In BOLERO-2, combining everolimus with exemestane significantly improved progression-free survival (medians: 7.8 vs 3.2 mo [local assessment]; P < 0.0001) without a statistically significant difference in overall survival (medians: 31.0 vs 26.6 mo; stratified log-rank P = 0.14) in patients with HR+, HER2− advanced breast cancer previously treated with a nonsteroidal aromatase inhibitor.
Keywords: everolimus, hormone-receptor-positive breast cancer, exemestane, overall survival
Abstract
Background
The BOLERO-2 study previously demonstrated that adding everolimus (EVE) to exemestane (EXE) significantly improved progression-free survival (PFS) by more than twofold in patients with hormone-receptor-positive (HR+), HER2-negative advanced breast cancer that recurred or progressed during/after treatment with nonsteroidal aromatase inhibitors (NSAIs). The overall survival (OS) analysis is presented here.
Patients and methods
BOLERO-2 is a phase III, double-blind, randomized international trial comparing EVE 10 mg/day plus EXE 25 mg/day versus placebo (PBO) + EXE 25 mg/day in postmenopausal women with HR+ advanced breast cancer with prior exposure to NSAIs. The primary end point was PFS by local investigator assessment; OS was a key secondary end point.
Results
At the time of data cutoff (3 October 2013), 410 deaths had occurred and 13 patients remained on treatment. Median OS in patients receiving EVE + EXE was 31.0 months [95% confidence interval (CI) 28.0–34.6 months] compared with 26.6 months (95% CI 22.6–33.1 months) in patients receiving PBO + EXE (hazard ratio = 0.89; 95% CI 0.73–1.10; log-rank P = 0.14). Poststudy treatments were received by 84% of patients in the EVE + EXE arm versus 90% of patients in the PBO + EXE arm. Types of poststudy therapies were balanced across arms, except for chemotherapy (53% EVE + EXE versus 63% PBO + EXE). No new safety concerns were identified.
Conclusions
In BOLERO-2, adding EVE to EXE did not confer a statistically significant improvement in the secondary end point OS despite producing a clinically meaningful and statistically significant improvement in the primary end point, PFS (4.6-months prolongation in median PFS; P < 0.0001). Ongoing translational research should further refine the benefit of mTOR inhibition and related pathways in this treatment setting.
Trial registration number
introduction
Systemic therapy with endocrine-directed agents for hormone-receptor-positive (HR+), human epidermal growth factor receptor-2-negative (HER2–) breast cancer is a standard component of treatment of most women [1, 2]. Although effective, it is not curative in patients with advanced disease, and eventually the disease progresses. Sequential treatment with an alternate endocrine therapy remains an option for patients with advanced disease (except when rapid symptom control or reduction in tumor burden is needed) but demonstrates limited efficacy, especially in patients previously treated with nonsteroidal aromatase inhibitors (NSAI) [3–5].
In the pivotal phase III BOLERO-2 trial, everolimus (EVE) plus exemestane (EXE) more than doubled progression-free survival (PFS) versus placebo (PBO) plus EXE in postmenopausal women with HR+, HER2– advanced breast cancer whose disease recurred/progressed during/after an NSAI {at the final PFS analysis after 18 months' median follow-up, median PFS in the overall population was 7.8 months (EVE + EXE) versus 3.2 months (PBO + EXE) by investigator review; hazard ratio (HR) = 0.45 [95% confidence interval (CI) = 0.38–0.54]; log-rank P < 0.0001} [6]. Adverse events with EVE were manageable [7] and quality of life was maintained [8]. This report presents the final overall survival (OS) analysis based on the protocol-specified event cutoff.
methods
patients
Eligibility criteria have been described in detail previously [9]. Postmenopausal women with HR+, HER2– metastatic/locally advanced breast cancer not amenable to curative treatment and progressing after anastrozole or letrozole (disease recurrence during/within 12 months after adjuvant treatment or progression during/within 1 month after treatment of advanced disease) were eligible.
