Abstract
Litsea cubeba (Lour.) Pers. is a promising industrial crop with fruits rich in essential oils. The chemical composition of essential oils of L. cubeba (EOLC) were determined for fruits harvested from eight regions in China. The overall essential oil content, obtained by hydrodistillation and analyzed by gas chromatography–mass spectrometry (GC-MS), ranged from 3.04% to 4.56%. In total, 59 compounds were identified, the dominant components being monoterpenes (94.4–98.4%), represented mainly by neral and geranial (78.7–87.4%). D-limonene was unexpectedly a lesser constituent (0.7–5.3%) in fruits, which differed from previous reports (6.0–14.6%). Several components were only detected in certain regions and compounds such as o-cymene and eremophilene have never before been reported in EOLC. These results demonstrate significant regional variation in the chemical composition of EOLC. This investigation provides important information with regard to the bioactivity, breeding work and industrial applications of L. cubeba.
Keywords: Litsea cubeba, essential oil, citral, D-limonene, varieties
1. Introduction
The genus Litsea (Lauraceae) is composed of ca. 622 species, distributed mainly in tropical and subtropical Australia, New Zealand, North America, South America, and Asia [1]. China alone has 74 species [2], among which Litsea cubeba (Lour.) Pers. is a perennial, dioecious tree or shrub, widespread in areas of southern China.
Like other plants of the genus Litsea, L. cubeba produces an essential oil (EOLC), which can be extracted from different parts of the plant, including the fruit, root and flower as well as stem and leaf, with significant diversity in composition and yield [3,4]. EOLC is a flowing, pale yellow liquid, with an intensely lemonlike, spicy aroma [5]. It has been widely employed in the food, chemical and medicinal industries. In China, the Ministry of Health has approved EOLC for use as a food additive in accordance with standard GB 2760-2007. Furthermore, EOLC has been used as a crude material for the manufacture of citral, vitamins A, E, and K, ionine, methylionone, and perfumes [6]. Extracts of L. cubeba have also been used in traditional Chinese medicine for the treatment of a variety of ailments [7]. Recently reports have demonstrated the bioactivities of EOLC, which include antibacterial [8], antifungal [9,10], acaricidal [11], insecticidal [12,13], antioxidant [14], and anticancer properties [15].
The biological activities of EOLC are attributed directly to the synergistic or antagonistic effects of its chemical composition. Therefore, intimate knowledge of the chemical composition of EOLC is important for understanding its role in biological systems. Several researchers have reported the essential oil compositions of L. cubeba from limited geographical areas. Twenty components were isolated from EOLC harvested from Hunan Province [16]. Zhou and Mo [17] also identified 20 constituents in EOLC taken from Guizhou Province, and 31 compounds were identified in EOLC from Guanxi Province [18]. However, each of these studies applied their own sets of extraction and purification conditions.
In general, the relative composition of essential oils varies remarkably with geographical position, climate conditions and several other factors [19]. To acquire more information, the current study determined the essential oil compositions of L. cubeba collected from different parts of China. This work lays a theoretical foundation for further pharmacological research to explore the properties of EOLC and provides a reference for later breeding of this plant.
2. Results
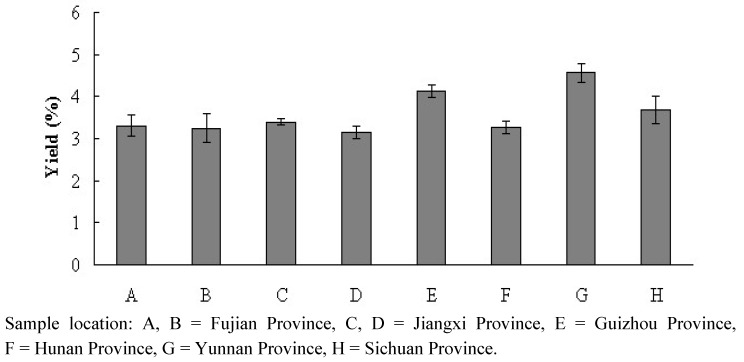
Plants were sampled primarily from hills and barren lands. Climatic information for these areas is provided in Table 1. Most were subtropical zones marked by warm and humid climates. Fresh fruits of L. cubeba afforded yellowish oils, with yields (mean of four replicates) ranging from 3.14% (D) to 4.56% (G) by dry weight (see Figure 1). In total, 59 individual components (41 monoterpenes, 15 sesquiterpenes, and three non-terpene compounds) were isolated and identified. Retention indices, relative percentages, and methods used for identification are listed in Table 2.
