardiovascular risk factors. The authors propose that either in vivo systemic treatment with an antioxidant and/or ex vivo treatment of MSCs with antioxidants could significantly increase the intended clinical benefit131.
A recent study by Rezabakhsh et al132 investigated the impact of T2D sera on the angiogenic differentiation capacity of primary healthy BM-MSCs. The study showed that T2D serum decreased the angiogenic properties of MSCs via direct effect on angiogenesis pathways or via induction of autophagy signaling132.
Taking all these considerations together, the pathophysiology of T2D and the associated changes in the bone marrow microenvironment seem to affect multiple aspects of BM-MSCs biology and function. T2D seems to exacerbate the impairment of these stem cells to an extent greater than T1D. It is however still largely unknown whether distinct mechanisms underlie BM-MSCs dysfunction in T1D compared to T2D36.
De Vyver et al36 argued that strategies focused on restoring stem/progenitor cells mobilization in autologous cell therapy are limited in that stem cell damage can occur at the bone marrow niche before mobilization into the peripheral blood. This hypothesis was also confirmed by an observation by Januszyk et al133, who affirmed that the pathogenesis of both T1D and T2D may deplete specific subpopulations of BM-MSCs and this defect cannot be corrected by restoring glucose homeostasis. In addition to affecting BM-MSCs viability and functional capacity, long term exposure to the pathological bone marrow niche environment can induce a certain degree of disease memory in MSCs100. Future studies are required to provide a strict assessment of the efficacy of MSCs transplantation in T1D, T2D, and related complications.
BOX 1. MSC-Derived exosomes and diabetes.
Exosomes (EXOs) are nanoscopic (30-100 nm) biological entities that are secreted as vesicles in the extracellular environment by many different types of cells59, including MSCs. MSC-derived exosomes (MSC-EXOs) are emerging as a new important paracrine mechanism for cell-to-cell communication, implicated in wound healing, injury and tissue repair. They are known to contain proteins, mRNAs and microRNAs60,61; moreover, they have immunostimulatory and immunoregulatory functions59,62,63. Certain EXOs and cargos present molecular signatures of pathological processes and could be implicated in the pathogenesis of multiple pancreatic diseases, such as T1D, T2D, diabetic nephropathy, diabetic retinopathy, gestational diabetes mellitus, and pancreatic cancer64,65. EXOs can be easily isolated from different body fluids collected by non-invasive methods and therefore have the potential to be utilized for the analysis of disease biomarkers. EXOs can also be easily collected from the supernatant of in vitro cell cultures. EXOs derived from MSC cultures were shown to promote regulatory T cell (T reg) activity, inhibit Effector T cell, natural killer (NK) cells and dendritic cells (DCs) activities66,67. The advantages of using EXOs instead of live cells are connected to their minimal immunogenicity (allowing an allogenic use), low inherent toxicity68, and potentially lower risk for tumor formation69. Moreover, because of their chemical composition and small size, EXOs may easily diffuse across the biological barriers reaching target cells. A common assumption in the context of T1D is that imbalances between Effector T cells and T regs, as well as DC presentation of islet auto-antigens, play a major role in the destruction of islet β cells70,71. The beneficial effect of MSCs for the treatment of T1D derives largely from their immune-modulatory and anti-inflammatory secretome. Therefore, MSC-EXOs might be employed as immune modulators in MHC-mismatched recipients, overcoming the potential immunogenicity of MSC in an allogenic setting68. EXOs/microvescicles derived from endothelial progenitor cells combined with islets can activate angiogenesis improving revascularization and pancreatic beta cell function72. The same study observed that EXOs/microvescicles also inhibited endothelial-leukocyte interaction. MSC-EXOs could have similar proangiogenic effects. Sheng et al73 showed the other side of the coin: insulinoma-derived EXOs contain diabetes-triggering autoantigens that may stimulate autoreactive T cells inducing inflammatory cytokine secretion and activating antigen-presenting cells. In accordance with this study, suggesting that exosomes could serve as triggering factors for specific autoimmunity events leading to diabetes, also Rahman et al74 and Lukic et al75 propose a possible causative role of the islet MSCs and their EXOs in triggering the islet-specific autoimmunity in the NOD mouse strain. During beta cell apoptosis in the islet, MSCs might be activated or recruited in islets to repair the damage and, therefore, could become a source of EXOs able to initiate autoimmune response74.
Footnotes
Conflict of interest
The Authors declare that they have no conflict of interests.
