Most adverse events with anti-programmed-cell-death protein 1 (PD-1)/PD-L1 agents are generally mild and reversible, yet some high-grade immune-related adverse events are managed with corticosteroids and other immune-modulating agents. The mechanisms underlying the development of these toxicities are the subject of ongoing studies.
Keywords: immune checkpoint antibody, anti-PD-1, anti-PD-L1, toxicity, adverse event
Abstract
Immune checkpoint antibodies that augment the programmed cell death protein 1 (PD-1)/PD-L1 pathway have demonstrated antitumor activity across multiple malignancies, and gained recent regulatory approval as single-agent therapy for the treatment of metastatic malignant melanoma and nonsmall-cell lung cancer. Knowledge of toxicities associated with PD-1/PD-L1 blockade, as well as effective management algorithms for these toxicities, is pivotal in order to optimize clinical efficacy and safety. In this article, we review selected published and presented clinical studies investigating single-agent anti-PD-1/PD-L1 therapy and trials of combination approaches with other standard anticancer therapies, in multiple tumor types. We summarize the key adverse events reported in these studies and their management algorithms.
introduction
Recent regulatory approvals for the anti-programmed cell death protein 1 (PD-1) immune checkpoint monoclonal antibodies (mAbs) nivolumab (BMS-936558) and pembrolizumab (MK-3475, previously lambrolizumab) for metastatic melanoma, and nivolumab for squamous nonsmall-cell lung carcinoma (NSCLC) serve to reinforce that immune-modulating mAbs have joined the list of standard and effective anticancer agents [1–4]. These agents, together with pidilizumab, and two anti-PD-L1 mAbs durvalumab (MEDI4736) and atezolizumab (MPDL3280A) that target the PD-1 ligand PD-L1, have demonstrated antitumor activity in a number of tumor types, including: renal cell carcinoma(RCC) [5], urothelial carcinoma [6], Hodgkin’s lymphoma [7], hepatocellular carcinoma (HCC) [8], head and neck carcinoma [9], and mismatch-repair-deficient colorectal cancer (CRC) [10]. These advances create a new set of challenges for clinicians, who must develop a working knowledge of the mode of action of these agents, their unique response kinetics, and importantly how to diagnose and effectively manage their toxicities.
Immune checkpoints are molecules involved in the maintenance of immunologic homeostasis and therefore help to maintain peripheral tolerance to self-molecules. Immune tolerance is critical in preventing excessive autoimmunity throughout life. Generally, tolerance is created through central tolerance in the thymus (during T-cell development) and peripheral tolerance (when self-antigens are encountered outside the thymus) [11]. A number of immune checkpoint molecules exist that may serve to either augment or inhibit an immune response. These include co-inhibitory molecules such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), PD-1, lymphocyte-activation gene 3, and T-cell immunoglobulin mucin-3, and co-stimulatory molecules such as: glucocorticoid-induced tumor necrosis factor receptor and OX40 (CD134, TNFRSF4, tumor necrosis factor receptor superfamily member 4). Tumor cells can escape from immune system destruction through many mechanisms, including the expression of immune suppressive molecules on their cell surface, secretion of soluble suppressive factors, and the recruitment of other suppressive immune cell populations to the tumor microenvironment [12]. The use of mAbs that block co-inhibitory immune checkpoint molecules, such as CTLA-4 and PD-1, may serve to increase a baseline T-cell-specific immune response that turns the immune system against the tumor [13]. However, a disruption in the functioning of immune checkpoint molecules can lead to imbalances in immunologic tolerance that result in an unchecked immune response. This may clinically manifest with autoimmune-like/inflammatory side-effects, which cause collateral damage to normal organ systems and tissues, including: the skin, gastrointestinal, hepatic, pulmonary, mucocutaneous, and endocrine systems [14]. Such adverse events, termed ‘immune-related adverse events’ (irAEs), have been the subject of much clinical interest and mechanistic research, and are also thought to be principally T-cell mediated [15]. Other immune cells may play a role in the development of irAEs, including B cells that secrete antibodies that may mediate toxicity [16, 17], granulocytes that secrete inflammatory mediators, and cytokines [16, 18]. Standard treatment algorithms for irAEs have been developed that utilize immune-modulating medications including corticosteroids, antihistamines, antitumor necrosis factor medications and calcineurin inhibitors, which may quell the inflammatory response, without eliminating the antitumor immune response [19].
In general, toxicities with anti-PD-1/PD-L1 mAbs appear to be less common and less severe when compared with anti-CTLA-4 mAbs, with reported grade 3–4 AEs ranging from 7% to 12% in patients receiving single-agent anti-PD-1/PD-L1 mAb [1–3], as opposed to 10%–18% of patients who receive single-agent anti-CTLA-4 mAb, in phase III studies [15, 20, 21]. In this review, we summarize the most commonly observed treatment-related irAEs associated with mAbs that target the PD-1/PD-L1 pathway from both single-agent and combination studies with standard anticancer agents, including: other immunotherapeutic agents, chemotherapy, targeted therapy, antiangiogenic agents, and radiation therapy (RT).
general adverse events of anti-PD-1/PD-L1 therapy
fatigue
The most common AE across studies incorporating anti-PD-1/PD-L1 agents is fatigue (Tables 1 and 2). In the first phase I studies published in 2012 of nivolumab and anti-PD-L1 mAb BMS-936559, 16%–24% of patients had treatment-related fatigue, and 1%–2% of these events were grade 3/4 in severity [22, 26]. Fatigue is consistently reported across single-agent studies, with an incidence of 16%–37% with anti-PD-1 agents [3, 25] and 12%–24% with anti-PD-L1 agents from selected studies [6, 33]. Clinical studies that combine anti-PD-1/PD-L1 agents with other immune checkpoint antibodies [23, 34–37], chemotherapy [39, 45], antiangiogenic agents [44–46], and targeted therapies [40–43] have reported slightly higher reported rates of fatigue, ranging from 21% to 71% (Table 2). Fatigue that occurs with other immunotherapeutic agents such as type I interferon therapy [47] may induce fatigue in association with other systemic symptoms such as influenza-like illness, suggestive of cytokine release [15]. In the case of anti-PD-1/PD-L1 agents, fatigue is usually mild and not associated with other systemic symptoms. A specific mechanism by which immune checkpoint antibodies may cause fatigue is currently not known, but this does not appear to be dose-related. A proportion of the patients with treatment-related fatigue may be presenting with early symptoms of hypothyroidism, a known endocrinopathy associated with immune checkpoint blockade therapy (see endocrine toxicities section).
Table 1.
Adverse events in selected single-agent studies with ant-PD-1/PD-L1 antibodies
| Agent | First author (year) | Phase | Tumor type | No. of patients receiving anti-PD-1/PD-L1 agent (N) | Therapy schedule | Treatment-related toxicities (grade 1–5) | Treatment-related grade 3–4 toxicities |
|---|---|---|---|---|---|---|---|
| Anti-PD-1 agent | |||||||
| Nivolumab | Topalian (2012) [22] | I | NSCLC; RCC; CRC; CRPC; melanoma | 296 | 0.1–10 mg/kg every 2 weeks for up to 2 years | Total: 70% (n = 207) Fatigue (24%, n = 72) Rash (12%, n = 36) Pruritus (10%, n = 28) Pneumonitis (9%, n = 3) Infusion reaction (9%, n = 3) Hypothyroidism (2%, n = 7) |
Total: 7% (n = 22) Hypothyroidism (<1%, n = 1) Pneumonitis (1%, n = 3) Diarrhea (1%, n = 3) AST elevation (1%, n = 2) ALT elevation (1%, n = 2) Rash (1%, n = 2) Infusion reaction (<1%, n = 1) |
