Abstract
The Global Program to Eliminate Lymphatic Filariasis (LF) relies on rapid diagnostic tests (RDTs) to determine where annual mass drug administration for LF is required and when it can be stopped. These tests detect a Wuchereria bancrofti glycoprotein in the blood of infected persons via a carbohydrate moiety recognized by the monoclonal antibodies AD12 and DH6.5. Loiasis cross-reactivity with LF RDTs has recently been recognized as a serious obstacle to LF elimination in loiasis-endemic areas. To better understand the nature of this cross-reactivity, we used the DH6.5 antibody to immunoaffinity purify Loa loa antigens from the sera of individuals with a positive RDT due to loiasis. Immunoblot analysis revealed many circulating AD12/DH6.5-reactive antigens, and proteomic analysis identified multiple L. loa proteins in LF RDT-positive loiasis sera. These included both secreted and somatic proteins, suggesting that they may be released by dying L. loa adult worms and/or microfilariae. Unlike the single high molecular weight W. bancrofti circulating filarial antigen that is reliably present in the blood of persons with bancroftian filariasis, reactive L. loa antigens appeared to be only transiently present in the blood of a subset of persons with loiasis. These key differences between the circulating antigens of W. bancrofti and L. loa can be used to differentiate positive results generated by both species and may lead to improved diagnostic tests for LF and loiasis.
Author summary
Lymphatic filariasis is a disfiguring parasitic infection tens of millions of people in more than 70 countries. The global effort to eliminate LF transmission via mass drug administration (MDA) relies on rapid diagnostic tests (RDTs) to identify infected individuals and map afflicted areas. This effort is complicated in loiasis-endemic nations of central Africa for two reasons. First, persons with heavy L. loa infections may suffer severe adverse events, including death, following treatment with MDA medications. Second, it is now clear that RDT testing for LF can be unreliable in areas with loiasis, since many L. loa-infected individuals, especially those with heavy infections, test positive by LF RDT in the absence of infection with W. bancrofti (the causative agent of LF in Africa). We report here the identity and characteristics of multiple L. loa antigens found in RDT-positive sera that bind to antibodies used in LF RDTs. Understanding the differences between these cross-reactive antigens and the circulating filarial antigen of W. bancrofti may lead to development of improved diagnostic tests for LF and loiasis to facilitate elimination of filarial infections in Sub-Saharan Africa.
Introduction
Lymphatic filariasis (LF) is a disabling and disfiguring disease caused by mosquito-borne, filarial (threadlike) parasitic worms. The Global Program to Eliminate LF (GPELF) reduced the at-risk population for LF from 1.2 billion to 789 million (a 46% reduction) between 2000 and 2012 by providing repeated, annual rounds of anti-filarial medications by mass drug administration (MDA). However, LF elimination in Africa lags behind other endemic regions with only a 25% reduction [1]. This is due in part to slow rollout of MDA in regions of central Africa co-endemic with Loa loa, because drugs used for MDA can cause severe adverse effects in people with loiasis [2].
The GPELF strategy relies on point of care rapid diagnostic tests (RDTs) to map regions endemic for LF and to determine when regions have successfully eliminated the disease. Two RDTs have been used to detect a circulating antigen of Wuchereria bancrofti, the filarial species that causes LF in Africa. These are the Binax NOW Filariasis immunochromatographic card test (ICT), and the Alere Filariasis Test Strip (FTS). The FTS is more stable, more sensitive, and less expensive than the ICT [3], but both are lateral flow assays that work as follows. Whole blood is applied to a sample pad containing colloidal gold-conjugated polyclonal antifilarial antibodies. The sample pad retains blood cells, while capillary action pulls the serum across the test strip and over a test line containing an immobilized IgM class monoclonal antibody called AD12. The AD12 antibody binds a carbohydrate epitope that is abundantly present on a high molecular weight (200–250 kDa) W. bancrofti circulating filarial antigen (CFA) [4, 5]. AD12 traps W. bancrofti CFA bound to the colloidal gold-labeled polyclonal antibodies to form a pink line that indicates a positive test result.
The molecular structure and saccharide composition of the carbohydrate epitope recognized by the AD12 antibody is unknown. This carbohydrate moiety, which we refer to as the AD12 epitope, appears to be specific to nematodes and is not phosphorylcholine [5]. A second IgM monoclonal antibody developed in the Weil laboratory, DH6.5, also recognizes the AD12 epitope [5], and we use these two antibodies interchangeably. Like the AD12 carbohydrate epitope, the exact identity of the high molecular weight W. bancrofti CFA remains unknown. It is clear, however, that it contains multiple AD12 epitopes per molecule, since capture of the molecule by DH6.5 in an ELISA format does not prevent AD12 / DH6.5 from binding to the non-bound surface of the molecule [6]. While other nematodes have antigens that contain the AD12 epitope [5], the ICT and FTS tests have been considered functionally specific for W. bancrofti infection, since until recently, such antigens had not been found circulating in the blood of persons with other infections.
It has now become clear, however, that some persons with loiasis, especially those with high L. loa microfilaria (Mf) counts, have positive LF RDT results [7–9]. This complicates mapping and monitoring for LF elimination programs in central Africa. Therefore, the purpose of this study was to identify filarial antigens in the blood of persons infected with L. loa that could cause false-positive LF RDT results. This information may lead to the development of improved diagnostic tests for both infections.
Materials and methods
Ethics statement
This research involved the testing of adult human sera. Sample collection was approved by the institutional review board of Washington University in St. Louis based on protocols 201512112 for Akonolinga/Awae and 201512016 for the East Region collections in Cameroon. The studies were also approved by the Cameroon National Ethics Committee and Ministry of Public Health. Written informed consent was obtained from all participants.The L. loa microfilariae harvested from baboons kindly provided by S. Wanji were approved by the Ministry of Scientific Research and Innovation of Cameroon (Research permit #028/MINRESI/B00/C00/C10/C12). Animal experiments were conducted within the guidelines stated by the animal care and use committee at the National Institutes of Health (USA).
Sample collection
We collected plasma and serum samples in two field studies (Fig 1). The first was conducted in the Akonolinga and Awae health districts (Central Region of Cameroon) in February 2016, for the specific purpose of obtaining serum samples from persons with loiasis that were positive in LF RDTs. The second study, conducted in the East Region of Cameroon in December 2016, was part of an effort to re-map areas of Cameroon for the presence of W. bancrofti and L. loa. Both study areas are endemic for L. loa and Mansonella perstans, but most likely not for W. bancrofti (Wanji and co-workers, pers. commun.). Neither area had received community-based ivermectin treatment or MDA for LF prior to these studies.
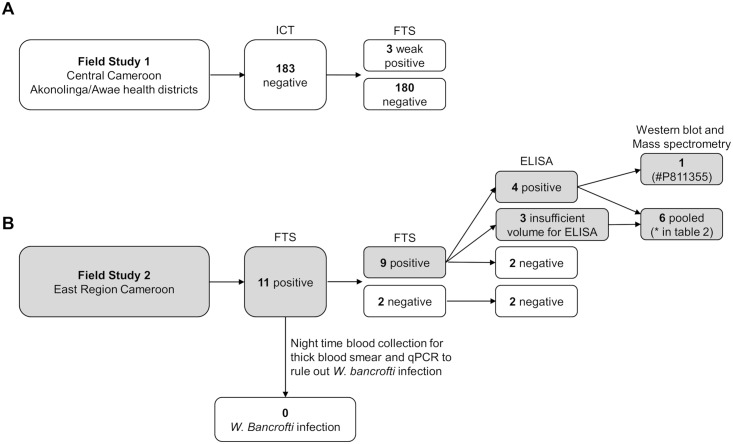
Fig 1. Field studies and samples tested.
