Abstract
Background
Combined inhibition of vascular, platelet-derived, and epidermal growth factor receptor (EGFR) pathways may overcome refractoriness to single agents in platinum-pretreated non-small-cell lung cancer (NSCLC).
Patients and methods
This randomized, double-blind, multicenter, phase II trial evaluated sunitinib 37.5 mg/day plus erlotinib 150 mg/day versus placebo plus erlotinib continuously in 4-week cycles. Eligible patients had histologically confirmed stage IIIB or IV NSCLC previously treated with one or two chemotherapy regimens, including one platinum-based regimen. The primary end point was progression-free survival (PFS) by an independent central review.
Results
One hundred and thirty-two patients were randomly assigned, and the median duration of follow-up was 17.7 months. The median PFS was 2.8 versus 2.0 months for the combination versus erlotinib alone (HR 0.898, P = 0.321). The median overall survival (OS) was 8.2 versus 7.6 months (HR 1.066, P = 0.617). Objective response rates (ORRs) were 4.6% and 3.0%, respectively. Sunitinib plus erlotinib was fairly well tolerated although most treatment-related adverse events (AEs) were more frequent than with erlotinib alone: diarrhea (55% versus 33%), rash (41% versus 30%), fatigue (31% versus 25%), decreased appetite (30% versus 13%), nausea (28% versus 14%), and thrombocytopenia (13% versus 0%).
Conclusions
The addition of sunitinib to erlotinib did not significantly improve PFS in patients with advanced, platinum-pretreated NSCLC.
Keywords: combination therapy, efficacy, erlotinib, non-small-cell lung cancer, safety, sunitinib
introduction
Treatments of advanced non-small-cell lung cancer (NSCLC) typically include platinum-based chemotherapy with or without a vascular endothelial growth factor (VEGF)-targeted monoclonal antibody [1]. Second-line treatments including the epidermal growth factor receptor (EGFR) inhibitor erlotinib are associated with modest prolongation of survival [2, 3]. Consequently novel treatment strategies for advanced NSCLC are required.
VEGFR and platelet-derived growth factor receptor (PDGFR) pathways are implicated in the pathogenesis of NSCLC. VEGFR-2 is the primary receptor involved in endothelial cell proliferation and migration, while VEGFR-3 plays a role in angiogenic sprouting and lymphangiogenesis, and PDGFR-α and -β are implicated in the growth and survival of vascular smooth muscle cells and pericytes [4–6]. The EGFR pathway has also been linked to cell proliferation, angiogenesis, and metastasis [7].
Sunitinib malate is an oral multitargeted tyrosine kinase inhibitor (TKI) of VEGFR-1, -2, and -3, PDGFR-α, PDGFR-β, stem-cell factor receptor (KIT), FMS-like tyrosine kinase 3 (FLT3), colony-stimulating factor 1 receptor (CSF-1R), and glial cell line-derived neurotrophic factor receptor (Rearranged during Transfection; RET) [8–13]. In a phase II trial, single-agent sunitinib provided clinical benefit with acceptable tolerability in patients with advanced, platinum-refractory NSCLC [14, 15].
Given the heterogeneity of NSCLC and potential crosstalk between signaling pathways implicated in tumor growth, angiogenesis and metastasis, combining targeted agents could improve the efficacy over single-target agents, and data from NSCLC xenograft models suggest that sunitinib may augment the antitumor activity of erlotinib [13, 16].
We conducted a randomized, double-blind, multicenter, phase II trial to evaluate the addition of sunitinib to erlotinib in patients with platinum-pretreated NSCLC. Data from 30 patients in lead-in cohorts of this study indicated acceptable safety and evidence of antitumor activity [17]. Here, we present the results of the randomized, phase II component of this trial (NCT00265317).
methods
patients
Patients ≥18 years old had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 and histologically confirmed stage IIIB (with malignant effusion) or IV NSCLC previously treated with one or two chemotherapy regimens, including one platinum-based regimen. Prior treatment with TKIs (except insulin-like growth factor receptor inhibitors) or cetuximab was not permitted. The exclusion criteria included a history of or current brain metastases or spinal cord compression, inadequate hepatic, hematologic or renal function, hemoptysis within 4 weeks before starting study treatment; uncontrolled hypertension and clinically significant cardiovascular disease during the preceding 12 months.
All patients provided written informed consent. The study was approved by the institutional review board of each center and carried out in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, and applicable local laws and regulatory requirements.
study design and treatment
Patients received sunitinib 37.5 mg/day on a continuous once-daily dosing schedule (or placebo) plus erlotinib 150 mg/day, in repeated 4-week cycles. Treatment was continued until the Response Evaluation Criteria in Solid Tumors (RECIST)-defined disease progression, unacceptable toxicity, or for up to 18 cycles (after which treatment assignment was unblinded and open-label access to sunitinib was offered).