All patients provided written informed consent before enrollment. The study was approved by the institutional review board at each participating center and was conducted in accordance with Good Clinical Practice, the Declaration of Helsinki, and other applicable local regulations. A steering committee supervised study conduct. An independent data and safety monitoring committee conducted semiannual safety reviews and reviewed interim efficacy results.
study design
BOLERO-2 was a multicenter, double-blind, randomized, PBO-controlled, international phase III study (NCT00863655). Patients were randomized (2 : 1) to blinded treatment with EVE 10 mg/day or matching PBO; all patients received open-label EXE 25 mg/day (N = 724) [9]. Patients were stratified by presence of visceral metastasis (yes/no) and sensitivity to previous hormonal therapy [yes/no; defined as ≥24 months' endocrine therapy before recurrence (adjuvant setting) or disease response/stabilization during ≥24 weeks of endocrine therapy (advanced disease)]. The primary end point was PFS as assessed by local investigator (per RECIST 1.0) [9]; OS was a key secondary end point. Safety assessments are described in the supplementary materials, available at Annals of Oncology online.
Poststudy anticancer therapies were recorded for at least the first treatment after discontinuation. After discontinuation of study treatment, all patients (unless they withdrew consent or were lost to follow-up) were followed continuously, at least every 3 months, for survival.
statistical analysis
Comparison of OS was a key secondary objective. Statistical power was based on an expected median OS with PBO + EXE of ∼24 and ∼8 months' prolongation of median OS to 32.4 months for EVE + EXE (corresponding to a 26% reduction in the hazard rate for OS). To detect an HR of 0.74 with 80% cumulative power, a maximum of 398 OS events were required to have occurred.
The distribution of OS, defined as the time from randomization to death from any cause, was compared between the two treatment groups using a stratified log-rank test within a 4-look Lan–DeMets group sequential design with O'Brien-Fleming-type boundary at one-sided 2.5% level of significance. The survival distribution function was estimated using the Kaplan–Meier method. Median OS and the corresponding 95% CIs for each treatment group were estimated. A stratified Cox regression was used to estimate the HR of OS and the associated 95% CIs. Post hoc analysis of postprogression survival duration, defined as time from disease progression to death, was assessed at the data cutoff for final OS analysis in patients who had progressed at the time of the final PFS analysis.
results
patients and study treatment disposition
Of the 724 patients enrolled between June 2009 and January 2011, 485 were assigned to EVE + EXE and 239 to PBO + EXE. Baseline disease/pretreatment characteristics were well balanced between arms [9]. Approximately 80% of patients received prior therapy for metastatic disease (including chemotherapy, 26%), and 20% received study treatment as initial therapy for metastatic disease.
At data cutoff for the OS analysis (3 October 2013; 39.3 months' median study follow-up), 11 patients (2.3%) receiving EVE + EXE and 2 patients (0.8%) receiving PBO + EXE were continuing study treatment (details provided in supplementary materials and Figure S1, available at Annals of Oncology online).
overall survival
At data cutoff for this analysis, 410 OS events had occurred [267 patients (55.1%), EVE + EXE versus 143 patients (59.8%), PBO + EXE]. Median OS durations were 31.0 months (95% CI 28.0–34.6), EVE + EXE versus 26.6 months (95% CI 22.6–33.1), PBO + EXE (Figure 1). Treatment with EVE + EXE did not significantly reduce the risk of death versus PBO + EXE (HR = 0.89; 95% CI 0.73–1.10; stratified log-rank P = 0.1426).
Figure 1.
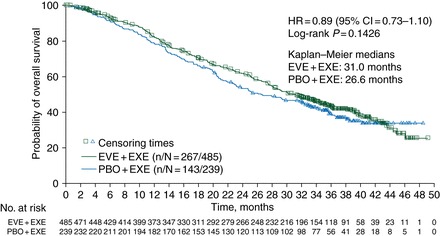
Kaplan–Meier estimates of overall survival. CI, confidence interval; EVE, everolimus; EXE, exemestane; HR, hazard ratio; PBO, placebo.
