Abstract
Purpose: RECIST have limitations when applied to potentially curable locally advanced squamous cell carcinoma of the head and neck (SCCHN). [18F]fluorodeoxyglucose–positron emission tomography (PET) scan may be useful in assessing treatment response and predicting patient outcome.
Patients and methods: We studied patients with previously untreated stages III–IVb SCCHN treated with primary concurrent chemoradiotherapy on five prospective clinical trials. Response was assessed by clinical exam, computed tomography (CT), and PET portions of combined PET–CT scan ∼8 weeks after completion of chemoradiotherapy.
Results: Fifty-three patients were analyzed. Complete response (CR) was demonstrated in 42 patients (79%) by clinical exam, 15 (28%) by CT, and 27 (51%) by PET. CR as assessed by PET, but not as assessed by clinical exam or CT using RECIST, correlated significantly with progression-free status (PFS) (P < 0.0001). The 2-year PFS for patients with CR and without CR by PET was 93% and 48%, respectively (P = 0.0002).
Conclusions: A negative PET scan on combined PET–CT after chemoradiotherapy is a powerful predictor of outcome in patients receiving curative chemoradiotherapy for SCCHN. PET–CT is indicated for response evaluation in this setting to improve the accuracy of post-treatment assessment by CT.
Keywords: chemoradiotherapy, head and neck cancer, objective response, PET–CT, RECIST
introduction
Head and neck cancer affects ∼47 000 individuals and causes 11 000 cancer deaths annually in the United States [1]. More than 90% of these cancers are squamous cell carcinomas, which are frequently related to smoking and alcohol use. Patients with squamous cell carcinoma of the head and neck (SCCHN) often present with advanced disease stages associated with significant local and/or regional spread of disease. Locally advanced SCCHN requires a multidisciplinary management approach and is potentially curable by means of combined modality treatment that may include surgery, radiotherapy (RT), and chemotherapy [2]. The role of concurrent chemoradiotherapy has been established by several randomized trials and meta-analyses [2, 3]. Despite appropriate treatment, local, regional, or distant disease recurrence occurs in more than half of patients treated for SCCHN with most relapses occurring during the first 2 years after treatment [4, 5].
Tumor response assessment methods routinely include clinical examination and radiographic imaging; however, current methods and criteria for tumor response assessment after definitive chemoradiotherapy have significant limitations. Response criteria guidelines, such as RECIST, utilize tumor measurements commonly obtained by anatomic imaging techniques, such as computed tomography (CT) or magnetic resonance imaging [6]. These criteria were primarily developed for the assessment of response in metastatic solid tumors treated with palliative systemic therapies. The definition of complete response (CR) requires complete disappearance of lesions, including lymph nodes. Nevertheless, subcentimeter nonpathologic lymphadenopathy can be found in normal necks or be a residual finding after shrinkage of pathologic lymph nodes after chemoradiotherapy. Therefore, defining CR using RECIST in this setting is challenging and lacks sensitivity.
Positron emission tomography (PET) permits assessment of metabolic activity within a target lesion. A variety of PET tracers have been developed to exploit metabolic differences between normal and cancer cells. Currently, the most widely available PET tracer is 2-fluoro-2-deoxy-D-glucose (FDG), a glucose analog. 2-[fluorine-18]fluoro-2-deoxy-D-glucose (FDG)–PET is potentially useful in detecting head and neck cancer recurrence even when disease is undetectable by clinical exam and other radiologic imaging [7]. Combined functional and anatomical imaging by means of combined PET–CT offers potential advantages in response assessment. In a prospective study from our institution, combined PET–CT had a higher sensitivity and specificity and yielded a superior receiver operating characteristic analysis by lesion when compared with PET alone and CT alone in patients known to have or suspected of having head and neck cancer [8]. The high diagnostic accuracy of PET–CT has also been shown to identify reliably the absence of nodal metastases and, thus, spare patients from unnecessary neck dissection [9, 10]. We have previously reported our preliminary experience with post-treatment PET–CT scan in 28 patients with SCCHN [11]. This study was conducted in a different cohort of patients of locally advanced SCCHN treated on clinical trials. We sought to evaluate the performance of PET–CT, CT alone, and physical exam in assessing tumor response and predicting long-term patient outcomes after curative chemoradiotherapy.