The primary end point of the double-blind phase was progression-free survival (PFS) assessed by an independent central radiologic review. Secondary end points included objective response rates (ORRs), duration of response, 1-year survival, overall survival (OS), safety, patient-reported outcomes (PROs), and associations between biomarkers and treatment outcome.
Randomization (1:1) was stratified by smoking history (never versus prior versus current) and EGFR status determined by immunohistochemistry or fluorescence in situ hybridization (positive versus negative versus unmeasured). A centralized randomization procedure (interactive response system accessible via telephone or internet) assigned patients to each treatment using a blocked randomization with a block size of 4 within each stratum. Patients, investigators, and the trial study team were blinded to treatment assignments.
study assessments
Tumor imaging by computed tomography or magnetic resonance was carried out at baseline, 8 and 12 weeks from the start of study medication, and every 8 weeks thereafter and whenever disease progression was suspected or to confirm response. Brain and bone scans were carried out at baseline and repeated if clinically indicated (regularly scheduled bone scans were required if bone metastases were present at baseline). Response measurements were carried out according to RECIST (version 1.0) [18]. PFS was defined as the time from randomization to the first documentation of objective tumor progression or death from any cause. The ORR was defined as the percentage of subjects with confirmed complete response (CR) or partial response (PR) relative to all randomly assigned patients. The duration of response was the time from first documentation of objective response to the time of tumor progression or death. One-year survival probability was based on Kaplan–Meier estimates from the date of randomization.
Safety was evaluated throughout the study for all patients receiving at least one dose of study treatment by assessment of adverse events [AEs; National Cancer Institute, Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0], laboratory abnormalities, physical examinations, electrocardiograms, and either multi-gated acquisition scans using red blood cells labeled with technetium-99m-pertechnate, or echocardiograms (carried out at baseline and as clinically indicated). PROs were measured each cycle using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) and supplemental lung cancer module (QLQ-LC13).
Exploratory analyses of tumor biopsies were established pre-hoc and included: immunohistochemistry for EGFR, EGFR gene copy number, EGFR (exons 18–21), and KRAS (exons 2 and 3, including codons 12, 13, and 61) mutational status, and mRNA expression profiling via multiplex XP-RT-PCR™ of gene transcripts relating to angiogenesis and tumor growth as well as targets of sunitinib [CSF-1R, PDGFR-α, PDGFR-β, KIT, FLT3], RET, VEGFR-1, VEGFR-2 and VEGFR-3, FGF (fibroblast growth factor), VEGF, and VEGF-C]. Methodological details are presented in the supplementary information, available at Annals of Oncology online.
Plasma samples were collected before dosing on day 1 of cycles 1–3 and stored at −70°C before analysis. Storage duration was within the period covered by stability evaluation for each analyte. VEGF-C, soluble (s) VEGFR-2, sVEGFR-3, and sKIT were analyzed under Good Laboratory Practice conditions using a validated enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN).
statistical analysis
The study was powered to detect a 50% improvement in median PFS from 10 weeks in the erlotinib alone arm to 15 weeks in the sunitinib plus erlotinib arm [3]. The target sample size was 126 patients, and 115 events were required to confer 80% power with a one-sided alpha of 0.1. An interim analysis of efficacy and safety was planned at 58 PFS events.
Efficacy end points and patient characteristics were evaluated in all randomly assigned patients (full analysis population). Treatment administration and safety were evaluated in all randomly assigned patients who received at least one dose of study medication (safety population). Response-related end points (PFS, ORR, and duration of response) were based on the independent, central review of tumor data.
PFS and OS were summarized using the Kaplan–Meier method; between-treatment comparisons for PFS and OS were conducted using one-sided log-rank tests. The Cox regression model was used to estimate hazard ratios (HRs) with two-sided 80% and 95% confidence intervals (CI) for PFS and OS, respectively. Log-rank tests and Cox regression models were used to explore the potential influences of patient/disease characteristics on PFS and OS. ORRs were compared between the treatment arms using Chi-square tests. Descriptive statistics were used for treatment administration and safety. Descriptive statistics and 95% CI for changes from baseline were calculated for PROs.
The significance of changes in soluble protein levels from baseline (expressed as ratios to baseline) was determined using the Wilcoxon signed–rank test. Concentrations of soluble proteins at baseline, and ratios to baseline at each time point, were compared between the treatment arms using the Wilcoxon rank sum test.