| Ansell (2015) [7] | I | Hodgkin's lymphoma | 23 | 3 mg/kg every 2 weeks | Total: 78% (n = 18) Rash (22%, n = 5) Thrombocytopenia (17%, n = 4) Fatigue (13%, n = 3) Pyrexia (13%, n = 3) |
Total: 22% (n = 5) Lipase elevation (4%, n = 1) Lymphopenia (4%, n = 1) MDSa (4%, n = 1) Pancreatitis (4%, n = 1) |
|
| Motzer (2014) [5] | II | RCC | 168 | 0.3, 2, or 10 mg/kg every 2 weeks | Total: 73% (n = 122) Fatigue (27%, n = 45) Rash (10%, n = 17) Pruritus (10%, n = 16) Hypothyroidism (12%, n = 10) AST elevation (5%, n = 8) ALT elevation (4%, n = 7) Pneumonitis (3%, n = 5) |
Total: 11% (n = 19) AST elevation (2%, n = 3) ALT elevation (2%, n = 3) Nausea (1%, n = 2) Hypothyroidism (<1%, n = 1) Pruritus (<1%, n = 1) Arthralgia (<1%, n = 1) |
|
| Sampson (2014) [23] | I | GBM | 20 (n = 10 single- agent arm) | Single-agent arm: 3 mg/kg every 2 weeks | Total: 60% (n = 6) Fatigue (30%, n = 3) Nausea (30%, n = 3) |
Total: 0% | |
| El-Khoueiry (2015) [8] | I/II | HCC | 47 | 0.1–10 mg/kg every 2 weeks | Total = 68% (n = 32) AST elevation (19%, n = 9) Lipase elevation (17%, n = 8) Rash (17%, n = 8) Amylase elevation (15%, n = 7) ALT elevation (15%, n = 7) |
Total = 19% (n = 9) AST elevation (11%, n = 5) ALT elevation (9%, n = 4) Lipase elevation (9%, n = 4) Fatigue (2%, n = 1) Anemia (2%, n = 1) |
|
| Gettinger (2015) [24] | I | NSCLC | 129 | 1, 3, or 10 mg/kg every 2 weeks | Total = 71% (n = 91) Fatigue (24%, n = 31) Decreased appetite (12%, n = 16) Diarrhea (10%, n = 13) Pyrexia (6%, n = 8) Pruritus (9%, n = 11) Pneumonitis (6%, n = 8) |
Total = 14% (n = 18) Fatigue (3%, n = 4) Pneumonitis (2%, n = 3) Low CD-4 cells (2%, n = 3) Diarrhea (<1%, n = 1) |
|
| Rizvi (2015) [25] | II | NSCLC (squamous) | 117 | 3 mg/kg every 2 weeks | Total = 74% (n = 87) Fatigue (33%, n = 38) Diarrhea (10%, n = 12) Rash (11%, n = 13) Pneumonitis (5%, n = 6) |
Total = 17% (n = 20) Fatigue (4%, n = 5) Diarrhea (3%, n = 3) Rash (1%, n = 1) Pneumonitis (3%, n = 4) |
|
| Weber (2015) [1] | III | Ipilimumab-refractory melanoma | 272 | 3 mg/kg every 2 weeks versus chemotherapy | Total = 67% (n = 181) Fatigue (25%, n = 67) Pruritus (16%, n = 43) Diarrhea (11%, n = 30) Nausea (9%, n = 25) |
Total = 9% (n = 24) Lipase elevation (1%, n = 3) ALT elevation (1%, n = 2) Anemia (1%, n = 2) Fatigue (1%, n = 2) |
|
| Brahmer [3] | III | NSCLC (squamous) | 131 | 3 mg/kg every 2 weeks versus docetaxel | Total = 58% (n = 76) Fatigue (16%, n = 21) Low appetite (14%, n = 11) Asthenia (10%, n = 13) Diarrhea (8% n = 10) Pneumonitis (5%, n = 6) Hypothyroidism (4%, n = 5) |
Total = 7% (n = 9) Fatigue (<1%, n = 1) Low appetite (<1%, n = 1) Leucopenia (<1%, n = 1) Pneumonitis (<1%, n = 1) Colitis (<1%, n = 1) Interstitial nephritis (<1%, n = 1) |
|
| BMS-39886 | Brahmer [26] | I | NSCLC; RCC; CRC; melanoma | 207 | 0.3–10 mg/kg every 2 weeks | Total = 61% (n = 126)b Fatigue (16%, n = 33) Infusion reaction (10%, n = 21) Diarrhea (9%, n = 19) Rash (9%, n = 14) Hypothyroidism (3%, n = 6) Adrenal insufficiency (2%, n = 3) |
Total = 5% (n = 11) Endocrine (1%, n = 2) Fatigue (<1%, n = 1) Pyrexia = 1 (<1%, n = 1) Diarrhea = 1 (<1%, n = 1) Myocarditis = 1 (<1%, n = 1) Sarcoidoisis = 1 (<1%, n = 1) |
| Pembrolizumab | Hamid (2012) [27] | I | Melanoma | 135 | 2 mg/kg every 3 weeks, or 10 mg/kg every 2 or 3 weeks | Total = 79% (n = 107) Fatigue (30%, n = 41) Rash (21%, n = 28) Pruritus (21%, n = 28) Diarrhea (20%, n = 27) |
Total = 13% (n = 17) Rash (2%, n = 3) Acute renal failure (1%, n = 2) AST elevation (1%, n = 2) Fatigue (1%, n = 2) |
| Le (2015) [10] | I | Metastatic carcinoma with or without mismatch repair deficiency | 41 | 10 mg/kg every 2 weeks | Total = 98% (n = 40) Fatigue (32%, n = 13) Diarrhea (24%, n = 10) Rash/pruritus (24%, n = 10) Hypophysitis/thyroiditis/ hypothyroidism (10%, n = 6) Pancreatitis (15%, n = 4) |
Total = 41% (n = 17) Lymphopenia (20%, n = 8) Hypoalbuminemia (10%, n = 4) Anemia (17%, n = 7) |
|
| Robert (2014) [28] | I | Ipilimumab-refractory melanoma | 173 | 2 or 10 mg/kg every 3 weeks | Total = 82% (n = 142) Fatigue (35%, n = 60) Pruritus (23%, n = 39) Rash (18%, n = 31) Diarrhea (13%, n = 22) Pneumonitis (2%, n = 3) Autoimmune hepatitis (<1%, n = 1) Hypothyroidism (4%, n = 7) |
Total = 12% (n = 20) Fatigue (3%, n = 5) Diarrhea (<1%, n = 1) Pneumonitis (<1%, n = 1) Hypophysitis (<1%, n = 1) Autoimmune hepatitis (<1%, n = 1) |
|
| Ribas (2015) [29] | II | Melanoma | 357 | 2 or 10 mg/kg every 3 weeks versus standard chemotherapy | Total = 71% (n = 252) Fatigue (26%, n = 92) Pruritus (22%, n = 79) Rash (8%, n = 29) Hypothyroidism (6% n = 22) Pneumonitis (1%, n = 3) |
Total = 13% (n = 45) Fatigue (<1%, n = 2) Myalgia (<1%, n = 2) Edema (<1%, n = 2) Colitis (<1%, n = 2) Hypophysitis (<1%, n = 2) Pneumonitis (<1%, n = 2) |
|
| Robert (2015) [2] | III | Untreated melanoma | 556 | 10 mg/kg every 2 or 3 weeks versus ipilimumab | Total = 76% (n = 423) Fatigue (20%, n = 111) Pruritus (14%, n = 79) Rash (14%, n = 77) Hypothyroidism (9%, n = 52) Hyperthyroidism (5%, n = 27) Colitis (3%, n = 15) Hepatitis (1%, n = 8) Pneumonitis (1%, n = 6) Uveitis (<0.1%, n = 4) |
Total = 12% (n = 65) Colitis (2%, n = 11) Diarrhea (n = 10) Hepatitis (n = 8) Hypophysitis (n = 2) Pneumonitis (n = 1) Type 1 diabetes (n = 1) |
|
| Garon (2015) [4] | I | NSCLC | 495 | 2 or 10 mg/kg every 3 weeks, or 10 mg/kg every 2 weeks | Total = 71% (n = 351) Fatigue (19%, n = 96) Pruritus (11%, n = 53) Low appetite (11%, n = 52) Rash (10%, n = 48) Infusion reaction (3%, n = 15) Hypothyroidism (7%, n = 34) Pneumonitis (4%, n = 18) |
Total = 10% (n = 47) Dyspnea (4%, n = 19) Pneumonitis (2%, n = 9) Low appetite (1%, n = 5) Fatigue (<1%, n = 4) Infusion reaction (<1%, n = 1) |
|
| Pidilizumab | Armand (2013) [30] | II | Lymphomac | 72 | 1.5 mg/kg every 42 days × 3 doses | Total = 96% (n = 69) Neutropenia (26%, n = 19) Fatigue (25%, n = 18) Respiratory infection (19%, n = 14) Diarrhea (17%, n = 12) Thrombocytopenia (14%, n = 10) |
Total = 54% (n = 39) Neutropenia (19%, n = 14) Thrombocytopenia (8%, n = 6) |
| Anti-PD-L1 agent | |||||||
| Durvalumab | Segal [31] | I | Multiple solid tumorsa | 346 | 10 mg/kg every 2 weeks × 1 year | Total = 39% (n = 135) Fatigue (13%, n = 45) Rash (9%, n = 30) Pneumonitis (1%, n = 5) AST/ALT elevation (4%, n = 13) Hypothyroidism (2%, n = 8) |
Total = 6% (n = 20) Fatigue (1%, n = 2) Rash (<1%, n = 1) AST/ALT elevation (1%, n = 3) Hypothyroidism (<1%, n = 1) |
| Rizvi (2015) [32] | I | NSCLC | 228 | 10 mg/kg every 2 weeks × 1 year | Total = 93% (n = 213) Fatigue = 18% Low appetite = 9% Nausea = 8% Hyperthyroidism (4%, n = 9) Diarrhea (7%, n = 15) Rash (8%, n = 17) Pneumonitis (1%, n = 3) |
Total = 53% (n = 121) Diarrhea (<1%, n = 1) Rash (0%, n = 0) Hyperthyroidism (<1%, n = 1) |
|
| Atezolizumab | Herbst (2014) [33] | I | Multiple solid tumors + hematologic malignancies | 277 | 0.01–20 mg/kg every 3 weeks | Total = 70% (n = 194) Fatigue (24%, n = 67) Low appetite (12%, n = 33) Rash (11%, n = 29) Influenza-like illness (6%, n = 16) AST/ALT elevation (4%, n = 10) Tumor lysis syndrome (<1%, n = 2) |
Total = 13% (n = 35) Fatigue (2%, n = 5) Low appetite (0%, n = 0) Rash (0%, n = 0) Influenza-like illness (<1%, n = 1) AST/ALT elevation (2%, n = 6) Tumor lysis syndrome (<1%, n = 2) |
| Powles (2014) [6] | I | Urothelial carcinoma | 68 | 15 mg/kg every 3 weeks × 1 year | Total = 57% (n = 39) Low appetite (12%, n = 8) Fatigue (12%, n = 8) Pyrexia (12%, n = 8) Influenza-like illness (4%, n = 3) Thrombocytopenia (3%, n = 2) |
Total = 4% (n = 3) Asthenia (1.5%, n = 1) Thrombocytopenia (1.5%, n = 1) Phosphorus elevation (1.5%, n = 1) |
|
aMDS, myelodysplastic syndrome.
bInvestigator-reported adverse events.
cIncluded patients with diffuse large B-cell lymphoma, primary mediastinal B-cell lymphoma and transformed indolent B-cell lymphoma
NSCLC, nonsmall-cell lung cancer; RCC, renal cell carcinoma; CRC, colorectal carcinoma; CRPC, castration-resistant prostate cancer; HCC, hepatocellular carcinoma; GBM, glioblastoma multiforme.