Median postprogression survival in patients who had progressed at the time of the final PFS analysis (n = 492) was similar in the EVE + EXE versus PBO + EXE arms [20.8 months (95% CI 17.3–23.3) versus 19.3 months (95% CI 15.9–23.9), respectively]. It should be noted that the population for this post hoc analysis differs from the final OS analysis population.
poststudy treatment
Chemotherapy and hormonal therapy were the most common poststudy treatments (supplementary Table S1, available at Annals of Oncology online). Fewer patients in the EVE + EXE arm received chemotherapy (53%) versus PBO + EXE (63%). Time from randomization to first chemotherapy or death was delayed with EVE + EXE [median 11.9 months (95% CI 10.45–13.08)] versus PBO + EXE [median 6.0 months (95% CI 5.09–7.39)].
safety
At the time of data cutoff, a higher proportion of patients discontinued EVE because of AEs (29%) versus PBO (5%; Table 1). Similarly, higher proportions of patients receiving EVE + EXE had grade 3/4 AEs and serious AEs (55% and 33%) versus patients receiving PBO + EXE (29% and 16%), consistent with earlier BOLERO-2 analyses [6, 9]. There were 26 on-treatment deaths: 22 (EVE + EXE) and 4 (PBO + EXE). In the EVE + EXE arm, 14 deaths were related to breast cancer progression and 8 were AE-related [pneumonia (n = 2); sepsis, Staphylococcus sepsis, tumor hemorrhage, transient ischemic attack, suicide, and renal failure (each n = 1)]. In the PBO + EXE arm, three deaths were related to breast cancer progression and one was AE-related (pneumonia). Analysis of AE-related on-treatment deaths failed to show any definite pattern for early (≤4 months) versus late incidents in the EVE + EXE arm (Table 1). The risk of on-treatment death is related to the duration that a patient remains on study treatment. Taking into account the total patient-years exposed, the annualized incidence rates of on-treatment death were ∼1.5-fold higher for EVE + EXE (5.8%) versus PBO + EXE (3.9%). Exposure-adjusted analyses based on patient age at randomization (supplementary Table S2, available at Annals of Oncology online) showed that the incidence of on-treatment deaths because of AEs was similar between arms (1.2% EVE + EXE versus 1.6% PBO + EXE) in patients <65 years of age, but higher in the EVE + EXE arm [3.8%, versus 0 (PBO + EXE)] in the ≥65-year-old group.
Table 1.
Summary of adverse eventsa
| Patients, n
(%) |
||
|---|---|---|
| EVE + EXE (n = 482) | PBO + EXE (n = 238) | |
| Serious adverse events | 157 (32.6) | 37 (15.5) |
| Suspected to be drug-related | 63 (13.1) | 4 (1.7) |
| Grade 3/4 adverse events | 266 (55.2) | 70 (29.4) |
| Suspected to be drug-related | 197 (40.9) | 20 (8.4) |
| Adverse events leading to treatment discontinuation | 140 (29.0) | 12 (5.0) |
| On-treatment deaths | ||
| Total | 22 (4.6) | 4 (1.7) |
| Related to disease progression | 14 (2.9) | 3 (1.3) |
| Related to adverse events | 8 (1.7) | 1 (0.4) |
| Total treatment exposure, patient-years | 378 | 103 |
| Exposure-adjusted on-treatment deaths (deaths per patient-year) | 22 (5.8) | 4 (3.9) |
| On-treatment deaths ≤4 months from randomization | ||
| Total | 11 (2.3) | 4 (1.7) |
| Related to disease progression | 6 (1.2) | 3 (1.3) |
| Related to adverse events | 5 (1.1) | 1 (0.4) |
| On-treatment deaths >4 months after randomization | ||
| Total | 11 (2.3) | 0 |
| Related to disease progression | 8 (1.7) | 0 |
| Related to adverse events | 3 (0.6) | 0 |
aDeaths occurring >28 days after end of treatment are not included.
