Abstract
Three crude oligosaccharides were respectively prepared by acid hydrolysis of three polysaccharides, which were water-extracted polysaccharide (WEP), sodium hydroxide-extracted polysaccharide (SEP) and acid-extracted polysaccharide (AEP) from the rhizomes of Dioscorea zingiberensis. Among the three oligosaccharides, the crude oligosaccharide prepared by acid hydrolysis of WEP was found to be the most efficient elicitor to enhance the production of palmarumycins C12 and C13 in liquid culture of endophytic fungus Berkleasmium sp. Dzf12. When OW was applied to the medium at 300 mg/L on day 3 of culture, the maximal yields of palmarumycin C12 (87.96 mg/L) and palmarumycin C13 (422.28 mg/L) were achieved on day 15 of culture, which were 9.83 and 3.24-fold in comparison with those (8.95 and 130.43 mg/L) of control, respectively. The results indicate that addition of the oligosaccharides from the host plant D. zingiberensis should be an effective strategy for enhancing production of palmarumycins C12 and C13 in liquid culture of endophytic fungus Berkleasmium sp. Dzf12.
Keywords: endophytic fungus, Berkleasmium sp. Dzf12, spirobisnaphthalene, palmarumycin C12, palmarumycin C13, crude oligosaccharide, polysaccharide, elicitation, Dioscorea zingiberensis
Abbreviations
- OW
crude oligosaccharide prepared by acid hydrolysis of the water-extracted polysaccharide
- OS
crude oligosaccharide prepared by acid hydrolysis of the sodium hydroxide-extracted polysaccharide
- OA
crude oligosaccharide prepared by acid hydrolysis of the acid-extracted polysaccharide
- WEP
water-extracted polysaccharide
- SEP
sodium hydroxide-extracted polysaccharide
- AEP
acid-extracted polysaccharide
- TFA
trifluoroacetic acid
1. Introduction
Plant endophytic fungi, which inhabit normal tissues of host plants without causing apparent symptoms of pathogenesis [1], are novel and rich sources of bioactive natural products [2,3,4,5]. In connection with our ongoing search for bioactive metabolites from an endophytic fungus Berkleasmium sp. Dzf12, isolated from the healthy rhizomes of the medicinal plant Dioscorea zingiberensis, five spirobisnaphthalenes have been successfully obtained that show antimicrobial activity [6]. Spirobisnaphthalenes are a group of fungal secondary metabolites, consisting of 1,8-dihydroxynaphthalene-derived spiroketal units linked to a second oxidized naphthalene moiety, that show a great variety of biological activities such as antitumor, antibacterial, antifungal, antileishmanial, enzyme-inhibitory, and other properties that suggest potential applications in agriculture, medicine and the food industry [7,8]. Among the spirobisnaphthalenes isolated from Berkleasmium sp. Dzf12, both palmarumycins C12 and C13 were found to be the predominant components. Besides our previous study [6], palmarumycin C12 has also been isolated from the fungi Coniothyrium sp. [9] and Sphaeropsidales sp. (F-24'707, Cladosporium chlorocephalum) [10]. Palmarumycin C12 showed antifungal activity on Ustilago violacea and Eurotium repens [9]. Palmarumycin C13 (also variously named Sch 53514, diepoxin ζ and cladospirone bisepoxide) has also been isolated from other fungi such as Coniothyrium sp. [9], a non-sporulating fungus LL-07F275 [11], Nattrassia mangiferae [12], Cladosporium chlorocephalum (F-24'707) [10,13,14,15]. Palmarumycin C13 exhibited obvious antibacterial and antifungal [6,11], anti-tumor activity and inhibitory activity on phospholipase D (PLD) [12].
In order to speed up application of palmarumycins C12 and C13, one of the most important approaches is to increase yields of palmarumycins C12 and C13 in fermentation culture of Berkleasmium sp. Dzf12. Many strategies have been developed to increase metabolite yield in microorganism or plant cultures, which include optimization of medium, utilization of two-phase culture systems, addition of precursors, as well as application of elicitation [16,17]. Among these, elicitation has been regarded as a convenient and effective approach [18,19,20]. The use of carbohydrates (i.e., polysaccharides and oligosaccharides) for enhancing the production of secondary metabolites in plant or fungal cultures is promising and could have potential applications [21,22,23]. In fungal cultures, addition of the oligosaccharides prepared from sodium alginate by partial acid hydrolysis to Penicillium chrysogenum cultures could increase the production of penicillin G and its biosynthetic intermediates [24]. In addition, changes in microbial cell morphology, sporulation, production of pigments and transcriptional activity of the penicillin biosynthesis genes (i.e., pcbAB, pcbC, and penDE) have also been reported in response to the addition of oligosaccharide elicitors [25,26]. In our previous study, an obvious enhancement of palmarumycin C13 accumulation was elicited in liquid culture of Berkleasmium sp. Dzf12 by water-extracted polysaccharide (WEP) at 400 mg/L, sodium hydroxide-extracted polysaccharide (SEP) at 100 mg/L, and acid-extracted polysaccharide (AEP) at 400 mg/L, which were prepared from the rhizomes of the host plant D. zingiberensis [27]. In this work, three crude oligosaccharides, namely OW, OS and OA, were respectively prepared by acid hydrolysis of three polysaccharides from the rhizomes of D. zingiberensis, which were WEP, SEP and AEP. The enhancing effects of the oligosaccharides on secondary metabolite production of Berkleasmium sp. Dzf12 were compared. To the best of our knowledge, there are no previous reports on the effect of the oligosaccharides as elicitors from the host plant on the accumulation of secondary metabolites in its endophytic fungus. The purpose of this study was to investigate the effects of oligosaccharides on palmarumycins C12 and C13 production in liquid culture of the endophytic fungus Berkleasmium sp. Dzf12. Secondly, it was to investigate whether these oligosaccharides have better stimulating effect on palmarumycin production than the precursor polysaccharides.