(A) Central Cameroon field study. Because the three FTS positive samples were very weakly positive by FTS and negative by ICT, we chose not to examine them further. (B) East Region study. Participants who were ICT-positive on initial screening were visited the following week for venous blood collection. Nine venous blood samples were FTS-positive. Two of these tested negative for filarial antigen by ELISA; the other seven were tested further by ELISA and/or western blot as indicated. Gray shading indicates the origin of the samples used in the proteomic analysis.
The goal of the Akonolinga/Awae study was to obtain serum samples containing cross-reactive L. loa antigens from at least ten persons with positive ICT tests due to loiasis. We planned a sample size of 200 participants, based on the assumption that about 10% of persons with L. loa microfilaremia would have a reactive test [7]. We specifically recruited adults with known loiasis in prior studies, but also invited adults with unknown infection status living in the same villages to participate in order to generate a biobank of loiasis positive and negative samples. Potential participants gathered at a central location in each village (usually the health center) and were informed of the purpose of the study. Consenting participants underwent day (10 AM to 2 PM) and night (10 PM to 2 AM) blood collections. Fresh fingerprick capillary blood was used for ICT testing (100 μL) and for preparation of thick blood smears (70 μL). Dried blood spots were collected for subsequent PCR testing (90 μL, applied to HemaSpot cartridges, Spot On Sciences, Inc., Austin, TX). Venous blood (~10 mL) was collected in K2 ethylenediaminetetraacetic acid (EDTA) vacutainer tubes (BD Biosciences, Franklin Lakes, NJ), stored overnight at 4°C, and then separated by centrifugation. Following separation, plasma was stored at 4°C for up to 3 days prior to transport to Yaoundé, then stored at -80°C. Dried blood spots and aliquots of plasma samples were shipped on dry ice to Washington University in St. Louis for further analysis. After finding that none of the participants in the first study were ICT-positive (see Results), we obtained sera from eleven FTS-positive participants of an initially unrelated study in the East Region of Cameroon. The purpose of the East Region study was to re-map the prevalence of LF and loiasis in Cameroon, and the primary results of that study will be published separately. The re-mapping study was conducted in the Lomie, Doume and Nguelemendouka health districts. Following a brief interview, consented individuals underwent serological and parasitological testing. Daytime (between 10 AM and 4 PM) capillary blood was tested for CFA by FTS (70 μL) and for Mf by thick calibrated thick blood film smears (TBS, 50 μL). Daytime venous blood (4 mL) was collected from FTS-positive individuals in vacutainer tubes without anticoagulant, and sera were separated using a centrifuge and stored in cryovials at -20°C for up to 7 days in the district facilities prior to transport to Buea, where they were stored at -80°C. Night TBS were prepared between 10 PM and 2 AM from participants that tested positive by FTS. Night blood dried onto filter paper was used for detection of parasite DNA by qPCR, as reported previously [7, 10]. Sera from the re-mapping study were shipped on dry ice to Washington University in St. Louis for further analysis. Loiasis-negative banked sera (collected outside of Africa) from persons with microfilaremic W. bancrofti infections [11, 12] were used as positive controls for CFA tests.
Detection of Mf in blood samples
Thick blood smears were stained with Giemsa, and the number of L. loa and M. perstans Mf were counted as previously described [13]. M. perstans were distinguished from L. loa based on morphology and size. Each slide was read by two experienced microscopists.
Quantitative PCR
qPCR was performed at Washington University as previously described [7]. Briefly, three blades of HemaSpot finger prick blood were used for DNA extraction by QIAamp DNA mini kits (Qiagen, Valencia, CA) Real-time PCR using Taqman Multiplex master mix (Applied Biosystems, Foster City, CA) was performed with an ABI Quant 6 instrument under standard conditions using primer and probe sequences as previously described [10, 14].
W. bancrofti RDTs
The ICT (BinaxNow Filariasis) and Filariasis Test Strip (FTS) (both purchased from Alere, Scarborough, ME) diagnostic tests for circulating filarial antigen were performed according to the manufacturer’s instructions and read 10 minutes after applying the sample to the sample pad [3]. Positive results were recorded with a semi-quantitative scale in which: 0 = no test (T) line visible (negative), 1 = the T line was visible but weaker than the control (C) line, 2 = the T line was equal to the C line, and 3 = the T line was stronger than the C line.
In vitro culture of L. loa
L. loa Mf were isolated and purified from venous blood collected from experimentally infected baboons [15]. Immature adult stage (L5) L. loa were isolated from Rag2-/-/IL2γc-/- BALB/c mice 90 days after subcutaneous inoculation of 100 L3 stage worms. Pools of parasites (40,000 Mf and 5 L5) were incubated in 400 μl serum-free RPMI medium supplemented with 2mM L-glutamine, 2 g/L Na2HCO3 with 10% (v/v) penicillin-streptomycin-neomycin mixture in a 37°C/ 5% CO2 incubator. Culture supernatants (ES) applied directly to FTS after incubation for 32 hours (Mf) or 24 hours (L5) to detect the presence of reactive antigens.
Filarial antigen ELISA
Detection of circulating filarial antigen by sandwich ELISA was performed as previously described [6]. This assay uses two IgM monoclonal antibodies, AD12 and DH6.5, both of which recognize the AD12 carbohydrate epitope. Reagents for ELISA, immunoprecipitation and immunoblot experiments were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise noted.
Competition assay for antibody to the AD12 epitope
Brugia malayi excretory secretory products (Bm-ES) were prepared by incubating adult female B. malayi worms (obtained from the Filariasis Research Reagent Resource Center, Athens, GA) in serum free RPMI 1640 media, supplemented with L-glutamine and penicillin/streptomycin (Gibco) in a 37°C/ 5% CO2 incubator. The spent media was collected every other day while worms were viable (approximately 2 weeks) and Bm-ES was concentrated by centrifugal filtration using an Amicon Ultra 3000 MWCO (Millipore) filter. Polyvinyl, U-bottom microtiter plates (Thermo Electron Corp., Milford, MA) were coated overnight at 37°C with 100 μl/well of Bm-ES product diluted to 1.25 μg/mL in 0.6M carbonate buffer, pH 9.6. After coating, wells were washed with phosphate buffered saline (PBS) containing 0.05% Tween 20 (PBS-T) then blocked with 200 μL 5% fetal calf serum in PBS-T. Wells were then incubated with 50 μL of a 1:5 dilution of test sera or unlabeled DH6.5 antibody (7μg/mL) for one hour at 37°C, washed with PBS-T to remove unbound antibody, and incubated with 100 μL peroxidase-conjugated AD12 antibody for one hour at 37°C. Wells were washed three times with PBS-T, and 100 μL O-phenylenediamine dihydrochloride (OPD) substrate added, developed for ten minutes, stopped by adding 30 μL of 4M sulfuric acid, and absorbance read at 492 nm.
