Abstract
Clinical outcomes for patients with a wide range of malignancies have improved substantially over the last two decades. Tyrosine kinase inhibitors (TKI) are potent signalling cascade inhibitors and have been responsible for significant advances in cancer therapy. By inhibiting vascular endothelial growth factor receptor- (VEGFR-) mediated tumour blood vessel growth, VEGFR-TKIs have become a mainstay of treatment for a number of solid malignancies. However, the incidence of VEGFR-TKI-associated cardiovascular toxicity is substantial and previously under-recognised. Almost all patients have an acute rise in blood pressure and the majority develop hypertension. They are associated with the development of left ventricular systolic dysfunction (LVSD), heart failure and, myocardial ischaemia and can have effects upon myocardial repolarisation. Consideration should be given to rigorous baseline assessment of patients prior to commencing VEGFR-TKIs, with careful attention paid to baseline cardiovascular risk factors. Baseline blood pressure measurement, electrocardiogram, and cardiac imaging should be performed routinely. Hypertension management currently follows national guidelines but there may be a future role for ET-1 antagonism in the prevention or treatment of VEGFR-TKI-associated hypertension. VEGFR-TKI-associated LVSD appears to be independent of dose and is reversible. Patients who develop LVSD and heart failure should be managed with conventional heart failure therapies but the role of prophylactic therapy is yet to be defined. Serial monitoring of left ventricular function and QT interval require better standardisation and co-ordinated care. Management of these complex patients requires collaborative, cardio-oncology care to allow the true therapeutic potential from cancer treatment while minimising competing cardiovascular effects.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in HEART editions and any other BMJPGL products to exploit all subsidiary rights.
Over the last two decades, clinical outcomes for patients with cancer have improved substantially. Approximately 50% of patients who develop cancer in any form will survive at least 10 years. [1] Tyrosine kinase inhibitors (TKIs) have accounted for a proportion of this success and these small molecule drugs have been developed to act against several primary signalling targets including epidermal growth factor, platelet-derived growth factor and breakpoint cluster region-Abelson murine leukaemia (Bcr-Abl). Vascular endothelial growth factor receptor- (VEGFR-)TKIs represent a major advance in the management of patients with a wide range of malignancies (Figure 1, Tables 1 and 2) and will form the basis of this review. This oncological success has been accompanied by new challenges, including the management of VEGFR-TKI-associated adverse cardiovascular effects. VEGFR-TKIs cause hypertension, left ventricular systolic dysfunction/heart failure, atherothrombosis and can also cause QT interval prolongation and dysrhythmia (Figure 2) [2], [3]. It is important to note that cardiovascular toxicity profiles of VEGFR-TKIs differ from those associated with TKIs directed primarily against other, non-VEGF, signal-transduction pathways.
Figure 1.
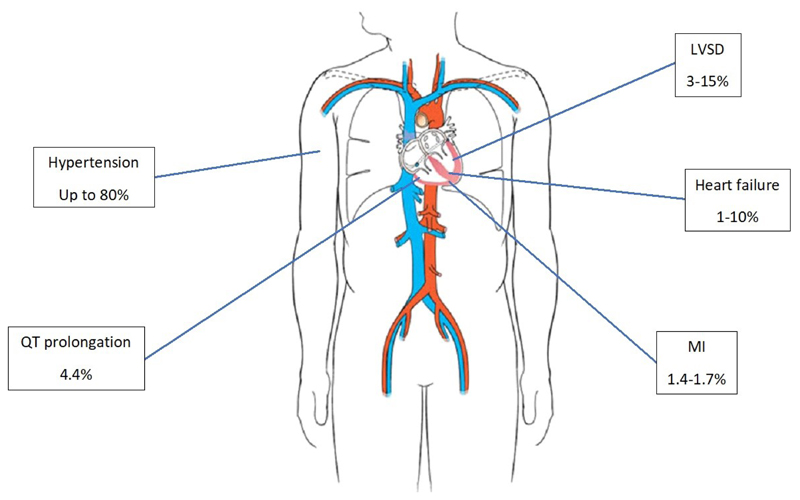
Estimated incidence of various cardiovascular toxicities associated with TKI therapy. [2]–[5], [9] LVSD – Left ventricular systolic dysfunction; MI – Myocardial infarction
Table 1.
Terms used to describe angiogenesis inhibitors and tyrosine kinase inhibitors. VEGFR – Vascular Endothelial Growth Factor Receptor, mAb – Monoclonal antibody, TKI – Tyrosine kinase inhibitor, Bcr-Abl – Breakpoint cluster region-Abelson murine leukaemia, EGFR – Epidermal Growth Factor Receptor
| Category | Examples | |
|---|---|---|
| Tyrosine Kinase Inhibitors (TKI) | Umbrella term for all small molecule inhibitors, directed against either single or multiple tyrosine kinases; primary targets include Bcr-Abl, EGFR and VEGFR | Bcr-Abl: imatinib |
| nilotinib | ||
| dasatinib | ||
| bosutinib | ||
| ponatinib | ||
| EGFR: gefitinib | ||
| lapatinib | ||
| erlotinib | ||
| afatinib | ||
| osimertinib | ||
| VEGFR: axitinib | ||
| cabozantinib | ||
| lenvatinib | ||
| nintedanib | ||
| pazopanib | ||
| regorafenib | ||
| sorafenib | ||
| sunitinib | ||
| tivozanib | ||
| vandetanib | ||
| Non-TKI VEGF Inhibitors | ||
| VEGF mAb | Monoclonal antibodies targeting circulating VEGF | bevacizumab |
| VEGFR mAb | Monoclonal antibodies targeting VEGF receptors | ramirucimabc |
| VEGF Trap | Mimic VEGF receptors and bind to circulating VEGF | aflibercept |
Table 2.