Table 2.
Incidence of adverse events in combination studies with ant-PD-1/PD-L1 antibodies and other therapies, across multiple solid tumors
| Agent | Author(s) | Phase | Tumor type | Total patients (N) | Treatment schedule | Treatment-related toxicities (grade 1–5) | Grade 3–4 treatment-related toxicities |
|---|---|---|---|---|---|---|---|
| Immune checkpoint antibodies | |||||||
| Nivolumab + ipilimumab | Wolchok et al. [34] | I | Melanoma | 86 (n = 53 concurrent, n = 33 sequenced) | Concurrent (N: 0.3–10 mg/kg every 3 weeks) + I (1–10 mg/kg every 3 weeks) then N + I every 3 months × 8 | Total = 93% (n = 49)a Rash (55%, n = 29) Pruritus (47%, n = 25) Fatigue (38%, n = 20) Diarrhea (34%, n = 18) Colitis (9%, n = 5) AST elevation (23%, n = 12) ALT elevation (21%, n = 11) |
Total = 53%a (n = 28)b Elevated lipase (13%, n = 7) AST elevation (13%, n = 7) ALT elevation (11%, n = 6) Diarrhea (6%, n = 3) Colitis (4%, n = 2) Rash (4%, n = 2) |
| Postow et al. [35] | II | Melanoma | 142 (n = 95 combination arm, n = 47 I-alone arm) | N (1 mg/kg every 3 weeks × 4, followed by 3 mg/kg every 2 weeks till progression/toxicity) + I (3 mg/kg every 3 weeks × 4) | Total = 91% (n = 86) Diarrhea (45%, n = 42) Rash (41%, n = 39) Colitis (23%, n = 22) AST elevation (22%, n = 21) ALT elevation (21%, n = 20) Hypothyroidism (16%, n = 15) Hypophysitis (12%, n = 11) |
Total = 54% (n = 51)b Colitis (17%, n = ) Diarrhea (11%, n = ) AST elevation (11%, n = 10) ALT elevation (7%, n = 7) Hypophysitis (7%, n = 3) Pneumonitis (2%, n = 2) |
|
| Larkin et al. [36] | III | Melanoma | 945 (N only = 316, N + I = 314, I alone = 315) | N (3 mg/kg every 3 weeks) versus I (3 mg/kg every 3 weeks × 4) versus N + I (N: 1 mg/kg × 4 doses + I then N: 3 mg/kg every 3 weeks × 4, or 3 mg/kg from cycle 3 on every 2 weeks) | Total = 96% (n = 299) Diarrhea (44%, n = 138) Fatigue (35%, n = 110) Pruritus (33%, n = 104) Rash (40%, n = 126) AST elevation (15%, n = 45) ALT elevation (18%, n = 55) Hypothyroidism (15%, n = 47) Colitis (12%, n = 37) |
Total = 55% (n = 172) Diarrhea (9%, n = 29) Fatigue (4%, n = 13) Pruritus (2%, n = 6) Rash (5%, n = 15) AST elevation (6%, n = 19) ALT elevation (8%, n = 26) Hypothyroidism (<1%, n = 1) Colitis (8%, n = 24) |
|
| Sampson et al. [23] | I | Glioblastoma multiforme | 20 (n = 10 combination arm) | Combination arm: N (1 mg/kg) + I (3 mg/kg every 3 weeks) followed by N (3 mg/kg every 2 weeks) | Total = 100% Fatigue (40%, n = 8) Diarrhea (35%, n = 7) AST elevation (25%, n = 5) High lipase (25%, n = 5) Vomiting (20%, n = 4) ALT elevation (20%, n = 4) |
Total = 70% (n = 7) Colitis (10%, n = 2) Hypothyroidism (10%, n = 2) Diarrhea (10%, n = 2) ALT elevation (10%, n = 2) Cholecystitis (5%, n = 1) Diabetic ketoacidosis (5%, n = 1) Elevated lipase (5%, n = 1) |
|
| Pembrolizumab + ipilimumab | Patnaik et al. [37] | I | NSCLC | 18 | P (2 or 10 mg/kg every 3 weeks) + I (1 or 3 mg/kg every 3 weeks × 4) + maintenance P | Total = 83% (n = 15) Fatigue (33%, n = 4) Low appetite (17%, n = 2) Pruritus (17%, n = 2) Rash (17%, n = 2) Myasthenia gravis (6%, n = 1) Myocarditis (6%, n = 1) Pneumonitis (6%, n = 1) Uveitis (6%, n = 1) |
Total = 17% (n = 3) Rash (17%, n = 2) Adrenal insufficiency (6%, n = 1) |
| MEDI4736 + tremelimumab | Antonia et al. [38] | Ib | NSCLC | 102 | M (3–20 mg/kg every 4 weeks or 10 mg/kg every 2 weeks) + T (1–3 mg/kg every 2 or 4 weeks × 6 doses) for 1 year | Total = 93% (n = 95) Diarrhea (27%, n = 28) Fatigue (26%, n = 27) Colitis (12%, n = 12) ALT elevation (10%, n = 10) AST elevation (6%, n = 6) Hypothyroidism (6%, n = 6) Pneumonitis (5%, n = 5) |
Total = 61% (n = 60) Diarrhea (8%, n = 8) Colitis = (9%, n = 9) ALT elevation (3%, n = 3) AST elevation (4%, n = 4) Myasthenia gravis (n = 1) Polymyositis (n = 1) Pneumonitis (4%, n = 4) Hypothyroidism (1%, n = 1) |
| Chemotherapy | |||||||
| Nivolumab + platinum-doublet chemotherapy | Antonia et al. [39] | I | NSCLC | 56 | N (10 mg/kg every 3 weeks or 5 mg/kg every 3 weeks) + chemotherapy × 4) + N alone (10 mg/kg every 3 weeks or 5 mg/kg every 3 weeks) | Total = 93% (n = 52) Fatigue (71%, n = 40) Nausea (46%, n = 26) Low appetite (36%, n = 20) Alopecia (30%, n = 17) Pneumonitis (13%, n = 7) |
Total = 45% (n = 25) Fatigue (5%, n = 3) Anemia (4%, n = 2) Rash (4%, n = 2) Acute renal failure (5%, n = 3) Pneumonitis (7%, n = 4) |
| Targeted therapy | |||||||
| Durvalumab + AZD9291 | Oxnard et al. [40] | Ib | EGFR-mutant T790M-positive NSCLC | 14 | M (3 or 10 mg/kg every 2 weeks) + A (80 mg daily) | Total: not reportedb Diarrhea (50%, n = 7) Vomiting (50%, n = 7) Anemia (45%, n = 6) Pneumonitis (21%, n = 3) |
Total: 1% (n = 2)b Neutropenia = 2 |
| Durvalumab + gefitinib | Creelan et al. [41] | Ib | NSCLC | 10 | M (3 or 10 mg/kg every 4 weeks) + G (250 mg daily) × 1 year | Total = 100% (n = 10) ALT elevation (50%, n = 5) AST elevation (50%, n = 5) Diarrhea (50%, n = 5) |
Total = 30% (n = 3) Dyspnea (1%, n = 1) Fatigue (1%, n = 1) ALT elevation (1%, n = 1) |
| Durvalumab + dabrafenib + trametinib | Ribas et al. [42] | Ib | BRAF-mutant and wild-type melanoma | 65 | M (3 or 10 mg/kg every 2 weeks) + D (150 mg b.i.d.) + T (2 mg q.d.) or M (10) + T or M (10) + T (× 6 weeks only) | Total = 98% (n = 64) Pyrexia (37%, n = 24) Chills (24%, n = 16) Arthralgia (17%, n = 11) Peripheral edema (17%, n = 11) Folliculitis (18%, n = 12) Pneumonitis (1%, n = 1) AST elevation (12%, n = 8) ALT elevation (10%, n = 7) Low ejection fraction (2%, n = 2) |
Total = 46% (n = 30) Pyrexia (2%, n = 2) Chills (3%, n = 1) Peripheral edema (5%, n = 3) AST elevation (8%, n = 2) ALT elevation (4%, n = 1) Low ejection fraction (9%, n = 2) Pneumonitis (0%, n = 0) |
| Pidilizumab + rituximab | Westin et al. [43] | II | Follicular lymphoma | 32 | P (3 mg/kg every 4 weeks × 4–12) + R (375 mg/m2 weekly × 4) | Total = 94% (n = 30) Anemia (47%, n = 14) Fatigue (43%, n = 13) Leucopenia (37%, n = 11) |
Total: 0% (n = 0) |
| Antiangiogenic therapy | |||||||
| Atezolizumab + bevacizumab | Sznol et al. [44] | Ib | RCC | 10 | B (15 mg/kg every 3 weeks) + A (20 mg/kg every 3 weeks) | Total: 80% (n = 8) Fatigue (40%, n = 4) Low appetite (30%, n = 3) Diarrhea (30%, n = 3) Arthalgia (20%, n = 2) |
Total: 0% (n = 0) |
| Atezolizumab + bevacizumab | Bendell et al. [45] | Ib | CRC | 14 (A + B) | A (20 mg/kg every 3 weeks) + B (15 mg/kg every 3 weeks) | Total = 79% (n = 11) Fatigue (21%, n = 3) Nausea (29% n = 4) Pyrexia (21%, n = 3) Decreased appetite (7%, n = 1) |
Total = 7% (n = 1) Neutropenia (7%, n = 1) |
| Nivolumab + sunitinib or pazopanib | Amin et al. [46] | Ib | RCC | 53 (N + S, n = 33, N + P, n = 20) | N (2–5 mg/kg every 3 weeks) + S (50 mg 4 weeks on, 2 weeks off) or P (800 mg daily) | Total: 100% (n = 53) Sunitinib: Fatigue (n = 27) Diarrhea (n = 20) ALT elevation (n = 13) AST elevation (n = 12) Acute renal failure (n = 4) Pneumonitis (n = 2) Pazopanib: Fatigue (n = 12) AST elevation (n = 6) ALT elevation (n = 5) |
Total: 77% (n = 41) Sunitinib: ALT elevation (18%, n = 6), AST elevation (9%, n = 3) Autoimmune nephritis (3%, n = 1) Pneumonitis (3%, n = 1) Pazopanib: AST elevation (20%, n = 4) ALT elevation (20%, n = 4) Fatigue (15%, n = 3) Diarrhea (20%, n = 4) |
| Antiangiogenic therapy + chemotherapy | |||||||
| Atezolizumab + bevacizumab + FOLFOX | Bendell et al. [45] | Ib | CRC | 30 (A + B + F) | A (14 mg/kg every 2 weeks) + B (10 mg/kg every 2 weeks) + F (standard doses, every 2 weeks) | Total = 100% (n = 30) Fatigue (47%, n = 14) Nausea (27% n = 8) Pyrexia (20%, n = 6) Decreased appetite (20%, n = 6) |
Total = 20% (n = 6) Neutropenia (7%, n = 2) AST elevation (7%, n = 2) ALT elevation (3%, n = 1) Diarrhea (3%, n = 1) |
aResults from concurrent arm only.
