Abstract
Background and aim
The progression of non-alcoholic fatty liver disease (NAFLD) to non-alcoholic steatohepatitis (NASH) is believed to be the driver for future development of fibrosis and cirrhosis. Nevertheless, there remains a clear deficit in non-invasive methods for the diagnosis of NASH. The aim of the present study was to evaluate the prevalence of portal lymphadenopathy (PL) in biopsy- proven NAFLD patients and to determine whether PL correlates with NAFLD stage and severity.
Methods
A retrospective study included biopsy-proven NAFLD patients with up to date (within one year) abdominal imaging by computed tomography (CT) and/or magnetic resonance imaging (MRI). Patients were clustered into three groups based on their NAFLD Activity Score (NAS): NAS1-2 (mild), NAS3-4 (moderate) and NAS≥5 (advanced). We Assessed for association between PL and other clinical and laboratory findings with NAS, NAS components and fibrosis.
Results
Seventy-five patients with NAFLD and no other competing etiologies for liver diseases or PL were included. The mean age was 50.7±14.84 years with male predominance (N = 47, 62.7%). Twenty-five (33.3%), 37 (49.3%) and 13 (17.3%) patients had mild, moderate and advanced NAS, respectively. PL significantly correlated with advanced NAS ≥ 5 (Fisher’s (F) 9.5, P = 0.009). Correlation was driven mainly by a link to hepatocytes ballooning (F of 5.9, P = 0.043). In addition, PL significantly correlated with portal inflammation (F 4.29, P = 0.038). As for hepatic fibrosis, the F test wasn’t significant, though spearman’s coefficient (SC) was significant (0.277, P = 0.012). On multivariate analysis, PL was identified as a sole predictor of advanced NAS score (Odds ratio of 2.68, P = 0.002). Incorporation of PL into noninvasive fibrosis scores improved their diagnostic yield.
Conclusion
PL predicts severity of NAFLD. Its presence may serve as a novel radiological marker for NAFLD/NASH differentiation and disease progression.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is characterized by hepatic fat accumulation and is largely a manifestation of metabolic syndrome [1]. The reported prevalence of NAFLD in obese people ranges between 75–100% [2]. Several studies have shown that almost 30% of the general population worldwide have radiological signs of fatty liver [3] [4] [5]. Currently, NAFLD is considered the most common cause worldwide for the development of chronic liver disease and liver cirrhosis [6]. The spectrum of NAFLD ranges from simple steatosis or isolated fatty liver, which is hepatic fat infiltration without inducing inflammation and without hepatocellular injury, to non-alcoholic steatohepatitis (NASH), characterized by hepatic fat infiltration coupled with hepatocyte inflammation. Studies have shown that approximately 20–30% of patients with NAFLD develop NASH, which is associated with increased morbidity and mortality [7]. NASH, in contrast to simple steatosis, was shown to be associated with an increased risk for development of cirrhosis and hepatocellular carcinoma (HCC) [8] [9] [10] [11].
To date, there is a scarcity of non-invasive tools that can predict the progression of NAFLD. The only reliable predictor is liver biopsy, an invasive and costly procedure [12].
The hepatic lymph originating in the sinusoids flow through the space of Disse and follows two major lymphatic pathways–the first traveling along the portal tracts to the hilum of the liver, and the second traveling along central veins and draining into mediastinal lymph nodes [13], the latter being the dominant through which drainage of about 80% of the hepatic lymph occurs. Normal hepatic lymph structures cannot be visualized by imaging, unless there is an underlying disease in proximity such as hepatic lymphoproliferative disease, hepatic lymphangitis, carcinomatosis or lymphocele, which are associated with the development of portal hepatic lymphadenopathy [14].
The aim of this study was to examine the prevalence and clinical significance of portal lymphadenopathy (PL) in NAFLD patients, and to determine whether its presence correlates with NAFLD severity, as assessed by NAFLD activity score (NAS), as well as with liver fibrosis.
Materials and methods
Study population
Study population was comprised of adult male and female patients who were diagnosed with NAFLD by liver biopsy between the years 2006 and 2016 and who had a computed tomography (CT) or a magnetic resonance imaging (MRI) imaging performed within 12 months before or after the performance of liver biopsy. We excluded patients with other causes of liver diseases, such as autoimmune hepatitis, viral hepatitis or who reported consumption of > 30 grams of alcohol per day. Patients who were treated with vitamin E or participated in clinical trials, had malignancy within 5 years before liver biopsy, had other causes of lymphadenopathy, such as infectious, neoplastic or autoimmune diseases, and pregnant patients at time of imaging or biopsy were all excluded.
