Abstract
The synthetic investigation of biologically active natural compounds serves two main purposes: (i) the total synthesis of alkaloids and their analogues; (ii) modification of the structures for producing more selective, more effective, or less toxic derivatives. In the chemistry of dimeric Vinca alkaloids enormous efforts have been directed towards synthesizing new derivatives of the antitumor agents vinblastine and vincristine so as to obtain novel compounds with improved therapeutic properties.
Keywords: antitumor therapy, vinblastine, vincristine, derivatives
1. Introduction
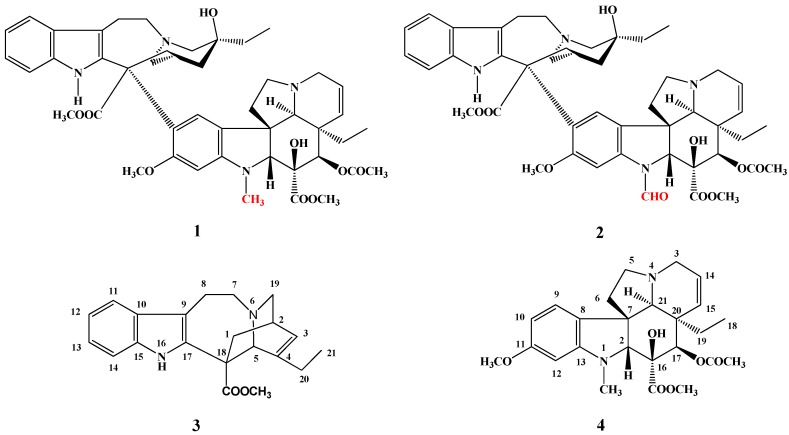
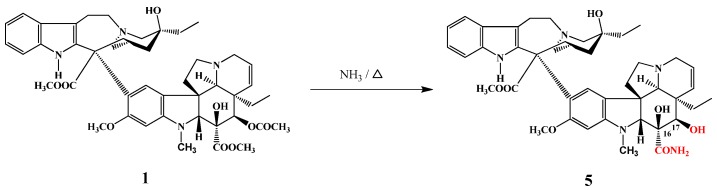
Vinblastine (1) and vincristine (2) are dimeric alkaloids (Figure 1) isolated from the Madagaskar periwinkle plant (Catharantus roseus), exhibit significant cytotoxic activity and are used in the antitumor therapy as antineoplastic agents.
In the course of cell proliferation they act as inhibitors during the metaphase of the cell cycle and by binding to the microtubules inhibit the development of the mitotic spindle. In tumor cells these agents inhibit the DNA repair and the RNA synthesis mechanisms, blocking the DNA-dependent RNA polymerase.
Figure 1.
Structures of vinblastine (1) and vincristine (2).
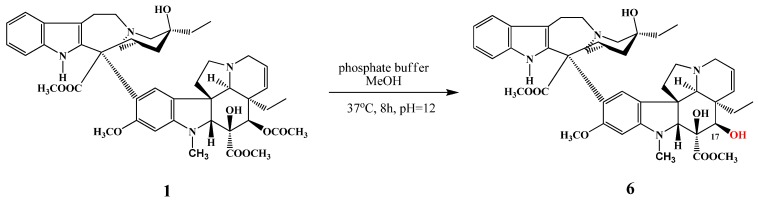
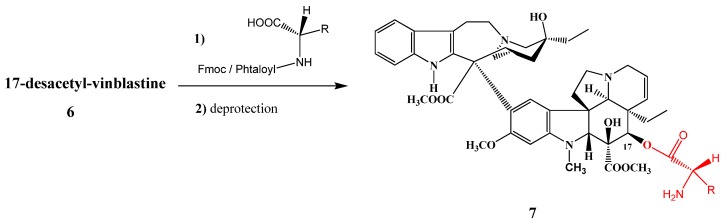
Vinblastine (1) has two monomer alkaloid parts: catharanthine (3) and vindoline (4). The difference between vinblastine (1) and vincristine (2) is that the former has a methyl while the latter has a formyl group on the indole nitrogen of the vindoline skeleton (Figure 1).
The chemical and biological characteristics of these dimeric alkaloids are presented in several reviews, of which only two publications are mentioned here [1,2]. The aim of our present work, however, is to highlight the different derivatives and derivatisations of vinblastine (1) and vincristine (2), focusing primarily on selected cases that stand out from the enormous literature data owing to their interesting chemistry or important biological effects.
2. Modification of Vinblastine
According to an Eli Lilly patent [3] the carboxylic ester group of vinblastine at position 16 was converted to amide 5 in a reaction with refluxing ammonia (Scheme 1).
Scheme 1.
Preparation of 17-desacetylvinblastine-16-amide (5).
Starting from the corresponding hydrazide, which was deacetylated at position 17, hydrogenolysis of the hydrazide resulted in the formation of 16-amide function [4]. 17-Desacetylvinblastine-16-amide (5), named VINDESINE, is an excellent antitumor agent, that can be used for the treatment of adenocarcinoma, lymphosarcoma and osteogen sarcinoma.
The 17-O-acetyl group of vinblastine was selectively hydrolysed by Thimmaiah and co-workers [5] using a phosphate buffer in methanol; thus 17-desacetylvinblastine (6) was successfully prepared in 95% yield (Scheme 2). Compound 6 can be considered as the active metabolite of vinblastine [6], because its activity is substantially higher than that of the prodrug vinblastine.
Scheme 2.
Selective desacetylation of vinblastine (1).
The same reaction was carried out by Brady [6] in 95% yield with a 1:3 hydrazine-methanol mixture at 20 °C for 20 h. The resulting 17-desacetylvinblastine (6) was allowed to react with amino acids protected on the N-terminal with Fmoc or Phth [(9-fluorenylmethyloxi)carbonyl) and phthtaloyl]; after coupling the products were deprotected and new derivatives 7 containing amino acids (e.g., glycine, leucine and proline) and different peptides attached to position 17 with an ester bond were obtained (Scheme 3). Compounds 7 showed significant effects against prostate cancer.
Scheme 3.
Amino acid derivatives of vinblastine.
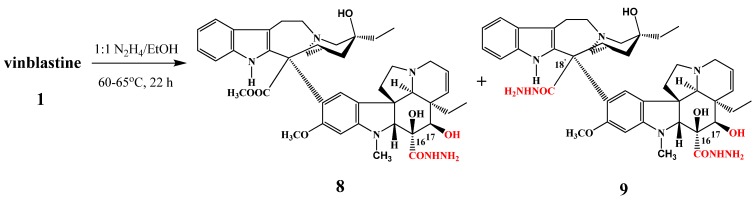
Under treatment of vinblastine with a 1:1 mixture of hydrazine and ethanol at 60–65 °C for 22 h two products were obtained (Scheme 4): 17-deacetyl-16-hydrazidevinblastine (8) and 17-deacetyl-16-hydrazide-18′-hydrazidevinblastine (9).
Scheme 4.
Hydrazide derivatives 8 and 9 of vinblastine (1).
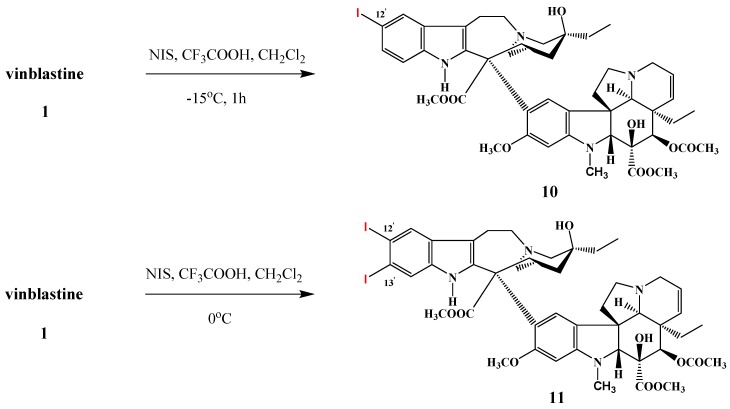
Scott et al. synthesized vinblastine derivatives substituted at the 12′- and 13′-positions of the catharanthine monomer (Scheme 5). In reactions with N-iodosuccinimide in dichloromethane solution in the presence of trifluoroacetic acid at −15 °C for 1 h, 12′-iodovinblastine (10) was isolated in high yield. By performing the iodination reaction at 0 °C 12′,13′-diiodovinblastine (11) was obtained in 25% yield.