Categories of VEGFR TKI drugs, their tyrosine kinase targets and indications. FGFR – Fibroblast Growth Factor Receptor; PDGFR – Platelet-derived Growth Factor Receptor; VEGFR – Vascular Endothelial Growth Factor Receptor.
| Agent | Target(s) | Cancer Type |
|---|---|---|
| Axitinib | VEGFR-1, -2, -3 | Metastatic renal cancer |
| Cabozantinib | VEGFR-2 | Medullary thyroid cancer |
| RET | Advanced renal cell cancer | |
| Lenvatinib | VEGFR-1, -2, -3 | Metastatic thyroid cancer |
| PDGFRα | Renal cell cancer | |
| c-Kit | ||
| RET | ||
| FGFR | ||
| Nintedanib | VEGFR-1, -2, -3 | Metastatic Non-small cell lung cancer |
| PDGFR | ||
| RET | ||
| FGFR | ||
| FLT3 | ||
| Pazopanib | VEGFR-1, -2, -3 | Advanced renal cell carcinoma |
| PDGFR | Advanced soft tissue sarcoma | |
| c-Kit | ||
| FGFR | ||
| Regorafenib | VEGFR-1, -2, -3 | Metastatic colorectal cancer |
| PDGFRβ | ||
| c-Kit | ||
| RET | ||
| FGFR | ||
| Sunitinib | VEGFR-1, -2, -3 | Gastrointestinal stromal tumour (GIST) |
| PDGFR | Advanced renal cell carcinoma | |
| Raf-1/ B-Raf | Advanced or metastatic pancreatic | |
| c-Kit | Neuroendocrine tumours | |
| RET | ||
| CSF-1R | ||
| FLT3 | ||
| Sorafenib | VEGFR-2,-3 | Hepatocellular carcinoma |
| PDGFR | Advanced renal cell carcinoma | |
| Raf-1/ B-Raf | ||
| c-Kit | ||
| FLT3 | ||
| Tivozinib | VEGFR-1, -2, -3 | Advanced renal cell carcinoma |
| Vandetanib | VEGFR-2 | Medullary thyroid cancer |
| PDGFRβ | ||
| RET | ||
Figure 2.
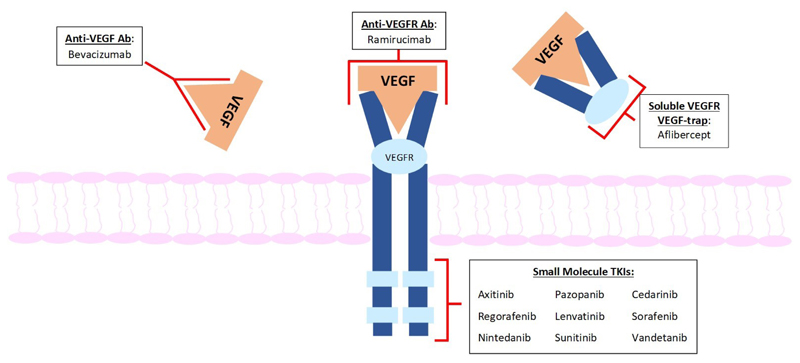
Mechanisms of action VSPIs. There are four main groups of VSPIs: 1) Monoclonal antibodies against VEGF: Bevacizumab was the first VSPI approved for use in a variety of solid tumours. It selectively binds to VEGF to inhibit its interaction with VEGF receptors 2) Small molecule inhibitors of intracellular tyrosine kinases (e.g. sunitinib, sorafenib): these agents are not VEGFR-2-specific but also inhibit a variety of other receptor tyrosine kinases. This increases anti-cancer efficacy but may also contribute to cardiovascular toxicity. 3) VEGF 'trap' (e.g. aflibercept): this recombinant fusion protein comprises VEGF- binding regions of VEGFR-1 and -2 4) Monoclonal VEGFR antibodies (e.g. ramirucimab): these target VEGFR2 receptors, to prevent VEGF-A binding.
Although potential for cardiovascular toxicity was identified early in drug development, rigorous patient selection in pivotal trials may have led to underestimation of the true impact in routine clinical practice. Additionally, no trials document long term safety follow-up despite some patients remaining on treatment for several years and potentially surviving several more. Therefore, it is likely that latent cardiovascular toxicity and that associated with chronic exposure have been under-reported. Some patients will have had previous chemotherapy or radiotherapy that can lower the threshold for subsequent VEGFR-TKI-associated cardiotoxicity [4]. The aim of providing high quality cardiovascular management should be to allow patients to continue safely receiving optimal doses of VEGFR-TKI therapy, minimising treatment interruption or dose reduction.
VEGF Signalling and Its Inhibition
Tumour growth is critically dependent upon a sufficient blood supply. As a solid tumour grows, the central core becomes hypoxic stimulating physiological tissue growth and repair pathways, including the release of angiogenic growth factors to allow new blood vessel formation (neo-angiogenesis). The VEGF pathway is central to this process and its inhibition has therefore become a major therapeutic target in cancer therapy. VEGF has multiple isoforms and binds to three tyrosine kinase receptors. It plays a pivotal role in endothelial cell proliferation and survival, vascular permeability, and angiogenesis by binding to VEGFR-2. [5]
In addition to their VEGF signalling effects, VEGFR-TKIs also inhibit a variable number of other tyrosine kinase targets. This broadens their therapeutic effects against an expanding range of malignancies but may also contribute to their adverse cardiovascular effects (Table 2). [6]
Hypertension
Hypertension is a class effect of VEGFR-TKI therapy and is the most common manifestation of cardiovascular toxicity. Almost every trial reports a treatment-associated rise in blood pressure (BP) and up to 80% of patients develop hypertension, either de novo or worsening of previously controlled high BP. [7] Registry data reveal that 73% of patients receiving targeted therapy (primarily VEGFR-TKIs) for renal cell cancer (RCC), developed cardiovascular toxicity, 55% of which was accounted for by hypertension. [8] VEGFR-TKI-associated hypertension can be severe and difficult to treat [7]–[9] but it is dose-dependent and reversible on discontinuing the VEGFR-TKI.
