Abstract
The hydroalcoholic extract of the steam bark of B. fagaroides var. fagaroides displayed potent cytotoxic activity against four cancer cell lines, namely KB (ED50 = 9.6 × 10−2 μg/mL), PC-3 (ED50 = 2.5 × 10−1 μg/mL), MCF-7 (ED50 = 6.6 μg/mL), and HF-6 (ED50 = 7.1 × 10−3 μg/mL). This extract also showed anti-tumour activity when assayed on mice inoculated with L5178Y lymphoma cells. Bioactivity-directed isolation of this extract, afforded seven podophyllotoxin-type lignans identified as podophyllotoxin (1), β-peltatin-A-methylether (2), 5′-desmethoxy-β-peltatin-A-methylether (3), desmethoxy-yatein (4), desoxypodophyllotoxin (5), burseranin (6), and acetyl podophyllotoxin (7) by 1D and 2DNMR and FAB-MS analyses, and comparison with reported values. All the isolated compounds showed potent cytotoxic activity in the cell lines tested, especially compound 3, which exhibited greater activity than camptothecin and podophyllotoxin against PC-3 (ED50 = 1.0 × 10−5 μg/mL), and KB (ED50 = 1.0 × 10−5 μg/mL). This is the first report of the isolation of podophyllotoxin and its acetate in a Bursera species.
Keywords: Bursera fagaroides var. fagaroides, lignans, podophyllotoxin, cytotoxic activity, antitumoral
1. Introduction
Podophyllotoxin (1) is one of the well-known bioactive naturally occurring aryltetralin lignans. This compound and its derivatives have great significance because of its biological activities, mainly as strong antineoplastic drugs and antiviral agents. Many semisynthetic derivatives of 1, developed and tested for anticancer activity, have resulted in the commercial production of three glucosidic cyclic acetals of epipodophyllotoxin, that is, etoposide, teniposide, and etopophos. They are currently used in chemotherapy for various types of cancer, including small cell lung cancer, testicular carcinoma, lymphoma, and Kaposi’s sarcoma [1,2,3]. Some reviews on its distribution, sources, applications, synthesis and structure-activity relationship of podophyllotoxin have been published [1,4,5].
The genus Bursera (Burseraceae), which comprises approximately 100 species distributed from the southwestern United States to Peru, predominates in the tropical dry forests of México where about 85 species coexist and some 75 of them are endemic [6,7,8]. Several species produce an aromatic resin known as “copal”, which has been commonly burnt as incense in religious activities all over the country since ancient times [9,10]. The chemical profile of these plants includes flavonoids [11,12], triterpenes [13,14], sesquiterpenes [15,16], diterpenes [17], and lignans [18,19,20,21,22,23,24,25,26,27].
In Mexican traditional medicine a taxa complex of three Bursera fagaroides varieties (B. fagaroides var. fagaroides, B. fagaroides var. elongata and B. fagaroides var. purpusii) is described [28], which are reputed to have antitumor activity [29,30]. These are wild trees endemic to México and known as “aceitillo”, “copal” and “cuajiote amarillo”. Previous studies made on B. fagaroides, without specifying the variety studied, demonstrated that the chloroform extract showed antitumoral activity in the Walker carcinoma 256 tumor system WA16 [18], and the ethanol extract showed immunomodulator and antitumoral activities in the mouse lymphoma L5178Y cell line [31]. On the other hand, the ethanol extract from the bark of this plant affects the levels of polyamines, as well as the activity of the enzyme ornithine decarboxylase (ODC) in vitro and on the growth of Entamoeba histolytica [32]. It was also studied for its immobilization and agglutination effects on human and mouse spermatozoa [33]. Two lignans, β-peltatin-A-methylether (2) and 5′-desmethoxy-β-peltatin-A-methylether (3) from this plant were active against the WA16 tumor system [18]. Recently four podophyllotoxin related lignans, including deoxypodophyllotoxin, morelensin, yatein, and desmethoxy-yatein, were isolated from the cytotoxic ethanol extract of the dried exudates [19].
On the basis of the therapeutic potential of this plant as herbal drug, and in order to define its cytotoxic potential, we undertook a bioassay-guided isolation of the cytotoxic principles present in the hydroalcoholic extract obtained from the stem bark of one of the three varieties of this complex: B. fagaroides var. fagaroides.
In this paper we report on the antitumor and potent cytotoxic activities of the hydroalcoholic extract (HA) of the steam bark of B. fagaroides var. fagaroides. Purification of this extract by bioassay-guided chromatographic methods afforded seven podophyllotoxin-type lignans, which showed important cytotoxic activities against KB (nasopharyngeal), HF-6 (colon), MCF-7 (breast), and PC-3 (prostate) cancer cell lines with ED50 values comparable to those displayed by camptothecin, podophyllotoxin and etoposide used as positive controls.
2. Results and Discussion
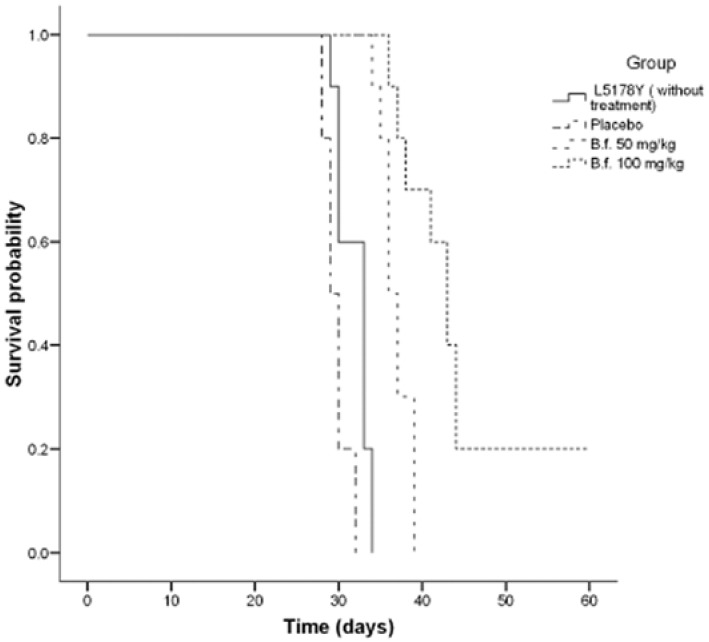
The intraperitoneal administration of 50 and 100 mg/Kg of the hydroalcoholic extract of the bark of B. fagaroides var. fagaroides (HA), on mice inoculated with 2 × 104 L5178Y lymphoma cells/mouse, showed an increase on the survival time (Figure 1). Mice with 2 × 104 L5178Y cells usually die within 30 days without treatment. When treated with the dose of 100 mg/Kg of HA extract over 15 days, the survival was significantly prolonged (p < 0.001) compared with the control groups. Median survival time for the group without HA treatment was of 29 days, while for those that received the dose of 50 and 100 mg/Kg, this time increased to 35 and 38 days, respectively. The survival of the 100 mg/Kg group was 50% better than the 50 mg/Kg group (p < 0.05). The best response was observed with the 100 mg/Kg/day dose, where the survival of treated mice was significantly prolonged (p < 0.001) compared with the placebo and control groups. This dose cured 26% of the treated mice. Survival for more than 60 days without a tumor was considered to be a ‘cure’ [31]. On the other hand, this extract also significantly inhibited the proliferation of KB (ED50 = 9.6 × 10−2 µg/mL), PC-3 (ED50 = 2.5 × 10−1 µg/mL), HF-6 (ED50 = 7.1 × 10−3 µg/mL) and MCF-7 (ED50 = 6.6 µg/mL) tumor cell lines (Table 1).