References
- 1.Pokrywczynska M, Lanzoni G, Ricordi C. From adult pancreatic islets to stem cells – regenerative strategies for the treatment of diabetes and its complications Principles of Regenerative Medicine 3rd Edition. 3rd Edition ed. Edited by Atala A, 2017. [Google Scholar]
- 2.Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med 1986; 314: 1360–1368. [DOI] [PubMed] [Google Scholar]
- 3.Eisenbarth GS. Banting Lecture 2009: an unfinished journey: molecular pathogenesis to prevention of type 1A diabetes. Diabetes 2010; 59: 759–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014; 383: 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson MA. The pathogenesis and natural history of type 1 diabetes. Cold Spring Eiarb Perspect Med 2012; 2 pii: a007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricordi C, Inverardi L, Domínguez-Bendala J. From cellular therapies to tissue reprogramming and regenerative strategies in the treatment of diabetes. Regen Med 2012; 7: 41–48. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro AM, Pokrywczynska M, Ricordi C Clinical pancreatic islet transplantation. Nat Rev Endocrinol 2017; 13: 268–277. [DOI] [PubMed] [Google Scholar]
- 8.Dominguez-Bendala J, Inverardi L, Ricordi C. Stem cell-derived islet cells for transplantation. Curr Opin Organ Transplant 2011; 16: 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricordi C, Goldstein JS, Balamurugan AN, Szot GL, Kin T, Liu C, Czarniecki CW, Barbara B, Bridges ND, Cano J, Clarke WR, Eggerman TL, Fiunsicker LG, Kaufman DB, Khan A, Lafontant DE, Linetsky E, Luo X, Markmann JF, Naji A, Korsgren O, Oberholzer J, Turgeon NA, Brandhorst D, Friberg AS, Lei J, Wang LJ, Wilhelm JJ, Willits J, Zhang X, Fiering BJ, Posselt AM, Stock PG, Shapiro AM. National Institutes of Fiealth-Sponsored Clinical Islet Transplantation Consortium Phase 3 Trial: Manufacture of a Complex Cellular Product at Eight Processing Facilities. Diabetes 2016; 65: 3418–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Beilin MD, Chaloner K, Czarniecki CW, Goldstein JS, Fiunsicker LG, Kaufman DB, Korsgren O, Larsen CP, Luo X, Markmann JF, Naji A, Oberholzer J, Posselt AM, Rickels MR, Ricordi C, Robien MA, Senior PA, Shapiro AM, Stock PG, Turgeon NA. Clinical Islet Transplantation C: Phase 3 Trial of Transplantation of Fiuman Islets in Type 1 Diabetes Complicated by Severe Fiypoglycemia. Diabetes Care 2016; 39: 1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baidal DA, Ricordi C, Berman DM, Alvarez A, Padilla N, Ciancio G, Linetsky E, Pileggi A, Alejandro R. Bioengineering of an Intraabdominal Endocrine Pancreas. N Engl J Med 2017; 376: 1887–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Belle T, von Fierrath M. Immunosuppression in islet transplantation. J Clin Invest 2008; 118: 1625–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inverardi L, Lanzoni G, Dominguez-Bendala J, Ricordi C. Mesenchymal Stromal Cells for Diabetes Mesenchymal Stromal Cells: Basic Biology and Clinical Application. Edited by Fiematti P, Keating A: Springer-Humana Press, 2013. [Google Scholar]
- 14.Lanzoni G, Oikawa T, Wang Y, Cui CB, Carpino G, Cardinal V, Gerber D, Gabriel M, Dominguez-Bendala J, Furth ME, Gaudio E, Alvaro D, Inverardi L, Reid LM. Concise review: clinical programs of stem cell therapies for liver and pancreas. Stem Cells 2013; 31: 2047–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 1974; 17: 331–340. [DOI] [PubMed] [Google Scholar]
- 16.Caplan AI. Mesenchymal stem cells. J Orthop Res 1991; 9: 641–650. [DOI] [PubMed] [Google Scholar]
- 17.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A 1999; 96: 10711–10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–147. [DOI] [PubMed] [Google Scholar]
- 19.Florwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A; International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005; 7: 393–395. [DOI] [PubMed] [Google Scholar]
- 20.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Florwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8: 315–317. [DOI] [PubMed] [Google Scholar]
