Summary
The nature of fluid dynamics within the brain parenchyma is a focus of intensive research. Of particular relevance is its participation in diseases associated with protein accumulation and aggregation in the brain, such as Alzheimer’s disease (AD). The meningeal lymphatic vessels of the central nervous system have recently been recognized as an important player in the complex circulation and exchange of soluble contents between the cerebrospinal fluid (CSF) and the interstitial fluid (ISF). In aging mammals, for example, impaired functioning of the meningeal lymphatic vessels can lead to accelerated accumulation of toxic amyloid beta protein in the brain parenchyma, thus aggravating AD-related pathology. Given that meningeal lymphatic vessels are functionally linked to paravascular influx/efflux of the CSF/ISF, and in light of recent findings that certain cytokines, classically perceived as immune molecules, exert neuromodulatory effects, it is reasonable to suggest that the activity of meningeal lymphatics could alter the accessibility of CSF-borne immune neuromodulators to the brain parenchyma, thereby altering their effects on the brain. Accordingly, in this Perspective we discuss the suggestion that the meningeal lymphatic system can be viewed as a novel player in neurophysiology.
In Brief paragraph
In this article, Da Mesquita et al. describe important features of the meningeal lymphatic system, its interaction with other pathways of macromolecule clearance in the brain and its role in the regulation of CNS immune response, cytokine signaling, neural cell function and behavior.
Introduction
Cerebrospinal fluid (CSF) recirculation within the central nervous system (CNS) happens through numerous different pathways. Recent revelations about a previously unappreciated meningeal lymphatic system of the CNS (Aspelund et al., 2015; Louveau et al., 2015b) have prompted a fresh look at some basic ideas about fluid, molecular, and cellular exchanges between different brain compartments, as well as a reassessment of the importance of these CNS-draining lymphatics for CNS homeostasis (Antila et al., 2017; Da Mesquita et al., 2018; Louveau et al., 2017; Ma et al., 2017). Accordingly, after a brief historical summary, we begin by introducing several new concepts and findings concerning the development and function of the meningeal lymphatic system, and its role in CSF drainage and as a modulator of paravascular mechanisms for macromolecular exchange (through the glymphatic route). We then discuss the relevance of the meningeal lymphatic system for aging-associated brain dysfunction, amyloid clearance in Alzheimer’s disease (AD) and cytokine signaling in the brain.
History of the discovery and rediscovery of meningeal lymphatic vessels
The existence of lymphatic vessels in the brain meninges was first mentioned towards the end of the 18th century by Paolo Mascagni, an Italian physician known for his unparalleled anatomical knowledge (Bucchieri et al., 2015). Despite the exceptional anatomical precision of Mascagni’s wax models of human body parts and organs (on display at the Josephinum Medical Museum in Vienna), his claim that lymphatic vessels are present in the brain meninges was discredited and evidently forgotten (Lukic et al., 2003). Almost two centuries later, another Italian scientist reported Mascagni’s ‘discovery’ of lymphatic vessels after inspecting samples of human dura (Lecco, 1953), and, in the 1960s, Csanda and colleagues (Foldi et al., 1966) described the existence of a lymphatic connection between the CNS and the periphery that was actually involved in drainage of CNS molecules. Those works were also met with skepticism by their contemporaries. At the end of the last century, Jicheng Li and colleagues, using the more robust scientific technique of scanning electron microscopy, claimed discovery of meningeal lymphatic vessels, which they named cerebral meningeal stomata, and suggested to be part of the cerebral prelymphatic capillary system (Li et al., 1996). However, given the available methodology, they could not be certain whether the round to oval stomata, which were localized between the mesothelial cells of the cerebral meninges (Li et al., 1996), were in fact lymphatic vessels.