patients and methods
patient selection
We included patients with locally advanced, stages III, IVa, or IVb SCCHN of any primary site excluding nasopharynx who were treated on five prospective clinical trials conducted at the University of Pittsburgh Cancer Institute from 2004 to 2007. Staging classification was carried out accordingly to the American Joint Committee on Cancer Staging system. The study protocols were approved by the University of Pittsburgh Institutional Review Board and all patients signed informed consent. We selected patients who completed treatment with definitive concurrent chemoradiotherapy according to each study protocol and underwent staging evaluation by PET–CT at baseline and ∼8 weeks after completion of chemoradiotherapy. Patients with evidence of early disease progression or who died before undergoing response evaluation (n = 3) were excluded from this analysis. From a total of 91 patients enrolled in these five protocols, 53 met our inclusion criteria.
imaging and interpretation
Combined PET–CT scanning was carried out using standard protocols [8] on one of two scanners: a Discovery ST or a Discovery VCT system (GE Medical System, Waukesha, WI). Serum glucose level did not exceed 200 mg/dl. One hour after receiving 10–17 mCi of FDG, each patient received nonionic iodinated contrast 125 ml at a rate of 2 ml/s, and a combined PET–CT was carried out. The helical CT portion of the examination used diagnostic parameters, including 120 kVp, 120 mA, and 30-s contrast delay. The axial images were reconstructed at 3.75-mm thickness, and multiplanar reconstructions, both fused and unfused, were available in coronal and sagittal planes. The dose of 18F-FDG ranged from 12 to 16 mCi. Patients were scanned with arms placed at the side, using shallow breathing. All images were reviewed by a radiologist (BFB) with >7 years of experience interpreting combined PET–CT of the head and neck who was unaware of the eventual clinical outcome of patients.
tumor response assessment
As part of the prospective clinical trials, patients underwent baseline PET–CT before treatment (within 4 weeks) and ∼8 weeks after chemoradiotherapy. Primary site and nodal response to treatment was assessed by (i) clinical exam, (ii) CT portion of PET–CT using RECIST, and (iii) PET portion of PET–CT. CR by clinical exam, including laryngoscopy and neck palpation, was defined as no clinical evidence of disease, whereas non-CR implied residual abnormalities either on laryngoscopy and/or on neck palpation. CR by CT using RECIST was defined as disappearance of all target lesions. Non-CR by CT encompassed partial response (PR), progressive disease (PD), and stable disease (SD). PR was defined as at least a 30% decrease in the sum of the longest diameter (LD) of target lesions using as reference the baseline sum LD. However, there was no requirement for confirmation of response. PD by CT was defined as at least a 20% increase in the sum of the LD of target lesions or the appearance of new lesions. SD was defined as neither sufficient shrinkage to qualify for PR nor sufficient increased measurement to qualify for PD using the smallest sum LD from pretreatment imaging.
CR by PET was defined as complete disappearance of FDG activity attributable to malignancy, without regard to the degree of CT response, as assessed on combined PET–CT. Patients without CR on PET (non-CR) were categorized as having either abnormal findings but unlikely to be malignant or abnormal findings likely to represent residual malignancy with recommendations to undergo tissue sampling as clinically appropriate [12, 13].
After completing chemoradiotherapy, patients were assessed according to the individual study protocol. In general, evaluation included clinical exam that usually included laryngoscopy every 1–3 months and imaging studies at 8 weeks and then every 3–6 months for the first 2 years unless more frequent evaluations were clinically indicated. Although evaluation by PET–CT was optional, most patients treated on these studies underwent PET–CT. Confirmatory scans were not routinely obtained. Criteria for tumor biopsy after chemoradiotherapy included persistent mucosal abnormality by clinical exam or imaging studies suspicious for persistent or recurrent disease. Patients with marked tumor response but subtle residual FDG activity on PET were usually followed closely and underwent biopsy or neck dissection as clinically indicated. The reference standard for disease progression was pathologic confirmation of recurrent cancer or unequivocal enlargement of disease on radiologic follow-up.