Immunoprecipitation
Five mg of antibody DH6.5 was directly conjugated to 1mL packed Affigel 10 beads (Bio-Rad, Hercules, CA) according to the manufacturer’s protocol. Fifty microliters of conjugated beads were mixed with 100–750 μl of each human serum sample (depending on the amount of sample available), then incubated at 4°C with rocking overnight. The beads were then washed four times with cold PBS, re-suspended in 1X NuPAGE LDS sample buffer (Invitrogen), and heated to 95°C for five minutes to release bound antigens. For L. loa ES products, one mL of in vitro culture supernatant was mixed with 50 μL DH6.5-conjugated beads and rocked at 4°C overnight. The beads were washed four times with cold PBS, four times with a high salt, detergent buffer (MIB-T buffer: 5mM HEPES, 1M NaCl, 1mM EDTA, 1mM EGTA, 10mM NaF, 2.4mM NaVO4, 0.5% Triton X-100 + protease inhibitor cocktail), then twice in cold PBS to remove the detergent. Antigens were released by heating in 1X NuPAGE LDS sample buffer as written above.
Polyacrylamide gel electrophoresis and western blot analysis
Proteins were resolved by SDS-PAGE using a 4–12% bis-tris NuPAGE gel (Invitrogen) and transferred to nitrocellulose membranes (Cell Signaling Technology, Danvers, MA). Membranes were incubated 1 hour at room temperature with PBS-T containing 5% nonfat, dried milk to block nonspecific binding and then incubated with a 1:1000 solution of peroxidase-conjugated AD12 antibody in blocking buffer for one hour at room temperature. Blots were washed three times in PBS-T, incubated with Clarity Western ECL substrate (Bio-Rad, Hercules, CA), and bound antibody detected by chemiluminescence using a Bio-Rad ChemiDoc instrument and Image Lab 5.2.1 software. The L. loa soluble antigen (Loa Ag) used as a positive control in the immunoblots was prepared by grinding adult L. loa worms in extraction buffer (10mM Tris pH 8.3, 2% sodium deoxycholate, 1mM PMSF, 1mM EDTA, 1mM EGTA, 25ug/mL TLCK protease inhibitor, 15ug/mL TPCK protease inhibitor). The adult worms were a gift from Dr. Vida Dennis, Tulane University.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS)
Immunoprecipitation samples were prepared for LC-MS/MS using the filter-aided sample preparation method [16]. Briefly, samples were reduced with DTT and buffer-exchanged into 8M urea/Tris, alkylated with 40mM iodoacetamide, then buffer-exchanged in several steps to 0.05M NH4HCO3 using a Microcon 30K filter concentrator (Millipore, Billerica, MA). Samples were treated with PNGase F for 1–3 hours to remove N-linked carbohydrates. The samples were then resuspended in urea/tris, reduced with TCEP, incubated with iodoacetamide to block reactive thiols, and buffer-exchanged back into 0.05M NH4HCO3. Trypsin, chymotrypsin, or both were added and samples were incubated overnight at 37°C, after which the digested peptides were centrifuged through the 30K filter and desalted using a Glygen C4 tip (Glygen, Columbia, MD). LC-ESI/MS/MS analysis was performed using a Q-Exactive Plus Hybrid Quadrupole-Orbitrap Plus mass spectrometer (ThermoFisher Scientific) coupled to an EASY-nanoLC 1000 system (ThermoFisher Scientific). The samples were loaded (2.5 μL) onto a 75 μm × 50 cm Acclaim PepMap 100 RP column (ThermoFisher Scientific). The peptides were eluted at a flow rate of 300 nL/min with an acetonitrile gradient in aqueous formic acid (0.1%) as mobile phase A. Peptide elution occurred in the following sequence: 0–4% buffer B (Acetonitrile containing 0.1% formic acid) for 1 min, 4–12% over 63 minutes, 12–22% B over 56 minutes, 22–30% B over 20 minutes, 30–70% B over 6 minutes, followed by increase in B to 95% B over 1 min and an isocratic wash at 95% B for 12 min. Full-scan mass spectra were acquired by the Orbitrap mass analyzer in the mass-to-charge ratio (m/z) of 375 to 1500 and with a mass resolving power set to 70,000. Twelve data-dependent high-energy collisional dissociations (HCD) were performed with a mass resolving power set to 35,000, a fixed first m/z 100, an isolation width of 1.2 m/z, and the normalized collision energy (NCE) setting of 27. The maximum injection time was 120 ms for parent-ion analysis and 120 ms for product-ion analysis. Target ions already selected for MS/MS were dynamically excluded for 30 sec. An automatic gain control (AGC) target value of 3 x 106 ions was used for full MS scans and 5 x 105 ions for MS/MS scans. Peptide ions with charge states of one or greater than seven were excluded from MS/MS acquisition. The resulting MS spectra were converted to Mascot generic format (MGF) using Proteome Discoverer v2.1.0.81. MGF files were submitted for peptide identification against target databases available for L. loa (Bioprojects PRJNA246086 [17] and PRJNA60051 [18] downloaded from WormBase ParaSite (parasite.wormbase.org) on Feb 27, 2017), contaminant databases from ENSEMBL for Human (Homo_sapiens.GRCh37.72 ENHU) and Mouse (Mus_musculus.GRCm38.72 ENMOU), and the cRAP database version (2012.01.01) for common contaminating peptides (http://www.thegpm.org/crap/). The search engine used was PEAKS Studio 8.0 build 20160908. The following parameters were used during database search: Oxidation of methionine was allowed as variable modification; carbamidomethylation of cysteine as a fixed modification; maximum of 3 missed cleavages; trypsin and chymotrypsin as the proteolytic enzymes depending on the sample; MS1 error tolerance of 20.0 ppm and MS2 error tolerance of 0.02Da. Qualifying peptides had a less than 1% false discovery rate and were absent from the combined control databases with human, mouse and common contaminating peptides.
Bioinformatic analysis
Blast2Go software was used to assign Gene Ontology (GO) terms to the combined immunoprecipitation (IP) dataset and to the whole genome of Loa (PRJNA246086) to build a reference database [19]. NetOGlyc 4.0 was used to predict O-glycosylation sites in the hits from the mass spec analysis. These results were compared with the O-glycosylation prediction of 500 randomly selected proteins from the genome to determine enrichment of O-glycoproteins [20]. The same approach used to predict N-glycosylation sites with NetNGlyc 1.0 [21]. SignalP 4.1 software was used to predict classical N-terminus secretion signals and SecretomeP 2.0 was used to predict non-classical secretion signals [22, 23]. Reciprocal BLAST searches with the B. malayi genome (BioProject PRJNA10729, parasite.wormbase.org) were used to identify B. malayi homologs of the L. loa antigens identified in the proteomics screen. The resulting dataset was used for a meta-analysis of B. malayi proteomics studies as a proxy to characterize the L. loa proteins.