2. Results and Discussion
2.1. Effects of Oligosaccharides OW, OS and OA on Mycelia Growth and Palmarumycin Production
The optimal concentrations of oligosaccharide elicitors were determined based on their effects on palmarumycin production. The addition time of oligosaccharides was determined on day 3 of culture according to the growth curves of Berkeasmium sp. Dzf12 in the liquid culture [21].
Table 1.
Effects of the oligosaccharides OW, OS and OA on mycelia growth and palmarumycins C12 and C13 production in liquid culture of Berkleasmium sp. Dzf12.
| Oligo. Conc. (mg/L) | Mycelia biomass (g dw/L) | C12 content in mycelia (mg/g dw) | C13 content in mycelia (mg/g dw) | C13 yield in broth (mg/L) | C12 plus C13 yield (mg/L) | |
|---|---|---|---|---|---|---|
| CK | 0 | 6.84 ± 0.26 e | 1.31 ± 0.08 h | 3.24 ± 0.18 f | 108.28 ± 6.00 e | 139.45 ± 5.20 g |
| OW | 200 | 11.48 ± 0.26 b | 5.40 ± 0.24 c | 8.21 ± 0.21 b | 229.06 ± 6.62 b | 385.28 ± 8.32 b |
| 400 | 13.90 ± 0.86 a | 6.20 ± 0.31 b | 9.92 ± 0.18 a | 245.40 ± 4.69 a | 469.33 ± 9.43 a | |
| 600 | 14.06 ± 0.25 a | 4.95 ± 0.18 d | 6.50 ± 0.32 c | 193.78 ± 10.41c | 369.53 ± 16.80 b | |
| OS | 100 | 8.69 ± 0.26 d | 2.42 ± 0.14 g | 4.21 ± 0.14 e | 169.23 ± 7.01 d | 226.90 ± 8.35 f |
| 200 | 9.24 ± 0.29 cd | 5.09 ± 0.19 cd | 6.88 ± 0.06 c | 232.16 ± 9.47 ab | 340.96 ± 14.79 c | |
| 400 | 9.55 ± 0.47 c | 8.06 ± 0.30 a | 5.22 ± 0.06 d | 179.05 ± 7.21 d | 305.83 ± 8.69 d | |
| OA | 200 | 8.80 ± 0.24 d | 2.63 ± 0.10 fg | 4.33 ± 0.16 e | 176.69 ± 8.76 d | 237.89 ± 9.33 f |
| 400 | 9.32 ± 0.37 cd | 3.44 ± 0.17 e | 6.29 ± 0.35 c | 241.72 ± 7.16 ab | 332.28 ± 5.92 c | |
| 600 | 9.82 ± 0.30 c | 2.93 ± 0.15 f | 5.15 ± 0.12 d | 205.61 ± 9.00 c | 283.56 ± 10.75 e | |
Note: CK, a control without addition of oligosaccharide; Oligo., oligosaccharide; C12, palmarumycin C12; C13, palmarumycin C13; The values are expressed as means ± standard deviations (n = 3). Different letters indicate significant differences among the treatments in each column including different oligosaccharides and their concentrations at p = 0.05 level.
The increase in palmarumycin production is due to not only the mycelia growth but also palmarumycin content of the fungus. The effects of three oligosaccharides (i.e., OW, OS and OA) on mycelia growth and palmarumycin production in Berkeasmium sp. Dzf12 liquid culture are presented in Table 1. All three oligosaccharides showed improving effects on mycelia growth and palmarumycin accumulation. The optimal concentrations to stimulate mycelia growth for OW, OS and OA were respectively at 600, 400 and 600 mg/L. However, the optimal concentrations to obviously increase total palmarumycin yield (palmarumycins C12 plus C13) for OW, OS and OA were respectively at 400, 200 and 400 mg/L.
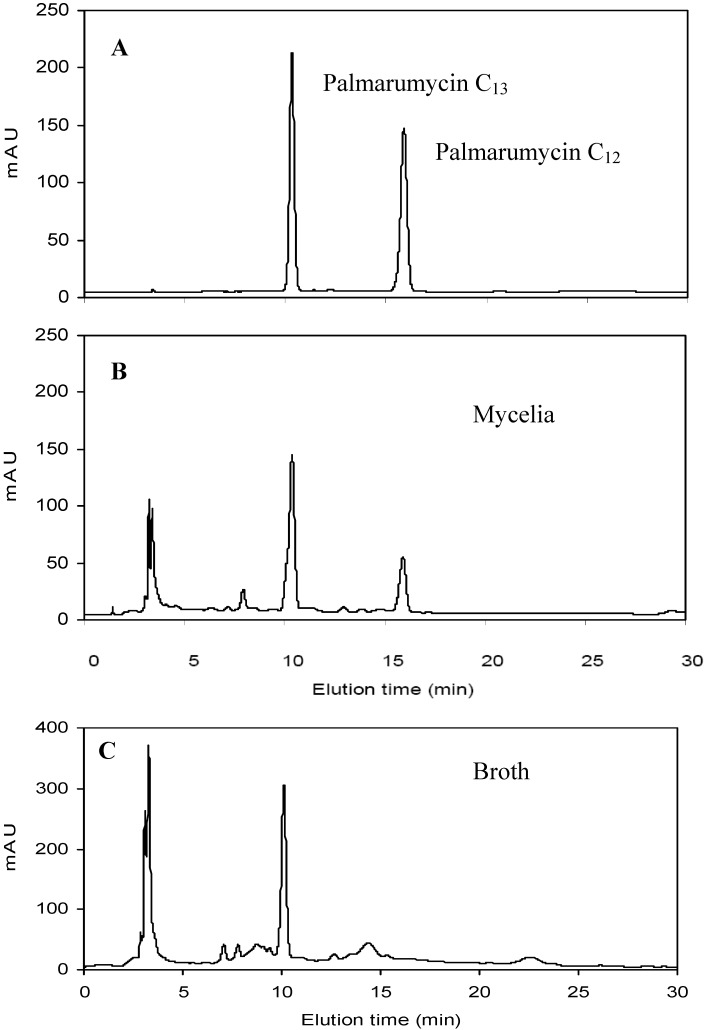
Among the three oligosaccharides, OW was the most effective elicitor to enhance mycelia growth and palmarumycin production. Feeding with 400 mg/L of OW on day 3 increased intracellular palmarumycin C12 yield by 8.60-fold (86.15 mg/L versus 8.97 mg/L), and total palmarumycin C13 yield (intracellular palmarumycin C13 in mycelia plus extracellular palmarumycin C13 in medium) by 1.94-fold (383.18 mg/L versus 130.48 mg/L). Correspondingly, total palmarumycin (palmarumycins C12 plus C13) yield of endophyte Dzf12 liquid cultures was increased to reach 469.33 mg/L, which was 3.37-fold of control yield (139.45 mg/L). Very interestingly, palmarumycin C12 was undetectable in the broth extract which means it was not secreted from the intracellular to the extracellular. The HPLC profiles of the extracts of mycelia and broth along with palmarumycins C12 and C13 standards are shown in Figure 1.