statistical methods
The Spearman correlation was used to assess the concordance of response (CR versus non-CR) among clinical exam, CT portion of PET–CT, PET portion of PET–CT, and also between response results and eventual documented disease progression. Time to progression (TTP) was calculated as the time interval between the start date of treatment (i.e. either induction chemotherapy or concurrent chemoradiotherapy per protocol) and the documented date of disease progression. Progression-free survival (PFS) was measured from the start date of treatment until the recorded date of disease progression or death. For patients without disease progression, follow-up was censored at the date of last evaluation. PFS was estimated by the Kaplan–Meier method and log-rank test was used to assess the difference in PFS between patient groups. Cox proportional hazards regression was further used to assess the relationship of PFS to other covariates, such as primary site, age, sex, and tumor differentiation [14]. Statistical analyses were carried out using SAS version 9 (SAS Institute Inc., Cary, NC). All reported P values are two sided.
results
patient characteristics
We reviewed medical records of 53 patients with previously untreated stage III or IV SCCHN without distant metastasis treated with primary chemoradiotherapy on five clinical trials conducted at the University of Pittsburgh Cancer Institute from 2004 to 2007 (Table 1). All patients had Eastern Cooperative Oncology Group performance status of zero or one. The median age was 54 years (range 20–72) (Table 1). Forty-three were males (81%) and 10 females (19%). Stage at time of diagnosis was III in 8 patients (15%) and stage IV (without distant metastasis) in 45 patients (85%). Primary disease site was the oropharynx in 28 (54%), oral cavity in 3 (6%), larynx in 11 (21%), hypopharynx in 6 (11%), nasal cavity in 1 (2%), and unknown primary in 4 patients (7%). Five different chemoradiotherapy regimens were utilized (Table 2). Thirty-one patients (58%) received RT concurrently with cisplatin and cetuximab; 11 (21%) with cetuximab and pemetrexed; 9 (17%) with docetaxel and erlotinib; and 1 with cisplatin and 1 with carboplatin. In addition, a total of 29 patients (55%) also received induction chemotherapy: 28 with cisplatin, docetaxel, and cetuximab followed by RT, cisplatin, and cetuximab. Almost all patients received an epidermal growth factor receptor (EGFR) inhibitor (96%). All but one of the patients received standard dose RT with 70 Gy in 35 fractions. Only two patients were eventually lost to follow-up.
Table 1.
Patient characteristics (N = 53)
| Age, median (range) | 54 (20–72) |
| Sex, n (%) | |
| Male | 43 (81) |
| Female | 10 (19) |
| Stage, n (%) | |
| III | 8 (15) |
| IV | 45 (85) |
| IVa | 41 (77) |
| IVb | 4 (8) |
| Primary site, n (%) | |
| Oropharynx | 28 (54) |
| Larynx | 11 (21) |
| Hypopharynx | 6 (11) |
| Oral cavity | 3 (6) |
| Unknown primary | 4 (7) |
| Nasal cavity | 1 (2) |
Table 2.
Chemoradiotherapy regimens
| Chemoradiotherapy regimen | n (%) |
| Cetuximab/cisplatina | 31 (58) |
| RT 70 Gy/35 fractions | |
| Cetuximab/pemetrexed | 11 (21) |
| RT 70 Gy/35 fractions | |
| Docetaxel/erlotinib | 9 (17) |
| RT 70 Gy/35 fractions | |
| Cisplatin | 1 (2) |
| RT 72 Gy (accelerated concomitant boost; 36 Gy/20 daily fractions then 36 Gy in b.i.d fractions) | |
| Carboplatin | 1 (2) |
| RT 70 Gy/35 fractions |
Twenty-nine patients also received induction chemotherapy.
RT, radiotherapy; b.i.d = twice a day.
response assessment by clinical exam, CT, and PET
All patients underwent a baseline PET–CT before treatment and a follow-up PET–CT after completion of cathode ray tube. Median interval between RT completion and first follow-up PET–CT was 55 days (range 42–81). All patients had response assessment by all three methods, clinical exam, CT portion of PET–CT, and PET portion of PET–CT.
CR was demonstrated in 42 patients (79%) by clinical exam, 15 patients (28%) by CT, and 27 patients (51%) by PET. Response assessment by clinical exam did not correlate with response assessment by PET (Spearman correlation coefficient 0.06, P = 0.69) or CT (Spearman correlation coefficient 0.22, P = 0.12). However, response assessment by CT correlated significantly and positively with response assessment by PET (Spearman correlation coefficient 0.45, P = 0.0007).
clinical outcomes
With a median follow-up of all alive patients of 28 months (range, 15–60 months), 17 of the 53 patients (32%) developed PD: 7 patients had locoregional recurrence only, 9 patients developed distant metastases only, and 1 patient had locoregional recurrence with synchronous distant metastasis. Median TTP for the 17 patients who eventually progressed was 10.6 months (range 3.5–29.5 months), and the median duration between the completion of chemoradiotherapy and date of PD was 7.0 months (range 1.8–27.4 months). Nine patients (17%) have died, all due to disease progression. A total of nine patients underwent an invasive procedure immediately after tumor response assessment: eight patients underwent neck dissection and one had a fine needle aspiration of a neck lymph node. Only one of those eight patients had pathologic evidence of residual squamous cell carcinoma; this patient died due to locoregional progression.
