Abstract
Background:
Pollution may play a role in population trends of declining semen quality and regional differences in time to pregnancy (TTP) in industrialized societies. Dioxins including 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) have been suspected. In 1976, an explosion near Seveso, Italy resulted in the highest TCDD exposure known in residential populations. Twenty years after, we conducted a retrospective cohort study, the Seveso Women’s Health Study.
Methods:
Of 981 participants, 472 women attempted pregnancy post-explosion, and 278 delivered a livebirth not due to contraceptive failure. Individual serum TCDD levels were measured from samples collected soon after the explosion and extrapolated to the conception attempt. We examined the relation of TCDD levels to TTP, parameterized as the monthly probability of conception within the first 12 months of trying, and to infertility, defined as conception ≥12 months of trying. We modeled fecundability with discrete time Cox proportional hazards regression and modeled fertility with logistic regression. We tested sensitivity of the conclusions to differing definitions of eligibility and outcome.
Results:
Median TCDD level was 50 ppt, median TTP was 2 months, and 17% reported taking ≥12 months to conceive. For every ten-fold increase in serum TCDD, we observed a 25% increase in TTP (adjusted-fecundability odds ratio = 0.75; 95% confidence interval (CI) 0.60, 0.95) and about a doubling in odds of infertility (adjusted odds ratio = 1.9; 95% CI 1.14, 3.22). Results were similar for extrapolated TCDD and sensitivity analyses.
Conclusions:
We found dose-related increases in TTP and infertility associated with individual serum TCDD levels in the women from Seveso, Italy. These findings may have important implications for fertility in industrialized areas.
INTRODUCTION
Pollution may play a role in population trends noted in declining semen quality1–3 and regional differences in time to pregnancy (TTP)4–6, in industrialized societies. One class of environmental compounds suspected to impact population fertility is endocrine disruptors which include dioxins.2 Dioxins, including 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), are highly lipophilic and extremely persistent in the human body and environment, and commonly found in industrialized areas as a waste product of combustion.7 Few studies have examined the relationship of endocrine disrupting compounds and fertility or TTP. Of these, most have suggested an association between higher exposure to polychlorinated biphenyls (PCBs) and longer TTP.8–11 However, most did not specifically examine the dioxin-like compounds nor use a direct measure of exposure.
On July 10, 1976, a trichlorophenol-manufacturing plant explosion near Seveso, Italy resulted in the highest TCDD exposure known in human residential populations.12 Twenty years later, the Seveso Women’s Health Study (SWHS) followed female residents of this area to determine if TCDD exposure had adverse effects on reproductive health. Individual TCDD exposure was quantified using serum samples collected soon after the explosion. We have previously reported that individual serum TCDD levels were associated with a small increased risk for earlier menarche among women less than five years old at the time of the explosion13, 14 and with an increase in menstrual cycle length among women who were premenarcheal at exposure.15 We furthermore observed a non-monotonic dose-related association of TCDD with earlier onset of natural menopause,16 but we failed to detect an association between TCDD levels and ovarian function17 or with spontaneous abortion.18 In the present study, we examine the association of individual serum TCDD with TTP and infertility. The results of this study may shed light on whether population decreases in fertility suggested in the literature may be related to dioxin exposure.
METHODS
Study Population
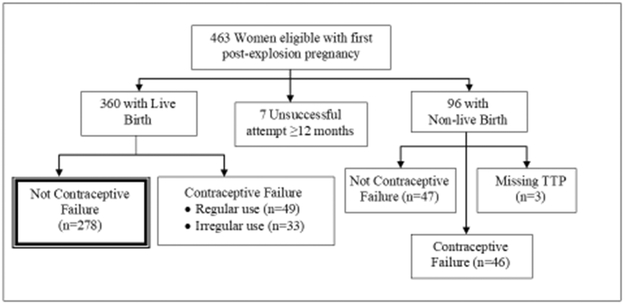
To be eligible for SWHS, women were newborn to 40 years old at the time of the explosion, had resided in the most highly contaminated area at the time of the explosion (Zones A or B), and had adequate volumes of stored sera collected soon after the explosion (see Eskenazi et al.19 for details). Women were interviewed between March 1996 and July 1998. Of the 981 participants, 745 reported a pregnancy, and 472 attempted pregnancy after the explosion. Nine women were excluded because they had fertility-related issues including history of infertility prior to the explosion, fertility drug use within 12 months of trying, or reported male fertility problems, yielding 463 eligible women for this analysis.
For the main analyses, we included only women who delivered a live birth that was not the result of contraceptive failure (N = 278) (see Figure 1). In other analyses, we included one or more of the following groups: women who delivered a live birth resulting from contraceptive failure (n = 49) or irregular use of contraception (n = 33); women with a non-contracepted pregnancy that resulted in a non-live birth (n = 47 (41 spontaneous abortions, 2 voluntary abortions, 4 ectopic pregnancies)); or women who delivered a non-live birth resulting from contraceptive failure (n=46 (9 spontaneous abortions, 37 voluntary abortions)). In addition, for some analyses, we included seven women who reported trying for at least 12 months to become pregnant, but never became pregnant and did not attribute this difficulty to a male fertility problem.
Figure 1.
Flow diagram of study sample. Seveso Women’s Health Study, Italy, 1996–1998.
Procedure
Study details are presented elsewhere.19 Briefly, participation included signed informed consent, structured personal interview, blood draw, and, for most women, a gynecologic examination and ultrasound. Medical records were requested for all gynecologic procedures/surgeries. Interviews were conducted in private by trained nurse-interviewers blinded to zone of residence and serum TCDD levels. Detailed information was collected about the first post-explosion pregnancy. TTP was determined from the question, “How many months did it take to become pregnant? In other words, for how many months had you been having sexual intercourse without doing anything to prevent pregnancy?” A calendar and pregnancy wheel were used to assist participants in recollection. If a couple had used contraception or the pregnancy did not result in a live birth, the participant was asked the same questions about the next pregnancy that had ended in a live birth. The Institutional Review Boards of participating institutions approved the study.
