In the retrospective analysis of two randomized trials, we demonstrated that in patients with advanced terminal cancers and opioid induced constipation, treatment with methylnaltrexone and even more so response to treatment are associated with prolonged survival compared to placebo or no response. Survival was not affected in patients with advanced terminal illness other than cancer.
Keywords: survival, methylnaltrexone, mu opioid receptor, opioids, cancer, constipation
Abstract
Background
Methylnaltrexone (MNTX), a peripherally acting μ-opioid receptor (MOR) antagonist, is FDA-approved for treatment of opioid-induced constipation (OIC). Preclinical data suggest that MOR activation can play a role in cancer progression and can be a target for anticancer therapy.
Patients and methods
Pooled data from advanced end-stage cancer patients with OIC, despite laxatives, treated in two randomized (phase III and IV), placebo-controlled trials with MNTX were analyzed for overall survival (OS) in an unplanned post hoc analysis. MNTX or placebo was given subcutaneously during the double-blinded phase, which was followed by the open-label phase, allowing MNTX treatment irrespective of initial randomization.
Results
In two randomized, controlled trials, 229 cancer patients were randomized to MNTX (117, 51%) or placebo (112, 49%). Distribution of patients' characteristics and major tumor types did not significantly differ between arms. Treatment with MNTX compared with placebo [76 days, 95% confidence interval (CI) 43–109 versus 56 days, 95% CI 43–69; P = 0.033] and response (laxation) to treatment compared with no response (118 days, 95% CI 59–177 versus 55 days, 95% CI 40–70; P < 0.001) had a longer median OS, despite 56 (50%) of 112 patients ultimately crossing over from placebo to MNTX. Multivariable analysis demonstrated that response to therapy [hazard ratio (HR) 0.47, 95% CI 0.29–0.76; P = 0.002) and albumin ≥3.5 (HR 0.46, 95% CI 0.30–0.69; P < 0.001) were independent prognostic factors for increased OS. Of interest, there was no difference in OS between MNTX and placebo in 134 patients with advanced illness other than cancer treated in these randomized studies (P = 0.88).
Conclusion
This unplanned post hoc analysis of two randomized trials demonstrates that treatment with MNTX and, even more so, response to MNTX are associated with increased OS, which supports the preclinical hypothesis that MOR can play a role in cancer progression. Targeting MOR with MNTX warrants further investigation in cancer therapy.
Clinical trials number
introduction
The peripheral μ-opioid receptor (MOR) antagonist methylnaltrexone (MNTX) has been approved by the US Food and Drug Administration and the European Medicines Agency since 2008 for the treatment of opioid-induced constipation (OIC) in patients with advanced illness who are receiving palliative care when response to laxative therapy has not been sufficient, and most recently for OIC in patients with chronic pain [1, 2]. Because MNTX has restricted passage through the blood–brain barrier, it can be given to patients with cancer who are receiving opioid therapy without affecting analgesia. Recent cellular, molecular, animal and human data [3–15] suggest that MOR may be a target for potential anticancer therapy. It has been proposed that MNTX might attenuate cancer progression [1, 4–6, 8, 11]. In animal models, MOR antagonists in clinically relevant doses reduced tumor growth in non-small-cell lung cancer (NSCLC), head and neck cancer, breast cancer and melanomas [11, 16–18]. MOR knockout mice showed decreased tumor growth and metastasis in NSCLC and melanoma [11, 18]. Infusion of MNTX also reduced growth and metastasis in the Lewis lung cancer model [11]. Additionally, polymorphisms in the MOR, which plausibly confers opioid resistance, demonstrated significantly improved survival in all stages of human breast cancer [19], although a recent animal study using breast cancer models failed to demonstrate an effect of opioids on tumor progression [20]. While there are limited data for humans, opioid use has been found as a co-factor in survival in advanced prostate cancer, and two recent retrospective studies demonstrated that perioperative opiate use is associated with decreased overall survival (OS) and increased recurrence in patients undergoing surgery for early stage NSCLC [13, 21, 22]. It was therefore hypothesized that peripheral antagonism of opioid-mediated effects may attenuate disease progression in cancer patients. Accordingly, we explored pooled data from two randomized, placebo-controlled registration trials in patients with advanced disease and examined those with cancer to identify whether MNTX given at regular clinical doses could influence survival during the trial period [23–25].
methods
patients and clinical trials
Starting in February 2003, adult patients with expected terminal illnesses, including advanced cancers, who experienced OIC refractory to laxatives, treated in the palliative care or hospice settings were enrolled in two placebo-controlled, double-blinded multicenter randomized clinical trials (NCT00401362 and NCT00672477) [23–25]. Patients had to be treated with regular opioids for at least 2 weeks with no change in the dose during the last at least 3 days.