Scheme 5.
Iodination reactions on vinblastine (1).
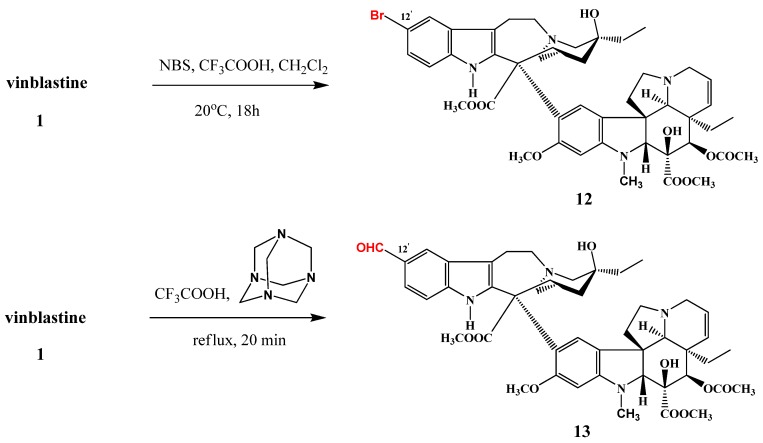
12′-Bromovinblastine (12) was prepared in 69% yield (Scheme 6) in the reaction of vinblastine (1) with N-bromosuccinimide. Reaction between hexamethylenetetramine and vinblastine under reflux for 20 min resulted in the corresponding formyl derivative (13) in 40% yield [7].
Scheme 6.
Bromination and formylation of vinblastine (1).
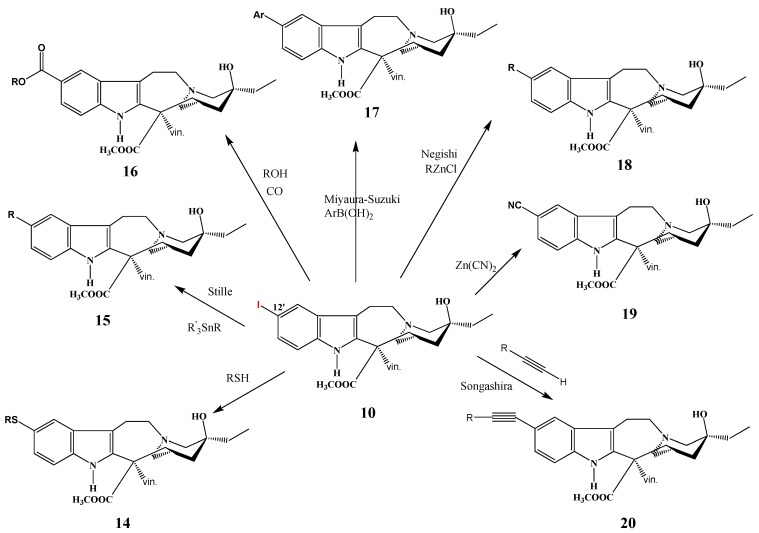
Numerous derivatives of vinblastine on the catharanthine part (compounds 14–20, Scheme 7) were prepared by the same research group from 12′-iodovinblastine (10), primarily by coupling reactions catalyzed by palladium (e.g., Stille, Songashira, Negishi) [8]. SAR of derivatives were investigated on HeLa (cervical cancer) and MCF-7 (breast cancer) cell lines and several derivatives showed promising anticancer activity in the P388 murine leukemia model.
Scheme 7.
Reactions of 12′-iodovinblastine (10).
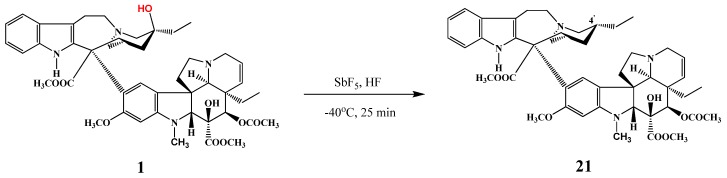
Selective removal of the 4′-hydroxy group of vinblastine was performed by Lafitte starting from vinblastine (1). In the reaction with antimony(V) pentafluoride and hydrogen fluoride at −40 °C for 25 min (Scheme 8) 4′-desoxyvinblastine (21) was obtained in 63% yield [9]. The same procedure resulted in the derivatives of anhydrovinblastine (32) and vinorelbine (33) with a reduced C=C double bond.
Scheme 8.
Reduction of vinblastine (1).
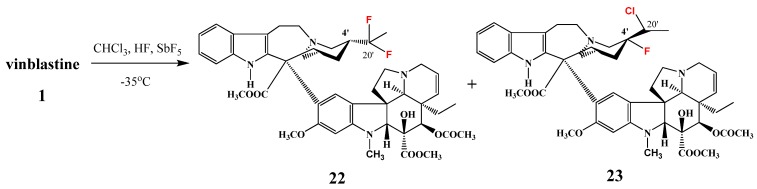
Fahy and co-workers also treated vinblastine (1) with antimony pentafluoride and hydrogen fluoride in chloroform at −35 °C and two new derivatives of vinblastine were isolated (Scheme 9): 4′-deoxy-20′,20′-difluorovinblastine (22) in 50% yield and 4′-deoxy-4′-fluoro-20′-chlorovinblastine (23) in 6% yield [10].
Scheme 9.
Fluorination reaction of vinblastine (1).
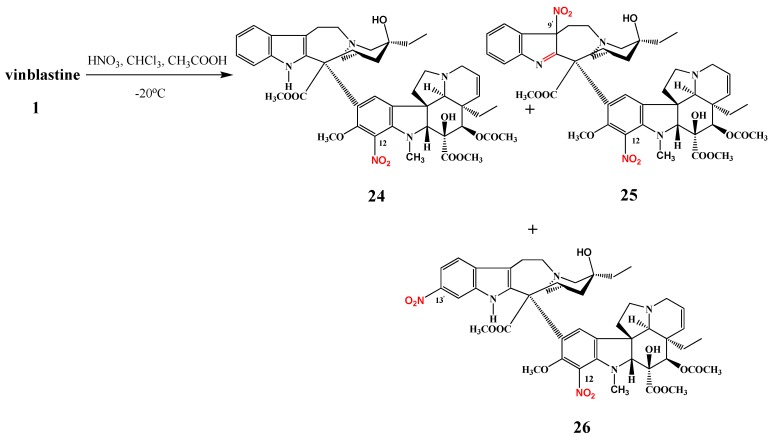
The nitration of vinblastine (1) was investigated by Szántay et al. [11]. Vinblastine (1) was allowed to react with nitric acid in an acetic acid/chloroform solution at −20 °C and three products were isolated (Scheme 10): 12-nitrovinblastine (24) in 49% yield, a 9′,12-dinitrovinblastine derivative 25 in 9% yield, and 12,13′-dinitrovinblastine (26) in 6% yield [12]. After reduction of the corresponding nitro derivatives some of the aminovinblastines obtained had important antitumor activity in non-small cell lung cancer, in breast and colon cancer and in leukemia.
Scheme 10.
Nitration of vinblastine (1).
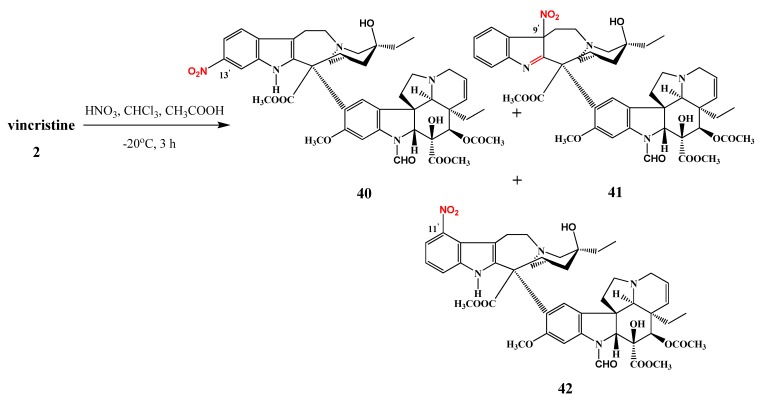
Vincristine (2) was also nitrated under similar conditions, and in this case the nitro group attacked the 11′-, 9′- or the 13′-positions. All of the derivatives, amongst which 13′-nitrovincristine (40) was the major product, were isolated [13].