results
patient characteristics and dosage
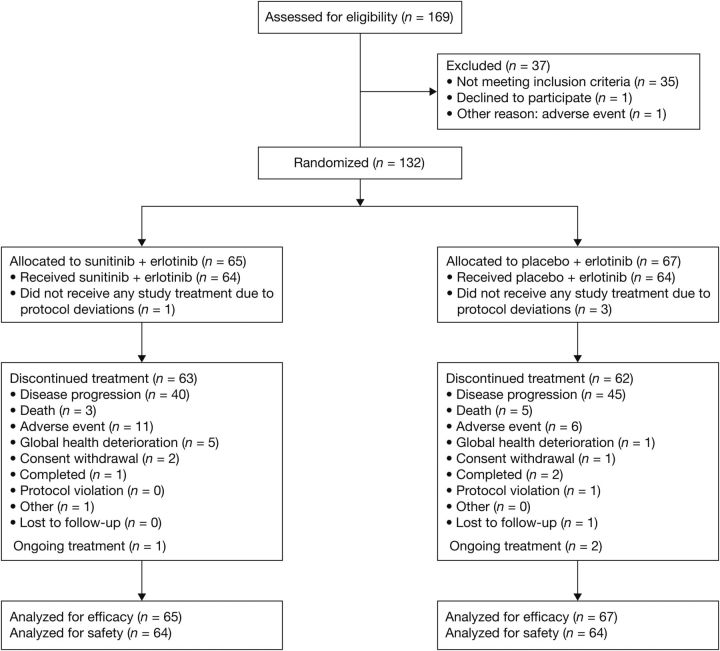
In total, 132 patients were randomized between January 2007 and January 2009, at 24 centers in the USA, Canada and Europe, and 64 patients were treated in each arm (Figure 1). Patient and tumor characteristics were balanced between arms, including EGFR gene copy number, amplification, and mutational status (Table 1). The most common histologies were adenocarcinoma, squamous cell carcinoma, and large-cell carcinoma. Most patients had received one prior chemotherapy (64.4%) or two prior chemotherapies (33.3%). Patients received a median of three treatment cycles in both the study arms; most dose delays, reductions and interruptions were attributed to AEs. There were more dose reductions and dose interruptions with the combination than with erlotinib treatment alone (Table 2).
Figure 1.
Patient disposition (CONSORT flow diagram).
Table 1.
Patient and disease characteristics at baseline
| Patient characteristic | Sunitinib + erlotinib (n = 65) | Placebo + erlotinib (n = 67) |
|---|---|---|
| Median age (range), years | 59 (37–79) | 61 (39–81) |
| Male, n (%) | 39 (60) | 45 (67) |
| Race, n (%)a | ||
| Caucasian | 63 (97) | 64 (96) |
| Asian | 1 (2) | 2 (3) |
| Other | 0 | 1 (1) |
| Smoking status, n (%)b | ||
| Current | 26 (40) | 24 (36) |
| Prior | 31 (48) | 33 (49) |
| Never | 7 (11) | 9 (13) |
| Unknown | 1 (2) | 1 (1) |
| Eastern Cooperative Oncology Group performance status (ECOG PS), n (%)c | ||
| 0 | 21 (32) | 21 (31) |
| 1 | 43 (66) | 45 (67) |
| Disease stage, n (%)a | ||
| IIIB | 1 (2) | 0 |
| IV | 63 (97) | 67 (100.0) |
| Histology, n (%)a | ||
| Adenocarcinoma | 36 (55) | 29 (43) |
| Squamous cell carcinoma | 15 (23) | 19 (28) |
| Large cell carcinoma | 6 (9) | 10 (15) |
| Bronchioloalveolar carcinoma | 0 | 2 (3) |
| Other/NOS | 7 (11) | 7 (10) |
| EGFR expression, n (%) | ||
| Positive | 29 (45) | 36 (54) |
| Negative | 19 (29) | 12 (18) |
| Unmeasuredd | 17 (26) | 19 (28) |
| EGFR gene copy number increased, n (%) | ||
| Positive | 0 | 1 (1) |
| Negative | 31 (48) | 28 (42) |
| Unmeasuredd | 34 (52) | 38 (57) |
| EGFR gene amplification, n (%) | ||
| No | 31 (48) | 29 (43) |
| Unmeasured | 34 (52) | 38 (57) |
| EGFR gene mutation, n (%) | ||
| Mutated | 4 (6) | 1 (1) |
| Wild type | 21 (32) | 19 (28) |
| Indeterminated | 40 (62) | 47 (70) |
| KRAS mutation status, n (%) | ||
| Mutated | 6 (9) | 4 (6) |
| Wild type | 22 (34) | 19 (28) |
| Indeterminated | 37 (57) | 44 (66) |
| Prior radiation therapy, n (%) | ||
| Neoadjuvant | 3 (5) | 3 (4) |
| Adjuvant | 6 (9) | 3 (4) |
| Palliative | 28 (43) | 29 (43) |
| Prior surgery (resection or exploratory thoracotomy), n (%) | 20 (31) | 15 (22) |
| Prior chemotherapy, n (%)a | ||
| 1 regimen | 39 (60) | 46 (69) |
| 2 regimens | 23 (35) | 21 (31) |
| ≥3 regimens | 1 (2) | 0 |
aData not collected from one subject in the sunitinib arm with no informed consent who was randomized in error.
bCurrent smoker includes subjects who stopped smoking <1 year before the first dose of study medication; prior smokers stopped smoking ≥1 year before the first dose of study medication.
cIn the erlotinib arm, 1 patient had ECOG PS of 2.
dIncludes patients where a tissue sample was not available (n = 14 for combination arm and n = 14 for erlotinib arm).