Figure 1.
HPLC profiles of the extracts of mycelia and broth for palmarumycins C12 and C13 analysis: palmarumycins C12 and C13 (A), mycelia extract from batch culture on day 13 (B), broth extract from batch culture on day 13 (C). Elution time (retention time) for palmarumycins C12 and C13 were 15.911 min and 10.326 min, respectively. Palmarumycin C12 was undetectable in the broth extract.
2.2. Effects of Oligosaccharide OW Addition Time on Mycelia Growth and Palmarumycin Production
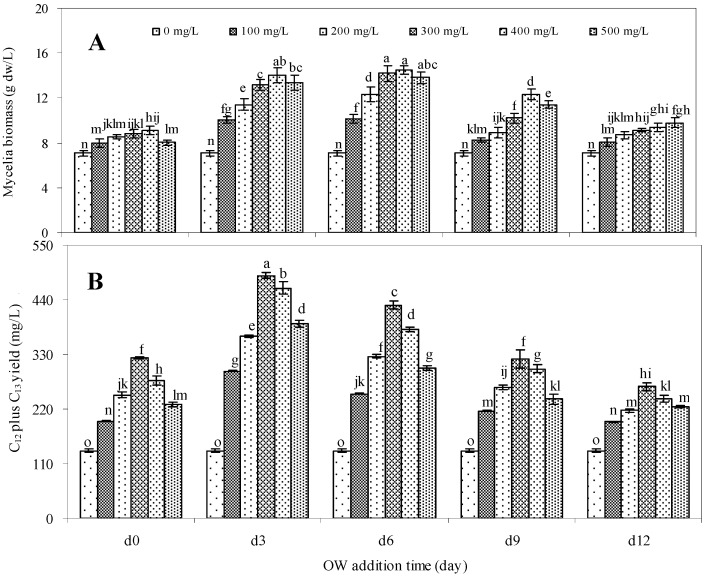
Addition time of the elicitor has been considered as a main factor affecting its elicitation effect on the biosynthesis of secondary metabolites [28,29]. The oligosaccharide OW obtained by acid hydrolysis of WEP was the most effective elicitor to improve palmarumycin biosynthesis. Hence, addition time of OW and its concentration were further optimized. As the 3-day-old cultures treated with 600 mg/L of OW could diminish palmarumycin production (Table 1), the highest concentration of OW in subsequent studies was limited at 500 mg/L.
Figure 2.
Effects of different concentrations of OW and its addition time on mycelia growth (A) and palmarumycins C12 plus C13 yield (B) in liquid culture of Berkleasmium sp. Dzf12. All cultures were harvested on day 13. The error bars represent standard deviations (n = 3). Different letters indicate significant differences among the treatments at p = 0.05 level.
Figure 2 shows the effects of OW on mycelia growth, palmarumycin content in mycelia, and palmarumycin yield in Berkleasmium sp. Dzf12 liquid cultures, which were dependent on both OW concentrations (100, 200, 300, 400 and 500 mg/L) and its addition time (added on day 0, 3, 6, 9 and 12). As shown in Figure 2A, when the cultures were fed with 400 mg/L of OW on day 6, the mycelia biomass was 2.05-fold of control (14.49 g dw/L versus 7.08 g dw/L). The palmarumycins C12 and C13 production was also effectively enhanced by OW. With 300 mg/L of OW fed on day 3, the highest contents of palmarumycins C12 (10.30 mg/g dw) and C13 (6.33 mg/g dw) in mycelia were obtained, which were respectively 3.12 and 4.83-fold in comparison with those produced in the control (3.40 and 1.31 mg/g dw). The total palmarumycin yield (palmarumycins C12 plus C13) was improved to reach 488.48 mg/L, which was about 3.60-fold of control yield (135.57 mg/L) (Figure 2B).
