Abstract
Chronic hepatitis is the most common hepatic disease in dogs. Copper accumulation is an important cause of chronic hepatitis in dogs; however, the etiology in most dogs cannot be determined. Clinical signs of chronic hepatitis are often non-specific; therefore, this disease is frequently diagnosed in an advanced stage that makes successful intervention less likely. Early diagnosis of chronic hepatitis in dogs would thus be beneficial. The identification of proteins that are differentially expressed in dogs with chronic hepatitis could contribute to the development of novel diagnostic markers for this disease and provide insight into its pathogenesis. The objective of this study was to identify novel proteins that are differentially expressed in the liver of dogs with chronic hepatitis. Hepatic tissue was collected from 8 healthy dogs during ovariohysterectomy and from 8 dogs with histologically confirmed chronic hepatitis. The proteome of the liver samples was extracted by mechanical disruption and detergent-based cell lysis and differentially labeled prior to analysis by 2-dimensional fluorescence difference gel electrophoresis. Spots with an absolute fold change value > 2.0 were selected for further analysis. Protein identification was achieved by nanoflow liquid chromatography tandem mass spectrometry. Differential expression of select proteins was validated by Western blot. Five protein spots were differentially expressed between patients with chronic hepatitis and healthy control dogs. From these 5 protein spots 11 proteins were identified. Differential expression of cytokeratin 18 and annexin 5 were confirmed by Western blot analysis. Differential protein expression was shown between dogs with chronic hepatitis and healthy control dogs. Upregulation of cytokeratin 18 in chronic hepatitis may suggest increased hepatocellular apoptosis and necrosis, whereas upregulation of annexin 5A suggests increased hepatocellular apoptosis. Further studies are needed to determine whether either protein has diagnostic utility.
Introduction
Chronic hepatitis is the most common type of liver disease in dogs with chronic hepatitis being the most frequently observed form [1]. Chronic hepatitis is associated with apoptosis/necrosis and an inflammatory infiltrate that is often mononuclear. This results in a repair process that can lead to hepatic fibrosis including end-stage liver disease characterized by fibrosis of the liver and the conversion of normal liver architecture into abnormal liver nodules with portal-central vascular anastomoses [2]. End-stage hepatic disease carries a poor long term prognosis with a reported median survival time of 0.4 months [3,4]. Chronic hepatitis, in contrast diagnosed at an earlier stage carries a reported median survival from diagnosis to death of 18.3–36.4 months [3,4]. Cases of chronic hepatitis are often diagnosed at an advanced stage where treatment is less likely to be effective and thus early identification of dogs with chronic hepatitis and hepatic fibrosis, ideally before clinical signs develop, would be beneficial.
The histological evaluation of a liver biopsy specimen continues to be the gold standard for the assessment of hepatocellular necrosis, inflammation, fibrosis, and for a definitive diagnosis of chronic hepatitis. The current lack of robust biomarkers for canine chronic hepatitis restricts the non-invasive evaluation of this disease. A number of non-invasive biomarkers of hepatocellular injury have been identified and validated in dogs and humans. For example, the most widely used biomarker for hepatocellular injury is serum alanine aminotransferase activity [5]. Alanine aminotransferase is predominantly found in the liver with lower level enzyme activities found in skeletal and cardiac muscle. Serum enzyme activity increases are associated with either reversible or irreversible hepatocellular damage [6]. Serum alanine aminotransferase activity has a reported sensitivity for detecting histopathologic evidence of hepatocellular injury in symptomatic dogs that varies from 60% - 76% [7]. Little is known about the sensitivity of biomarkers including alanine aminotransferase to detect hepatobiliary injury in the subclinical disease phase. A recent study by Dirksen et al. found that serum alanine aminotransferase and alkaline phosphatase activities and serum bile acid concentrations have a sensitivity for detecting histologic evidence of copper-associated chronic hepatitis in the subclinical phase of 71%, 35%, and 13% respectively [8]. Additionally, serum ALT activity can also be increased due to extrahepatic diseases such as pancreatitis, enteritis, and endocrinopathies [1]. Therefore, a more sensitive and specific marker of hepatocellular injury in dogs with chronic hepatitis would be beneficial. There is also an unmet need for novel non-invasive diagnostic tests for hepatic fibrosis, hepatic function, and hepatic neoplasia in dogs.
Proteomics is the large-scale characterization of the protein complement of a cell, tissue, or organism and has emerged as a powerful tool in biomarker discovery. Chronic inflammatory hepatobiliary disease has been demonstrated to result in changes to proteins found in hepatic tissues in humans [9]. There is one proteomic study of the canine liver to the authors knowledge. This study evaluated the soluble liver proteome from bull terriers affected with inherited lethal acrodermatitis [10]. Proteomic analysis of canine hepatic tissue from dogs with chronic hepatitis may lead to the identification of novel candidate biomarkers that could later be evaluated in serum or urine as well as having the potential to provide new insights into the pathogenesis of this disease.