Data collection
Imaging files and pathology slides were retrieved from the central radiology system and the pathology department archive of the Hadassah university hospital which is a tertiary referral center. Blood tests results were retrieved from patients records and were all performed at time of liver biopsy. Patients demographic and clinical data were retrieved from the electronic patient record.
Study design
A retrospective, cross sectional design was utilized. All pathology slides and imaging files were reviewed by a blinded senior radiologist and pathologist. NAS was used to evaluate NASH severity according to three components: 1) Steatosis, 2) Hepatocytes ballooning and 3) Lobular inflammation [15]. Patients with portal lymph nodes larger than 10 mm were considered to have PL. Included patients were clustered into three groups according to their NAS. Patient with NAS of 1–2, likely to have simple steatosis without steatohepatitis, were labeled as "mild disease", those with NAS 3–4, likely to have steatohepatitis, as "moderate disease", and those with NAS of 5–8, likely with advanced steatohepatitis, as severe disease [16]
We assessed association between PL and other clinical and laboratory findings with NAS, with each of its components, with portal inflammation and with hepatic fibrosis. Dependent variables were patients laboratory, imaging (presence of lymphadenopathy) and clinical characteristics while independent variables were NAS, its components, portal inflammation and fibrosis stage. The Hadassah-Hebrew university medical center institutional review board (IRB) approval was obtained. Trial registration number: 0131-16-HMO. Written informed consent was waived by the IRB due to the retrospective non-interventional design of the study.
Statistical analysis
Characteristics of participants were presented with descriptive statistics as arithmetic means (±SD) or range for continuous variables, or as percentages for categorical variables. Association between continuous variables (patients' blood tests and calculated scores) and histological findings was measured by Pearson correlation and were validated by Mann-Witney when the distribution of the continuous variables was not normal.
The association between categorical variables (background illness and presence of lymphadenopathy vs. histological findings) was tested with χ2 and Fisher’s exact test as appropriate. We used Generalized Linear Models with lymphadenopathy as the dependent binary logistic variable. The non-lymphadenopathy participants served as the control group. A multivariate analysis included age and suspected confounders that showed an association with NAS or one of its components in the univariate analysis at the p < .05 level of significance. Linear regression was utilized to formulate a formula implementing lymphadenopathy into the traditional fibrosis scores. Analyses were done with IBM-SPSS software, version 23.
Results
Baseline characteristic of the patient’s cohort
Seventy-five patients with biopsy-proven NAFLD were included in the study. Twenty-five patients (33.3%) had mild disease (NAS 1–2), thirty-seven (49.3%) had moderate disease (NAS 3–4) and thirteen (17.3%) had advanced disease (NAS ≥ 5). The mean ± standard deviation (SD) for age of study subjects was 50.73±14.84 (range 18–81). Forty-seven patients (62.7%) were male. The average age and the male\female ratio were similar among all the 3 NAS groups (P = NS). PL as per imaging was found in 32/75 (42.7%) of patients (Table 1). Comparing patients with PL with those without PL, we found that the averages of NAS score, HB, LI and PI were significantly higher among patients with PL. Table 2 show baseline characteristic of positive PL population as compared to negative PL.
Table 1. Clinical and demographic characteristics of study participants.
| Patients number | 75 |
| Mean age ± SD (range) | 50.73±14.84 (18–81) |
| Male\Female | 47\28 |
| HTN N(%) | 20(26.7) |
| T2-DM N(%) | 22(29.3) |
| Hyperlipidemia N(%) | 16(21.3) |
| Cirrhosis N(%) | 15(20) |
| IHD N(%) | 8 (10.7) |
| PL N(%) | 32 (42.7) |
Table 2. Baseline characteristics of PL+ and PL- groups.