Figure 1.
Antitumor activity of Bursera fagaroides var. fagaroides HA extract evaluated for the survival rate of mice with lymphoma L5178Y, comparing the control groups (without or placebo treatments) and versus treated groups with 50 and 100 mg/Kg/day/15 days (n = 10). All of them by Kaplan-Maier estimation of survival were different (p < 0.001).
Table 1.
ED50 Values (μg/mL) of Extract, Fractions, and Active Compounds Isolated from B. fagaroides var. fagaroides against four human cancer cell lines.
| Compound | KB | PC-3 | MCF-7 | HF-6 |
|---|---|---|---|---|
| HA | 9.6 × 10−2 ± 0.07 | 2.5 × 10−1 ± 0.03 | 6.6 ± 0.01 | 7.1 × 10−3 ± 0.1 |
| F-1 | 6.0 × 10−3 ± 0.08 | 1.0 × 10−5 ± 0.006 | 8.8 × 10−1 ± 0.03 | 4.3 × 10−3 ± 0.04 |
| F-2 | 1.3 × 10−1 ± 0.02 | 1.0 × 10−5 ± 0.004 | 8.2 × 10−1 ± 0.03 | 3.6 × 10−2 ± 0.02 |
| F-1-1 | 3.94 × 10−1 ± 0.08 | 1.0 × 10−5 ± 0.1 | 8.1 ± 0.1 | 8.0 × 10−5 ± 0.03 |
| F-1-2 | 3.5 × 10−1 ± 0.02 | 7.8 × 10−4 ± 0.06 | 1.3 ± 0.08 | 6.5 × 10−3 ± 0.01 |
| F-2-1 | 1.9 × 10−1 ± 0.01 | 4.2 × 10−3 ± 0.02 | >20 | 3.5 × 10−2 ± 0.02 |
| F-2-2 | 3.2 ± 0.01 | 2.0 ± 0.1 | >20 | 2.9 ± 0.05 |
| F-2-3 | 1.0 × 10−2 ± 0.01 | 5.5 × 10−3 ± 0.01 | 2.5 × 10−7 ± 0.03 | 2.6 × 10−3 ± 0.001 |
| 1 | 1.91 × 10−6 ± 0.01 | 0.95 ± 0.005 | 1.04 × 10−5 ± 0.031 | 1.8 × 10−4 ± 0.01 |
| 2 | 0.189 ± 0.01 | 0.085 ± 0.005 | 0.798 ± 0.01 | 3.8 × 10−2 ± 0.01 |
| 3 | 1.0 × 10−5 ± 0.02 | 1.0 × 10−5 ± 0.004 | 1.02 × 10−4 ± 0.005 | 0.40 ± 0.01 |
| 4 | 0.4 ± 0.03 | 1.7 × 10−3 ± 0.01 | 0.4 ± 0.01 | 0.68 ± 0.01 |
| 5 | 1.5 ± 0.01 | 2.0 × 10−3 ± 0.003 | 1.25 ± 0.01 | 1.23 ± 0.01 |
| 6 | 2.89 ± 0.009 | 2.0 × 10−3 ± 0.005 | 3.68 ± 0.08 | 2.89 ± 0.006 |
| 7 | 1.03 ± 0.01 | 5.0 × 10−3 ± 0.005 | >4 | 2.41 ± 0.004 |
| Camptothecin | 1.58 × 10−3 ± 0.01 | 0.96 ± 0.006 | 1.28 × 10−4 ± 0.01 | 5.5 × 10−6 ± 0.01 |
| Podophyllotoxin | 8.7 × 10−5 ± 0.003 | 0.85 ± 0.009 | 9.9 × 10−5 ± 0.005 | 7.6 × 10−3 ± 0.05 |
| Etoposide | 25 × 10−3 ± 0.002 | 5.6 × 10−3 ± 0.0005 | 0.54 ± 0.009 | 0.091 ± 0.02 |
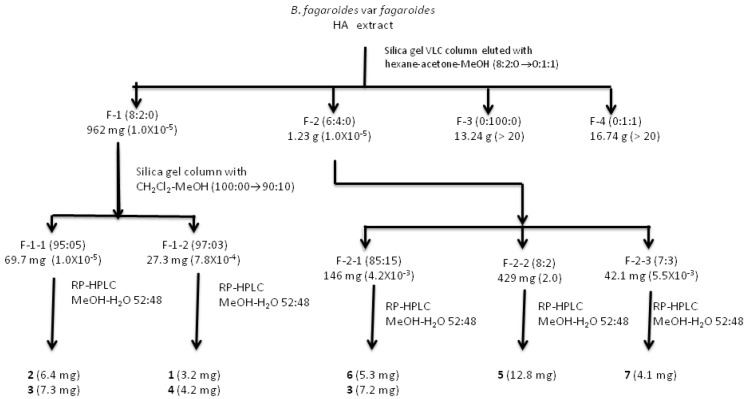
Bioassay-guided isolation procedures, using the activity against KB, HF-6, MCF-7, and PC-3 cancer cell lines were carried out to define active components in this plant. Figure 2 shows the chromatographic fractionation of the HA extract monitored by the cytotoxic activity against PC-3 cells. Chromatographic fractionation of the HA extract afforded four fractions, two of which (F-1 and F-2), displayed potent cytotoxic activity against the four tested cell lines, principally against PC-3, both with ED50 values (1 × 10−5 μg/mL) greater than that displayed by the therapeutic drugs camptothecin (0.96 μg/mL), and etoposide (5.6 × 10−3 μg/mL) used as positive controls (Table 1).
Figure 2.
Fractionation tree diagram of the HA extract, monitored by the cytotoxic activity against PC-3 cell line in culture (ED50 values in μg/mL in square brackets).
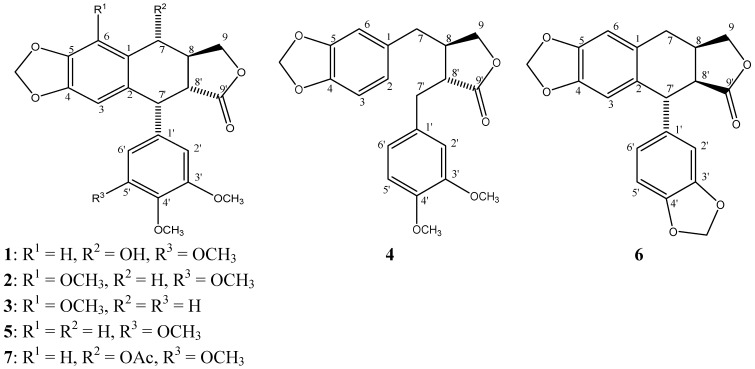
Chromatographic purification of F-1 afforded β-peltatin-A-methylether (2), 5′-desmethoxy-β-peltatin-A-methylether (3), desmethoxy-yatein (4), desoxypodophyllotoxin (5); while purification of F-2 afforded podophyllotoxin (1), burseranin, (6), and acetyl podophyllotoxin (7), which were identified by comparing their spectroscopic data with those previously described in the literature. The purity of isolated compounds was determined to be above of 95%, based on the peak areas of their HPLC chromatograms, as well as by their 1H-NMR spectra. The structures of these compounds are shown in Figure 3.