The aim of this study was to identify proteins that are differentially expressed between hepatic tissue from dogs with chronic hepatitis and healthy control dogs. We hypothesized that dogs with chronic hepatitis will exhibit differential hepatic protein expression compared to healthy control dogs.
Materials and methods
Patients and procedures
Eight canine patients undergoing liver biopsy for routine diagnostic purposes that were histologically confirmed to have chronic hepatitis and 8 healthy control dogs undergoing ovariohysterectomy were enrolled at the Texas A&M University Veterinary Medical Teaching Hospital between September 2014 –August 2017. Informed client consent was obtained for each patient and the study was approved by the Texas A&M University Institutional Animal Care and Use Committee (Animal Use Protocol #2014–320 and 2015–0043). Patients with suspected chronic hepatitis underwent laparoscopic liver biopsy and 5 to 8, 5 mm closed cup forceps liver biopsy specimens were collected. The same 5 mm closed cup forceps were used to collect 3–4 liver biopsy specimens from healthy dogs at the time of ovariohysterectomy. One biopsy specimen, approximately 0.5 g of liver tissue from each patient was flash frozen in liquid nitrogen within 30 minutes of acquisition and stored at -80°C. One liver biopsy specimen was collected in a sterile red top tube for copper quantification by atomic adsorption spectroscopy (expressed as μg/g dry weight) and another was collected and stored in a sterile specimen cup for aerobic and anaerobic culture and susceptibility testing. The remaining liver tissue from dogs in each group was fixed in neutral buffered formalin for routine histological processing. Histological sections from formalin-fixed paraffin embedded tissues were stained with hematoxylin and eosin, picrosirius red, and rhodamine. The diagnosis of chronic hepatitis and healthy control were based on clinical signs, routine serum biochemistry panels, and histological assessment of liver specimens by a board-certified veterinary pathologist according to the World Small Animal Veterinary Association Liver Standardization Group Guidelines[11]. The stage of hepatic fibrosis, the grade of necroinflammatory activity, and the semiquantitative assessment of copper content were assessed using a previously published scoring scheme[2,12].
2-Dimensional fluorescence difference gel electrophoresis
Protein processing and labeling
Each liver biopsy specimen was placed in a 1.5 mL ground glass pestle and tube tissue grinder and homogenized in 10 mM Tris-HCL/1% CHAPS buffer. The overall protein concentration was determined by Bradford protein assay using bovine serum albumin as a standard. The proteome from each liver sample was precipitated with choloroform/methanol and dissolved in 1 mL of DIGE labeling buffer (30 mM Tris, 7 M urea, 2M Thiourea, 4% CHAPS, pH 8.5 buffer) [13]. Samples were fluorescently labeled by reacting 50 μg of protein with 200 pmol CyDye DIGE Fluors (GE Healthcare). One sample was labeled with Cy3 while the other was labeled with Cy5 to allow for differential labeling. A pooled sample containing equal amounts of each sample was labeled with Cy2. The labeling reactions were quenched with 10 mM lysine.
2- Dimensional difference gel electrophoresis
Cy2-, Cy3- and Cy5-labeled samples were mixed together, and rehydration buffer was added (7 M urea, 2 M thiourea, 4% CHAPS, 0.5% pharmalytes (GE Healthcare), 40 mM dithiothreitol (DTT), and 0.002% bromophenol blue) to a final volume of 450 μl and used to rehydrate an immobilized pH gradient strip (24 cm; pH 3–10NL; GE Healthcare) by passive diffusion at 20°C for 12h. Isoelectric focusing was performed on an IPG Phor 3 horizontal electrophoresis system (GE Healthcare) with a program of 0.5 kV for 1h, holding at 0.5 kV for 5 h, ramping to 1 kV over 1 h, ramping to 8 kV over 3 h, holding at 8 kV until 60 kV*h, and holding at 0.5 kV for 4 h. Each focused strip was then equilibrated in two steps: 15 minutes in a reducing equilibration buffer (6 M urea, 50 mM Tris-HCl, pH 8.8 with 30% (v/v) glycerol, 2% (w/v) SDS, 0.01 bromophenol, and 10 mg/mL DTT), followed by 15 minutes in an alkylating equilibration buffer where DTT was replaced by 25 mg/mL iodoacetamine. The equilibrated IPG strips were then placed directly on top of polymerized 12% SDS gels and sealed with an agarose sealing solution (25 mM Tris, 192 mM, glycine, 0.1% SDS, 0.5% (w/v) agarose, and 0.02% bromophenol blue). Gels were run in cooled tanks with DIGE buffer on an Ettan Dalt-6 (GE Healthcare) at 1 W per gel until the bromophenol blue dye front reached the bottom of the gel.