- 21.Anzalone R, Lo Iacono M, Loria T, Di Stefano A, Giannuzzi P, Farina F, La Rocca G. Wharton’s jelly mesenchymal stem cells as candidates for beta cells regeneration: extending the differentiative and immunomodulatory benefits of adult mesenchymal stem cells for the treatment of type 1 diabetes. Stem Cell Rev 2011; 7: 342–363. [DOI] [PubMed] [Google Scholar]
- 22.da Silva Meirelles L, Caplan Al, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 2008; 26: 2287–2299. [DOI] [PubMed] [Google Scholar]
- 23.Sheng H, Wang Y, Jin Y, Zhang Q, Zhang Y, Wang L, Shen B, Yin S, Liu W, Cui L, Li N. A critical role of IF-Ngamma in priming MSC-mediated suression of T cell proliferation through up-regulation of B7-H1. Cell Res 2008; 18: 846–857. [DOI] [PubMed] [Google Scholar]
- 24.Boissel L, Tuncer HH, Betancur M, Wolfberg A, Klingemann H. Umbilical cord mesenchymal stem cells increase expansion of cord blood natural killer cells. Biol Blood Marrow Transplant 2008; 14: 1031–1038. [DOI] [PubMed] [Google Scholar]
- 25.Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells, immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy 2009; 11: 377–391. [DOI] [PubMed] [Google Scholar]
- 26.Marigo I, Dazzi F. The immunomodulatory properties of mesenchymal stem cells. Semin Immunopathol 2011; 33: 593–602.’ [DOI] [PubMed] [Google Scholar]
- 27.Bunnell BA, Betancourt AM, Sullivan DE. New concepts on the immune modulation mediated by mesenchymal stem cells. Stem Cell Res Ther 2010; 1: p. 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kronsteiner B, Wolbank S, Peterbauer A, Flackl C, Redl FI, van Griensven M, Gabriel C. Fluman mesenchymal stem cells from adipose tissue and amnion influence T-cells depending on stimulation method and presence of other immune cells. Stem Cells Dev 2011; 20: 2115–2126. [DOI] [PubMed] [Google Scholar]
- 29.Siegel G, Schäfer R, Dazzi F. The immunosuppressive properties of mesenchymal stem cells. Transplantation 2009; 87: S45–49. [DOI] [PubMed] [Google Scholar]
- 30.Meirelles LaS, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 2009; 20: 419–427. [DOI] [PubMed] [Google Scholar]
- 31.Dominguez-Bendala J, Lanzoni G, Inverardi L, Ricordi C. Concise review: mesenchymal stem cells for diabetes. Stem Cells Transl Med 2012; 1: 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bosi CA, Lanzoni G, Pugliese A. Clinical trials of mesenchymal stem cell transplantation in patients with type 1 diabetes and systemic lupus erythematosus: is it time for larger studies? CellR4 2016; 4: e2134. [Google Scholar]
- 33.Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K. Preserved beta-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes 2015; 64: 587–592. [DOI] [PubMed] [Google Scholar]
- 34.Cai J, Wu Z, Xu X, Liao L, Chen J, Huang L, Wu W, Luo F, Wu C, Pugliese A, Pileggi A, Ricordi C, Tan J. Umbilical cord mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: a pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. Diabetes care 2016; 39: 149–157. [DOI] [PubMed] [Google Scholar]
- 35.Stephen J, Bravo EL, Colligan D, Fraser AR, Petrik J, Campbell JD. Mesenchymal stromal cells as multifunctional cellular therapeutics—a potential role for extracellular vesicles. Transfus Apher Sci 2016; 55: 62–69. [DOI] [PubMed] [Google Scholar]
- 36.van de Vyver M Intrinsic mesenchymal stem cell dysfunction in diabetes mellitus: implications for autologous cell therapy. Stem Cells Dev 2017; 26: 1042–1053. [DOI] [PubMed] [Google Scholar]
- 37.Caplan AI, Correa D: The MSC: an injury drugstore. Cell Stem Cell 2011; 9: 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci 2009; 1176: 101–117. [DOI] [PubMed] [Google Scholar]
- 39.Ge W, Jiang J, Arp J, Liu W, Garcia B, Wang H: Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation 2010; 90: 1312–1320’ [DOI] [PubMed] [Google Scholar]
- 40.Sioud M, Mobergslien A, Boudabous A, Floisand Y. Mesenchymal stem cell-mediated T cell suppression occurs through secreted galectins. Int J Oncol 2011; 38: 385–390. [DOI] [PubMed] [Google Scholar]