Clinical Consequences of VEGFR-TKI-Associated Hypertension
An acute rise in BP in patients not previously ‘conditioned’ to the effects of hypertension can precipitate acute end-organ complications, such as stroke, myocardial ischaemia, heart failure and acute kidney injury at a lower threshold than might be expected in patients with long-standing hypertension. [10] This is relevant as VEGFR-TKI-associated hypertension, develops within hours to days of starting therapy. Therefore, prior to introducing a VEGFR-TKI, a comprehensive assessment for pre-existing cardiovascular disease is important and management of pre-existing hypertension optimised. Early recognition of VEGFR-TKI-associated hypertension and prompt initiation of treatment remains fundamental. The development of VEGFR-TKI-associated hypertension is associated with better cancer outcomes but, importantly, anti-hypertensive treatment does not modify the anti-cancer effect. [11]
Although rare (<1% of patients), VEGFR-TKIs have been associated with the development of posterior reversible leucoencephalopathy. [12], [13]This presents with headache, confusion, seizures and visual impairment. Magnetic resonance imaging of the brain reveals characteristic posterior fossa changes on T2-weighted imaging reflecting oedema. The underlying pathophysiology seems to be related to the combination of hypertension, impaired cerebral auto-regulation and cerebrovascular permeability/endothelial dysfunction. Importantly, if this condition is diagnosed early, hypertension treated promptly and VEGFR-TKI withdrawn, there is a favourable prognosis.
As patients survive longer and receive VEGFR-TKIs for prolonged periods, the chronic end-organ effects of hypertension need careful consideration. However, long-term follow-up data are lacking.
Mechanism of VEGFR-TKI-Associated Hypertension
Mechanisms underlying the development of hypertension during VEGFR-TKI therapy remain incompletely defined. The acute increase in BP upon VEGFR-TKI treatment, and its reduction upon VEGFR-TKI cessation, [14] suggest that changes in vascular tone are of fundamental importance. Rarefaction, a reduction in capillary density, is also notable [15] but whether this is a cause or a consequence of VEGFR-TKI-associated hypertension remains unclear.
Reduced nitric oxide (NO) bioavailability is a potentially important factor in VEGFR-TKI-associated hypertension. VEGF stimulates NO release, while VEGF inhibition is associated with decreased NO generation. In patients treated with VEGFR-TKI, plasma levels of nitrate and its metabolites are reduced, but return to baseline following withdrawal of treatment. [16] VEGFR-TKI therapy is also associated with increased production of potent vasoconstrictor, endothelin-1 (ET-1). [14] Pre-clinical data in swine demonstrate the effective reversal of acute VEGFR-TKI-associated hypertension by ET-1 receptor antagonism.[17] There may, therefore, be a role for ET-1 receptor antagonists in the treatment, or prevention, of VEGFR-TKI-related hypertension. However, this has yet to be proven clinically. Recent studies have identified oxidative stress as another mechanism for VEGFR-TKI-induced vascular dysfunction in hypertension. [18]
While the renin-angiotensin-aldosterone (RAA) system is critically implicated in the pathophysiology of essential hypertension, there is no convincing evidence that it plays a major role in VEGFR-TKI-associated hypertension. [19] VEGFR-TKI therapy is associated with decreased renin activity in experimental models [16], [20] and angiotensin-converting enzyme (ACE) inhibition has a limited impact on VEGFR-TKI-related hypertension when compared with calcium-channel antagonism in these models. [21] Consistent with these pre-clinical findings, patients treated with sunitinib had a rise in BP of around 15mmHg but with a 60% decrease in plasma renin and no change in aldosterone levels [22] which may reflect a secondary down-regulation of the RAA system. [20], [21]
Assessment and Treatment of VEGFR-TKI-Associated Hypertension
The Cardiovascular Toxicities Panel of the National Cancer Institute provide guidance on the assessment and management of VEGFR-TKI-associated hypertension [23] which is also highlighted in a European Society of Cardiology position paper. [3] BP should be monitored by the oncology team frequently (weekly during the first cycle) and subsequently 2- to 3-weekly. Home BP monitoring has been recommended during treatment but this may not always be feasible. [24]
Patients with a BP of ≥140/90 mmHg should receive anti-hypertensive treatment. [24], [25] Choice of anti-hypertensive agents generally follows national guidelines for first-line treatment of hypertension and there is currently no clinical evidence of superiority of one agent over another (Table 3). [26] However, non-dihydropyridine calcium channel antagonists such as verapamil and diltiazem inhibit cytochrome P450 3A4 and should be avoided because of the potential for consequent VEGFR-TKI toxicity [3], [11]. ACE inhibitors or angiotensin receptor blockers (ARBs) may be of benefit in patients with left ventricular systolic dysfunction (LVSD) or proteinuria induced by VEGFR-TKIs. However, given the limited evidence for the RAA system in the pathophysiology of VEGFR-TKI-associated hypertension, pathophysiologically-targeted treatment is notably absent. Multiple agents are frequently required to achieve satisfactory control and although agents such as nebivolol and long-acting nitrates improve BP control in VEGFR-TKI-associated hypertension, [27] alternative anti-hypertensives have not been extensively evaluated in this setting (Tables 3 and 4).
Table 3.
Anti-hypertensive agents for treatment of TKI-associated hypertension. The choice of anti-hypertensive agent generally follows national guidelines for first-line treatment of hypertension and there is currently no clinical evidence to suggest superiority of one agent over another. Non-dihydropyridine calcium channel blockers such as diltiazem and verapamil should be avoided as they can lead to TKI toxicity. COPD – Chronic obstructive pulmonary disease.
| Anti-hypertensive Treatment | Advantages | Disadvantages |
|---|---|---|
| ACE Inhibitors / Angiotensin receptor antagonists | Beneficial effects in patients with LVSD or proteinuria Quick onset of action |
Caution in renal impairment and nephrectomy RAA axis not substantially implicated in TKI-associated hypertension |
| Dihydropyridine calcium channel blockers | Vasodilator action effective in TKI-hypertension | Can exacerbate fluid retention Slower onset of action |
| Beta-blockers | Beneficial effects in patients with LVSD Vasodilator action effective in TKI-hypertension |
Contraindicated in asthma/COPD and decompensated HF |
| Diuretics | Effective in elderly patients | Caution in renal impairment and nephrectomy May cause electrolyte disturbance |
Table 4.