Image acquisition and analysis
Gel images were obtained on a Typhoon FLA 9500 (GE Healthcare) at an excitation wavelength of 473 nm for Cy2, 532 nm for Cy3, and 635 nm for Cy5 labeled samples. The resolution was set at 100 μm. DeCyder 2-D Differential Analysis Software (v 6.5, GE Healthcare) was utilized for data analysis. The software subtracts the background signal using a subtraction algorithm and quantifies spot intensity based on normalized spot volume. The normalization is ratiometric with Cy3 and Cy5 images being expressed as a ratio of the corresponding Cy2 internal standard protein spot. Further, DeCyder was also used to match spots between gels and determine significant changes in protein expression and the absolute fold change ratio. Absolute fold change ratio was derived from the normalized spot volume standardized against the intragel standard providing a measure of the magnitude of expression differences between identified spots. Protein spots present in the gels were matched using Decyder software from which a pick list was generated. Spot detection was verified visually. Statistical significance was determined by a two-sided student's t-test. Post-hoc testing was performed using Bonferroni’s multiple comparisons test and significance was set at q < 0.05. Proteins with an absolute fold change value > 2.0 between dogs with chronic hepatitis and healthy controls were selected for further analysis. All gels were fixed overnight in 10% methanol and 7.5% acetic acid.
Spot picking and protein processing
Ettan Spot Handling Workstations (GE Healthcare, Chicago, IL USA) were used to cut out the selected protein spots from a protein gel, perform an in-gel tryptic digestion with recombinant porcine trypsin (Promega, Madison, WI) and extract the peptides from the gel. Extracted trypsin peptides were concentrated by SpeedVac. Protein identification was achieved by nanoflow liquid chromatography tandem mass spectrometry. Subsequently, peptides from the MS were identified using the MASCOT search engine. The MASCOT program (v2.2) searched the mouse genome using the following parameters for protein identification: 1) one missed cleavage by trypsin; 2) monoisotopic peptide masses; 3) peptide mass tolerance of 1.2 Da; and 4) fragment mass tolerance of 0.8 Da. Further, oxidation of methionine (variable modification) and carbamidomethylation (fixed modification) of cysteine were taken into consideration by MASCOT in the protein identification. Peptides were matched to proteins at a minimum of two peptides. Protein identification was verified by Scaffold (Proteome Software, Portland, OR).
Western blotting
Select proteins (annexin A5 and cytokeratin 18) that were identified in spots that showed differential expression in hepatic tissue from dogs with chronic hepatitis (based on the 2D-DIGE results) were validated using standard Western blotting techniques. The following antibodies were used: annexin V (1:1000, rabbit polyclonal, Abcam, Cambridge, MA, USA) and cytokeratin 18 (1:20000, mouse monoclonal, Abcam, Cambridge, MA, USA). Liver samples were homogenized in 10 mM Tris-HCL/1% CHAPS buffer. The protein concentration was determined with the Bradford protein assay using a bovine serum albumin standard. Equal protein loading for each lane was confirmed by immunoblotting for anti-Lamin B1 (1:5000, rabbit monoclonal, Abcam, Cambridge, MA, USA). Protein samples were boiled following addition of Laemmli loading dye, separated on precast gels (BIO-RAD 8–16% Mini-PROTEAN TGX), and transferred to polyvinylidene difluoride (PVDF) membranes. PVDF membranes were blocked in 5% milk 0.05% Tween—Tris buffered saline (TTBS) overnight at 4°C, incubated with primary antibodies diluted in 5% milk 0.05% TTBS overnight at 4°C. Membranes were washed in 5% milk 0.05% TTBS and incubated with an HRP-conjugated anti-rabbit or goat IgG 1/20000 dilution in 5% milk 0.05% TTBS. PVDF membranes were washed in 5% milk 0.05% TTBS, incubated in ECL-plus, and the signal was detected using an Amersham Imager 600 (GE Healthcare, Chicago, IL USA). Densitometry was performed using the ImageQuantTM TL (GE Healthcare Life Sciences, Chicago, IL USA) software.
Statistical analysis
Differences between groups were analyzed for statistical significance by two-sided student's T-test. Post-hoc testing was performed using Bonferroni’s multiple comparisons test. Significance was set at q < 0.05.
Results
Dog demographics and clinical characteristics
The demographics and clinical characteristics for the dogs enrolled into the study are shown in Table 1.