bAll case adverse events.
GBM, glioblastoma multiforme; HNSCC, head and neck squamous cell carcinoma; CRC, colorectal carcinoma; RCC, renal cell carcinoma; EGFR, epidermal growth factor receptor; N, nivolumab, I, ipilimumab; P, pembrolizumab; A, atezolizumab; B, bevacizumab; F, FOLFOX chemotherapy (5-flurouracil bolus and continuous infusion, leucovorin, oxaliplatin).
pyrexia, chills, infusion reactions
Fever, chills, and infusion reactions have been described across multiple modalities of immunotherapeutic anticancer agents, including cancer vaccines, adoptive T-cell therapy, chimeric antigen receptor T cells, cytokines, and immune-modulating antibodies [15]. The mechanism underlying the development of these toxicities is postulated to be due to cytokine release and nonspecific activation of an immune response [48]. Fevers and chills may be managed supportively with antipyretics such as acetaminophen or nonsteroidal anti-inflammatory drugs at the time of development of the toxicity, and to prevent the recurrence of infusion reactions, as needed [48]. In cases of grade 3 infusion reactions, patients may also receive antihistamines and corticosteroid medications intravenously at the time of the hypersensitivity reaction as required, in line with prior experience with ipilimumab [49]. Infusion reactions with agents that target the PD-1/PD-L1 pathway are very rare, accounting for <1% of AEs in phase III studies [1–3, 36].
organ-specific adverse events with single-agent anti-PD-1/PD-L1 agents
dermatologic toxicities
Skin rash is the most common irAE associated with immune checkpoint mAb therapy, and typically occurs after the second cycle in the patient's clinical course [14, 15]. A variety of clinical presentations of rash can manifest including: maculopapular, papulopustular, Sweet's syndrome, follicular, or urticarial dermatitis. In a pooled safety analysis of melanoma patients with dermatological AEs such as rash, pruritus, and vitiligo, toxicities were observed in 34% of patients who received nivolumab [50], and 39% of patients who received pembrolizumab [2]. Of note, a direct comparison of pembrolizumab with ipilimumab in the latter study demonstrated a higher incidence of vitiligo of ∼10% in pembrolizumab-treated patients versus 2% in ipilimumab-treated patients [2].
With anti-PD-1/PD-L1 mAb, a maculopapular rash is most commonly observed. However, rarer rashes have been described, including lichenoid (e.g. lichenoid dermatitis) [51], and bullous disorders including bullous pemphigoid [52] (correspondence with J. Naidoo et al.), Stevens Johnson syndrome, and toxic epidermal necrolysis [14], which are of special interest due to their severity and potentially life-threatening consequences. It has been postulated in some cases that the underlying mechanism for the development of this toxicity may be due to the effect of blockade of a common antigen, co-expressed on a patient's tumor cells, and those of the dermo-epidermal junction and/or other levels of the skin [53]. Infrequently, cases involving the oral mucosa may be seen with the development of oral lichenoid mucositis (correspondence with M. E. Lacouture et al.). Additional reported mucosal toxicities include: oral mucositis, gingivitis, and sicca syndrome-like symptoms, which can be managed with supportive care.
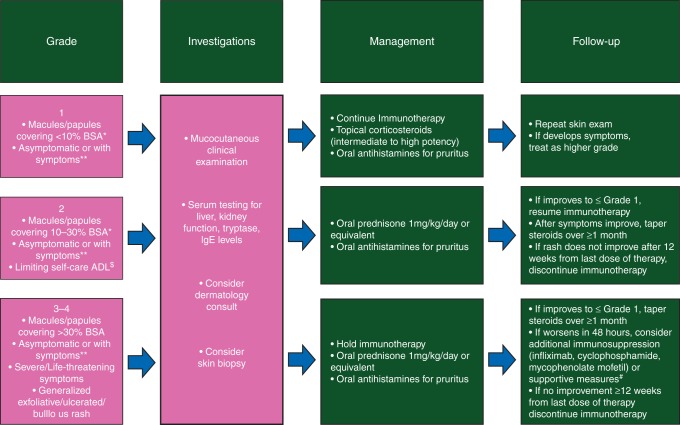
The prototypical maculopapular rash seen with anti-PD-1/PD-L1 mAb may be managed successfully with topical or oral corticosteroids, depending on severity, along with oral antipruritic agents (e.g. antihistamines, GABA agonists, NK-1 receptor inhibitors, antidepressants) in patients with pruritus. Early dermatologic evaluation is recommended for any atypical rashes, those that do not improve after interventions, involvement of the oral mucosa, or in patients with grade 3 events. Standard dermatologic evaluation usually involves a clinical assessment with or without a skin biopsy, and laboratory evaluation of kidney and liver function, as well as serum levels of tryptase and immunoglobulin E. Histologic evaluation often reveals an interface, perivascular, and periadnexal lymphocytic dermatitis, with few plasma cells and eosinophils (correspondence with V. R. Belum et al.). In grade 2 cases with intolerable symptoms, or grade 3 cases, immune checkpoint blockade may be temporarily held until toxicities are grade ≤1 in severity. Permanent discontinuation of therapy due to dermatologic toxicity has been reported in <5% of patients in clinical studies [14]. An algorithm for diagnosis and treatment of checkpoint mAB-induced dermatologic AEs is provided in Figure 1.
Figure 1.
Adapted management algorithm for skin rash with immune checkpoint blockade. *BSA, body surface area, **Symptoms as per CTCAE version 4.0. For example: pruritus, burning and skin tightness. $Additional supportive measures: this denotes the use of, for example, prophylatic antibiotics and management in the burns unit.
diarrhea/colitis
Diarrhea and colitis lie along a clinical spectrum where diarrhea is defined as increased stool frequency, and colitis involves symptoms of abdominal pain and either clinical or radiologic evidence of colonic inflammation [14]. Diarrhea/colitis with CTLA-4 blockade treatment usually occurs 6–8 weeks after commencement of therapy [15], with an incidence of grade 3/4 colitis of ∼5% in late phase studies with these agents [21, 54], and 1%–3% in reported studies of anti-PD-1/PD-L1 mAb alone [2, 3, 27, 29, 33]. Pathologic features of ipilimumab-related colitis include both neutrophilic and lymphocytic infiltrates [55], while biopsy-proven colitis with anti-PD-1/PD-L1 therapy has not yet been reported. Mild or grade 1 colitis can be managed with the American Dietary Association's colitis diet and antidiarrheal medications including atropine and oral diphenoxylate hydrochloride [14]. Worsening or persistent diarrhea for more than 3 days should prompt early investigations to rule out an infectious cause, withholding of the anti-CTLA-4 mAb, antidiarrheal medications, intervention with oral corticosteroids, as well as endoscopic or radiologic evaluation to confirm the diagnosis. In clinically severe cases or those that do not respond to the above interventions, patients may be admitted to hospital for intravenous corticosteroids (methylprednisolone 1–2 mg/kg total daily dose) and additional immunosuppression with anti-TNF medicines, such as infliximab, which is administered at a dose of 5 mg/kg [56–58]. Infliximab is typically recommended if intravenous corticosteroids are not effective within approximately the first 3 days, and can be repeated 2 weeks after the initial dose if symptoms persist. The cornerstone of effective colitis management is early intervention, as colitis-related mortality is associated with delayed reporting, noncompliance with an antidiarrheal regimen, and lack of drug withholding [59]. A randomized study of prophylactic budesonide in patients with melanoma treated with ipilimumab did not demonstrate a reduction in the incidence of diarrhea, and is not recommended for use to prevent diarrhea [60]. However, some patients report symptomatic benefit from using budesonide to treat mild diarrhea.
endocrine toxicities
Immune-related toxicities affecting the endocrine glands have been widely described with anti-CTLA-4 mAb, and now to a lesser extent, anti-PD-1/PD-L1 therapy [61, 62]. Typical endocrine irAEs seen with anti-CTLA-4 mAb include: hypophysitis, hypothyroidism, hyperthyroidism, thyroiditis, and adrenal insufficiency. Establishing a diagnosis of endocrine dysfunction can be clinically challenging, as these AEs may manifest with nonspecific symptoms such as fatigue and headache. Hypophysitis is diagnosed by biochemical testing of the pituitary-hypothalamic (prolactin), pituitary–thyroid (T4, TSH), pituitary–gonadal axes (LH, FSH), and pituitary–adrenal axes (ACTH, cortisol), as well as radiologic evidence of pituitary inflammation in selected cases [14]. The incidence of hypophysitis with single-agent anti-PD-1/PD-L1 mAb therapy ranges from 1% to 6% (Table 1) and 2% to 10% in selected combination studies (Table 2). Recovery of endocrine function for the gonadal axis has been reported in 57% of men [63], and recovery of the thyroid axis in 37%–50% of cases in selected studies [64–66]. Primary adrenocortical insufficiency is treated with glucocorticoid replacement, which may be life-long. In rare cases, patients may present with an adrenal crisis that requires hospitalization, endocrinology consultation, intravenous corticosteroid replacement, and aggressive fluid and electrolyte replacement. The immunologic mechanism underlying the development of hypophysitis is postulated to be due to humoral immunity against the pituitary gland, with involvement of the complement system [17].