- 41.Han Z, Jing Y, Zhang S, Liu Y, Shi Y, Wei L. The role of immunosuppression of mesenchymal stem cells in tissue repair and tumor growth. Cell Biosci 2012; 2: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 2013; 13: 392–402. [DOI] [PubMed] [Google Scholar]
- 43.Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant 2016; 25: 829–848. [DOI] [PubMed] [Google Scholar]
- 44.Carrion FA, Figueroa FE. Mesenchymal stem cells for the treatment of systemic lupus erythematosus: is the cure for connective tissue diseases within connective tissue? Stem Cell Res Ther 2011; 2: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.English K Mesenchymal stem cells to promote islet transplant survival. Curr Opin Organ Transplant 2016; 21: 568–573. [DOI] [PubMed] [Google Scholar]
- 46.Solari MG, Srinivasan S, Boumaza I, Unadkat J, Harb G, Garcia-Ocana A, Feili-Hariri M. Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long-term islet allograft survival and sustained normoglycemia. J Autoimmun 2009; 32: 116–124. [DOI] [PubMed] [Google Scholar]
- 47.Figliuzzi M, Cornolti R, Perico N, Rota C, Morigi M, Remuzzi G, Remuzzi A, Benigni A. Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant Proc 2009; 41: 1797–1800. [DOI] [PubMed] [Google Scholar]
- 48.Jacobson S, Kumagai-Braesch M, Tibell A, Svensson M, Flodström-Tullberg M. Co-transplantation of stromal cells interferes with the rejection of allogeneic islet grafts. Ann NY Acad Sci 2008; 1150: 213–216. [DOI] [PubMed] [Google Scholar]
- 49.Longoni B, Szilagyi E, Quaranta P, Paoli GT, Tripodi S, Urbani S, Mazzanti B, Rossi B, Fanci R, Demontis GC, Marzola P, Saccardi R, Cintorino M, Mosca F. Mesenchymal stem cells prevent acute rejection and prolong graft function in pancreatic islet transplantation. Diabetes Technol Ther 2010; 12: 435–446. [DOI] [PubMed] [Google Scholar]
- 50.Berman DM, Willman MA, Han D, Kleiner G, Kenyon NM, Cabrera O, Karl JA, Wiseman RW, O’Connor DH, Bartholomew AM, Kenyon NS. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes 2010; 59: 2558–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cantarelli E, Pellegrini A, Citro A, Sordi V, Piemonti L Bone Marrow- and Cord Blood-Derived Stem Cell Transplantation for Diabetes. CellR4 2015; 3: el408. [Google Scholar]
- 52.Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes 2015; 64: 587–592. [DOI] [PubMed] [Google Scholar]
- 53.Pileggi A, Ricordi C, Alessiani M, Inverardi L: Factors influencing Islet of Langerhans graft function and monitoring. Clin Chim Acta 2001; 310: 3–16. [DOI] [PubMed] [Google Scholar]
- 54.Ezquer ME, Ezquer FE, Arango-Rodríguez ML, Conget PA. MSC transplantation: a promising therapeutic strategy to manage the onset and progression of diabetic nephropathy. Biol Res 2012; 45: 289–296. [DOI] [PubMed] [Google Scholar]
- 55.Jackson WM, Nesti LJ, Tuan RS. Concise review: clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl Med 2012; 1: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li M, Ikehara S. Bone-marrow-derived mesenchymal stem cells for organ repair. Stem Cells Int 2013; 2013: 132642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang R, Han Z, Zhuo G, Qu X, Li X, Wang X, Shao Y, Yang S, Han ZC. Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: a pilot study. Front Med 2011; 5: 94–100. [DOI] [PubMed] [Google Scholar]
- 58.El-Badawy A, El-Badri N. Clinical Efficacy of Stem Cell Therapy for Diabetes Mellitus: A Meta-Analysis. PLoS One 2016; 11: e0151938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009; 9: 581–593. [DOI] [PubMed] [Google Scholar]
- 60.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9: 654–659. [DOI] [PubMed] [Google Scholar]
- 61.Vella LJ, Sharpies RA, Nisbet RM, Cappai R, Hill AF. The role of exosomes in the processing of proteins associated with neurodegenerative diseases. Eur Biophys J 2008; 37: 323–332. [DOI] [PubMed] [Google Scholar]
- 62.Admyre C, Johansson SM, Qazi KR, Filén JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J Immunol 2007; 179: 1969–1978. [DOI] [PubMed] [Google Scholar]
- 63.Bruno S, Deregibus MC, Camussi G. The secretome of mesenchymal stromal cells: Role of extracellular vesicles in immunomodulation. Immunol Lett 2015; 168: 154–158. [DOI] [PubMed] [Google Scholar]