Table 1. Demographics and clinical characteristics of dogs enrolled into the study.
| Patient | Disease | Agea | Sex | Breed | FIS | AS | HVS | CuS | Q Liver Cu | ALT | ALKP | TBILI | GGT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 227448 | Chronic Hepatitis | 12 | SF | Labrador Retriever | 4 | 2 | 1 | 1 | 667 | 466 | 557 | 0.2 | 16 |
| 229439 | Chronic Hepatitis | 6 | CM | Doberman Pincher | 3 | 1 | 0 | 2 | 196 | 989 | 283 | 0.3 | 12 |
| 203392 | Chronic Hepatitis | 9 | M | Labrador Retriever | 2 | 2 | 2 | 0 | 463 | 577 | 230 | 0.2 | <10 |
| 231640 | Chronic Hepatitis | 5 | M | Doberman Pincher | 2 | 2 | 2 | 3 | 3450 | 750 | 334 | 0.2 | 18 |
| 229216 | Chronic Hepatitis | 4 | SF | German Shorthaired | 1 | 2 | 1 | 2 | 1080 | 231 | 98 | 0.4 | <10 |
| 220269 | Chronic Hepatitis | 11 | SF | Labradoodle | 2 | 2 | 2 | 3 | 1230 | 663 | 179 | 0.3 | 22 |
| 175660 | Chronic Hepatitis | 14 | CM | Rat Terrier | 3 | 0 | 3 | 0 | 170 | 476 | 327 | <0.1 | 12 |
| 231108 | Chronic Hepatitis | 9 | F | Rottweiler Dog | 4 | 1 | 0 | 0 | 257 | 121 | 122 | 0.2 | 8 |
| 229461 | Healthy Control | 0.5 | F | Catahoula Hog Dog | 0 | 0 | 2 | 181 | |||||
| 211715 | Healthy Control | 6 | F | Greyhound Dog | 0 | 0 | 0 | 168 | |||||
| 227893 | Healthy Control | 1 | F | Chihuahua | 0 | 0 | 0 | 280 | |||||
| 229383 | Healthy Control | 5 | F | Am. Staffordshire Terrier | 0 | 0 | 2 | 153 | |||||
| 229057 | Healthy Control | 1 | F | Labrador Retriever | 0 | 0 | 1 | 124 | |||||
| 229382 | Healthy Control | 3 | F | Walker Coonhound | 0 | 0 | 0 | 96.3 | |||||
| 231847 | Healthy Control | 0.5 | F | Labrador Retriever | 0 | 0 | 0 | 316 | |||||
| 229270 | Healthy Control | 0.5 | F | Australian Shepherd | 0 | 0 | 0 | 158 |
aAge (years)
SF–Spayed female, M–Male, CM–Castrated male, F–Intact female, M–Intact male, FIS–Fibrosis score, AS–Activity score, HVS–Hepatocellular vacuolation score, CuS–Copper Score, Q Liver Cu–Quantitative liver copper, ALT–Alanine transaminotransferase, ALKP–Alkaline phosphatase, GGT–Gamma-glutamyl transferase
Liver biopsy specimens
All liver biopsies were assessed to be of adequate diagnostic quality with > 12 portal triads per biopsy specimen [14]. The liver biopsy specimens of control dogs were free from any remarkable histopathological changes.
Identification of candidate biomarkers for chronic hepatitis
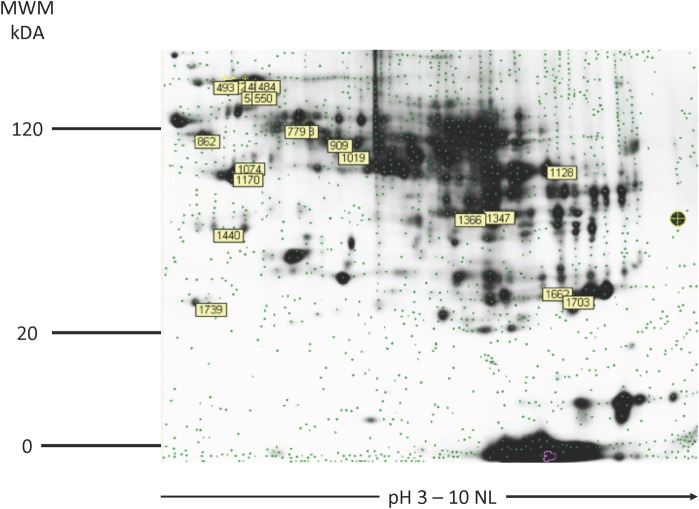
Liver tissue specimens from eight dogs with chronic hepatitis and eight healthy control dogs were adequate to show a reproducible liver proteome spot pattern of approximately 2,500 spots. Each spot could contain 1 or multiple proteins. Labeled spots were selected for analysis (Fig 1). Image analysis revealed 5 differentially expressed protein spots (q < 0.05 and fold change > 2.0) that were selected for protein identification.
Fig 1. 2-D gel image of the master gel with labeled picked proteins spots.
Proteome pattern of canine liver tissue by means of 2D –DIGE. For protein analysis, proteins were labeled with Cy2, Cy3, and Cy5, separated in the first dimension using an immobilized pH gradient, and subsequently, in the second dimension, by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Protein spots were detected using a fluorescence scanner (MWM–molecular weight marker; kDA–kilodalton, NL–nonlinear).