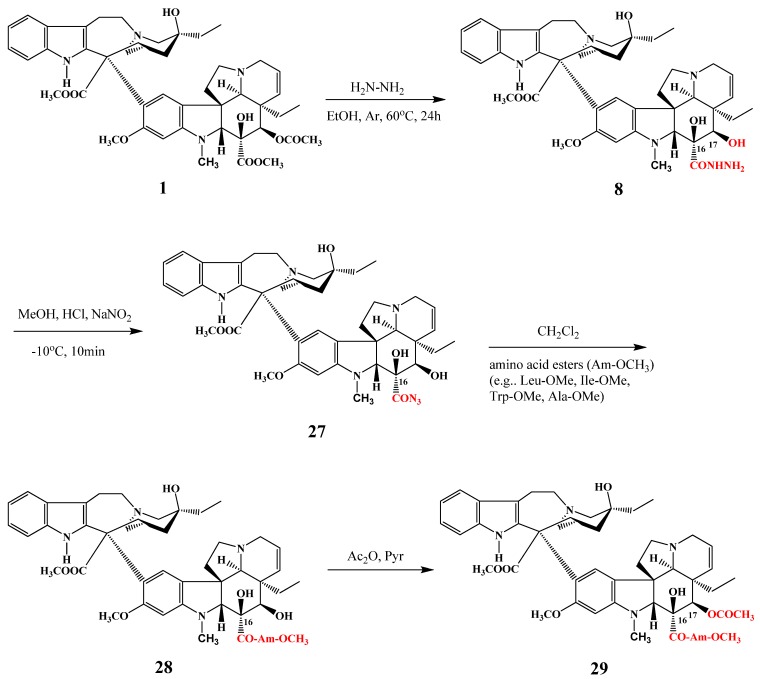
Rao and co-workers synthesized 17-desacetylvinblastine-16-hydrazide (8) from vinblastine (1), then the azide 27 was prepared and without isolation of the intermediate azide, vinblastine (1) was coupled with different amino acids through their amino group giving compounds 28 (Scheme 11). Finally, in most cases, the 17-hydroxy substituent was acetylated again to give 29 [14]. Most of the new compounds showed cytotoxic activity against P388 and L1210 leukemia, melanoma, breast cancer and small cell lung cancer [15].
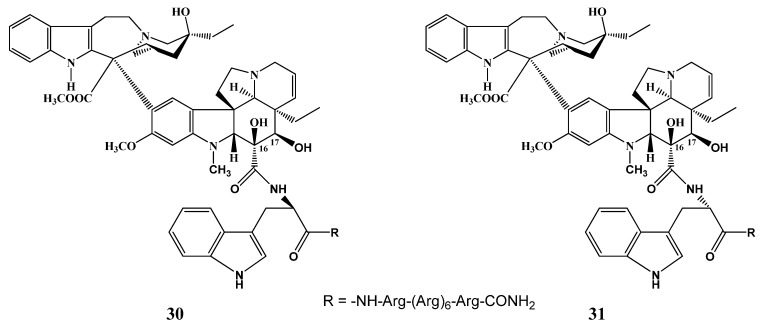
D- and L-Tryptophan derivatives of the 16-position of desacetylvinblastine were conjugated through the carboxyl group with oligoarginine octapeptide as a carrier peptide at the N-terminus (compounds 30,31) by Bánóczi et al. [16] (Scheme 12). One of the stereoisomers 30 or 31 showed a selective cytotoxic effect against the HL-60 human leukemia cells of higher proliferation rate.
Scheme 11.
Coupling of vinblastine (1) with amino acids.
Scheme 12.
Vinblastine derivatives conjugated with carrier peptide.
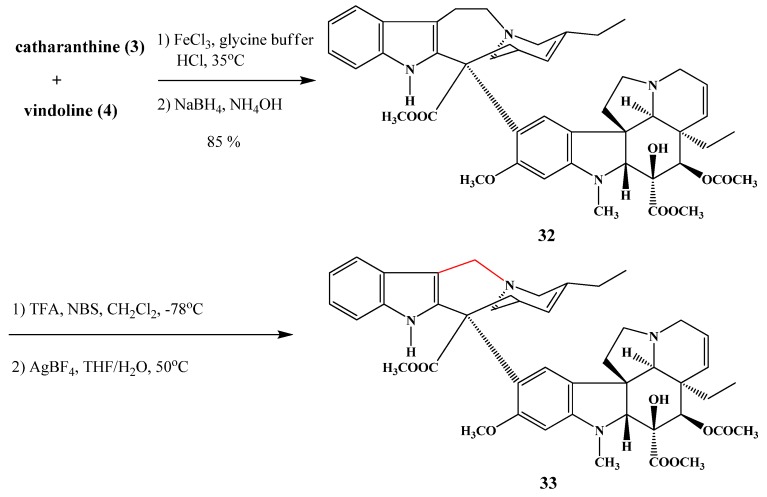
Ngo et al. [17] synthesized vinorelbine (33) in two steps from anhydrovinblastine (32), prepared by the coupling of vindoline (4) and catharanthine (3) (Scheme 13). This derivative is a potent antitumor agent in the treatment of non-small cell lung cancer [18]. In addition vinorelbine (33) and anhydrovinblastine (32) were hybridized through the 17-hydroxy group of the vindoline part with colchicine, podophyllotoxine and baccatine III to investigate the effect of the new molecules on the polymerisation of tubuline [19].
Scheme 13.
Synthesis of vinorelbine (33).
Ngo and his research group hybridized the cleavamine moiety of anhydrovinblastine (32) and vinorelbine (33) on the tertiary amine part with the antimitotic cyclopeptide phomopsin-A and obtained some potent inhibitors of the microtubules assembly and derivatives of good cytotoxicity against KB human cell lines [17]. Vinblastine (1) was also conjugated with a folic acid unit by the azide coupling method presented by Vlahov et al. [20].
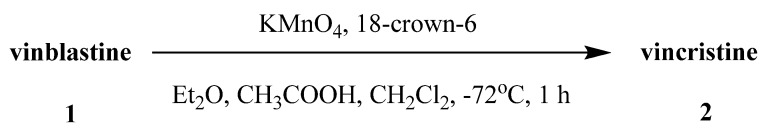
A number of vinblastine congeners were synthesized by Kuehne and co-workers, first of all by changing the piperidine ring of catharanthine (3), which were potent against leukemia and colon cancer cell lines. Vinblastine (1) was successfully oxidized to vincristine (2) (Scheme 14) by the same research group, using potassium permanganate and 18-crown-6 phase transfer catalyst [21].
Scheme 14.
Oxidation of vinblastine (1) to vincristine (2).
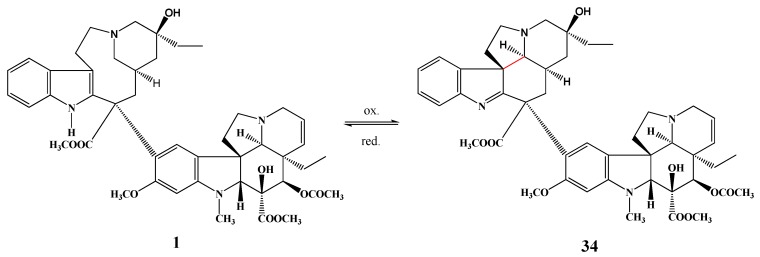
Szántay et al. prepared cyclovinblastine (34) by oxidation of vinblastine (1). The ring transformation reaction of cyclovinblastine (Scheme 15) and cyclovincristine was also investigated and the structure of the products was identified [22].
Scheme 15.
Ring transformation reaction.