Table 1.
Climate and geographic habitats of Litsea cubeba.
| Latitude(°) | Longitude(°) | Elevation(m) | T max(°C) | T min(°C) | T average(°C) | Precipitation(mm) | |
|---|---|---|---|---|---|---|---|
| A | 27.15 N | 118.27 E | 609 | 40.7 | −7.2 | 18.7 | 1662.9 |
| B | 25.97 N | 117.41 E | 495 | 39.8 | −6.0 | 19.3 | 1563.8 |
| C | 25.23 N | 115.30 E | 271 | 40.0 | −3.8 | 19.4 | 1461.2 |
| D | 27.39 N | 114.35 E | 260 | 39.9 | −5.5 | 17.6 | 1643.6 |
| E | 27.35 N | 105.43 E | 1608 | 36.2 | −10.9 | 12.7 | 899.5 |
| F | 26.34 N | 111.80 E | 137 | 39.7 | −7.0 | 17.8 | 1425.7 |
| G | 24.42 N | 100.74 E | 2068 | 37.3 | −1.4 | 18.6 | 1131.6 |
| H | 28.19 N | 104.51 E | 823 | 39.5 | −1.7 | 17.8 | 1063.1 |
Elevation is given as an average: Tmax = Annual extreme maximum temperature; T min = Annual extreme minimum temperature; Taverage = Annual average temperature; Precipitation = Annual precipitation. Locations of L. cubeba: A, B = Fujian Province; C, D = Jiangxi Province; E = Guizhou Province; F = Hunan Province; G = Yunnan Province; H = Sichuan Province.
Figure 1.
Essential oil yields of Litsea cubeba harvested from different areas.
Of the monoterpene constituents, most were oxygenated, including 28 of the monoterpenes in oil A (97.4% of the total oil), 26 in oil B (98.4% of the oil), 30 in oil C (96.5% of the oil), 23 in oil D (97.3% of the oil), 28 in oil E (98.0% of the oil), 23 in oil F (98.4% of the oil), 20 in oil G (94.6% of the oil), and 26 in oil H (94.4% of the oil). The predominant compounds were neral and geranial, accounting for 78.7% (G) to 87.4% (E) of the total oil of L. cubeba. Note that, neral and geranial are cis-trans isomers of citral. Neral always occurred in higher quantities than geranial. The citral content in sample G was relatively low, despite a higher relative yield of oil.
Table 2.
Essential oil compositions of Litsea cubeba harvested from different areas.