Thyroid dysfunction associated with anti-CTLA-4 mAb occurs typically after two to four infusions, may be transient, but in many cases may be permanent [67]. The timing of onset with anti-PD-1/PD-L1 mAb has not been formally reported. Mechanisms underlying the development of this AE are not fully understood, but may be due to the development of antithyroglobulin or antithyroid peroxidase antibodies [66]. In rare cases, Grave's disease may arise due to the development of anti-TSH-receptor antibodies [68]; however, antibodies do not develop in all cases [61]. Hypothyrodisim is managed with thyroid hormone replacement, and hyperthyroidism is managed with standard antithyroid pharmacotherapy. In cases of thyroiditis, patients may develop initial hyperthyroidism that can be treated with β-blockers in symptomatic cases, followed by hypothyroidism that develops later, and usually requires thyroid hormone replacement [62]. As most endocrinopathies can be treated successfully with hormone replacement, immune checkpoint therapy is not usually discontinued.
hepatic toxicities
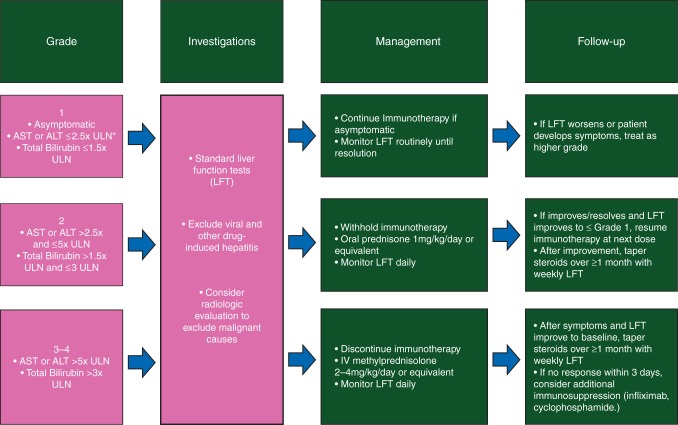
Hepatic AEs with immune checkpoint blockade consist mainly of asymptomatic elevations in AST and ALT levels [14]. Anti-CTLA-4 mAbs are associated with elevated AST and ALT levels in 10% of patients or less [20, 21, 54, 69]. With anti-PD-1/PD-L1 mAb, this is 5% or less in reported studies, with grade 3/4 events occurring in 1%–2% of patients [31, 33]. Interestingly, higher rates of AST/ALT elevation of ∼20% have been reported with single-agent anti-PD-1 therapy in HCC [8], with the anti-PD-1 and anti-CTLA-4 mAb combination [34], or when anti-CTLA-4 mAb were combined with targeted therapy or chemotherapy [70, 71]. In addition, nivolumab combined with either sunitinib or pazopanib was associated with 9%–20% grade 3/4 AST/ALT elevations in metastatic RCC [46]. Pathologic appearances of immune checkpoint-induced hepatitis with ipilimumab have been reported, with panlobular hepatitis, perivenular infiltrates, or infiltrates surrounding the primary biliary ducts [72]. Radiologic appearances include hepatomegaly, periportal edema, and periportal lymphadenopathy [73]. Hepatitis with anti-CTLA-4 therapy occurs approximately at 8–12 weeks after starting therapy [15]; however, this has not been reported in the context of anti-PD-1/PD-L1 therapy. An algorithm for the diagnostic investigations and management of suspected immune-related hepatitis adapted from guidelines used across anti-PD-1/PD-L1 studies is depicted in Figure 2. Treatment for immune-related hepatitis involves a corticosteroid taper for a minimum of 3 weeks [14], and occasionally additional immune suppression with mycophenolate mofetil 500–1000 mg b.i.d. or antithymocyte globulin, which has been used successfully in one case [74]. Infliximab should not be used for hepatitis as it confers its own risk of hepatotoxicity.
Figure 2.
Adapted management algorithm for hepatitis with immune checkpoint blockade. *ULN, upper limit of normal.
pneumonitis
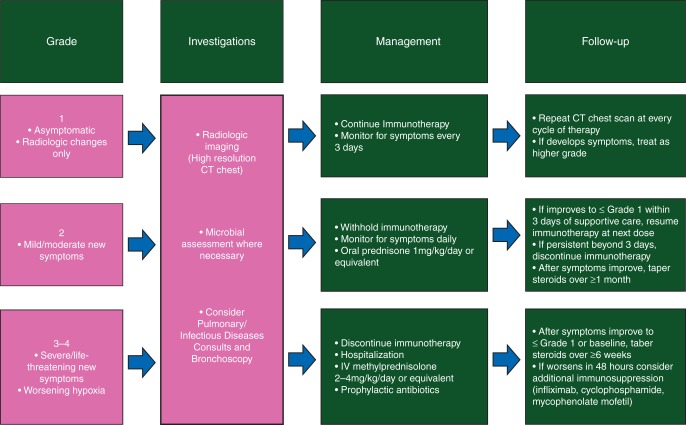
Pneumonitis is broadly defined as inflammation of the lung parenchyma, and has been described in <10% of patients receiving anti-PD-1/PD-L1 therapy either alone or in combination, and appears to occur more commonly in patients with lung cancer [3, 4, 24, 32] (Tables 1 and 2). This toxicity led to three treatment-related deaths in an early phase study of nivolumab [22]. Interestingly, this AE was not described in major studies of anti-CTLA-4 mAb alone, where pulmonary toxicities such as sarcoid-like granulomatous reactions [75] and obstructive pneumonia were reported [76]. The incidence of pneumonitis may be higher in studies where anti-PD-1/PD-L1 mAb are combined with other agents also known to carry a risk of pneumonitis, such as standard chemotherapy agents and targeted therapies. Radiologic appearances of pneumonitis have been reported in three cases and were consistent with an acute interstitial pneumonia/acute respiratory distress syndrome-type pattern [77]. Timing of development of pneumonitis appears to exhibit a wide range, with patients in this small series developing pneumonitis between 7.4 and 24.3 months after initiating therapy. Patients with suspected pneumonitis may present with shortness of breath, cough, fever, or chest pain. An algorithm for the diagnostic investigations and management of suspected immune-related pneumonitis adapted from guidelines used across anti-PD-1/PD-L1 studies is depicted in Figure 3. Standard diagnostic algorithms recommend radiologic investigation with a chest computed tomography scan. In cases of grade 2 or higher pneumonitis, consultations from infectious diseases and pulmonology physicians can be considered, in order to to rule out overt infection and malignant lung infiltration, as well as lung function testing and bronchoscopy. Management is guided by clinical symptoms, such that mild cases are managed by withholding therapy, and higher grade cases may be managed with oral or intravenous corticosteroids. Severe cases require hospitalization for intravenous corticosteroids, and other forms of immunosuppression may be used such as infliximab, cyclophosphamide, or mycophenolate mofetil [14].
Figure 3.
Adapted management algorithm for pneumonitis with immune checkpoint blockade.
rare toxicities with anti-PD-1/PD-L1 agents
neurologic syndromes
Isolated cases of myasthenia gravis have been reported in studies combining anti-PD-1/PD-L1 mAb with anti-CTLA-4 mAb [37, 38, 78]. Single-agent ipilimumab therapy was associated with a number of neurologic syndromes such as transverse myelitis [79], enteric neuropathy [80], and aseptic meningitis [81], and a case of Guillain–Barre syndrome that led to treatment-related fatality [82]. Patients should be managed with corticosteroids and neurologic consultation, and intravenous immunoglobulin or plasmapheresis may be of benefit [14].
ocular toxicity
Uveitis has been reported in patients receiving both single-agent [2] and combination therapies with anti-PD-1 and anti-CTLA-4 mAb [37], as well as ipilimumab alone [83]. Patients who develop uveitis are typically managed with topical corticosteroid solutions in consultation with an ophthalmologist, with consideration for oral corticosteroids in grade 3/4 cases [14].
renal toxicity
Isolated cases of interstitial nephritis have been reported with both single-agent anti-PD-1 therapy [27] and the combination of nivolumab and ipilimumab [34]. Pathologic appearances of interstitial nephritis as a result of anti-PD-1/PD-L1 mAb to our knowledge have not been reported; however, ipilimumab-related interstitial nephritis may exhibit pathologic appearances consistent with lupus nephritis [84] or granulomatous nephritis [85, 86]. Three cases of grade 3 acute renal failure were reported in a phase I study of nivolumab plus platinum-doublet chemotherapy in NSCLC and were deemed related to study therapy [39]. The clinical course is usually one of asymptomatic, gradually rising creatinine, and most patients improve with use of corticosteroids.