It thus took more than two hundred years, and the eventual application of state-of-the-art techniques, for Mascagni’s initial observations to be confirmed by a sufficiently detailed structural and functional characterization of meningeal lymphatic vessels (Louveau et al., 2015b). This odyssey serves as a good example of the rejection of new paradigms by the contemporary community simply because they do not fit the prevailing dogma―which, in the present case, was based on the universally held conception of the CNS as an ‘immuneprivileged’ organ having no direct communication or interaction with the immune system, at least under healthy conditions. Parenthetically, this begs an intriguing question: how many other rejected ‘discoveries’ are awaiting rebirth?
The bona fide lymphatic vessels existing in the meninges of the CNS express the classical markers of lymphatic endothelial cells (LECs), namely vascular endothelial growth factor receptor 3 (VEGFR3), prospero homeobox protein 1 (Prox1), podoplanin, lymphatic vessel endothelial hyaluronan receptor 1, C-C motif chemokine ligand 21 and CD31, and can efficiently drain both molecules and immune cells from the subarachnoid space into the CLNs (Aspelund et al., 2015; Louveau et al., 2015b). Ever since their characterization in mice, publications on the subject have reported that meningeal lymphatic vessels are evolutionarily conserved and are found also in fish, rats, non-human primates and humans (Absinta et al., 2017; Bower et al., 2017; Jung et al., 2017). In the developing embryo, peripheral lymphatic vessels sprout from venous vasculature, by differentiation of venous endothelial cells into LECs, through Prox1- and VEGFR3-dependent mechanisms (Alitalo, 2011; Alitalo et al., 2005). On the contrary, in rodents, meningeal lymphatics develop and mature after birth and respond to vascular endothelial growth factor C (VEGF-C), but not to VEGF-D (Antila et al., 2017). However, as seen in peripheral lymphatics (Joukov et al., 1996; Karkkainen et al., 2004), blocking of the interaction of VEGF-C with one of its receptors (particularly VEGFR3) impairs the development of brain meningeal lymphatic vessels (Antila et al., 2017). In peripheral tissues, cells and molecules are first guided into smaller caliber initial lymphatic vessel capillaries (initial lymphatics) and then into precollector and larger collector lymphatic vessels, which are equipped with valves (specialized structures expressing integrin-α9) that prevent backflow of lymph constituents. Initial lymphatics are permeable to cells and debris/molecules due to the existence of flap-like openings between the single layer of LECs, which is made possible due to discontinuous button-like junctions between LECs (Alitalo, 2011; Alitalo et al., 2005; Baluk et al., 2007). When compared to peripheral lymphatics, meningeal lymphatics are composed of a less ramified network of thin-walled initial lymphatic vessels (without integrin-α9-expressing valves) that converge and exit the cranium along particular anatomical structures, namely along the retroglenoid vein and sigmoid sinus and along the meningeal portions of the pterygopalatine artery (Aspelund et al., 2015; Louveau et al., 2015b). In adult mice, diffusible solutes (in this case Evans blue) injected into the CSF that fills the cisterna magna were shown to drain first into the deep cervical lymph nodes (dCLNs) and later into superficial cervical lymph nodes (sCLNs) (Louveau et al., 2015b). Molecular tracers injected into the mouse brain parenchyma were detected, after one hour, in the draining dCLNs but not in the sCLNs (Aspelund et al., 2015), suggesting that ISF and CSF molecules in mice are initially drained into dCLNs and only later into sCLNs. Evans blue injected into the nasal mucosa, however, could not be detected in the dCLNs 30 minutes later (Louveau et al., 2015b), pointing to slower drainage by the nasal lymphatics and/or a possible alternative drainage route. Still lacking, however, is a comprehensive characterization of the cellular players in meningeal lymphatic system formation and maintenance, and of the lymphatic network that connects the CNS-draining initial lymphatics to the CLNs. We also need to broaden our knowledge of meningeal lymphatic network complexity in rodents, non-human primates and humans (Absinta et al., 2017). It will be interesting to evaluate whether this network of lymphatics is more complex in organisms that have more convoluted brains, higher cortical neuronal density and that display multifaceted cognitive behaviors (Azevedo et al., 2009; Mota and Herculano-Houzel, 2015).