CR as predictor of disease progression
Using clinical exam for response assessment, we found that 12 of 42 patients (29%) with CR progressed versus 5 of 11 patients (45%) without CR (P = 0.29) (Table 3). When CR was assessed by CT portion of PET–CT and applying RECIST definitions, 4 of 15 patients (27%) with CR progressed versus 13 of 38 patients (34%) without CR (P = 0.6). However, when assessment was made by PET portion of PET–CT, only 2 of 27 (7%) patients with CR progressed compared with 15 of 26 (58%) patients without CR (P < 0.0001). Of the two patients with CR by PET who progressed, one developed distant metastasis and the other both locoregional recurrence and distant metastasis. The Spearman correlation coefficient between PET response and eventual documented disease progression was 0.54, whereas there was no significant correlation between response by clinical exam or CT and disease progression (Table 3).
Table 3.
Assessment of CR after chemoradiotherapy (n = 53)
| Assessment of CR | Total number of patients | Number of patients with progression (rate) | P valuea |
| Clinical exam | |||
| CR | 42 | 12 (29%) | 0.29 |
| Non-CR | 11 | 5 (45%) | |
| CT (RECIST) | |||
| CR | 15 | 4 (27%) | 0.6 |
| Non-CR | 38 | 13 (34%) | |
| PET | |||
| CR | 27 | 2 (7%) | <0.0001b |
| Non-CR | 26 | 15 (58%) | |
By Spearman's correlation coefficient.
Spearman's correlation coefficient = 0.54.
CR, complete response; Non-CR, noncomplete response; PET, positron emission tomography.
The sensitivity, specificity, positive predictive value, and negative predictive value of a positive PET after chemoradiotherapy were 88.2%, 69.4%, 57.7%, and 92.6%, respectively.
Of 26 patients with non-CR by PET, 21 patients had a CR or PR and 5 SD or PD as assessed by CT scan. Ten out of 21 patients with CR or PR by CT progressed, however, all 5 patients with SD (n = 3) or PD (n = 2) by CT had documented disease progression.
progression-free survival
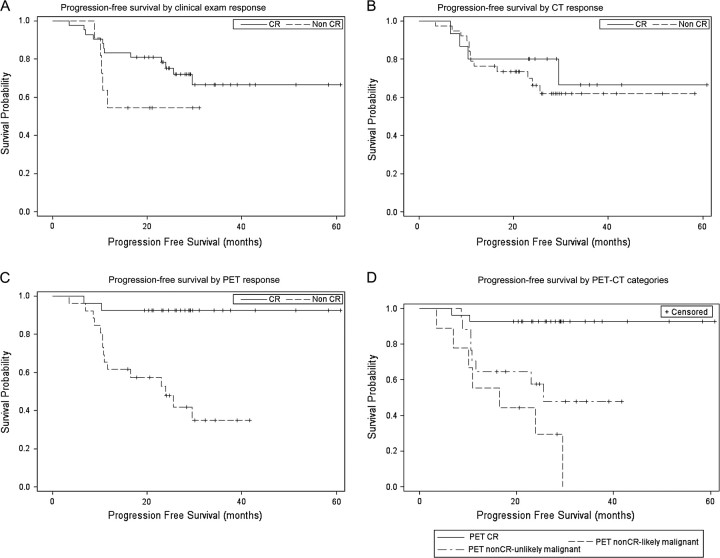
The corresponding PFS distributions in patients with and without CR by clinical exam, CT, and PET were estimated by the Kaplan–Meier method (Figure 1). There was no significant difference in PFS between patients with or without CR as assessed by CT or clinical exam (Figure 1A and B). However, patients with CR by PET had a better PFS compared with those without CR by PET (P = 0.0002, log-rank test) (Figure 1C). The 2-year PFS for patients with CR by PET was 92.6% versus 47.9% for patients without CR by PET.