pancreatic toxicities
Elevations in lipase levels have been reported in studies of both anti-CTLA-4 and anti-PD-1/PD-L1 mAb [1, 7, 8]. These are usually asymptomatic laboratory abnormalities that can be monitored without immunosuppressive therapy. Pancreatitis has been reported infrequently in studies of anti-CTLA-4 [87] and anti-PD-1 agents [7, 10, 28]; therefore, clinical suspicion of pancreatitis should prompt assessment of amylase and lipase. Routine assessment of these enzymes in asymptomatic patients is not required outside of clinical trials and may be detrimental if inappropriate discontinuation of therapy occurs as a result [14].
combinations of anti-PD-1/PD-L1 agents with other anticancer agents
other immunotherapy
Combination studies of anti-PD-1/PD-L1 agents with other immunotherapeutic agents are currently underway in multiple tumor types. The combination of ipilimumab and nivolumab was first studied in a phase I trial of 86 patients with pretreated malignant melanoma and demonstrated a 40% objective response rate by modified World Health Organization criteria, with 30% of patients (n = 16) exhibiting responses of >80% in the concurrent arm [34]. These data paved the way for phase II [35] and III studies [36] of this combination in advanced melanoma, and the exploration of similar combinations in other tumor types. While response rates were impressive in these studies, toxicity was notably increased. The majority (83%–89%) of patients in the combination arm of the melanoma studies required either topical or oral immunosuppressive therapy for irAEs, and these events led to treatment-related drug discontinuation in 36%–47% of all patients on the combination arm [35, 36]. However, 80%–100% of the patients treated with immunosuppressive medications had their irAE completely resolve, or return to baseline. This combination has also been studied in 10 patients with recurrent glioblastoma multiforme (GBM) after standard therapy with surgery, RT, and temozolomide (Table 2) [23]. All patients receiving ipilimumab and nivolumab in this study experienced an AE, and four patients discontinued therapy due to AEs. Similar combinations have been studied in pretreated NSCLC: pembrolizumab plus ipilimumab in 56 patients [37], and durvalumab plus tremelimumab in 102 patients [38]. Preliminary results presented in 2015 demonstrated similar toxicity data, with 83%–93% of patients experiencing treatment-related AEs, and up to 61% of patients experiencing grade 3/4 AEs, with some rarer irAEs reported including polymyositis (n = 1), myocarditis (n = 1), and myasthenia gravis (n = 2) [37, 38].
chemotherapy
A number of multiarm phase I studies are currently underway in NSCLC and other solid tumors, aimed at investigating the safety and tolerability of combining anti-PD-1/PD-L1 mAb with standard chemotherapeutic agents (NCT01454102, NCT02039674, NCT01633970). In NSCLC, a four-arm study examined the combination of single-agent nivolumab with one of three possible platinum-doublet chemotherapy regimens at standard doses (cisplatin/gemcitabine, cisplatin/pemetrexed, and carboplatin/paclitaxel) [39]. High rates of all AEs (93%) and grade 3/4 AEs (43%) were seen, where the treatment arm with the highest rate of toxicity was nivolumab 10 mg/kg with carboplatin/paclitaxel (n = 11/15, 73%) [39]. Eleven patients discontinued treatment due to AEs, of which eight (17%) were grade 3/4 (pneumonitis: n = 3, 15%; acute renal failure: n = 3, 15%) [39]. Certain cytotoxic chemotherapeutic agents are thought to have immunogenic properties, such as 5-flurouracil which may decrease myeloid-derived suppressor cells (MDSC) [88] and increase effector T cells at the tumor microenvironment [89], and oxaliplatin which induces immunogenic cell death [90]. These effects form the basis of a phase I study of the combination of FOLFOX chemotherapy, bevacizumab, and atezolizumab in metastatic CRC [45]. Preliminary results of this study demonstrate that 80% of patients (n = 24/30) receiving the three-drug combination experienced a treatment-related AE, 20% of which (n = 6/30) were grade 3/4 in severity.
targeted therapy
The majority of patients treated with targeted therapies eventually develop acquired resistance to these agents through a number of mechanisms [91–93], one of which is postulated to be immune escape via the PD-1/PD-L1 and other immune checkpoint pathways [94]. In patients with epidermal growth factor receptor (EGFR)-mutant NSCLC with confirmed positivity of the T790M resistance mutation, the combination of an oral irreversible selective EGFR tyrosine kinase inhibitor (TKI) AZD9291 with durvalumab was investigated in 14 patients [40]. Toxicities with the combination included: diarrhea (50%), vomiting (50%), anemia (45%), and three cases of pneumonitis (21%). A similar study examined the combination of durvalumab and nonselective EGFR-TKI gefitinib in heavily pretreated patients, regardless of EGFR mutation status. This study demonstrated promising clinical activity with the combination, with mild treatment-related AEs in all patients, most commonly AST/ALT elevation (50%, n = 5 each) [41]. In melanoma, a three-arm study of durvalumab with the BRAF-inhibitor dabrafenib and MEK-inhibitor trametinib either concomitantly or sequentially with trametinib alone is under investigation in BRAF-mutant and wild-type melanoma [42]. Toxicities associated with dabrafenib (pyrexia, chills, arthralgia) or trametinib (peripheral edema and acneiform rash) did not appear to be increased with the addition of the anti-PD-L1 agent (Table 2). Lastly, the anti-PD-1 mAb pidilizumab has been studied as a single agent in a phase II study in lymphoma [30], and in combination with anti-CD20 mAb rituximab in a phase II study in follicular lymphoma [43]. In the combination study, the majority of patients had grade 1–2 AEs (94%), including anemia, fatigue, and leukopenia. Overall, combination approaches with anti-PD-1/PD-L1 mAb and targeted agents appear to have increased rates of toxicity, depending on the targeted agents used. Specific anti-PD-1/PD-L1 toxicities do not appear to be increased; however, our experience with these combinations is early and limited.
antiangiogenic agents
Antiangiogenic agents including mAb aimed at vascular endothelial growth factor (VEGF), such as bevacizumab, and multitargeted TKIs have been combined with anti-PD-1/PD-L1 mAb in early phase clinical studies. In metastatic RCC, nivolumab has been combined with both sunitinib (n = 33) and pazopanib (n = 20) in a phase I study [46]. Grade 3/4 treatment-related AEs were reported in 73% (n = 24/33) of patients who received nivolumab plus sunitinib and 60% (n = 12/20) patients who received nivolumab plus pazopanib. These led to treatment discontinuation in 24% (n = 8/33) of the sunitinib patients and 20% (n = 4/20) of the pazopanib patients. In addition to antiangiogenic effects, blockade of VEGF has been reported to possess immunomodulatory effects such as promoting increased effector T-cell trafficking [95, 96], and reducing MDSCs, T-regulatory cells, and suppressive cytokines at the tumor microenvironment [97]. The combination of bevacizumab and atezolizumab has been studied in metastatic clear-cell RCC and metastatic CRC, without any exacerbation of known bevacizumab AEs [44, 45]. This combination is currently being studied in the phase II setting in RCC (NCT01984242).
radiation therapy
Ionizing radiation is known to lead to immunogenic death of cancer cells in the tumor microenvironment [98], which can in turn result in proimmunogenic effects such as increased antigen presentation by tumor cells, increased chemokine release, and recruitment of effector T cells to the tumor microenvironment; as well as less favorable immunologic effects, such as impaired dendritic cell function [99, 100], increases in tumor-associated macrophages and T-regulatory cells [101, 102]. Combining RT and immunotherapy may create opportunities to synergize these effects, as well as generate an antitumor effect outside the irradiated field, termed the abscopal effect [103, 104]. This phenomenon is postulated to be mediated by cross-priming of cytotoxic T cells [103]. The combination of 8 Gy of external-beam RT delivered to one to three osseous metastases plus ipilimumab was investigated in metastatic castration-resistant prostate cancer, and deemed to be safe and tolerable [105]. In this study, ipilimumab 3–10 mg/kg demonstrated similar rates of toxicity with or without the addition of RT. In the 10 mg/kg ipilimumab ± RT expansion cohort, toxicities included diarrhea (54%), colitis (22%), rash (32%), and pruritus (20%); and grade 3/4 irAEs included colitis (16%) and hepatitis (10%) [105]. Studies aimed at determining the safety and efficacy of RT combined with anti-PD-1/PD-L1 mAb are currently underway, with no reported toxicity data.
anti-PD-1/PD-L1 therapy in patients with pre-existing autoimmune or infectious diseases
Typical exclusion criteria for treatment with immune checkpoint mAb in clinical trials include autoimmune conditions that require immune suppression above a certain daily dosage. This is based on murine models where fatal autoimmune conditions were unmasked during anti-CTLA-4 therapy [106, 107]. A select number of patients with known autoimmune diseases have received ipilimumab safely outside of clinical studies [108, 109], including patients with prior organ transplant [110, 111]. However, general conclusions regarding treatment with immune checkpoint mAb in large numbers of such patients is unknown. In addition, patients with hepatitis B and C infection have not been suitable candidates for these therapies in the past, due to a theoretical concern for worsening viral infection. However, comparable rates of toxicity have been reported in a single-agent nivolumab study in HCC, which included patients with hepatitis B and C infection [8]. Furthermore, ongoing studies are evaluating the role of checkpoint blockade in HIV-associated malignancies such as HPV-associated squamous cell carcinomas (NCT2408861, NCT02255097).