2.3. Kinetics of Mycelia Growth and Palmarumycin Accumulation after Treatment with Oligosaccharide OW
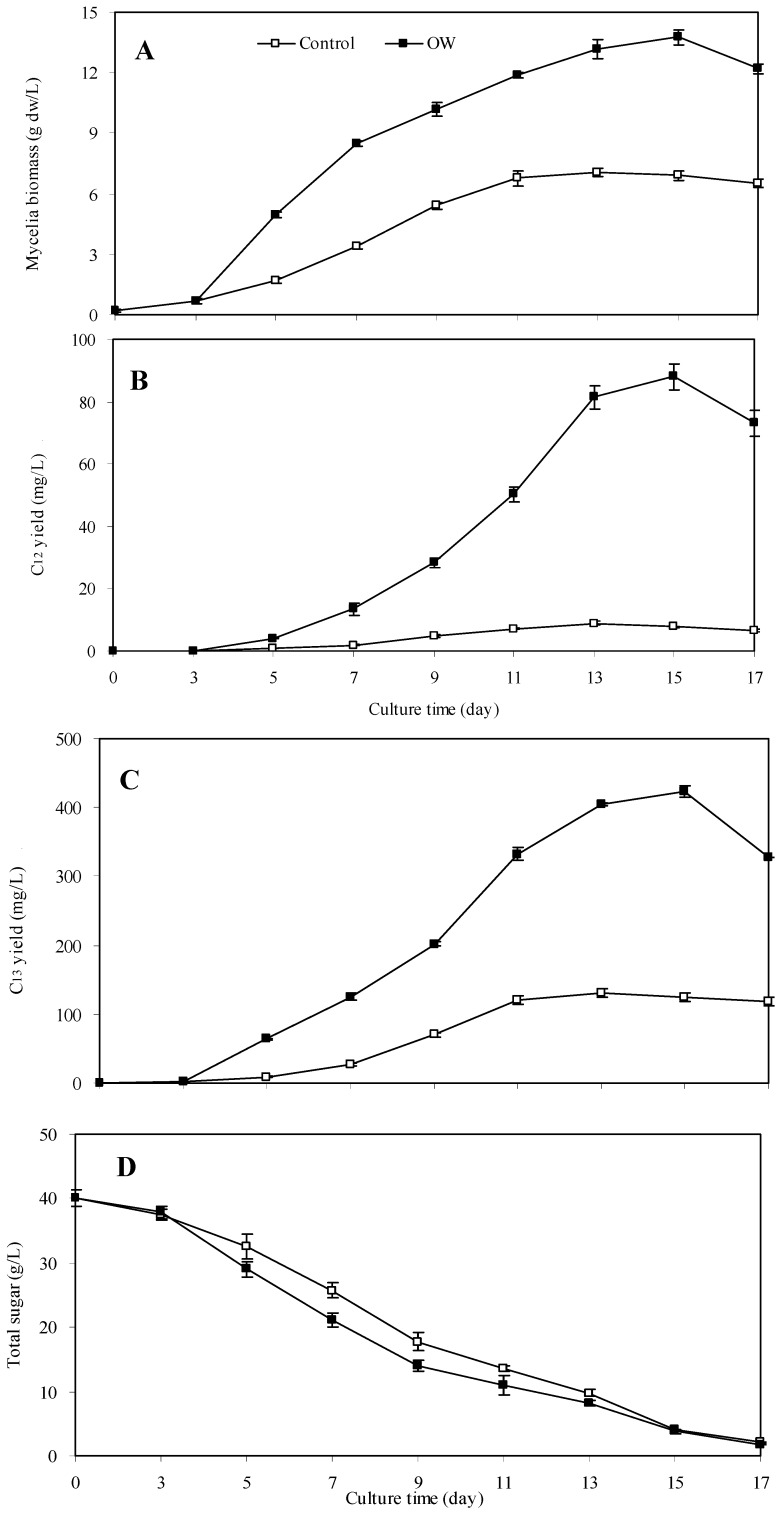
The maximal palmarumycin accumulation was obtained when the cultures were treated with OW at 300 mg/L on day 3 (Figure 2). So the kinetic studies of mycelia growth, palmarumycin accumulation and glucose consumption in liquid culture of Berkleasmium sp. Dzf12 elicited by OW at 300 mg/L added on day 3 were investigated, which are shown in Figure 3.
Figure 3.
Kinetics of mycelia growth (A), palmarumycin C12 accumulation (B), palmarumycin C13 accumulation (C), and total sugar content (D) after treatment with OW at 300 mg/L on day 3 in liquid culture of Berkleasmium sp. Dzf12. The period of culture was in 17 days. The error bars represent standard deviations (n = 3).
The maximal mycelia biomass (13.76 g dw/L) was obtained on day 15, about 1.95-fold of control (7.04 g dw/L) (Figure 3A On day 15 of culture, palmarumycin C12 yield reached the maximum (87.96 mg/L), and palmarumycin C13 yield also reached the highest value (422.28 mg/L) (Figure 3B and C). The total palmarumycin yield was increased to the maximum on day 15 of culture, which was 510.24 mg/L, about 3.66-fold of control (139.38 mg/L). As shown in Figure 3D, the total sugar was consumed at a slightly higher rate with OW elicitation on day 5, and the trend maintained till day 11 in comparison with the control. The mycelia growth against total sugar consumption was 0.23 g dw/g for the control and 0.37 g dw/g for the cultures treated with OW, while the corresponding total palmarumycin yield on total sugar consumption was 4.61 mg/g (for the control) and 14.09 mg/g (for the cultures treated with OW). It indicated that the increase in both mycelia growth and palmarumycin yield was due to the elicitation of OW added in trace amounts (mg/L) but not as carbon source [24,30].
3. Experimental
3.1. Endophytic Fungus and Culture Conditions
The endophytic fungus Berkleasmium sp. Dzf12 (GenBank accession number EU543255) was isolated from the healthy rhizomes of the medicinal plant Dioscorea zingiberensis C. H. Wright (Dioscoreaceae) in our previous study [6,31]. The living culture has been deposited in China General Microbiological Culture Collection Center (CGMCC) under the number of CGMCC 2476. It was also maintained on potato dextrose agar (PDA) slants at 25 °C. For preparation of the inoculum, 4 disks (about 5 mm) of the mycelia of Dzf12 were transferred into each Erlenmeyer flask containing 100 mL of potato dextrose broth. The flasks were then placed on a rotary shaker at 150 rpm and 25 °C for 4 days. All the experiments were carried out in Erlenmeyer flasks (150 mL) containing 30 mL of fermentation medium, where liquid medium composition was (in g/L): glucose 40, peptone 10, KH2PO4 1.0, MgSO4·7H2O 0.5, FeSO4·7H2O 0.05, pH 6.5. Three percent (v/v) of seed suspension cultures was inoculated. The Erlenmeyer flasks were incubated in darkness on a rotary shaker at 150 rpm and 25 °C.