| PL + | PL - | P value | |
|---|---|---|---|
| Number | 32 | 43 | |
| Mean age ± SD (range) | 50±15.3 (19–78) | 51.2±14.6 (15–81) | 0.3 |
| Male\Female N | 20\12 | 27\16 | 0.48 |
| HTN N(%) | 13 (40.6) | 5 (11.6) | 0.033 |
| T2-DM N(%) | 12 (37) | 12 (27.9) | 0.1 |
| Hyperlipidemia N(%) | 7 (21.8) | 7 (16.3) | 0.2 |
| Cirrhosis N(%) | 8 (25) | 8 (18.6) | 0.4 |
| IHD N(%) | 7 (21.8) | 2 (4.6) | 0.06 |
| Mean ALT (U/L) ± SD (range) | 51.3±45.4 (8–236) | 52.6 ±46.3 (8–226) | 0.45 |
| Mean AST (U/L) ± SD (range) | 45.7±29.2 (15–137) | 50.9±50 (9–265) | 0.33 |
| Mean GGT (U/L) ± SD (range) | 112.6±134.2 (16–618) | 96.5±78 (20–348) | 0.28 |
| Mean ALK (U/L) ± SD (range) | 91.1±38.5 (45–201) | 108.3±96.5 (11–659) | 0.17 |
| Mean T. Bili (umol/L) ± SD (range) | 20.3±25.7 (3–137) | 15.7±16.3 (3–84) | 0.17 |
| Average NAS score (range) | 3.62 (1–6) | 2.74 (1–6) | 0.003 |
| Average steatosis score (range) | 1.84 (1–3) | 1.6 (0–3) | 0.08 |
| Average HB (range) | 1.06 (0–2) | 0.67 (0–2) | 0.008 |
| Average LI (range) | 0.72 (0–2) | 0.46 (0–1) | 0.02 |
| Average fibrosis (range) | 2.25 (0–4) | 1.53 (0–4) | 0.008 |
| Average PI (range) | 1.12 (0–2) | 0.81 (0–2) | 0.008 |
NAS distribution and laboratory findings
The distribution of NAS, NAS components, fibrosis stage and laboratory findings are shown in Table 3. The NAS in our cohort ranged from 1–6 as no patient had a NAS of 7 and 8 points. The mean NAS was 3.12±1.4. The mean ± SD for steatosis, hepatocytes ballooning (HB), lobular inflammation (LI), fibrosis and portal inflammation (PI) grade in our cohort were 1.71 ± 0.73, 0.84 ± 0.7, 0.57 ± 0.57, 1.84 ±1.28 and 0.95 ± 0.56, respectively (Table 3).
Table 3. Descriptive statistics of NAS, laboratory findings and fibrosis scores.
| Parameters | Mean (±SD) | Range |
|---|---|---|
| NAS | 3.12 ± 1.4138 | 1–6 |
| Steatosis | 1.71 ± 0.73 | 0–3 |
| HB | 0.84 ± 0.7 | 0–2 |
| LI | 0.57 ± 0.57 | 0–2 |
| Fibrosis | 1.84 ± 1.28 | 0–4 |
| PI | 0.95 ± 0.56 | 0–2 |
| ALT(U/L) | 52.08 ± 45.6 | 8–236 |
| AST (U/L) | 48.54 ± 42.05 | 9–265 |
| ALK (U/L) | 100.79 ± 76.75 | 11–659 |
| GGT (U/L) | 104.14 ± 107.65 | 16–618 |
| T. Bili (umol/L) | 17.74 ± 20.93 | 3–137 |
| WBC (109/L) | 7.09 ± 2.12 | 3.2–16.4 |
| HgB (gr %) | 13.51 ± 1.9 | 7.8–17.2 |
| INR | 1.19 ± 0.3 | 1–2.4 |
| Plts (109/L)) | 189.45 ± 84.27 | 33–457 |
| CRP (mg %) | 0.73 ± 0.76 | 0–3 |
| FIB-4 score | 3.11 ± 2.91 | 0.45–12 |
| APRI score | 0.92 ± 0.71 | 0.1–3.1 |
Association of demographic, clinical and laboratory findings with NAS and its sub-scores
The results of univariate analysis are shown in Table 4. Age was found to be negatively correlated and alanine aminotransferase (ALT) positively correlated with steatosis (P = 0.022 and P = 0.004, respectively). Higher total bilirubin (TB), international normalized ration (INR) and C-reactive protein (CRP) and reduced platelet (PLT) count were all associated with increased HB (P = 0.025, P = 0.027, P = 0.026 and P = 0.013, respectively). Higher INR, white blood cells (WBC) and lower hemoglobin (HgB) were positively correlated with LI (P = 0.027, P = 0.048 and P = 0.001, respectively). Furthermore, higher Age, TB, and INR levels correlated positively with advanced stages of fibrosis (P = 0.013, P = 0.001 and P = 0.001 respectively), while ALT, Hgb and PLTs negatively correlated with higher stages of fibrosis (P = 0.017, P = 0.013 and P = 0.0001, respectively). There was a trend for correlation between age and ALT with higher NAS (P = 0.056 and P = 0.07, respectively) (Table 4). We found no significant correlation between background illness–ischemic heart disease (IHD), Type 2 Diabetes Mellitus (T2-DM), hypertension (HTN) and dyslipidemia and NAS. On the other hand, T2-DM had significant correlation with fibrosis stage (P = 0.027) and borderline correlation with LI (P = 0.051). HTN was found to correlate with LI (P = 0.04).