conclusion
Three immune checkpoint antibodies are now FDA-approved for the treatment of metastatic melanoma (ipilimumab, pembrolizumab, and nivolumab) and lung cancer (nivolumab), and a clear knowledge of the toxicities of these agents is vital to achieving their safe delivery outside of clinical trials. Anti-PD-1/PD-L1 mAb appears to be generally less toxic compared with anti-CTLA-4 mAb, with a slightly different toxicity profile that includes organ-specific inflammatory conditions such as pneumonitis rather than colitis. With increasing use of these agents and an awareness of what toxicities to expect and how to manage them, morbidity as well as mortality associated with severe irAEs, appear to be waning. However, use of these agents in new tumor types and combination approaches with standard anticancer agents that carry their own toxicities may result in an increase in the incidence of AEs and facilitate the emergence of new irAEs. As our familiarity with these agents grows, we may expand the patient population we deem acceptable to receive these treatments and learn more about their effects on different patient populations. Further research is required to advance our understanding of the mechanisms underlying the development of these toxicities, why they occur in particular patients, and improve upon current management strategies.
disclosure
MAP: Research funding: Bristol-Myers Squibb, Consulting: Bristol-Myers Squibb. JDW: Research funding and Consulting: Bristol-Myers Squibb, Medimmune/AstraZeneca, Genentech/Roche. All remaining authors declare no conflicts of interest.
references
- 1. Weber JS, D'Angelo SP, Minor D et al. . Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015; 16: 375–384. [DOI] [PubMed] [Google Scholar]
- 2. Robert C, Schachter J, Long GV et al. . Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372: 2521–2532. [DOI] [PubMed] [Google Scholar]
- 3. Brahmer J, Reckamp KL, Baas P et al. . Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garon EB, Rizvi NA, Hui R et al. . Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- 5. Motzer RJ, Rini BI, McDermott DF et al. . Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol 2015; 33: 1430–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Powles T, Eder JP, Fine GD et al. . MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515: 558–562. [DOI] [PubMed] [Google Scholar]
- 7. Ansell SM, Lesokhin AM, Borrello I et al. . PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015; 372: 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El-Khoueiry AB, Melero I, Crocenzi TS et al. . Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209–040. J Clin Oncol 2015; 33: 15s. [Google Scholar]
- 9. Segal NH, Ou SI, Balmanoukian AS et al. . Safety and efficacy of MEDI4736, an anti-PD-L1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort. J Clin Oncol 2015; 33: 15s. [Google Scholar]
- 10. Le DT, Uram JN, Wang H et al. . PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372: 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haanen JB, Thienen H, Blank CU. Toxicity patterns with immunomodulating antibodies and their combinations. Semin Oncol 2015; 42: 423–428. [DOI] [PubMed] [Google Scholar]
- 12. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dunn GP, Bruce AT, Ikeda H et al. . Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3: 991–998. [DOI] [PubMed] [Google Scholar]
- 14. Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book 2015; 35: 76–83. [DOI] [PubMed] [Google Scholar]
- 15. Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of immunotherapy for the practitioner. J Clin Oncol 2015; 33: 2092–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Good-Jacobson KL, Szumilas CG, Chen L et al. . PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol 2010; 11: 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iwama S, De Remigis A, Callahan MK et al. . Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med 2014; 6: 230ra245. [DOI] [PubMed] [Google Scholar]
- 18. Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology 2012; 1: 1223–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horvat TZ, Adel NG, Dang TO et al. . Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015; 33: 3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ribas A, Kefford R, Marshall MA et al. . Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol 2013; 31: 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hodi FS, O'Day SJ, McDermott DF et al. . Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Topalian SL, Hodi FS, Brahmer JR et al. . Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366: 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sampson JH, Vlahovic G, Sahebjam S et al. . Preliminary safety and activity of nivolumab and its combination with ipilimumab in recurrent glioblastoma (GBM): CHECKMATE-143. J Clin Oncol 2015; 33: 15_suppl3010. [Google Scholar]
- 24. Gettinger SN, Horn L, Gandhi L et al. . Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2015; 33: 2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rizvi NA, Mazieres J, Planchard D et al. . Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015; 16: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brahmer JR, Tykodi SS, Chow LQ et al. . Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366: 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamid O, Robert C, Daud A et al. . Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robert C, Ribas A, Wolchok JD et al. . Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014; 384: 1109–1117. [DOI] [PubMed] [Google Scholar]
- 29. Ribas A, Puzanov I, Dummer R et al. . Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015; 16: 908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Armand P, Nagler A, Weller EA et al. . Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol 2013; 31: 4199–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Segal NH, Antonia SJ, Brahmer JR et al. . Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. J Clin Oncol 2014; 32: 5s. [Google Scholar]
- 32. Rizvi NA, Brahmer JR, Ou SI et al. . Safety and clinical activity of MEDI4736, an anti-programmed cell death-ligand 1 (PD-L1) antibody, in patients with non-small cell lung cancer (NSCLC). J Clin Oncol 2015; 33: 15s. [Google Scholar]
- 33. Herbst RS, Soria JC, Kowanetz M et al. . Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wolchok JD, Kluger H, Callahan MK et al. . Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Postow MA, Chesney J, Pavlick AC et al. . Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015; 372: 2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Larkin J, Chiarion-Sileni V, Gonzalez R et al. . Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patnaik AM, Socinski MA, Gubens MA et al. . Phase 1 study of pembrolizumab (pembro; MK-3475) plus ipilimumab (IPI) as second-line therapy for advanced non-small cell lung cancer (NSCLC): KEYNOTE-021 cohort D. J Clin Oncol 2015; 33: 15s. [Google Scholar]
- 38. Antonia SJ, Goldberg SB, Balmanoukian AS et al. . Phase Ib study of MEDI4736, a programmed cell death ligand-1 (PD-L1) antibody, in combination with tremelimumab, a cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) antibody, in patients (pts) with advanced NSCLC. J Clin Oncol 2015; 33: 15s. [Google Scholar]
- 39. Antonia SJ, Brahmer JR, Gettinger SN et al. . Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with platinum-based doublet chemotherapy (PT-DC) in advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2015; 32: 5s. [Google Scholar]
- 40. Oxnard GR, Ramalingam SS, Ahn M et al. . Preliminary results of TATTON, a multi-arm phase Ib trial of AZD9291 combined with MEDI4736, AZD6094 or selumetinib in EGFR-mutant lung cancer. J Clin Oncol 2015; 33: 15s. [DOI] [PubMed] [Google Scholar]
- 41. Creelan BC, Chow LQ, Kim D et al. . Safety and tolerability results from a phase I study of MEDI4736, a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib in patients (pts) with non-small-cell lung cancer (NSCLC). J Clin Oncol 2015; 33: 15s. [Google Scholar]
- 42. Ribas A, Butler M, Lutzky J et al. . Phase I study combining anti-PD-L1 (MEDI4736) with BRAF (dabrafenib) and/or MEK (trametinib) inhibitors in advanced melanoma. J Clin Oncol 2015; 33: 15s. [Google Scholar]
- 43. Westin JR, Chu F, Zhang M et al. . Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol 2014; 15: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sznol M, McDermott MF, Jones SF et al. . Phase Ib evaluation of MPDL3280A (anti-PDL1) in combination with bevacizumab (bev) in patients (pts) with metastatic renal cell carcinoma (mRCC). J Clin Oncol 2015; 33: 15s. [Google Scholar]
- 45. Bendell JC, Powderly JD, Lieu CH et al. . Safety and efficacy of MPDL3280A (anti-PDL1) in combination with bevacizumab (bev) and/or FOLFOX in patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol 2015; 33: 15s. [Google Scholar]
- 46. Amin A, Plimack ER, Infante JR et al. . Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC). J Clin Oncol 2014; 32: 5s. [Google Scholar]
- 47. Kirkwood JM, Bender C, Agarwala S et al. . Mechanisms and management of toxicities associated with high-dose interferon alfa-2b therapy. J Clin Oncol 2002; 20: 3703–3718. [DOI] [PubMed] [Google Scholar]
- 48. Schwartz RN, Stover L, Dutcher J. Managing toxicities of high-dose interleukin-2. Oncology (Williston Park) 2002; 16: 11–20. [PubMed] [Google Scholar]
- 49. Momtaz P, Park V, Panageas KS et al. . Safety of infusing ipilimumab over 30 minutes. J Clin Oncol 2015June 29 [epub ahead of print], doi: 10.1200/JCO.2015.61.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weber JS SJ, Topalian S, Schadendorf D et al. . Safety profile of nivolumab (NIVO) in patients (pts) with advanced melanoma (MEL): a pooled analysis. J Clin Oncol 2015; 33: 15s. [DOI] [PubMed] [Google Scholar]
- 51. Joseph RW, Cappel M, Goedjen B et al. . Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res 2015; 3: 18–22. [DOI] [PubMed] [Google Scholar]
- 52. Carlos G, Anforth R, Chou S et al. . A case of bullous pemphigoid in a patient with metastatic melanoma treated with pembrolizumab. Melanoma Res 2015; 25: 265–268. [DOI] [PubMed] [Google Scholar]
- 53. Krenacs T, Kiszner G, Stelkovics E et al. . Collagen XVII is expressed in malignant but not in benign melanocytic tumors and it can mediate antibody induced melanoma apoptosis. Histochem Cell Biol 2012; 138: 653–667. [DOI] [PubMed] [Google Scholar]
