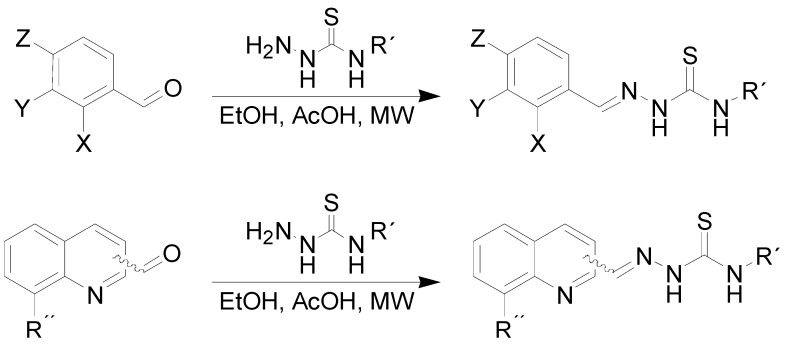
Abstract
Two series of thiosemicarbazone-based iron chelators (twenty-seven compounds) were designed and synthesized using a microwave-assisted approach. Quinoline and halogenated phenyl were selected as parent scaffolds on the basis of a similarity search. The lipophilicity of the synthesized compounds was measured using HPLC and then calculated. Primary in vitro screening of the synthesized compounds was performed against eight pathogenic fungal strains. Only a few compounds showed moderate activity against fungi, and (E)-2-(quinolin-2-ylvinyl)-N,N-dimethylhydrazine-carbothioamide appeared to be more effective than fluconazole against most of the fungal strains tested. Antiproliferative activity was measured using a human colon cancer cell line (HCT-116). Several of the tested compounds showed submicromolar antiproliferative activity. Compounds were also tested for their activity related to the inhibition of photosynthetic electron transport (PET) in spinach (Spinacia oleracea L.) chloroplasts. The structure-activity relationships are discussed for all of the compounds.
Keywords: thiosemicarbazones, lipophilicity, photosynthetic electron transport inhibition, spinach chloroplasts, iron chelators, in vitro antifungal activity, in vitro anticancer activity
1. Introduction
Iron is an element of great importance for virtually all living organisms. It is present in various biological processes such as metabolic pathways, reproduction or photosynthesis. On the basis of its importance, the iron-sulphur theory of the origin of life has been proposed [1,2]. Despite being so pervasive, iron rarely exists alone or in the form of simple cations. It is commonly chelated by larger organic molecules such as porphyrin. This form is present in hemoglobin or in most of the metabolic enzymes of cytochrome P450 [3]. Clearly, all living organisms have their ways of acquiring and managing such an important element. Any alteration of or damage to the iron metabolism system may lead to serious diseases. In humans, this may cause hemochromatosis or iron deficiency anemia [4]. As a target for therapy, iron is used mainly in the treatment of iron overload using iron chelators such as deferoxamine (DFO, also known as desferrioxamine B) [5]. More recently, iron chelators have been proposed for cancer treatment [5,6,7,8,9,10] because rapidly proliferating cancer cells have a higher demand for this element compared to the normal cells [5,11]. Although the exact mechanism of the anticancer activity of iron chelators remains unknown, several possible modes of action can be implemented, e.g., the inhibition of iron uptake [12] and the inhibition of ribonucleotide reductase (RR) [13] or the formation of reactive oxygen species (ROS) [14].
Other potential therapeutic or agricultural application of iron chelators may be derived from processes or pathways that are dependent on iron in pathological organisms. Fungi and bacteria require an appropriate amount of iron for correct growth, and apparently to develop their resistance to drugs [15,16]. Pathogenic microbes have evolved their unique way of acquiring this important metal from a host organism through the overexpression of Fe receptors or the utilization of siderophores [17]. If the iron level is reduced, fungi lose some of their virulence and have a decreased potency to invade the epithelium. Hence, lactoferrin and deferoxamine have been tested for their antifungal potency. Early attempts have consisted in the co-administration of lactoferrin with conventional antifungals such as amphotericin B [18,19]. DFO was tested as an ancillary drug with amphotericin B in antifungal therapy in a clinical trial [20,21]. The study was terminated prematurely due to an increased mortality in patients who had taken DFO when compared to those who had taken a placebo. However, this seems to be an effect of the more complex prerequisites connected with the unknown site of administration of DFO rather than with synergic toxicity. Nevertheless, this is a trigger to search for structurally new iron chelators with an antifungal activity. Several reports describing the synthesis and antifungal activity of thiosemicarbazones have been published [22,23,24,25,26]. Li et al. described a series of benzyl thiosemicarbazones whose substitution with a chlorine atom triggered the activity of mushroom tyrosinase [27]. Moreover, chlorine derivatives of benzaldehyde 4-phenyl-3-thiosemicarbazone and their cyclization products were tested against Toxoplasma gondii and several bacterial strains [28]. Some of these derivatives were more active than chloramphenicol and rifampicin.
In this paper, some disubstituted benzaldehyde thiosemicarbazones are described along with their anticancer and antifungal activity. These structures were tested on the basis of a former study on antifungal compounds [29,30,31,32,33] and quinolone-based thiosemicarbazones with anticancer activity [34]. Figure 1 illustrates the fragment-based similarity of the compounds investigated with known and effective iron chelators [5,35,36,37]. The design of compounds with polypharmacological activity was the aim of our investigation. Such compounds may be of great value in anticancer therapy where fungal infections are very common. Furthermore, these compounds, due to their simple and cheap synthesis, are suitable for agricultural use. However, antifungal agents such as azoles used for crop protection raise some concerns, e.g., triggering resistance [38] and have a negative impact on the environment. In fact, iron is also important in plants where it plays a crucial role in photosystems I and II as a part of ferredoxin [39] and cytochrome b559 [40,41,42,43]. Taking this aspect into account, thiosemicarbazone iron chelators may exert some unwanted effects on the green parts of plants. Thus, the compounds prepared for the inhibition of photosynthetic electron transport were also tested.
Figure 1.
(A) Structures of known iron chelators 3-AP [5], 311 [35], Dp44mT [36,37]. (B) investigated benzylidenethiosemicarbazones 3a–o, quinolinylvinylthiosemicarbazones 4a–l.
2. Results and Discussion
2.1. Chemistry
Compounds 3a–o were obtained as shown in Scheme 1. Microwave irradiation was found to be helpful for enhancing the yield of the syntheses and the purity of the final products (the purity of all of the compounds was assessed using HPLC and was higher than 98%, while the overall average yield exceeded 80%). All of the synthesized compounds were in the E-configuration, which was confirmed using 1H-NMR spectroscopy (the signal of the NH group was in the 10–11 ppm range, in comparison to Z-isomer, which possesses a characteristic NH signal in the 14–15 ppm range), the COSY and NOESY experiments and by crystal structure as was reported recently [44]. Exemplary spectra are available in Supplementary Material for this article.
Scheme 1.
Synthesis of the discussed benzylidenethiosemicarbazones 3a–o and quinolinylvinylthiosemicarbazones 4a–l.
2.2. Lipophilicity
Lipophilicity is a property that has a major effect on absorption, distribution, metabolism, excretion and toxicity properties as well as on pharmacological activity because drugs cross biological membranes through passive transport, which is strongly dependent on their lipophilicity. Lipophilicity has been studied and applied as an important drug property for decades. The lipophilicity of the compounds studied was determined using RP-HPLC as a capacity factor logarithm (log k) and calculated as log P/Clog P using two commercially available software programs (ChemOffice and ACD/LogP) [45,46]. The results for benzylidenethiosemicarbazones 3a–o are shown in Table 1, they are summarized for quinolinylvinylthiosemicarbazones 4a–l in Table 2 and are illustrated for both series in Figure 2.
Table 1.