Altogether 5 differentially expressed protein spots contained 11 different proteins (Table 2). Spot 1440 showed a 3.18-fold increase in abundance (for chronic hepatitis dogs compared to healthy control dogs; q = 0.00095) and contained annexin 5, regucalin, and haptoglobin (Table 1). For protein disulfide–isomerase A4, from spot 472 a 2.33-fold increase was detected (q = 0.0000325). Additionally, we found a 2.24-fold increase in abundance of spot 779 that contained formimidoyltransferase-cyclodeaminase isoform X1, annexin A6, and UDP-glucose 6-dehydrogenase (q = 0.00033) (Table 2).
Table 2. Summary of proteins identified from differentially expressed spots.
| Spot | Identified Protein | GENE | GO Biological Process | Fold ▽ | Peptide No. | q Value | P Value |
|---|---|---|---|---|---|---|---|
| 1047 | Succinyl CoA | SUCLG1 | cellular metabolic process | 2.75 | 10.00 | 0.031 | 0.0062 |
| 1047 | Keratin, Type II Cytoskeletal 8 | KRT8 | hepatocyte apoptotic process | 2.75 | 6.00 | 0.031 | 0.0062 |
| 1047 | Keratin, Type I Cytoskeletal 18 | KRT18 | hepatocyte apoptotic process | 2.75 | 7.00 | 0.031 | 0.0062 |
| 1440 | Annexin A5 | ANXA5 | negative regulation of apoptotic process | 3.18 | 6.00 | 0.00095 | 0.00019 |
| 1440 | Regucalcin | RGN | L-ascorbic acid biosynthetic process | 3.18 | 8.00 | 0.00095 | 0.00019 |
| 1440 | Haptoglobin | HP | acute—phase response/antioxidant activity | 3.18 | 3.00 | 0.00095 | 0.00019 |
| 460 | Protein Disulfide-Isomerase A4 | PDIA4 | cell redox homeostasis | 2.24 | 3.00 | 0.00295 | 0.00059 |
| 460 | Gamma-glutamyltransferase 2 | TGM3 | protein tetramerization | 2.24 | 3.00 | 0.00295 | 0.00059 |
| 472 | Protein Disulfide-Isomerase A4 | PDIA4 | cell redox homeostasis | 2.33 | 3.00 | 3.25E-005 | 6.50E-006 |
| 779 | Formimidoyltransferase-cyclodeaminase | FTCD | cellular metabolic process | 2.24 | 7.00 | 3.30E-004 | 6.60E-005 |
| 779 | Annexin A6 | ANXA6 | apoptotic signaling pathway | 2.24 | 6.00 | 3.30E-004 | 6.60E-005 |
| 779 | UDP-glucose 6-dehydrogenase | UDGH | glycosaminoglycan biosynthetic process | 2.24 | 4.00 | 3.30E-004 | 6.60E-005 |
Analysis of cytokeratin 18 and annexin 5 in the liver of dogs with chronic hepatitis using immunoblotting
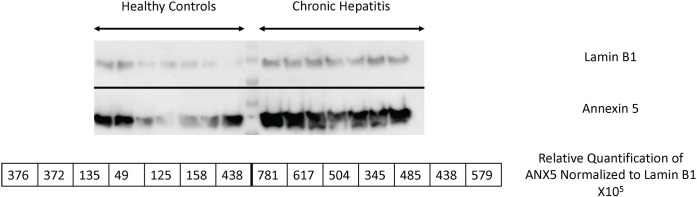
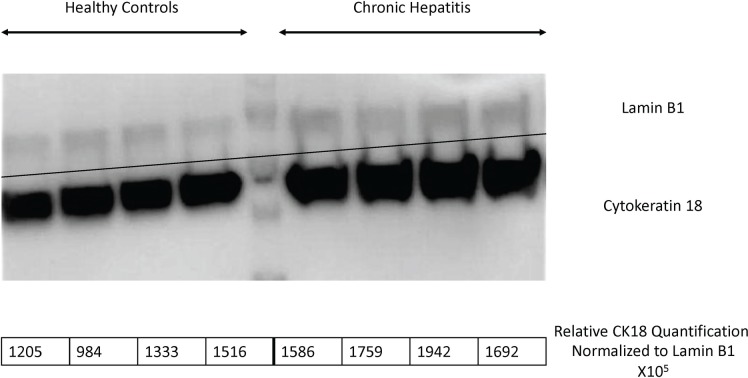
Two proteins were selected for Western blot analysis. The proteins, cytokeratin 18 and annexin 5 were confirmed to contribute to the increased relative abundance in the liver proteome of dogs with chronic hepatitis (n = 7, n = 4) compared to healthy controls (n = 7, n = 4), respectively (Table 2; Table 3; Fig 2 and Fig 3). Not all dogs were used to conserve the limited quantity of available protein.