Table 4. Correlation of laboratory variables and age with NAS, NAS components, fibrosis stage and portal inflammation- univariate analysis.
| AGE | ALT | AST | GGT | ALK | T. Bili | INR | CRP | WBC | HgB | PLT | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NAS | Pearson | -.221 | .214 | .199 | -.057 | -.082 | .195 | .174 | .263 | .209 | -.220 | -.096 |
| p* | 0.056 | 0.070 | 0.165 | 0.676 | 0.491 | 0.098 | 0.139 | 0.122 | 0.075 | 0.060 | 0.413 | |
| HEPATOCYTE BALLOONING | Pearson | -.102 | .029 | .056 | -.170 | -.138 | .262 | .257 | .372 | .077 | -.134 | -.287 |
| p* | 0.384 | 0.808 | 0.698 | 0.207 | 0.244 | .025 | .027 | .026 | 0.513 | 0.255 | 0.013 | |
| LOBULAR INFLAMMATION | Pearson | -.085 | .060 | .180 | .160 | .093 | .207 | .258 | .069 | .231 | -.366 | -.017 |
| p* | 0.468 | 0.617 | 0.211 | 0.236 | 0.436 | 0.078 | .027 | 0.690 | 0.048 | 0.001 | 0.887 | |
| STEATOSIS | Pearson | -.264 | .336 | .200 | -.087 | -.099 | -.036 | -.113 | .109 | .145 | -.006 | .101 |
| p* | 0.022 | 0.004 | 0.164 | 0.520 | 0.402 | 0.765 | 0.337 | 0.526 | 0.218 | 0.957 | 0.391 | |
| FIBROSIS STAGE | Pearson | .286 | -.279 | -.058 | .069 | -.070 | .367 | .675 | .271 | -.055 | -.288 | -.532 |
| p* | 0.013 | 0.017 | 0.689 | 0.607 | 0.559 | 0.001 | 0.001 | 0.110 | 0.640 | 0.013 | 0.001 | |
| PORTAL INFLAMMATION | Pearson | .165 | -.108 | -.139 | .027 | -.146 | .071 | .254 | .111 | -.296 | -.061 | -.323 |
| p* | 0.156 | 0.361 | 0.334 | 0.840 | 0.217 | 0.551 | 0.029 | 0.521 | 0.01 | 0.606 | 0.005 | |
*2 tailed P value
green shadowing indicates positive correlation, red shadowing indicates negative correlation
Association of portal lymphadenopathy with NAS, portal inflammation and fibrosis
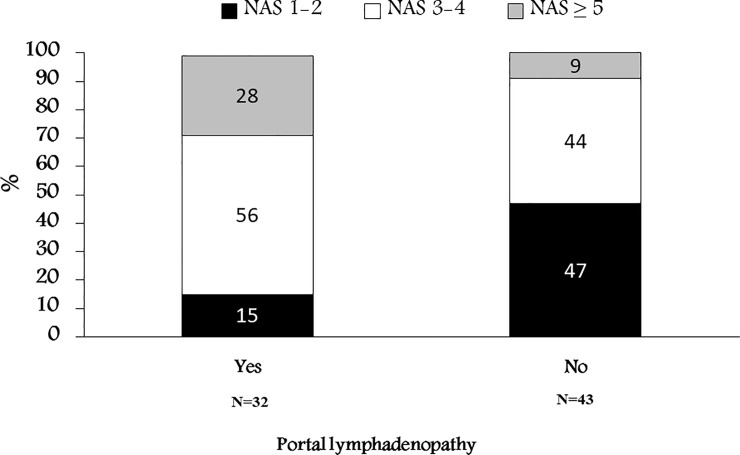
On univariate analysis, there was a significant association between the presence of PL and the severity of NAS (Fig 1, Table 5) (P = 0.009). Only 9% of patients without PL had advanced NAS compared to 28% of patients with PL. Of patients with positive PL, only 15% had mild NAS. The discriminative power of PL was low in the moderate NAS, as 44% did not have PL as compared to 56% who had PL. When further analyzing the correlation with the NAS sub-scores, we found a statistically significant correlation between PL and HB (P = 0.043), as only 18.8% of patients with PL didn’t had HB as compared to 44.2% of patients without PL who didn’t have HB. Notably, there was only a trend for correlation between PL and LI (P = 0.075), while no correlation was found with steatosis (P = 0.1). The correlation between PL and fibrosis was not significant as per Fisher’s test (P = 0.146) but spearman’s coefficient showed significant correlation (P = 0.012), implying some relation do exist (Table 5). By performing further analysis utilizing one-way-Anova, a positive correlation between PL and fibrosis was clearly seen (P = 0.016).