EVE, everolimus; EXE, exemestane; PBO, placebo.
discussion
Endocrine therapy provides long-term disease control with limited adverse effects in women with early HR+ breast cancer and among patients with disease recurrence after adjuvant therapy or progression after first-line treatment. Patients whose disease is initially well controlled by endocrine treatment are eligible for several sequential lines of endocrine therapy, but eventually the disease becomes unresponsive.
Two decades of translational research have highlighted at least four potential mechanisms of endocrine treatment failure—loss of estrogen receptor (ER) expression (often linked to epigenetic silencing), ER mutations (now shown with deep DNA sequencing to be not a rare phenomenon in advanced disease), altered expression of ER co-regulators at the level of the ER transcription machinery, and upregulation of alternative signal transduction pathways [10–16]. This last mechanism has been subjected to extensive testing in the last decade, with mixed results summarized below.
While trials of endocrine therapy given with/without anti-HER2 drugs have shown modest PFS improvements [17, 18], studies combining endocrine agents with either anti-HER1 or anti-insulin-like growth factor 1 (IGF-1)R agents have been negative [19–21]. Exploitation of the well-documented cross-talk between the ER and the PI3K/AKT/mTOR pathways [22–24] also has been explored as a strategy for circumventing or delaying endocrine resistance, with mTOR inhibition being the first strategy subjected to randomized clinical testing. Temsirolimus failed to enhance sensitivity to first-line endocrine therapy with letrozole in a large, PBO-controlled, phase III trial for advanced disease [n = 1112; similar median PFS (9 months) in both arms] [25].
These disappointing results did not discourage testing of another mTOR inhibitor, EVE, in combination with two different endocrine agents and in three different clinical scenarios. All these trials generated positive signals, supporting a role for EVE in enhancing the efficacy of endocrine therapy in endocrine-naive patients and in patients exposed to prior NSAIs. In the randomized phase II study of neoadjuvant EVE + letrozole versus PBO + letrozole, the addition of EVE marginally improved the sonographic response rate (68% versus 59%, respectively; P = 0.062), but markedly enhanced antiproliferative response (defined as natural logarithm of percentage positive Ki67 <1 on day 15 versus baseline; 57% versus 30%, respectively; P < 0.01) [26]. In TAMRAD, a randomized phase II trial of EVE+ tamoxifen versus tamoxifen alone in patients with advanced disease pre-exposed to aromatase inhibitors, the addition of EVE was associated with a 4-month improvement in time to progression (HR = 0.54, 95% CI 0.36–0.81) [27]. The BOLERO-2 trial, updated herein, is by far the most positive and robust phase III trial conducted to date showing a clinically meaningful benefit from inhibiting a signal transduction pathway distinct from ER signaling: median PFS was prolonged from 3.2 months with PBO + EXE to 7.8 months with EVE + EXE (HR = 0.45; log-rank P < 0.0001) with increased but manageable toxicity [6].
The lack of a statistically significant survival benefit from the addition of EVE to EXE in the BOLERO-2 study may be explained by many reasons. First, BOLERO-2 was not powered to detect a realistic OS advantage of 4–6 months: instead, with the chosen sample size based on the primary end point of PFS, the trial had 80% power to declare statistical significance only with an optimistic 8-month OS improvement. It should nonetheless be noted that the median OS for EVE + EXE (31 months) represents the longest reported thus far in the post-NSAI setting [5, 28]. Second, there was a small imbalance in poststudy salvage chemotherapy (more often used in the control versus experimental arm). This is a well-known risk of randomized trials conducted relatively early in the metastatic setting and makes any effect on OS notoriously difficult to demonstrate, with the exception of anti-HER2 antibody-based strategies in HER2+ disease, which affect the tumor microenvironment through their immunologic mechanism of action [29]. It is also likely that, despite the PBO-controlled design of BOLERO-2, clinicians could easily guess which patient was not receiving EVE (e.g. through absence of stomatitis); this might have encouraged initiation of more aggressive treatments (e.g. chemotherapy) after progression on EXE alone, a control therapy that has been criticized for its low efficacy in the clinical context of this trial. The third potential explanation resides in tumor biology: when mTOR complex 1 (mTORC-1) is inhibited by drugs such as EVE, a negative intracellular feedback loop between mTORC-1 and the IGF-1 signaling axis is released, leading to paradoxical activation of AKT [30, 31]. Although yet to be investigated in the clinic, such AKT activation might impair response to subsequent salvage therapies (not recorded in the BOLERO-2 database).