3.2. Preparation of Oligosaccharides
Three crude oligosaccharides, OW (6.20 g), OS (5.25 g), OA (5.10 g) were respectively obtained by acid hydrolysis of 15 g of three freeze-dried polysaccharides, which were water-extracted polysaccharide (WEP), sodium hydroxide-extracted polysaccharide (SEP) and acid-extracted polysaccharide (AEP). These polysaccharides were prepared from the rhizomes of D. zingiberensis as described previously [27]. The oligosaccharides were prepared according to the methods of Zhang et al. [19] and Li et al. [29]. Each polysaccharide (WEP, SEP and AEP) was placed into a glass bottle treated with 2.17 mol/L trifluoroacetic acid (TFA) at 90 °C for 3 h in a water bath. The bottles were shaken every half hour and placed on an ice bath at the end of hydrolysis. The acid was removed under reduced pressure by repeated evaporation with MeOH. Three volumes of 95% EtOH were added to the hydrolysates to precipitate the non-hydrolyzed polysaccharides. The supernatant was concentrated. The values of anthrone-positive carbohydrate were 99.56%, 98.84% and 99.01% for the crude oligosaccharides from WEP, SEP and AEP, respectively. The obtained oligosaccharides were subjected to thin-layer chromatography (TLC) detection with n-butanol-ethyl acetate-acetic acid-water (3.0:0.5:1.7:4.1, v/v) as the developing agent. The reference oligosaccharides were kindly supplied by Prof. Shilin Wang of Kunming Institute of Botany, Chinese Academy of Sciences. The degrees of polymerization (DPs) of the oligosaccharides were at ranges of 2 to 12. The oligosaccharides were stored as solutions at -20°C, and sterilized by filtration before use.
3.3. Application of Oligosaccharides
Stock solutions (100 mg of carbohydrate equivalent per milliliter) were prepared by dissolving each crude oligosaccharide (i.e., OW, OS and OA) in distilled water, and the pH was adjusted to 6.5. The solutions were sterilized by filtrating through a microfilter (pore size, 0.45 µm), diluted with sterile water into different concentrations, and then stored at 4 °C before use. Based on our previous research (data not shown), the oligosaccharide solutions were added to 3-day-old cultures with the final concentrations of 200, 400 and 600 mg/L for OW or OA, and 100, 200 and 400 mg/L for OS, respectively. As OW was found to be the most effective elicitor (data shown in Table 1), OW was applied in the next experiments at five concentrations (100, 200, 300, 400 and 500 mg/L) on days 0, 3, 6, 9 and 12 of culture, respectively. Furthermore, the kinetics of the mycelia growth and palmarumycin accumulation was investigated when OW was added on day 3 of culture at a concentration of 300 mg/L. Each flask was worked up separately.
3.4. Determination of Mycelia Biomass
The mycelia of Berkleasmium sp. Dzf12 were separated from the liquid medium by filtration under vacuum and rinsed thoroughly with distilled water, and then dried at 50–55 °C in an oven to a constant dry weight (dw).
3.5. Extraction and Quantification of Palmarumycins C12 and C13
Palmarumycin extraction and determination was carried out as previously described [21,27,32]. Briefly, 50 mg of dry mycelia powder was added into a tube with 5 mL of methanol-chloroform (9:1, v/v), and then subjected to ultrasonic treatment (three times, 60 min each). After removal of the solid by filtration, the filtrate was evaporated to dryness and re-dissolved in 1 mL of methanol. For quantitative analysis of palmarumycins C12 and C13 in broth, 5 mL of the culture broth without mycelia was evaporated to dryness and extracted with 5 mL of methanol-chloroform (9:1, v/v) in an ultrasonic bath (three times, 60 min each), and the liquid extract was then evaporated to dryness and re-dissolved in 1 mL of methanol.
Palmarumycin content was analyzed by the high performance liquid chromatography (HPLC) system (Shimadzu, Japan), which consisted of two LC-20AT solvent delivery units, an SIL-20A autosampler, an SPD-M20A photodiode array detector, and CBM-20Alite system controller. The reversed-phase Agilent TC-C18 column (250 mm × 4.6 mm i.d., particle size 5 μm) was used for separation by using a mobile phase of methanol-H2O (50:50, v/v) at a flow rate of 1 mL/min. The temperature was maintained at 40 °C, and UV detection at 226 nm. The sample injection volume was 10 μL. The LCsolution multi-PDA workstation was employed to acquire and process chromatographic data. Palmarumycins C12 and C13 were detected and quantified with the standards prepared according to the method of Cai et al. [6].