Table 3. Patient allocation for Western blot analysis.
| Patient ID# | Disease | FIS | Agea | Sex | Breed | WB CK18 | WB ANX5 |
|---|---|---|---|---|---|---|---|
| 227448 | Chronic Hepatitis | 4 | 12 | SF | Labrador Retriever | X | |
| 229439 | Chronic Hepatitis | 3 | 6 | CM | Doberman Pincher | X | X |
| 203392 | Chronic Hepatitis | 2 | 9 | M | Labrador Retriever | X | X |
| 231640 | Chronic Hepatitis | 2 | 5 | M | Doberman Pincher | X | X |
| 229216 | Chronic Hepatitis | 1 | 4 | SF | German Shorthaired Pointer | X | |
| 220269 | Chronic Hepatitis | 2 | 11 | SF | Labradoodle | X | |
| 175660 | Chronic Hepatitis | 3 | 14 | CM | Rat Terrier | X | |
| 231108 | Chronic Hepatitis | 4 | 9 | F | Rottweiler Dog | X | |
| 229461 | Healthy | 0 | 1 | F | Catahoula Hog Dog | ||
| 211715 | Healthy | 0 | 6 | F | Greyhound Dog | X | X |
| 227893 | Healthy | 0 | 1 | F | Chihuahua | X | X |
| 227893 | Healthy | 0 | 5 | F | Am. Staffordshire Terrier | X | X |
| 229057 | Healthy | 0 | 1 | F | Labrador Retriever | X | |
| 229382 | Healthy | 0 | 3 | F | Treeing Walker Coonhound | X | X |
| 231847 | Healthy | 0 | 1 | F | Labrador Retriever | X | |
| 229382 | Healthy | 0 | 1 | F | Australian Shepherd | X |
aAge (years)
SF–Spayed female, M–Male, CM–Castrated male, F–Intact female, M–Intact male, FIS–Fibrosis score, WB–Western blot, CK18 –Cytokeratin 18, ANX5 –Annexin 5
Fig 2. Relative abundance of annexin 5 in the hepatic tissue of dogs with chronic hepatitis by Western blot analysis.
Annexin 5 was assessed in the liver proteome of 7 dogs with chronic hepatitis and compared to 7 healthy control dogs. Lamin was used as a loading control.
Fig 3. Abundance of cytokeratin 18 in the hepatic tissue of dogs with chronic hepatitis by Western blot analysis.
Cytokeratin 18 was assessed in the liver proteome of 4 dogs with chronic hepatitis and compared to 4 healthy control dogs. Lamin was used as a loading control.
Discussion
Our study utilized a gel-based, quantitative proteomics approach to identify differentially expressed proteins in the liver of dogs with chronic hepatitis compared to healthy control dogs. We detected 2,500 protein spots in the liver biopsy samples from dogs. We identified 5 protein spots that showed differential expression with an absolute fold change of at least 200% (2.00 absolute fold change) representing 11 proteins was significantly different between groups. These proteins warrant investigation as potential biomarkers of canine chronic hepatitis. Several of the identified proteins such as cytokeratin 18, cytokeratin 8, annexin 5, and annexin 6 are involved with hepatocyte apoptosis. Only cytokeratin 18 and annexin 5 were validated by Western blot in this study. All other identified proteins need to be validated to confirm these differences.
Hepatocellular apoptosis is a key event in the pathophysiology of many chronic liver diseases of dogs and humans and it is associated with liver fibrogenesis and the development of cirrhosis[15–18]. The effector caspases are activated in the final common pathway of apoptosis. Cytokeratin 18 is the major intermediate filament protein in the liver and the most prominent substrate of caspases during hepatocellular apoptosis[19]. Apoptotic cell death of hepatocytes is associated with release of caspase‐cleaved CK18 fragments in the bloodstream and in contrast, the cytosolic pool of uncleaved CK18 is released from hepatocytes during necrosis[20]. Multiple studies have assessed human serum or plasma CK18 concentrations and suggested the potential use of CK18 fragments as a noninvasive biomarker for the diagnosis or staging of chronic liver disease[21]. Furthermore, several studies of nonalcoholic fatty liver disease in humans have demonstrated that caspase activation and liver cell apoptosis were prominent features of this disease. Additionally, the degree of apoptosis correlated with the severity of hepatitis and the stage of fibrosis[22]. Serum CK18 is a marker of hepatocyte apoptosis in humans and correlates with histologic activity more accurately than serum alanine aminotransferase[23]. Annexin 5A (ANXA5) is a negatively charged phospholipid-binding protein that is widely used as a marker of apoptosis. Annexin 5A binds to negatively charged phospholipids, such as phosphatidylserine that are exposed on the cell surface during apoptosis[24,25]. Increased hepatic annexin 5A expression could therefore indicate ongoing apoptosis. Annexin A5 and CK18 have not been previously evaluated in canine liver disease. The apoptotic pathway may be altered in canine chronic hepatitis and other proteins in this pathway should be further investigated in a targeted study.