Figure 1.
(A) Progression-free survival (PFS) by clinical exam response (log-rank test, P value = 0.14). (B) PFS by computed tomography (CT) response (log-rank test, P value = 0.56). (C) PFS by positron emission tomography (PET) response (log-rank test, P value = 0.0002). (D) PFS by PET-CT categories (log-rank test, P value < .0001). CR, complete response; non-CR, noncomplete response.
Of the 26 patients without CR by PET, 17 were considered to have radiographic findings on combined PET–CT unlikely to be malignant (per subjective review by an expert radiologist), whereas 9 patients were considered to have radiographic findings likely to be malignant. Therefore, by using combined PET–CT findings, we identified three separate groups of patients with distinct PFS (P < 0.0001, log-rank test): (i) PET–CR with 2-year PFS of 92.6%; (ii) PET non-CR unlikely to be malignant with 2-year PFS of 57.5%; and (iii) PET non-CR likely to be malignant with 2-year PFS of 29.6% (Figure 1D).
The effect of covariates, including primary site, age, sex, and tumor differentiation, was assessed by Cox proportional hazards regression model. There was no significant effect on PD detected among the covariates. Also, there was no significant difference in PFS between patients treated on different chemoradiotherapy regimens or those who received or not induction chemotherapy (P = 0.10, log-rank test).
discussion
Our study provides evidence that supports the role of combined PET–CT for tumor response assessment in patients with locally advanced SCCHN treated with curative-intent chemoradiotherapy. We found that CR as assessed by the PET portion of combined PET–CT carried out ∼8 weeks after completion of definitive chemoradiotherapy for locally advanced SCCHN correlates strongly with improved patient outcome. The 2-year PFS in patients with or without CR by PET was 93% versus 48% (P = 0.0002). On the other hand, CR as assessed by clinical exam or CT portion of PET–CT was not predictive of PFS. Although PET had a high negative predictive value (93%), its positive predictive value was suboptimal (58%). Therefore, additional prognostic criteria are needed to predict the outcome of patients who have a positive PET 8 weeks after chemoradiotherapy in order to identify earlier those patients who need salvage therapy. In our series, all three patients with non-CR by PET and SD by RECIST developed disease progression. It is reasonable to assume that SD in the setting of a positive PET indicates gross persistent disease and poor patient outcome; however, the number of patients in this subgroup was very small to allow us to draw any definitive conclusions. Furthermore, subjective review of combined PET–CT assisted in identifying two distinct groups among patients with non-CR by PET: those with residual findings deemed unlikely to be malignant (n = 17, 2-year PFS 57.5%) and those with findings likely to be malignant (n = 9, 2-year PFS 29.6%).
The present study evaluated different methods of assessing tumor response after chemoradiotherapy for SCCHN. Although CT findings correlated with PET findings, CR as assessed by CT did not predict disease progression. This may be because RECIST are not sensitive enough in defining CR since complete disappearance of small residual nonpathologic appearing lymph nodes is required to assign a CR assessment. In addition, postradiation changes in the primary site may obscure tumor measurements. The addition of PET to CT has been shown to change management in both newly diagnosed and treated patients with SCCHN [15, 16]. Combined PET–CT has been shown to have higher diagnostic accuracy in patients known to have or suspected of having head and neck cancer [8]. In that study, negative predictive value with PET–CT was 99% compared with 93% for PET alone and 83% for CT alone. PET–CT also improved radiologist’s confidence in diagnosing previously indefinite lesions seen on PET alone and CT alone. Another study showed that the sensitivity of combined PET–CT was 95% and the specificity was 60% for localizing tumor recurrence [17]. Zimmer et al., who evaluated 66 patients with stages I–IV SCCHN treated with various modalities including surgery, showed that PET–CT had a high negative predictive value for residual disease and a favorable impact on patient management by obviating unnecessary surgery [18]. Complete metabolic response was also associated with improved PFS and overall survival in that study. Moreover, our group showed that PET–CT had a diagnostic accuracy of 91% in detecting eventual progression in the neck, which led to a deferred elective neck dissection in 86% of patients with N2 or greater SCCHN treated with initial nonsurgical approaches [9]. Based on our experience at the University of Pittsburgh, we consider combined PET–CT, especially using diagnostic CT scans with i.v. contrast, as providing optimal anatomical correlation of abnormalities and best diagnostic accuracy.