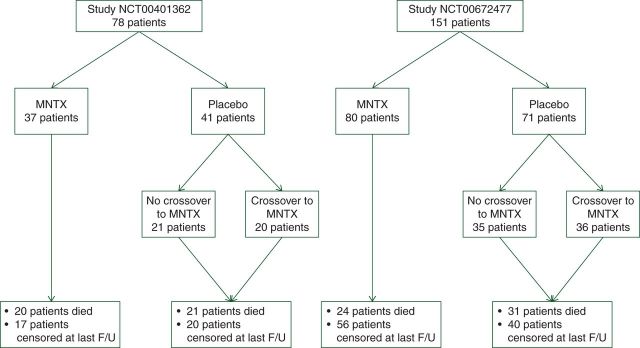
Patients with advanced cancers treated with MNTX or placebo in the above-mentioned randomized trials (Figure 1) were analyzed in the unplanned, post hoc survival analysis and appropriate data including demographic, cancer types, laboratory data (albumin), response to MNTX and dates of treatment initiation, death or the last follow-up were abstracted from the clinical trial databases.
Figure 1.
Enrollment of cancer patients to two placebo-controlled randomized trials (studies: NCT00401362 and NCT00672477) of methylnaltrexone (MNTX) in patients with advanced illnesses treated with regular opioids with opioid-induced constipation.
MNTX responder analysis
In both studies, MNTX responders were defined as patients achieving a laxative response within 4 h of at least two of the first four doses of MNTX. This definition was also applied to patients in the combined analysis. Data for placebo patients who elected to participate in the open-label MNTX treatment phase were collected from the first four doses of the open-label treatment. Data for the MNTX-treated patients were collected from the first four doses of the double-blind phase of the study.
statistical analysis
OS was defined as the time interval from the treatment initiation to the date of death or the date of the last follow-up, whichever occurred first. OS was estimated using the Kaplan-Meier method and compared among the subgroups of patients using a log-rank test [26]. The Cox proportional hazards regression models were constructed to assess the association between patient characteristics and OS [27]. All tests were two-sided, and P values of <0.05 were considered statistically significant. The Bonferroni correction for multiple comparisons was not applied as we tested only one hypothesis. All statistical analyses were carried out using SPSS 21 computer software (SPSS, Chicago, IL, USA). The Cochrane Collaboration's tool was used for assessing the risk of bias [28].
results
patients
The combined dataset from the two studies contained 363 patients including 229 patients with advanced cancers and 134 patients with other advanced illnesses, who were treated with regular opioids and experienced OIC. Of the 229 cancer patients, 117 (51%) were treated with MNTX and 112 (49%) were treated with placebo during the double-blind portion of the study (Figure 1). Cancer patients in both treatment arms presented with a similar distribution of cancer types (supplementary Table S1, available at Annals of Oncology online). Lung (25%), prostate (13%), breast (10%) and pancreatic (7%) cancers were the most frequent types. Treatment arms did not have any major imbalances for gender, age, albumin level or tumor type with the exception of uterine cancer (only enrolled to the placebo arm). Among the non-cancer patients, chronic obstructive pulmonary disease (29%), cardiovascular disease (27%), neurodegenerative diseases (12%) and congestive heart failure (12%) were the most frequently reported conditions. Non-cancer disease distribution in each of the treatment arms was also well balanced.