Figure 3.
Structures of compounds 1–7.
Podophyllotoxin (1) has traditionally been isolated from Podophyllum peltatum and Podophyllum emodi, but it has been found in around 20 genera such as Diphylleia, Dysosma, Catharanthus, Polygala, Anthriscus, Linum, Hyptis, Teucrium, Nepeta, Thuja, Juniperus, Cassia, Haplophyllum, Commiphora, and Hernandia [4]; β-peltatin-A-methylether (2) has been found in: Juniperus phoenicea [34], B. permollis [26], B. fagaroides [18], B. simaruba [35], Anthriscus sylvestris [36], Libocedrus plumose [37], and some Linum species and cultures [38,39,40,41], and its activity against A431, BC1, Col 2, HT, KB, LNCaP, Lu 1, Mel 2, U373, ZR-75-1 cancer cell lines [26], as well as its antitumor activity in the WA16 tumor system [18], have been described; 5′-desmethoxy-β-peltatin-A-methylether (3) has only been reported from B. fagaroides with activity against the WA16 tumor system [18]; desmethoxy-yatein (4) showed activity against P388 lymphocytic leukemia cell line [42], and has been isolated from Hernandia ovigera [43,44], H. nymphaeifolia [42], Bursera schlechtendalii [24], and B. fagaroides [19]; desoxypodophyllotoxin (5) is the most widespread aryltetralin lignan, and has been found in a great variety of plants belonging to various genera such as Libocedrus [37], Linum [41], Bursera [19], Podophyllum [45,46,47], Anthriscus [36], Diphylleia [47], Dysosma [48], Hernandia [42], among others, and its cytotoxic activity is well known [4]; burseranin (6) has been only described as a constituent of B. graveolens, and showed activity against HT1080 cell line [22]; acetyl podophyllotoxin (7) is a constituent of Podophyllum, and has been obtained from podophyllotoxin for structure-activity studies [1]. Until now, Bursera has been reported to contain only podophyllotoxin-related lignans, and this is the first report of the presence of podophyllotoxin (1) and its acetate 7 in a Bursera species.
Evaluation of the cytotoxic activity of the isolated compounds against the human cancer cell lines KB, PC-3, MCF-7, and HF-6 showed that, except for compounds 6 and 7, all the isolated compounds displayed high activity (Table 1). The results showed that compound 3 exhibited the most potent cytotoxicity against PC-3 cells, with ED50 value of 1.0 × 10−5 µg/mL; whereas podophyllotoxin (1) displayed the most potent cytotoxiciy against KB and MCF-7 cells with ED50 values of 1.91 × 10−6, and 1.04 × 10−5 μg/mL, respectively. Remarkably, the cytotoxic activities of compounds 2–6 toward PC-3 cells were greater than those of podophyllotoxin (1), as well as the anticancer chemotherapy drugs camptothecin and etoposide (Table 1). On the other hand, acetyl podophyllotoxin (7), was the only compound that was not active towards MCF-7 cells, and together with burseranin (6), showed slight activity against KB, and HF-6 tumor cell lines, showing better activity against PC-3 (5.0 × 10−3, and 2.0 × 10−3 μg/mL, respectively), than etoposide. Compared with 1, compound 2 displayed moderate activity against KB, PC-3 and MCF-7, with ED50 values ranging from 0.08 to 0.79 μg/mL, and showed the best activity against HF-6 cells with ED50 = 9.1 × 10−2. Compound 3, displayed high activity against KB (ED50 = 1.0 × 10−5 μg/mL), PC-3 (ED50 = 1.0 × 10−5 μg/mL), and MCF-7 (ED50 = 1.02 × 10−4 μg/mL), and was moderately active against HF-6 (ED50 = 0.40 μg/mL). Compounds 4 and 5 displayed similar activities against the tested cell lines; the best activity was observed against PC-3 with ED50 values of 1.7 × 10−3, and 2.0 × 10−3 μg/mL respectively. It is interesting to point out that all of the lignans isolated from B. fagaroides var. fagaroides were active against PC-3 cells.
The cytotoxic activity of podophyllotoxin (1), desoxypodophyllotoxin (5) and their congeners is well known [49,50,51,52]. Some structure-activity relationship studies, using several podophyllotoxin analogues, showed that the core structure of deoxypodophyllotoxin (5) is responsible for this cytotoxicity. The extra methoxy group on the 6-position in 5′-desmethoxy-β-peltatin-A-methylether (3) significantly changed the in vitro cytotoxicity when compared to desoxypodophyllotoxin (5). Compounds 4 and 6 which do not have the core structure of 5, displayed less potent activity against KB, MCF-7 and HF-6, and were more selective against PC-3 cell line (Table 1). Results here obtained confirm the structure-activity relationships previously described, and provide new cytotoxic data for compounds 3, 4, 6 and 7 that complement the knowledge of this type of compounds.
Although the cytotoxicity of podophyllotoxin (1) and desoxypodophyllotoxin (5) is well known, the cytotoxicity of 3, 4, 6, and 7 against the four cell lines tested, and of 2 against PC-3, MCF-7, and HF-6, is reported here for the first time.
3. Experimental
3.1. General
NMR spectra were acquired on a Varian Unity NMR spectrometer operating at 400 MHz for 1H and 100 MHz for 13C nuclei. Chemical shifts are listed in parts per million (ppm), referenced to CDCl3 and were made on the basis of 1H-1H gCOSY, 1H-1H TOCSY, NOESY, gHSQC and gHMBC spectral analysis as required. NMR experiments performed in CDCl3 are referenced to Me4Si (0 ppm). FABMS spectra in a matrix of m-nitrobenzyl alcohol or glycerol were recorded on a JEOL JMX-AX 505 HA mass spectrometer. All reagents and solvents used were analytical grade. Optical rotations were acquired with a Perkin-Elmer 241MC polarimeter (10 cm, 1 mL cell) at the sodium D line. High Performance Liquid Chromatography (HPLC) was performed using a Waters Delta Prep 4000 Module System equipped with a Waters 717 plus Autosampler and 996 Photodiode Array Detector (Waters Co., Milford, MA, USA), and a Xterra prep C18 column (5 μm, 7.8 × 100 mm) with MeOH-H2O (52:48) as the isocratic eluent system, UV detection at 215 nm and a flow rate of 1 mL/min.
3.2. Plant Material
The bark of B. fagaroides var. fagaroides (H.B.K.) Engl. was collected in the village of Capula between Zacapu and Quiroga, Michoacán, México. Its identification was made at the herbarium of the Instituto Mexicano del Seguro Social (registration number-12 051 IMSSM) and the Institute of Botany, University of Guadalajara (IBUG-140 748), México.