Results
Loss of ICT cross-reactivity in residents of Akonolinga/Awae
Prior filariasis surveys in the Akonolinga and Awae health districts in Central Cameroon area had shown 1–5% ICT positivity with a W. bancrofti microfilaremia prevalence of 0.23%. This result suggested that most, if not all, of the ICT positives were due to L. loa cross reactivity [24]. In February 2016, we collected daytime and nighttime blood samples from 183 persons. This included 89 persons who had tested positive for loiasis in prior studies, and 18 who were previously ICT positive (tested in either 2013 or 2015; S1 Table). One hundred eleven participants (59%) had L. loa microfilaremia in blood collected during the day, and 71 (41%) of these also had nocturnal L. loa microfilaremia. No participant had W. bancrofti microfilaremia by microscopy and only one sample, which was FTS negative, tested positive for W. bancrofti DNA by qPCR. Surprisingly, none of the participants were ICT positive, including the 18 who had been ICT positive in prior studies, even though L. loa Mf counts in these participants had not decreased (Table 1). We also tested plasma from each participant by FTS; and only three samples were weakly positive (1+ test line).
Table 1. Central Cameroon field study L. loa and W. bancrofti test results by prior ICT status.
| Prior ICT * | ICT+ | FTS+ | L. loa PCR+ | Wb PCR+ | Median L. loa Mf/mL (IQR) | |
|---|---|---|---|---|---|---|
| Prior results | Current results | |||||
| ICT+ (N = 18) | 0 | 1 | 15 (83%) | 0 | 11,870 (5,240–32,540) |
17,540 (12,120–36,620) |
| ICT- (N = 30) | 0 | 0 | 13 (43%) | 0 | 2,720 (840–6,900) |
2,000 (340–4,360) |
| No prior (N = 135) | 0 | 2 | 38 (28%) | 1 | N/A | 40 (0–3,880) |
*Prior ICT tests were in either 2013 (N = 24) or 2015 (N = 24)
Loss of reactivity is not due to masking antibody
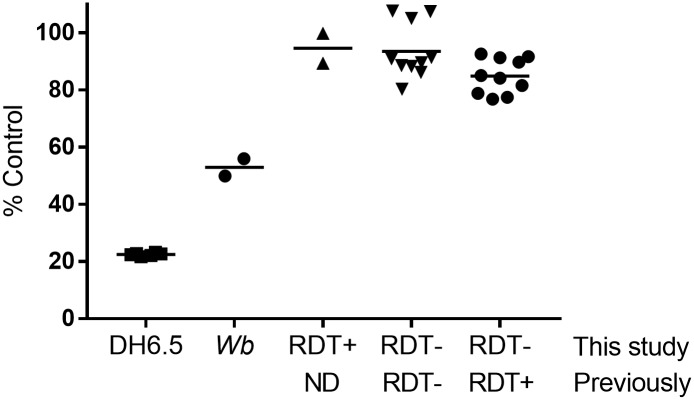
It has been observed that the absence of circulating filarial antigen in some W. bancrofti-infected individuals is related to the host humoral response to the AD12 epitope [6]. To investigate whether the lack of W. bancrofti RDT cross-reactivity in the previously ICT-positive participants was due to development of antibodies against the AD12 epitope in these persons, we tested a subset of samples for anti-AD12 epitope antibody by competition ELISA. None of the samples reduced binding of the AD12 antibody to AD12 epitopes by more than 20%, regardless of the current or prior ICT/FTS status of the individual (Fig 2). This suggests that the lack of RDT-positivity in the previously positive samples is not due to the presence of AD12 epitope-masking antibodies.
Fig 2. Loiasis patient sera lack antibodies specific to the AD12 carbohydrate epitope.
Serum samples were tested for the ability to block AD12 binding to B. malayi antigens that contain the AD12 epitope. The horizontal line denotes the mean value for each group.
East Region samples
Given the lack of cross-reactive antigenemia in the Akonolinga/Awae participants, we also tested banked sera from eleven ICT-positive participants in an integrated LF re-mapping study conducted in the East Region of Cameroon in 2016. These participants all had L. loa microfilaremia (Table 2) but no W. bancrofti Mf by nocturnal thick blood smear and no detectable W. bancrofti DNA by qPCR. All 11 participants also had M. perstans microfilaremia. Nine of the eleven sera were FTS positive, and four had detectable AD12 epitope-containing antigen by CFA ELISA (Table 2 and Fig 1).
Table 2. Summary of Mf burden and antigenemia in ICT/FTS cross-reactive L. loa infected individuals from East Region Cameroon mapping study.
| Sample ID# | L. loa (Mf/mL) | FTS score | CFA (ng/mL) | M. perstans (Mf/mL) |
|---|---|---|---|---|
| P811473 | 13600 | 0 | 0 | 1200 |
| P811092 | 41650 | 0 | 0 | 900 |
| P811331* | 9750 | 1 | 5.8 | 1150 |
| P811493* | 7700 | 1 | ND | 1950 |
| P811461* | 21450 | 1 | 81.4 | 8650 |
| P811408* | 44350 | 1 | ND | 6650 |
| P811377* | 74350 | 1 | 86.3 | 1800 |
| P811161 | 50100 | 1 | 0 | 550 |
| P811264 | 16800 | 2 | 0 | 950 |
| P811134* | 59500 | 2 | ND | 1000 |
| P811355 | 82950 | 2 | >400 | 900 |
*Serum pooled in further analyses
ND = insufficient sample volume to conduct CFA ELISA
Western blot analysis of cross-reactive sera
We tested FTS-positive samples by western blot to visualize antigens reactive with AD12. Sample P811355, which had the highest antigen level, was analyzed separately; six other samples with weaker antigen signals by FTS were pooled (see Table 2) for analysis. We captured AD12 epitope-containing antigens by incubating these sera with agarose beads conjugated to DH6.5 monoclonal antibody, which recognizes the same carbohydrate epitope as AD12, and then detected the bound proteins by western blot using horseradish peroxidase-conjugated AD12 antibody. Both sample 811355 and the pooled serum sample contained many reactive proteins including a major band at ~80 kDa. This pattern is very different from that seen with sera from W. bancrofti-infected persons (Fig 3). Serum samples from LF RDT-negative loiasis serum, including five individuals from the Akonolinga/Awae field study who were previously W. bancrofti RDT positive but negative at the time of our study, were negative for AD12 containing antigens by western blot.
Fig 3. Loiasis sera positive by LF RDT contain multiple AD12 epitope-containing antigens.
(A) AD12 western blot showing reactive bands in pooled sera from 13 individuals with W. bancrofti infection (Wb CFA+) or 12 uninfected individuals (CFA-). (B) Antigens from sample P811355 (355), and pooled cross-reactive sera. Soluble L. loa antigen (Loa Ag, 0.5 μg) served as a positive control. Because the antigen level was much higher in sample P811355 than in the pooled sample, different chemiluminescent exposure times were used for the same blot (45 seconds for soluble L. loa antigen and P811355; 15 minutes for the pooled serum sample).