Laboratory Analyses
TCDD was measured in archived sera by high-resolution gas chromatography/high-resolution mass spectrometry methods.20 Values are reported on a lipid-weight basis in parts per trillion (ppt).21 Details of serum sample selection are presented elsewhere.19 The analytic sample consists of 463 women. We measured TCDD in sera collected in 1976 or 1977 for 431 women; between 1978 and 1981 for 13 women; and in 1996 or 1997 for 19 women with insufficient volume in earlier samples. For women with detectable post-1977 TCDD measurements (n=27), the TCDD level was back-extrapolated to 1976 using the Filser Model.22 For non-detectable values (n=58), a serum TCDD level of one-half the limit of detection was assigned.23
Statistical Analyses
Serum TCDD was analyzed both as a continuous variable (log10 TCDD) and as a four category variable. The cut-point for the lowest group was set at ≤20 ppt (body burden ≈ 4 ng/kg), since 15–20 ppt was the average TCDD level in serum pools collected from unexposed Italian women in 1976.24 The three remaining categories were defined by calculating tertiles of exposure >20 ppt in our main analytic population of 278 women, giving groups: ≤20, 20.1–44.4, 44.5–100.0, >100 ppt. For the extrapolated values, four quartile groups were created: <6, 6.0–14.2, 14.3–40.7, >40.7 ppt.
Because the relevant exposure may be the body burden at the time a woman was attempting to become pregnant rather than the initial dose, we also estimated TCDD levels at the time each woman initiated her attempt to become pregnant by extrapolating from her serum TCDD level measured near the explosion. Specifically, for women who were 16 years old or less at the time of explosion, we used a physiologically-based toxicokinetic model,22 and for women who were older than 16 years, we used a fist-order kinetic model assuming a 9-year half-life.25 TCDD extrapolated to pregnancy attempt (heretofore referred to as “extrapolated TCDD”) was considered both continuously (log10) and categorically, divided into quartiles based on the main analytic population of 278 women.
“Infertility” was defined as ≥12 months time to pregnancy. We employed multiple logistic regression to analyze the association between serum TCDD and infertility. To analyze the association between serum TCDD and TTP, we estimated fecundability odds ratios (fOR) using Cox proportional hazards models adapted for discrete time data. The fOR can be viewed as the odds of conceiving in a given cycle per 10-fold increase in TCDD (log10TCDD) or for each category of exposure relative to the referent exposure group. A fOR less than 1.0 indicates reduced fecundability or longer TTP. TTP was measured in months. TTP was censored at 13 months, but alternate censoring scenarios were also explored (see below).
To check for possible biases and investigate the consistency of our findings, we conducted several additional sensitivity analyses, as recommended by Joffe.26 Models were repeated: 1) including the seven women who had not achieved pregnancy by the time of interview, censored at 13 months; 2) excluding women who reported conceiving in the first cycle; 3) expanding the population to include pregnancies resulting from contraceptive failure; 4) expanding the population to include non-live births; 5) changing the censoring time to 14, 10, or 7 months. When included in sensitivity analyses, contraceptive failures were coded with a TTP of 0 or 1. For women reporting use of contraception “not quite regularly”, TTP was assigned a value of 0 for some sensitivity analyses and for others was calculated as one-half the reported duration of irregular use.
Covariates were included in Cox models if they changed the coefficient for log10 TCDD by ≥10% or if they were independently associated with TTP (p<0.10). For simplicity, the same covariates were used in the models for infertility after we checked that no additional covariates were important in these models. Covariates retained include maternal age, maternal smoking in the year before conception, parity, menstrual cycle irregularity, oral contraceptive (OC) use in the year before attempt, paternal age near the time of conception, and history of reproductive and endocrine conditions including pelvic infection, thyroid or urogenital problems. Additional covariates that we considered but did not keep in the final model included marital status, frequency of sexual intercourse, menstrual cycle length, whether actively trying or “not concerned” about becoming pregnant, age at menarche, maternal and paternal education, history of Cesarean-section, alcohol and coffee consumption, paternal smoking around the time of conception, and zone of the father at the time of the explosion. For the seven women who did not become pregnant, we imputed paternal age at the time of the initial attempt to become pregnant from maternal age and age married. We evaluated the form of the dose–response curve with fractional polynomials.27
We repeated the main analysis with the following variations: adding frequency of sexual intercourse as a covariate (n=275); limiting the population to primiparous women (n=224), to those who were less than eight years old at explosion (n=19), to those who were premenarcheal at explosion (n=59), and to women not using oral contraceptives within a year of trying (n=201). All statistical analyses were performed using STATA 8.0 (Stata Corporation, College Station, TX.
RESULTS
Demographic characteristics of the 278 women who had a post-explosion index pregnancy are presented in Table 1. Women averaged 17 years of age at the time of explosion, 26 years at the time they began trying to become pregnant, and 38 years at the time of interview. Almost all (99%) were less than 36 years old and most (81%) were primiparous at the time of the index pregnancy. About 27% smoked and 28% used OCs in the year before the pregnancy. Fathers of the index pregnancy averaged 30 years of age at the time of trying, and 23% were reported by their partners to have lived in Zones A or B at the time of the explosion.
Table 1.