| Components | Peak area (%) | Identification b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RI a | A | B | C | D | E | F | G | H | |||
| 1 | α-Pinene | 930 | 0.2 | 0.1 | 0.3 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | GC-MS,RI |
| 2 | Camphene | 944 | 0.1 | t c | 0.2 | 0.1 | t | 0.1 | 0.1 | GC-MS,RI | |
| 3 | β-Phellandrene | 970 | t | 0.4 | t | t | t | t | 0.1 | GC-MS,RI | |
| 4 | β-Pinene | 972 | 0.3 | 0.2 | 0.3 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | GC-MS,RI |
| 5 | Methyl heptenone | 987 | 0.8 | 0.5 | 0.3 | 0.5 | 0.5 | 0.2 | 0.6 | 0.9 | GC-MS,RI |
| 6 | 2,3-Dehydro-1,8-cineole | 989 | t | 0.1 | GC-MS,RI | ||||||
| 7 | β-Myrcene | 991 | 0.7 | 0.3 | 0.8 | 0.6 | 0.3 | 0.5 | 0.5 | 0.6 | GC-MS,RI |
| 8 | 2-Methyl-6-hepten-1-ol | 995 | t | 0.1 | GC-MS,RI | ||||||
| 9 | α-Phellandrene | 1003 | 0.2 | t | GC-MS,RI | ||||||
| 10 | 3-Carene | 1008 | t | GC-MS,RI | |||||||
| 11 | α-Terpinene | 1015 | t | GC-MS,RI | |||||||
| 12 | p-Cymene | 1020 | t | GC-MS,RI | |||||||
| 13 | o-Cymene | 1022 | t | GC-MS,RI,RT | |||||||
| 14 | D-limonene | 1026 | 5.0 | 2.5 | 5.3 | 4.1 | 0.7 | 3.1 | 1.3 | 2.4 | GC-MS,RI,RT |
| 15 | Cineole | 1028 | 0.4 | 0.4 | 0.2 | 0.4 | 0.1 | 0.3 | 0.2 | 0.1 | GC-MS,RI |
| 16 | β-( E)-Ocimene | 1038 | t | 0.1 | t | GC-MS,RI | |||||
| 17 | β-( Z)-Ocimene | 1047 | 0.1 | t | 0.3 | t | GC-MS,RI | ||||
| 18 | γ-Terpinolene | 1056 | t | GC-MS,RI | |||||||
| 19 | ( Z)-β-Terpineol | 1066 | t | GC-MS,RI | |||||||
| 20 | Terpinolene | 1085 | 0.1 | t | t | t | t | GC-MS,RI | |||
| 21 | (+)-4-Carene | 1086 | t | t | GC-MS,RI | ||||||
| 22 | Linalool | 1101 | 1.5 | 1.5 | 1.2 | 1.3 | 1.3 | 1.4 | 1.4 | 1.6 | GC-MS,RI |
| 23 | ( Z)-Limonene oxide | 1131 | t | t | GC-MS,RI | ||||||
| 24 | ( E)-Limonene oxide | 1136 | t | t | 0.1 | GC-MS,RI | |||||
| 25 | Camphor | 1140 | t | GC-MS,RI | |||||||
| 26 | Isopulegol | 1143 | 1.1 | t | t | GC-MS,RI | |||||
| 27 | Citronellal | 1153 | 0.6 | 0.8 | 1.8 | 1.3 | 1.5 | 6.2 | 1.3 | GC-MS,RI | |
| 28 | Borneol | 1163 | 0.2 | 0.1 | 0.2 | GC-MS,RI | |||||
| 29 | Verbenol | 1165 | 1.4 | 1.2 | 1.4 | 1.4 | 1.3 | 1.3 | 1.5 | 1.8 | GC-MS,RI |
| 30 | Terpinen-4-ol | 1175 | 0.1 | 0.3 | 0.1 | 0.1 | 0.1 | t | 0.1 | GC-MS,RI | |
| 31 | Isopulegone | 1183 | 2.1 | 1.8 | 1.9 | 2.1 | 1.8 | 1.9 | 2.0 | 2.5 | GC-MS,RI |
| 32 | α-Terpineol | 1189 | 0.4 | 0.4 | 0.2 | 0.4 | 0.1 | 0.4 | 0.2 | 0.2 | GC-MS,RI |
| 33 | cis-Piperitol | 1199 | t | t | GC-MS,RI | ||||||
| 34 | cis-Carveol | 1205 | 0.1 | t | t | 0.1 | t | t | t | GC-MS,RI | |
| 35 | ( S)-Citronellol | 1223 | t | t | GC-MS,RI | ||||||