Structure of aryl compounds 3a–o and comparison of the calculated lipophilicities (log P/Clog P) with determined log k values.
 | ||||||
|---|---|---|---|---|---|---|
| Comp. | R1 | R2 | R3 | log k | log P/Clog P ChemOffice | log P ACD/LogP |
| 3a | 2,3-Cl | H | H | 0.6892 | 2.81/3.473 | 3.22 ± 0.38 |
| 3b | 2,3-Cl | H | CH3 | 0.7357 | 3.33/3.177 | 3.14 ± 0.59 |
| 3c | 2,3-Cl | CH3 | CH3 | 0.7214 | 3.71/3.533 | 3.11 ± 0.60 |
| 3d | 2,3-Cl | H | C2H5 | 0.7958 | 3.67/3.706 | 3.68 ± 0.59 |
| 3e | 2,3-Cl | H | C6H5 | 0.9230 | 5.00/5.266 | 4.90 ± 0.59 |
| 3f | 3,4-Cl | H | H | 0.6974 | 2.81/3.473 | 3.28 ± 0.38 |
| 3g | 3,4-Cl | H | CH3 | 0.7563 | 3.33/3.177 | 3.21 ± 0.59 |
| 3h | 3,4-Cl | CH3 | CH3 | 0.7555 | 3.71/3.533 | 3.17 ± 0.60 |
| 3i | 3,4-Cl | H | C2H5 | 0.8415 | 3.67/3.706 | 3.74 ± 0.59 |
| 3j | 3,4-Cl | H | C6H5 | 0.9774 | 5.00/5.266 | 4.96 ± 0.59 |
| 3k | 4-Br | H | H | 0.6580 | 2.53/2.952 | 2.79 ± 0.39 |
| 3l | 4-Br | H | CH3 | 0.6993 | 3.05/2.734 | 2.72 ± 0.61 |
| 3m | 4-Br | CH3 | CH3 | 0.6784 | 3.42/3.090 | 2.68 ± 0.62 |
| 3n | 4-Br | H | C2H5 | 0.7568 | 3.39/3.263 | 3.25 ± 0.61 |
| 3o | 4-Br | H | C6H5 | 0.8772 | 4.71/4.823 | 4.47 ± 0.61 |
Table 2.
Structure of heteroaryl compounds 4a–l and comparison of calculated lipophilicities (log P/Clog P) with determined log k values.
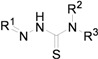 | ||||||
|---|---|---|---|---|---|---|
| Comp. | R1 | R2 | R3 | log k | log P/Clog P ChemOffice | log P ACD/LogP |
| 4a | 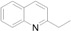 |
H | CH3 | 0.6329 | 2.73/1.968 | 2.06 ± 0.59 |
| 4b |  |
CH3 | CH3 | 0.6184 | 3.10/2.114 | 2.02 ± 0.60 |
| 4c |  |
H, | C2H5 | 0.6892 | 3.06/2.497 | 2.59 ± 0.59 |
| 4d |  |
H | C6H5 | 0.7553 | 4.39/4.057 | 3.81 ± 0.59 |
| 4e | 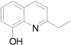 |
H | CH3 | 0.6236 | 2.34/2.064 | 1.85 ± 0.84 |
| 4f | 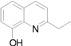 |
CH3 | C6H5 | 0.5927 | 2.71/2.163 | 1.81 ± 0.85 |
| 4g |  |
H | C2H5 | 0.6810 | 2.67/2.593 | 2.38 ± 0.84 |
| 4h |  |
H | C6H5 | 0.7548 | 4.00/4.153 | 3.60 ± 0.84 |
| 4i | 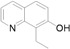 |
H | CH3 | 0.6193 | 1.91/2.131 | 1.62 ± 1.09 |
| 4j |  |
CH3 | CH3 | 0.5831 | 2.29/2.163 | 1.58 ± 1.10 |
| 4k |  |
H | C2H5 | 0.6539 | 2.25/2.660 | 2.15 ± 1.09 |
| 4l |  |
H | C6H5 | 0.6748 | 3.58/4.220 | 3.37 ± 1.09 |
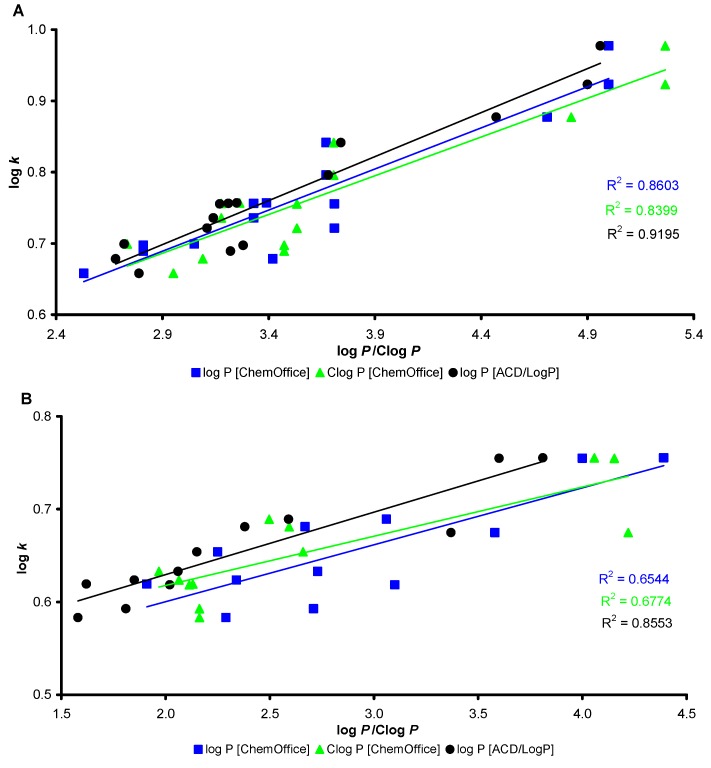
Figure 2.
Comparison of log P/Clog P data calculated using two programs with log k values found experimentally. (A) match of calculated data with log k values of 3a–o found experimentally; (B) match of calculated data with log k values of 4a–l found experimentally.
Reversed phase high performance liquid chromatography (RP-HPLC) methods have become popular and are widely used for lipophilicity measurements. The general procedure is the measurement of the directly accessible retention time under isocratic conditions using varying amounts of methanol as an organic modifier in the mobile phase using RP columns and calculating the logarithm of the capacity factors (log k). Log k is the logarithm of the capacity factors in chromatographic approaches that is related to the partitioning of a compound between a mobile and a (pseudo-)stationary phase. Log k is used as the lipophilicity index converted to log P scale.
Commercially available chemical software does not resolve the various lipophilicity values of individual positional isomers, etc. The software calculates lipophilicity contributions according to different internal databases/libraries and uses different approaches. It can be concluded that for small, highly functionalized molecules with many heteroatoms, a number of intermolecular forces and intramolecular interactions are typical. The more of these interactions that can be expected, the less the predictability of common software can be, thus experimental lipophilicity determination is of great importance. Therefore, we decided to perform our measurements using the water-methanol system as the mobile phase. Log k derived from RP-HPLC retention factors and computational log P values are given in the manuscript and biological data are related to log k data because log k data specify lipophilicity within the series of compounds more precisely than the available chemical software.
The results obtained with all the compounds show that the experimentally-determined lipophilicities (log k) of the compounds discussed are relatively in accordance with the calculated values of compounds 3a–o and 4a–l as is shown in Figure 2. The minimum match of experimental (log k) versus calculated (log P) values was found for ChemOffice. Generally, benzylidenethiosemicarbazones 3a–o showed a higher match (see Figure 2A) than quinolinylvinylthiosemicarbazones 4a–l (see Figure 2B), which indicates inter- and intramolecular interactions in the last mentioned series.
Lipophilicity calculations of these small and highly functionalized molecules do not cover inter- and intramolecular interactions that can be found by experimental lipophilicity determination. As was expected, quinolinylvinylthiosemicarbazones 4a–l showed a lower lipophilicity (log k) than benzylidene-thiosemicarbazones 3a–o. Within the series of benzylidenethiosemicarbazones, the determined log k values increased as follows: 4-Br (3k–o) < 2,3-Cl (3a–e) < 3,4-Cl (3f–j), while within the series of quinolinylvinylthiosemicarbazones, the determined log k values increased in the following order: 7-hydroxyquinolin-8-yl (4i–l) < 8-hydroxyquinolin-2-yl (4e–h) < quinoline-2-yl (4a–d). The influence of R2 and R3 substituents on lipophilicity is as follows: 2H < 2CH3 < H, CH3 < H, C2H5 < H, C6H5. Based on the above-mentioned data, it can be assumed that compounds 4e–l are possible tautomeric isomers. This seems to be in agreement with our former findings [47]. Therefore, it can be concluded that log k values specify lipophilicity within individual series of the compounds studied more precisely than the calculated log P/Clog P data.
2.3. Biological Activities
The compounds under investigation can be divided into two groups based on their chemical structure: Group 1 included benzylidenethiosemicarbazones 3a–o and Group 2 contained quinolinylvinylthiosemicarbazones 4a–l. The compounds showed a wide range of biological activities and some interesting structure-activity relationships were observed. All of the results are summarized in Table 3.
Table 3.