Select characteristics of the 278 women with a non-contracepted post-explosion pregnancy resulting in a live birth, Seveso Women’s Health Study, Italy, 1996–98.
| Time to Pregnancy | TCDD (ppt) | ||
|---|---|---|---|
| Freq. (%) | Crude fOR (95% CI) | median (IQR) | |
| Total | 278 (100.0) | 49.7 (25, 117) | |
| Maternal age (years) at pregnancy attempt | 25.7 ± 3.8 | 0.99 (0.95, 1.03) | |
| ≤25 | 133 (47.8) | 1.00 | 46.2 (23, 93) |
| 26–30 | 114 (41.0) | 0.97 (0.71, 1.33) | 60.3 (27, 142) |
| 31–35 | 29 (10.4) | 0.76 (0.45, 1.29) | 43.9 (24, 119) |
| ≥36 | 2 (0.7) | 1.09 (0.85, 1.41) | 26.4 (23, 29) |
| Maternal age (years)at explosion | 17.2 ± 6.2 | 0.99 (0.96, 1.01) | |
| 0–10 | 27 (9.7) | 1.12 (0.70, 1.79) | 81.2 (44, 288) |
| 11–20 | 149 (53.6) | 1.00 | 53.4 (28, 112) |
| 21–30 | 94 (33.8) | 0.86 (0.62, 1.21) | 41.2 (22, 85) |
| 31–40 | 8 (2.9) | 2.55 (0.77, 3.14) | 36.6 (26, 115) |
| Maternal age (years) at interview | 37.7 ± 6.2 | 0.98 (0.96, 1.01) | |
| 21–30 | 20 (7.2) | 1.11 (0.66, 1.86) | 92.6 (44, 205) |
| 31–40 | 150 (54.0) | 1.00 | 50.2 (27, 122) |
| 41–50 | 98 (35.3) | 0.83 (0.60, 1.16) | 44.8 (22, 88) |
| 51–60 | 10 (3.6) | 1.94 (0.84, 4.49) | 28.6 (24, 86) |
| Parity at time of explosion | |||
| Nulliparous | 224 (80.6) | 1.00 | 53.5 (28, 124) |
| Parous | 54 (19.4) | 1.12 (0.75, 1.66) | 34.3 (22, 81) |
| Marital status | |||
| No | 17 (6.1) | 1.00 | 45.6 (29, 99) |
| Yes | 261 (93.9) | 0.75 (0.37, 1.50) | 49.9 (25, 117) |
| Education<required | |||
| No | 222 (79.9) | 1.00 | 49.7 (26, 123) |
| Yes | 56 (20.1) | 1.06 (0.72, 1.54) | 49.3 (23, 90) |
| No | 203 (73.0) | 1.00 | 49.9 (27, 123) |
| Yes | 75 (27.0) | 0.76 (0.55, 1.05) | 45.4 (21, 102) |
| OC use past year | |||
| No | 201 (72.3) | 1.00 | 48.0 (24, 93) |
| Yes | 77 (27.7) | 1.16 (0.86, 1.56) | 60.2 (33, 157) |
| Irregular cycle | |||
| No | 247 (88.9) | 1.00 | 50.6 (26, 117) |
| Yes | 31 (11.2) | 0.63 (0.40, 1.00) | 33.3 (15, 122) |
| History of reproductive or endocrine conditions | |||
| No | 247 (88.9) | 1.00 | 49.9 (25, 109) |
| Yes | 31 (11.2) | 0.59 (0.38, 0.90) | 48.7 (22, 163) |
| Paternal Age (years) | 30.2 ± 4.6 | 0.94 (0.91, 0.98) | |
| ≤25 | 34 (12.2) | 1.44 (0.73, 2.84) | 45.0 (26, 124) |
| 26–30 | 133 (47.8) | 1.00 | 52.8 (25, 90) |
| 31–35 | 78 (28.1) | 0.93 (0.67, 1.28) | 51.4 (26, 163) |
| ≥36 | 33 (11.9) | 0.44 (0.27, 0.72) | 41.9 (22, 81) |
| Father zone A/B | |||
| No | 186 (66.9) | 1.00 | 52.9 (27, 135) |
| Yes | 64 (23.0) | 0.90 (0.63, 1.29) | 51.9 (25, 88) |
| Unknown | 28 (10.1) | 1.29 (0.89, 1.88) | 37.5 (12, 67) |
The median TCDD concentration measured in blood collected around the time of the explosion for the 278 women was 50 ppt (interquartile range (IQR) = 25, 117) and the median TCDD level extrapolated to the time of conception was 13.4 ppt (IQR = 5.3, 38.2). Median serum TCDD levels at explosion were higher in women who were nulliparous and in those who used OCs in the year before the pregnancy (Table 1). Age at explosion for the entire Seveso cohort24 and in this study population is negatively associated with serum TCDD levels, with the youngest women having the highest levels. The associations between TCDD levels and parity and oral contraceptives likely reflect this association of TCDD with age.
The median (IQR) time to the index pregnancy for the 278 women was two (1, 7) months. Table 1, shows odds ratios for fecundability (monthly probability of conception). Lower fecundability was associated with smoking (fOR = 0.76), having irregular cycles (fOR = 0.63) or a history of reproductive or endocrine conditions (fOR = 0.59), and older paternal age (fOR = 0.94 per year).
Table 2 shows the association of time to pregnancy with initial and extrapolated TCDD levels, with and without adjustment for covariates. In the adjusted analysis with log10 serum TCDD as a continuous variable, a 10-fold increase in TCDD is associated with a 25% decrease in the per cycle probability of conception (adjusted fOR (a-fOR) = 0.75; 95% confidence interval (CI) 0.60, 0.95 per ten-fold increase in serum TCDD). A fractional polynomial in log10 TCDD indicated that no model (up to four knots) fit better than this linear model (data not shown). In the adjusted categorical model of TCDD, with a reference category of ≤20 ppt, categories of 20.1–44.4, 44.5–100, and >100 ppt were associated with decreases in a-fOR (1- a-fOR) of 19%, 29%, and 37%, respectively. The results were similar after controlling for frequency of sexual intercourse or eliminating adjustment for parity prior to the explosion, OC use in the year before attempt, or irregular cycles.