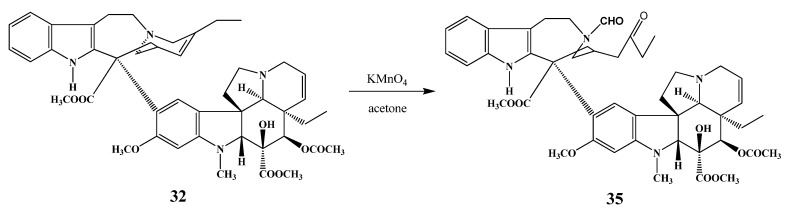
Kutney et al. prepared vinamidine (35) by the reaction of anhydrovinblastine (32) with potassium permanganate (Scheme 16) in acetone solution [23].
Scheme 16.
Preparation of vinamidine (35).
Bornmann and Kuehne elaborated the total synthesis of vinamidine (35) and some similar alkaloids [24]. In the course of the reaction procedure a new tetracyclic key intermediate was synthesized, which could be used for preparation of other similar alkaloids.
3. Changes in the Structure of Vincristine
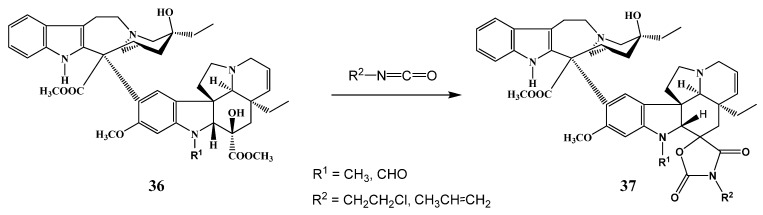
Spiro-oxazolidinedione substituted derivatives (Scheme 17) of vincristine [and similarly of vinblastine (37)] were synthesized starting from the corresponding 17-deacetoxy basic skeletons [25]. The key step of the reaction procedure was the reaction of the dimers 36 with isocyanates. In the course of the synthesis compounds were obtained with excellent cytotoxic activity and the new derivatives were active against leukemia.
Scheme 17.
Spiro-substituted dimer alkaloids.
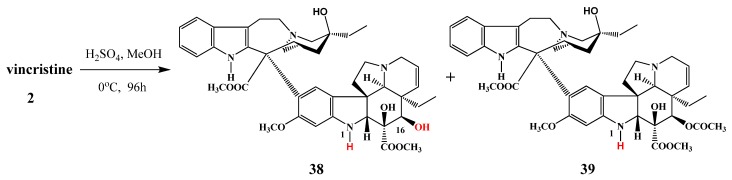
Thimmaiah et al. treated vincristine (2) with sulfuric acid in methanol (Scheme 18) and 17-deacetyl-N-desformylvincristine (60%) (38) and N-deformylvincristine (40%) (39) were isolated [5].
Scheme 18.
Deacetylation of vincristine (2).
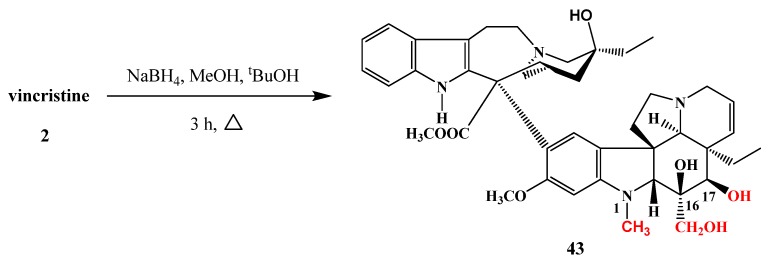
The reduced derivative of vincristine (43) was prepared by Szántay and his co-workers (Scheme 19). The reduction was carried out with sodium borohydride in a mixture of alcohols in 63% yield [26].
Scheme 19.
Reduction of vincristine (2).
The same research group investigated the nitration reaction also of vincristine (2) and three of the products were isolated (Scheme 20): 13′-nitrovincristine (40) as a major products (60%), a derivative of 9′-nitrovincristine (41) (31%) and 11′-nitrovincristine (42) in 5% yield [12].
Scheme 20.
Nitration of vincristine (2).
Scott et al. synthesized vincristine derivatives substituted in the catharanthine part at the aromatic ring in positions of 12′ and of 13′ (Scheme 21).
Scheme 21.
Iodinated derivatives of vincristine (2).
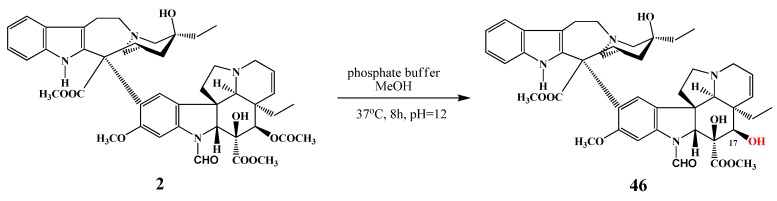
The reaction between vincristine (2) and N-iodosuccinimide in dichloromethane in the presence of trifluoroacetic acid at −15 °C for 1 h resulted in 12′-iodovincristine (44) in 91% yield. When this reaction was carried out at 0 °C, 12′,13′-diiodovincristine (45) was obtained in 18% yield [7]. Thimmaiah et al., similarly to vinblastine (1), succeded in realizing the selective hydrolysis of vincristine (2) in the position of 17 (Scheme 22). Thus, 17-deacetylvincristine (46) was prepared in the reaction with phosphate buffer in methanol at pH 12 in 95% yield [5]. Cyclovinblastine (34) was successfully oxidized to cyclovincristine by Szántay with cromyl acetate [27].
Scheme 22.
Selective deacetylation of vincristine (2).
Ahn et al. prepared N-formylcatharinine with enzymatic methods. This compound differs from vinamidine in position 1 of the vindoline part containing a formyl group instead of a methyl substituent [28].
Scheme 23.
Detailed synthesis of vinflunine (58).
4. Modifications on the Catharanthine Skeleton
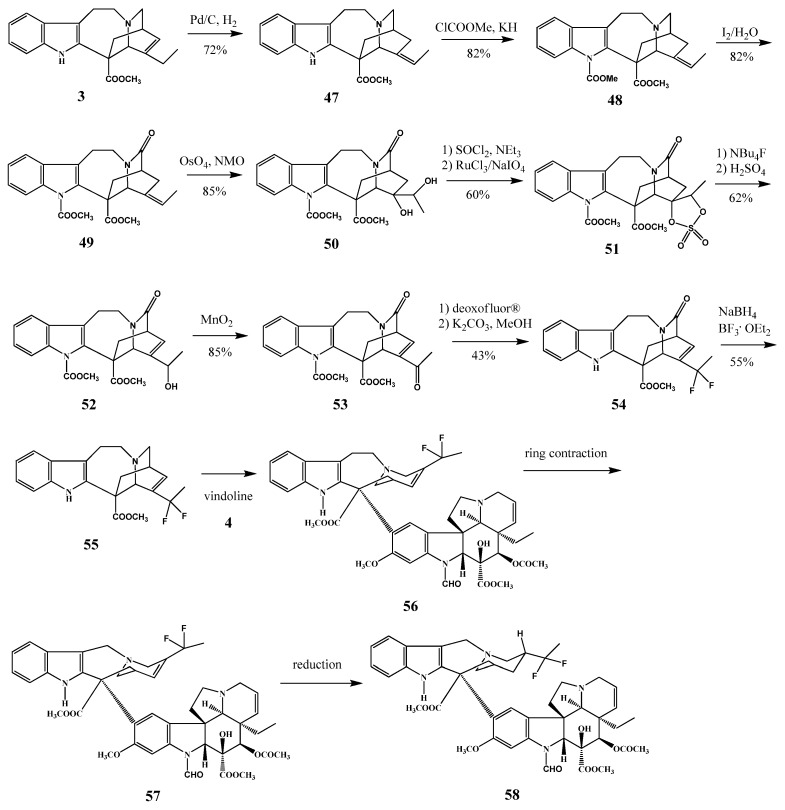
Moisan and his co-workers synthesized 20,20-difluorocatharanthine (55) starting from catharanthine (3) (Scheme 23). The latter difluoro derivative, when coupled with vindoline resulted in vinflunine (Javlor) (58) which is a vinorelbine (33) derivative [29]. The pharmacology of this new fluorinated Vinca alkaloid was fully investigated [30] and Phase III results are also presented [31].