MNTX and survival
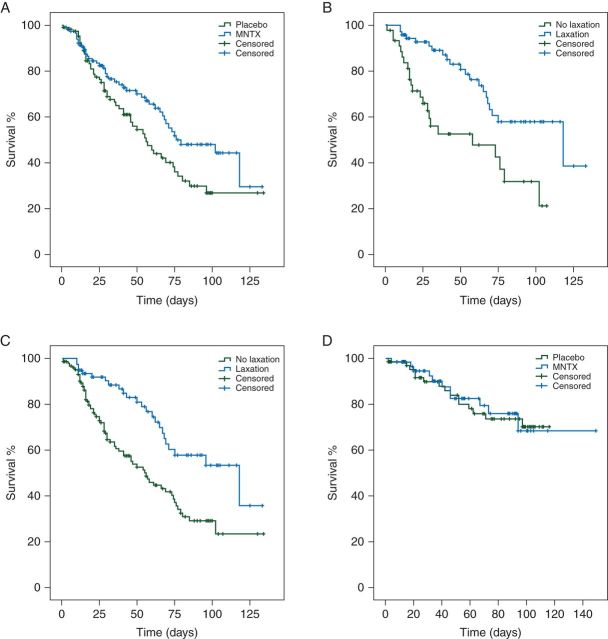
Cancer patients treated with MNTX (n = 117) had a longer median OS compared with 112 cancer patients treated with placebo [76 days, 95% confidence interval (CI) 43–109 versus 56 days, 95% CI 43–69, P = 0.033; hazard ratio (HR) 0.63, 95% CI 0.42–0.95; Figure 2A]. Patients treated with MNTX were more likely to have a response (laxation within 4 h after the first administration) compared with those treated with placebo (72/117, 62% versus 5/112, 4%, P < 0.001). In 117 patients treated with MNTX, 72 patients who responded to MNTX had a longer median OS compared with patients without a response to MNTX (n = 45) (118 days, 95% CI 46–190 versus 58 days, 95% CI 2.7–113, P = 0.001; HR 0.37, 95% CI 0.20–0.67; Figure 2B). Furthermore, 77 patients (MNTX, 72; placebo, 5) with a response to treatment also had a longer median OS compared with 152 patients (MNTX, 45; placebo, 107) without a response (118 days, 95% CI 59–177 versus 55 days, 95% CI 40–70; P < 0.001; HR 0.42, 95% CI 0.26–0.68; Figure 2C). Furthermore, we analyzed 134 patients with advanced illness other than cancer treated in these randomized studies and there was no difference in OS between MNTX versus placebo (P = 0.88, Figure 2D).
Figure 2.
(A) Patients (n = 117, blue) treated with methylnaltrexone (MNTX) had a longer median overall survival (OS) compared with patients (n = 112, green) treated with placebo [76 days, 95% confidence interval (CI) 43–109 versus 56 days, 95% CI 43–69; P = 0.033]. (B) In the MNTX arm, patients (n = 72, blue) with response to MNTX (laxation within 4 h after the first administration) had a longer median OS compared with patients (n = 45, green) without a response or treated with placebo (118 days, 95% CI 46–190 versus 58 days, 95% CI 3–113; P = 0.001). (C) Patients (n = 77, blue) with a response to treatment (laxation within 4 h after the first administration) had a longer median OS compared with patients (n = 152, green) without a response (118 days, 95% CI 59–177 versus 55 days, 95% CI 40–70; P < 0.001). (D) In disease other than cancer, patients (n = 62, blue) treated with MNTX had a similar OS compared with patients (n = 72, green) treated with placebo (medians not reached; P = 0.88).
To assess the significance of our observations regarding MNTX, we compared these findings with the effect of albumin. Albumin levels <3.5 g/dl have been found to be an independent prognostic factor for survival in cancer patients refractory to standard therapies referred for phase I clinical trials [29, 30]. Albumin levels were available for 223 of cancer patients; 128 patients had albumin levels ≥3.5 g/dl, which resulted in longer OS compared with 95 patients with albumin <3.5 g/dl (76 days, 95% CI 55–97 versus 41 days, 95% CI 30–52; P < 0.001; supplementary Figure S1, available at Annals of Oncology online). We found no difference in OS with respect to age and gender. In multivariable analysis stratified by study (study NCT00401362 versus study NCT00672477), which included albumin (≥3.5 versus <3.5 g/dl), treatment (MNTX versus placebo) or response to treatment (response versus no response), albumin ≥3.5 g/dl (HR 0.46, 95% CI 0.30–0.69, P < 0.001) and response to treatment (HR 0.47, 95% CI 0.29–0.76, P = 0.002) were independent prognostic factors for longer OS. Treatment with MNTX, regardless of response, had a trend toward a longer OS (HR 0.68, 95% CI 0.45–1.03, P = 0.07; Table 1).
Table 1.