Proteomic analysis of L. loa antigens immunoprecipitated from human serum with monoclonal antibody DH6.5
We next analyzed immunoaffinity-purified antigens from sample 811355 and the pooled sera by protease digestion and LC-MS/MS, matching MS spectra to both available L. loa genomes [17, 18]. After reconciling duplicated annotations between the two genomes, 220 L. loa proteins with two or more unique peptides were detected in the 811355 sample; ten were detected in the pooled sera. Seven proteins were detected in both samples. Table 3 lists the most abundant proteins identified; a list of all identified proteins is provided in S2 Table.
Table 3. Proteins identified in cross-reactive L. loa sera.
| Unique peptides (total spectral counts) | Description | ID1 | LOAG ID2 | C. elegans ortholog3 | |
|---|---|---|---|---|---|
| 811355 | Pooled | ||||
| 76 (93) | 2 (2) | Myosin heavy chain 4 | EN70_1344 | - | unc-54 |
| 29 (63) | 21 (30) | Hypothetical zinc metalloprotease | EN70_10459 | - | nas-14 |
| 21 (30) | 2 (2) | Sugar transporter | EN70_10241 | LOAG_09566 | fgt-1 |
| 18 (21) | 3 (3) | Tropomyosin | - | LOAG_17659 | lev-11 |
| 2 (2) | 1 (12) | Hypothetical zinc metalloprotease | EN70_104634 | - | nas-14 |
| 2 (19) | 1 (12) | Hypothetical zinc metalloprotease | EN70_10462 | - | nas-14 |
| 2 (17) | 5 (5) | Myosin heavy chain | - | LOAG_18869 | unc-54 |
| 41 (53) | - | Paramyosin | EN70_1595 | LOAG_05509 | unc-15 |
| 31 (34) | - | Mesocentin | - | LOAG_01560 | dig-1 |
| 30 (40) | - | Chaperonin GroEL | EN70_9299 | LOAG_17523 | hsp-60 |
| 30 (34) | - | ATP synthase subunit beta | EN70_3459 | - | atp-2 |
| 24 (24) | - | Spectrin protein 1 | EN70_4597 | LOAG_16411 | add-1 |
| 22 (34) | - | Galectin-2 | EN70_3647 | LOAG_01794 | lec-2 |
| 21 (28) | - | Heat shock protein 90 | EN70_561 | - | daf-21 |
| 21 (26) | - | ADP/ATP carrier protein | EN70_6911 | LOAG_07239 | ant-1.1 |
| 20 (20) | - | Spectrin beta chain | EN70_174 | LOAG_04011 | unc-70 |
| 19 (21) | - | Myosin light chain | EN70_10990 | LOAG_04932 | mlc-3 |
| 18 (26) | - | Myosin regulatory light chain 1 | EN70_948 | LOAG_03316 | mlc-1 |
| 18 (20) | - | Calcium-transporting ATPase | EN70_10268 | - | sca-1 |
| 17 (21) | - | Phosphate carrier protein | EN70_6829 | LOAG_00237 | F01G4.6 |
| 15 (17) | - | Sodium/potassium-transporting ATPase subunit alpha | EN70_7308 | LOAG_07650 | catp-4 |
| 14 (17) | - | ATP synthase subunit alpha | EN70_7528 | LOAG_16780 | H28O16.1 |
| 12 (13) | - | Hypothetical protein, adhesion domains | EN70_5928 | - | dig-1 |
| 12 (12) | - | Chaperone DnaK | EN70_547 | LOAG_16336 | hsp-6 |
| 11 (14) | - | Elongation factor 1-alpha | EN70_10934 | LOAG_08753 | eef-1A.1 |
| 11 (13) | - | Disorganized muscle protein 1 | EN70_901 | LOAG_02014 | dim-1 |
| 11 (13) | - | Enolase | EN70_8669 | - | enol-1 |
1Bioproject PRNJA246086 (Talon et al 2014)
2Bioproject PRNJA60051 (Desjardin et al 2013)
3parasite.wormbase.org
4Nine peptides shared between EN70_10462 and EN70_10463
Bioinformatic analysis
To further characterize the loiasis antigens captured by DH6.5 immuno-purification, we performed a gene ontology (GO) enrichment analysis using Blast2Go software [19, 25] with the published L. loa proteome as a comparator [17]. At least one GO term could be assigned for 206 of the 220 (94%) loiasis proteins identified. Table 4 shows the top ten GO terms enriched in each category. Compared to the whole L. loa proteome, our identified proteins were enriched for cytoplasmic, organelle, and cytoskeletal proteins. The over-represented molecular functions included structural and protein binding activity, as well as nucleoside enzymatic activity and purine nucleoside binding. The biological processes enriched in the IP were largely concerned with reproduction and early development.
Table 4. GO enrichment analysis of mass spectrometry hits.
| GO ID | GO Name | Fold Change | p-value1 |
|---|---|---|---|
| Cellular Compartment | |||
| GO:0005737 | cytoplasm | 2.0 | 2.3E-17 |
| GO:0044422 | organelle part | 2.2 | 8.1E-16 |
| GO:0044446 | intracellular organelle part | 2.2 | 2.3E-14 |
| GO:0044444 | cytoplasmic part | 2.1 | 6.9E-14 |
| GO:0099512 | supramolecular fiber | 4.4 | 1.0E-11 |
| GO:0099081 | supramolecular polymer | 4.4 | 1.2E-11 |
| GO:0043228 | non-membrane-bounded organelle | 2.4 | 1.2E-11 |
| GO:0043232 | intracellular non-membrane-bounded organelle | 2.4 | 1.2E-11 |
| GO:0099080 | supramolecular complex | 4.3 | 1.4E-11 |
| GO:0030016 | Myofibril | 7.4 | 6.3E-11 |
| Molecular Function | |||
| GO:0005198 | structural molecule activity | 3.9 | 1.8E-11 |
| GO:0005515 | protein binding | 2.1 | 2.5E-08 |
| GO:0017111 | nucleoside-triphosphatase activity | 2.8 | 2.9E-07 |
| GO:0016462 | pyrophosphatase activity | 2.8 | 4.4E-07 |
| GO:0016818 | hydrolase activity, acting on acid anhydrides, in phosphorus-containing anhydrides | 2.7 | 4.9E-07 |
| GO:0003735 | structural constituent of ribosome | 4.0 | 5.1E-07 |
| GO:0016817 | hydrolase activity, acting on acid anhydrides | 2.7 | 5.4E-07 |
| GO:0035639 | purine ribonucleoside triphosphate binding | 1.9 | 2.5E-06 |
| GO:0001883 | purine nucleoside binding | 1.9 | 2.6E-06 |
| GO:0032550 | purine ribonucleoside binding | 1.9 | 2.6E-06 |
| Biological Processes | |||
| GO:0009792 | embryo development ending in birth or egg hatching | 3.5 | 1.4E-23 |
| GO:0002119 | nematode larval development | 3.3 | 1.1E-21 |
| GO:0002164 | larval development | 3.3 | 1.2E-21 |
| GO:0009790 | embryo development | 3.2 | 1.9E-21 |
| GO:0009791 | post-embryonic development | 3.3 | 3.1E-21 |
| GO:0000003 | reproduction | 2.7 | 6.2E-20 |
| GO:0048608 | reproductive structure development | 4.7 | 5.1E-19 |
| GO:0061458 | reproductive system development | 4.7 | 5.1E-19 |
| GO:0045137 | development of primary sexual characteristics | 4.6 | 2.5E-17 |
| GO:0008406 | gonad development | 4.6 | 2.5E-17 |
1based on binomial distribution
Analysis with secretion signal prediction software SignalP, which searches for classical N-terminus secretion signals, and SecretomeP, which searches for non-classical secretion signals, predicted that only 69 of 220 (31%) of the loiasis proteins are likely to be secreted [22, 23]. In comparison, 246 of 500 (49%, 95% CI: 45%– 54%) randomly selected proteins from the L. loa genome are predicted to be secreted by these algorithms. Because SecretomeP and SignalP were designed to predict mammalian, not filarial, protein secretion, we also compared the proteins identified in our screen to the well-characterized B. malayi secretome [26–28]. One hundred five of the 220 L. loa proteins we identified have clear B. malayi homologs. Eighteen (17%) of these were present in the B. malayi secretome, twelve of which are reportedly secreted by Mf, but not by adult worms. Nearly all (92 of 105, 88%) of the B. malayi homologues show evidence of expression in the B. malayi proteome, and the 71 of 92 (77%), are expressed in all stages examined [29]. Taken together, these analyses suggest that most of proteins detected by our screen in loiasis sera are not secreted, and that their source may be either Mf or adult worms (or both).