IC50 [μmol/L] values related to PET inhibition in spinach chloroplasts of the substituted thiosemicarbazones in comparison with 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) as the standard; in vitro antifungal activity (IC80/IC50 [μmol/L]) of compounds compared to fluconazole (FLU) as the standard; in vitro antiproliferative activity IC50 [μmol/L] of compounds compared to doxorubicin as the standard. ND = not determined due to precipitation during the experiment or interaction with 2,6-dichlorophenol-indophenol (DCPIP).
| Comp. | PET IC50 | 1,2 MIC (a IC80 / b IC50) [µmol/L] | HCT-116 IC50 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CA a | CT a | CK a | CG a | TB a | AF b | AC b | TM b | |||
| 24 h | 24 h | 24 h | 24 h | 24 h | 24 h | 24 h | 72 h | |||
| 48 h | 48 h | 48 h | 48 h | 48 h | 48 h | 48 h | 120 h | |||
| 3a | ND | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | 41.5 ± 1.7 3 |
| 3b | 170.1 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >60 |
| 3c | ND | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | - |
| 3d | ND | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | 58.5 ± 0.2 3 |
| 3e | 499.3 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >60 |
| 3f | 283.3 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | 46.0 ± 0.9 |
| 3g | 135.6 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | 55.5 ± 6.2 |
| 3h | 329.3 | 15.62 | 500 | 500 | 500 | 125 | >500 | >500 | 31.25 | 47.0 ± 1.3 |
| 31.25 | 500 | 500 | 500 | 500 | >500 | >500 | 62.5 | |||
| 3i | 280.2 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | 48.5 ± 2.8 |
| 3j | 425.9 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >60 |
| 3k | 586.4 | 500 | >500 | >500 | >500 | 500 | >500 | >500 | >500 | >60 |
| 500 | >500 | >500 | >500 | 500 | >500 | >500 | >500 | |||
| 3l | ND | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >60 |
| 3m | 594.6 | 62.5 | >500 | >500 | >500 | 15.62 | >500 | >500 | 62.5 | 47.5 ± 2.4 |
| 250 | >500 | >500 | >500 | 62.5 | >500 | >500 | 62.5 | |||
| 3n | ND | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >60 3 |
| 3o | ND | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >60 |
| 4a | ND | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >25 |
| 4b | 1368 | 1.95 | 1.95 | 3.9 | 1.95 | 1.95 | 3.9 | 3.9 | 3.9 | 4.86 ± 1.48 3 |
| 1.95 | 7.81 | 3.9 | 1.95 | 1.95 | 3.9 | 3.9 | 3.9 | |||
| 4e | 302 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | 1.71 ± 0.34 3 |
| 4f | ND | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | - |
| 4h | ND | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | 24.97 ± 4.29 3 |
| >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | |||
| 4i | ND | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >25 3 |
| >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | |||
| 4j | 520.4 | 125 | >250 | >250 | 125 | 125 | >250 | >250 | 125 | 20.75 ± 5.34 3 |
| >250 | >250 | >250 | 125 | >250 | >250 | >250 | 125 | |||
| 4k | ND | >500 | >500 | >500 | 62.5 | >500 | >500 | >500 | 62.5 | >25 3 |
| >500 | >500 | >500 | 500 | >500 | >500 | >500 | 62.5 | |||
| 4l | ND | 31.25 | 125 | 31.25 | 15.62 | 15.62 | 125 | 15.62 | 15.62 | 16.28 ± 1.69 3 |
| 62.5 | 125 | 125 | 31.25 | 125 | 125 | 62.5 | 15.62 | |||
| DCMU | 1.9 | - | - | - | - | - | - | - | - | |
| FLU | - | 0.06 | 0.12 | 3.91 | 0.98 | 0.24 | >125 | >125 | 1.95 | - |
| 0.12 | >125 | 15.62 | 3.91 | 0.48 | >125 | >125 | 3.91 | |||
| DXR | - | - | - | - | - | - | - | - | - | 10 ± 1.1 |
1 The MIC determination was performed according to the CLSI reference protocol: a M27-A2 for yeasts (IC80 value) and b M38-A for moulds (IC50 value); CA = Candida albicans, CT = Candida tropicalis, CK = Candida krusei, CG = Candida glabrata, TB = Trichosporon beigelii, AF = Aspergillus fumigatus, AC = Absidia corymbifera, and TM = Trichophyton mentagrophytes. 2 All compounds were tested for short and long term activity. When inactive only one value is presented. 3 Synthesis and anticancer activity described elsewhere [34,44]. HCT-116 human colon cancer cells, DXR- doxorubicin as the standard.
2.3.1. Inhibition of Photosynthetic Electron Transport (PET) in Spinach Chloroplasts
The activity of all of the evaluated derivatives related to the inhibition of photosynthetic electron transport (PET) in spinach (Spinacia oleracea L.) chloroplasts was moderate or rather low relative to the standard (see Table 3). Diuron® (DCMU), a marketed herbicide with its activity mechanism directed to photosystem II, was used as the standard. The compounds within Group 2 could not be evaluated for their structure-activity relationships because the majority of these compounds showed no PET-inhibiting activity. (E)-2-(3,4-Dichlorobenzylidene)-N-methylhydrazinecarbothioamide (3g) expressed the highest PET-inhibiting activity (IC50 = 135.6 µmol/L) within Group 1.
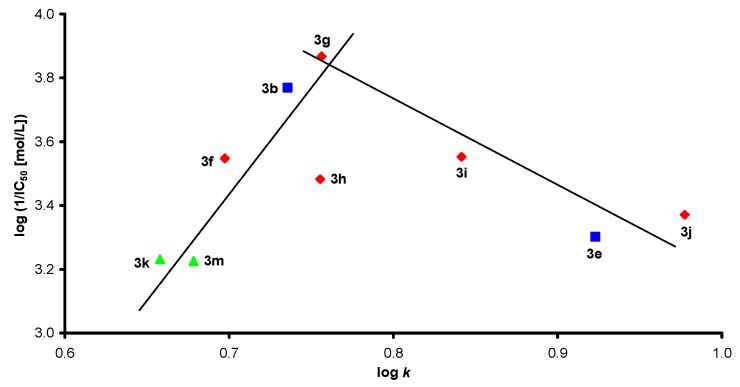
Figure 3 illustrates the dependence of the PET-inhibiting activity, expressed by the negative logarithm of the log (1/IC50[mol/L]) value on the lipophilicity, expressed as log k, of benzylidenethiosemicarbazones 3a–o. Although the dependence seems to be clear, it is not possible to draw a simple relationship. The regression with parabolic function did not provide a good correlation. This suggests that lipophilicity is only one of the important factors. Nevertheless, despite the relatively low inhibitory activity of the compounds studied, the correlations between log (1/IC50) and the lipophilicity showed a bilinear course: in the range of log k = 0.6580 (3k) to log k = 0.7563 (3g) PET-inhibiting activity linearly increased with increasing compound lipophilicity; however, with a further increase in lipophilicity, the inhibitory activity declined. Generally, 4-Br-benzylidenethiosemi-carbazones 3k–o showed the worst PET inhibition. Disubstitution by 3,4-Cl of the benzylidene ring seems to be more advantageous than disubstitution by 2,3-Cl of the benzylidene moiety. It can be stated that within the 3,4-Cl-benzylidenethiosemicarbazones 3f–j N,N-disubstitution caused a decrease in PET inhibition (compare 3g/3h). Based on these facts, it can be concluded that PET-inhibiting activity would probably be negatively influenced by bulky or long-chain alkyls or by the substitution of the second hydrogen of the terminal amino moiety.
Figure 3.
Relationships between PET inhibition log (1/IC50) [mol/L] in spinach chloroplasts and lipophilicity of studied benzylidenethiosemicarbazones 3a–o.
2.3.2. In Vitro Antifungal Susceptibility Testing
The antifungal activity of the compounds studied was tested against several pathogenic fungi and the results of the screening are shown in Table 3. The values are correlated to the standard drug fluconazole. The majority of the compounds studied appeared to be inactive against the fungal strains tested. Nevertheless, some of them, 3h, 3m, and 4l, showed moderate activity, especially against Candida albicans, Trichosporon beigelii, and Trichophyton mentagrophytes. (E)-2-(Quinolin-2-ylvinyl)-N,N-dimethylhydrazinecarbothioamide (4b) expressed the strongest activity among all of the compounds tested. Compound 4b was more active than fluconazole against Aspergillus fumigatus and Absidia corymbifera. Furthermore, this compound showed an interesting pattern of long terminal activity and was active at a comparable level for 24 h and 48 h protocols. The (Candida tropicalis and Candida krusei) activity of fluconazole decreased significantly for some fungi.