3.6. Physicochemical and Spectroscopic Data of Palmarumycins C12 and C13
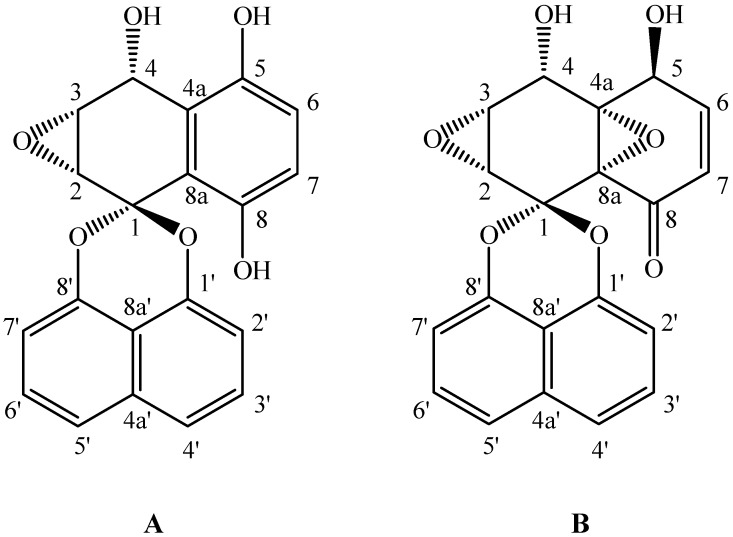
Palmarumycin C12 was obtained as a white amorphous powder (MeOH), and mp 199-201 °C. Its molecular formula was determined as C20H14O6 (m/z 373.06805 [M+Na]+, calcd. 373.06826) by its HR-ESI-MS. 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 9.21 (1H, br. s, 5-OH), 8.81 (1H, s, 8-OH), 7.59 (lH, d, J = 8.2 Hz, 4'-H), 7.57 (lH, d, J = 8.2 Hz, 5'-H), 7.51 [1H, pseudo-t (dd), J = 7.4, 8.5 Hz, 3'-H], 7.49 [1H, pseudo-t (dd), J = 7.4, 8.5 Hz, 6'-H], 7.01 (1H, d, J = 7.4 Hz, 2'-H), 6.96 (1 H, d, J = 7.4 Hz, 7'-H), 6.81 (1H, d, J = 8.7 Hz, 7-H), 6.76 (1H, d, J = 8.7 Hz, 6-H), 6.46 (1H, br. s, 4-OH), 5.31 (l H, d, J = 2.6 Hz, 4-H), 3.64 (1H, d, J = 4.3 Hz, 2-H), 3.61 (1H, dd, J = 2.9, 4.3 Hz, 3-H). 13C-NMR (DMSO-d6, 100 MHz) δ (ppm): 97.2 (C-1), 52.4 (C-2), 54.0 (C-3), 64.4 (C-4), 121.5 (C-4a), 148.6 (C-5), 117.4 (C-6), 117.4 (C-7), 150.0 (C-8), 118.3 (C-8a), 147.4 (C-1'), 108.8 (C-2'), 127.9 (C-3'), 120.3 (C-4'), 133.9 (C-4a'), 120.1 (C-5'), 127.7 (C-6'), 108.6 (C-7'), 147.4 (C-8'), 112.1 (C-8a'). The structure (shown in Figure 4) of palmarumycin C12 was confirmed by comparison with literature data [9,33].
Palmarumycin C13 was obtained as colorless crystals (MeOH), mp 230 °C. Its molecular formula was determined as C20H14O7 (m/z 405.03720 [M+K]+, calcd. 405.03711) by its HR-ESI-MS. 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 7.64 (lH, d, J = 7.4 Hz, 4'-H), 7.62 (lH, d, J =7.4 Hz, 5'-H), 7.52 [2H, pseudo-t (dd), J = 7.4, 8.5 Hz, 3'-H, 6'-H], 7.10 (1H, d, J = 7.5 Hz, 2'-H), 7.04 (1H, d, J = 7.6 Hz, 7'-H), 6.74 (1H, dd, J = 4.9, 10.5 Hz, 6-H), 6.24 (1H, d, J = 7.8 Hz, 4-OH), 6.00 (1H, d, J = 7.5 Hz, 5-OH), 5.88 (1H, d, J = 2.2, 10.5 Hz, 7-H), 4.98 (lH, d, J = 7.6 Hz, 4-H), 4.69 (1H, m, 5-H), 3.17 (2H, d, J = 5.2 Hz, 2-H, 3-H). 13C-NMR (DMSO-d6, 100 MHz) δ (ppm):95.1 (C-1), 52.7 (C-2), 55.2 (C-3), 59.7 (C-4), 70.7 (C-4a), 60.6 (C-5), 125.3 (C-6), 144.8 (C-7), 188.8 (C-8), 62.2 (C-8a), 145.2 (C-l'), 109.1 (C-2'), 127.9 (C-3'), 120.7 (C-4'), 133.8 (C-4a'), 120.7 (C-5'), 127.7 (C-6'), 108.7 (C-7'), 145.5 (C-8'), 111.5 (C-8a'). The structure (shown in Figure 4) of palmarumycin C13 was confirmed by comparison with literature data [9,34].
Figure 4.
Chemical structures of palmarumycins C12 (A) and C13 (B).
3.7. Measurement of Total Sugar Content
Total sugar content in the medium was determined by anthrone test using glucose as the reference [18]. According to the method of Zhu et al. [35], mycelia growth against total sugar consumption was calculated as: (maximum mycelia biomass—Initial mycelia biomass)/(initial total sugar concentration—Residual total sugar concentration when the maximal mycelia biomass was obtained). Palmarumycin yield against total sugar consumption was calculated as: (maximum total palmarumycin yield—Initial total palmarumycin yield)/(initial total sugar concentration—Residual total sugar concentration when the maximal total palmarumycin yield was obtained).
3.8. Statistical Analysis
All experiments were carried out in triplicate, and the results were represented by their mean values and the standard deviations (SD). The data were submitted to analysis of variance (one-way ANOVA) to detect significant differences by PROC ANOVA of SAS version 8.2. The term significant has been used to denote the differences for which p ≤ 0.05.