Since our purification strategy involved capturing with a monoclonal antibody specific for the AD12 carbohydrate epitope, we examined the predicted glycosylation status of the L. loa proteins identified in human serum samples. Eighty-three percent were predicted by NetOGlyc 4.0 [20] to have O-linked glycosylation sites, compared to 77% (95% CI: 74%– 82%) of a random set of 500 L. loa proteins. NetNGlyc 1.0 software [21] predicted that 47% of the L. loa proteins we identified may have N-linked glycosylation, compared to 18% (95% CI: 14%–21%) of the randomly selected L. loa proteins. Thus, the majority of proteins identified in our screen were, as expected, predicted to be glycoproteins. The absence of predicted glycosylation sites on a minority (20/220, 9%) of the identified proteins suggests that a fraction of the proteins we identified are not decorated with the AD12 glycan epitope.
Analysis of excretory-secretory products from cultured L. loa worms
Both L. loa Mf and explanted L5 (adult) worms cultured ex vivo secrete antigens that cross-react with W. bancrofti RDTs [15]. To identify these secreted antigens and to determine if they were among those present in patient sera, we examined the excretory/secretory (ES) products from adult female worms and from Mf cultured in vitro. Using DH6.5-conjugated beads to capture AD12 epitope-containing antigens, we detected several AD12-reactive antigens in the culture supernatants of adult female worms by western blot, but none were detected in Mf supernatants (Fig 4). LC MS/MS analysis identified four L. loa proteins in the adult (L5) supernatants that met the pre-specified requirement of having at least two unique peptides detected. Only one of these proteins, the L. loa ortholog of filarial antigen Av33, was also detected in loiasis sera (Table 5).
Fig 4. L. loa secretes glycoproteins reactive with monoclonal antibody AD12.
Western blot of AD12 glycoproteins immunoaffinity purified from 1mL of culture supernatant (ES products) from L. loa adult worms (L5) or microfilaria (Mf). Soluble L. loa antigen (Loa Ag, 0.5 μg) served as a positive control.
Table 5. Proteins identified in L5 stage L. loa excretory/secretory products.
Discussion
Reactivity of circulating L. loa antigens with W. bancrofti RDTs is an impediment to successful LF elimination in loiasis-endemic areas in Central Africa, because a CFA prevalence of 1% or higher is considered to be evidence that the area is endemic for LF and should receive MDA [30]. In this study we sought to identify L. loa antigens that are responsible for false-positive results in LF RDTs. This work led to several unexpected observations. First, RDT cross-reactivity was absent in many persons previously ICT-positive despite persistence of high L. loa microfilarial loads. The mechanism of loss of cross-reactivity is unclear, but it does not appear to be due to masking of the AD12 epitope by antibodies. It also seems unlikely that changes in the performance characteristics of the ICT were responsible; we contacted Alere and were assured no changes in the manufacturing process or quality control testing of the ICT were made between 2013 and 2016. Second, unlike bancroftian filariasis, where RDT positivity is associated with a single circulating filarial glycoprotein, cross-reactive loiasis sera contain multiple AD12 epitope-containing L. loa antigens. Most of these antigens are not predicted to be secreted and are related to intracellular processes and structures. In addition, most are not specific to any particular stage of the filarial lifecycle. Only one of the L. loa antigens identified in cross-reactive sera was also detected in excretory/secretory products of ex vivo cultured L. loa adults.
These results suggest that the release of cross-reactive L. loa antigens occurs by a process or processes quite different from that of the W. bancrofti CFA [5]. We believe that the most likely mechanism for loiasis cross-reactivity with W. bancrofti RDTs is death of L. loa Mf and/or adult worms associated with release of multiple AD12 glycoproteins into circulation and that imbalance between release and clearance of these antigens accounts for their detection in the circulation of some loiasis patients. It is known that not all loiasis patients with active infection (as indicated by the presence of eye worms) have circulating L. loa Mf, and this is believed to be due to immune clearance of Mf. Perhaps immune-mediated clearance of Mf results in intermittent release of AD12 epitope-containing antigens. Another explanation would be that the antigens are released by the intermittent death of adult worms. Either of these mechanisms would be consistent with the observation that although there is a correlation between microfilarial load and RDT-positivity, not all persons with cross-reactive RDTs due to loiasis have detectable microfilaremia [7].
It is important to recognize that while the ICT and FTS rapid diagnostic tests detect a specific high molecular weight W. bancrofti glycoprotein in serum samples from persons infected with W. bancrofti, they do so by recognizing a carbohydrate epitope (the AD12 epitope) that is not unique to W. bancrofti. This epitope is present on multiple proteins in lysates of Dirofilaria and Brugia [5, 6]. While AD12 epitope-containing proteins are present in somatic antigen preparations and ES products of L. loa Mf and L3 larval stages [15], the developmental stage from which the cross-reactive antigens in serum originate remains unclear. Our set of 220 L. loa proteins resembles microfilarial or immature uterine expression signatures based on number of matches and total spectral counts from a stage specific study of B. malayi [29] and corresponds well with a L. loa Mf transcription analysis [18]. It seems likely that at least some of the proteins we detected are of microfilarial origin, which contrasts with Bancroftian filariasis in which the dominant circulating W. bancrofti antigen is produced primarily by adult worms [5, 31].
Several prior studies have reported circulating L. loa antigens in the blood [32–34] and urine [35] of persons with loiasis. Our study differed in that we sought to specifically capture the antigens responsible for W. bancrofti RDT cross-reactivity. It is not surprising, therefore, that our approach identified different circulating L. loa antigens compared to prior studies. For example, out of the 18 L. loa proteins identified by Drame et al. in urine from one L. loa microfilaremic individual, only two, peptidase M16 inactive domain-containing protein (LOAG_04876) and pyruvate kinase (LOAG_17249), were also found in our analysis [35].