Summary of risk factors, screening and investigations, and potential management options for the main cardiovascular toxicities associated with TKIs. ACE – Angiotensin-converting enzyme; ARB – Angiotensin receptor blocker; CAD – Coronary artery disease; DAPT – Dual anti-platelet therapy; LVEF – Left ventricular ejection fraction; LVSD – Left ventricular systolic dysfunction; MI – Myocardial infarction; NT-proBNP – N-terminal pro b-type natriuretic peptide; PVD – peripheral vascular disease.
| Toxicity | Risk factors | Investigations / Screening | Management |
|---|---|---|---|
| Hypertension | Age (>65) Pre-existing hypertension Pre-existing vascular disease (stroke / MI / PVD) Diabetes Mellitus |
Monitor weekly during first cycle 2- to 3- weekly thereafter Home blood pressure monitoring where possible |
Control existing hypertension ACE inhibitor / ARB Dihydropyridine calcium channel blocker Beta blocker Diuretics Dose reduction / discontinuation of TKI with severe hypertension NOT verapamil or diltiazem |
| LV dysfunction | Pre-existing heart failure / LVSD Significant CAD Pre-existing hypertension Valvular heart disease Previous anthracycline exposure |
Baseline imaging assessment Serial monitoring at 1 month and every 3 months on TKI Role for biomarker testing not yet defined (Troponin / NT-proBNP) |
ACE inhibitor/ARB and beta blocker ± mineralocorticoid receptor antagonist in patients with heart failure Consider ACE inhibitor/beta blocker in asymptomatic LVSD Discontinuation of TKI with heart failure or significant reduction in LVEF |
| Myocardial infarction | Age (>65) Pre-existing CAD |
Consider stress testing/coronary angiography in presence of potentially ischaemic symptoms at baseline | Anti-platelet primary prevention should be avoided Safest shortest duration of DAPT after percutaneous coronary intervention should be sought Discontinuation/interruption of TKI following MI |
|
QT Prolongation |
Age (>65) Electrolyte imbalance Hypothyroidism QT-prolonging drugs |
Baseline ECG and electrolyte monitoring Serial monitoring |
Withdraw QT-prolonging drugs Temporary withdrawal of TKI with QTc >500ms or increase of >60ms Discontinuation of TKI with Torades de Pointes |
Some VEGFR-TKI treatment regimens have off-periods during which VEGFR-TKIs are temporarily withheld. For example, sunitinib is conventionally given for 4 weeks followed by a 2-week break (Figure 3). During this time, vigilance to possible symptomatic rebound hypotension is important. [28] Dose reduction or temporary withdrawal of anti-hypertensive agents may be required. [7]
Figure 3.
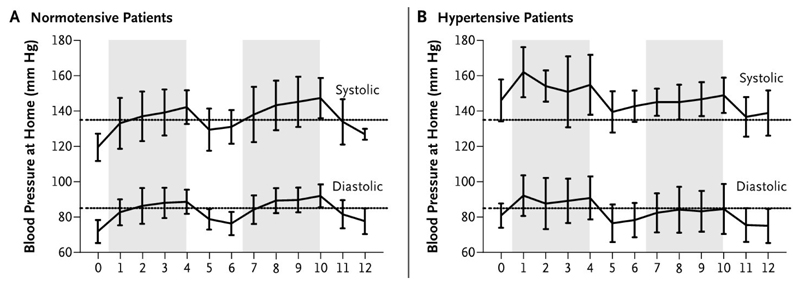
Changes in systolic and diastolic Blood Pressure. Change in mean blood pressure as measured by teletransmitted results of home monitoring in patients with metastatic renal-cell carcinoma treated with two cycles of sunitinib at a dose of 50 mg daily for 4 weeks (shaded area), followed by 2 weeks without treatment. The results are shown separately for patients who were normotensive (Panel A) and those who were hypertensive (Panel B) before starting sunitinib treatment. (Azizi M et al. N Engl J Med 2008;358:95-97, with permission).
Prior to commencing treatment with a VEGFR-TKI, referral for cardio-oncology review should be considered in patients with a history of hypertension and this is particularly important for patients with sub-optimal BP control or hypertension-related end-organ dysfunction. [29] The development of VEGFR-TKI-associated hypertension that is not easily controlled with a single agent, or where there is evidence of end-organ damage should prompt referral to a cardio-oncologist. However, there appears to be wide variation in practice around the globe. Although there are no guidelines recommending thresholds for discontinuation of VEGFR-TKI therapy, severe hypertension may require dose reduction or withdrawal, but this should generally be considered a last resort. Decision-making and management requires input from both the oncologist and cardiologist and needs to take account of cancer and cardiovascular risks, and whether there is an alternative effective cancer therapy that can be used in place of a VEGFR-TKI.
Left Ventricular Systolic Dysfunction
The spectrum of VEGFR-TKI-associated cardiotoxicity ranges from asymptomatic LVSD, to heart failure (HF), cardiogenic shock and death.[30], [31] Obtaining an accurate incidence of LVSD or HF with VEGFR-TKI use has been limited and that reported may underestimate the reality. The definition and reporting of cardiac toxicity has been inconsistent and, despite more robust definitions of HF and better reporting of events in clinical trials, [32] long term follow-up data remain sparse. There is often overlap between symptoms that may reflect HF with those that are related to cancer. [30]
Meta-analysis of trials of VEGFR-TKIs including 10,647 patients reveals a combined incidence of asymptomatic LVSD and HF of 2.4%. 1.2% developed symptomatic heart failure. Notably, there was no apparent difference in risk of cardiotoxicity between relatively specific VEGFR-TKIs (e.g. axitinib) and those directed against a broader range of tyrosine kinases (e.g. sunitinib, sorafenib, vandetanib and pazopanib) [31]. In a randomised controlled trial comparing pazopanib with sunitinib for the treatment of RCC, both agents were associated with a 1% incidence of HF and 9% of patients in each group had a ≥15% decline in left ventricular ejection fraction (LVEF) over a median duration of 8 months. [33] In the large ASSURE trial of 1599 patients with RCC treated with sunitinib, sorafenib or placebo, a reduction in LVEF >15% to a value below the lower limit of normal occurred in 1.8% and 1.4% for sunitinib and sorafenib respectively, and in 0.9% receiving placebo over six months [34].