| 36 | Nerol | 1230 | 0.8 | 1.3 | 0.5 | 0.6 | 1.0 | 0.2 | 0.9 | 0.7 | GC-MS,RI |
| 37 | ( R)-Citronellol | 1233 | 0.1 | 0.2 | 0.3 | 0.2 | 0.3 | 0.2 | 0.8 | 0.5 | GC-MS,RI |
| 38 | Isogeraniol | 1237 | t | t | GC-MS,RI | ||||||
| 39 | Neral | 1245 | 35.7 | 36.3 | 34.7 | 35.7 | 37.4 | 37.0 | 34.2 | 35.4 | GC-MS,RI,RT |
| 40 | Piperitone | 1253 | t | t | 0.1 | t | t | t | t | t | GC-MS,RI |
| 41 | Geraniol | 1258 | 1.4 | 2.6 | 0.7 | 1.5 | 1.4 | 0.8 | 0.7 | 0.4 | GC-MS,RI |
| 42 | Geranial | 1276 | 45.9 | 48.0 | 45.9 | 47.2 | 50.0 | 49.5 | 44.4 | 46.2 | GC-MS,RI,RT |
| 43 | Geranic acid | 1359 | 0.1 | t | 0.2 | 0.1 | 0.2 | t | 0.2 | GC-MS,RI | |
| 44 | Copaene | 1371 | 0.1 | t | GC-MS,RI | ||||||
| 45 | Geranyl acetate | 1384 | t | GC-MS,RI | |||||||
| 46 | β-Elemene | 1388 | 0.1 | t | t | GC-MS,RI | |||||
| 47 | β-Caryophyllene | 1420 | 0.3 | 0.1 | 0.3 | 0.3 | 0.1 | 0.3 | 0.1 | 0.8 | GC-MS,RI |
| 48 | β-Farnesene | 1456 | 0.4 | 2.3 | 2.0 | GC-MS,RI | |||||
| 49 | α-Caryophyllene | 1462 | t | 0.1 | 0.1 | t | t | t | GC-MS,RI | ||
| 50 | Germacrene D | 1485 | t | t | GC-MS,RI | ||||||
| 51 | γ-Elemene | 1496 | t | t | GC-MS,RI | ||||||
| 52 | Eremophilene | 1502 | t | GC-MS,RI,RT | |||||||
| 53 | Germacrene A | 1503 | 0.1 | t | GC-MS,RI | ||||||
| 54 | β-Bisabolene | 1510 | t | GC-MS,RI | |||||||
| 55 | Cadinene | 1526 | t | 0.1 | GC-MS,RI | ||||||
| 56 | Caryophyllene oxide | 1586 | 0.1 | t | 0.2 | 0.1 | 0.1 | 0.1 | t | 0.3 | GC-MS,RI |
| 57 | Humulene epoxide II | 1613 | t | GC-MS,RI | |||||||
| 58 | Selina-6-en-4-ol | 1624 | t | GC-MS,RI | |||||||
| 59 | α-Cadinol | 1662 | t | GC-MS,RI | |||||||
| Total compounds 59 | 35 | 30 | 40 | 29 | 33 | 31 | 26 | 34 | |||
| Monoterpene hydrocarbons | 6.6 | 3.5 | 7.0 | 5.2 | 1.6 | 3.9 | 2.1 | 3.3 | |||
| Oxygenated monoterpenes | 90.7 | 95.0 | 89.5 | 92.2 | 96.4 | 94.5 | 92.5 | 91.1 | |||
| Sesquiterpene hydrocarbons | 0.3 | 0.1 | 0.6 | 0.5 | 0.5 | 0.4 | 2.4 | 2.9 | |||
| Oxygenated sesquiterpenes | 0.1 | t | 0.2 | 0.1 | 0.1 | 0.1 | t | 0.3 | |||
| Non-terpenes | 0.8 | 0.5 | 0.3 | 0.5 | 0.5 | 0.2 | 0.7 | 0.9 | |||
| Total identified | 98.7 | 99.1 | 97.6 | 98.3 | 99.0 | 99.2 | 97.8 | 98.5 | |||
a Retention index on a Hewlett Packard 5MS column; b GC-MS = identification based on a high-quality match of mass spectra; RI = Retention index compared with those in the literature; RT = Retention time compared with authentic compounds; c t = Trace component (<0.1%). Sample location: A, B = Fujian Province, C, D = Jiangxi Province, E = Guizhou Province, F = Hunan Province, G = Yunnan Province, H = Sichuan Province.