Our study had some significant limitations. First, the difficulty of obtaining cross-reactive sera and the need to pool the samples that were not strongly positive has resulted in characterization of only two samples. Whether these findings are representative of all persons with W. bancrofti RDT cross-reactivity due to loiasis will require further study. Second, since all the sera we analyzed were from persons co-infected with M. perstans, it is impossible to rule out detection of some Mansonella proteins in our analyses. It seems unlikely, however, that cross-reactive Mansonella proteins were prominent, since M. perstans Mf counts were 2.5 to 92 times lower than L. loa Mf counts in the samples we analyzed (see Table 2). Furthermore, prior studies found no correlation between M. perstans microfilariemia and ICT or FTS positivity among patients with loiasis [7–9]. It would be interesting to see how many of the proteins identified in our MS analysis might also be part of the M. perstans proteome; unfortunately, there is no M. perstans genome available to address this issue at this time.
Second, we cannot be certain which of the antigens we identified actually contribute to RDT cross-reactivity. The immunoprecipitation conditions we used to capture cross-reactive antigen (4°C rocking overnight) differ from the RDTs (room temperature, 10 minutes). We chose overnight incubation in order to capture as many cross-reactive antigens as possible for downstream analysis. It is possible that shorter incubation times would capture fewer antigens. Additionally, because these antigens are glycoproteins, it is impossible to correlate the apparent MW, based on gel electrophoresis, with the proteins identified by shotgun mass spectrometry. Our data therefore identify L. loa antigens present in cross-reactive sera, but do not establish which antigens are necessary and/or sufficient to cause a cross-reactive RDT. The presence of a predominant ~80 kDa band in both the #811355 sera and the pooled sera suggests it may be a major contributor. Future studies with larger sample numbers will be necessary to address these questions.
One perplexing aspect of W. bancrofti RDT positivity in loiasis is that only a small percentage of samples from persons with loiasis cross-react. Our Akonolinga and Awae field study adds another piece to this puzzle, since it demonstrates that loiasis cross-reactivity may vary over time despite persistence of microfilaremia. As mentioned above, we feel the most plausible explanation for this is an imbalance between the intermittent release of L. loa antigens and the capacity of the host to clear these antigens. The number and varied intracellular locations and diverse functions of the antigens detected in cross-reactive sera suggest that the antigens are coming from dead or dying parasites and not being excreted or secreted from living worms.
In conclusion, this study has provided new information on cross-reactivity of CFA tests for LF in persons with loiasis. Prospective studies examining the duration of RDT cross-reactivity in persons with loiasis (and the factors associated with its development and resolution) may help to clear up unresolved issues. Our results also demonstrate a method for distinguishing between lymphatic filariasis and loiasis based on patterns of AD12-reactive antigens in human sera revealed by western blot. Additional research will be required to develop improved tests for the specific diagnosis of both of these infections.
Supporting information
(XLSX)
(XLSX)
Acknowledgments
We would like to thank the members of the Weil, Townsend and Mitreva labs for advice and thoughtful discussion. This study made use of the NIH / NIGMS Biomedical Mass Spectrometry Resource at Washington University in St. Louis, MO.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study made use of the National Institute of Health/National Institute of General Medical Sciences (NIH / NIGMS) Biomedical Mass Spectrometry Resource at Washington University in St. Louis, MO, which is supported by National Institutes of Health / National Institute of General Medical Sciences Grant # 8P41GM103422. This work was supported by National Institute for Allergy and Infectious Disease (NIAID) grant K08AI121422 (PJB), and by funds from Washington University in St. Louis (collection of Akonolinga/Awae samples) and an NIH Loan Repayment Programs (NIAID) award. Further support was provided by the Coalition for Operational Research on Neglected Tropical Diseases (COR-NTD, http://www.ntdsupport.org/cor-ntd, collection of East Region samples, awarded to SW and PUF) and the Bill and Melinda Gates Foundation (https://www.gatesfoundation.org/, grant OPPGH 5342 awarded to PUF and GW). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Hooper PJ, Chu BK, Mikhailov A, Ottesen EA, Bradley M. Assessing progress in reducing the at-risk population after 13 years of the global programme to eliminate lymphatic filariasis. PLoS Negl Trop Dis. 2014;8(11):e3333 10.1371/journal.pntd.0003333 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardon J, Gardon-Wendel N, Demanga N, Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350(9070):18–22. Epub 1997/07/05. 10.1016/S0140-6736(96)11094-1 . [DOI] [PubMed] [Google Scholar]
- 3.Weil GJ, Curtis KC, Fakoli L, Fischer K, Gankpala L, Lammie PJ, et al. Laboratory and field evaluation of a new rapid test for detecting Wuchereria bancrofti antigen in human blood. Am J Trop Med Hyg. 2013;89(1):11–5. Epub 2013/05/22. 10.4269/ajtmh.13-0089 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weil GJ, Lammie PJ, Weiss N. The ICT Filariasis Test: A rapid-format antigen test for diagnosis of bancroftian filariasis. Parasitol Today. 1997;13(10):401–4. . [DOI] [PubMed] [Google Scholar]
- 5.Weil GJ, Liftis F. Identification and partial characterization of a parasite antigen in sera from humans infected with Wuchereria bancrofti. J Immunol. 1987;138(9):3035–41. . [PubMed] [Google Scholar]
- 6.Weil GJ, Jain DC, Santhanam S, Malhotra A, Kumar H, Sethumadhavan KV, et al. A monoclonal antibody-based enzyme immunoassay for detecting parasite antigenemia in bancroftian filariasis. J Infect Dis. 1987;156(2):350–5. Epub 1987/08/01. . [DOI] [PubMed] [Google Scholar]