- 64.Garcia-Contreras M, Ricordi C, Robbins P, Oltra E. Exosome in the pathogenesis, diagnosis and treatment of pancreatic diseases. CellR4 2014; 2: e807. [PMC free article] [PubMed] [Google Scholar]
- 65.Garcia-Contreras M, Brooks RW, Boccuzzi L, Robbins PD, Ricordi C. Exosomes as biomarkers and therapeutic tools for type 1 diabetes mellitus. Eur Rev Med Pharmacol Sci 2017; 21: 2940–2956. [PubMed] [Google Scholar]
- 66.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 2014; 14: 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett 2012; 147: 47–54. [DOI] [PubMed] [Google Scholar]
- 68.Carpanetto A, Gai C, Favaro E, Zanone MM, Camussi G. Potential immune modulatory action of mesenchymal stem cell-derived extracellular vesicles in type 1 diabetes. Int J Stem Cell Res Ther 2015; 2. [Google Scholar]
- 69.Crivelli B, Chlapanidas T, Perteghella S, Lucarelli E, Pascucci L, Brini AT, Ferrero I, Marazzi M, Pessina A, Torre ML; Italian Mesenchymal Stem Cell G. Mesenchymal stem/stromal cell extracellular vesicles: from active principle to next generation drug delivery system. J Control Release 2017; 104–117. [DOI] [PubMed] [Google Scholar]
- 70.Fiorina P, Voltarelli J, Zavazava N. Immunological applications of stem cells in type 1 diabetes. Endocr Rev 2011; 32: 725–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morel PA. Dendritic cell subsets in type 1 diabetes: friend or foe? Front Immunol 2013; 4: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cantaluppi V, Biancone L, Figliolini F, Beltramo S, Medica D, Deregibus MC, Galimi F, Romagnoli R, Salizzoni M, Tetta C, Segoloni GP, Camussi G. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transplant 2012;21:1305–1320. [DOI] [PubMed] [Google Scholar]
- 73.Sheng H, Hassanali S, Nugent C, Wen L, Hamilton-Williams E, Dias P, Dai YD. Insulinoma-released exosomes or microparticles are immunostimulatory and can activate autoreactive T cells spontaneously developed in non-obese diabetic mice. J Immunol 2011; 187: 1591–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rahman MT, Regn D, Bashratyan R, Dai YD Exosomes released by islet-derived mesenchymal stem cells trigger autoimmune responses in NOD mice. Diabetes 2014; 63: 1008–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lukic ML, Pejnovic N, Lukic A. New insight into early events in type 1 diabetes: role for islet stem cell exosomes. Diabetes 2014; 63: 835–837. [DOI] [PubMed] [Google Scholar]
- 76.Panepucci RA, Siufi JL, Silva WA, Proto-Siquiera R, Neder L, Orellana M, Rocha V, Covas DT, Zago MA. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells 2004; 22: 1263–1278. [DOI] [PubMed] [Google Scholar]
- 77.Noël D, Caton D, Roche S, Bony C, Lehmann S, Casteilla L, Jorgensen C, Cousin B. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp Cell Res 2008; 314: 1575–1584. [DOI] [PubMed] [Google Scholar]
- 78.Burk J, Gittel C, Heller S, Pfeiffer B, Paebst F, Ahrberg AB, Brehm W. Gene expression of tendon markers in mesenchymal stromal cells derived from different sources. BMC Res Notes 2014; 7: 826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 2011; 9: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parker AM, Katz AJ. Adipose-derived stem cells for the regeneration of damaged tissues. Expert Opin Biol Ther 2006; 6: 567–578. [DOI] [PubMed] [Google Scholar]
- 81.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006; 24: 1294–1301. [DOI] [PubMed] [Google Scholar]
- 82.Ballen KK, Barker JN, Stewart SK, Greene MF, Lane TA; American Society of Blood and Marrow Transplantation. Collection and preservation of cord blood for personal use. Biol Blood Marrow Transplant 2008; 14: 356–363. [DOI] [PubMed] [Google Scholar]
- 83.Jeon YJ, Kim J, Cho JH, Chung HM, Chae JI Comparative analysis of human mesenchymal stem cells derived from bone marrow, placenta, and adipose tissue as sources of cell therapy. J Cell Biochem 2016; 117: 1112–1125. [DOI] [PubMed] [Google Scholar]