Fig 1. Distribution (%) of NAS according to the status of Portal lymphadenopathy.
Table 5. Correlation of PL with NAS, NAS components and fibrosis-univariate analysis.
| NAS | HEPATOCYTE BALLOONINBG | LOBULAR INFLAMMATION | STEATOSIS | HEPATIC FIBROSIS | ||
|---|---|---|---|---|---|---|
| PORTAL LYMPHADENOPATHY | FISHER'S | 9.566 | 5.980 | 4.700 | 3.500 | 6.671 |
| p | 0.009 | 0.043 | 0.075 | 0.296s | 0.146 | |
| SPEARMAN’S COEFFICIENT | 0.356 | 0.280 | 0.198 | 0.174 | 0.292 | |
| p | 0.002 | 0.015 | 0.086 | 0.143 | 0.012 | |
Multivariate analysis
All parameters that were significantly correlated with NAS sub-scores or showed a trend for association with NAS as assessed by univariate analysis were included in the multivariate model (Table 6). PL was identified as the only significant predictor of advanced NAS (Odds ratio (OR) = 2.68, P = 0.002) (Table 6). Not being diagnosed with T2-DM had a protective effect against high NAS (OR = 0.027, P = 0.01). Increased age and PLT count were borderline associated with decreased NAS. PL was also significantly associated with the presence of PI (OR = 1.29, P = 0.033) and hepatic fibrosis (OR = 1.5, P = 0.033) but showed only a trend for association with HB (P = 0.08) and LI (P = 0.06). PL was not associated with degree of steatosis (P = 0.11).
Table 6. Correlation of demographic, clinical and laboratory variables with NAS -Multivariate analysis.
| Sig.* | EXP(B)$ | 95% Confidence Interval | |
|---|---|---|---|
| AGE | 0.01 | 0.97 | 0.94–0.99 |
| WBC | 0.23 | 1.09 | 0.95–1.26 |
| HgB | 0.91 | 0.99 | 0.80–1.22 |
| Plts | 0.01 | 0.99 | 0.99–1.00 |
| INR | 0.85 | 0.89 | 0.26–3.06 |
| PL | 0.002 | 2.68 | 1.42–5.07 |
| ALT | 0.93 | 1.00 | 0.99–1.01 |
| TB | 0.24 | 1.01 | 0.99–1.03 |
| CRP | 0.43 | 1.22 | 0.74–2.03 |
| [T2-DM = 0]¥ | 0.01 | 0.27 | 0.10–0.71 |
| [T2-DM = 1] | 1.00 | ||
| [HTN = 0]¥ | 0.20 | 1.89 | 0.72–4.95 |
| [HTN = 1] | 1.00 |
* Wald Chi-Square
¥ 0 denotes patient does not have disease
$ signifies odds ratio
Correlation of portal lymphadenopathy with laboratory findings and demographics
We found a significant correlation between PL and HTN (OR = 3.5, P = 0.033). While, only a trend for correlation was seen with IHD (OR = 4.7, P = 0.06). However, no correlation was seen with age, T2-DM and hyperlipidemia (P = NS). There was no correlation between HTN and fibrosis (P = 0.6). In the other hand, we found that HTN correlated with LI (P = 0.04), while no correlation was seen with steatosis or HB (P = 0.4). Additionally, the presence of PL didn’t correlate with liver enzymes, TB and white blood count, while we noticed a decreased PLT count in patients with PL (P = 0.05). No correlation between CRP and PL was noted.
Diagnostic performance for NASH
Using a NAS cutoff of ≥3, the sensitivity, specificity, positive predictive value (PPV) and negative predictive values (NPV) for the diagnosis of NASH stood at 54%, 80%, 84% and 46%, respectively. Using a cutoff of > = 5 yielded sensitivity, specificity, PPV and NPV of 69%, 62%, 28% and 91%.