ECOG, Eastern Cooperative Oncology Group; NOS, not otherwise specified.
Table 2.
Study drug exposure
| Sunitinib + erlotinib (n =
64) |
Placebo + Erlotinib (n =
64) |
|||
|---|---|---|---|---|
| Sunitinib | Erlotinib | Placebo | Erlotinib | |
| Median cycles started (range) | 3 (1–18) | 3 (1–18) | ||
| Median days study treatment administered (range) | 58.5 (1–473) | 59.0 (1–470) | 84.5 (3–511) | 84.0 (3–504) |
| Patients with cycle delaysa, n (%) | 18 (28) | 14 (22) | 6 (9) | 7 (11) |
| Cycle delays due to adverse eventsb | 10 (16) | 6 (9) | 3 (5) | 2 (3) |
| Patients with dose reductions, n (%) | 15 (23) | 14 (22) | 6 (9) | 7 (11) |
| Dose reductions due to adverse eventsb | 13 (20) | 13 (20) | 6 (9) | 6 (9) |
| Patients with dose interruptionsc, n (%) | 17 (27) | 18 (28) | 9 (14) | 11 (17) |
| Dose interruptions due to adverse eventsb | 16 (25) | 15 (23) | 8 (13) | 10 (16) |
| Mean relative dose intensity, % (SD) | 91.2 (13.01) | 90.7 (14.36) | 96.1 (10.34) | 94.8 (10.48) |
aDelay ≥4 days in starting the next cycle.
bIncludes reason ‘adverse events and other’.
cMissed doses in the middle of a cycle.
SD, standard deviation.
antitumor activity
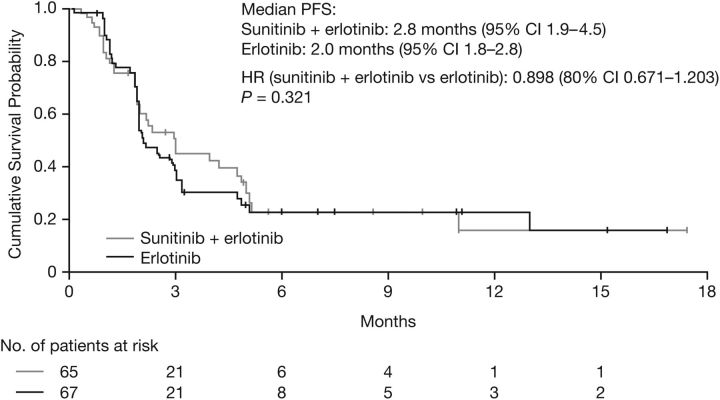
The median follow-up was 17.7 months. Of 115 planned PFS events, only 78 (n = 36 for the combination, n = 42 for erlotinib alone) were observed based on the central third-party review. The number was lower than expected due to a greater than anticipated dropout rate (balanced between the study arms) and withdrawal of subjects based on investigator-assessed disease progression that was not subsequently confirmed by a central review. The reasons for dropout included AEs (9%), protocol violations (4%), global deterioration of health status (2%), refusal to continue treatment for reason other than AE (2%), and lost to follow–up (1%). Withdrawals due to investigator-assessed progression not subsequently confirmed occurred in 12 subjects on the combination arm and 6 subjects on the erlotinib arm. Median PFS was 2.8 months [95% confidence interval (CI) 1.9–4.5] for the combination and 2.0 months (95% CI 1.8–2.8) for erlotinib [HR 0.898 (80% CI 0.671–1.203, P = 0.321; Figure 2].
Figure 2.
Kaplan–Meier estimates of progression-free survival (PFS).
The median OS was 8.2 months (95% CI 5.7–11.3) for the combination and 7.6 months (95% CI 5.3–13.4) for erlotinib (HR 1.066; 95% CI 0.705–1.612, P = 0.617). Approximately 30% of patients were censored in the analysis, primarily because they were in follow-up at the time of data cut-off. The probability of survival at 1 year was 0.32 (95% CI 0.197–0.443) and 0.42 (95% CI 0.301–0.542), respectively. No patient subsets had statistically significant improvements in PFS or OS.