Superacids were used in some cases to carry out the fluorination reactions [32], and the mechanism of the superacidic fluorination was investigated in detail [33]. The preparation of the vinflunine (58) was also elaborated directly by fluorination of vinorelbine (33) or by fluorination of anhydrovinblastine (32) and then with C′-ring contraction [34].
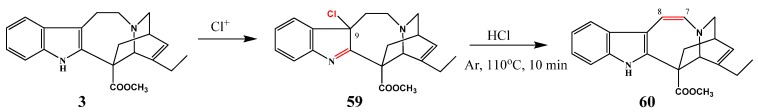
Andriamialisoa et al. successfully prepared 9-chlorocatharanthine (59), and starting from this compound Δ7-catharanthine (60) was obtained (Scheme 24) [35]. Further reactions resulted in 7′-nor derivatives.
Scheme 24.
Preparation and reaction of 9-chlorocatharanthine (59).
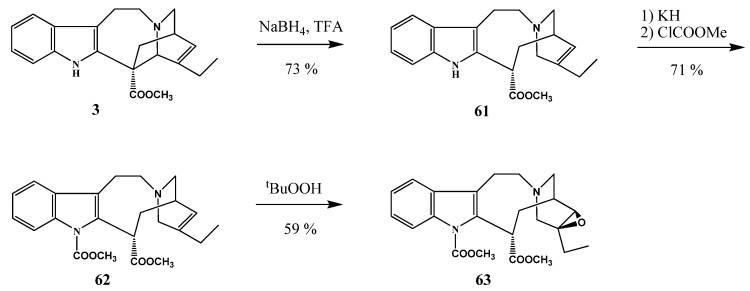
Catharanthine 63 containing an oxirane ring (Scheme 25) was prepared in the reaction of compound 62 with tert-butyl peroxide by Hardouin and his co-workers [36] who used this intermediate in the synthesis of leurosine. The latter was converted to anhydrovinblastine (32) which can be considered as the key intermediate for the synthesis of vinorelbine (33).
Scheme 25.
Building in an oxirane ring to catharanthine (3).
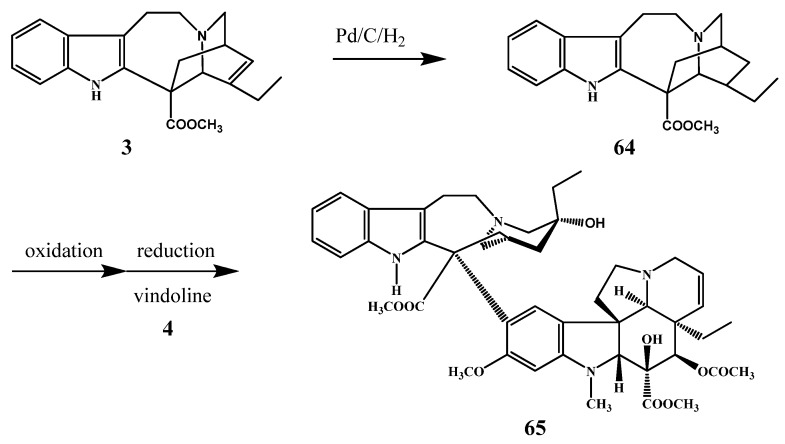
Catharanthine (3) was at first hydrogenated catalytically by Potier (Scheme 26) obtaining the saturated derivative 63 and after coupling with vindoline (4), leurosidine (65) was obtained [37]. Tam et al. prepared a number of derivatives of catharanthine and then these new derivatives were coupled with vindoline (4) (see Section 6. Coupling reactions) [38].
Scheme 26.
Preparation of leurosidine (65).
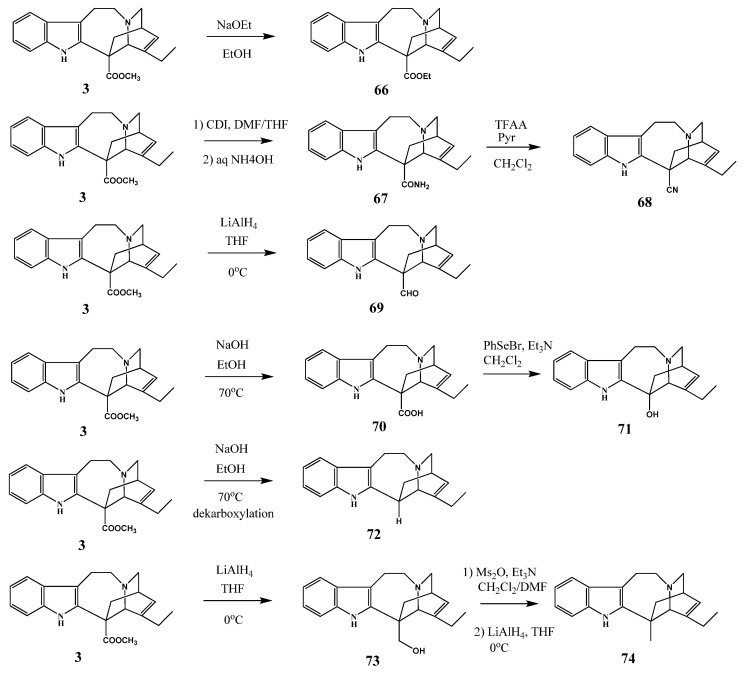
Scheme 27.
New derivatives of catharanthine.
In the course of the mentioned research project the ester group in position 18 of catharanthine was converted to other acid derivatives [38], e.g., amide, nitrile, and to different substituents such as aldehyde, hydroxy, alkyl, etc. by standard methods (Scheme 27). The aim of this work was to obtain new dimer alkaloids substituted in the catharanthine monomer.
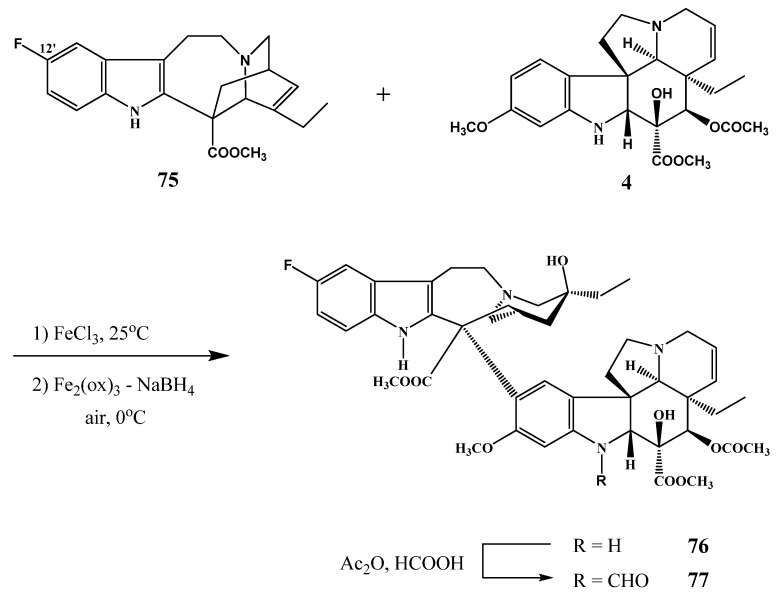
Numerous derivatives of vinblastine and vincristine were prepared by Boger [39]. These compounds were synthesized by the coupling of catharanthine substituted at the aromatic ring in position 12′ and vindoline (4) (Scheme 28). The new derivatives contained different substituents (nitro, amino, halogene, nitrile, alkyl, alkoxy, and thioalkyl), but compounds 76 and 77 proved to be the best antitumor molecules in the case of both sensitive and resistent human colon cancer cells.
Scheme 28.
Fluoro-substituted dimeric alkaloids.
5. Derivatizations of Vindoline
According to the literature the monomer Vinca alkaloid vindoline (4) was generally presumed and found to be ineffective against cell proliferations. Reactions and derivatives of vindoline for the synthesis of vinblastine were scarcely investigated. The reactivity of the aromatic ring of vindoline was presented by Szántay et al. [40], but the new vindoline derivatives were not used for the preparation of dimer alkaloids.