3.3. Extraction and Isolation
The stem bark from B. fagaroides var. fagaroides was dried under dark conditions at room temperature for 10 days. The dry material (1,420 g) was milled to obtain 2–5 mm particles and then extracted by successive percolation with n-hexane (3 L) and then with 70% aqueous methanol (MeOH, 3 L) at room temperature (three times). The obtained extracts were evaporated to dryness with a rotary evaporator under reduced pressure producing 6.14 g (0.43% yield) of n-hexane, and 33 g (6.51% yield) of hydroalcoholic dried extract, respectively. The hydroalcoholic extract was fractionated by percolation on a vacuum liquid chromatography column of silica gel (47 g) eluting with n-hexane-acetone-MeOH mixtures of increasing polarity to yield four fractions: F-1, 962 mg (8:2:0, 1.5 L), F-2, 1.23 g (6:4:0, 1.5 L), F-3, 13.24 g (0:100:0, 1.5 L), and F-4, 16.74 g (0:1:1, 3.5 L).
F-1 was chromatographed on silica gel (28.8 g) with a gradient mixture of CH2Cl2-MeOH (100:0→9:1) to give two active fractions: F-1-1 (69.7 mg), eluted with CH2Cl2-MeOH (95:5), and F-1-2 (27.3 mg), eluted with CH2Cl2-MeOH (97:3). An aliquot of 22.5 mg of F-1-1 was subjected to reverse-phase HPLC, to afford 6.4 mg of 5′-desmethoxy-β-peltatin-A methylether (3, tR = 3.38 min), and 7.3 mg of desmethoxy-yatein (4, tR = 4.21 min). The yields were based on peak areas of the HPLC chromatogram. An aliquot of 10.2 mg of F-1-2 was subjected to reverse-phase HPLC with the same conditions than F-1-1 to yield 3.2 mg of β-peltatin-A methylether (2, tR = 13.95 min) and 4.2 mg of deoxypodophyllotoxin (5, tR = 10.55 min). The yields were based on peak areas of the HPLC chromatogram.
F-2 was subjected to column chromatography packed with silica gel (50 g) and eluted with a gradient system of n-hexane-acetone (98:2→60:40) obtaining 110 fractions of 25 mL each. One of the active fractions (F-2-1, 0.146 g), eluted with 85:15 n-hexane-acetone, was subjected to column chromatography on silica gel (4.5 g), eluted with a gradient of n-hexane-acetone (95:5→8:2) to give 18.5 mg of a mixture of two compounds which were purified by HPLC with MeOH-H2O 52:48 as the isocratic eluent system, to afford 5.3 mg of acetyl podophyllotoxin (7, tR = 8.08 min), and 7.2 mg of desmethoxy-yatein (4, tR = 4.10 min). An aliquot of the second fraction, F-2-2 (429 mg), eluted with 8:2 n-hexane:acetone, was further purified by HPLC providing 12.8 mg of burseranin (6, tR = 7.2 min). F-2-3, eluted with 7:3 n-hexane-acetone, was purified by HPLC with MeOH-H2O 52:48 as the isocratic eluent system to yield 4.1 mg of podophyllotoxin (1, tR = 15.2 min). All the isolated compounds were identified using 1D and 2D NMR, optical rotation (OR), and HRMS analyses, and comparison with reported values.
3.4. Spectral Data
Podophyllotoxin (1). White amorphous powder; purity = 98%; [α]24D -133° (c 0.012, CHCl3); IR (KBr) γmax 2932, 1778.9, 1727, 1241.0, 937.7 cm−1; 1H-NMR (CDCl3) δ 7.11 (1H, s, H-6), 6.50 (1H, s, H-3), 6.37 (2H, s, H-2′, H-6′), 5.97 (2H, dd, J = 6, 1.6 Hz, -O-CH2-O-), 4.61 (3H, m, H-7β, H-9α, H-7′), 4.07 (1H, dd, J = 10.8, 6 Hz, H-9β), 3.80 (3H, s, CH3O-4′), 3.75 (6H, s, CH3O-3′, CH3O-5′), 2.83 (2H, m, H-8, H-8′); 13C-NMR (CDCl3) δ 174.63 (C-9′), 152.83 (C-3′, C-5′), 148.03 (C-5), 147.91 (C-4), 137.49 (C-4′), 135.63 (C-1′), 133.37 (C-2), 131.39 (C-1), 110.01 (C-3), 108.66 (C-2′, C-6′), 106.5 (C-6), 101.66 (O-CH2-O), 71.54 (C-9), 60.96 (CH3O-4′), 56.6 (CH3O-3′, CH3O-5′), 45.52 (C-8), 45.53 (C-8′), 44.31 (C-7′), 40.99 (C-7); positive FAB-MS m/z 415 (20) [M + H]+, 413 (81), 391 (59), 355 (22), 327 (41), 467 (36), 239 (31), 221(90), 207 (100), 205 (57). These data match those in the literature [53].
β-Peltatin A methyl ether (2). White amorphous powder; purity = 96%; [α]24D -113° (c 0.011, CHCl3); 1H-NMR (CDCl3) δ 6.8 (2H, s, H-2′, H-6′), 6.2 (1H, s, H-3), 5.8 (2H, s, O-CH2-O), 4.5 (1H, d, J = 4.4 Hz, H-7′), 4.4 (1H, dd, J = 8.8 Hz, H-9α), 3.9 (3H, s, CH3O-6), 3.8 (1H, dd, J = 10.4 Hz, H-9β), 3.7 (3H, s, CH3O-4′), 3.68 (6H, s, CH3O-3′, CH3O-5′), 3.1 (1H, dd, J = 4.8, 16 Hz, H 7β), 2.6 (1H, m, H-8′), 2.58 (1H, m, H-8), 2.4 (1H, dd, J = 10.4, 16 Hz, H-7α); 13C-NMR (CDCl3) δ 175.2 (C-9′), 152.9 (C-3′, C-5′), 148.6 (C-4), 141.1 (C-6), 136.4 (C-1′, C-4′), 135.1 (C-5), 132.0 (C-2), 121.2 (C-1), 109.0 (C-2′, C-6′), 104.7 (C-3), 101.2 (O-CH2-O), 72.6 (C-9), 59.6 (CH3O-6), 56.7 (CH3O-3′, CH3O-5′), 55.9 (CH3O-4′) 47.6 (C-8′), 44.1 (C-7′), 32.7 (C-8), 27.8 (C-7); positive FAB-MS m/z 428 [M + H]+ (66), 400 (13), 261 (32), 203 (15), 181 (24), 149 (51), 81 (100), 55 (98). These data match those in the literature [18,35].