Table 2.
Association between serum TCDD levels and time to pregnancy for 278 pregnancies among women who tried to become pregnant after the explosion, Seveso Women’s Health Study, Italy, 1996–1998.
| Crude | Adjusted* | ||
|---|---|---|---|
| N | fOR (95% CI) | fOR (95% CI) | |
| Serum TCDD (ppt) | |||
| Continuous | |||
| log10 TCDD | 278 | 0.81 (0.65, 1.02) | 0.75 (0.60, 0.95) |
| Categorical | |||
| ≤20 | 52 | 1.00 | 1.00 |
| 20.1–44.4 | 76 | 0.96 (0.61, 1.52) | 0.81 (0.52, 1.25) |
| 44.5–100 | 75 | 0.88 (0.57, 1.36) | 0.71 (0.46, 1.09) |
| >100 | 75 | 0.76 (0.50, 1.16) | 0.63 (0.42, 0.96) |
| Extrapolated TCDD (ppt) | |||
| Continuous | |||
| log10 TCDD | 278 | 0.77 (0.61, 0.96) | 0.73 (0.58, 0.94) |
| Quartiles | |||
| <6.0 | 70 | 1.00 | 1.00 |
| 6.0–14.2 | 69 | 1.45 (0.95, 2.22) | 1.49 (0.95, 2.33) |
| 14.3–40.7 | 70 | 1.00 (0.66, 1.52) | 0.95 (0.61, 1.47) |
| >40.7 | 69 | 0.79 (0.53, 1.17) | 0.76 (0.49, 1.17) |
Adjusted for parity, maternal and paternal age, irregular menstrual cycle, oral contraceptive use in past year, smoking, and history of gynecological or chronic health condition.
The results were similar for analyses with extrapolated TCDD levels. We observed a 27% decrease in fOR for every ten-fold increase in extrapolated TCDD (a-fOR = 0.73; 95% CI 0.58, 0.94). In the categorical analysis, with a reference category of <6 ppt, the categories 6.0–14.2, 14.3–40.7, and >40.7 ppt had a-fORs of 1.49, 0.95, and 0.76, respectively. Although there is a suggestion of a rise, then fall, in the dose-response curve, the fractional polynomial model indicated the linear model provided the best fit (data not shown).
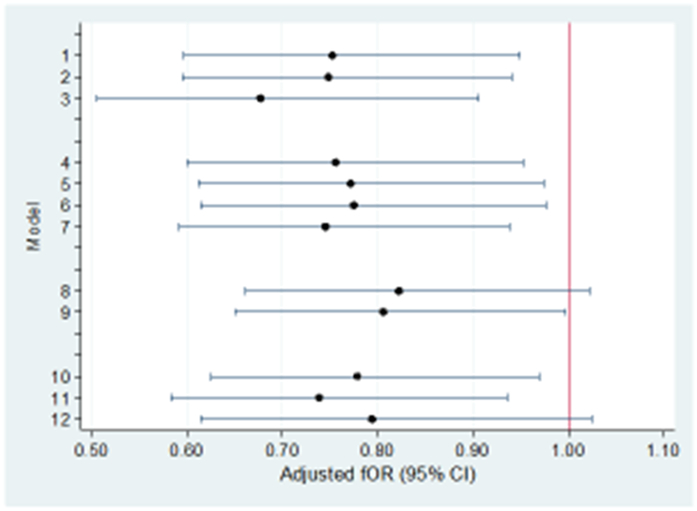
Figure 2 shows the fecundability odds ratios from the model with continuous (log10) serum TCDD, adjusted for covariates (also shown in Table 2), for 12 different definitions of the population and outcomes. Population 1 (Figure 2) is that analyzed in Table 2, referred to as the “main population”. The other scenarios vary in their inclusion or exclusion of: women who never conceived; women who conceived in the first month of trying; irregular users of contraception; outcomes other than live births; and month of censorship. Results for all scenarios were similar to those shown in Table 2 for the main population. Ten-fold increases in serum TCDD were associated with decreased a-fORs ranging from 0.68 to 0.82. Similar results were observed when extrapolated TCDD was used in place of measured TCDD (data not shown).
Figure 2.
Sensitivity analyses for log10 serum TCDD levels and time to pregnancy, Seveso Women’s Health Study, Italy, 1996–1998.
We repeated analyses with log10 serum TCDD for other subpopulations. Although numbers were small, the results were generally similar to those above. For example, among women who were premenarcheal at the time of the explosion, the a-fOR was 0.78 (95% CI 0.45, 1.35; n = 59); among those who were eight years old or younger, the unadjusted fOR was 0.70 (95% CI 0.18, 2.67; n=19). Among primiparous women (n=224), the a-fOR was 0.76 (95% CI 0.59, 0.97); and among women who had not used OCs within the year prior to trying to conceive (n=201), the results were also similar (a-fOR = 0.83; 95% CI 0.61, 1.11).
Forty-nine of 285 women (17%) reported taking twelve months or longer to conceive their first post-explosion pregnancy. (This numerator excludes women who conceived late, but attributed this to reproductive problems in their partners.) Table 3 reports the crude and adjusted odds ratios (a-OR) for infertility. With a ten-fold increase in serum TCDD level, we found nearly a doubling in the odds of infertility (a-OR = 1.9; 95% CI 1.14, 3.22). When serum TCDD levels were categorized, the trend for increasing odds of infertility was significant. The results were similar for extrapolated TCDD levels.