- 7.Bakajika DK, Nigo MM, Lotsima JP, Masikini GA, Fischer K, Lloyd MM, et al. Filarial antigenemia and Loa loa night blood microfilaremia in an area without bancroftian filariasis in the Democratic Republic of Congo. Am J Trop Med Hyg. 2014;91(6):1142–8. 10.4269/ajtmh.14-0358 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wanji S, Amvongo-Adjia N, Koudou B, Njouendou AJ, Chounna Ndongmo PW, Kengne-Ouafo JA, et al. Cross-Reactivity of Filariais ICT Cards in Areas of Contrasting Endemicity of Loa loa and Mansonella perstans in Cameroon: Implications for Shrinking of the Lymphatic Filariasis Map in the Central African Region. PLoS neglected tropical diseases. 2015;9(11):e0004184 10.1371/journal.pntd.0004184 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pion SD, Montavon C, Chesnais CB, Kamgno J, Wanji S, Klion AD, et al. Positivity of Antigen Tests Used for Diagnosis of Lymphatic Filariasis in Individuals Without Wuchereria bancrofti Infection But with High Loa loa Microfilaremia. Am J Trop Med Hyg. 2016;95(6):1417–23. 10.4269/ajtmh.16-0547 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao RU, Atkinson LJ, Ramzy RM, Helmy H, Farid HA, Bockarie MJ, et al. A real-time PCR-based assay for detection of Wuchereria bancrofti DNA in blood and mosquitoes. Am J Trop Med Hyg. 2006;74(5):826–32. Epub 2006/05/12. . [PMC free article] [PubMed] [Google Scholar]
- 11.Ismail MM, Weil GJ, Jayasinghe KS, Premaratne UN, Abeyewickreme W, Rajaratnam HN, et al. Prolonged clearance of microfilaraemia in patients with bancroftian filariasis after multiple high doses of ivermectin or diethylcarbamazine. Trans R Soc Trop Med Hyg. 1996;90(6):684–8. Epub 1996/11/01. . [DOI] [PubMed] [Google Scholar]
- 12.Thomsen EK, Sanuku N, Baea M, Satofan S, Maki E, Lombore B, et al. Efficacy, Safety, and Pharmacokinetics of Coadministered Diethylcarbamazine, Albendazole, and Ivermectin for Treatment of Bancroftian Filariasis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2016;62(3):334–41. Epub 2015/10/22. 10.1093/cid/civ882 . [DOI] [PubMed] [Google Scholar]
- 13.Gass K, Beau de Rochars MV, Boakye D, Bradley M, Fischer PU, Gyapong J, et al. A multicenter evaluation of diagnostic tools to define endpoints for programs to eliminate bancroftian filariasis. PLoS Negl Trop Dis. 2012;6(1):e1479 Epub 2012/01/25. 10.1371/journal.pntd.0001479 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fink DL, Kamgno J, Nutman TB. Rapid molecular assays for specific detection and quantitation of Loa loa microfilaremia. PLoS Negl Trop Dis. 2011;5(8):e1299 Epub 2011/09/14. 10.1371/journal.pntd.0001299 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wanji S, Amvongo-Adjia N, Njouendou AJ, Kengne-Ouafo JA, Ndongmo WP, Fombad FF, et al. Further evidence of the cross-reactivity of the Binax NOW(R) Filariasis ICT cards to non-Wuchereria bancrofti filariae: experimental studies with Loa loa and Onchocerca ochengi. Parasit Vectors. 2016;9:267 Epub 2016/05/07. 10.1186/s13071-016-1556-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nature methods. 2009;6(5):359–62. 10.1038/nmeth.1322 . [DOI] [PubMed] [Google Scholar]
- 17.Tallon LJ, Liu X, Bennuru S, Chibucos MC, Godinez A, Ott S, et al. Single molecule sequencing and genome assembly of a clinical specimen of Loa loa, the causative agent of loiasis. BMC Genomics. 2014;15:788 Epub 2014/09/14. 10.1186/1471-2164-15-788 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desjardins CA, Cerqueira GC, Goldberg JM, Dunning Hotopp JC, Haas BJ, Zucker J, et al. Genomics of Loa loa, a Wolbachia-free filarial parasite of humans. Nat Genet. 2013;45(5):495–500. Epub 2013/03/26. 10.1038/ng.2585 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gotz S, Garcia-Gomez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, et al. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36(10):3420–35. Epub 2008/05/01. 10.1093/nar/gkn176 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013;32(10):1478–88. Epub 2013/04/16. 10.1038/emboj.2013.79 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta R, Jung E, Brunak S. Prediction of N-glycosylation sites in human proteins. 2004.
- 22.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nature methods. 2011;8(10):785–6. Epub 2011/10/01. 10.1038/nmeth.1701 . [DOI] [PubMed] [Google Scholar]
- 23.Bendtsen JD, Jensen LJ, Blom N, Von Heijne G, Brunak S. Feature-based prediction of non-classical and leaderless protein secretion. Protein Eng Des Sel. 2004;17(4):349–56. Epub 2004/04/30. 10.1093/protein/gzh037 . [DOI] [PubMed] [Google Scholar]
- 24.Nana-Djeunga HC, Tchatchueng-Mbougua JB, Bopda J, Mbickmen-Tchana S, Elong-Kana N, Nnomzo’o E, et al. Mapping of Bancroftian Filariasis in Cameroon: Prospects for Elimination. PLoS Negl Trop Dis. 2015;9(9):e0004001 Epub 2015/09/10. 10.1371/journal.pntd.0004001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–6. Epub 2005/08/06. 10.1093/bioinformatics/bti610 . [DOI] [PubMed] [Google Scholar]
- 26.Hewitson JP, Harcus YM, Curwen RS, Dowle AA, Atmadja AK, Ashton PD, et al. The secretome of the filarial parasite, Brugia malayi: proteomic profile of adult excretory-secretory products. Mol Biochem Parasitol. 2008;160(1):8–21. Epub 2008/04/29. 10.1016/j.molbiopara.2008.02.007 . [DOI] [PubMed] [Google Scholar]
- 27.Moreno Y, Geary TG. Stage- and gender-specific proteomic analysis of Brugia malayi excretory-secretory products. PLoS Negl Trop Dis. 2008;2(10):e326 Epub 2008/10/30. 10.1371/journal.pntd.0000326 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennuru S, Semnani R, Meng Z, Ribeiro JM, Veenstra TD, Nutman TB. Brugia malayi excreted/secreted proteins at the host/parasite interface: stage- and gender-specific proteomic profiling. PLoS Negl Trop Dis. 2009;3(4):e410 Epub 2009/04/09. 10.1371/journal.pntd.0000410 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennuru S, Meng Z, Ribeiro JM, Semnani RT, Ghedin E, Chan K, et al. Stage-specific proteomic expression patterns of the human filarial parasite Brugia malayi and its endosymbiont Wolbachia. Proc Natl Acad Sci U S A. 2011;108(23):9649–54. Epub 2011/05/25. 10.1073/pnas.1011481108 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO. Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Geneva, Switzerland: WHO Press; 2006. [Google Scholar]
- 31.Chanteau S, Moulia-Pelat JP, Glaziou P, Nguyen NL, Luquiaud P, Plichart C, et al. Og4C3 circulating antigen: a marker of infection and adult worm burden in Wuchereria bancrofti filariasis. J Infect Dis. 1994;170(1):247–50. Epub 1994/07/01. . [DOI] [PubMed] [Google Scholar]
- 32.Jaoko WG. Loa loa antigen detection by ELISA: a new approach to diagnosis. East Afr Med J. 1995;72(3):176–9. Epub 1995/03/01. . [PubMed] [Google Scholar]
- 33.Walker-Deemin A, Kombila M, Mouray H, Rondi ML, Nguiri C, Richard-Lenoble D. Detection of circulating antigens in Gabonese patients with Loa loa filariasis. Trop Med Int Health. 1996;1(6):772–8. Epub 1996/12/01. . [DOI] [PubMed] [Google Scholar]
- 34.Walker-Deemin A, Ferrer A, Gauthier F, Kombila M, Richard-Lenoble D. Identification and specificity of a 38 kDa Loa loa antigenic fraction in sera from high-microfilaraemic Gabonese patients. Parasitol Res. 2004;92(2):128–32. Epub 2003/12/03. 10.1007/s00436-003-1024-1 . [DOI] [PubMed] [Google Scholar]
- 35.Drame PM, Meng Z, Bennuru S, Herrick JA, Veenstra TD, Nutman TB. Identification and Validation of Loa loa Microfilaria-Specific Biomarkers: a Rational Design Approach Using Proteomics and Novel Immunoassays. MBio. 2016;7(1):e02132–15. Epub 2016/02/18. 10.1128/mBio.02132-15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.