Multivariable model for overall survival (Cox regression)
| Variable | Hazard ratio | 95% confidence interval | P value |
|---|---|---|---|
| All patients (MNTX 117, placebo 112) | |||
| Albumin: ≥3.5 versus <3.5 g/dl | 0.46 | 0.30–0.69 | <0.001 |
| Treatment: MNTX versus placebo | 0.68 | 0.45–1.03 | 0.07 |
| Response to MNTX: laxation versus no laxation | 0.47 | 0.29–0.76 | 0.002 |
| Excluding crossover to MNTX (MNTX 117, placebo 56) | |||
| Albumin: ≥3.5 versus <3.5 g/dl | 0.40 | 0.24–0.67 | <0.001 |
| Treatment: MNTX versus placebo | 0.32 | 0.18–0.57 | <0.001 |
| Response to MNTX: laxation versus no laxation | 0.34 | 0.20–0.58 | <0.001 |
| Patients on placebo (crossover to MNTX 56, placebo 56) | |||
| Albumin: ≥3.5 versus <3.5 g/dl | 0.54 | 0.31–0.95 | 0.03 |
| Treatment: MNTX versus placebo | 0.15 | 0.07–0.31 | <0.001 |
Statistical significant values are in bold.
MNTX, methylnaltrexone.
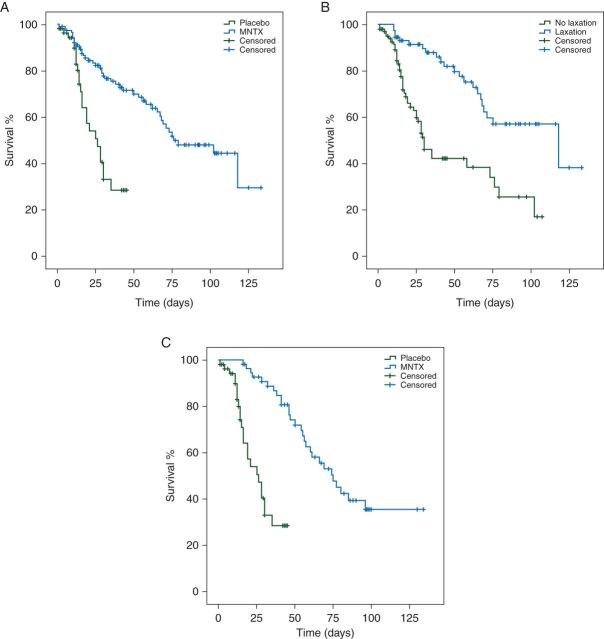
Additional support for an effect of MNTX on survival emerges from analysis accounting for 56 of 112 patients treated with placebo, who crossed over to the MNTX treatment arm in the open-label phase. First, we carried out analysis, which excluded 56 patients, who crossed over from placebo to MNTX; 117 patients treated with MNTX had a longer median OS compared with 56 patients treated with placebo without subsequent crossover to MNTX (76 days, 95% CI 43–109 versus 26 days, 95% CI 17–35, P < 0.001; HR 0.29, 95% CI 0.16–0.50; Figure 3A). Furthermore, 73 patients with response to treatment also had a longer median OS compared with 100 patients without response (118 days, 95% CI 46–190 versus 30 days, 95% CI 23–37, P < 0.001; HR 0.31, 95% CI 0.18–0.52; Figure 3B). In multivariable analysis, which included albumin (≥3.5 versus <3.5 g/dl), treatment (MNTX versus placebo) or response to treatment (response versus no response), albumin ≥3.5 g/dl (HR 0.40, 95% CI 0.24–0.67, P < 0.001), response to treatment (HR 0.34, 95% CI 0.20–0.58, P < 0.001) and treatment with MNTX (HR 0.32, 95% CI 0.18–0.57, P < 0.001) were independent prognostic factors for longer OS (Table 1). Furthermore, we investigated if patients who crossed over from placebo to MNTX had different outcomes compared with those who did not. Patients (n = 56) who crossed over to MNTX had a longer median OS compared with 56 patients treated with placebo who did not (75 days, 95% CI 59–91 versus 26 days, 95% CI 17–35, P < 0.001; HR 0.14, 95% CI 0.07–0.30; Figure 3C). In multivariable analysis, which included albumin (≥3.5 versus <3.5 g/dl) and treatment (MNTX versus placebo), treatment with MNTX (HR 0.15, 95% CI 0.07–0.31, P < 0.001) and albumin ≥3.5 g/dl (HR 0.54, 95% CI 0.31–0.95, P = 0.03 were independent prognostic factors for longer OS (Table 1).