As most compounds showed only a moderate or no activity, no thorough structure-activity relationships could be established. According to the results presented in Table 3, it can be concluded that compounds with a quinoline nucleus, i.e., quinolinylvinylthiosemicarbazones (Group 2) seem to be more effective antifungals than Group 1, i.e., benzylidenethiosemicarbazones. Based on recent results [32], it was expected that 8-hydroxyquinolinylvinylthiosemicarbazones would possess a higher antifungal activity than antifungal tests showed. It can be stated, contrary to PET-inhibiting activity, that N,N-disubstitution of the terminal amino moiety by two methyl groups is crucial for a high antifungal effect. This fact corresponds to results obtained by Opletalova et al. [26].
2.3.3. In Vitro Antiproliferative Activity
The antiproliferative activity of all of the compounds was examined against the human colon cancer (HCT-116) cell line using an MTS assay. The HCT-116 p53 wild type cell line was chosen on the basis of our former experience and some results on the activity of quinoline-based thiosemicarbazones that was published recently. Namely, compounds 4a–l have been tested previously for their wide-ranging anticancer activity [34]. The antiproliferative activity of the compounds evaluated was assessed using Doxorubicin as a reference compound. In general, quinoline-based thiosemicarbazones 4 seem to have a much better anticancer activity than halogenophenyl compounds 3. Thus, the quinoline nucleus can be defined as being crucial for antiproliferative activity. A more detailed analysis of the anticancer activity of quinoline-based thiosemicarbazone is available [34]. Benzylidenethiosemicarbazones were much less active against the cells tested. Compound 3a appeared to be the most active in this assay. Nevertheless, some more general conclusions can be drawn from the gathered data. Dichlorosubstituted compounds are in general more active than monobromo equivalents. The free amino group or substitution pattern with small substituents such as methyl or ethyl are favorable for activity. According to our former results the chelating ability of the compounds may be one of the prerequisites of the antiproliferative activity. In this study we have measured the UV-VIS spectra of the compounds in presence of various concentration of Fe3+ cations to observe its chelating potency (supplementary material). Isosbestic points are observable in case of more active compounds like 4a–l series while in 3a–o only minor or no interaction between ligand and metal can be noticed. Compound 4b one of the most active against HCT-116 cells and in antifungal assays gave clear isosbestic points at 375 nm. However as 4j give also two points at 330 and 385 nm, iron chelating ability should be regarded only as one of the factor influencing the biological activity.
3. Experimental
3.1. General
All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Princeton Chemicals Ltd. (Luton, Bedfordshire, UK). Kieselgel 60, 0.040–0.063 mm (Merck, Darmstadt, Germany) was used for the column chromatography. Syntheses were performed in a CEM DISCOVERY microwave reactor (Matthews, NC, USA) with temperature and pressure control. TLC experiments were performed on alumina-backed silica gel 40 F254 plates (Merck, Darmstadt, Germany). The plates were illuminated under UV (254 nm) and evaluated in an iodine vapor. The melting points were determined on an Optimelt MPA100 instrument (SRS, USA) and are uncorrected. Infrared (IR) spectra were recorded on a Smart MIRacle™ ATR ZnSe for Nicolet™ Impact 410 FT-IR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The spectra were obtained by the accumulation of 256 scans with 2 cm−1 resolution in the region of 4000–600 cm−1. All 1H and 13C-NMR spectra were recorded on a Bruker Avance III 400 MHz FT-NMR spectrometer (400 MHz for 1H and 100 MHz for 13C, BrukerBioSpin Comp., Karlsruhe, Germany). Chemicals shifts are reported in ppm (δ) using internal Si(CH3)4 as the reference with diffuse, easily exchangeable signals being omitted. HR-MS (EI) analysis was performed on a Finnigan MAT95 spectrometer (ThermoFinnigan, San Jose, CA, USA) for all new compounds.
3.2. Synthesis
3.2.1. General Procedure for Synthesis of Thiosemicarbazones 3a–o
Equimolar quantities of an appropriate thiosemicarbazide and benzaldehyde derivative were mixed and 2 drops of glacial acetic acid were added. The resulting mixture was heated in a microwave reactor at 85 °C for 12 min (max microwave power 75 W). After cooling, the precipitated solids were filtered off, washed with ether and crystallized from methanol. The compounds 3a–o studied are presented in Table 1.
(E)-2-(2,3-Dichlorobenzylidene)hydrazinecarbothioamide (3a). Yield: 78%, 1H-NMR (d6-DMSO, 400 MHz, ppm): 11.69 (bs, 1H, NH), 8.49 (s, 1H, CH), 8.36 (s, 1H, NH), 8.30 (d, J = 8.0 Hz, 1H, ArH), 8.18 (bs, 1H, NH), 7.66 (d, J = 7.9 Hz, 1H, ArH), 7.38 (t, J = 8.0 Hz, 1H, ArH). 13C-NMR (d6-DMSO, 100 MHz, ppm): 178.8, 138.2, 134.2; 132.6, 131.6, 131.2, 128.5, 126.5. MP: 226–227 °C HR-MS (EI): 246.9745 (calc. for C8H7Cl2N3S: 246.9738).
(E)-2-(2,3-Dichlorobenzylidene)-N-methylhydrazinecarbothioamide (3b). Yield: 74%, 1H-NMR (d6-DMSO, 400 MHz, ppm): 11.76 (s, 1H, NH), 8.68 (d, J = 4.4 Hz, 1H, NH), 8.49 (s, 1H, CH), 8.29 (d, J = 8.0 Hz, 1H, ArH), 7.67 (d, J = 7.9 Hz, 1H, ArH), 7.41 (t, J = 7.9 Hz, 1H, ArH), 3.02 (d, J = 4.5 Hz, CH3). 13C-NMR (d6-DMSO, 100 MHz, ppm): 178.5, 137.7, 134.5, 132.5, 131.4, 131.3, 128.4, 126.2, 31.4. MP: 223–224 °C, HR-MS (EI): 260.9891 (calc. for C9H9Cl2N3S: 260.9894).
(E)-2-(2,3-Dichlorobenzylidene)-N,N-dimethylhydrazinecarbothioamide (3c). Yield: 69%, 1H-NMR (d6-DMSO, 400 MHz, ppm): 11.27 (s, 1H, NH), 8.64 (s, 1H, CH), 7.92 (dd, J1 = 7.9 Hz, J2 = 1.4 Hz, 1H, ArH), 7.66 (dd, J1 = 7.9 Hz, J2 = 1.5 Hz, 1H, ArH), 7,42 (t, J = 8.0 Hz, 1H, ArH), 3.31 (s, 6H, CH3). 13C-NMR (d6-DMSO, 100 MHz, ppm): 180.8, 140.0, 134.7, 132.7, 131.5, 131.0, 128.8, 125.7, 42.4. MP: 145–146 °C, HR-MS (EI): 275.0047 (calc. for C10H11Cl2N3S: 275.0051).
(E)-2-(2,3-Dichlorobenzylidene)-N-ethylhydrazinecarbothioamide (3b). Synthesis and more detailed analysis of the structure is available [44]. Yield: 67%, 1H-NMR (d6-DMSO, 400 MHz, ppm): 11.70 (s, 1H, NH), 8.70 (m, 1H, NH), 8.49 (s, 1H, CH), 8.31 (d, J = 7.9 Hz, 1H, ArH), 7.67(d, J = 7.9 Hz, 1H, ArH), 7.41 (t, J = 7.9 Hz, 1H, ArH), 3.70–3.52 (m, 2H, CH2), 1.16 (t, J = 7.0 Hz, 3H, CH3). 13C-NMR (d6-DMSO, 100 MHz, ppm): 177.4, 137.6, 134.4, 132.5, 131.5, 131.3, 128.5, 126.02, 38.9, 14.7. MP: 213–214 °C HR-MS (EI): 275.0054 (calc. for C10H11Cl2N3S: 275.0051), IR: 3349 (νNH), 3142 (νPhH), 2983 (νCH), 1584 (νC=N), 1542 (νC-N), 1520 (νCH), 1452 (νCH/NH), 1416 (νCH3), 1311 (νCNH), 1264 (νΝ−C/δNH), 1160(νN-N), 1108 (ωΝΗ), 1042 (ρNH), 929 , 906 (δΝCS), 782 (νCS), 737, 704 (ring), 626 (δΝ−C-N). UV-VIS (methanol; logε): 323.0 (3.96), 237.0 (3.67), 204.0 (3.91).