- 84.Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Müller B, Vallejo M, Thomas MK, Habener JF. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes 2001; 50: 521–533. [DOI] [PubMed] [Google Scholar]
- 85.Guo XR, Wang XL, Li MC, Yuan YH, Chen Y, Zou DD, Bian LJ, Li DS. PDX-1 mRNA-induced reprogramming of mouse pancreas-derived mesenchymal stem cells into insulin-producing cells in vitro. Clin Exp Med 2015; 15: 501–509. [DOI] [PubMed] [Google Scholar]
- 86.Davani B, Ikonomou F, Raaka BM, Geras-Raaka E, Morton RA, Marcus-Samuels B, Gershengorn MC. Eluman islet-derived precursor cells are mesenchymal stromal cells that differentiate and mature to hormone-expressing cells in vivo. Stem Cells 2007; 25: 3215–3222. [DOI] [PubMed] [Google Scholar]
- 87.Zanini C, Bruno S, Mandili G, Baci D, Cerutti F, Cenacchi G, Izzi F, Camussi G, Forni M. Differentiation of mesenchymal stem cells derived from pancreatic islets and bone marrow into islet-like cell phenotype. PLoS One 2011; 6: e28175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fee S, Jeong S, Fee C, Oh J, Kim SC. Mesenchymal Stem Cells Derived from Fluman Exocrine Pancreas Spontaneously Express Pancreas Progenitor-Cell Markers in a Cell-Passage-Dependent Manner. Stem Cells Int 2016; 2016: 2142646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sordi V, Pellegrini S, Krampera M, Marchetti P, Pessina A, Ciardelli G, Fadini G, Pintus C, Pantè G, Piemonti F. Stem cells to restore insulin production and cure diabetes. Nutr Metab Cardiovasc Dis 2017; 27: 583–600. [DOI] [PubMed] [Google Scholar]
- 90.Griffin MD, Ryan AE, Alagesan S, Fohan P, Treacy O, Ritter T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol Cell Biol 2013; 91: 40–51. [DOI] [PubMed] [Google Scholar]
- 91.Davies EC, Aim JJ, Fleldring N, Moll G, Gavin C, Batsis I, Qian H, Sigvardsson M, Nilsson B, Kyllonen FE, Salmela KT, Carlsson PO, Korsgren O, Fe Blanc K. Type 1 diabetes mellitus donor mesenchymal stromal cells exhibit comparable potency to healthy controls in vitro. Stem Cells Transl Med 2016; 5: 1485–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Fima KA, de Oliveira GF, Yaochite JN, Pinheiro DG, de Azevedo JT, Silva WA, Covas DT, Couri CE, Simões BP, Voltarelli JC, Oliveira MC, Malmegrim KC. Transcriptional profiling reveals intrinsic mRNA alterations in multipotent mesenchymal stromal cells isolated from bone marrow of newly-diagnosed type 1 diabetes patients. Stem Cell Res Ther 2016; 7: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yaochite JN, de Fima KW, Caliari-Oliveira C, Palma PV, Couri CE, Simões BP, Covas DT, Voltarelli JC, Oliveira MC, Donadi EA, Malmegrim KC. Multipotent mesenchymal stromal cells from patients with newly diagnosed type 1 diabetes mellitus exhibit preserved in vitro and in vivo immunomodulatory properties. Stem Cell Res Ther 2016; 7: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dazzi F, Krampera M. Mesenchymal stem cells and autoimmune diseases. Best Pract Res Clin Flaematol 2011; 24: 49–57. [DOI] [PubMed] [Google Scholar]
- 95.Dong QY, Chen F, Gao GQ, Wang F, Song J, Chen B, Xu YX, Sun F. Allogeneic diabetic mesenchymal stem cells transplantation in streptozotocin-induced diabetic rat. Clin Invest Med 2008; 31: E328–337. [DOI] [PubMed] [Google Scholar]
- 96.Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, Fa Rosa S, Selig M, Godwin J, Faw K, Placidi C, Smith RN, Capella C, Rodig S, Adra CN, Atkinson M, Sayegh MH, Abdi R. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol 2009; 183: 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fuz-Crawford P, Kurte M, Bravo-Alegría J, Contreras R, Nova-Famperti E, Tejedor G, Noël D, Jorgensen C, Figueroa F, Djouad F, Carrión F. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther 2013; 4: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Casiraghi F, Azzollini N, Cassis P, Imberti B, Morigi M, Cugini D, Cavinato RA, Todeschini M, Solini S, Sonzogni A, Perico N, Remuzzi G, Noris M. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol 2008; 181: 3933–3946. [DOI] [PubMed] [Google Scholar]
- 99.Madec AM, Mallone R, Afonso G, Abou Mrad E, Mesnier A, Eljaafari A, Thivolet C. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia 2009; 52: 1391–1399. [DOI] [PubMed] [Google Scholar]