Retrospective data from patients undergoing treatment with a VEGFR-TKI at Stanford University revealed a similar incidence of HF and these real world patients were also systematically screened for the development of asymptomatic LVSD or rise in brain natriuretic peptide. Of 217 patients treated with a VEGFR-TKI, 21.6% developed elevated plasma levels of NT-proBNP (>300 pg/mL or a 100% increase from a previously elevated level) and 9.6% had ≥10% decline in LVEF during treatment. [7]
VEGFR-TKI-associated LVSD is at least partially reversible. In a randomised controlled trial of sunitinib versus placebo in the treatment of imatinib-resistant gastro-intestinal stromal tumours 28% of patients had a reduction of LVEF by ≥10%. [35] A steady decline in LVEF was observed with each of four cycles over a 24-week period. 5 of 6 patients with HF had an improvement in LVEF in response to HF therapies. Endomyocardial biopsies from these patients demonstrated mitochondrial abnormalities but not apoptosis nor fibrosis, further suggesting a reversible process. [35] More recently, 90 patients with RCC receiving sunitinib were followed prospectively with echocardiographic and biomarker assessment. 9.7% of patients had a decline in LVEF by ≥10% from baseline to a value <50%. Eight of the nine patients who developed cardiotoxicity, did so within the first cycle of treatment. Importantly, however, with sunitinib dose reduction and/or the institution of anti-hypertensive medication, LV dysfunction was at least partially reversible and non-progressive over 33 weeks. [36]
Pathophysiology of LVSD in patients treated with VEGFR-TKI
Mechanisms underlying VEGFR-TKI-associated cardiac dysfunction appear to reflect direct myocardial toxicity amplified by hypertension. Mitochondrial dysfunction and inhibition of AMP-kinase may be important. VEGF plays a central role in the myocardial hypertrophic response to hypertension and VEGFR-TKIs appear to accelerate the process of decompensation from left ventricular hypertrophy to dilatation and HF. [37] These on-target effects of VEGFR-TKIs reflect the overlap between tyrosine kinases expressed in both the heart and the tumour. However, VEGFR-TKIs act at a range of different pathways and off-target effects occur from their limited selectivity. [38] As such, given the variety in range and specificity of tyrosine kinases targeted by individual small molecule inhibitors it may be an oversimplification to consider any cardiotoxic action as a class effect. [30]
Prophylaxis, Monitoring and Treatment
Risk factors for the development of VEGFR-TKI-associated LVSD or HF are outlined in Table 4. The development of cardiac dysfunction appears to be independent of dose or treatment duration. [39]
The American Society of Echocardiography and European Association of Cardiovascular Imaging recommend a baseline echocardiogram, with follow-up at 1 month and every 3 months while on VEGFR-TKI therapy. However, they concede that this recommendation is based upon opinion and lacks a firm evidence base. [40] There is currently wide variation in local practice, but imaging assessment is important for patients at higher baseline risk for LV dysfunction and particularly those with other potential cancer treatment options. The early development of cardiotoxicity demonstrated with sunitinib suggests that screening should be focused to the early cycles of therapy but the onset of cardiotoxicity with other VEGFR-TKIs may differ. A low threshold for imaging assessment of LV function is vital in patients with symptoms suggestive of HF, particularly given the potentially reversible nature of VEGFR-TKI-associated LVSD.
The role of cardiac biomarkers for the prediction and diagnosis of VEGFR-TKI-associated cardiotoxicity remains undefined. Notably, in the prospective assessment of patients receiving sunitinib, 18.9% of patients had elevation of high sensitivity troponin or natriuretic peptides but this did not correspond to an echocardiographically detectable decline in LVEF [36]. It is unclear whether this reflects a true disconnect between LVSD and humoral biomarkers or insufficient sensitivity of echocardiography to detect subtle alterations in myocardial function.
Patients with LVSD at baseline or with risk factors for the development of VEGFR-TKI-associated LVSD (Table 4) should be referred for cardiology review prior to commencing VEGFR-TKI. Those who develop HF or LVSD while receiving VEGFR-TKI treatment should be seen on an urgent basis by a cardiologist, preferentially with cardio-oncology expertise. They should receive conventional therapy including a beta blocker, ACE inhibitor/ARB and potentially a mineralocorticoid receptor antagonist. The decision to interrupt, postpone or switch VEGFR-TKIs in the face of cardiotoxicity is complex and requires careful weighing of potential oncologic benefits against cardiac effects and specialist cardio-oncology management is vital. However, the development of VEGFR-TKI-associated LVSD should prompt the interruption of VEGFR-TKI therapy and introduction of ACE inhibitor/ARB and beta blockade (Table 4), although there is a complete lack of evidence to guide therapy. For those patients with recovery of left ventricular function, resumption of treatment with VEGFR-TKI may be considered. [41]
Patients with significantly impaired LV function at baseline were excluded from most pivotal VEGFR-TKI trials and, where feasible, alternative treatment approaches should be considered for such patients. There is no evidence to support the routine prophylactic use of therapies such as ACE inhibitors or beta blockers.