5′-Desmethoxy-β-peltatin A methylether (3). White amorphous powder; purity = 99%; [α]24D -140° (c 0.018, CHCl3); 1H-NMR (CDCl3) δ 6.9 (1H, d, J = 2.4 Hz, H-2′), 6.8 (1H, d, J = 8.4 Hz, H-5′), 6.4 (1H, dd, J = 8, 2 Hz, H-6′), 6.2 (1H, s, H-3), 5.9 (2H, d, J = 4.8 Hz, O-CH2-O), 4.5 (1H, d, J = 4.4 Hz, H-7′), 4.37 (1H, t, J = 6.8 Hz, H-9α), 4.0 (3H, s, CH3O-6), 3.85 (1H, dd, J = 10 Hz, H-9β), 3.8 (3H, s, CH3O-3′), 3.7 (3H, s, CH3O-4′), 3.1 (1H, dd, J = 4.8, 16 Hz, H-7α), 2.6 (1H, m, H-8′), 2.4 (1H, m, H-8), 2.3 (1H, dd, J = 10.4, 16 Hz, H-7β); 13C NMR (CDCl3) δ 175.4 (C-9′), 148.5 (C-5), 148.47 (C-4), 148.1 (C-6), 140.9 (C-4′), 134.9 (C-1′), 133.94 (C-3′), 132.2 (C-2), 129.0 (C-1), 122.8 (C-6′), 114.7 (C-2′), 110.5 (C-5′), 104.6 (C-3), 101.1 (O-CH2-O), 72.6 (C-9), 59.6 (CH3O-6), 56.2 (CH3O-3′), 56.0 (CH3O-4′), 47.5 (C-8), 43.5 (C-7′), 32.4 (C-8′), 29.9 (C-7); positive FAB-MS m/z 370 (18) [M + H]+, 313 (5), 279 (7), 257 (4), 178 (4), 149 (100), 95 (51), 57 (79). These data match those in the literature [18].
5′-Desmethoxyyatein (4). White amorphous powder; purity = 95%; [α]24D -20° (c 0.018, CHCl3); 1H-NMR (CDCl3) δ 6.7 (1H, d, J = 7.6 Hz, H-6′), 6.67 (1H, d, J = 8 Hz, H-5′), 6.66 (1H, s, H-2′), 6.45 (1H, d, J = 7.6 Hz, H-3), 6.44 (1H, d, J = 8 Hz, H-2), 6.43 (1H, s, H-6), 5.9 (2H, d, J = 4 Hz, O-CH2-O), 4.1 (1H, dd, J = 7.2, 8.8 Hz, H-9β), 3.9 (3H, s, CH3O-3′), 3.84 (3H, s, CH3O-4′), 3.8 (1H, m, H-9α), 2.9 (1H, dd, J = 4.8 Hz, H-7′), 2.6 (1H, dd, J = 6.8 Hz, H-7β), 2.5 (2H, m, H-7α, H-8′), 2.48 (1H, m, H-8); 13C NMR (CDCl3) δ 178.8 (C-9′), 149.3 (C-3′), 148.2 (C-4′), 148.1 (C-5), 146.6 (C-4), 131.8 (C-1), 130.4 (C-1′), 121.8 (C-2), 121.6 (C-6′), 112. (C-2′), 109.0 (C-3), 111.4 (C-5′), 108.5 (C-6), 101.3 (CH2O2), 71.37 (C-9), 56.10 (CH3O-3′), 56.18 (CH3O-4′), 46.8 (C-8′), 41.3 (C-8), 38.6 (C-7), 34.9 (C-7′); positive FAB-MS m/z 370 (18) [M + H]+, 313 (5), 279 (7), 257 (4), 178 (4), 149 (100), 95 (51), 57 (79). These data match those in the literature [24].
Desoxypodophyllotoxin (5). White amorphous powder; purity = 99%; [α]24D -104° (c 0.018, CHCl3); 1H-NMR (CDCl3) δ 7.1 (1H, s, H-6), 6.5 (1H, s, H-3), 6.37 (2H, s, H-2′, H-6′), 6.0 (2H, d, J = 1.2 Hz, O-CH2-O), 4.6 (1H, m, H-9α, H-7′), 4.0 (1H, dd, J = 10.8 Hz, H-9β), 3.8 (3H, s, CH3O-4′), 3.7 (6H, s, CH3O-3′, CH3O-5′), 2.8 (3H, m, H-7α, H-7β, H-8, H-8′); 13C-NMR (CDCl3) δ 174.6 (C-9′), 152.8 (C-3′, C-5′) 148.0 (C-5), 147.9 (C-4), 137.5 (C-4′), 135.6 (C-1′), 133.4 (C-2), 131.4 (C-1), 110.1 (C-3), 108.6 (C-6), 106.5 (C-6′, C-2′) 101.6 (O-CH2-O), 71.5 (C-9), 60.9 (CH3O-4′), 56.5 (CH3O-3′), 56.5 (CH3O-5′), 45.5 (C-8), 45.5 (C-8′), 44.3 (C-7′), 40.9 (C-7). These data match those in the literature [19].
Burseranin (6). White amorphous powder; purity = 96%; [α]24D +34° (c 0.012, CHCl3); 1H-NMR (CDCl3) δ 6.7 (1H, d, J = 8.4 Hz, H-5′), 6.6 (1H, d, J = 8.0 Hz, H-6′), 6.59 (1H, s, H-2′), 6.3 (1H, s, H-3), 5.9 (2H, s, O-CH2-O-3′,4′), 5.8 (2H, s,), 4.4 (1H, dd, J = 9.2 Hz, H-9α), 4.3 (1H, d, J = 2.8 Hz, H-7′), 3.92 (3H, s, CH3O-6), 3.9 (1H, dd, J = 6.2 Hz, H-9β), 3.2 (1H, m, H-8′), 2.9 (1H, m, H-8), 2.7 (2H, m, H-7); 13C-NMR (CDCl3) δ 178.0 (C-9′), 147.9 (C-4), 147.5 (C-4′), 141.9 (C-3′), 140.5 (C-6), 135.2 (C-5), 131.8 (C-2), 121.0 (C-1′), 120.7 (C-1), 119.8 (C-6′), 108.3 (C-2′, C-5´), 104.1 (C-3), 100.72 (O-CH2-O-4,5), 100.7 (O-CH2-O-3′,4′), 73.0 (C-9), 59.5 (CH3O-6), 46.0 (C-8′), 44.7 (C-7′), 32.3 (C-8), 24.1 (C-7); positive FAB-MS m/z 382 [M + H]+ (0.4), 368 (0.4), 283 (0.6), 203 (0.5), 154 (40), 136 (35), 95 (75), 69 (91), 55 (100). These data match those in the literature [22].
Acetyl podophyllotoxin (7). White amorphous powder; purity = 99%; [α]24D -146.0° (c 0.011, CHCl3); 1H-NMR (CDCl3) δ 6.7 (1H, s, H-6), 6.5 (1H, s, H-3), 6.3 (2H, s, H-2′, H-6′), 5.9 (2H, d, J = 1 Hz, O-CH2-O), 5.8 (1H, d, J = 8.4 Hz, H-7β), 4.5 (1H, d, J = 4 Hz, H-7′), 4.3 (1H, dd, J = 6.2, 9.0 Hz, H-9α), 4.1 (1H, dd, J = 9.4 Hz, H-9β), 3.75 (3H, s, CH3O-4′), 3.7 (6H, s, CH3O-3′, CH3O-5′), 2.8 (2H, m, H-8, H-8′), 2.1 (3H, s, CH3CO); 13C-NMR (CDCl3) δ 173.7 (C-9′), 171.4 (CH3CO), 152.6 (C-3′, C-5′), 147.6 (C-4), 148.1 (C-5), 137.1 (C-4′), 134.9 (C-1′), 132.4 (C-1), 128.3 (C-2), 109.8 (C-6), 108.1 (C-2′, C-6′), 107.1 (C-3), 101.7 (O-CH2-O), 73.7 (C-9), 71.5 (C-7), 60.9 (CH3O-4′), 56.28 (CH3O-3′, CH3O-5′), 45.7 (C-7′), 43.8 (C-8′), 38.8 (C-8), 21.3 (CH3CO); positive FAB-MS m/z 456 [M + H]+ (58), 397 (21), 313 (9), 229 (7), 185 (18), 154 (53), 136 (48), 95 (41), 77 (75), 55 (100), 41 (98). These data match those in the literature [19].