- 100.Madhira SF, Challa SS, Chalasani M, Nappanveethl G, Bhonde RR, Ajumeera R, Venkatesan V. Promise(s) of mesenchymal stem cells as an in vitro model system to depict pre-diabetic/diabetic milieu in WNIN/GR-Ob mutant rats. PFoS One 2012; 7: e48061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sulpice E, Ding S, Muscatelli-Groux B, Bergé M, Flan ZC, Plouet J, Tobelem G, Merkulova-Rainon T. Crosstalk between the VEGF-A and F1GF signalling pathways in endothelial cells. Biol Cell 2009; 101: 525–539. [DOI] [PubMed] [Google Scholar]
- 102.Kitamura K, Iwanami A, Nakamura M, Yamane J, Watanabe K, Suzuki Y, Miyazawa D, Shibata S, Funakoshi FI, Miyatake S, Coffin RS, Nakamura T, Toyama Y, Okano FI. Flepatocyte growth factor promotes endogenous repair and functional recovery after spinal cord injury. J Neurosci Res 2007; 85: 2332–2342. [DOI] [PubMed] [Google Scholar]
- 103.Tamama K, Fan VH, Griffith EG, Blair HC, Wells A. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells 2006; 24: 686–695. [DOI] [PubMed] [Google Scholar]
- 104.Krampera M, Pasini A, Rigo A, Scupoli MT, Tecchio C, Malpeli G, Scarpa A, Dazzi F, Pizzolo G, Vinante F. F1B-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood 2005; 106: 59–66. [DOI] [PubMed] [Google Scholar]
- 105.Wang Y, Weil BR, Flerrmann JF, Abarbanell AM, Tan J, Markel TA, Kelly ML, Meldrum DR MEK, p38, and PI-3K mediate cross talk between EGFR and TNFR in enhancing hepatocyte growth factor production from human mesenchymal stem cells. Am J Physiol Cell Physiol 2009; 297: C1284–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.De Fuca A, Gallo M, Aldinucci D, Ribatti D, Famura F, D’Alessio A, De Filippi R, Pinto A, Normanno N. Role of the EGFR ligand/receptor system in the secretion of angiogenic factors in mesenchymal stem cells. J Cell Physiol 2011; 226: 2131–2138. [DOI] [PubMed] [Google Scholar]
- 107.Shin F, Peterson DA. Impaired therapeutic capacity of autologous stem cells in a model of type 2 diabetes. Stem Cells Transl Med 2012; 1: 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Phadnis SM, Ghaskadbi SM, Flardikar AA, Bhonde RR. Mesenchymal stem cells derived from bone marrow of diabetic patients portrait unique markers influenced by the diabetic microenvironment. Rev Diabet Stud 2009; 6: 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kojima FI, Fujimiya M, Matsumura K, Nakahara T, Flara M, Chan F. Extrapancreatic insulin-producing cells in multiple organs in diabetes. Proc Natl Acad Sci US A 2004; 101: 2458–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaiser N, Feibowitz G, Nesher R. Glucotoxicity and beta-cell failure in type 2 diabetes mellitus. J Pediatr Endocrinol Metab 2003; 16: 5–22. [DOI] [PubMed] [Google Scholar]
- 111.Ribot J, Caliaperoumal G, Paquet J, Boisson-Vidal C, Petite H, Anagnostou F. Type 2 diabetes alters mesenchymal stem cell secretome composition and angiogenic properties. J Cell Mol Med 2017; 21: 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pacini S, Petrini I. Are MSCs angiogenic cells? New insights on human nestin-positive bone marrow-derived multipotent cells. Front Cell Dev Biol 2014; 2: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Watt SM, Gullo F, van der Garde M, Markeson D, Camicia R, Khoo CP, Zwaginga JJ The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br Med Bull 2013; 108: 25–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res 2004; 94: 678–685. [DOI] [PubMed] [Google Scholar]
- 115.Shigematsu S, Yamauchi K, Nakajima K, Iijima S, Aizawa T, Flashizume K IGF-1 regulates migration and angiogenesis of human endothelial cells. Endocr J 1999; 46 Suppl: S59–62. [DOI] [PubMed] [Google Scholar]
- 116.Middleton K, Jones J, Lwin Z, Coward JI. Interleukin-6: an angiogenic target in solid tumours. Crit Rev Oncol Hematol 2014; 89: 129–139. [DOI] [PubMed] [Google Scholar]
- 117.Salcedo R, Ponce ML, Young F1A, Wasserman K, Ward JM, Kleinman F1K, Oppenheim JJ, Murphy WJ Fluman endothelial cells express CCR2 and respond to MCP-E direct role of MCP-1 in angiogenesis and tumor progression. Blood 2000; 96: 34–40. [PubMed] [Google Scholar]