This is the first study to evaluate the liver proteome of dogs with chronic hepatitis. However, limitations of our study include the heterogeneity of the dogs in the chronic hepatitis group with one copper-associated chronic hepatitis case and 7 idiopathic chronic hepatitis cases. Examining specific subpopulations of dogs with chronic hepatitis may be advantageous. Another limitation is the fact that we only looked at proteins with an absolute fold change of > 2.0 between groups to avoid false discovery, which eliminated a number of proteins from further analysis. Another limitation is the use of only female dogs as a control group; however, the impact of this limitation is unknown. The healthy control group dogs were significantly younger than the chronic hepatitis dogs due to selection bias. The impact of this limitation is unknown, although, age-related changes in the liver proteome have been described in rats[26]. Finally, this initial study has a limited sample size that could have led to type II error. Further work that includes measurement in blood or urine and validation in a diverse clinical population will be required to establish whether the identified proteins have any diagnostic utility.
Proteomic evaluation of liver tissue from dogs with chronic hepatitis demonstrated differential protein expression compared to healthy controls. The increased expression of two proteins involved in the apoptotic pathway, cytokeratin 18 and annexin 5 was confirmed with Western blotting in these tissues. Further work will be required to establish whether these proteins have diagnostic utility over currently available biomarkers.
Supporting information
Annexin 5 was assessed in the liver proteome of 7 dogs with chronic hepatitis compared to 7 healthy controls. Lamin was used as a loading control. The unedited image is provided as a supplemental figure.
(TIFF)
Cytokeratin 18 was assessed in the liver proteome of 4 dogs with chronic hepatitis compared to 4 healthy controls. Lamin was used as a loading control. The unedited image is provided as a supplemental figure.
(TIFF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Poldervaart JH, Favier RP, Penning LC, van den Ingh TSGAM, Rothuizen J. Primary hepatitis in dogs: a retrospective review (2002–2006). J Vet Intern Med. 2009;23: 72–80. 10.1111/j.1939-1676.2008.0215.x [DOI] [PubMed] [Google Scholar]
- 2.Cullen JM, van den Ingh T, Bunch SE, Rothuizen J. WSAVA Standards for clinical and histological diagnosis of canine and feline liver disease. 2006. [Google Scholar]
- 3.Sevelius E. and Diagnosis prognosis of chronic hepatitis and cirrhosis in dogs. Journal of Small Animal Practice. 1995;36: 521–528. 10.1111/j.1748-5827.1995.tb02801.x [DOI] [PubMed] [Google Scholar]
- 4.Raffan E, McCallum A, Scase TJ, Watson PJ. Ascites is a negative prognostic indicator in chronic hepatitis in dogs. J Vet Intern Med. 2009;23: 63–66. 10.1111/j.1939-1676.2008.0230.x [DOI] [PubMed] [Google Scholar]
- 5.Dirksen K, Verzijl T, van den Ingh TSGAM, Vernooij JCM, van der Laan LJW, Burgener IA, et al. Hepatocyte-derived microRNAs as sensitive serum biomarkers of hepatocellular injury in Labrador retrievers. TVJ. W.B. 2016;211: 75–81. 10.1016/j.tvjl.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 6.Lawrence YA, Steiner JM. Laboratory Evaluation of the Liver. Veterinary Clinics: Small Animal Practice. Elsevier; 2017;47: 539–553. 10.1016/j.cvsm.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 7.Center SA. Interpretation of Liver Enzymes. Veterinary Clinics: Small Animal Practice. Elsevier; 2007;37: 297–333. 10.1016/j.cvsm.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 8.Dirksen K, Burgener IA, Rothuizen J, van den Ingh TSGAM, Penning LC, Spee B, et al. Sensitivity and specificity of plasma ALT, ALP, and bile acids for hepatitis in labrador retrievers. J Vet Intern Med. 2017;31: 1017–1027. 10.1111/jvim.14716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mölleken C, Sitek B, Henkel C, Poschmann G, Sipos B, Wiese S, et al. Detection of novel biomarkers of liver cirrhosis by proteomic analysis. Hepatology. 2008;49: 1257–1266. 10.1002/hep.22764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grider A, Mouat MF, Mauldin EA, Casal ML. Analysis of the liver soluble proteome from bull terriers affected with inherited lethal acrodermatitis. MGM. 2007;92: 249–257. 10.1016/j.ymgme.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Administration FAD. Guidance for industry: bioanalytical method validation US Department of Health and Human Services, Washington, DC: 2001. [Google Scholar]