Phase III clinical trials of targeted therapies for advanced solid tumors have typically used PFS as a primary end point, with OS being an additional end point in each study. This reflects pragmatic recognition of the potentially confounding effects of poststudy antineoplastic therapies on OS, especially in cancers associated with multiple available treatments and relatively long survival durations. Recent phase III trials of vemurafenib in advanced melanoma and crizotinib in advanced lung cancer showed significant PFS benefits but inconsistent effects on OS, even after patient selection based on expression of molecular targets for these agents [32, 33]. Nonetheless, these are considered major advances in melanoma and lung cancer, respectively (additional details and perspective provided in supplementary materials, available at Annals of Oncology online). Demonstrating survival benefit in patients with HR+ advanced breast cancer has been generally challenging, and significant OS benefits with endocrine therapy in patients with HR+ advanced breast cancer have been restricted to studies of first-line interventions for advanced disease [34–36]. More recently, the CONFIRM study in patients who had received prior hormonal therapy for advanced disease reported a survival advantage with fulvestrant 500 versus 250 mg after prolonged follow-up (median OS, 26.4 versus 22.3 months; HR = 0.81; nominal P = 0.02) [37]. However, this OS benefit was not considered statistically significant because of the post hoc nature of this analysis.
The absence of biomarkers predicting response to mTOR inhibitors and other trial design parameters (e.g. study size, 2 : 1 randomization, near-universal use of poststudy therapies) make it unsurprising that BOLERO-2 did not show a significant OS benefit with EVE + EXE versus PBO + EXE. Nonetheless, BOLERO-2 represents a clear step forward in a series of attempts to expand and prolong the use of endocrine therapy in HR+ advanced breast cancer. Future efforts to improve treatment outcomes in patients with HR+ breast cancer include investigating the introduction of EVE earlier in the disease course (two adjuvant trials ongoing: NCT01805271 and NCT01674140), developing improved endocrine agents (namely, new ER downregulators possibly active against mutant forms of ER) [14], and developing other phase III, dual-inhibition strategies showing early encouraging signals [inhibitors of histone deacetylase (HDAC) [38], fibroblast growth factor receptor (FGFR) [39], or CDK4-6 [40]].
Meanwhile, the decision to prescribe EVE + EXE in advanced breast cancer following progression on NSAIs must be individualized, taking into account the benefit-risk profile and the patient's preference. Additionally, efforts at identifying robust biomarkers of EVE efficacy and toxicity must be pursued.
funding
This study was sponsored by Novartis Pharmaceuticals Corporation (no grant number). Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation.