4. Conclusions
In this work, the oligosaccharides prepared from the polysaccharides isolated from the rhizomes of D. zingiberensis were successfully applied to the liquid cultures of Berkleasmium sp. Dzf12 for enhancing the production of palmarumycins C12 and C13. There were some reports about the oligosaccharide elicitation of fungal secondary metabolite production, such as the oligosaccharides derived from sodium alginate and locust-bean gum [24,25]. However, there are few reports about the effects of the oligosaccharides from plants on the secondary metabolism of their endophytic fungi. Oligosaccharides OW, OS and OA from D. zingiberensis had obvious enhancing effects on palmarumycin biosynthesis in Berkleasmium sp. Dzf12, although the elicitation mechanism was not clear. As the oligosaccharide employed in this study was a mixture, further separation and identification of the bioactive oligosaccharide monomers are necessary. The results indicate that enhancement of palmarumycin accumulation in liquid culture of Berkleasmium sp. Dzf12 by the oligosaccharides from host plant D. zingiberensis could be an effective strategy for large-scale production of palmarumycins C12 and C13 in the future. By comparing with our previous study on the enhancing effects of the polysaccharides from D. zingiberensis on palmarumycin C13 production [27], the oligosaccharide (i.e., OW) has a better stimulating effect than its corresponding polysaccharide (i.e., WEP). This indicated that the oligosaccharide fragments in polysaccharide may be the active components to stimulate secondary metabolism that should be further studied.
Acknowledgements
This work was co-financed by the grants from the National Natural Science Foundation of China (31071710), the program for Changjiang Scholars and Innovative Research Team in University of China (IRT1042), and the Natural Science Foundation of Beijing (6092015).
Footnotes
Sample Availability: Samples of the oligosaccharides are available from the authors.
References and Notes
- 1.Aly A.H., Debbab A., Kjer J., Proksch P. Fungal endophytes from higher plants: A prolific source of phytochemicals and other bioactive natural products. Fungal Divers. 2010;41:1–16. [Google Scholar]
- 2.Schulz B., Boyle C., Draeger S., Rommert A.K., Krohn K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002;106:996–1004. [Google Scholar]
- 3.Strobel G., Daisy B., Castillo U., Harper J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004;67:257–268. doi: 10.1021/np030397v. [DOI] [PubMed] [Google Scholar]
- 4.Kharwar R.N., Mishra A., Gond S.K., Stierle A., Stierle D. Anticancer compounds derived from fungal endophytes: Their importance and future challenges. Nat. Prod. Rep. 2011;28:1208–1228. doi: 10.1039/c1np00008j. [DOI] [PubMed] [Google Scholar]
- 5.Zhao J., Shan T., Mou Y., Zhou L. Plant-derived bioactive compounds produced by endophytic fungi. Mini-Rev. Med. Chem. 2011;11:159–168. doi: 10.2174/138955711794519492. [DOI] [PubMed] [Google Scholar]
- 6.Cai X., Shan T., Li P., Huang Y., Xu L., Zhou L., Wang M., Jiang W. Spirobisnaphthalenes from the endophytic fungus Dzf12 of Dioscorea zingiberensis and their antimicrobial activities. Nat. Prod. Commun. 2009;4:1469–1472. [PubMed] [Google Scholar]
- 7.Zhou L., Zhao J., Shan T., Cai X., Peng Y. Spirobisnaphthalenes from fungi and their biological activities. Mini-Rev. Med. Chem. 2010;10:977–989. doi: 10.2174/138955710792007178. [DOI] [PubMed] [Google Scholar]
- 8.Cai Y.S., Guo Y.W., Krohn K. Structure, bioactivities, biosynthetic relationships and chemical synthesis of the spirodioxynaphthalene. Nat. Prod. Rep. 2010;27:1840–1870. doi: 10.1039/c0np00031k. [DOI] [PubMed] [Google Scholar]
- 9.Krohn K., Michel A., Floerke U., Aust H.-J., Draeger S., Schulz B. Palmarumycins C1-C16 from Coniothyrium sp.: Isolation, structure elucidation, and biological activity. Liebigs Ann. Chem. 1994;11:1099–1108. [Google Scholar]
- 10.Bode H.B., Walker M., Zeeck A. Secondary metabolites by chemical screening. 42 Cladospirones B to I from Sphaeropsidales sp. F-24'707 by variation of culture conditions. Eur. J. Org. Chem. 2000;18:3185–3193. [Google Scholar]
- 11.Schlingmann G., West R.R., Milne L., Pearce C.J., Carter G.T. Diepoxins, novel fungal metabolites with antibiotic activity. Tetrahedron Lett. 1993;34:7225–7228. [Google Scholar]
- 12.Chu M., Truumees I., Patel M.G., Gullo V.P., Blood C., King I., Pai J.K., Puar M.S. A novel class of anti-tumor metabolites from the fungus Nattrassia mangiferae. Tetrahedron Lett. 1994;35:1343–1346. [Google Scholar]
- 13.Thiergardt R., Hug P., Rihs G., Peter H.H. Cladospirone bisepoxide-a novel fungal metabolite structure determination. Tetrahedron Lett. 1994;35:1043–1046. [Google Scholar]
- 14.Thiergardt R., Rihs G., Hug P., Peter H.H. Cladospirone bisepoxide: Definite structure assignment including absolute configuration and selective chemical transformations. Tetrahedron. 1995;51:733–742. [Google Scholar]
- 15.Petersen F., Moerker T., Vanzanella F., Peter H.H. Production of cladosporine bisepoxide, a new fungal metabolite. J. Antibiot. 1994;47:1098–1103. doi: 10.7164/antibiotics.47.1098. [DOI] [PubMed] [Google Scholar]
- 16.Zhou L.G., Wu J.Y. Development and application of medicinal plant tissue cultures for production of drugs and herbal medicinals in China. Nat. Prod. Rep. 2006;23:789–810. doi: 10.1039/b610767b. [DOI] [PubMed] [Google Scholar]