The ORR was 4.6% (95% CI 0.96–12.90) for the combination and 3.0% (95% CI 0.36–10.37) for erlotinib (P = 0.624); no CR was observed. Durations of responses (censored at the time of analysis) were 14.1+, 3.6+, and 1.0+ months for the three patients with PR in the combination arm and 10.9+ and 3.7+ months for the two patients with PRs in the erlotinib arm. One of these patients (the 14.1+ month responder) had a confirmed EGFR mutation; the others were wild-type, indeterminate or not reported.
safety
Combination treatment was associated with more severe side effects than erlotinib alone, particularly grade 3 toxic effects. With the combination, the most common treatment-related AEs were diarrhea, rash, and fatigue. With erlotinib, the incidence of fatigue was similar, while diarrhea and rash were less frequent (Table 3). Other treatment-related AEs that differed between the study arms included: decreased appetite, nausea, thrombocytopenia, and pruritus. Two treatment-related grade 4 AEs occurred with the combination [ischemia and thrombocytopenia (reported as an AE but not noted in the hematologic laboratory data; supplementary Table 1, available at Annals of Oncology online)]; three grade 4 treatment-related AEs occurred with erlotinib (ulcer hemorrhage, dehydration, and pulmonary embolism).
Table 3.
Treatment-related AEs experienced by ≥10% of patients in either treatment arm
| Sunitinib + erlotinib (n =
64)a |
Placebo + erlotinib (n =
64) |
|||||
|---|---|---|---|---|---|---|
| Adverse event, n (%) | Any grade | Grade 3 | Grade 4 | Any grade | Grade 3 | Grade 4 |
| Diarrhea | 35 (55) | 11 (17) | 0 | 21 (33) | 1 (2) | 0 |
| Rash | 26 (41) | 5 (8) | 0 | 19 (30) | 2 (3) | 0 |
| Fatigue | 20 (31) | 6 (9) | 0 | 16 (25) | 2 (3) | 0 |
| Decreased appetite | 19 (30) | 3 (5) | 0 | 8 (13) | 0 | 0 |
| Dry skin | 18 (28) | 0 | 0 | 15 (23) | 0 | 0 |
| Nausea | 18 (28) | 3 (5) | 0 | 9 (14) | 0 | 0 |
| Dysgeusia | 12 (19) | 0 | 0 | 6 (9) | 0 | 0 |
| Mucosal inflammation | 11 (17) | 1 (2) | 0 | 6 (9) | 0 | 0 |
| Vomiting | 10 (16) | 0 | 0 | 7 (11) | 1 (2) | 0 |
| Thrombocytopenia | 8 (13) | 3 (5) | 1 (2) | 0 | 0 | 0 |
| Pruritus | 7 (11) | 0 | 0 | 14 (22) | 0 | 0 |
| Acne | 7 (11) | 0 | 0 | 8 (13) | 0 | 0 |
| Dermatitis acneiform | 6 (9) | 0 | 0 | 11 (17) | 0 | 0 |
| Exfoliative rash | 3 (5) | 0 | 0 | 7 (11) | 0 | 0 |
aA grade 5 intracranial hemorrhage was reported in one patient 31 days post dose (erlotinib and sunitinib). It was not considered an on-study event because it occurred after the 28-day post-treatment window; however, it was considered related to both erlotinib and sunitinib.
Treatment-related serious AEs included diarrhea [combination: 8% (grade 3); erlotinib: 2% (grade 3)], gastrointestinal hemorrhage [combination only: 2% (grade 2), 2% (grade 3)], and dehydration [erlotinib only: 2% (grade 3), 2% (grade 4)].
Treatment-related AEs resulting in study discontinuation were fatigue, nausea, acute pancreatitis, thrombocytopenia, ischemia, diarrhea, esophagitis, and pulmonary hemorrhage (n = 1 each) for the combination; and alveolar proteinosis, vomiting, and deep vein thrombosis (n = 1 each) for erlotinib.
There were 20 on-study deaths: 9 in the combination arm (n = 8 disease progression, n = 1 suicide) and 11 in the erlotinib arm (n = 8 disease progression, n = 2 cardiopulmonary failure, n = 1 pulmonary edema). All were judged to be unrelated to treatment. One patient in the combination arm died of intracranial hemorrhage 31 days after the last dose of study medication, which was considered treatment-related.