The chemical compounds present in all of the oils were neral, geranial, α-pinene, β-pinene, methyl heptenone, β-myrcene, D-limonene, cineole, linalool, citronellal, verbenol, isopulegone, α-terpineol, (R)-citronellol, piperitone, geraniol, β-caryophyllene and caryophyllene oxide. D-limonene prevailed in the third oil extracted from L. cubeba samples A, C, D, F, but was substituted for geraniol, isopulegone, citronellal and isopulegone in the oils of samples B, E, G, and H, respectively. Other constituents were only identified in samples obtained from certain regions. These unique compounds included o-cymene, camphor, selina-6-en-4-ol, α-cadinol in oil A, α-terpinene, γ-terpinolene and (Z)-β-terpineol in oil B, geranyl acetate and eremophilene in oil F, 3-carene and p-cymene in oil G, and β-bisabolene and humulene epoxide II in oil H. Only trace amounts of these unique compounds were shared among samples.
3. Discussion
Unexpectedly, d-limonene was present in EOLC only as a minor constituent (0.7–5.3%), which differs significantly from the findings of previous studies [20,21,22], where it was a primary component (6.0–14.6%). In addition, certain trace compounds, such as o-cymene and eremophilene had never before been found in oil obtained from L. cubeba friut. o-Cymene has, however, been found in the leaf oil of Litsea glutinosa in trace amounts of less than 0.1% [23], and eremohpilene has been reported in other Lauraceae plants [24].
As expected, both quantitative and qualitative differences in composition were observed among the essential oils, all of which contained citral as the primary ingredient. Even EOLCs from the same province exhibited significant differences in composition. Even plants gathered at the same stage of development and from within the same locality and ecological environment have shown possible differences in their essential oils [25]. EOLC composition may ultimately result from gene-environment interactions.
EOLC is natural product, consisting primarily of terpenes with some non-terpene compounds. Terpenes were thought to be products of detoxification and overflow metabolism until several were confirmed to be repellents or attractants to other organisms. This led to the belief that terpenes play an important role in antagonistic or mutualistic interactions between organisms [26]. Synthesis-related enzyme genes of some terpenes in EOLC have been partially functionally characterized [27]. Nevertheless, we still know relatively little about their actual bioactivity. Biological activity can vary depending on the composition of the essential oil [28]. Therefore, the bioactivity of EOLC may depend on the area from which it is harvested.
Note that the current study examined the chemical compositins of L. cubeba oil taken from several provinces in China. Preliminary information does exist, however, on the chemical fingerprint of L. cubeba from those provinces and will be the focal point of a future study. The powerful tools provided by modern molecular biology, chemical biology, and analytical chemistry promise to yield a much more comprehensive view of the terpenes associated with bioactivity.
4. Experimental
4.1. Plant Materials
Aerial parts of L. cubeba were randomly collected in September 2011 from the plant’s natural habitat: Jianou and Yongan of Fujian Province (A, B), Anyuan and Fenyi of Jiangxi Province (C, D), Bijie of Guizhou Province (E), Yongzhou of Hunan Province (F), Jingdong of Yunnan Province (G) and Changning of Sichuan Province (H). Only fresh, mature, and healthy fruits were chosen as samples. All samples were transported immediately to the lab and air-dried at ambient temperature prior to essential oil extraction. The fruits of L. cubeba were authenticated morphologically and microscopically by Vice-Prof. Shengni Tian, Anhui Agriculture University, China. Voucher specimens have been deposited in the Herbarium of the College of Life Science, Anhui Agriculture University, China.