- 17.Schneider P., Misiek M., Hoffmeister D. In vivo and in vitro production options for fungal secondary metabolites. Mol. Pharmaceut. 2008;5:234–242. doi: 10.1021/mp7001544. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J., Zhou L., Wu J. Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl. Microbiol. Biotechnol. 2010;87:137–144. doi: 10.1007/s00253-010-2443-4. [DOI] [PubMed] [Google Scholar]
- 19.Zhang R., Li P., Xu L., Chen Y., Sui P., Zhou L., Li J. Enhancement of diosgenin production in Dioscorea zingiberensis cell culture by oligosaccharide elicitor from its endophytic fungus Fusarium oxysporum Dzf17. Nat. Prod. Commun. 2009;4:1459–1462. [PubMed] [Google Scholar]
- 20.Li P., Mou Y., Shan T., Xu J., Li Y., Lu S., Zhou L. Effects of polysaccharide elicitors from endophytic Fusarium oxysporium Dzf17 on growth and diosgenin production in cell suspension culture of Dioscorea zingiberensis. Molecules. 2011;16:9003–9016. doi: 10.3390/molecules16119003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J., Zheng B., Li Y., Shan T., Mou Y., Lu S., Li P., Zhou L. Enhancement of diepoxin ζ production by yeast extract and its fractions in liquid culture of Berkleasmium-like endophytic fungus Dzf12 from Dioscorea zingiberensis. Molecules. 2011;16:847–856. doi: 10.3390/molecules16010847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ariyo B., Tamerler C., Bucke C., Keshavarz T. Enhanced penicillin production by oligosaccharides from batch cultures of Penicillium chrysogenum in stirred-tank reactors. FEMS Microbiol. Lett. 1998;166:165–170. doi: 10.1111/j.1574-6968.1998.tb13198.x. [DOI] [PubMed] [Google Scholar]
- 23.Tamerler C., Ariyo B., Bucke C., Keshavarz T. Effect of mannan and alginate oligosaccharides on production in bioreactors of penicillin G and its biosynthetic intermediates. Ann. Microbiol. 2001;51:53–60. [Google Scholar]
- 24.Ariyo B., Bucke C., Keshaverz T. Alginate oligosaccharides as enhancers of penicillin production in cultures of Penicillium chrysogenum. Biotechnol. Bioeng. 1997;53:17–20. doi: 10.1002/(SICI)1097-0290(19970105)53:1<17::AID-BIT3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 25.Radman R., Bucke C., Keshavarz T. Elicitor effects on Penicillium chrysogenum morphology in submerged cultures. Biotechnol. Appl. Biochem. 2004;40:229–233. doi: 10.1042/BA20040062. [DOI] [PubMed] [Google Scholar]
- 26.Liu G., Casqueiro J., Gutierrez S., Kosalkova K., Castillo N.I., Martin J.F. Elicitation of penicillin biosynthesis by alginate in Penicillium chrysogenum, exerted on pcbAB, pcbC, and penDE genes at the transcriptional level. J. Microbiol. Biotechnol. 2001;11:812–818. [Google Scholar]
- 27.Li Y., Li P., Mou Y., Zhao J., Shan T., Ding C., Zhou L. Enhancement of diepoxin ζ production in liquid culture of endophytic fungus Berkleasmium sp. Dzf12 by polysaccharides from its host plant Dioscorea zingiberensis. World J. Microbiol. Biotechnol. 2012;28 doi: 10.1007/s11274-11011-10940-y. [DOI] [PubMed] [Google Scholar]
- 28.Zhu L.W., Tang Y.J. Significance of protein elicitor isolated from Tuber melanosporum on the production of ganoderic acid and Ganoderma polysaccharides during the fermentation of Ganoderma lucidum. Bioproc. Biosyst. Eng. 2010;33:999–1005. doi: 10.1007/s00449-010-0424-8. [DOI] [PubMed] [Google Scholar]
- 29.Li P., Mao Z., Lou J., Li Y., Mou Y., Lu S., Peng Y. Enhancement of diosgenin in Dioscorea zingiberensis cell cultures by oiligosaccharides from its endophytic fungus Fusarium oxysporum Dzf17. Molecules. 2011;16:10631–10644. doi: 10.3390/molecules161210631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy T., Parra R., Radman R., Roy I., Harrop A., Dixon K., Keshavarz T. Novel application of oligosaccharides as elicitors for the enhancement of bacitracin A production in cultures of Bacillus licheniformis. Enzyme Microb. Technol. 2007;40:1518–1523. doi: 10.1016/j.enzmictec.2006.10.030. [DOI] [Google Scholar]
- 31.Zhang R., Li P., Zhao J., Yin C., Zhou L. Endophytic fungi from Dioscorea zingiberensis and their effects on the growth and diosgenin production of the host plant cultures. Nat. Prod. Res. Dev. 2010;22:11–15. [Google Scholar]
- 32.Zhao J., Li Y., Shan T., Mou Y., Zhou L. Enhancement of diepoxin ζ production with in situ resin adsorption in mycelial liquid culture of the endophytic fungus Berkleasmium sp. Dzf12 from Dioscorea zingiberensis. World J. Microbiol. Biotechnol. 2011;27:2753–2758. doi: 10.1007/s11274-011-0750-2. [DOI] [Google Scholar]
- 33.Barrett A.G.M., Blaney F., Campbell A.D., Hamprecht D., Meyer T., White A.J.P., Witty D., Williams D.J. Unified route to the palmarumycin and preussomerin natural products. Enantioselective synthesis of (-)-preussomerin G. J. Org. Chem. 2002;67:2735–2750. doi: 10.1021/jo0110247. [DOI] [PubMed] [Google Scholar]
- 34.Schlingmann G., Matile S., Berova N., Nakanishi K., Carter G.T. Absolute stereochemistry of the diepoxins. Tetrahedron. 1996;52:435–446. [Google Scholar]
- 35.Zhu L.W., Zhong J.J., Tang Y.J. Significance of fungal elicitors on the production of ganoderic acid and Ganoderma polysaccharides by the submerged culture of medicinal mushroom Ganoderma lucidum. Process Biochem. 2008;43:1359–1370. doi: 10.1016/j.procbio.2008.08.003. [DOI] [Google Scholar]