Table 3.
Association between serum TCDD levels and infertility based on 285 women who tried to become pregnant after the explosion, Seveso Women’s Health Study, Italy, 1996–1998.
| Crude | Adjusted* | ||
|---|---|---|---|
| Infertile/Total | OR (95% CI) | OR (95% CI) | |
| Serum TCDD (ppt) | |||
| Continuous | |||
| log10 TCDD | 49/285 | 1.78 (1.10, 2.90) | 1.92 (1.14, 3.22) |
| Categorical | |||
| ≤20 | 6/53 | 1.00 | 1.00 |
| 20.1–44.4 | 9/76 | 1.05 (0.35, 3.16) | 1.14 (0.36, 3.64) |
| 44.5–100 | 16/78 | 2.02 (0.73, 5.56) | 2.45 (0.83, 7.25) |
| >100 | 18/78 | 2.35 (0.86, 6.39) | 2.77 (0.95, 8.10) |
| Extrapolated TCDD (ppt) | |||
| Continuous | |||
| log10 TCDD | 49/285 | 1.85 (1.14, 2.99) | 2.04 (1.22, 3.41) |
| Quartiles | |||
| <6.0 | 9/70 | 1.00 | 1.00 |
| 6.0–14.2 | 10/74 | 1.06 (0.40, 2.78) | 1.28 (0.46, 3.52) |
| 14.3–40.7 | 12/71 | 1.38 (0.54, 3.51) | 1.81 (0.66, 4.96) |
| >40.7 | 18/70 | 2.35 (0.97, 5.66) | 2.80 (1.09, 7.18) |
Adjusted for parity, maternal and paternal age, irregular menstrual cycle, oral contraceptive use in past year, smoking, and history of gynecological or chronic health condition.
DISCUSSION
This study is the first to examine the relation of dioxin exposure in women to reduced fecundability and fertility. We found that a 10-fold increase in TCDD was associated with a 25% reduction in the monthly probability of conception in the 12 months preceding the first pregnancy post-explosion and a doubling of odds that the pregnancy took 12 or more months to conceive. These findings were robust in that we found similar reductions in fertility when we considered dose extrapolated to the time of conception or included different sub-populations in sensitivity analyses. Although the average level of TCDD exposure at the time of the explosion was high, the average levels at the time of conception are at the high end of the range of current background levels reported in Europe.28 Thus, these results support the hypothesis that dioxin exposure may play a role in reduced fertility in industrialized areas where it is a widespread contaminant.
Previous studies have examined the relationship of persistent organochlorines and female fertility, with inconsistent results. Axmon et al.8 examined the relationship of serum PCB-153 and 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) levels and fecundability in women from Greenland, Warsaw and Kharkiv and in a cohort of Swedish fisherman’s wives. Neither PCB-153 nor DDE is dioxin-like, but PCB-153 correlates with TCDD in the population. Axmon et al. found an association with TTP only in women from Greenland, where both PCB-153 and DDE were strongly correlated; it was not possible to separate out the individual effects of the compounds. In another study, Law et al.10 used stored serum specimens from the Collaborative Perinatal Project to examine the association of TTP and serum measures of DDE and 1,1,1-trichloro-2,2’-bis(p-chlorophenyl)ethane (DDT) and 11 PCBs, two of which were dioxin-like. They found that TTP increased for women in the highest exposure group for both total PCBs (fOR = 0.65; 95% CI 0.36, 1.18) and for DDE (fOR = 0.65; 95% CI 0.32, 1.31), compared to women in the lowest group, but there were no congener-specific associations or associations with DDT. Other studies have examined women who had high levels of PCBs and other organochlorines because they ate contaminated fish. In the New York Angler Cohort, individual serum levels were not measured, but TTP was associated with frequency and number of years of fish consumption and with PCB intake estimated from species, frequency of consumption, and portion size.9 In contrast to these studies, a study of fisherman wives/sisters in Sweden,29 where PCB-153 serum levels were back-extrapolated to the pregnancy, showed a decrease in TTP in the highest exposed groups.
As mentioned, no previous study of time to pregnancy and infertility has studied dioxin. The study of these outcomes with exposure most similar to ours examined the women of Yucheng in Taiwan, who were poisoned by PCB-contaminated cooking oil in 1979.11 Serum samples from a subset of the population taken more than a decade later revealed high levels of polychlorinated dibenzofurans and total PCBs. The authors compared 186 Yucheng women to 226 unexposed controls, and found that fecundability decreased by about 10% in the Yucheng women and that the odds of infertility doubled (OR= 2.3). In the small subset of Yucheng women with earlier measured PCB serum levels, there was also a decrease in fecundability in the higher group compared to the lower PCB group.
The results of the present study of women from Seveso are corroborated, in part, by the recent results of semen and hormone analyses of males from Seveso.30 These analyses found decreases in semen quality in those males who were <10 years old at the time of the explosion. The fact that we do not observe a relationship of fecundability and husbands’ residence in Zones A or B (based on the wives report) may be due to the small sample size. Slama et al.31 found that several thousand couples are needed to show that declines in sperm concentration produce a noticeable change on time to conception.