4.2. Chemicals and Reagents
All chemicals and reagents were of analytical grade. Standards were supplied by Sigma-Aldrich Company (Steinheim, Germany).
4.3. Isolation of Essential Oils
The dried vegetable matter (100 g) was subjected to hydrodistillation using a Clevenger-type apparatus to extract essential oil. The Clevenger-type apparatus consisted of a 2,000-mL flask, a vertical tube, a condenser, a measuring tube with stopcock, and a return tube. The return tube connected the bottom of the measuring tube to the vertical tube, which combined with the top of the condenser. The flask was filled with 1,500 mL of distilled water and heated by an electric heating mantle. The extraction time was 5 h, after which no more essential oil was obtained. The volatile distillate was dried over anhydrous sodium sulfate and stored at 4 °C until subsequent analysis.
4.4. GC-MS Analysis
GC-MS analysis was carried out on an Agilent 6890N gas chromatograph coupled to a mass spectrometer (Agilent 5975B, Santa Clara, CA, USA) using a fused silica capillary column (HP-5MS) coated with polydimethylsiloxane (19091 S-433) (30 m × 0.25 mm internal diameter, 0.25 μm film thickness). The column temperature was set at 50 °C for 2 min, ramped at a rate of 3 °C/min to 120 °C for 2 min, then increased to 250 °C at 15 °C/min for 5 min. Helium was used as the carrier gas at a flow rate of 1 mL/min. The sample was diluted in the ether (1:10) and a volume of 1.0 μL was injected. The injector was held at 220 °C and operated in the split mode at a ratio of 1:40 for each sample. The MS operating parameters were an ionization voltage of 70 eV, ion source temperature of 230 °C, and electron multiplier energy of 1,024 V.
4.5. Qualitative and Quantitative Analysis
Compound identifications were based on comparisons their mass spectra with the mass spectra obtained from a MS database (NIST 08) and by comparisons of the RI with values reported in the literature [29,30] or against values obtained on authentic compounds. A homologous series of n-alkanes (C7–C30) were run under the same operating conditions as the essential oil to determine RIs. The relative amounts of individual components were calculated via peak area normalization.
4.6. Data Analysis
Yields of essential oil are expressed as mean values ±1 standard deviation. Graphs were drawn in SigmaPlot v10.0. Images were identically processed using Adobe Photoshop CS5 software.
5. Conclusions
EOLCs harvested from various areas of China were obtained by hydrodistillation of L. cubeba and analyzed by GC-MS. Fifty-nine components were identified. Citral was the most abundant component in all of the oils. The relative composition of EOLC varied with the region from which the sample was collected. For the first time, eremophilene and o-cymene were identified in EOLC.
Acknowledgements
This study was financially supported by the State Forestry Administration, China (2011-01) and the Science Technology Department of Zhejiang Province, China (2009c32107).
Footnotes
Sample Availability: Samples of the essential oils are available from the authors.