Figure 3.
(A) Patients (n = 117, blue) treated with methylnaltrexone (MNTX) had a longer median overall survival (OS) compared with patients (n = 56, green) treated with placebo without subsequent crossover to MNTX (76 days, 95% CI 43–109 versus 26 days, 95% CI 17–35; P < 0.001). (B) Patients (n = 73, blue) with a response to treatment (laxation within 4 h after the first administration) had a longer median OS compared with patients (n = 100, green) without a response and crossover to MNTX (118 days, 95% CI 46–190 versus 30 days, 95% CI 23–37; P < 0.001). (C) Patients (n = 56, blue) who crossed over from placebo to MNTX had a longer median OS compared with patients (n = 56, green) treated with placebo without subsequent crossover to MNTX (75 days, 95% CI 59–91 versus 26 days, 95% CI 17–35; P < 0.001).
We also analyzed the MNTX effect on survival in the four largest tumor subgroups, which included lung (supplementary Figure S2A, available at Annals of Oncology online), prostate (supplementary Figure S2B, available at Annals of Oncology online), breast (supplementary Figure S2C, available at Annals of Oncology online) and pancreatic cancers (supplementary Figure S2D, available at Annals of Oncology online). Small sample sizes for each cancer types limited the power of the analyses; however, in 16 patients with pancreatic cancer, 8 patients treated with MNTX had a trend to longer median OS compared with 8 patients treated with placebo (76 versus 28 days; P = 0.14; supplementary Figure S2D, available at Annals of Oncology online). Of interest, there was no death among 4 patients with response to therapy, which translated to improved OS compared with 12 patients without response (medians not calculated; P = 0.035).
risk of bias assessment
The risk of bias was assessed using the Cochrane Collaboration's tool for assessing the risk of bias in randomized trials [28]. Neither study was designed to show the OS difference. Therefore, we identified a possible unclear risk of selection bias in random sequence generation. Also, both studies followed patients only for a few weeks and 58% of patients (62% in the MNTX arm and 54% in the placebo arm) were censored at the time of the last follow-up, which could introduce a high risk of attrition bias due to incomplete outcome data (supplementary Table S2, available at Annals of Oncology online).
discussion
In a retrospective combined post hoc analysis of two independent randomized placebo-controlled clinical trials, we demonstrated that, in patients with advanced terminal cancers and OIC, treatment with MNTX (76 versus 56 days, P = 0.033) and, even more so, response to treatment (118 versus 55 days, P < 0.001) are associated with prolonged OS compared with placebo or no response. Our data suggest an effect of MNTX on OS that is as significant as, but independent from, albumin.
While our observations of longer OS in patients with MNTX could be partially explained by direct effects improving gut function, or an indirect effect such as immunosuppression, we favor a direct effect of the drug on tumors as an explanation of our observations. Although opioids have long been known to induce immune suppression, studies on nude mice show that their effect on tumor growth and metastasis persists [31]. While an effect of MNTX on improved gut function is consistent with our responder analysis in cancer patients, the lack of a similar improvement in survival in non-cancer patients makes this a less probable explanation. Therefore, we favor the hypothesis that the improved survival we have observed with MNTX response is a direct effect of the drug on cellular targets related to the MOR and its pathway [1, 3–8, 10, 11, 31, 32].
There has been emerging preclinical literature suggesting a relationship between opioids, MOR and cancer progression. For instance, morphine in clinically applicable concentrations was found to stimulate angiogenesis in breast cancer xenograft models and increase vascular permeability of human pulmonary microvascular endothelial cells and dermal microvascular endothelial cells, and such effects were blocked by MNTX [3, 5, 8]. Also, in vitro opioid-mediated MOR activation can induce an epithelial–mesenchymal transition in NSCLC, which can be attenuated by MNTX or MOR silencing [10, 11]. In clinically relevant doses, MOR antagonists reduced tumor growth in NSCLC, head and neck cancer, melanoma and breast cancer in vivo models [11, 16–18].