3.5. Cytotoxicity Assay
The in vitro cytotoxicity was measured by the sulphorhodamine B (SRB) (MP Biomedicals, LLC) protein staining assay [54,55] using KB (nasopharyngeal), HF-6 (colon), MCF7 (breast), and PC-3 (prostate) cancer cell lines. The cell cultures were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 5,000 units/mL penicillin, 5 mg/mL streptomycin, 7.5% NaHCO3, and cultured in a 96-well microtiter plate (104 cells/mL, 190 μL/well) at 37 °C in a 5% CO2-air atmosphere (100% humidity). The cells at the log phase of growth were treated in triplicate (n = 3) with different concentrations of the test compounds (0.16, 0.8, 4 and 20 μg/mL), and incubated for 72 h. The cell concentration was determined by protein analysis. The optical density was measured at 590 nm with an ELISA-Reader (Molecular Devices, SPECTRA max plus 384). Results were expressed as the concentration that inhibits 50% of control growth after the incubation period (IC50). The values were estimated from a semi-log plot of the extract concentration (μg/mL) against the percentage of viable cells. Camptothecin, etoposide, and podophyllotoxin were included as positive standards.
3.6. Antitumor Activity
Male BALB/c mice (6–8 weeks old, 22–26 g) were provided by the Centro de Investigación Biomedica de Occidente (CIBO-IMSS). A lymphoma L5178Y cell line was used derived from a thymic lineage (haplotype H-2d) tumor induced in DBA/2 mouse by methyl-cholanthrene adopted to an ascetic form, and maintained by intraperitoneal (i.p.) transplantation of 10 × 106 cells/mouse every 15 days in syngenic BALB/c mice [31]. For this study, all procedures involving animals were performed according to protocols approved by NOM-062-ZOO-1999. Animals were inoculated i.p. with 0.1 mL of suspension of fresh ascitic fluid, containing L5178Y lymphoma (2 × 104) cells/mouse on day zero. Treatment with HA extract started 24 h after inoculation at doses of 50 or 100 mg/kg oral rout/day during 15 days, each group containing five mice and were observed during 60 days.
3.7. Statistical Analysis
The results were analyzed using one-way ANOVA followed by Kaplan-Meier estimation of survival and Cox’s regression through the statistical package SPSS V.15.
4. Conclusions
Bioassay-guided isolation of the hydroalcoholic extract obtained from the steam bark of B. fagaroides var. fagaroides identified a family of seven related lignans, among which podophyllotoxin (1) and acetyl podophyllotoxin (7) are described by the first time in Bursera. The presence of podophyllotoxin (1), together with six other related lignans in the cytotoxic extract of B. fagaroides var. fagaroides is noteworthy. In summary the cytotoxic and antitumor activities observed for B. fagaroides var. fagaroides are ascribable to the lignans present in this extract. Investigation of the podophyllotoxin-related lignans obtained from B. fagaroides var. fagaroides may lead to new cytostatic compounds, which could serve as the basis for new anti-tumor drugs.
Acknowledgments
Partial financial support from CONACYT, México (Grants No. 82851, 80980 and LN 56431) and UNAM (Grants IX201110, IN219309) is acknowledged. A. M. R. S. thanks CONACYT for fellowship (219701).
Footnotes
Sample Availability: Samples of the compounds 1–7 are available from the authors.
References
- 1.Xu H., Lv M., Tian X. A review on hemisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins: 2003–2007. Curr. Med. Chem. 2009;16:327–349. doi: 10.2174/092986709787002682. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava V., Negi A.S., Kumar J.K., Gupta M.M., Khanuja S.P.S. Plant-based anticancer molecules: A chemical and biological profile of some important leads. Bioorg. Med. Chem. 2005;13:5892–5908. doi: 10.1016/j.bmc.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann J.T., Lipp H.P. Camptothecin and podophyllotoxin derivatives: Inhibitors of topoisomerase I and II-Mechanisms of action, pharmacokinetics and toxicity profile. Drug Saf. 2006;29:209–230. doi: 10.2165/00002018-200629030-00005. [DOI] [PubMed] [Google Scholar]
- 4.Gordaliza M., Garcia P.A., del Corral J.M., Castro M.A., Gomez-Zurita M.A. Podophyllotoxin: Distribution, sources, applications and new cytotoxic derivatives. Toxicon. 2004;44:441–459. doi: 10.1016/j.toxicon.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Botta B., Monache G.D., Misiti D., Vitali A., Zappia G. Aryltetralin lignans: Chemistry, pharmacology and biotransformations. Curr. Med. Chem. 2001;8:1363–1381. doi: 10.2174/0929867013372292. [DOI] [PubMed] [Google Scholar]
- 6.Becerra J.X. Evolution of Mexican Bursera (Burseraceae) inferred from ITS, ETS, and 5S nuclear ribosomal DNA sequences. Mol. Phylogenet. Evol. 2003;26:300–309. doi: 10.1016/S1055-7903(02)00256-7. [DOI] [PubMed] [Google Scholar]
- 7.Becerra J.X., Venable D.L. Nuclear ribosomal DNA phylogeny and its implications for evolutionary trends in Mexican Bursera (Burseraceae) Am. J. Bot. 1999;86:1047–1057. doi: 10.2307/2656622. [DOI] [PubMed] [Google Scholar]
- 8.Rzedowski J., Kruze H. Algunas tendencias evolutivas en Bursera (Burseraceae) Taxon. 1979;28:103–116. doi: 10.2307/1219565. [DOI] [Google Scholar]
- 9.Peter C.M., Purata S.E., Chibnik M., Brosi B.J., López A.M., Amrosio M. The life and times of Bursera glabrifolia (H.B.K.) Engl. In México: A parable for ethnobotany. Econ. Bot. 2003;57:431–441. [Google Scholar]
- 10.Case R.J., Tucker A.O., Maciarello M.J., Wheeler K.A. Chemistry and ethnobotany of commercial incense copals, copal blanco, copal oro, and copal negro, of North America. Econ. Bot. 2003;57:189–202. doi: 10.1663/0013-0001(2003)057[0189:CAEOCI]2.0.CO;2. [DOI] [Google Scholar]