References and Notes
- 1.Agrawal N., Choudhary A.S., Sharma M.C., Dobhal M.P. Chemical constituents of plants from the genus Litsea. Chem. Biodivers. 2011;8:223–243. doi: 10.1002/cbdv.200900408. [DOI] [PubMed] [Google Scholar]
- 2.Li X.W., Li J., Huang P.H., Wei F.N., Cui H.B., Werff H.V.D. Lauraceae. In: Wu Z.Y., Raven P.H., Hong D.Y., editors. Flora of China. Vol. 7. Science Press; Beijing, China: Missouri Botanical Garden Press; St. Louis, MO, USA: 2008. pp. 118–141. [Google Scholar]
- 3.Choudhury S., Ahmed R., Barthel A., Leclercq P.A. Composition of the stem, flowers and fruit oils of Litsea cubeba Pers. from two locations of Assam, India. J. Essent. Oil Res. 1998;10:381–386. [Google Scholar]
- 4.Zhao O., Zhou J.W., Ban D.M. Analysis of volatile oil from different parts of Litsea cubeba (in Chinese) J. Chin. Med. Mater. 2010;33:1417–1419. [PubMed] [Google Scholar]
- 5.Luo M., Jiang L.K., Zou G.L. Acute and genetic toxicity of essential oil extracted from Litsea cubeba (Lour.) Pers. J. Food Prot. 2005;68:581–588. doi: 10.4315/0362-028x-68.3.581. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Z., Akhtar Y., Bradbury R., Zhang X., Isman M.B. Comparative toxicity of essential oils of Litsea pungens and Litsea cubeba and blends of their major constituents against the cabbage looper, Trichoplusiani. J. Agric. Food Chem. 2009;57:4833–4837. doi: 10.1021/jf900274r. [DOI] [PubMed] [Google Scholar]
- 7.Mao A.A., Wetten A., Fay M.F., Caligari P.D.S. In vitro propagation of Litsea cubeba (Lours.) Pers., a multipurpose tree. Plant Cell Rep. 2000;19:263–267. doi: 10.1007/s002999900099. [DOI] [PubMed] [Google Scholar]
- 8.Wang H.W., Liu Y.Q. Chemical composition and antibacterial activity of essential oils from different parts of Litsea cubeba. Chem. Biodivers. 2010;7:229–235. doi: 10.1002/cbdv.200800349. [DOI] [PubMed] [Google Scholar]
- 9.Luo M., Jiang L.K., Huang Y.X., Xiao M., Li B., Zou G.L. Effects of citral on Aspergillus flavus spores by quasi-elastic light scattering and multiplex microanalysis techniques. Acta Bioch. Bioph. Sin. 2004;36:277–283. doi: 10.1093/abbs/36.4.277. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y., Jiang J.Z., Luobu Q.M., Yan X.J., Zhao J.X., Yuan H.Z., Qin Z.H., Wang M.G. The fungicidal terpenoids and essential oil from Litsea cubeba in Tibet. Molecules. 2010;15:7075–7082. doi: 10.3390/molecules15107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pumnuan J., Chandrapatya A., Insung A. Acaricidal activities of plant essential oils from three plants on the mushroom mite, Luciaphorus perniciosus Rack (Acari: Pygmephoridae) Pakistan J. Zool. 2010;42:247–252. [Google Scholar]
- 12.Amer A., Mehlhorn H. Repellency effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol. Res. 2006;99:478–490. doi: 10.1007/s00436-006-0184-1. [DOI] [PubMed] [Google Scholar]
- 13.Noosidum A., Prabaripai A., Chareonviriyaphap T., Chandrapatya A. Excito-repellency properties of essential oils from Melaleuca leucadendron L., Litsea cubeba (Lour.) Persoon, and Litsea salicifolia (Nees) on Aedes aegypti (L.) mosquitoes. J. Vector Ecol. 2008;33:305–312. doi: 10.3376/1081-1710-33.2.305. [DOI] [PubMed] [Google Scholar]
- 14.Hwang J.K., Choi E.M., Lee J.H. Antioxidant activity of Litsea cubeba. Fitoterapia. 2005;76:684–686. doi: 10.1016/j.fitote.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Ho C.L., Jie-Pinge O., Liu Y.C., Hung C.P., Tsai M.C., Liao P.C., Wang E.I., Chen Y.L., Su Y.C. Compositions and in vitro anticancer activities of the leaf and fruit oils of Litsea cubeba from Taiwan. Nat. Prod. Commun. 2010;5:617–620. [PubMed] [Google Scholar]