Clinical data demonstrating an effect of μ-opioids on cancer growth and metastasis remain limited but are supportive of our hypothesis. A small study with 34 NSCLC patients demonstrated that samples of adjacent non-malignant tissue had lower MOR expression on immunohistochemistry compared with tumor samples, and tumor samples from patients with metastatic disease had the highest MOR expression [7]. In addition, in patients with breast cancer, the MOR A118G mutation, which confers relative resistance to opioids, had improved OS at all stages with heterozygotes manifesting two-fold improved survival, and homozygotes a four-fold improvement [19]. That might plausibly explain why a large prospective study with 34 188 patients did not identify association between opioid use and breast cancer recurrence [33]. A retrospective study with 480 patients with advanced prostate cancer reported that higher opioid requirements were associated with shorter progression-free survival [13]. Finally, retrospective analysis of perioperative opioid use in cancer surgery demonstrated improved survival in patients with regional versus general anesthesia in patients with breast and prostate cancer, and similar data were reported from a large meta-analysis, which included 47 000 patients with cancer [13, 34, 35]. Two recent studies in lung cancer surgery related perioperative opiate use to cancer recurrence [21, 22].
Our results provide evidence suggesting the possible role of opioids and MOR in cancer progression. Interestingly, the impact on survival appeared to be larger in patients with response to MNTX (laxation), which can be perhaps understood as a pharmacodynamic marker. In our study, 50% of patients crossed over from placebo to MNTX, which could have influenced survival outcomes favoring the placebo arm. We carried out analysis in which patients crossing over from placebo to MNTX were excluded and found that, in this subanalysis, patients treated with MNTX had nearly tripled OS compared with those treated with placebo (76 versus 26 days, P < 0.001). Of interest, even patients treated on placebo lived longer if they crossed over to MNTX (75 versus 26 days, P < 0.001).
To our knowledge, this is the first human demonstration of improved OS for cancer patients following treatment with peripheral opioid antagonists. While tertiary opioid antagonists have been clinically available for over 70 years, the development of peripheral opioid antagonists that can be administered to cancer patients requiring μ-opioids for pain or during surgery without affecting analgesia or precipitating withdrawal may represent an important therapeutic adjunct. Moderate to severe pain, which requires opioid administration, affects up to 70%–80% of patients with advanced cancers and are widely used in surgery [36, 37]. There has been a major focus in anesthesia as to whether the type of anesthetic used can influence tumor recurrence. MNTX has been safely given to more than 500 colectomy patients without affecting perioperative analgesia in two proposed randomized clinical trials [38]. The widespread use of opioids in cancer surgery and cancer care underscores the possible clinical relevance of our observations, which, if confirmed, could transform patient care.
Our study also has several limitations. First, the study was retrospective. Secondly, even though data were abstracted from two randomized trials, these studies were neither designed nor stratified to look at survival end points; therefore, despite both groups appearing to be well-balanced, our observations need to be validated in prospective studies. Thirdly, both randomized trials followed patients only for several weeks and as a consequence of that 58% of patients (62% in the MNTX arm and 54% in the placebo arm) were censored at the date of the last follow-up. Fourthly, our results were obtained from patients treated with regular opioids. It is plausible that MOR overexpression can contribute to cancer progression even in the absence of opioids and this needs to be investigated in future studies [7]. Also the type of opioid and dose were not included in the analysis. Fifthly, our statistical analysis was not adjusted for multiple comparisons; however, we believed it was appropriate since we tested a single hypothesis if MNTX and its effect have any impact on OS. Finally, it still remains unclear whether MOR can mediate disease progression irrespective of tumor type or whether this mechanism is more important for certain specific cancers. Our findings suggest that lung and pancreatic cancer may be good targets for further investigations.
In conclusion, our data suggest that MNTX use in advanced cancer patients treated with opioids can prolong survival, plausibly through attenuation of opioid-mediated MOR signaling. In a recent review article, we hypothesized such an effect [1]. While our findings are in patients with advanced malignancies, the hypothesis that μ-opioid antagonism may have a potential therapeutic value also extends to earlier tumors and to the perioperative period [39, 40]. Our findings should be interpreted as preliminary, hypothesis-generating and insufficient to mandate a change in clinical practice, where pain control remains an important issue [41]. Prospective clinical studies to confirm the role of MNTX in patients with advanced cancers are merited to confirm the clinical relevance of our findings.
funding
This study received no funding.
disclosure
JM is one of the developers of methylnaltrexone and receives royalties from The University of Chicago through Progenics, to whom methylnaltrexone was initially licensed. JM is also a paid consultant for Salix Pharmaceuticals, who has the worldwide sales rights of methylnaltrexone, through Progenics. LKJ was an employee of Salix Pharmaceuticals, Inc. during the duration of the study. JM and PAS also have patents related to methylnaltrexone. All remaining authors have declared no conflicts of interest.