Recently, changes in RECIST were proposed and included incorporation of new guidelines for measurement of nodal disease and assignment of CR in lymph nodes (short axis of <1 cm is no longer considered residual disease), redefinition of the number of lesions needed to assess tumor burden, and clarifications in the methods of assessment [19]. Although unidimensional anatomic measurement methods, such as CT, remain standard for response assessment, the new guidelines have permitted the addition of PET to complement dedicated CT during routine surveillance for progression.
The timing of PET after completion of RT correlates with its diagnostic accuracy. PET scans carried out >1 month after completion of radiation therapy have been shown to have a significantly higher sensitivity and negative predictive value than those carried out within 1 month [20]. Similarly, a PET scan done at 4 months after definitive RT was found to have a higher accuracy for detecting SCCHN recurrence compared with PET done 1 month after treatment [21]. In a previous separate retrospective analysis of SCCHN patients from our center, the diagnostic accuracy of PET–CT was 76.5% when carried out between 4 and 8 weeks postradiation therapy versus 100% when carried out later than 8 weeks [11]. Therefore, it has been our institutional preference to carry out after chemoradiotherapy response evaluation at 8 weeks. Other authors have recommended that PET–CT be carried 10–12 weeks after chemoradiotherapy [22] after finding an accuracy of PET–CT of 77% when examined 8–12 weeks after chemoradiotherapy as opposed to 95% when done >12 weeks after chemoradiotherapy. Monitoring of disease at subsequent time points (e.g. 3 months later) may offer additional information and provide a stronger correlation. However, detection of persistent disease is more meaningful when carried out earlier in time in order to increase the chances that any salvage treatment will be efficacious. In a recent study, PET–CT carried out 8 weeks after completion of primary RT with or without chemotherapy yielded a higher positive predictive value at detecting recurrence specifically in high-risk patients defined as those with human papillomavirus-negative, nonoropharyngeal primaries, and history of alcohol and tobacco use [23].
Since our patients were not treated with surgery, we cannot provide a correlation with pathologic response. However, in each case, disease recurrence was documented by biopsy. In general, we elected to closely follow patients with responsive disease but subtle residual FDG activity on PET. Any suspicious findings were evaluated by biopsy as clinically indicated.
Although our study was retrospective, all patients were treated in the context of therapeutic clinical trials, and clinical and radiologic data were collected prospectively as outlined in these protocols. On the other hand, since our study was conducted in selected patients of whom almost all were treated with multiagent EGFR inhibitor-containing chemoradiotherapy regimens on clinical protocols, results may not be applicable to unselected patients with SCCHN treated with standard therapies.
In conclusion, our study underscores the limitations of response assessment by standard RECIST and clinical exam in predicting disease progression in patients with locally advanced SCCHN. PET negativity after chemoradiotherapy is a powerful predictor of outcome in patients with locally advanced SCCHN, regardless of response using CT scan alone. We recommend that combined PET–CT with diagnostic CT scan with intravenous contrast be routinely utilized for tumor assessment before and after primary chemoradiotherapy for locally advanced SCCHN.
disclosure
None of the authors declare conflicts of interest.