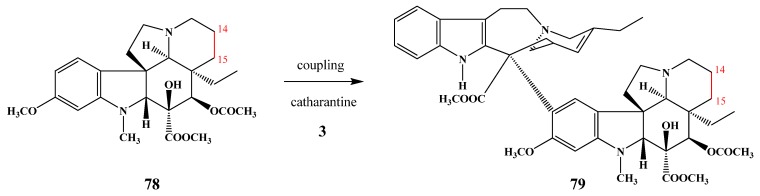
Kutney et al. [41] and Ishikawa [42] synthesized 14,15-dihydrovindoline (78), and its coupling with catharanthine resulted in 14,15-dihydroanhydrovinblastine (79) (Scheme 29). Meanwhile 14,15-dihydrovinblastine was simply prepared by catalytic hydrogenation of vinblastine, and its mechanism of action was different from that of the original vinblastine and the antitumor activity was rather low [43].
Scheme 29.
Coupling with saturated vindoline.
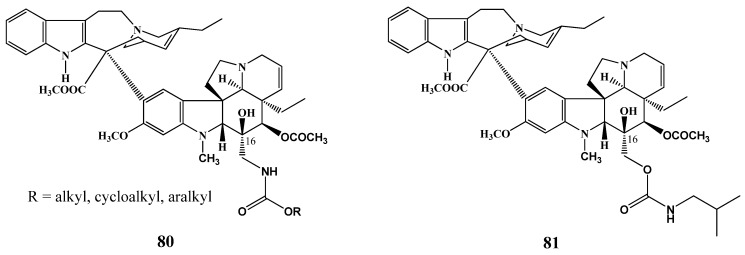
One of the examples is the synthesis of carbamates of vindoline in position 16 (Scheme 30) and after coupling with catharanthine (3) new derivatives 80 and 81 of vinblastine were obtained which had important anticancer activity against human non-small cell lung cancer and on human cervix epithelial adenocarcinoma cell lines [44].
Scheme 30.
Carbamates of vinblastine.
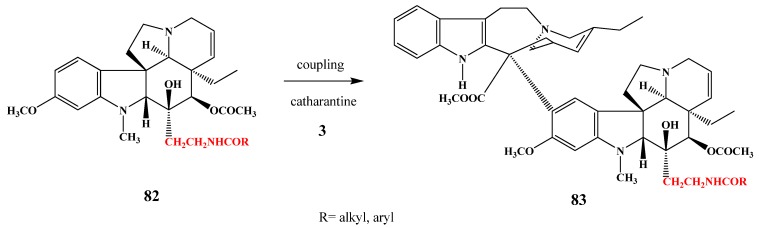
Amide substituted anhydrovinblastines 83 were synthesized by building in acylated aminoethyl substituents in position 16 of the vindoline monomer 82 (Scheme 31). After coupling with catharanthine (3) new derivatives showed proliferation inhibition against HeLa cells [45].
Scheme 31.
Amide substitued anhydrovinblastine derivatives.
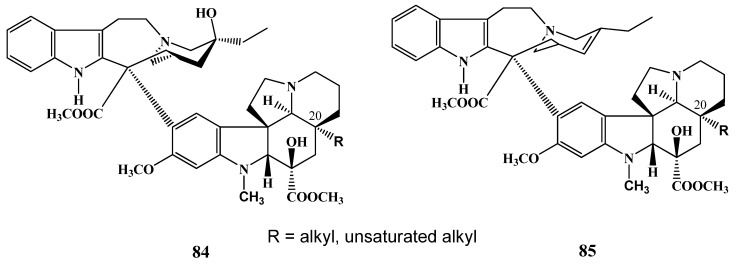
The C-20 ethyl substituent of vindoline was changed to alkyl and alkene groups by another research group [46]. The new vindoline derivatives were coupled with catharanthine (3) resulting in new C-20 alkyl and alkenyl vinblastines 84 and 85 (Scheme 32). Biological investigations on colon cell lines were presented discussing the role of the absence or the presence of the C-20 ethyl group on the surrounding tubulin binding site.
Scheme 32.
Modification of C-20 ethyl substitutent of vindoline.
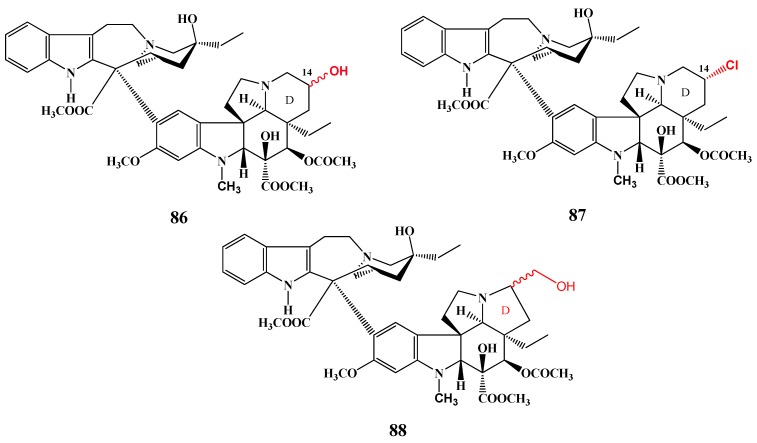
Sasaki and his co-workers synthesized new derivatives of vindoline substituted in the D-ring (Scheme 33). After coupling by the usual way with catharanthine (3), new vinblastine derivatives were obtained containing a hydroxy group (compound 86) or chloro atom (compound 87) in the vindoline moiety. Moreover, a new derivative 88 was also prepared containing a five membered D-ring in place of the six-membered ring in the vindoline part of the dimer [47].
Scheme 33.
Vinblastines substituted at the vindoline D-ring.
6. Coupling Reactions
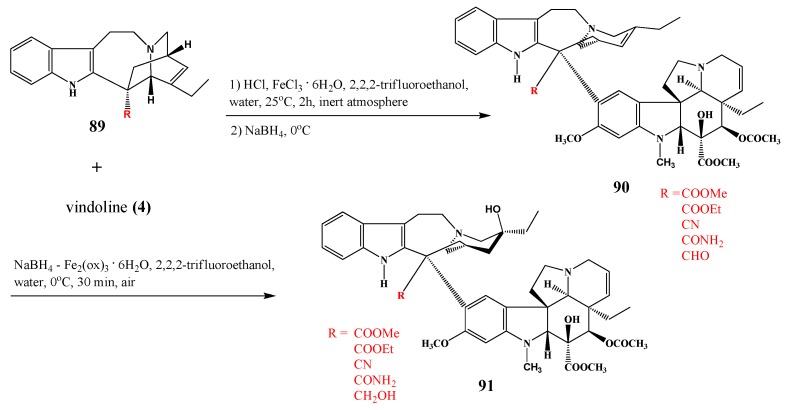
In this section only some tipical example resulting in new derivatives of dimeric alkaloids are mentioned from the many coupling reactions presented in the literature. Tam et al. [38] successfully coupled in two reaction steps the vindoline part with catharanthine substituted in the position 18′ shown before (cf. Scheme 27). The anhydro intermediates 90 were then oxidized to the expected target molecules, which are vinblastines 91 substituted in the catharanthine monomer (Scheme 34).
Scheme 34.
Coupling vindoline (4) with substituted catharanthines.
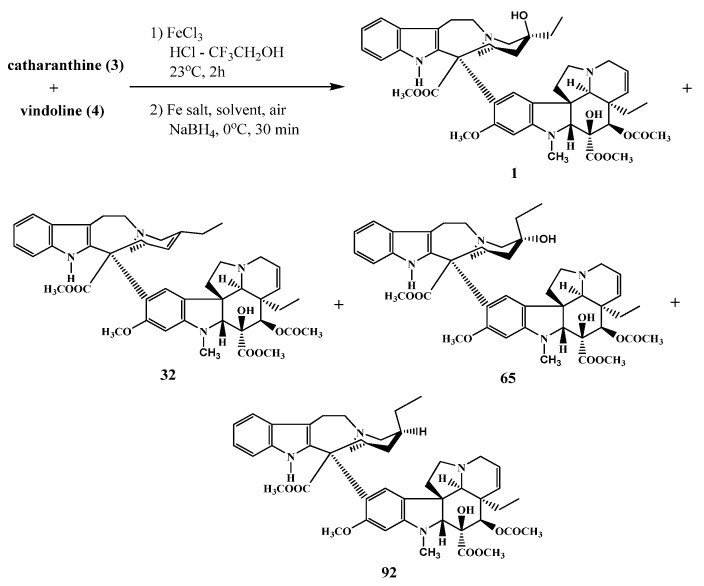
Ishikawa and his research group [42] elaborated the preparation of vinblastine by coupling catharanthine and vindoline in one step (Scheme 35). Depending the kind of Fe salt and the solvents used, products [vinblastine (1), anhydrovinblastine (32), leurosidine (65) and desoxyleurosidine (92), respectively] and their yields could be changed
Scheme 35.