- 54. Wolchok JD, Neyns B, Linette G et al. . Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010; 11: 155–164. [DOI] [PubMed] [Google Scholar]
- 55. Beck KE, Blansfield JA, Tran KQ et al. . Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol 2006; 24: 2283–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Merrill SP, Reynolds P, Kalra A et al. . Early administration of infliximab for severe ipilimumab-related diarrhea in a critically ill patient. Ann Pharmacother 2014; 48: 806–810. [DOI] [PubMed] [Google Scholar]
- 57. Minor DR, Chin K, Kashani-Sabet M. Infliximab in the treatment of anti-CTLA4 antibody (ipilimumab) induced immune-related colitis. Cancer Biother Radiopharm 2009; 24: 321–325. [DOI] [PubMed] [Google Scholar]
- 58. Pages C, Gornet JM, Monsel G et al. . Ipilimumab-induced acute severe colitis treated by infliximab. Melanoma Res 2013; 23: 227–230. [DOI] [PubMed] [Google Scholar]
- 59. Weber JS, Dummer R, de Pril V et al. . Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 2013; 119: 1675–1682. [DOI] [PubMed] [Google Scholar]
- 60. Weber J, Thompson JA, Hamid O et al. . A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res 2009; 15: 5591–5598. [DOI] [PubMed] [Google Scholar]
- 61. Ryder M, Callahan M, Postow MA et al. . Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer 2014; 21: 371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Corsello SM, Barnabei A, Marchetti P et al. . Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab 2013; 98: 1361–1375. [DOI] [PubMed] [Google Scholar]
- 63. Blansfield JA, Beck KE, Tran K et al. . Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother 2005; 28: 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dillard T, Yedinak CG, Alumkal J, Fleseriu M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary 2010; 13: 29–38. [DOI] [PubMed] [Google Scholar]
- 65. Juszczak A, Gupta A, Karavitaki N et al. . Ipilimumab: a novel immunomodulating therapy causing autoimmune hypophysitis: a case report and review. Eur J Endocrinol 2012; 167: 1–5. [DOI] [PubMed] [Google Scholar]
- 66. Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol 2011; 164: 303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Di Giacomo AM, Biagioli M, Maio M. The emerging toxicity profiles of anti-CTLA-4 antibodies across clinical indications. Semin Oncol 2010; 37: 499–507. [DOI] [PubMed] [Google Scholar]
- 68. Borodic G, Hinkle DM, Cia Y. Drug-induced Grave’s disease from CTLA-4 receptor suppression. Ophthal Plast Reconstr Surg 2011; 27: e87–e88. [DOI] [PubMed] [Google Scholar]
- 69. Bernardo SG, Moskalenko M, Pan M et al. . Elevated rates of transaminitis during ipilimumab therapy for metastatic melanoma. Melanoma Res 2013; 23: 47–54. [DOI] [PubMed] [Google Scholar]
- 70. Ribas A, Hodi FS, Callahan M et al. . Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med 2013; 368: 1365–1366. [DOI] [PubMed] [Google Scholar]
- 71. Robert C, Thomas L, Bondarenko I et al. . Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364: 2517–2526. [DOI] [PubMed] [Google Scholar]
- 72. Kleiner DE, Berman D. Pathologic changes in ipilimumab-related hepatitis in patients with metastatic melanoma. Dig Dis Sci 2012; 57: 2233–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kim KW, Ramaiya NH, Krajewski KM et al. . Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Invest New Drugs 2013; 31: 1071–1077. [DOI] [PubMed] [Google Scholar]
- 74. Chmiel KD, Suan D, Liddle C et al. . Resolution of severe ipilimumab-induced hepatitis after antithymocyte globulin therapy. J Clin Oncol 2011; 29: e237–e240. [DOI] [PubMed] [Google Scholar]
- 75. Berthod G, Lazor R, Letovanec I et al. . Pulmonary sarcoid-like granulomatosis induced by ipilimumab. J Clin Oncol 2012; 30: e156–e159. [DOI] [PubMed] [Google Scholar]
- 76. Barjaktarevic IZ, Qadir N, Suri A et al. . Organizing pneumonia as a side effect of ipilimumab treatment of melanoma. Chest 2013; 143: 858–861. [DOI] [PubMed] [Google Scholar]
- 77. Nishino M, Sholl LM, Hodi FS et al. . Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med 2015; 373: 288–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Loochtan AI, Nickolich MS, Hobson-Webb LD. Myasthenia gravis associated with ipilimumab and nivolumab in the treatment of small cell lung cancer. Muscle Nerve 2015; 52: 307–308. [DOI] [PubMed] [Google Scholar]
- 79. Bot I, Blank CU, Boogerd W, Brandsma D. Neurological immune-related adverse events of ipilimumab. Pract Neurol 2013; 13: 278–280. [DOI] [PubMed] [Google Scholar]
- 80. Bhatia S, Huber BR, Upton MP, Thompson JA. Inflammatory enteric neuropathy with severe constipation after ipilimumab treatment for melanoma: a case report. J Immunother 2009; 32: 203–205. [DOI] [PubMed] [Google Scholar]
- 81. Liao B, Shroff S, Kamiya-Matsuoka C, Tummala S. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol 2014; 16: 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wilgenhof S, Neyns B. Anti-CTLA-4 antibody-induced Guillain-Barre syndrome in a melanoma patient. Ann Oncol 2011; 22: 991–993. [DOI] [PubMed] [Google Scholar]
- 83. Robinson MR, Chan CC, Yang JC et al. . Cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma: a new cause of uveitis. J Immunother 2004; 27: 478–479. [DOI] [PubMed] [Google Scholar]
- 84. Fadel F, El Karoui K, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med 2009; 361: 211–212. [DOI] [PubMed] [Google Scholar]
- 85. Izzedine H, Gueutin V, Gharbi C et al. . Kidney injuries related to ipilimumab. Invest New Drugs 2014; 32: 769–773. [DOI] [PubMed] [Google Scholar]
- 86. Thajudeen B, Madhrira M, Bracamonte E, Cranmer LD. Ipilimumab granulomatous interstitial nephritis. Am J Ther 2015; 22: e84–e87. [DOI] [PubMed] [Google Scholar]
- 87. Di Giacomo AM, Danielli R, Guidoboni M et al. . Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother 2009; 58: 1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vincent J, Mignot G, Chalmin F et al. . 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 2010; 70: 3052–3061. [DOI] [PubMed] [Google Scholar]
- 89. Michaud M, Martins I, Sukkurwala AQ et al. . Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 2011; 334: 1573–1577. [DOI] [PubMed] [Google Scholar]
- 90. Tesniere A, Schlemmer F, Boige V et al. . Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2010; 29: 482–491. [DOI] [PubMed] [Google Scholar]
- 91. Sierra JR, Cepero V, Giordano S. Molecular mechanisms of acquired resistance to tyrosine kinase targeted therapy. Mol Cancer 2010; 9: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jancarikova D, Pesek M, Benesova L et al. . Acquired resistance of pulmonary adenocarcinoma to initially successful targeted therapy due to EGFR mutation T790M. Anticancer Res 2007; 27: 1879–1882. [PubMed] [Google Scholar]
- 93. Jackman D, Pao W, Riely GJ et al. . Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol 2010; 28: 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Garrido G, Rabasa A, Garrido C et al. . Preclinical modeling of EGFR-specific antibody resistance: oncogenic and immune-associated escape mechanisms. Oncogene 2014; 33: 3129–3139. [DOI] [PubMed] [Google Scholar]
- 95. Manning EA, Ullman JG, Leatherman JM et al. . A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res 2007; 13: 3951–3959. [DOI] [PubMed] [Google Scholar]
- 96. Shrimali RK, Yu Z, Theoret MR et al. . Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res 2010; 70: 6171–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Roland CL, Lynn KD, Toombs JE et al. . Cytokine levels correlate with immune cell infiltration after anti-VEGF therapy in preclinical mouse models of breast cancer. PLoS One 2009; 4: e7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Vatner RE, Cooper BT, Vanpouille-Box C et al. . Combinations of immunotherapy and radiation in cancer therapy. Front Oncol 2014; 4: 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Merrick A, Errington F, Milward K et al. . Immunosuppressive effects of radiation on human dendritic cells: reduced IL-12 production on activation and impairment of naive T-cell priming. Br J Cancer 2005; 92: 1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Liao YP, Wang CC, Butterfield LH et al. . Ionizing radiation affects human MART-1 melanoma antigen processing and presentation by dendritic cells. J Immunol 2004; 173: 2462–2469. [DOI] [PubMed] [Google Scholar]
- 101. Ahn GO, Tseng D, Liao CH et al. . Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci USA 2010; 107: 8363–8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lugade AA, Moran JP, Gerber SA et al. . Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 2005; 174: 7516–7523. [DOI] [PubMed] [Google Scholar]
- 103. Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953; 26: 234–241. [DOI] [PubMed] [Google Scholar]
- 104. Postow MA, Callahan MK, Barker CA et al. . Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366: 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Slovin SF, Higano CS, Hamid O et al. . Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol 2013; 24: 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wang HB, Shi FD, Li H et al. . Anti-CTLA-4 antibody treatment triggers determinant spreading and enhances murine myasthenia gravis. J Immunol 2001; 166: 6430–6436. [DOI] [PubMed] [Google Scholar]
- 107. Luhder F, Hoglund P, Allison JP et al. . Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med 1998; 187: 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kyi C, Carvajal RD, Wolchok JD, Postow MA. Ipilimumab in patients with melanoma and autoimmune disease. J Immunother Cancer 2014; 2: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pedersen M, Andersen R, Norgaard P et al. . Successful treatment with ipilimumab and interleukin-2 in two patients with metastatic melanoma and systemic autoimmune disease. Cancer Immunol Immunother 2014; 63: 1341–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Morales RE, Shoushtari AN, Walsh MM et al. . Safety and efficacy of ipilimumab to treat advanced melanoma in the setting of liver transplantation. J Immunother Cancer 2015; 3: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lipson EJ, Bodell MA, Kraus ES, Sharfman WH. Successful administration of ipilimumab to two kidney transplantation patients with metastatic melanoma. J Clin Oncol 2014; 32: e69–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]