Myocardial Ischemia
Although VEGFR-TKIs are associated with both thrombotic and haemorrhagic complications, the risk of thrombotic events predominate. [42], [43] The risk of arterial thrombosis is greater than that of venous thrombo-embolism [44], [45] and many trials report an increased incidence of myocardial ischaemia and acute coronary syndrome but these are reported inconsistently. The incidence is variable and depends upon the underlying cancer and its stage. Meta-analysis reveals an incidence of arterial thromboembolic events of 1.4% and 1.7% associated with the use of sorafenib and sunitinib, respectively [44]. In a major randomised controlled trial of 903 patients with advanced RCC, 3% of patients receiving sorafenib suffered myocardial ischemia or infarction compared with <1% receiving placebo. [46]
Treatment and Prevention of Cardiac Ischaemia
One meta-analysis, primarily of patients treated for RCC, reported an incidence of 16.7% of all-grade bleeding with sunitinib and sorafenib. 2.4% of events were considered to be ‘high-grade’. [47] However, a more recent meta-analysis of trials including a wider range of underlying malignancies suggests that the risk of bleeding is primarily ‘low-grade’ with epistaxis being particularly frequent (10.8% in VEGFR-TKI treated patients versus 2.2% in controls). Although 13.4% of high grade events were accounted for by cerebral haemorrhage this was not statistically different from control patients and the small numbers involved limit major conclusions. Gastrointestinal haemorrhage was also not significantly different between VEGFR-TKI-treated patients and controls (2.6% versus 3.6% respectively). The risk of haemorrhagic events varies depending upon the underlying tumour type and is increased by the use of combination VEGFR-TKI therapy. [48]
Concerns about VEGFR-TKI-associated bleeding pose a dilemma when considering the use of anti-platelet agents in the treatment or prevention of ischaemic events. [44] In patients who require percutaneous coronary intervention, strategies to allow a shorter period of dual anti-platelet therapy should be sought. There are no data to support the routine use of anti-platelets as an anti-ischaemic primary preventative strategy (Table 4).
QT Interval Prolongation
QT prolongation with VEGFR-TKIs is reported but varies widely by individual drugs (Table 5). Vandetanib is most associated with this effect, with up to 8% of patients exhibiting a corrected QT (QTc) interval duration of >500 ms. [3] Meta-analysis of VEGFR-TKI trials found an incidence of 4.4% of all-grade QTc prolongation when compared to non-TKI therapy. The incidence of QTc >500ms was low, and ventricular arrhythmias and sudden death were scarce. [2] However, the incidence is likely to be higher in patients not being treated in a clinical trial and VEGFR-TKI-associated QTc prolongation, torsades de pointes ventricular arrhythmia and sudden death have been reported. [49] The mechanism for QT prolongation, which is also seen with other ATP-mimetic TKIs is probably distinct from the VEGFR-targeted effects and may be related to interaction with the myocardial human ether-a-go-go related gene (hERG) potassium channels. [50]
Table 5.
Incidence of QT prolongation with TKIs.
| TKI Agent | Average QT prolongation (ms) | Increase in QTc >60ms (%) | QTc >500ms (%) | Torsades de pointes (%) |
|---|---|---|---|---|
| Axitinib | <10 | N/a | N/a | N/a |
| Cabozantinib | 10-15 | N/a | N/a | N/a |
| Pazopanib | N/a | N/a | 2 | <0.3 |
| Sorafenib | 8-13 | N/a | N/a | N/a |
| Sunitinib | 9.6-15.4 | 1-4 | 0.5 | <0.1 |
| Vandetanib | 36 | 12-15 | 4.3-8 | Described, % N/a |
Care should be taken to avoid co-prescription of other drugs that may prolong the QT interval and to avoid or correct electrolyte abnormalities. A baseline ECG should be performed in all patients due to start treatment with a VEGFR-TKI. For patients treated with vandetanib, the package insert specifically recommends monitoring of the QT interval at baseline, 2-4 weeks, and 8-12 weeks after starting treatment and every 3 months thereafter. [23] Package inserts for other VEGFR-TKIs are less proscriptive regarding timing of QT interval assessment but state that this should be assessed ‘periodically’. [23]
It is recommended that treatment should be suspended if QTc is >500 ms or increases by >60 ms from baseline. The risk of torsades de pointes is substantially greater above these thresholds, and these patients should be referred to a cardio-oncology service for management. [3]
Conclusions
TKIs of the VEGF-receptor signalling pathway have had a major impact in the treatment of a wide range of cancers and indications for their use have increased substantially. However, they are associated with a range of cardiovascular adverse effects including hypertension, LVSD/HF, atherothrombosis and QT interval prolongation. Clinical trial estimates of these effects have been variable, partly reflecting inconsistent inclusion and definition of cardiovascular endpoints. Furthermore, patients receiving VEGFR-TKIs often have substantially more comorbidity than those included in clinical trials, putting them at further risk of adverse effects.
Evidence to guide the best approach in the assessment and treatment of VEGFR-TKI-associated cardiovascular effects is limited but rigorous baseline cardiovascular risk assessment remains key, with particular focus on blood pressure control. The overarching goal should be to allow the continued administration of optimal doses of VEGFR-TKI wherever possible, often with the co-administration of cardiovascular medicines. Decision-making requires close interaction between the oncologist and cardiologist, often via a dedicated cardio-oncology clinic. Such collaborative care should be considered as a basic standard to allow patients to achieve the true therapeutic potential from cancer treatment whilst minimising competing cardiovascular effects.
Funding Statement
This article is funded by the BHF centre of excellence award (award number: RE/13/5/30177) and the BHF Chair award (award number: CH/12/429762).
Footnotes
Contributorship Statement: The idea for the article originally came from Dr Lang, and Dr Dobbin performed the literature search and wrote the article, with input from all other authors. Dr Lang is the guarantor for the article, and ultimately had control over the decision to publish.
Competing Interests: There are no conflicts of interest to declare.