Coupling in a one-step reaction.
7. Conclusions
Over the years a number of research teams have performed extensive and valuable work to synthesize new derivatives of vinblastine and vincristine. Modifications in the vindoline skeleton or in the catharanthine moiety resulted in a number of new antitumor agents with more selectivity or less toxic properties. The mechanism of activity of Vinca alkaloids was investigated using these new derivatives and some new important results were found in connection with the tubulin polymerisation system. Currently, the structure of these dimers still seems to be an inexhaustible source of further research in this field of chemistry and therapy.
Acknowledgements
The authors are grateful to Gedeon Richter Plc for financial assistance.
References
- 1.Brossi A., Suffness M. The Alkaloids. Vol. 37. Academic Press Inc.; New York, NY, USA: 1990. Antitumor Bisindol Alkaloids from Catharanthus Roseus (L.) pp. 1–240. [Google Scholar]
- 2.Bölcskei H., Szabó L., Szántay C. Synthesis of vinblastine derivatives. Front. Nat. Prod. Chem. 2005;1:43–49. doi: 10.2174/1574089054583849. [DOI] [Google Scholar]
- 3.Eli Lilly Company, Neue Aminderivate von Vinblastin, Leurosidin und Leurocristin und Verfahren zu ihrer Herstellung. 22415980. DE Patent. 1974 [Chem. Abstr. 1974, 82, 579967b]
- 4.Barnett C.J., Cullinan G.J., Gerzon K., Hoying R.C., Jones W.E., Newlon W.M., Poore G.A., Robison R.L., Sweeney M.J., Todd G.C. Structure-activity relationships of dimeric Catharanthus alkaloids. 1. Deacetylvinblastine amide (vindesine) sulfate. J. Med. Chem. 1978;21:88–96. doi: 10.1021/jm00199a016. [DOI] [PubMed] [Google Scholar]
- 5.Thimmaiah K.N., Lloyd W.D., Sethi V.S. A simple method for the chemical modification of antitumor Catharanthus Vinca alkaloids. Ind. J. Chem. Sect. B: Org. Chem. Med. Chem. 1990;29:678–680. [Google Scholar]
- 6.Brady S.F., Pawluczyk J.M., Lumma P.K., Feng D.-M., Wai J.M., Jones R., DeFeo-Jones D., Wong B.K., Miller-Stein C., Lin J.H., et al. Design and synthesis of a pro-drug of vinblastine targeted at treatment of prostate cancer with enhanced efficacy and reduced systemic toxicity. J. Med. Chem. 2002;45:4706–4715. doi: 10.1021/jm020139f. [DOI] [PubMed] [Google Scholar]
- 7.Scott I.L., Ralph J.M., Voss M.E. Vinca derivatives. 2005/55939. WO Patent. 2005
- 8.Voss M.E., Ralph J.M., Xie D., Manning D.D., Chen X., Frank A.J., Leyhane A.J., Liu L., Stevens J.M., Budde C., et al. Synthesis and SAR of Vinca alkaloid analogues. Bioorg. Med. Chem. Lett. 2009;19:1245–1249. doi: 10.1016/j.bmcl.2008.12.077. [DOI] [PubMed] [Google Scholar]
- 9.Lafitte C., Jouannetaud M.-P., Jacquesy J.-C., Fahy J., Duflos A. Stereoselective ionic hydrogenation of Vinca alkaloids and vinorelbine in Superacids: An access to 4′R-reduced analogs. Tetrahedron Lett. 1998;39:8281–8282. [Google Scholar]
- 10.Fahy J., Duflos A., Ribet J.-P., Jacquesy J.-C., Berrier C., Jouannetaud M.-P., Zunino F. Vinca alkaloids in superacid media: A method for creating a new family of antitumor derivatives. J. Am. Chem. Soc. 1997;119:8576–8577. doi: 10.1021/ja971864w. [DOI] [Google Scholar]
- 11.Bölcskei H., Szántay C.Jr., Mák M., Balázs M., Szántay C. New antitumor derivatives of vinblastine (in Hungarian) Acta Pharm. Hung. 1998;68:87–93. [PubMed] [Google Scholar]
- 12.Szabó L., Bölcskei H., Baitz-Gács E., Mák M., Szántay C. Synthesis of Vinca alkaloids and related compounds, part XCVI. Nitration study of vinblastine-type bisindole alkaloids. Arch. Pharm. (Weinheim, Germany) 2001;334:399–405. doi: 10.1002/1521-4184(200112)334:12<399::AID-ARDP399>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 13.Szabó L., Szántay C., Gács-Baitz E., Mák M. Synthesis of Vinca alkaloids and related compounds. LXXVIII. A potentionally biologically useful reduction of nitro-vincristine. Tetrahedron Lett. 1995;36:5265–5266. [Google Scholar]
- 14.Rao K.S.P.B., Collard M.-P.M., Dejonghe J.P.C., Atassi G., Hannart J.A., Trouet A. Vinblastin-23-oyl amino acid derivatives: Chemistry, physicochemical data, toxicity, and antitumor activities against P388 and L1210 leukemias. J. Med. Chem. 1985;28:1079–1088. doi: 10.1021/jm00146a017. [DOI] [PubMed] [Google Scholar]
- 15.Hendriks H.R., Langdon S., Berger D.P., Breistol K., Fiebig H.H., Fodstad O., Schwartsmann G. Comparative antitumour activity of vinblastine-isoleucinate and related vinca alkaloids in human tumour xenografts. Eur. J. Cancer. 1992;28:767–773. doi: 10.1016/0959-8049(92)90112-F. [DOI] [PubMed] [Google Scholar]
- 16.Bánóczi Z., Gorka-Kereskényi Á., Reményi J., Orbán E., Hazai L., Tőkési N., Oláh J., Ovádi J., Béni Z., Háda V., et al. Synthesis and in vitro antitumor effect of vinblastine derivative-oligoarginine conjugates. Bioconjug. Chem. 2010;21:1948–1955. doi: 10.1021/bc100028z. [DOI] [PubMed] [Google Scholar]
- 17.Ngo Q.A., Roussi F., Cormier A., Thoret S., Knossow M., Guénard D., Guéritte F. Synthesis and biological evaluation of Vinca alkaloids and phomopsin hybrids. J. Med. Chem. 2009;52:134–142. doi: 10.1021/jm801064y. [DOI] [PubMed] [Google Scholar]
- 18.Trédaniel J., Staudacher L., Teixeira L., Sebbagh S., Bucquet S., Hennequin C. Pharmacotherapy update: Vinorelbine in the treatment non-small-cell lung cancer. Clin. Med. Insights: Ther. 2010;2:179–188. [Google Scholar]
- 19.Passrella D., Giardini A., Peretto B., Fontana G., Sacchetti A., Silvani A., Ronchi C., Cappalletti G., Cartelli D., Borlak J., et al. Inhibitors of tubulin polymerization: Synthesis and biological evaluation of hybrids of vindoline, anhydrovinblastine and vinorelbine with thiocolchicine, podophyllotoxin and baccatin III. Bioorg. Med. Chem. 2008;16:6269–6285. doi: 10.1016/j.bmc.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Vlahov I.R., Santhapuram H.K.R., Kleindl P.J., Howard S.J., Stanford K.M., Leamon C.P. Design and regioselective synthesis of a new generation of targeted chemotherapeutics. Part 1: EC145, a folic acid conjugate of desacetylvinblastine monohydrazide. Bioorg. Med. Chem. Lett. 2006;16:5093–5096. doi: 10.1016/j.bmcl.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 21.Kuehne M.E., Bornmann W.G., Markó I., Qin Y., LeBoulluec K.L., Frasier D.A., Xu F., Mulamba T., Ensinger C.L., Borman L.S., et al. Synthesis and biological evaluation of vinblastine congeners. Org. Biomol. Chem. 2003;1:2120–2136. doi: 10.1039/b209990j. [DOI] [PubMed] [Google Scholar]