As was observed for the men in Seveso, women who were younger at the time of TCDD exposure may be among the most susceptible, given that their reproductive systems were not fully mature, and in the Seveso cohort, the children had the highest serum TCDD levels.24 Nevertheless, we observed little difference in fecundability by age at exposure, perhaps because of the relatively small number of pregnancies in the younger subgroup when they were interviewed twenty years after the explosion. We are currently conducting a follow-up study, which will add 10 years of reproductive experience for these women. We note, however, that the population of greatest susceptibility may not be the women, but their daughters. Previous studies of other endocrine disruptors reported that exposure during in utero development of the ovary may be the most sensitive stage to affect subsequent fertility.32
An increase in length of time to pregnancy can result from multiple mechanisms including a direct effect on oocyte reserve, on hormones which may impact ovulation or maintenance of the corpus luteum, or an increase in undetected spontaneous abortions resulting from failure in implantation or embryo development. There is evidence that TCDD can affect each of these processes. In animal studies, TCDD has been shown to alter ovarian function, including steroidogenesis and ovulation.33, 34 Although we found no relation of TCDD with current ovarian function in the Seveso cohort,17 TCDD exposure in rats has been associated with morphologic changes in the ovary, inhibition of follicular maturation and rupture, and altered cyclicity with disruption of the estrous cycle.33, 35–37 Altered hormone levels have also been reported with TCDD exposure in rats and primates.34, 35, 38 In addition, although we found no relation between TCDD exposure and clinically-recognized spontaneous abortion in Seveso,18 TCDD exposure has been shown to impact early embryo development.39 Thus, the potential for dioxin exposure to affect fertility and fecundability is biologically plausible.
One of the strengths of this study is that the findings in sensitivity analyses were robust regardless of inclusion criteria such as planning status, contraceptive use, birth outcome, or other criteria. Another unique strength of this study is that we analyzed levels of individual serum TCDD in samples drawn close to the time of the explosion. Also, reports of pregnancy outcome were not likely to be biased by knowledge of exposure, because participants and interviewers were blinded to TCDD levels and because all participants lived in exposed zones.
The present study has a number of limitations. First, although we measured TCDD in blood collected near the time of the explosion, we do not know the exact level at the time the woman attempted conception. We were able, however, to estimate this dose by extrapolation, and the analyses of extrapolated and measured levels yielded similar results. Because we analyzed first post-explosion pregnancies, the accuracy of the extrapolation could not be affected by lactation or subsequent pregnancies. It could, however, be affected in ways we cannot fully consider such as body weight changes. Second, few women had very low TCDD exposure, because all lived in exposed zones. Moreover, we previously showed that background levels of other dioxin-like compounds were high.24 Thus, our findings may underestimate the true relationship of TCDD and fecundability and infertility. Third, pregnancy histories were recorded an average of 12 years following the index pregnancy, raising the possibility of inaccurate recall. However, Joffe et al.40 found that even more than 14 years after a pregnancy, women can fairly accurately report their time to conception. And inaccuracies in reported times to conception were not likely to be related to exposure, because participants and interviewers were blinded to exposure, as described above.
Retrospective report of TTP has been used in a number of previous studies examining the potential effects of environmental chemicals.8–11 To address the potential biases and inadequacies of this type of study, Joffe et al.41 recommended a number of steps, including the use of discrete-time survival analysis, sensitivity analyses, a study sample representative of the underlying population, and a well-designed questionnaire. We have attempted to adopt these recommendations.
In conclusion, the present study demonstrated dose-related increases in time to pregnancy and infertility associated with individual serum TCDD levels in the female population from Seveso, Italy. These findings were robust to inclusion/exclusion criteria. It is unclear by what mechanism TCDD may affect this population, because time to pregnancy is the end-product of multiple and potentially unknown factors. Given that the serum levels extrapolated to the time of the pregnancy are at the high end of the range observed in some European countries, the results of this study may have important implications for fertility in industrialized areas.
Key:
Inclusion or exclusion of infertile phases
-
1
main population (live births not due to contraceptive failure) n=278
-
2
main + unsuccessful attempts (censored at 13 months) n=285
-
3
main - women who conceive in the first month of trying n=170
Inclusion or exclusion of use of contraception
-
4
main + live births resulting from contraceptive failure among regular contraception users (TTP=0) n=327
-
5
main + live births resulting from contraceptive failure among irregular contraception users (TTP= duration of irregular use/2) n=311
-
6
main + live births resulting from contraceptive failure among regular contraception users (TTP=0) and irregular contraception users (TTP=duration of irregular use/2) n=360
-
7
main + live births resulting from contraceptive failure among regular and irregular contraception users (TTP=0) n=360
Treatment of nonbirth outcomes
-
8
main + non-live births not resulting from contraceptive failure n=325
-
9
main + unsuccessful attempts (censored at 13 months) + non-live births not resulting from contraceptive failure n=332
Censoring
-
10
main (censor at 14 months) n=278
-
11
main (censor at 10 months) n=278
-
12
main (censor at 7 months) n=278
Acknowledgements:
We gratefully acknowledge Dr. Stefania Casalini for coordinating data collection at Hospital of Desio and Dr. Donald Patterson and Wayman Turner (CDC) for serum TCDD measurements.
Sources of Financial Support: This study was supported by Grant Numbers R82471 from the U.S. Environmental Protection Agency, R01 ES07171 and F06 TW02075–01 from the National Institutes of Health, EA-M1977 from the Endometriosis Association, 2P30-ESO01896–17 from the National Institute of Environmental Health Sciences, and #2896 from Regione Lombardia and Fondazione Lombardia Ambiente, Milan, Italy.
Contributor Information
Brenda Eskenazi, School of Public Health, University of California at Berkeley, Berkeley, CA.
Marcy Warner, School of Public Health, University of California at Berkeley, Berkeley, CA.
Amy Marks, School of Public Health, University of California at Berkeley, Berkeley, CA.
Steven Samuels, School of Public Health, State University of New York at Albany, Albany, NY.
Larry Needham, Division of Environmental Health Laboratory Science, National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Paolo Brambilla, Department of Laboratory Medicine, University of Milano-Bicocca, School of Medicine, Hospital of Desio, Desio, Italy.