(E)-2-(2,3-dichlorobenzylidene)-N-phenylhydrazinecarbothioamide (3b). Yield: 79%, 1H-NMR (d6-DMSO, 400 MHz, ppm): 12.08 (bs, 1H, NH), 10.26 (s, 1H, NH), 8.62 (s, 1H, CH), 8.47 (d, J = 9.3 Hz, 1H, ArH), 7.68 (t, J = 7.1 Hz, 1H, ArH), 7.55 (d, J = 7.7 Hz, 2H, ArH), 7.43–7.37 (m, 3H, ArH), 7.23 (t, J = 7.6 Hz, 1H, ArH). 13C-NMR (d6-DMSO, 100 MHz, ppm): 176.9, 139.5, 138.9, 134.3, 132.6, 131.8, 131.5, 128.6, 126.8, 126.6, 126.0. MP: 203–204 °C, HR-MS (EI): 323.0066 (calc. for C14H11Cl2N3S: 323.0051).
(E)-2-(3,4-Dichlorobenzylidene)hydrazinecarbothioamide (3f). Synthesis and more detailed analysis of the structure is available [44]. Yield: 85%, 1H-NMR (d6-DMSO, 400 MHz, ppm): 11.56 (s, 1H, NH), 8.28 (s, 1H, NH), 8.24 (d, J = 1.7 Hz, 1H, ArH), 8.00 (s, 1H, CH), 7.72 (dd, J = 8.4, 1.8 Hz, 1H, ArH), 7.64 (d, J = 8.3 Hz, 1H, ArH). 13C-NMR (d6-DMSO, 100 MHz, ppm): 178.7, 139.9, 135.6, 132.3, 132.3, 131.2, 128.7, 128.2. MP: 205–206 °C [34]. IR: 3395, 3256 (νNH), 3154 (νPhH), 2992 (νCH), 1596 (δNH2), 1538 (νC=N), 1471 (δCH/NH), 1291 (νΝ−Χ/δNH), 1129 (νN-N), 1100 (ωΝΗ), 1065 (ρNH), 1031(νring), 938(δCH), 870, 823 (νCS), 625 (δΝ−C-N). UV-VIS (MeOH; log ε): 320.0 (4.01), 239.0 (3.63), 203.0 (3.92).
(E)-2-(3,4-Dichlorobenzylidene)-N-methylhydrazinecarbothioamide (3f). Yield: 89% 1H-NMR (d6-DMSO, 400 MHz, ppm): 11.63 (s, 1H, NH), 8.68 (d, J = 3.8 Hz, 1H, NH), 8.20 (s, 1H, CH), 8.00 (s, 1H, ArH), 7.69 (dd, J1 = 23.9, J2 = 8.3 Hz, 2H), 3.03 (d, J = 4.3 Hz, 3H). 13C-NMR (d6-DMSO, 100 MHz, ppm): 178.4, 139.4, 135.7, 132.2, 132.2, 131.2, 128.5, 128.1, 31.62. MP: 202–203 °C. HR-MS (EI): 260.9902 (calc. for C9H9Cl2N3S: 260.9894).
(E)-2-(3,4-Dichlorobenzylidene)-N,N-dimethylhydrazinecarbothioamide (3h). Yield: 79% 1H-NMR (d6-DMSO, 400 MHz, ppm): 11.12 (s, 1H, NH), 8.17 (s, 1H, CH), 7.86 (d, J = 1.7 Hz, 1H, ArH), 7.70–7.63 (m, 1H, ArH), 3.30 (s, 6H, CH3). 13C-NMR (d6-DMSO, 100 MHz, ppm): 181.0, 141.5, 136.0, 132.1, 132.0, 131.5, 128.4, 127.0, 42.5. MP: 138–139 °C HR-MS (EI): 275.0048 (calc. for C10H11Cl2N3S: 275.0051).
(E)-2-(3,4-Dichlorobenzylidene)-N-ethylhydrazinecarbothioamide (3i). Yield: 83%, 1H-NMR (d6-DMSO, 400 MHz, ppm): 11.56 (s, 1H, NH), 8.73 (t, J = 5.7 Hz, 1H, NH), 8.19 (d, J = 1.7 Hz, 1H, ArH), 8.00 (s, 1H, CH), 7.78–7.70 (m, 1H, ArH), 7.67 (d, J = 8.3 Hz, 1H), 3.61 (m, 2H, CH2), 1.16 (t, J = 7.1 Hz, 3H, CH3). 13C-NMR (d6-DMSO, 100 MHz, ppm): 177.3, 139.5, 135.6, 132.2, 132.2, 131.3, 128.6, 128.1, 38.8, 15.0. MP: 177–178 °C, HR-MS (EI): 275.0058 (calc. for C10H11Cl2N3S: 275.0051).
(E)-2-(3,4-Dichlorobenzylidene)-N-phenylhydrazinecarbothioamide (3j). Yield: 86%, 1H-NMR (d6-DMSO, 400 MHz, ppm): 11.96 (s, 1H, NH), 10.27 (s, 1H, NH), 8.35 (s, 1H, NH), 8.12 (s, 1H, Ar), 7.82 (dd, J = 8.4, 1.7 Hz, 1H, ArH), 7.68 (d, J = 8.3 Hz, 1H, ArH), 7.53 (d, J = 7.7 Hz, 2H, Ph), 7.39 (t, J = 7.8 Hz, 2H, Ph), 7.24 (t, J = 7.4 Hz, 1H, Ph). 13C-NMR (d6-DMSO, 100 MHz, ppm): 176.9, 140.6, 139.5, 135.4, 132.5, 132.3, 131.2, 128.9, 128.6, 126.9, 126.1. MP: 200–201 °C [28].
(E)-2-(4-Bromobenzylidene)hydrazinecarbothioamide (3k). Yield: 69% 1H-NMR (d6-DMSO, 400 MHz, ppm): 11.48 (s, 1H, NH), 8.23 (s, 1H, NH), 8.07 (s, 1H, NH), 8.02 (s, 1H, CH), 7.77 (d, J = 8.5 Hz, 2H, ArH), 7.59 (d, J = 8.5 Hz, 2H, ArH). 13C-NMR (d6-DMSO, 100 MHz, ppm): 178.6, 141.4, 134.0, 132.1, 129.6, 123.5. MP: 222 °C [48].
(E)-2-(4-bromobenzylidene)-N-methylhydrazinecarbothioamide (3l). Yield: 72%; 1H-NMR (d6-DMSO, 400 MHz, ppm): 11.54 (s, 1H, NH), 8.57 (d, J = 4.0 Hz, 1H), 8.01 (s, 1H, CH), 7.77 (d, J = 8.3 Hz, 2H, ArH), 7.61 (d, J = 8.4 Hz, 2H, ArH), 3.02 (d, J = 4.4 Hz, 3H, CH3). 13C-NMR (d6-DMSO, 100 MHz, ppm): 178.3, 140.8, 134.1, 132.0, 129.4, 123.4, 31.2. MP: 204–205 °C [49].
(E)-2-(4-bromobenzylidene)-N,N-dimethylhydrazinecarbothioamide (3m). Yield: 61%, 1H-NMR (d6-DMSO, 400 MHz, ppm): 11.00 (s, 1H, NH), 8.17 (s, 1H, CH), 7.71–7.45 (m, 4H, ArH), 3.29 (s, 6H, CH3). 13C-NMR (d6-DMSO, 100 MHz, ppm): 181.0, 143.0, 134.4, 132.2, 129.0, 123.1, 42.6. MP: 150–151 °C [50].
(E)-2-(4-Bromobenzylidene)-N-ethylhydrazinecarbothioamide (3n). Synthesis and more detailed analysis of the structure is available [44]. Yield: 78%, 1H-NMR (d6-DMSO, 400 MHz, ppm): 11.47 (s, 1H, NH), 8.61 (t, J = 5.8 Hz, 1H, NH), 8.02 (s, 1H, CH), 7.77 (d, J = 8.5 Hz, 2H, ArH), 7.61 (d, J = 8.5 Hz, 2H, ArH), 3.63–3.56 (m, J = 7.0 Hz, 2H, CH2), 1.15 (t, J = 7.1 Hz, 3H, ArH). 13C-NMR (d6-DMSO, 100 MHz, ppm): 177.2, 140.9, 134.1, 132.0, 129.6, 123.4, 38.8, 15.0. MP: 199–200 °C, HR-MS(EI): 284.9944 (calc. for C10H12BrN3S Exact Mass: 284,9935), IR: 3363 (νNH), 3139 (νPhH), 2980 (νCH), 1589 (νC=N), 1538 (νC-N), 1522 (δCH), 1485 (δCH/NH), 1397 (δCNH), 1293 (νΝ−C/δNH), 1104 (νN-N), 1081 (ωΝΗ), 1066 (ρNH), 1006 (νring), 921 (δCH), 816 (νCS), 623 (δΝ−C-N). UV-VIS (MeOH, log ε): 330 (sh), 319.5 (3.98), 233.0 (3.65), 218 (sh), 202.5 (3.91).