biomarker analysis
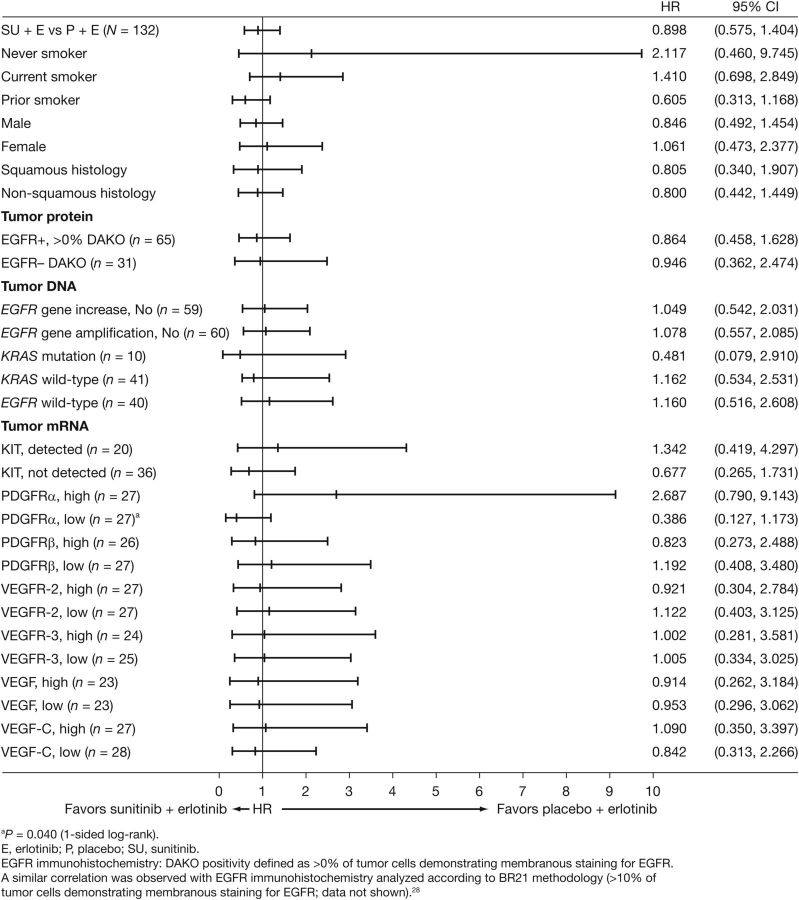
No significant differences in PFS were observed between the study arms according to tumor EGFR (assessed by protein expression, gene mutation, and gene copy number) or KRAS mutation status (Figure 3). In patients with KRAS mutations (n = 6 in the combination arm and n = 4 in the erlotinib arm), the HR for the addition of sunitinib was 0.481 (95% CI, 0.079–2.910). In patients with low (< median) tumor PDGFR-α RNA expression levels, PFS favored the combination (n = 16 versus n = 11; HR = 0.386, 95% CI: 0.127–1.173, one-sided P = 0.040, unadjusted for multiplicity).
Figure 3.
Subgroup analyses of progression-free survival (PFS).
Plasma levels of sVEGFR-2, sVEGFR-3, and sKIT decreased significantly from baseline with the combination (P < 0.0001), while no significant changes from baseline were observed with erlotinib (Supplementary Figure 1, available at Annals of Oncology online). With the combination, there were no significant differences in PFS for any soluble protein that was assessed. With erlotinib, PFS was significantly prolonged in patients with high (≥ median) baseline sKIT levels (HR = 0.406, P = 0.005), and in patients having low (< median) sVEGFR-3 ratios to baseline at cycle 2 day 1 (HR = 2.652, P = 0.006) and at cycle 3 day 1 (HR = 2.673, P = 0.016).
The relationship between soluble protein levels and OS was explored in a post hoc analysis. In the combination arm, OS was significantly prolonged in patients with low (< median) baseline VEGF-C levels (HR = 2.105, P = 0.033; Supplementary Figure 2, available at Annals of Oncology online) and in patients with high (≥ median) sVEGFR-3 ratios to baseline at cycle 2 day 1 (HR = 0.435, P = 0.038). No other statistically significant associations between OS and soluble protein levels were observed.
patient-reported outcomes
There were no clinically or statistically significant changes in global health status, quality of life or functioning scales in either treatment arm, suggesting that global health-related quality of life and functioning were maintained for patients receiving sunitinib plus erlotinib (data not shown).
discussion
This phase II study did not demonstrate a PFS difference with sunitinib plus erlotinib (2.8 months, 95% CI 1.9–4.5) versus erlotinib alone (2.0 months, 95% CI 1.8–2.8) in patients with platinum-pretreated, advanced NSCLC (HR 0.898, P = 0.321). In addition, no significant difference was observed for OS (HR 1.066, 95% CI 0.705–1.612, P = 0.617). A recently reported phase III trial of sunitinib plus erlotinib in treatment-refractory advanced NSCLC also showed no significant effect on OS compared with erlotinib alone, but did demonstrate a significant improvement in PFS (a secondary end point) [19].
Exploratory analyses by clinical subsets indicated no effect of combination treatment on PFS. Additionally, there are currently no validated biomarkers that predict clinical activity with sunitinib treatment for NSCLC. BATTLE I, a phase II biomarker-driven study, suggested that patients with NSCLC and KRAS mutations may be more likely to benefit from treatment with sorafenib, while those with EGFR mutation/copy number gain may do worse [20]. Similarly, exploratory analyses in this study showed an HR of 0.481 for the addition of sunitinib in patients with KRAS mutations. However, the small number of subjects (n = 10) and the corresponding large confidence interval (0.079–2.910) limit confidence in the interpretation. Interestingly, a treatment-related difference in PFS favoring the combination was observed for patients with low-tumor PDGFR-α RNA levels (HR 0.386, P = 0.040). Amplification of chromosomal segment 4q12 in NSCLC tumors has been previously attributed to PDGFR-α and KIT copy number gains, and PDGFR-α and KIT have been implicated as potential oncogenes [21]. The relevance of these findings requires additional investigation.