disclosure
MP is a board member for PharmaMar; is a consultant to Sanofi, Astellas, Amgen, Bristol-Myers Squibb (BMS), Boehringer Ingelheim, Roche, Synthon, and Bayer; has obtained grant support for her institution from Pfizer, Amgen, Bayer, Boehringer Ingelheim, BMS, GlaxoSmithKline (GSK), Novartis, Roche, and Sanofi; and has received honoraria from Bayer, BMS, Boehringer Ingelheim, Roche, Amgen, Sanofi, and AstraZeneca. GNH has served as a consultant to Antigen Express, Novartis, and Pfizer, and is currently conducting research sponsored by Novartis. MC is a consultant to Novartis and Servier, and has received honoraria from Novartis. KIP has received consulting fees from Sanofi, AstraZeneca, Pfizer, Roche, Amgen, Novartis, GSK, Boehringer Ingelheim, Genomic Health, and Eisai; has received speaker's fees from Novartis; and has received travel funding from Novartis, Roche, and AstraZeneca. FL has nothing to disclose. YI has received grant support and honoraria from Chugai, Novartis, Pfizer, Parexel, Boehringer Ingelheim, Daiichi Sankyo, Taiho, and Eisai. SN is an advisor to AstraZeneca and Novartis, and has received grant support and honoraria from AstraZeneca, BMS, Chugai, GSK, Novartis, Pfizer, Sanofi, Taiho, Daiichi Sankyo, and Takeda. AP has nothing to disclose. HSR has received grant support to the regents of the University of California from Pfizer, Novartis, and Merck, and has received travel support from Novartis. ID's clinical trial unit receives a yearly grant from Novartis for general purposes. HAB has nothing to disclose. LP has been a member on an Advisory Committee for Roche, Novartis, GSK, and Amgen; has received travel expense reimbursement from Novartis, Roche, and GSK; and has received research grant support from Roche. PN has nothing to disclose. MG has received research support from Sanofi, Novartis, Roche, GSK, Pfizer, and Smith Medical; is a consultant to AstraZeneca, Novartis, and Accelsiors; and has received honoraria (speaking, advisory boards, etc.) and travel support from Amgen, Novartis, GSK, AstraZeneca, Roche, and Nanostring Technologies. MS has nothing to disclose. CW is an employee of Novartis. JF is an employee of Novartis and holds Novartis stock. WF is an employee of Novartis. TT is an employee of Novartis with stock. JB is a consultant for Novartis.
Supplementary Material
acknowledgements
We thank Shalini Murthy, ProEd Communications, Inc.®, for her medical editorial assistance with this manuscript.
references
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer, v2.2014. www.nccn.org. 3 April 2014, date last accessed. [DOI] [PubMed]
- 2.Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1) Breast. 2012;21:242–252. doi: 10.1016/j.breast.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664–1670. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- 4.Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28:4594–4600. doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
- 5.Johnston SR, Kilburn LS, Ellis P, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol. 2013;14:989–998. doi: 10.1016/S1470-2045(13)70322-X. [DOI] [PubMed] [Google Scholar]
- 6.Yardley DA, Noguchi S, Pritchard KI, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30:870–884. doi: 10.1007/s12325-013-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rugo HS, Pritchard KI, Gnant M, et al. Incidence and time course of everolimus-related adverse events in postmenopausal women with hormone receptor-positive advanced breast cancer: insights from BOLERO-2. Ann Oncol. 2014;25:808–815. doi: 10.1093/annonc/mdu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burris HA, III, Lebrun F, Rugo HS, et al. Health-related quality of life of patients with advanced breast cancer treated with everolimus plus exemestane versus placebo plus exemestane in the phase 3, randomized, controlled, BOLERO-2 trial. Cancer. 2013;119:1908–1915. doi: 10.1002/cncr.28010. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giuliano M, Schifp R, Osborne CK, Trivedi MV. Biological mechanisms and clinical implications of endocrine resistance in breast cancer. Breast. 2011;20(Suppl 3):S42–S49. doi: 10.1016/S0960-9776(11)70293-4. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Becerra R, Santos N, Diaz L, Camacho J. Mechanisms of resistance to endocrine therapy in breast cancer: focus on signaling pathways, miRNAs and genetically based resistance. Int J Mol Sci. 2012;14:108–145. doi: 10.3390/ijms14010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeselsohn R, Yelensky R, Buchwalter G, et al. Emergence of constitutively active estrogen receptor-alpha mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res. 2014;20:1757–1767. doi: 10.1158/1078-0432.CCR-13-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson DR, Wu YM, Vats P, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45:1446–1451. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toy W, Shen Y, Won H, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45:1439–1445. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Galan J, Torres-Torres B, Nunez MI, et al. ESR1 gene promoter region methylation in free circulating DNA and its correlation with estrogen receptor protein expression in tumor tissue in breast cancer patients. BMC Cancer. 2014;14:59. doi: 10.1186/1471-2407-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 17.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27:5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 19.Mauriac L, Cameron D, Dirix L, et al. Results of randomized phase II trial combining Iressa® (gefitinib) and arimidex in women with advanced breast cancer (ABC). EORTC protocol 10021. Cancer Res. 2009;69(2 Suppl 1) abstract 6133. [Google Scholar]
- 20.Osborne CK, Neven P, Dirix LY, et al. Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clin Cancer Res. 2011;17:1147–1159. doi: 10.1158/1078-0432.CCR-10-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robertson JF, Ferrero JM, Bourgeois H, et al. Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone-receptor-positive breast cancer: a randomised, controlled, double-blind, phase 2 trial. Lancet Oncol. 2013;14:228–235. doi: 10.1016/S1470-2045(13)70026-3. [DOI] [PubMed] [Google Scholar]
- 22.Campbell RA, Bhat-Nakshatri P, Patel NM, et al. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 23.Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible AKT activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002;1:707–717. [PubMed] [Google Scholar]
- 24.Simoncini T, Hafezi-Moghadam A, Brazil DP, et al. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolff AC, Lazar AA, Bondarenko I, et al. Randomized phase III placebo-controlled trial of letrozole plus oral temsirolimus as first-line endocrine therapy in postmenopausal women with locally advanced or metastatic breast cancer. J Clin Oncol. 2013;31:195–202. doi: 10.1200/JCO.2011.38.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630–2637. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 27.Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30:2718–2724. doi: 10.1200/JCO.2011.39.0708. [DOI] [PubMed] [Google Scholar]
- 28.Chia S, Piccart M, Gradishar W. Fulvestrant versus Exemestane Following non-Steroidal Aromatase Inhibitor Failure: First Overall Survival Data from the EFECT Trial. San Antonio, TX: San Antonio Breast Cancer Symposium (Poster 2091).; 2007. [Google Scholar]
- 29.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan X, Harkavy B, Shen N, et al. Rapamycin induces feedback activation of AKT signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 31.LoRusso PM. Mammalian target of rapamycin as a rational therapeutic target for breast cancer treatment. Oncology. 2013;84:43–56. doi: 10.1159/000343063. [DOI] [PubMed] [Google Scholar]
- 32.McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323–332. doi: 10.1016/S1470-2045(14)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 34.Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med. 2012;367:435–444. doi: 10.1056/NEJMoa1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufmann M, Bajetta E, Dirix LY, et al. Exemestane is superior to megestrol acetate after tamoxifen failure in postmenopausal women with advanced breast cancer: results of a phase III randomized double-blind trial. The Exemestane Study Group. J Clin Oncol. 2000;18:1399–1411. doi: 10.1200/JCO.2000.18.7.1399. [DOI] [PubMed] [Google Scholar]
- 36.Howell A, Robertson JFR, Abram P, et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol. 2004;22:1605–1613. doi: 10.1200/JCO.2004.02.112. [DOI] [PubMed] [Google Scholar]
- 37.Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106:djt337. doi: 10.1093/jnci/djt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yardley DA, Ismail-Khan RR, Melichar B, et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol. 2013;31:2128–2135. doi: 10.1200/JCO.2012.43.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andre F, Bachelot T, Campone M, et al. Targeting FGFR with dovitinib (TKI258): preclinical and clinical data in breast cancer. Clin Cancer Res. 2013;19:3693–3702. doi: 10.1158/1078-0432.CCR-13-0190. [DOI] [PubMed] [Google Scholar]
- 40.Finn RS, Crown JP, Lang I, et al. Phase II study of palbociclib (PD-0332991) + letrozole vs letrozole alone in first-line ER+/HER2− advanced breast cancer. Ann Oncol. 2013;24(Suppl 9):ix32–ix33. abstract O31–030. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