(E)-2-(4-bromobenzylidene)-N-phenylhydrazinecarbothioamide (3o). Yield: 68%, 1H-NMR (d6-DMSO, 400 MHz, ppm): 11.88 (s, 1H, NH), 10.17 (s, 1H, NH), 8.13 (s, 1H, CH), 7.89 (d, J = 8.5 Hz, 2H, ArH), 7.63 (d, J = 8.5 Hz, 2H, ArH), 7.56 (d, J = 7.6 Hz, 2H, Ph), 7.38 (t, J = 7.8 Hz, 2H, Ph), 7.22 (t, J = 7.4 Hz, 1H, Ph). 13C-NMR (d6-DMSO, 100 MHz, ppm):176.6, 142.0, 139.5, 133.9, 132.1, 130.0, 128.5, 126.5, 125.9, 123.7. MP: 197–198 °C [49].
3.2.2. General Procedure for the Synthesis of Thiosemicarbazones 4a–l
All quinoline-based thiosemicarbazones were prepared according to a recently published procedure [34]. Equimolar quantities of an appropriate thiosemicarbazide and benzaldehyde derivative were mixed and 2 drops of glacial acetic acid were added. The resulting mixture was heated in a microwave reactor at 85 °C for 12 min (max microwave power 75 W). After cooling, the precipitated soli were filtered and washed with ether and crystallized from methanol. The compounds 4a–l studied are presented in Table 2.
3.3. Lipophilicity Determination Using HPLC (Capacity Factor k/Calculated log k)
A Waters Alliance 2695 XE HPLC separation module and a Waters Photodiode Array Detector 2996 (Waters Corp., Milford, MA, USA) were used. A Symmetry® C18 5 μm, 4.6 × 250 mm, Part No. WAT054275 (Waters Corp.) chromatographic column was used. The HPLC separation process was monitored using Empower™ 2 Chromatography Data Software, Waters 2009 (Waters Corp.). A mixture of MeOH p.a. (55%) and H2O-HPLC-Mili-Q Grade (45%) was used as a mobile phase. The total flow of the column was 0.9 mL/min, injection volume 30 μL, column temperature 30 °C and sample temperature 10 °C. A detection wavelength of 210 nm was chosen. A KI methanolic solution was used for the determination of dead time (tD). Retention times (tR) were measured in minutes. The capacity factors k were calculated using the Empower™ 2 Chromatography Data Software according to the formula k = (tR − tD)/tD, where tR is the retention time of the solute, whereas tD denotes the dead time obtained using an unretained analyte. Log k, calculated from the capacity factor k, is used as the lipophilicity index converted to log P scale. The log k values of the individual compounds are shown in Table 1 and Table 2.
3.4. Lipophilicity Calculations
Log P, i.e., the logarithm of the partition coefficient for n-octanol/water, was calculated using the programs ChemOffice [45] and ACD/LogP [46]. Clog P values (the logarithm of n-octanol/water partition coefficient based on established chemical interactions) were generated using ChemOffice software [45]. The results are shown in Table 1 and Table 2.
3.5. Study of the Inhibition Photosynthetic Electron Transport (PET) in Spinach Chloroplasts
Chloroplasts were prepared from spinach (Spinacia oleracea L.) according to Masarovicova and Kralova [51]. The inhibition of the photosynthetic electron transport (PET) in spinach chloroplasts was determined spectrophotometrically (Genesys 6, Thermo Fisher Scientific, Waltham, MA, USA) using an artificial electron acceptor 2,6-dichlorophenol-indophenol (DCIPP) according to Kralova et al. [52] and the rate of photosynthetic electron transport was monitored as a photoreduction of DCPIP. The measurements were carried out in a phosphate buffer (0.02 mol/L, pH 7.2) containing sucrose (0.4 mol/L), MgCl2 (0.005 mol/L) and NaCl (0.015 mol/L). The chlorophyll content was 30 mg/L in these experiments and the samples were irradiated (~100 W/m2 with 10 cm distance) with a halogen lamp (250 W) using a 4 cm water filter to prevent warming of the samples (suspension temperature 22 °C). The compounds studied were dissolved in DMSO due to their limited water solubility. The applied DMSO concentration (up to 4%) did not affect the photochemical activity in the spinach chloroplasts. The inhibitory efficiency of the compounds studied was expressed by IC50 values, i.e., by the molar concentration of the compounds that caused a 50% decrease in the oxygen evolution rate relative to the untreated control. The comparable IC50 value for a selective herbicide 3-(3,4-dichlorophenyl)-1,1-dimethylurea, DCMU (Diurone®) was about 1.9 μmol/L. The results are summarized in Table 3.
3.6. In Vitro Antifungal Susceptibility Testing
The broth microdilution test [53,54,55] was used for the assessment of the in vitro antifungal activity of the synthesized compounds against Candida albicans ATCC 44859 (CA), Candida tropicalis 156 (CT), Candida krusei ATCC 6258 (CK), Candida glabrata 20/I (CG), Trichosporon beigelii 1188 (TB), Aspergillus fumigatus 231 (AF), Absidia corymbifera 272 (AC), and Trichophyton mentagrophytes 445 (TM). Fluconazole (FLU) was used as the standard since it is a clinically used antimycotic drug. The procedure was performed using a twofold dilution of the compounds in RPMI 1640 (Sevapharma a.s., Prague, Czech Republic) buffered to pH 7.0 with 0.165 mol of 3-morpholino-propane-1-sulfonic acid (MOPS, Sigma, Prague, Czech Republic). The final concentrations of the compounds ranged from 500 to 0.975 μmol/L. Drug-free controls were included. The determination of minimum inhibitory concentration (MIC) was performed according to the Clinical and Laboratory Standards Institute (CLSI, formerly NCCLS) reference protocol M27-A2 for yeasts (IC80 value) and M38-A for moulds (IC50 value). IC80 and IC50 were defined as an 80% resp. 50% or greater reduction in growth in comparison with the control. The values of MICs were determined after 24 and 48 h of static incubation at 35 °C. The final MICs for T. mentagrophytes, were determined after 72 and 120 h of incubation. The results are summarized in Table 3.
3.7. Cell Culture and in Vitro Antiproliferative Activity
The human colon cancer cell line HCT116 with the wild type p53 gene (p53+/+) were obtained from Maria Sklodowska-Curie Memorial Cancer Center in Gliwice, Poland. Briefly, the cells were seeded in 96-well plates (3 × 103 cells/well) in cellular proliferation assays 24 h before the addition of the chelators. The assay was performed following a 72 h incubation with varying concentrations of the agents. Each compound was tested in triplicate in a single experiment with each experiment being repeated 3 times. After 72 h incubation with the compounds being investigated, 20 µL of MTS solution was added to each well (100 µL) and incubated for 1 h at 37 °C. The optical densities of the samples were analyzed at 490 nm. Results were expressed as a percentage of the control. The inhibitory concentration (IC50) was defined as the compound concentration necessary to reduce the absorbance to 50% of the untreated control. The results are summarized in Table 3.
4. Conclusions
A series of fifteen substituted benzylidenethiosemicarbazones and twelve substituted quinolinylvinylthiosemicarbazones were designed, prepared and characterized. The synthesis of these compounds was carried out in microwave conditions, which improves the yield and purity of products isolated. The prepared compounds were tested for their ability to inhibit photosynthetic electron transport (PET) in spinach chloroplasts (Spinacia oleracea L.), for their antifungal activity and for their anticancer activity against human colon cancer. All of the compounds were primarily designed as potential thiosemicarbazone-based iron chelators. All of the compounds discussed showed moderate PET inhibition as well as an antifungal effect. (E)-2-(quinolin-2-ylvinyl)-N,N-dimethylhydrazine-carbothioamide (4b) expressed the strongest activity among all of the compounds tested; it was comparable to or more active than fluconazole against Aspergillus fumigatus, Absidia corymbifera, Candida tropicalis and Candida krusei. Compounds 4b and 4e expressed micromolar antiproliferative activity better than standard doxorubicin. According to the results, the quinoline nucleus and the N,N-dimethylhydrazinecarbothioamide chain can be considered as basic structural fragments for high antiproliferative and antifungal activity. Iron chelation was assumed in UV-VIS spectra analysis. According to the results we can concluded that iron chelating ability of the compounds is one of factors necessary for the activity.