Plasma levels of sVEGFR-2, sVEGFR-3, and sKIT decreased significantly from baseline in the combination arm (P < 0.0001), but not in the erlotinib arm. These observations were consistent with studies of sunitinib in other tumor types [22–24], and suggest that sunitinib pharmacodynamics are not attenuated by co-administration with erlotinib. Comparable reductions in levels of these plasma proteins were not observed in patients treated with erlotinib alone, consistent with the target profile of this EGFR inhibitor. Interestingly, lower baseline levels of VEGF-C were associated with longer OS in patients receiving combination treatment, consistent with the association of low baseline levels of VEGF-C and longer PFS observed in sunitinib trials in RCC [25, 26].
Sunitinib plus erlotinib was fairly well tolerated, although grade 3 and 4 toxic effects were more common than with erlotinib alone. The most common AEs were consistent with previous reports from studies of single-agent sunitinib or erlotinib, and no unexpected AEs were observed [3, 14, 15]. Treatment-related AEs were more frequent with the combination than with erlotinib, including diarrhea, rash, anorexia, nausea, and thrombocytopenia. Dose delays, reductions, and interruptions therefore occurred more often in the combination arm.
Co-inhibition of VEGF and EGFR pathways in refractory NSCLC has been investigated in other clinical trials. Vandetanib, a dual VEGFR and EGFR inhibitor, was investigated in four phase III trials in combination with chemotherapy (pemetrexed or docetaxel) or best supportive care [27–30]. Only one of these trials, ZODIAC (vandetanib in combination with docetaxel as second-line therapy in patients with advanced NSCLC) met its primary end point of prolonging PFS compared with docetaxel alone [29]. However, there was no significant effect on OS. As with the ZEAL trial (vandetanib plus pemetrexed) [30], the OS results may have been confounded by differences between groups in post-progression therapies. Similarly, the addition of bevacizumab to erlotinib in the second-line setting did not prolong OS or PFS in the phase III BeTa Lung trial, despite promising results in two phase II trials [31–33]. These trials indicate the difficulties of identifying novel, effective treatment combinations for unselected patients with refractory NSCLC.
Improving clinical outcomes in patients with recurrent NSCLC remains challenging. This study showed no difference between the treatment arms in PFS or OS in such patients. Some individuals may benefit from angiogenesis inhibition plus EGFR inhibition, but advances in molecular markers will be needed to identify the likely responders to this and other targeted combinations.
funding
This study was sponsored by Pfizer, Inc. CH was a paid contractor of Pfizer during the development of this manuscript and the analysis and interpretation of the data. [no grant number]
disclosure
PB and MAS both received research funding from Pfizer; PB and CAB served on advisory boards for Pfizer; GB Jr received research funding from and served in an advisory role to Bayer; C.AB received honoraria from Pfizer for serving on advisory boards; RCC, CSH, PS, LMT, TU, and JAW are/were employees of Pfizer; RCC, FG, CSH, PS, LMT, and JAW all hold/held stock in Pfizer; CG served on advisory boards and was a member of the speakers bureau for Roche, Merck-Serono, Eli Lilly, and Amgen-Dompé; FG has served as a speaker for Roche; HJMG, EJ, and EC have no conflicts of interest to declare.
Supplementary Material
acknowledgements
The authors thank the participating patients, their families, and the global network of investigators, research nurses, study coordinators, and operations staff. Zhixiao Wang (Pfizer Oncology, New York, NY, ) contributed to the analysis and interpretation of patient-reported outcomes. Medical writing support was provided by Siân Marshall and Susanne Gilbert at ACUMED® (Tytherington, UK) and funded by Pfizer Inc.