- 118.Tatti O, Vehviläinen P, Lehti K, Keski-Oja J. MT1-MMP releases latent TGF-betal from endothelial cell extracellular matrix via proteolytic processing of LTBP-T Exp Cell Res 2008; 314: 2501–2514. [DOI] [PubMed] [Google Scholar]
- 119.Todorovic V, Rifkin DB. LTBPs, more than just an escort service. J Cell Biochem 2012; 113: 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kale S, Raja R, Thorat D, Soundararajan G, Patil TV, Kundu GC. Osteopontin signaling upregulates cyclooxygenase-2 expression in tumor-associated macrophages leading to enhanced angiogenesis and melanoma growth via α9β1 integrin. Oncogene 2014; 33: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 121.Berchem G, Glondu M, Gleizes M, Brouillet JP, Vignon F, Garcia M, Liaudet-Coopman E. Cathepsin-D affects multiple tumor progression steps in vivo: proliferation, angiogenesis and apoptosis. Oncogene 2002; 21; 5951–5955. [DOI] [PubMed] [Google Scholar]
- 122.Jian J, Zheng Z, Zhang K, Rackohn TM, Flsu C, Levin A, Enjamuri DR, Zhang X, Ting K, Soo C. Fibromodulin promoted in vitro and in vivo angiogenesis. Biochem Biophys Res Commun 2013; 436: 530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Monnier VM, Mustata GT, Biemel KL, Reihl O, Lederer MO, Zhenyu D, Sell DR. Cross-linking of the extracellular matrix by the maillard reaction in aging and diabetes: an update on “a puzzle nearing resolution”. Ann N Y Acad Sci 2005; 1043: 533–544. [DOI] [PubMed] [Google Scholar]
- 124.Yan J, Tie G, Park B, Yan Y, Nowicki PT, Messina LM. Recovery from hind limb ischemia is less effective in type 2 than in type 1 diabetic mice: roles of endothelial nitric oxide synthase and endothelial progenitor cells. J Vase Surg 2009; 50: 1412–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fleeschen C, Lehmann R, Flonold J, Assmus B, Aicher A, Walter DH, Martin FI, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation 2004; 109: 1615–1622. [DOI] [PubMed] [Google Scholar]
- 126.Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res 2008; 102: 1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.MacKenzie TC, Flake AW. Fluman mesenchymal stem cells: insights from a surrogate in vivo assay system. Cells Tissues Organs 2002; 171: 90–95. [DOI] [PubMed] [Google Scholar]
- 128.Urbich C, Dimmeler S. Risk factors for coronary artery disease, circulating endothelial progenitor cells, and the role of FlMG-CoA reductase inhibitors. Kidney Int 2005; 67: 1672–1676. [DOI] [PubMed] [Google Scholar]
- 129.Amin AH, Abd Elmageed ZY, Nair D, Partyka MI, Kadowitz PJ, Belmadani S, Matrougui K. Modified multipotent stromal cells with epidermal growth factor restore vasculogenesis and blood flow in ischemic hind-limb of type II diabetic mice. Lab Invest 2010; 90: 985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yan J, Tie G, Wang S, Messina KE, DiDato S, Guo S, Messina LM. Type 2 diabetes restricts multipotency of mesenchymal stem cells and impairs their capacity to augment postischemic neovascularization in db/db mice. J Am Heart Assoc 2012; 1: e002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yan J, Tie G, Xu TY, Cecchini K, Messina LM. Mesenchymal stem cells as a treatment for peripheral arterial disease: current status and potential impact of type II diabetes on their therapeutic efficacy. Stem Cell Rev 2013; 9: 360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rezabakhsh A, Cheraghi O, Nourazarian A, Hassanpour M, Kazemi M, Ghaderi S, Faraji E, Rahbarghazi R, Avci Ç, Bagca BG, Garjani A. Type 2 diabetes inhibited human mesenchymal stem cells angiogenic response by over-activity of the autophagic pathway. J Cell Biochem 2017; 118: 1518–1530. [DOI] [PubMed] [Google Scholar]
- 133.Januszyk M, Sorkin M, Glotzbach JP, Vial IN, Maan ZN, Rennert RC, Duscher D, Thangarajah H, Longaker MT, Butte AJ, Gurtner GC. Diabetes irreversibly depletes bone marrow-derived mesenchymal progenitor cell subpopulations. Diabetes 2014; 63: 3047–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]