Paolo Mocarelli, Department of Laboratory Medicine, University of Milano-Bicocca, School of Medicine, Hospital of Desio, Desio, Italy.
REFERENCES
- 1.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305(6854):609–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skakkebaek NE, Jorgensen N, Main KM, Rajpert-De Meyts E, Leffers H, Andersson AM, Juul A, Carlsen E, Mortensen GK, Jensen TK, Toppari J. Is human fecundity declining? Int J Androl. 2006. February;29(1):2–11. [DOI] [PubMed] [Google Scholar]
- 3.Auger J, Kunstmann J, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. New England Journal of Medicine. 1995;332(5):281–5. [DOI] [PubMed] [Google Scholar]
- 4.Juul S, Karmaus W, Olsen J. Regional differences in waiting time to pregnancy: pregnancy-based surveys from Denmark, France, Germany, Italy and Sweden. The European Infertility and Subfecundity Study Group. Hum Reprod. 1999. May;14(5):1250–4. [DOI] [PubMed] [Google Scholar]
- 5.Jensen TK, Slama R, Ducot B, Suominen J, Cawood EH, Andersen AG, Eustache F, Irvine S, Auger S, Jouannet P, Vierula M, Jorgensen N, Toppari J, Skakkebaek NE, Keiding N, Spira A. Regional differences in waiting time to pregnancy among fertile couples from four European cities. Hum Reprod. 2001. December;16(12):2697–704. [DOI] [PubMed] [Google Scholar]
- 6.Foster WG, Neal MS, Han MS, Dominguez MM. Environmental contaminants and human infertility: hypothesis or cause for concern? J Toxicol Environ Health B Crit Rev. 2008. March;11(3–4):162–76. [DOI] [PubMed] [Google Scholar]
- 7.IARC. Polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 1997;69:33–342. [PMC free article] [PubMed] [Google Scholar]
- 8.Axmon A, Thulstrup AM, Rignell-Hydbom A, Pedersen HS, Zvyezday V, Ludwicki JK, Jonsson BA, Toft G, Bonde JP, Hagmar L. Time to pregnancy as a function of male and female serum concentrations of 2,2’4,4’5,5’-hexachlorobiphenyl (CB-153) and 1,1-dichloro-2,2-bis (p-chlorophenyl)-ethylene (p,p’-DDE). Hum Reprod. 2006. March;21(3):657–65. [DOI] [PubMed] [Google Scholar]
- 9.Buck GM, Vena JE, Schisterman EF, Dmochowski J, Mendola P, Sever LE, Fitzgerald E, Kostyniak P, Greizerstein H, Olson J. Parental consumption of contaminated sport fish from Lake Ontario and predicted fecundability. Epidemiology. 2000. July;11(4):388–93. [DOI] [PubMed] [Google Scholar]
- 10.Law DC, Klebanoff MA, Brock JW, Dunson DB, Longnecker MP. Maternal serum levels of polychlorinated biphenyls and 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) and time to pregnancy. Am J Epidemiol. 2005. September 15;162(6):523–32. [DOI] [PubMed] [Google Scholar]
- 11.Yang CY, Wang YJ, Chen PC, Tsai SJ, Guo YL. Exposure to a mixture of polychlorinated biphenyls and polychlorinated dibenzofurans resulted in a prolonged time to pregnancy in women. Environ Health Perspect. 2008. May;116(5):599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mocarelli P, Pocchiari F, Nelson N. Preliminary report: 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure to humans--Seveso, Italy. Morbidity and Mortality Weekly Report. 1988;37(48):733–6. [PubMed] [Google Scholar]
- 13.Warner M, Eskenazi B. TCDD and Puberty: Warner and Eskenazi respond. Environ Health Perspect. 2005. January;113(1):A18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warner M, Samuels S, Mocarelli P, Gerthoux PM, Needham L, Patterson DG Jr., Eskenazi B. Serum dioxin concentrations and age at menarche. Environ Health Perspect. 2004. September;112(13):1289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eskenazi B, Warner M, Mocarelli P, Samuels S, Needham LL, Patterson DG Jr., Lippman S, Vercellini P, Gerthoux PM, Brambilla P, Olive D. Serum dioxin concentrations and menstrual cycle characteristics. Am J Epidemiol. 2002. August 15;156(4):383–92. [DOI] [PubMed] [Google Scholar]
- 16.Eskenazi B, Warner M, Marks AR, Samuels S, Gerthoux PM, Vercellini P, Olive DL, Needham L, Patterson D Jr., Mocarelli P. Serum dioxin concentrations and age at menopause. Environ Health Perspect. 2005. July;113(7):858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warner M, Eskenazi B, Olive DL, Samuels S, Quick-Miles S, Vercellini P, Gerthoux PM, Needham L, Patterson DG, Mocarelli P. Serum dioxin concentrations and quality of ovarian function in women of Seveso. Environ Health Perspect. 2007. March;115(3):336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eskenazi B, Mocarelli P, Warner M, Chee W, Gerthoux P, Samuels S, Needham L, Patterson D. Maternal serum dioxin levels and birth outcomes in women of Seveso, Italy. Environ Health Perspect. 2003;111(7):947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eskenazi B, Mocarelli P, Warner M, Samuels S, Vercellini P, Olive D, Needham L, Patterson D, Brambilla P. Seveso Women’s Health Study: a study of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproductive health. Chemosphere. 2000;40(9–11):1247–53. [DOI] [PubMed] [Google Scholar]
- 20.Patterson D, Hampton L, Lapeza C, Belser W, Green V, Alexander L, Needham L. High-resolution gas chromatographic/high-resolution mass spectrometric analysis of human serum on a whole-weight and lipid basis for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Anal Chem. 1987;59(15):2000–5. [DOI] [PubMed] [Google Scholar]