References
- [1].Quaresma M, Coleman MP, Rachet B. 40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971-2011: A population-based study. Lancet. 2015;385(9974):1206–1218. doi: 10.1016/S0140-6736(14)61396-9. [DOI] [PubMed] [Google Scholar]
- [2].Ghatalia P, Je Y, Kaymakcalan MD, Sonpavde G, Choueiri TK. QTc interval prolongation with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Br J Cancer. 2015;112(2):296–305. doi: 10.1038/bjc.2014.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zamorano JL, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: The task force for cancer treatments and cardiovascular toxicity of the european society of cardiology (ESC. Russian Journal of Cardiology. 2017;143(3):105–139. doi: 10.1002/ejhf.654. [DOI] [PubMed] [Google Scholar]
- [4].Abdel-Qadir H, Ethier J-L, Lee DS, Thavendiranathan P, Amir E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: A systematic review and meta-analysis. Cancer Treat Rev. 2017;53:120–127. doi: 10.1016/j.ctrv.2016.12.002. [DOI] [PubMed] [Google Scholar]
- [5].Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17(10):611–625. doi: 10.1038/nrm.2016.87. [DOI] [PubMed] [Google Scholar]
- [6].Maurea N, et al. Pathophysiology of cardiotoxicity from target therapy and angiogenesis inhibitors. J Cardiovasc Med. 2016;17:S19–S26. doi: 10.2459/JCM.0000000000000377. [DOI] [PubMed] [Google Scholar]
- [7].Small HY, Montezano AC, Rios FJ, Savoia C, Touyz RM. Hypertension due to antiangiogenic cancer therapy with vascular endothelial growth factor inhibitors: understanding and managing a new syndrome. Can J Cardiol. 2014;30(5):534–43. doi: 10.1016/j.cjca.2014.02.011. [DOI] [PubMed] [Google Scholar]
- [8].Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail. 2013;1(1):72–78. doi: 10.1016/j.jchf.2012.09.001. [DOI] [PubMed] [Google Scholar]
- [9].Robinson ES, et al. Rapid development of hypertension and proteinuria with cediranib, an oral vascular endothelial growth factor receptor inhibitor. Clin J Am Soc Nephrol. 2010;5(3):477–83. doi: 10.2215/CJN.08111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guiga H, et al. Hospital and out-of-hospital mortality in 670 hypertensive emergencies and urgencies. J Clin Hypertens. 2017;19(11):1137–1142. doi: 10.1111/jch.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rini BI, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103(9):763–773. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Asaithambi G, Peters BR, Hurliman E, Moran BP, Khan AS, Taylor RA. Posterior reversible encephalopathy syndrome induced by pazopanib for renal cell carcinoma. J Clin Pharm Ther. 2013;38(2):175–176. doi: 10.1111/jcpt.12031. [DOI] [PubMed] [Google Scholar]
- [13].Tlemsani C, et al. Posterior reversible encephalopathy syndrome induced by anti-VEGF agents. Target Oncol. 2011;6(4):253–258. doi: 10.1007/s11523-011-0201-x. [DOI] [PubMed] [Google Scholar]
- [14].De Jesus-Gonzalez N, et al. Regorafenib induces rapid and reversible changes in plasma nitric oxide and endothelin-1. Am J Hypertens. 2012;25(10):1118–1123. doi: 10.1038/ajh.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Van Der Veldt AAM, et al. Reduction in skin microvascular density and changes in vessel morphology in patients treated with sunitinib. Anticancer Drugs. 2010;21(4):439–446. doi: 10.1097/CAD.0b013e3283359c79. [DOI] [PubMed] [Google Scholar]
- [16].Robinson ES, et al. Suppression of the nitric oxide pathway in metastatic renal cell carcinoma patients receiving vascular endothelial growth factor-signaling inhibitors. Hypertension. 2010;56(6):1131–1136. doi: 10.1161/HYPERTENSIONAHA.110.160481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kappers MHW, et al. Sunitinib-induced systemic vasoconstriction in swine is endothelin mediated and does not involve nitric oxide or oxidative stress. Hypertension. 2012;59(1):151–157. doi: 10.1161/HYPERTENSIONAHA.111.182220. [DOI] [PubMed] [Google Scholar]
- [18].Neves KB, et al. VEGFR (Vascular Endothelial Growth Factor Receptor) Inhibition Induces Cardiovascular Damage via Redox-Sensitive Processes. Hypertension. 2018 doi: 10.1161/HYPERTENSIONAHA.117.10490. HYPERTENSIONAHA.117.10490. [DOI] [PubMed] [Google Scholar]
- [19].Lankhorst S, Saleh L, Danser AHJ, Van Den Meiracker AH. Etiology of angiogenesis inhibition-related hypertension. Current Opinion in Pharmacology. 2015;21:7–13. doi: 10.1016/j.coph.2014.11.010. [DOI] [PubMed] [Google Scholar]
- [20].Te Riet L, Van Esch JHM, Roks AJM, Van Den Meiracker AH, Danser AHJ. Hypertension: Renin-Angiotensin-Aldosterone System Alterations. Circulation Research. 2015;116(6):960–975. doi: 10.1161/CIRCRESAHA.116.303587. [DOI] [PubMed] [Google Scholar]
- [21].Curwen JO, Musgrove HL, Kendrew J, Richmond GHP, Ogilvie DJ, Wedge SR. Inhibition of vascular endothelial growth factor-A signaling induces hypertension: Examining the effect of cediranib (recentin; AZD2171) treatment on blood pressure in rat and the use of concomitant antihypertensive therapy. Clin Cancer Res. 2008;14(10):3124–3131. doi: 10.1158/1078-0432.CCR-07-4783. [DOI] [PubMed] [Google Scholar]
- [22].Remuzzi G, Perico N, Benigni A. New therapeutics that antagonize endothelin: Promises and frustrations. Nature Reviews Drug Discovery. 2002;1(12):986–1001. doi: 10.1038/nrd962. [DOI] [PubMed] [Google Scholar]
- [23].Steingart RM, et al. Management of cardiac toxicity in patients receiving vascular endothelial growth factor signaling pathway inhibitors. Am Heart J. 2012;163(2):156–163. doi: 10.1016/j.ahj.2011.10.018. [DOI] [PubMed] [Google Scholar]