- 12.van den Ingh TSGAM, Rothuizen J, Cupery R. Chronic active hepatitis with cirrhosis in the Doberman Pinscher. Veterinary Quarterly. 2011;10: 84–89. 10.1080/01652176.1988.9694154 [DOI] [PubMed] [Google Scholar]
- 13.Wessel D, Flügge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Analytical Biochemistry. 1984;138: 141–143. 10.1016/0003-2697(84)90782-6 [DOI] [PubMed] [Google Scholar]
- 14.Kemp SD, Zimmerman KL, Panciera DL, Monroe WE, Leib MS, Lanz OI. A comparison of liver sampling techniques in dogs. J Vet Intern Med. 2015;29: 51–57. 10.1111/jvim.12508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribeiro PS, Cortez-Pinto H, Solá S, Castro RE, Ramalho RM, Baptista A, et al. Hepatocyte apoptosis, expression of death receptors, and activation of NF-κB in the liver of nonalcoholic and alcoholic steatohepatitis patients. The American Journal of Gastroenterology 2004;99: 1708–1717. 10.1111/j.1572-0241.2004.40009.x [DOI] [PubMed] [Google Scholar]
- 16.Bexfield NH, Andres-Abdo C, Scase TJ, Constantino-Casas F, Watson PJ. Chronic hepatitis in the English springer spaniel: clinical presentation, histological description and outcome. Veterinary Record. 2011;169: 415–415. 10.1136/vr.d4665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thornburg LP. Histomorphological and immunohistochemical studies of chronic active hepatitis in doberman pinschers. Vet Pathol. 2016;35: 380–385. 10.1177/030098589803500507 [DOI] [PubMed] [Google Scholar]
- 18.Bantel H, Schulze-Osthoff K. Apoptosis in hepatitis C virus infection. Cell Death and Differentiation 2003. 10.1038/sj.cdd.4401119 [DOI] [PubMed] [Google Scholar]
- 19.Linder S, Havelka AM, Ueno T, Shoshan MC. Determining tumor apoptosis and necrosis in patient serum using cytokeratin 18 as a biomarker. Cancer Letters. 2004;214: 1–9. 10.1016/j.canlet.2004.06.032 [DOI] [PubMed] [Google Scholar]
- 20.Leers MPG, Kölgen W, Björklund V, Bergman T, Tribbick G, Persson B, et al. Immunocytochemical detection and mapping of a cytokeratin 18 neo‐epitope exposed during early apoptosis. The Journal of Pathology. 1999;187: 567–572. 10.1002/(SICI)1096-9896(199904)187:5<567::AID-PATH288>3.0.CO;2-J [DOI] [PubMed] [Google Scholar]
- 21.YILMAZ Y. Systematic review: caspase‐cleaved fragments of cytokeratin 18 –the promises and challenges of a biomarker for chronic liver disease. Alimentary Pharmacology & Therapeutics. 2009;30: 1103–1109. 10.1111/j.1365-2036.2009.04148.x [DOI] [PubMed] [Google Scholar]
- 22.Lebensztejn DM, Wierzbicka A, Socha P, Pronicki M, Skiba E, Werpachowska I, et al. Cytokeratin-18 and hyaluronic acid levels predict liver fibrosis in children with non-alcoholic fatty liver disease. Acta Biochimica Polonica. 2011;58. [PubMed] [Google Scholar]
- 23.Tsutsui M, Tanaka N, Kawakubo M, Sheena Y, Horiuchi A, Komatsu M, et al. Serum fragmented cytokeratin 18 levels reflect the histologic activity score of nonalcoholic fatty liver disease more accurately than serum alanine aminotransferase levels. Journal of Clinical Gastroenterology. 2010;44: 440 10.1097/MCG.0b013e3181bdefe2 [DOI] [PubMed] [Google Scholar]
- 24.Hawkins TE, Das D, Young B, Moss SE. DT40 cells lacking the Ca2+-binding protein annexin 5 are resistant to Ca2+-dependent apoptosis. PNAS. 2002;99: 8054–8059. 10.1073/pnas.132598099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayes MJ, Moss SE. Annexins and disease. Biochemical and Biophysical Research Communications. 2004;322: 1166–1170. 10.1016/j.bbrc.2004.07.124 [DOI] [PubMed] [Google Scholar]
- 26.Ori A, Toyama BH, Harris MS, Bock T, Iskar M, Bork P, et al. Integrated transcriptome and proteome analyses reveal organ-specific proteome deterioration in old rats. Cell Systems. 2015;1: 224–237. 10.1016/j.cels.2015.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Annexin 5 was assessed in the liver proteome of 7 dogs with chronic hepatitis compared to 7 healthy controls. Lamin was used as a loading control. The unedited image is provided as a supplemental figure.
(TIFF)
Cytokeratin 18 was assessed in the liver proteome of 4 dogs with chronic hepatitis compared to 4 healthy controls. Lamin was used as a loading control. The unedited image is provided as a supplemental figure.
(TIFF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.