Supplementary Material
references
- 1. Singleton PA, Moss J, Karp DD et al. . The mu opioid receptor: a new target for cancer therapy? Cancer 2015; 121: 2681–2688. [DOI] [PubMed] [Google Scholar]
- 2. Moss J, Rosow CE. Development of peripheral opioid antagonists’ new insights into opioid effects. Mayo Clin Proc 2008; 83: 1116–1130. [DOI] [PubMed] [Google Scholar]
- 3. Gupta K, Kshirsagar S, Chang L et al. . Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res 2002; 62: 4491–4498. [PubMed] [Google Scholar]
- 4. Singleton PA, Garcia JG, Moss J. Synergistic effects of methylnaltrexone with 5-fluorouracil and bevacizumab on inhibition of vascular endothelial growth factor-induced angiogenesis. Mol Cancer Ther 2008; 7: 1669–1679. [DOI] [PubMed] [Google Scholar]
- 5. Singleton PA, Lingen MW, Fekete MJ et al. . Methylnaltrexone inhibits opiate and VEGF-induced angiogenesis: role of receptor transactivation. Microvasc Res 2006; 72: 3–11. [DOI] [PubMed] [Google Scholar]
- 6. Singleton PA, Mambetsariev N, Lennon FE et al. . Methylnaltrexone potentiates the anti-angiogenic effects of mTOR inhibitors. J Angiogenes Res 2010; 2: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singleton PA, Mirzapoiazova T, Hasina R et al. . Increased mu-opioid receptor expression in metastatic lung cancer. Br J Anaesth 2014; 113(Suppl. 1): i103–i108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singleton PA, Moreno-Vinasco L, Sammani S et al. . Attenuation of vascular permeability by methylnaltrexone: role of mOP-R and S1P3 transactivation. Am J Respir Cell Mol Biol 2007; 37: 222–231. [DOI] [PubMed] [Google Scholar]
- 9. Leo S, Nuydens R, Meert TF. Opioid-induced proliferation of vascular endothelial cells. J Pain Res 2009; 2: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lennon FE, Mirzapoiazova T, Mambetsariev B et al. . The mu opioid receptor promotes opioid and growth factor-induced proliferation, migration and epithelial mesenchymal transition (EMT) in human lung cancer. PLoS One 2014; 9: e91577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mathew B, Lennon FE, Siegler J et al. . The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg 2011; 112: 558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schlagenhauff B, Ellwanger U, Breuninger H et al. . Prognostic impact of the type of anaesthesia used during the excision of primary cutaneous melanoma. Melanoma Res 2000; 10: 165–169. [PubMed] [Google Scholar]
- 13. Zylla D, Gourley BL, Vang D et al. . Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer 2013; 119: 4103–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zylla D, Kuskowski MA, Gupta K, Gupta P. Association of opioid requirement and cancer pain with survival in advanced non-small cell lung cancer. Br J Anaesth 2014; Oct 10. pii: aeu351. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Juneja R. Opioids and cancer recurrence. Curr Opin Support Palliat Care 2014; 8: 91–101. [DOI] [PubMed] [Google Scholar]
- 16. McLaughlin PJ, Stucki JK, Zagon IS. Modulation of the opioid growth factor ([Met(5)]-enkephalin)-opioid growth factor receptor axis: novel therapies for squamous cell carcinoma of the head and neck. Head Neck 2012; 34: 513–519. [DOI] [PubMed] [Google Scholar]
- 17. Nguyen J, Luk K, Vang D et al. . Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer. Br J Anaesth 2014; 113(Suppl 1): i4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boehncke S, Hardt K, Schadendorf D et al. . Endogenous mu-opioid peptides modulate immune response towards malignant melanoma. Exp Dermatol 2011; 20: 24–28. [DOI] [PubMed] [Google Scholar]
- 19. Bortsov AV, Millikan RC, Belfer I et al. . mu-Opioid receptor gene A118G polymorphism predicts survival in patients with breast cancer. Anesthesiology 2012; 116: 896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doornebal CW, Vrijland K, Hau CS et al. . Morphine does not facilitate breast cancer progression in two preclinical mouse models for human invasive lobular and HER2(+) breast cancer. Pain 2015; 156: 1424–1432. [DOI] [PubMed] [Google Scholar]