- 21.Akins J, Waldrep K, Bernett J. The estimation of total serum lipids by a completely enzymatic summation method. Clin Chim Acta. 1989;184(3):219–26. [DOI] [PubMed] [Google Scholar]
- 22.Kreuzer PE, Csanády GA, Baur C, Kessler W, Päpke O, Greim H, Filser JG. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and congeners in infants. A toxicokinetic model of human lifetime body burden by TCDD with special emphasis on its uptake by nutrition. Archives of Toxicology. 1997;71(6):383–400. [DOI] [PubMed] [Google Scholar]
- 23.Hornung R, Reed L. Estimation of average concentration in the presence of non-detectable values. Appl Occup Environ Hyg. 1990;5:48–51. [Google Scholar]
- 24.Eskenazi B, Mocarelli P, Warner M, Needham L, Patterson D, Samuels S, Turner W, Gerthoux P, Brambilla P. Relationship of serum TCDD concentrations and age at exposure of female residents of Seveso, Italy. Environ Health Perspect. 2004. January;112(1):22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pirkle J, Wolfe W, Patterson D, Needham L, Michalek J, Miner J, Peterson M, Philips D. Estimates of the half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Vietnam veterans of Operation Ranch Hand. Journal of Toxicology and Environmental Health. 1989;27:165–71. [DOI] [PubMed] [Google Scholar]
- 26.Joffe M, Key J, Best N, Keiding N, Scheike T, Jensen TK. Studying time to pregnancy by use of a retrospective design. Am J Epidemiol. 2005. July 15;162(2):115–24. [DOI] [PubMed] [Google Scholar]
- 27.Royston P, Altman D. Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling. Applied Statistics. 1994;43:429–67. [Google Scholar]
- 28.Warner M, Eskenazi B, Patterson DG, Clark G, Turner WE, Bonsignore L, Mocarelli P, Gerthoux PM. Dioxin-Like TEQ of women from the Seveso, Italy area by ID-HRGC/HRMS and CALUX. J Expo Anal Environ Epidemiol. 2005. July;15(4):310–8. [DOI] [PubMed] [Google Scholar]
- 29.Axmon A, Rylander L, Stromberg U, Jonsson B, Nilsson-Ehle P, Hagmar L. Polychlorinated biphenyls in serum and time to pregnancy. Environ Res. 2004. October;96(2):186–95. [DOI] [PubMed] [Google Scholar]
- 30.Mocarelli P, Gerthoux PM, Patterson DG Jr., Milani S, Limonta G, Bertona M, Signorini S, Tramacere P, Colombo L, Crespi C, Brambilla P, Sarto C, Carreri V, Sampson EJ, Turner WE, Needham LL. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ Health Perspect. 2008. January;116(1):70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slama R, Kold-Jensen T, Scheike T, Ducot B, Spira A, Keiding N. How would a decline in sperm concentration over time influence the probability of pregnancy? Epidemiology. 2004. July;15(4):458–65. [DOI] [PubMed] [Google Scholar]
- 32.Cohn BA, Cirillo PM, Wolff MS, Schwingl PJ, Cohen RD, Sholtz RI, Ferrara A, Christianson RE, van den Berg BJ, Siiteri PK. DDT and DDE exposure in mothers and time to pregnancy in daughters. Lancet. 2003. June 28;361(9376):2205–6. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Johnson D, Rozman K. Reproductive effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in female rats: Ovulation, hormonal regulation, and possible mechanism(s). Toxicology and Applied Pharmacology. 1995;133:321–7. [DOI] [PubMed] [Google Scholar]
- 34.Moran FM, Tarara R, Chen J, Santos S, Cheney A, Overstreet JW, Lasley BL. Effect of dioxin on ovarian function in the cynomolgus macaque (M. fascicularis). Reprod Toxicol. 2001. Jul-Aug;15(4):377–83. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Johnson D, Rozman K. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on estrous cyclicity and ovulation in female Sprague-Dawley rats. Toxicology Letters. 1995;78:219–22. [DOI] [PubMed] [Google Scholar]
- 36.Roby KF. Alterations in follicle development, steroidogenesis, and gonadotropin receptor binding in a model of ovulatory blockade. Endocrinology. 2001. June;142(6):2328–35. [DOI] [PubMed] [Google Scholar]
- 37.Salisbury TB, Marcinkiewicz JL. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin and 2,3,4,7,8-pentachlorodibenzofuran reduces growth and disrupts reproductive parameters in female rats. Biol Reprod. 2002. June;66(6):1621–6. [DOI] [PubMed] [Google Scholar]
- 38.Chaffin C, Peterson R, Hutz R. In utero and lactational exposure of female Holtzman rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin: Modulation of the estrogen signal. Biology of Reproduction. 1996;55:62–7. [DOI] [PubMed] [Google Scholar]
- 39.Guo Y, Hendrickx AG, Overstreet JW, Dieter J, Stewart D, Tarantal AF, Laughlin L, Lasley BL. Endocrine biomarkers of early fetal loss in cynomolgus macaques (Macaca fascicularis) following exposure to dioxin. Biology of Reproduction. 1999;60(3):707–13. [DOI] [PubMed] [Google Scholar]
- 40.Joffe M, Villard L, Li Z, Plowman R, Vessey M. A time to pregnancy questionnaire designed for long term recall: validity in Oxford, England. J Epidemiol Community Health. 1995. June;49(3):314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joffe M, Paranjothy S, Fielder H, Lyons R, Palmer S. Use of time to pregnancy in environmental epidemiology and surveillance. J Public Health (Oxf). 2008. June;30(2):178–85. [DOI] [PubMed] [Google Scholar]