Acknowledgements
This study was supported by the grant from the Polish Ministry of Science R 0504303, by the Slovak Grant Agency VEGA Grant No. 1/0612/11 and by Sanofi-Aventis Pharma Slovakia. M.S. was supported by a TWING fellowship and NCN grant DEC-2011/01/N/NZ4/01166. A.M.-W. appreciates the support of the UPGOW fellowship, NCN grant N405/068440 and Dokto-RIS studentship.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/17/11/13483/s1.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Wachtershauser G. Before enzymes and templates: Theory of surface metabolism. Microbiol. Rev. 1988;52:452–484. doi: 10.1128/mr.52.4.452-484.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wachtershauser G. Groundworks for an evolutionary biochemistry: The iron-sulphur world. Prog. Biophys. Mol. Biol. 1992;58:85–201. doi: 10.1016/0079-6107(92)90022-X. [DOI] [PubMed] [Google Scholar]
- 3.Danielson P.B. The cytochrome P450 superfamily: Biochemistry, evolution and drug metabolism in humans. Curr. Drug Metab. 2002;3:561–597. doi: 10.2174/1389200023337054. [DOI] [PubMed] [Google Scholar]
- 4.Pietrangelo A., Schilsky M. Metal storage disorders. Forward. Semin. Liver Dis. 2011;31:231–232. doi: 10.1055/s-0031-1286053. [DOI] [PubMed] [Google Scholar]
- 5.Kalinowski D.S., Richardson D.R. The evolution of iron chelators for the treatment of iron overload disease and cancer. Pharmacol. Rev. 2005;57:547–583. doi: 10.1124/pr.57.4.2. [DOI] [PubMed] [Google Scholar]
- 6.Kalinowski D.S., Richardson D.R. Future of toxicology-iron chelators and differing modes of action and toxicity: The changing face of iron chelation therapy. Chem. Res. Toxicol. 2007;20:715–720. doi: 10.1021/tx700039c. [DOI] [PubMed] [Google Scholar]
- 7.Richardson D.R., Kalinowski D.S., Lau S., Jansson P.J., Lovejoy D.B. Cancer cell iron metabolism and the development of potent iron chelators as anti-tumour agents. Biochim. Biophys. Acta. 2009;1790:702–717. doi: 10.1016/j.bbagen.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Richardson D.R., Tran E.H., Ponka P. The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents. Blood. 1995;86:4295–4306. [PubMed] [Google Scholar]
- 9.Richardson D.R. The therapeutic potential of iron chelators. Expert Opin. Investig. Drugs. 1999;8:2141–2158. doi: 10.1517/13543784.8.12.2141. [DOI] [PubMed] [Google Scholar]
- 10.Yu Y., Kalinowski D.S., Kovacevic Z., Siafakas A.R., Jansson P.J., Stefani C., Lovejoy D.B., Sharpe P.C., Bernhardt P.V., Richardson D.R. Thiosemicarbazones from the old to new: Iron chelators that are more than just ribonucleotide reductase inhibitors. J. Med. Chem. 2009;52:5271–5294. doi: 10.1021/jm900552r. [DOI] [PubMed] [Google Scholar]
- 11.Richardson D.R., Baker E. The uptake of iron and transferrin by the human malignant melanoma cell. Biochim. Biophys. Acta. 1990;1053:1–12. doi: 10.1016/0167-4889(90)90018-9. [DOI] [PubMed] [Google Scholar]
- 12.Richardson D.R., Baker E. Two mechanisms of iron uptake from transferrin by melanoma cells. The effect of desferrioxamine and ferric ammonium citrate. J. Biol. Chem. 1992;267:13972–13979. [PubMed] [Google Scholar]
- 13.Thelander L., Gräslund A., Thelander M. Continual presence of oxygen and iron required for mammalian ribonucleotide reduction: Possible regulation mechanism. Biochem. Biophys. Res. Commun. 1983;110:859–865. doi: 10.1016/0006-291X(83)91040-9. [DOI] [PubMed] [Google Scholar]
- 14.Yuan J., Lovejoy D.B., Richardson D.R. Novel di-2-pyridyl-derived iron chelators with marked and selective antitumor activity: In vitro and in vivo assessment. Blood. 2004;104:1450–1458. doi: 10.1182/blood-2004-03-0868. [DOI] [PubMed] [Google Scholar]
- 15.Prasad T., Chandra A., Mukhopadhyay C.K., Prasad R. Unexpected link between iron and drug resistance of Candida spp.: Iron depletion enhances membrane fluidity and drug diffusion, leading to drug-susceptible cells. Antimicrob. Agents Chemother. 2006;50:3597–3606. doi: 10.1128/AAC.00653-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holbein B.E., Mira de Orduña R. Effect of trace iron levels and iron withdrawal by chelation on the growth of Candida albicans and Candida vini. FEMS Microbiol. Lett. 2010;307:19–24. doi: 10.1111/j.1574-6968.2010.01956.x. [DOI] [PubMed] [Google Scholar]
- 17.Nevitt T. War-Fe-re: Iron at the core of fungal virulence and host immunity. Biometals. 2011;24:547–558. doi: 10.1007/s10534-011-9431-8. [DOI] [PubMed] [Google Scholar]
- 18.Kuipers M.E., Beljaars L., van Beek N., de Vries H.G., Heegsma J., van Den Berg J.J.M., Meijer D.K.F., Swart P.J. Conditions influencing the in vitro antifungal activity of lactoferrin combined with antimycotics against clinical isolates of Candida. Impact on the development of buccal preparations of lactoferrin. Acta Pathol. Microbiol. Immunol. Scand. 2002;110:290–298. doi: 10.1034/j.1600-0463.2002.100403.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuipers M.E., de Vries H.G., Eikelboom M.C., Meijer D.K., Swart P.J. Synergistic fungistatic effects of lactoferrin in combination with antifungal drugs against clinical Candida isolates. Antimicrob. Agents Chemother. 1999;43:2635–2641. doi: 10.1128/aac.43.11.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spellberg B., Ibrahim A.S., Chin-Hong P.V., Kontoyiannis D.P., Morris M.I., Perfect J.R., Fredricks D., Brass E.P. The deferasirox-ambisome therapy for mucormycosis (DEFEAT Mucor) study: A randomized, double-blinded, placebo-controlled trial. J. Antimicrob. Chemother. 2012;67:715–722. doi: 10.1093/jac/dkr375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnelly J.P., Lahav M. Deferasirox as adjunctive therapy for mucormycosis. J. Antimicrob. Chemother. 2012;67:201–202. doi: 10.1093/jac/dkr540. [DOI] [PubMed] [Google Scholar]
- 22.Konstantinovic S., Radovanovic B., Sovilj S., Stanojevic S. Antimicrobial activity of some isatin-3-thiosemicarbazone complexes. J. Serb. Chem. Soc. 2008;73:7–13. doi: 10.2298/JSC0801007K. [DOI] [Google Scholar]
- 23.Kizilcikli I., Kurt Y.D., Akkurt B., Genel A.Y., Birteksoz S., Otuk G., Ulkuseven B. Antimicrobial activity of a series of thiosemicarbazones and their Zn(II) and Pd(II) complexes. Folia Microbiol. 2007;52:15–25. doi: 10.1007/BF02932132. [DOI] [PubMed] [Google Scholar]
- 24.Kulandaivelu U., Padmini V.G., Suneetha K., Shireesha B., Vidyasagar J.V., Rao T.R.KN.J., Basu A., Jayaprakash V. Synthesis, antimicrobial and anticancer activity of new thiosemicarbazone derivatives. Arch. Pharm. 2011;344:84–90. doi: 10.1002/ardp.201000201. [DOI] [PubMed] [Google Scholar]
- 25.Pervez H., Iqbal M.S., Tahir M.Y., Nasim F.H., Choudhary M.I., Khan K.M. In vitro cytotoxic, antibacterial, antifungal and urease inhibitory activities of some N4-substituted isatin-3-thiosemicarbazones. J. Enzym. Inhib. Med. Chem. 2008;23:848–854. doi: 10.1080/14756360701746179. [DOI] [PubMed] [Google Scholar]
- 26.Opletalova V., Kalinowski D.S., Vejsova M., Kunes J., Pour M., Jampilek J., Buchta V., Richardson D.R. Identification and characterization of thiosemicarbazones with antifungal and antitumor effects: Cellular iron-chelation mediating cytotoxic activity. Chem. Res. Toxicol. 2008;21:1878–1889. doi: 10.1021/tx800182k. [DOI] [PubMed] [Google Scholar]