- 21. Cata JP, Keerty V, Keerty D et al. . A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non-small cell lung cancer resection. Cancer Med 2014; 3: 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maher DP, Wong W, White PF et al. . Association of increased postoperative opioid administration with non-small-cell lung cancer recurrence: a retrospective analysis. Br J Anaesth 2014; 113(Suppl. 1): i88–i94. [DOI] [PubMed] [Google Scholar]
- 23. Slatkin N, Thomas J, Lipman AG et al. . Methylnaltrexone for treatment of opioid-induced constipation in advanced illness patients. J Support Oncol 2009; 7: 39–46. [PubMed] [Google Scholar]
- 24. Bull J, Wellman CV, Israel RJ et al. . Fixed-dose subcutaneous methylnaltrexone in patients with advanced illness and opioid-induced constipation: results of a randomized, placebo-controlled study and open-label extension. J Palliat Med 2015; 18: 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomas J, Karver S, Cooney GA et al. . Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med 2008; 358: 2332–2343. [DOI] [PubMed] [Google Scholar]
- 26. Kaplan E, Meier P. Nonparametric estimator from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 27. Cox D. Regression models and life tables (with discussion). J R Stat Soc B 1972; 34: 187–220. [Google Scholar]
- 28. Higgins JP, Altman DG, Gotzsche PC et al. . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arkenau HT, Barriuso J, Olmos D et al. . Prospective validation of a prognostic score to improve patient selection for oncology phase I trials. J Clin Oncol 2009; 27: 2692–2696. [DOI] [PubMed] [Google Scholar]
- 30. Arkenau HT, Olmos D, Ang JE et al. . Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the Royal Marsden Hospital experience. Br J Cancer 2008; 98: 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lennon FE, Mirzapoiazova T, Mambetsariev B et al. . Overexpression of the mu-opioid receptor in human non-small cell lung cancer promotes Akt and mTOR activation, tumor growth, and metastasis. Anesthesiology 2012; 116: 857–867. [DOI] [PubMed] [Google Scholar]
- 32. Lennon FE, Moss J, Singleton PA. The mu-opioid receptor in cancer progression: is there a direct effect? Anesthesiology 2012; 116: 940–945. [DOI] [PubMed] [Google Scholar]
- 33. Cronin-Fenton DP, Heide-Jorgensen U, Ahern TP et al. . Opioids and breast cancer recurrence: a Danish population-based cohort study. Cancer 2015; 121: 3507–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Exadaktylos AK, Buggy DJ, Moriarty DC et al. . Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology 2006; 105: 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen WK, Miao CH. The effect of anesthetic technique on survival in human cancers: a meta-analysis of retrospective and prospective studies. PLoS One 2013; 8: e56540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oosten AW, Oldenmenger WH, Mathijssen RH, van der Rijt CC. A systematic review of prospective studies reporting adverse events of commonly used opioids for cancer-related pain: a call for the use of standardized outcome measures. J Pain 2015; 16: 935–946. [DOI] [PubMed] [Google Scholar]
- 37. Ventafridda V, Tamburini M, Caraceni A et al. . A validation study of the WHO method for cancer pain relief. Cancer 1987; 59: 850–856. [DOI] [PubMed] [Google Scholar]
- 38. Yu CS, Chun HK, Stambler N et al. . Safety and efficacy of methylnaltrexone in shortening the duration of postoperative ileus following segmental colectomy: results of two randomized, placebo-controlled phase 3 trials. Dis Colon Rectum 2011; 54: 570–578. [DOI] [PubMed] [Google Scholar]
- 39. Cassinello F, Prieto I, del Olmo M et al. . Cancer surgery: how may anesthesia influence outcome? J Clin Anesth 2015; 27: 262–272. [DOI] [PubMed] [Google Scholar]
- 40. Cata JP, Lasala J, Bugada D. Best practice in the administration of analgesia in postoncological surgery. Pain Manage 2015; 5: 273–284. [DOI] [PubMed] [Google Scholar]
- 41. Paice JA. Cancer pain management: strategies for safe and effective opioid prescribing. J Natl Compr Canc Netw 2016; 14: 695–697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.