Impact on the diagnostic yield of fibrosis scores
The ALT to PLT ratio (APRI) score and Fibrosis-4 (FIB4) score were found to significantly correlate with fibrosis stage (Pearson’s coefficient 0.42 and 0.52, respectively, both P<0.001). Via a generalized linear model, we found that addition of the status of PL (absent = 0 or present = 1) to APRI score significantly enhanced the diagnostic yield. Having a high APRI score, presence of PL increased the odds for hepatic fibrosis (Regression coefficient of 0.575, P = 0.45). The status of PL can be incorporated in a new regression equation: 0.963+0.688*APRI+0.575*PL (1 or 0). This equation require validation in further prospective trials. As for FIB-4 the increment in diagnostic yield didn’t reach statistical significance.
Discussion
In the present study, we found that the presence of PL as assessed by CT/MRI imaging, strongly correlated with a more advanced stage of NAFLD, as defined by NAS of ≥ 5. Furthermore, within the NAS components, we found that PL significantly correlated with HB, while only a trend was seen with LI and no correlation was seen with simple steatosis. Using a NAS cutoff of 5, the status of PL can be utilized to rule out NASH with a NPV of 91%. Addition of the status of PL improves the diagnostic yield of non-invasive fibrosis scores.
The spectrum of NALFD ranges from simple hepatic steatosis, which usually remains stable over the years, [17] [18], to steatohepatitis and subsequent liver cirrhosis and HCC, with increased mortality if liver transplantation is not performed [19] [20] [21]. While patients with simple steatosis do not require close observation, patients with steatohepatitis and fibrosis require close monitoring for the development of liver cirrhosis. Steatohepatitis signifies a transition state from relatively benign isolated fatty liver to parenchymal inflammation and scar formation. Thus, identifying the presence and severity of liver inflammation in patients with NAFLD is of critical importance in guiding the subsequent disease management. Currently, the only mean of accurately assessing the degree of hepatic inflammation and fibrosis in NAFLD patients is via liver biopsy. Several non-invasive, non-radiological scoring scales for NAFLD, including NAFLD fibrosis score [15], APRI score [22], FIB-4 index [23] and BARD score [24] have recently been incorporated into clinical practice, but still suffer from some limitations and are used to assess the probability of fibrosis, not inflammation [25].
A recent study claimed that the presence of HB provides a more confident diagnosis of NASH [26]. Interestingly, while PL clearly associated with NAS, on univariate analysis the only significant association with NAS components was with HB with only a trend towards association with LI. However on multivariate analysis the association was a bit weaker and below significance. A previous study showed a positive correlation between CRP level and histologically proven hepatic inflammation [27]. In the present study, CRP correlated only with HB, but not with NAS.
The observed correlation between PL and PI coupled with the similar CRP level among the three NAS groups and the absence of leukocytosis might suggest that the presence of PL is secondary to the development of steatohepatitis and not secondary to systemic inflammatory reaction. We also showed that PL significantly correlated with advanced fibrosis stage as being assessed by histology and by the non-invasive FIB-4 and APRI scores. These score are limited by their sensitivity and specificity [23]. In our study we showed that the addition of PL to APRI score significantly enhance the diagnostic performance of fibrosis. Thus, it might be worthful to incorporate PL in those scores.
An unexplained finding was the correlation between systemic arterial hypertension and PL. Recent studies reported that the incidence of HTN increases in NAFLD severity stage and its rate is gradually increased with advancing NAFLD [28]. In our study, we assume that the correlation between systemic arterial HTN and PL is an indirect sign of advanced NASH stages since PL was shown to be a strong predictor of more severe form of fatty liver disease, thus, HTN is a comorbid disease associated with advanced NASH rather than a cause of PL. In addition, we found that the grade LI was higher in both T2-DM and HTN, while the stage of fibrosis was associated with the diagnosis of T2-DM, thus emphasizing the need for in-depth evaluation of NAFLD in patients with T2-DM and HTN. PL didn’t correlate with liver enzymes (ALT, aspartate transaminase (AST), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALK), TB) and WBC. Notably, there was a strong trend for correlation with low PLT count as compared to patients without PL (P = 0.05). This observation might also underscore the importance of PL as a predictor for advanced NAFLD and fibrosis stage which might reflect the development of subclinical portal hypertension as manifested by decreased PLT count.