- 22.Honty K., Demeter Á., Szántay C.Jr., Hollósi M., Kolonits P., Szántay C. Synthesis of Vinca alkaloids and related compounds. Part XCIII. Skeletal rearrangement of cyclovinblastine derivatives: Formation of a novel bisindole system. Heterocycles. 1995;50:169–194. [Google Scholar]
- 23.Kutney J.P., Balsevich J., Worth B.R. Studies on the synthesis of bisindole alkaloids. XV. A synthesis of vinamidine (catharinine) Heterocycles. 1978;11:69–73. [Google Scholar]
- 24.Bornmann W.G., Kuehne M.E. A common intermediate providing syntheses of Ψ-tabersonine, coronaridine, iboxyphylline, ibophyllidine, vinamidine, and vinblastine. J. Org. Chem. 1992;57:1752–1760. doi: 10.1021/jo00032a029. [DOI] [Google Scholar]
- 25.Orosz F., Comin B., Rais B., Puigjaner J., Cascante M., Kovács J., Tárkányi G., Ács T., Keve T., Ovádi J. New bis-indol derivatives: Chemical, biochemical and cellular studies. Br. J. Cancer. 1999;79:1356–1365. doi: 10.1038/sj.bjc.6690218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bölcskei H., Szántay C.Jr., Mák M., Balázs M., Szántay C. New antitumor hydroxymethyl derivatives of vinblastine. J. Indian Chem. Soc. 1997;74:904–907. [Google Scholar]
- 27.Szántay C. Indole alkaloids in human medicine. Pure Appl. Chem. 1990;62:1299–1302. doi: 10.1351/pac199062071299. [DOI] [Google Scholar]
- 28.Ahn S.H., Duffel M.W., Rosazza J.P.N. Oxidations of vincristine catalyzed by peroxidase and ceruloplasmin. J. Nat. Prod. 1997;60:1125–1129. doi: 10.1021/np970226o. [DOI] [PubMed] [Google Scholar]
- 29.Moisan L., Comesse S., Giovanelli E., Rousseau B., Doris E., Hellier P. Fluorinated catharanthine derivatives, their preparation and their utilisation as Vinca dimeric alkaloid precursors. 2008/034882. WO Patent. 2008
- 30.Kruczynski A., Barret J.-M., Etiévant C., Colpaert F., Fahy J., Hill B.T. Antimitotic and tubulin-interacting properties of vinflunine, a novel fluorinated Vinca alkaloid. Biochem. Pharmacol. 1998;55:635–648. doi: 10.1016/S0006-2952(97)00505-4. [DOI] [PubMed] [Google Scholar]
- 31.Krzakowski M., Ramlau R., Jassem J., Szczesna A., Zatloukal P., Von Pawel J., Sun X., Bennoung J., Santoro A., Biesma B., et al. Phase III trial comparing vinflunine with docetaxel in second-line advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy. J. Clin. Oncol. 2010;28:2167–2173. doi: 10.1200/JCO.2009.23.4146. [DOI] [PubMed] [Google Scholar]
- 32.Fahy J., Duflos A., Ribet J.-P., Jacquesy J.-C., Berrier C., Jouannetaud M.-P., Zunino F. Vinca alkaloids in superacidic media: A method for creating a new family of antitumor derivatives. J. Am. Chem. Soc. 1997;119:8576–8577. doi: 10.1021/ja971864w. [DOI] [Google Scholar]
- 33.Giovanelli E., Leroux S., Moisan L., Carreyre H., Thuéry P., Buisson D.-A., Meddour A., Coustard J.-M., Thibaudeau S., Rousseau B., et al. On the elucidation of the mechanism of Vinca alkaloid fluorination in superacidic medium. Org. Lett. 2011;13:4116–4119. doi: 10.1021/ol201637m. [DOI] [PubMed] [Google Scholar]
- 34.Jacquesy J.-C., Berrier C., Jouannetaud M.-P., Zunino F., Fahy J., Duflos A., Ribet J.-P. Fluorination in superacids: A novel access to biologically active compounds. J. Fluorine Chem. 2002;114:139–142. doi: 10.1016/S0022-1139(02)00019-2. [DOI] [Google Scholar]
- 35.Andriamialisoa R.Z., Langlois N., Langlois Y., Potier P. Compesés antitumoraux du groupe de la vinblastine: Nouvelle méthode de préparation. Tetrahedron. 1980;36:3053–3060. doi: 10.1016/0040-4020(80)88032-X. [DOI] [Google Scholar]
- 36.Hardouin C., Doris E., Rousseau B., Mioskowski C. Selective deoxygenation of leurosine: Concise access to anhydrovinblastine. J. Org. Chem. 2002;67:6571–6574. doi: 10.1021/jo0202942. [DOI] [PubMed] [Google Scholar]
- 37.Potier P. Synthesis of the antitumor dimeric indole alkaloids from Catharanthus species (vinblastine group) J. Nat. Prod. 1979;43:72–86. doi: 10.1021/np50007a004. [DOI] [Google Scholar]
- 38.Tam A., Gotoh H., Robertson W.M., Boger D.L. Catharanthine C16 substituent effects on the biomimetic coupling with vindoline: Preparation and evaluation of a key series of vinblastine analogues. Bioorg. Med. Chem. Lett. 2010;20:6408–6410. doi: 10.1016/j.bmcl.2010.09.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gotoh H., Duncan K.K., Robertson W.M., Boger D.L. 10′-Fluorovinblastine and 10′-fluorovincristine: Synthesis of a key series of modified Vinca alkaloids. ACS Med. Chem. Lett. 2011;2:948–952. doi: 10.1021/ml200236a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szabó L., Hazai L., Lengyel M., Szántay C.Jr., Sánta Z., Kalaus G., Szántay C. Aromatic electrophilic substitutions on vindoline. Heterocycles. 2007;71:1553–1563. doi: 10.3987/COM-07-11049. [DOI] [Google Scholar]
- 41.Kutney J.P., Balsevich J., Honda T., Liao P.-H., Thiellier H.P.M., Worth B.R. Total synthesis of indole and dihydroindole alkaloids. XVI. Derivatives of vinblastine and vincristine: Change of functionality in the vindoline unit. Can. J. Chem. 1978;56:2560–2566. [Google Scholar]
- 42.Ishikawa H., Colby D.A., Seto S., Va P., Tam A., Kakei H., Rayl T.J., Hwang I., Boger D.L. Total synthesis of vinblastine, vincristine, related natural products, and key structural analogues. J. Am. Chem. Soc. 2009;131:4904–4916. doi: 10.1021/ja809842b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noble R.L., Beer T., McIntyre R.W. Biological effects of dihydroviblastine. Cancer. 1967;20:885–890. doi: 10.1002/1097-0142(1967)20:5<885::AID-CNCR2820200549>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 44.Shao Y., Ding H., Tang W., Lou L., Hu L. Synthesis and structure-activity relationships study of novel anti-tumor carbamate anhydrovinblastine analogues. Bioorg. Med. Chem. 2007;15:5061–5075. doi: 10.1016/j.bmc.2007.05.045. [DOI] [PubMed] [Google Scholar]
- 45.Song W., Lei M., Zhao K., Hu L., Meng Y., Guo D., Liu X., Hu L. Ceric ammonium nitrate-promoted oxidative coupling reaction for the synthesis and evaluation of a series of anti-tumor amide anhydrovinblastine analogs. Bioorg. Med. Chem. Lett. 2012;22:387–390. doi: 10.1016/j.bmcl.2011.10.114. [DOI] [PubMed] [Google Scholar]
- 46.Va P., Campbell E.L., Robertson W.M., Boger D.L. Total synthesis and evaluation of a key series of C5-substituted vinblastine derivatives. J. Am. Chem. Soc. 2010;132:8489–8495. doi: 10.1021/ja1027748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasaki Y., Kato D., Boger L. Asymmetric total synthesis of vindorosine, vindoline, and key vinblastine analogues. J. Am. Chem. Soc. 2010;132:13533–13544. doi: 10.1021/ja106284s. [DOI] [PMC free article] [PubMed] [Google Scholar]