- [24].Maitland ML, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. Journal of the National Cancer Institute. 2010;102(9):596–604. doi: 10.1093/jnci/djq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lenihan DJ, Kowey PR. Overview and management of cardiac adverse events associated with tyrosine kinase inhibitors. Oncologist. 2013;18(8):900–8. doi: 10.1634/theoncologist.2012-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].NICE. Hypertension in adults: diagnosis and management. Guidel - Summ Clin Guidel Prim care. 2017:59–65. no. August 2011. [Google Scholar]
- [27].Copur MS, Obermiller A. An algorithm for the management of hypertension in the setting of vascular endothelial growth factor signaling inhibition. Clinical Colorectal Cancer. 2011;10(3):151–156. doi: 10.1016/j.clcc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- [28].Eigenbrodt ML, Rose KM, Couper DJ, Arnett DK, Smith R, Jones D. Orthostatic Hypotension as a Risk Factor for Stroke : The Atherosclerosis Risk in Communities (ARIC) Study, 1987-1996. Stroke. 2000;31(10):2307–2313. doi: 10.1161/01.str.31.10.2307. [DOI] [PubMed] [Google Scholar]
- [29].Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: Cardio-oncology. Mayo Clinic Proceedings. 2014;89(9):1287–1306. doi: 10.1016/j.mayocp.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ky B, Vejpongsa P, Yeh ETH, Force T, Moslehi JJ. Emerging paradigms in cardiomyopathies associated with cancer therapies. Circ Res. 2013;113(6):754–764. doi: 10.1161/CIRCRESAHA.113.300218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ghatalia P, et al. Congestive heart failure with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Critical Reviews in Oncology/Hematology. 2015;94(2):228–237. doi: 10.1016/j.critrevonc.2014.12.008. [DOI] [PubMed] [Google Scholar]
- [32].National Institute of Cancer. Common Terminology Criteria for Adverse Events ( CTCAE ), Version 4.0. DCTD, CTI, NIH, DHHS; 2009. [Google Scholar]
- [33].Motzer RJ, et al. Pazopanib versus Sunitinib in Metastatic Renal-Cell Carcinoma. N Engl J Med. 2013;369(8):722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- [34].Haas NB, et al. Effects of adjuvant sorafenib and sunitinib on cardiac function in renal cell carcinoma patients without overt metastases: Results from ASSURE, ECOG 2805. Clin Cancer Res. 2015;21(18):4048–4054. doi: 10.1158/1078-0432.CCR-15-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chu TF, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370(9604):2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Narayan V, et al. Prospective Evaluation of Sunitinib-Induced Cardiotoxicity in Patients with Metastatic Renal Cell Carcinoma. Clin Cancer Res. 2017;23(14):3601–3609. doi: 10.1158/1078-0432.CCR-16-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS, Walsh K. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension. 2006;47(5):887–893. doi: 10.1161/01.HYP.0000215207.54689.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Moslehi JJ. Cardiovascular Toxic Effects of Targeted Cancer Therapies. N Engl J Med. 2016;375(15):1457–1467. doi: 10.1056/NEJMra1100265. [DOI] [PubMed] [Google Scholar]
- [39].Cardinale D, et al. Anthracycline-Induced Cardiomyopathy. Clinical Relevance and Response to Pharmacologic Therapy. J Am Coll Cardiol. 2010;55(3):213–220. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- [40].Plana JC, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15(10):1063–1093. doi: 10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schmidinger M. Understanding and managing toxicities of vascular endothelial growth factor (VEGF) inhibitors. European Journal of Cancer, Supplement. 2013;11(2):172–191. doi: 10.1016/j.ejcsup.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kuenen BC, et al. Analysis of coagulation cascade and endothelial cell activation during inhibition of vascular endothelial growth factor/vascular endothelial growth factor receptor pathway in cancer patients. Arterioscler Thromb Vasc Biol. 2002;22(9):1500–1505. doi: 10.1161/01.atv.0000030186.66672.36. [DOI] [PubMed] [Google Scholar]
- [43].Herrmann J, Lerman A. An update on cardio-oncology. Trends in Cardiovascular Medicine. 2014;24(7):285–295. doi: 10.1016/j.tcm.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Choueiri TK, Schutz FAB, Je Y, Rosenberg JE, Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: A systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28(13):2280–2285. doi: 10.1200/JCO.2009.27.2757. [DOI] [PubMed] [Google Scholar]
- [45].Schutz FAB, Je Y, Azzi GR, Nguyen PL, Choueiri TK. Bevacizumab increases the risk of arterial ischemia: A large study in cancer patients with a focus on different subgroup outcomes. Ann Oncol. 2011;22(6):1404–1412. doi: 10.1093/annonc/mdq587. [DOI] [PubMed] [Google Scholar]
- [46].Escudier B, et al. Sorafenib in Advanced Clear-Cell Renal-Cell Carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- [47].Je Y, Schutz FaB, Choueiri TK. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. Lancet Oncol. 2009;10(10):967–974. doi: 10.1016/S1470-2045(09)70222-0. [DOI] [PubMed] [Google Scholar]
- [48].Qi WX, et al. Incidence and risk of hemorrhagic events with vascular endothelial growth factor receptor tyrosine-kinase inhibitors: An up-to-date meta-analysis of 27 randomized controlled trials. Annals of Oncology. 2013;24(12):2943–2952. doi: 10.1093/annonc/mdt292. [DOI] [PubMed] [Google Scholar]
- [49].Shah RR, Morganroth J, Shah DR. Cardiovascular safety of tyrosine kinase inhibitors: With a special focus on cardiac repolarisation (QT Interval) Drug Safety. 2013;36(5):295–316. doi: 10.1007/s40264-013-0047-5. [DOI] [PubMed] [Google Scholar]
- [50].Kloth JSL, et al. Incidence and relevance of QTc-interval prolongation caused by tyrosine kinase inhibitors. Br J Cancer. 2015;112(6):1011–1016. doi: 10.1038/bjc.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]