Acknowledgments
Preliminary results of this study were presented at the annual meeting of the American Society of Clinical Oncology in 2008.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pignon JP, le Maitre A, Bourhis J. Meta-analyses of chemotherapy in head and neck cancer (MACH-NC): an update. Int J Radiat Oncol Biol Phys. 2007;69:S112–S114. doi: 10.1016/j.ijrobp.2007.04.088. [DOI] [PubMed] [Google Scholar]
- 4.Leemans CR, Tiwari R, Nauta JJ, et al. Recurrence at the primary site in head and neck cancer and the significance of neck lymph node metastases as a prognostic factor. Cancer. 1994;73:187–190. doi: 10.1002/1097-0142(19940101)73:1<187::aid-cncr2820730132>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.Ritoe SC, Krabbe PF, Kaanders JH, et al. Value of routine follow-up for patients cured of laryngeal carcinoma. Cancer. 2004;101:1382–1389. doi: 10.1002/cncr.20536. [DOI] [PubMed] [Google Scholar]
- 6.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 7.Lowe VJ, Boyd JH, Dunphy FR, et al. Surveillance for recurrent head and neck cancer using positron emission tomography. J Clin Oncol. 2000;18:651–658. doi: 10.1200/JCO.2000.18.3.651. [DOI] [PubMed] [Google Scholar]
- 8.Branstetter BF, Blodgett TM, Zimmer LA, et al. Head and neck malignancy: is PET/CT more accurate than PET or CT alone? Radiology. 2005;235:580–586. doi: 10.1148/radiol.2352040134. [DOI] [PubMed] [Google Scholar]
- 9.Nayak JV, Walvekar RR, Andrade RS, et al. Deferring planned neck dissection following chemoradiation for stage IV head and neck cancer: the utility of PET-CT. Laryngoscope. 2007;117:2129–2134. doi: 10.1097/MLG.0b013e318149e6bc. [DOI] [PubMed] [Google Scholar]
- 10.Yao M, Smith RB, Graham MM, et al. The role of FDG PET in management of neck metastasis from head-and-neck cancer after definitive radiation treatment. Int J Radiat Oncol Biol Phys. 2005;63:991–999. doi: 10.1016/j.ijrobp.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 11.Andrade RS, Heron DE, Degirmenci B, et al. Posttreatment assessment of response using FDG-PET/CT for patients treated with definitive radiation therapy for head and neck cancers. Int J Radiat Oncol Biol Phys. 2006;65:1315–1322. doi: 10.1016/j.ijrobp.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal V, Branstetter BF, Johnson JT. Indications for PET/CT in the head and neck. Otolaryngol Clin North Am. 2008;41:23–49. doi: 10.1016/j.otc.2007.10.005. , v. [DOI] [PubMed] [Google Scholar]
- 13.Shankar LK, Hoffman JM, Bacharach S, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–1066. [PubMed] [Google Scholar]
- 14.Klein JP, Moeschberger ML. In Survival Analysis. 2nd edition. New York: Springer; 2005. Semiparametric proportional hazards regression with fixed covariates; pp. 243–287. [Google Scholar]
- 15.Gordin A, Daitzchman M, Doweck I, et al. Fluorodeoxyglucose-positron emission tomography/computed tomography imaging in patients with carcinoma of the larynx: diagnostic accuracy and impact on clinical management. Laryngoscope. 2006;116:273–278. doi: 10.1097/01.mlg.0000197930.93582.32. [DOI] [PubMed] [Google Scholar]
- 16.Gordin A, Golz A, Keidar Z, et al. The role of FDG-PET/CT imaging in head and neck malignant conditions: impact on diagnostic accuracy and patient care. Otolaryngol Head Neck Surg. 2007;137:130–137. doi: 10.1016/j.otohns.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Zimmer LA, Snyderman C, Fukui MB, et al. The use of combined PET/CT for localizing recurrent head and neck cancer: the Pittsburgh experience. Ear Nose Throat J. 2005;84 104, 106, 108–110. [PubMed] [Google Scholar]
- 18.Connell CA, Corry J, Milner AD, et al. Clinical impact of, and prognostic stratification by, F-18 FDG PET/CT in head and neck mucosal squamous cell carcinoma. Head Neck. 2007;29:986–995. doi: 10.1002/hed.20629. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Ryan WR, Fee WE, Jr, Le QT, Pinto HA. Positron-emission tomography for surveillance of head and neck cancer. Laryngoscope. 2005;115:645–650. doi: 10.1097/01.mlg.0000161345.23128.d4. [DOI] [PubMed] [Google Scholar]
- 21.Greven KM, Williams DW, III, McGuirt WF, Sr, et al. Serial positron emission tomography scans following radiation therapy of patients with head and neck cancer. Head Neck. 2001;23:942–946. doi: 10.1002/hed.1136. [DOI] [PubMed] [Google Scholar]
- 22.Ong SC, Schoder H, Lee NY, et al. Clinical utility of 18F-FDG PET/CT in assessing the neck after concurrent chemoradiotherapy for locoregional advanced head and neck cancer. J Nucl Med. 2008;49:532–540. doi: 10.2967/jnumed.107.044792. [DOI] [PubMed] [Google Scholar]
- 23.Moeller BJ, Rana V, Cannon BA, et al. Prospective risk-adjusted [18F]Fluorodeoxyglucose positron emission tomography and computed tomography assessment of radiation response in head and neck cancer. J Clin Oncol. 2009;27:2509–2515. doi: 10.1200/JCO.2008.19.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]