- 11.Nakanishi T., Inatomi Y., Satomi A., Yamada T., Fukatsu H., Murata H. New luteolin 3-O-acylated rhamnosides from leaves of Bursera graveolens. Heterocycles. 2003;60:2077–2083. doi: 10.3987/COM-03-9822. [DOI] [Google Scholar]
- 12.Souza M.P., Machado M.I.L., Braz-Filho R. Six flavonoids from Bursera leptophloeos. Phytochemistry. 1989;28:2467–2470. doi: 10.1016/S0031-9422(00)98007-5. [DOI] [Google Scholar]
- 13.Peraza-Sánchez S.R., Salazar-Aguilar N.E., Peña-Rodríguez L.M. A New triterpene from the resin of Bursera simaruba. J. Nat. Prod. 1995;58:271–274. doi: 10.1021/np50116a019. [DOI] [PubMed] [Google Scholar]
- 14.Syamasundar K.V., Mallavarapu G.R. Two triterpenoid lactones from the resin of Bursera delpechiana. Phytochemistry. 1995;40:337–339. [Google Scholar]
- 15.Barreira E.S., Queiroz-Monte F.J., Braz-Filho R. A New furanosesquiterpene from Bursera leptophloeos. Nat. Prod. Lett. 1996;8:285–289. doi: 10.1080/10575639608044909. [DOI] [Google Scholar]
- 16.Noge K., Becerra J.X. Germacrene D, a common sesquiterpene in the genus Bursera (Burseraceae) Molecules. 2009;14:5289–5297. doi: 10.3390/molecules14125289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Gutiérrez H.A., Cerda-García-Rojas C.M., Hernández-Hernández J.D., Román-Marín L.U., Joseph-Nathan P. Oxygenated verticillene derivatives from Bursera suntui. Phytochemistry. 2008;69:2844–2848. doi: 10.1016/j.phytochem.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Bianchi E., Sheth K., Cole J.R. Antitumor agents from Bursera fagaroides (Burseraceae). (Beta-peltatin-A-methylether and 5′-desemethoxy-beta-peltatin-A-methylether) Tetrahedron Lett. 1969;10:2759–2762. doi: 10.1016/s0040-4039(01)88262-9. [DOI] [PubMed] [Google Scholar]
- 19.Velazquez-Jimenez R., Torres-Valencia J.M., Cerda-Garcia-Rojas C.M., Hernandez-Hernandez J.D., Roman-Marin L.U., Manriquez-Torres J.J., Gomez-Hurtado M.A., Valdez-Calderon A., Motilva V., Garcia-Maurino S., et al. Absolute configuration of podophyllotoxin related lignans from Bursera fagaroides using vibrational circular dichroism. Phytochemistry. 2011;72:2237–2243. doi: 10.1016/j.phytochem.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Maldini M.T., Montoro P., Piacente S., Pizza C. Phenolic Compounds from Bursera simaruba Sarg. Bark: Phytochemical investigation and quantitative analysis by tandem mass spectrometry. Phytochemistry. 2009;70:641–649. doi: 10.1016/j.phytochem.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Jutiviboonsuk A., Zhang H., Tan G.T., Ma C., Hung N.V., Cuong N.M., Bunyapraphatsara N., Soejarto D.D., Fong H.H.S. Bioactive constituents from roots of Bursera tonkinensis. Phytochemistry. 2005;66:2745–2751. doi: 10.1016/j.phytochem.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Nakanishi T., Inatomi Y., Murata H., Shigeta K., Iida N., Inada A., Murata J., Farrera M.A., Iinuma M., Tanaka T., Tajima S., Oku N. A new and known cytotoxic aryltetralin-type lignans from stems of Bursera graveolens. Chem. Pharm. Bull. 2005;53:229–231. doi: 10.1248/cpb.53.229. [DOI] [PubMed] [Google Scholar]
- 23.Cole J.R., Bianchi E., Trumbull E.R. Antitumor agents from Bursera microphylla (Burseraceae) II: Isolation of a new lignan-burseran. J. Pharm. Sci. 1969;58:175–176. doi: 10.1002/jps.2600580204. [DOI] [PubMed] [Google Scholar]
- 24.McDoniel P.B., Cole J.R. Antitumor activity of Bursera schlechtendalii (Burseraceae): Isolation and structure determination of two new lignans. J. Pharm. Sci. 1972;61:1992–1994. doi: 10.1002/jps.2600611224. [DOI] [PubMed] [Google Scholar]
- 25.Peraza-Sanchez S.R., Peña-Rodríguez L.M. Isolation of picropolygamain from the resin of Bursera simaruba. J. Nat. Prod. 1992;55:1768–1771. doi: 10.1021/np50090a009. [DOI] [PubMed] [Google Scholar]
- 26.Wickramaratne D.B.M., Mar W., Chai H., Castlllo J.J., Farnsworth N.R., Soejarto D.D., Cordell G.A., Pezzuto I.M., Kinghorn A.D. Cytotoxic constituents of Bursera permollis. Planta Med. 1995;61:80–81. doi: 10.1055/s-2006-958008. [DOI] [PubMed] [Google Scholar]
- 27.Jolad S.D., Wiedhopf R.M., Cole J.R. Cytotoxic agents from Bursera morelensis (Burseraceae): Deoxypodophyllotoxin and a new lignan, 5′-desmethoxydeoxypodophyllotoxin. J. Pharm. Sci. 1977;66:892–893. doi: 10.1002/jps.2600660647. [DOI] [PubMed] [Google Scholar]
- 28.Rzedowski J.R.M.L., de Rzedowski G.C. Inventario del conocimiento taxonómico, así como de la diversidad y del endemismo regionales de las especies Mexicanas de Bursera (Burseraceae) Acta Botánica Mexicana. 2005;70:85–111. [Google Scholar]
- 29.Aguilar A., Camacho J.R., Chino S., Jacquez P., López M.E. Información Etnobotánica. Instituto Mexicano del Seguro Social; México D.F., Mexico: 1994. Herbario medicinal del instituto mexicano del seguro social; p. 28. [Google Scholar]
- 30.Hernández F. Historia de las Plantas de Nueva España. Universidad Nacional Autónoma de México; México D.F., Mexico: 1942. pp. 551–553. [Google Scholar]
- 31.Puebla-Pérez A.M., Huacuja L., Rodríguez G., Lozoya X., Zaitseva-Petrovna G., Villaseñor-García M. Cytotoxic and antitumor activity from Bursera fagaroides ethanol extract in mice with L5178Y lymphoma. Phytother. Res. 1998;12:545–548. doi: 10.1002/(SICI)1099-1573(199812)12:8<545::AID-PTR349>3.0.CO;2-S. [DOI] [Google Scholar]
- 32.Rosas-Arreguin P., Arteaga-Nieto P., Reynoso-Orozco R., Villagomez-Castro J.C., Sabanero-Lopez M., Puebla-Perez A.M., Calvo-Mendez C. Bursera fagaroides, Effect of an ethanolic extract on ornithine decarboxylase (ODC) activity in vitro and on the growth of Entamoeba histolytica. Exp. Parasitol. 2008;119:398–402. doi: 10.1016/j.exppara.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Huacuja R.L., Delgado N.M., Carranco L.A., Reyes L.R., Rosado G.A. Agglutinating and immobilizing activity of an ethanol extract of Bursera fagaroides on human and other mammalian spermatozoa. Arch. Invest. Med. (Mex) 1990;21:393–398. [PubMed] [Google Scholar]