- 16.Ma X.Y, Huang X.H, Hua S.M., Chen Y.Z. A study on essential oil of Litsea cubeba (Lour.) pers. (in Chinese) J. Instrum. Anal. 1986;5:22–26. [Google Scholar]
- 17.Zhou X., Mo B.B. The study on chemical components of volatile oil which from Litsea cubeba (Lour.) Pers. growing in Guizhou (in Chinese) J. GuiZhou Univ. 2001;18:45–47. [Google Scholar]
- 18.Zhou Y.H., Wang L.S., Liu X.M. GC-MS analysis of Litsea cubeba oil obtained in GuangXi (in Chinese) Biomass Chem. Eng. 2003;37:19–21. [Google Scholar]
- 19.Arruda M., Viana H., Rainha N.R., Rosa J.S., Nogueira J.M.F., Barreto M.C. Anti-acetylcholinesterase and antioxidant activity of essential oils from Hedychium gardnerianum Sheppard ex Ker-Gawl. Molecules. 2012;17:3082–3092. doi: 10.3390/molecules17033082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu L.S., Wang Y.D., Du M.H., Zhang J.P. Characterization of the volatiles and active components in ethanol extracts of fruits of Litsea cubeba (Lour.) by gas chromatography-mass spectrometry (GC-MS) and gas chromatography-olfactometry (GC-O) J. Med. Plants Res. 2011;5:3298–3303. [Google Scholar]
- 21.Seo S.M., Kin J., Lee S.G., Shin C.H., Shin S.C., Park I.K. Fumigant antitermitic activity of plant essential oils and components from ajowan (Trachyspermum ammi), allspice (Pimenta dioica), caraway (Carum carvi), dill (Anethum graveolens), geranium (Pelargonium graveolens), and litsea (Litsea cubeba) oils against Japanese termite (Reticulitermes speratus Kolbe) J. Agric. Food Chem. 2009;57:6596–6602. doi: 10.1021/jf9015416. [DOI] [PubMed] [Google Scholar]
- 22.Xiang Z.B., Chen H.S., Xia C.Y., Huang M.Z. Chemical composition and antibacterial activity research of essential oil from Litsea cubeba (in Chinese) Chin. Tradit. Pat. Med. 2008;30:1514–1516. [Google Scholar]
- 23.Nguyen T.H., Tran D.H., Do N.D., Tran H.T. Chemical composition of the leaf oil of Litsea glutinosa (Lour.) C. B. Rob. from Ha Tinh province. 2010;26:161–164. [Google Scholar]
- 24.Mediouni Ben Jemâa J., Tersim N., Toudert K.T., Khouja M.L. Insecticidal activities of essential oils from leaves of Laurus nobilis L. from Tunisia, Algeria and Morocco, and comparative chemical composition. J. Stored Prod. Res. 2012;48:97–104. [Google Scholar]
- 25.Conforti F., Menichini F., Formisano C., Rigano D., Senatore F., Arnold N.A., Piozzi F. Comparative chemical composition, free radical-scavenging and cytotoxic properties of essential oils of six Stachys species from different regions of the Mediterranean Area. Food Chem. 2009;116:898–905. doi: 10.1016/j.foodchem.2009.03.044. [DOI] [Google Scholar]
- 26.Langenheim J.H. Higher plant terpenoids: A phytocentric overview of their ecological roles. J. Chem. Ecol. 1994;20:1223–1280. doi: 10.1007/BF02059809. [DOI] [PubMed] [Google Scholar]
- 27.Chang Y.T., Chu F.H. Molecular cloning and characterization of monoterpene synthases from Litsea cubeba (Lour.) Persoon. Tree Genet. Genomes. 2011;7:835–844. [Google Scholar]
- 28.Leung A.Y., Foster S. Encyclopedia of Common Natural Ingredients Used in Foods, Drugs, and Cosmetics. 2nd. John Wiley & Sons Press; New York, NY, USA: 1996. p. 456. [Google Scholar]
- 29.Park I.K., Kim J., Lee S.G., Shin S.C. Nematicidal activity of plant essential oils and components from ajowan (Trachyspermum ammi), allspice (Pimenta dioica) and litsea (Litsea cubeba) essential oils against pine wood nematode (bursaphelenchus xylophius) J. Nematol. 2007;39:275–279. [PMC free article] [PubMed] [Google Scholar]
- 30.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. Allured Publications; Carol Stream, IL, USA: 1995. [Google Scholar]