- 27.Li Z.-C., Chen L.-H., Yu X.-J., Hu Y.-H., Song K.-K., Zhou X.-W., Chen Q.-X. Inhibition kinetics of chlorobenzaldehyde thiosemicarbazones on mushroom tyrosinase. J. Agric. Food Chem. 2010:12537–12540. doi: 10.1021/jf1033625. [DOI] [PubMed] [Google Scholar]
- 28.de Aquino T.M., Liesen A.P., da Silva R.E., Lima V.T., Carvalho C.S., de Faria A.R., de Araujo J.M., de Lima J.G., Alves A.J., de Melo E.J.T., et al. Synthesis, anti-Toxoplasma gondii and antimicrobial activities of benzaldehyde 4-phenyl-3-thiosemicarbazones and 2-[(phenylmethylene)hydrazono]-4-oxo-3-phenyl-5-thiazolidineacetic acids. Bioorg. Med. Chem. 2008;16:446–456. doi: 10.1016/j.bmc.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Jampilek J., Musiol R., Finster J., Pesko M., Carroll J., Kralova K., Vejsova M., O’Mahony J., Coffey A., Dohnal J., Polanski J. Investigating biological activity spectrum for novel styrylquinazoline analogues. Molecules. 2009;14:4246–4265. doi: 10.3390/molecules14104246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jampilek J., Musiol R., Pesko M., Kralova K., Vejsova M., Carroll J., Coffey A., Finster J., Tabak D., Niedbala H., et al. Ring-substituted 4-hydroxy-1H-quinolin-2-ones: Preparation and biological activity. Molecules. 2009;14:1145–1159. doi: 10.3390/molecules14031145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musiol R., Serda M., Hensel-Bielowka S., Polanski J. Quinoline-based antifungals. Curr. Med. Chem. 2010;17:1960–1973. doi: 10.2174/092986710791163966. [DOI] [PubMed] [Google Scholar]
- 32.Musiol R., Jampilek J., Buchta V., Silva L., Niedbala H., Podeszwa B., Palka A., Majerz-Maniecka K., Oleksyn B., Polanski J. Antifungal properties of new series of quinoline derivatives. Bioorg. Med. Chem. 2006;14:3592–3598. doi: 10.1016/j.bmc.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Musiol R., Jampilek J., Nycz J.E., Pesko M., Carroll J., Kralova K., Vejsova M., O’Mahony J., Coffey A., Mrozek A., Polanski J. Investigating the activity spectrum for ring-substituted 8-hydroxyquinolines. Molecules. 2010;15:288–304. doi: 10.3390/molecules15010288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serda M., Kalinowski D.S., Mrozek-Wilczkiewicz A., Musiol R., Szurko A., Ratuszna A., Pantarat N., Kovacevic Z., Merlot A.M., Richardson D.R., Polanski J. Synthesis and characterization of quinoline-based thiosemicarbazones and correlation of cellular iron-binding efficacy to anti-tumor efficacy. Bioorg. Med. Chem. Lett. 2012;22:5527–5531. doi: 10.1016/j.bmcl.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 35.Green D.A., Antholine W.E., Wong S.J., Richardson D.R., Chitambar C.R. Inhibition of malignant cell growth by 311, a novel iron chelator of the pyridoxal isonicotinoyl hydrazone class: effect on the R2 subunit of ribonucleotide reductase. Clin. Cancer Res. 2001;7:3574–3579. [PubMed] [Google Scholar]
- 36.Richardson D.R., Sharpe P.C., Lovejoy D.B., Senaratne D., Kalinowski D.S., Islam M., Bernhardt P.V. Dipyridyl thiosemicarbazone chelators with potent and selective antitumor activity form iron complexes with redox activity. J. Med. Chem. 2006;49:6510–6521. doi: 10.1021/jm0606342. [DOI] [PubMed] [Google Scholar]
- 37.Kalinowski D.S., Yu Y., Sharpe P.C., Islam M., Liao Y.T., Lovejoy D.B., Kumar N., Bernhardt P.V., Richardson D.R. Design, synthesis, and characterization of novel iron chelators: Structure−activity relationships of the 2-benzoylpyridine thiosemicarbazone series and their 3-nitrobenzoyl analogues as potent antitumor agents. J. Med. Chem. 2007;50:3716–3729. doi: 10.1021/jm070445z. [DOI] [PubMed] [Google Scholar]
- 38.Musiol R., Kowalczyk W. Azole antimycotics-A highway to new drugs or a dead end? Curr. Med. Chem. 2012;19:1378–1388. doi: 10.2174/092986712799462621. [DOI] [PubMed] [Google Scholar]
- 39.Roche J.L., Boyd P.W., McKay R.M.L., Geider R.J. Flavodoxin as an in situ marker for iron stress in phytoplankton. Nature. 1996;382:802–805. doi: 10.1038/382802a0. [DOI] [Google Scholar]
- 40.Pospisil P. Enzymatic function of cytochrome b559 in photosystem II. J. Photochem. Photobiol. B. 2011;104:341–347. doi: 10.1016/j.jphotobiol.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Hansch R., Mendel R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl) Curr. Opin. Plant Biol. 2009;12:259–266. doi: 10.1016/j.pbi.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Nouet C., Motte P., Hanikenne M. Chloroplastic and mitochondrial metal homeostasis. Trends Plant Sci. 2011;16:395–404. doi: 10.1016/j.tplants.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Pilon M., Cohu C.M., Ravet K., Abdel-Ghany S.E., Gaymard F. Essential transition metal homeostasis in plants. Curr. Opin. Plant Biol. 2009;12:347–357. doi: 10.1016/j.pbi.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Serda M., Malecki J.G., Mrozek-Wilczkiewicz A., Musiol R., Polanski J. Microwave assisted synthesis, X-ray crystallography and DFT calculations of selected aromatic thiosemicarbazones. J. Mol. Struct. 2012 in press. [Google Scholar]
- 45.CS ChemOfficeUltra, version 12.0; The World’s Premier Desktop Chemistry Software. CambridgeSoft; Cambridge, MA, USA: 2008. [Google Scholar]
- 46.ACD/LogP version 1.0; Software used for calculating logP values. Advanced Chemistry Development; Toronto, ON, Canada: 2006. [Google Scholar]
- 47.Musiol R., Jampilek J., Podeszwa B., Finster J., Tabak D., Dohnal J., Polanski J. RP-HPLC determination of lipophilicity in series of quinoline derivatives. Cent. Eur. J. Chem. 2009;7:586–597. doi: 10.2478/s11532-009-0059-2. [DOI] [Google Scholar]
- 48.Taft R.W. In: Steric Effects in Organic Chemistry. Newman M.S., editor. John Willey & Sons; New York, NY, USA: 1956. pp. 556–675. [Google Scholar]
- 49.Dimmock J.R., Puthucode R.N., Lo M.S., Quail J.W., Yang J., Stables J.P. Structural modifications of the primary amino group of anticonvulsant aryl semicarbazones. Pharmazie. 1996;51:83–88. [PubMed] [Google Scholar]
- 50.Walter W., Rohloff C. Oxidation-products of carbothioamides. 39. Configuration and hindered rotation of 4-monodisubstituted and 4,4-disubstituted thiosemicarbazone S,S,S-trioxides. Justus Liebigs Ann. Chem. 1977;447-462 [Google Scholar]
- 51.Masarovicova E., Kralova K. Approaches to measuring plant photosynthesis activity. In: Pessarakli M., editor. Handbook of Photosynthesis. 2nd. Taylor & Francis Group; Boca Raton, FL, USA: 2005. pp. 617–656. [Google Scholar]
- 52.Kralova K., Sersen F., Sidoova E. Photosynthesis inhibition produced by 2-alkylthio-6-R-benzothiazoles. Chem. Pap. 1992;46:348–350. [Google Scholar]
- 53.Sheehan D.J., Espinel-Ingroff A., Steele M., Webb C.D. Antifungal susceptibility testing of yeasts: A brief overview. Clin. Infect. Dis. 1993;17:494–500. doi: 10.1093/clinids/17.Supplement_2.S494. [DOI] [PubMed] [Google Scholar]
- 54.Clinical Laboratory Standards Institute (CLSI) Reference Method for Broth Dilution Testing of Yeasts: Approved Standard M27-A2. CLSI; Wayne, PA, USA: 2002. [Google Scholar]
- 55.Clinical Laboratory Standards Institute (CLSI) Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts: Approved Guideline M44-A. CLSI; Wayne, PA, USA: 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.