references
- 1.Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non-small-cell lung cancer. J Thorac Oncol. 2008;3 doi: 10.1056/NEJMoa061884. Abstract p.S302. [DOI] [PubMed] [Google Scholar]
- 2.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd FA, Rodrigues PJ, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 4.O'Byrne KJ, Koukourakis MI, Giatromanolaki A, et al. Vascular endothelial growth factor, platelet-derived endothelial cell growth factor and angiogenesis in non-small-cell lung cancer. Br J Cancer. 2000;82(8):1427–1432. doi: 10.1054/bjoc.1999.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J, Ustach C, Kim HR. Platelet-derived growth factor signaling and human cancer. J Biochem Mol Biol. 2003;36(1):49–59. doi: 10.5483/BMBRep.2003.36.1.049. [DOI] [PubMed] [Google Scholar]
- 6.Lohela M, Bry M, Tammela T, et al. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21(2):154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 7.De Luca A, Carotenuto A, Rachiglio A, et al. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol. 2008;214(3):559–567. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- 8.Abrams TJ, Lee LB, Murray LJ, et al. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2(5):471–78. [PubMed] [Google Scholar]
- 9.Kim DW, Jo YS, Jung HS, et al. An orally administered multitarget tyrosine kinase inhibitor, SU11248, is a novel potent inhibitor of thyroid oncogenic RET/papillary thyroid cancer kinases. J Clin Endocrinol Metab. 2006;91(10):4070–4076. doi: 10.1210/jc.2005-2845. [DOI] [PubMed] [Google Scholar]
- 10.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9(1):327–337. [PubMed] [Google Scholar]
- 11.Murray LJ, Abrams TJ, Long KR, et al. SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis. 2003;20(8):757–766. doi: 10.1023/B:CLIN.0000006873.65590.68. [DOI] [PubMed] [Google Scholar]
- 12.O'Farrell AM, Abrams TJ, Yuen HA, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101(9):3597–3605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 13.Pfizer Inc. 2011 Data on file. [Google Scholar]
- 14.Novello S, Scagliotti GV, Rosell R, et al. Phase II study of continuous daily sunitinib dosing in patients with previously treated advanced non-small cell lung cancer. Br J Cancer. 2009;101(9):1543–1548. doi: 10.1038/sj.bjc.6605346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Socinski MA, Novello S, Brahmer JR, et al. Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol. 2008;26(4):650–656. doi: 10.1200/JCO.2007.13.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Govindan R, Blumenschein G, Jr, Groen HJM, et al. Phase III trial of sunitinib and erlotinib in advanced/metastatic non-small cell lung cancer. Presented at the 3rd Latin American Conference on Lung Cancer; October 9–10; Vina del Mar, Chile. 2008. [Google Scholar]
- 17.Blumenschein GR, Ciuleanu T, Robert F, et al. Sunitinib plus erlotinib for the treatment of advanced/metastatic non-small cell lung cancer (NSCLC): A lead-in study. J Thorac Oncol. 2012;7:1406–1416. doi: 10.1097/JTO.0b013e31825cca1c. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Scagliotti GV, Krzakowski M, Szczesna A, et al. Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non–small-cell lung cancer: a phase III trial. J Clin Oncol. 2012;30:2070–2078. doi: 10.1200/JCO.2011.39.2993. [DOI] [PubMed] [Google Scholar]
- 20.Herbst RS, Blumenschein G, Kim E, et al. Sorafenib treatment efficacy and KRAS biomarker status in the Biomarker-Integrated Approaches of Targeted Therapy for Lung Cancer Elimination (BATTLE) trial. J Clin Oncol. 2010;28:15s. [Google Scholar]
- 21.Ramos AH, Dutt A, Mermel C, et al. Amplification of chromosomal segment 4q12 in non-small cell lung cancer. Cancer Biol Ther. 2009;8(21):2042–2050. doi: 10.4161/cbt.8.21.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faivre S, Raymond E, Boucher E, et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol. 2009;10(8):794–800. doi: 10.1016/S1470-2045(09)70171-8. [DOI] [PubMed] [Google Scholar]
- 23.DePrimo SE, Bello CL, Smeraglia J, et al. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J Transl Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45(11):1959–1968. doi: 10.1016/j.ejca.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Harmon C, Figlin R, Hutson T, et al. Circulating protein biomarkers of sunitinib (SU) and interferon-α (IFN-α) efficacy in treatment (Tx)-naive patients (pts) with metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2011;29(suppl) 10525. [Google Scholar]
- 26.Rini BI, Michaelson MD, Rosenberg JE, et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol. 2008;26(22):3743–3748. doi: 10.1200/JCO.2007.15.5416. [DOI] [PubMed] [Google Scholar]
- 27.Natale RB, Thongprasert S, Greco FA, et al. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2011;29:1059–1066. doi: 10.1200/JCO.2010.28.5981. [DOI] [PubMed] [Google Scholar]
- 28.De Boer R, Arrieta O, Yang CH, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2011;29:1067–1074. doi: 10.1200/JCO.2010.29.5717. [DOI] [PubMed] [Google Scholar]
- 29.Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11(7):619–626. doi: 10.1016/S1470-2045(10)70132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J, Hirsh V, Park K, et al. Vandetanib versus placebo in patients with advanced non-small cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase III trial (ZEPHYR) J Clin Oncol. 2012;30:1114–1121. doi: 10.1200/JCO.2011.36.1709. [DOI] [PubMed] [Google Scholar]
- 31.Herbst RS, Johnson DH, Mininberg E, et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23(11):2544–2555. doi: 10.1200/JCO.2005.02.477. [DOI] [PubMed] [Google Scholar]
- 32.Herbst RS, O'Neill VJ, Fehrenbacher L, et al. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non-small-cell lung cancer. J Clin Oncol. 2007;25(30):4743–4750. doi: 10.1200/JCO.2007.12.3026. [DOI] [PubMed] [Google Scholar]
- 33.Herbst R, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet. 2011;377:1846–1854. doi: 10.1016/S0140-6736(11)60545-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.