The non-invasive evaluation scales are typically used to evaluate fibrosis in NASH patients, and there remains a need for scores evaluating the inflammatory component in NAFLD/NASH. Previous animals studies have shown that lipopolysaccharides were elevated in animal models of NAFLD with progression to NASH [29] [30]. Previously, ALT has been used as an indicator of NASH [31]. In our study we showed that ALT but not AST correlated with higher NAS score ≥5 on univariate, but not multivariate analysis. However, previous study has questioned the predictive role of ALT for steatohepatitis and showed that even patients with NASH had normal ALT level [32]. Thus, the role of liver enzymes in histological prediction of NAFLD is still debated. Other biochemical markers (higher TB level and INR) were associated with advanced fibrosis and NAS score.
To date, there have been no reports addressing PL in predicting evolution from NAFLD to NASH. In our study, we found that PL predicts more severe hepatocytes ballooning, NASH as assessed by advanced NAS score ≥ 5, higher PI and fibrosis grades.
The present study had several limitations. It was a retrospective study with a relatively small sample size. A major limitation for every day practice is that ultrasonographic assessment of portal lymphadenopathy was not evaluated. Several laboratory parameters were missing for some patients. Finally, the study was performed in a single center. In the other hand, the definite diagnosis of NASH by means of liver biopsy assessed by senior liver pathologist, stood as the main strength of our study.
In conclusion, portal lymphadenopathy, as assessed by CT/MRI, is significantly associated with advanced stages of NAFLD, as reflected by NAS score and by fibrosis stage, and with PI, HTN and low PLT count–all pointing to advanced disease. Sensitivity and specificity are comparable to current non-invasive (and mostly non-available) measures of NASH, but absence of PL have high NPV for NASH. Addition of the status of PL to common noninvasive scores of fibrosis improves their diagnostic yield. Our results, provided they are supported by larger studies, suggest that there is a role for addressing the status of portal lymphadenopathy in NAFLD patients and calls for examining the rule of ultrasonography for this purpose.
Supporting information
(XLSX)
Abbreviations
- NAFLD
Non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- PL
portal lymphadenopathy
- CT
computed tomography
- MRI
magnetic resonance imaging
- NAS
NAFLD activity score
- F
Fisher’s
- SC
spearman’s coefficient
- HCC
hepatocellular carcinoma
- IRB
institutional review board
- SD
standard deviation
- HB
hepatocytes ballooning
- LI
lobular inflammation
- PI
portal inflammation
- ALT
alanine aminotransferase
- TB
total bilirubin
- INR
international normalized ratio
- CRP
C-reactive protein
- PLT
platelet
- WBC
white blood cells
- HgB
hemoglobin
- IHD
ischemic heart disease
- T2-DM
Type 2 Diabetes Mellitus
- HTN
hypertension
- OR
Odds ratio
- PPV
positive predictive value
- NPV
negative predictive values
- APRI
ALT to PLT ratio
- FIB-4
fibrosis-4 score
- AST
aspartate transaminase
- GGT
gamma-glutamyl transferase
- ALK
alkaline phosphatase
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–85. 10.1111/j.1365-2036.2011.04724.x . [DOI] [PubMed] [Google Scholar]
- 2.Erickson SK. Nonalcoholic fatty liver disease. J Lipid Res. 2009;50 Suppl:S412–6. 10.1194/jlr.R800089-JLR200 ; PubMed Central PMCID: PMCPMC2674744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark JM, Diehl AM. Defining nonalcoholic fatty liver disease: implications for epidemiologic studies. Gastroenterology. 2003;124(1):248–50. 10.1053/gast.2003.50032 . [DOI] [PubMed] [Google Scholar]
- 4.Fan JG, Zhu J, Li XJ, Chen L, Li L, Dai F, et al. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol. 2005;43(3):508–14. 10.1016/j.jhep.2005.02.042 . [DOI] [PubMed] [Google Scholar]
- 5.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–95. 10.1002/hep.20466 . [DOI] [PubMed] [Google Scholar]
- 6.Bzowej NH. Nonalcoholic steatohepatitis: the new frontier for liver transplantation. Curr Opin Organ Transplant. 2018. 10.1097/MOT.0000000000000502 . [DOI] [PubMed] [Google Scholar]
- 7.Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–71. 10.1146/annurev-pathol-121808-102132 . [DOI] [PubMed] [Google Scholar]
- 8.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–23. 10.1002/hep.25762 . [DOI] [PubMed] [Google Scholar]