- 34.Cairnes D.A., Ekundayo O., Kingston D.G. Plant anticancer agents. X. Lignans from Juniperus Phoenicea. J. Nat. Prod. 1980;43:493–497. doi: 10.1021/np50010a010. [DOI] [PubMed] [Google Scholar]
- 35.Noguera B., Díaz E., García M.V., San Feliciano A., López-Perez J.L., Israel A. Anti-Inflammatory activity of leaf extract and fractions of Bursera simaruba (L.) Sarg (Burseraceae) J. Ethnopharmacol. 2004;92:129–133. doi: 10.1016/j.jep.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Hendrawati O., Woerdenbag H.J., Michiels P.J.A., Aantjes H.G., van Dam A., Kayser O. Identification of lignans and related compounds in Anthriscus sylvestris by LC–ESI-MS/MS and LC-SPE–NMR. Phytochemistry. 2011;72:2172–2179. doi: 10.1016/j.phytochem.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Perry N.B., Foster L.M. Antitumour lignans and cytotoxic resin acids from a New Zealand gymnosperm, Libocedrus plumose. Phytomedicine. 1994;1:233–237. doi: 10.1016/S0944-7113(11)80070-X. [DOI] [PubMed] [Google Scholar]
- 38.Berlin J., Wray V., Mollenschott C., Sasse F. Formation of β-peltatin-A methyl ether and coniferin by root cultures of Linum flavum. J. Nat. Prod. 1986;49:435–439. doi: 10.1021/np50045a008. [DOI] [PubMed] [Google Scholar]
- 39.Kranz K., Petersen M. Peltatin 6-O-methyltransferase from suspension cultures of Linum nodiflorum. Phytochemistry. 2003;64:453–458. doi: 10.1016/S0031-9422(03)00196-1. [DOI] [PubMed] [Google Scholar]
- 40.Federolf K., Alfermann A.W., Fuss E. Aryltetralin-Lignan formation in two different cell suspension cultures of Linum album: Deoxypodophyllotoxin 6-hydroxylase, a key enzyme for the formation of 6-methoxypodophyllotoxin. Phytochemistry. 2007;68:1397–1406. doi: 10.1016/j.phytochem.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt T.J., Hemmati S., Klaes M., Konuklugil B., Mohagheghzadeh A., Ionkova I., Fuss E., Alfermann A.W. Lignans in flowering aerial parts of Linum species–chemodiversity in the light of systematics and phylogeny. Phytochemistry. 2010;71:1714–1728. doi: 10.1016/j.phytochem.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 42.Pettit G.R., Meng Y., Gearing R.P., Herald D.L., Pettit R.K., Doubek D.L., Chapuis J.C., Tackett L.P. Antineoplastic agents. 522. Hernandia peltata (Malaysia) and Hernandia nymphaeifolia (Republic of Maldives) J. Nat. Prod. 2004;67:214–220. doi: 10.1021/np030125s. [DOI] [PubMed] [Google Scholar]
- 43.Ito C., Itoigawa M., Ogata M., Mou X.Y., Tokuda H., Nishino H., Furukawa H. Lignans as anti-tumor-promoter from the seeds of Hernandia ovigera. Planta Med. 2001;67:166–168. doi: 10.1055/s-2001-11501. [DOI] [PubMed] [Google Scholar]
- 44.Ito C., Matsui T., Wu T.-S., Furukawa H. Isolation of 6,7-demethylenedesoxypodophyllotoxin from Hernandia ovigera. Chem. Pharm. Bull. 1992;40:1318–1321. doi: 10.1248/cpb.40.1318. [DOI] [Google Scholar]
- 45.Jackson D.E., Dewick P.M. Aryltetralin lignans from Podophyllum hexandrum and Podophyllum peltatum. Phytochemistry. 1984;23:1147–1152. [Google Scholar]
- 46.Jackson D.E., Dewick P.M. Tumour-inhibitory aryltetralin lignans from Podophyllum pleianthum. Phytochemistry. 1985;24:2407–2409. doi: 10.1016/S0031-9422(00)83052-6. [DOI] [Google Scholar]
- 47.Broomhead A.J., Dewick P.M. Tumour-inhibitory aryltetralin lignans in Podophyllum versipelle, Diphylleia cymosa and Diphylleia grayi. Phytochemistry. 1990;29:3831–3837. doi: 10.1016/0031-9422(90)85342-D. [DOI] [Google Scholar]
- 48.Jiang R.-W., Zhou J.-R., Hon P.-M., Li S.-L., Zhou Y., Li L.-L., Ye W.-C., Xu H.-X., Shaw P.-C., But P.P.-H. Lignans from Dysosma Wersipellis with inhibitory effects on prostate cancer cell lines. J. Nat. Prod. 2007;70:283–286. doi: 10.1021/np060430o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hadimani S.B., Tanpure R.P., Bhat S.V. Asymmetric total synthesis of (–)-podophyllotoxin. Tetrahedron Lett. 1996;37:4791–4794. doi: 10.1016/0040-4039(96)00937-9. [DOI] [Google Scholar]
- 50.Middel O., Woerdenbag H.J., Van Uden W., Van Oeveren A., Jansen J.F.G.A., Feringa B.L., Konings A.W.T., Pras N., Kellogg R.M. Synthesis and cytotoxicity of novel lignans. J. Med. Chem. 1995;38:2112–2117. doi: 10.1021/jm00012a010. [DOI] [PubMed] [Google Scholar]
- 51.Cho S.J., Tropsha A., Suffness M., Cheng Y.C., Lee K.H. Antitumor agents. 163. Three-dimensional quantitative structure-activity relationship study of 4′-O-demethylepipodophyllotoxin analogs using the modified CoMFA/q2-GRS approach. J. Med. Chem. 1996;39:1383–1395. doi: 10.1021/jm9503052. [DOI] [PubMed] [Google Scholar]
- 52.Thurston L.S., Irie H., Tani S., Han F.-S., Liu Z.-C., Cheng Y.-C., Lee K.-H. Antitumor agents. 78. Inhibition of human DNA topoisomerase II by podophyllotoxin and α-peltatin analogs. J. Med. Chem. 1986;29:1547–1550. doi: 10.1021/jm00158a042. [DOI] [PubMed] [Google Scholar]
- 53.Hartwell J.L., Schrecker A.W. Components of podophyllin V. The constitution of podophyllotoxin. J. Am. Chem. Soc. 1951;73:2909–2916. doi: 10.1021/ja01150a143. [DOI] [Google Scholar]
- 54.Geran R.I.N.H., Macdonald M.M., Schumacher A.M., Abbott B.J. Protocols for screening chemical agents and natural products against animal tumours and other biological systems. Cancer Chemother. Rep. 1972;3:51–61. [Google Scholar]
- 55.Houghton P., Fang R., Techatanawat I., Steventon G., Hylands P.J., Lee C.C. The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods. 2007;42:377–387. doi: 10.1016/j.ymeth.2007.01.003. [DOI] [PubMed] [Google Scholar]