- 9.Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11(1):1–16, vii. 10.1016/j.cld.2007.02.009 . [DOI] [PubMed] [Google Scholar]
- 10.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11(1):74–80. . [DOI] [PubMed] [Google Scholar]
- 11.Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet. 1995;346(8981):987–90. . [DOI] [PubMed] [Google Scholar]
- 12.Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L, American Association for the Study of Liver D, United States F, et al. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology. 2015;61(4):1392–405. 10.1002/hep.27678 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka M, Iwakiri Y. The Hepatic Lymphatic Vascular System: Structure, Function, Markers, and Lymphangiogenesis. Cell Mol Gastroenterol Hepatol. 2016;2(6):733–49. 10.1016/j.jcmgh.2016.09.002 ; PubMed Central PMCID: PMCPMC5240041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pupulim LF, Vilgrain V, Ronot M, Becker CD, Breguet R, Terraz S. Hepatic lymphatics: anatomy and related diseases. Abdom Imaging. 2015;40(6):1997–2011. 10.1007/s00261-015-0350-y . [DOI] [PubMed] [Google Scholar]
- 15.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846–54. 10.1002/hep.21496 . [DOI] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. 10.1002/hep.20701 . [DOI] [PubMed] [Google Scholar]
- 17.Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22(6):1714–9. . [PubMed] [Google Scholar]
- 18.Dam-Larsen S, Franzmann M, Andersen IB, Christoffersen P, Jensen LB, Sorensen TI, et al. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53(5):750–5. 10.1136/gut.2003.019984 ; PubMed Central PMCID: PMCPMC1774026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123(1):134–40. . [DOI] [PubMed] [Google Scholar]
- 20.Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43(4):682–9. 10.1002/hep.21103 . [DOI] [PubMed] [Google Scholar]
- 21.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–73. 10.1002/hep.21327 . [DOI] [PubMed] [Google Scholar]
- 22.Kruger FC, Daniels CR, Kidd M, Swart G, Brundyn K, van Rensburg C, et al. APRI: a simple bedside marker for advanced fibrosis that can avoid liver biopsy in patients with NAFLD/NASH. S Afr Med J. 2011;101(7):477–80. . [PubMed] [Google Scholar]
- 23.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7(10):1104–12. 10.1016/j.cgh.2009.05.033 ; PubMed Central PMCID: PMCPMC3079239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57(10):1441–7. 10.1136/gut.2007.146019 . [DOI] [PubMed] [Google Scholar]
- 25.Verhaegh P, Bavalia R, Winkens B, Masclee A, Jonkers D, Koek G. Noninvasive Tests Do Not Accurately Differentiate Nonalcoholic Steatohepatitis From Simple Steatosis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16(6):837–61. 10.1016/j.cgh.2017.08.024 . [DOI] [PubMed] [Google Scholar]
- 26.Caldwell S, Lackner C. Perspectives on NASH Histology: Cellular Ballooning. Ann Hepatol. 2017;16(2):182–4. 10.5604/16652681.1231550 . [DOI] [PubMed] [Google Scholar]
- 27.Shimomura Y, Takaki A, Wada N, Yasunaka T, Ikeda F, Maruyama T, et al. The Serum Oxidative/Anti-oxidative Stress Balance Becomes Dysregulated in Patients with Non-alcoholic Steatohepatitis Associated with Hepatocellular Carcinoma. Intern Med. 2017;56(3):243–51. 10.2169/internalmedicine.56.7002 ; PubMed Central PMCID: PMCPMC5348446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sung KC, Wild SH, Byrne CD. Development of new fatty liver, or resolution of existing fatty liver, over five years of follow-up, and risk of incident hypertension. J Hepatol. 2014;60(5):1040–5. 10.1016/j.jhep.2014.01.009 . [DOI] [PubMed] [Google Scholar]
- 29.Harte AL, da Silva NF, Creely SJ, McGee KC, Billyard T, Youssef-Elabd EM, et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond). 2010;7:15 10.1186/1476-9255-7-15 ; PubMed Central PMCID: PMCPMC2873499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moschen AR, Kaser S, Tilg H. Non-alcoholic steatohepatitis: a microbiota-driven disease. Trends Endocrinol Metab. 2013;24(11):537–45. 10.1016/j.tem.2013.05.009 . [DOI] [PubMed] [Google Scholar]
- 31.Schindhelm RK, Diamant M, Dekker JM, Tushuizen ME, Teerlink T, Heine RJ. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Rev. 2006;22(6):437–43. 10.1002/dmrr.666 . [DOI] [PubMed] [Google Scholar]
- 32.Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37(6):1286–92. 10.1053/jhep.2003.50229 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.