CSF homeostasis
The main components of the CSF, which fills the brain ventricles and the subarachnoid spaces, are produced by the choroid plexus (Johanson et al., 2008). Besides modulating CSF composition this highly vascularized epithelium, which is present in all four brain ventricles, constitutes the blood−CSF barrier, one of the interface barriers of the CNS (Ghersi-Egea et al., 2018; Johanson et al., 2008). Epithelial cells of the choroid plexus secrete some of the main CSF proteins, such as transthyretin (Schreiber et al., 1990), and express different ionic and water transporters, such as aquaporin-1, both at the basolateral membranes (on the blood side) and at the apical membranes (on the CSF side), thus tightly regulating CSF ionic and hydrostatic properties (Ghersi-Egea et al., 2018; Johanson et al., 2008; Oshio et al., 2005; Saunders et al., 2013). Owing to size-restricted filtration at the brain’s barriers, the protein contents of the CSF and the ISF are lower than those of plasma; nevertheless, some blood-borne ions and molecules (such as the hormone leptin) are able to reach both the CSF and the ISF via selective transport mechanisms present in epithelial cells of the choroid plexus and in endothelial cells of the bloodbrain barrier (BBB) (Ghersi-Egea et al., 2018; Hammock and Milhorat, 1976; Zhao et al., 2015a; Zlokovic et al., 2000). The composition of the CSF can also be modulated by brain parenchymal cells, which secrete molecules that can be transported from the ISF into the CSF sink through a paravascular route (Cserr and Ostrach, 1974; Iliff et al., 2012), also termed the glymphatic route (discussed below).
The total CSF volume is renewed about 11 times a day in young-adult rats and about 4 times a day in healthy humans (Preston, 2001; Silverberg et al., 2001). CSF flow within the ventricles is pulsatile, being influenced by respiratory and cardiac pulsation (Ridgway et al., 1987; Takizawa et al., 2017), and directionality of the ventricular CSF flow is influenced by the beating of cilia from ependymal cells that ensheathe the ventricular wall (Faubel et al., 2016). According to the traditional view of CSF homeostasis, the molecular contents of the CSF are cleared via three routes: via arachnoid granulations into the meningeal sinus (based on evidence from ex-vivo experiments using dogs and non-human primates (Pollay and Welch, 1962; Welch and Pollay, 1961)); via lymphatics of the nasal mucosa into the CLNs (after crossing the cribriform plate and following a poorly characterized route along the olfactory nerves (Bradbury and Westrop, 1983; Johnston et al., 2004; Kida et al., 1993)); and via transporters and receptors present on the apical side of the choroid plexus epithelium (Ghersi-Egea et al., 2018; Johanson et al., 2008; Zlokovic et al., 2000; Zlokovic et al., 1996). This traditional concept of CSF homeostasis has now been challenged, however, by recently acquired knowledge about meningeal lymphatic vessels; specifically, that they have access to the CSF and, under healthy conditions, continuously drain its molecular and cellular contents (Figure 1) into the CLNs (Aspelund et al., 2015; Louveau et al., 2015b, 2018). Thus, contrary to the proposed direct system for venous CSF outflow through arachnoid granulations (Pollay and Welch, 1962; Welch and Pollay, 1961)], recent studies (using in-vivo imaging techniques) show that molecular tracers present in the CSF are largely drained by the lymphatic vessels of the meninges (Absinta et al., 2017; Aspelund et al., 2015; Louveau et al., 2015b; Ma et al., 2017), highlighting the importance of this draining lymphatic route for the clearance of molecules from the brain. However, to further reinforce the observations obtained using the mouse model, it would be interesting to use larger mammals such as dogs or sheep (where the meningeal arachnoid granulations are actually detectable), to measure the differential contribution of the meningeal lymphatic vessels and of the arachnoid granulations-venous sinus route to CSF outflow from the CNS.
Figure 1. Cytoarchitecture of the meninges, brain vasculature and pathways of paravascular recirculation.
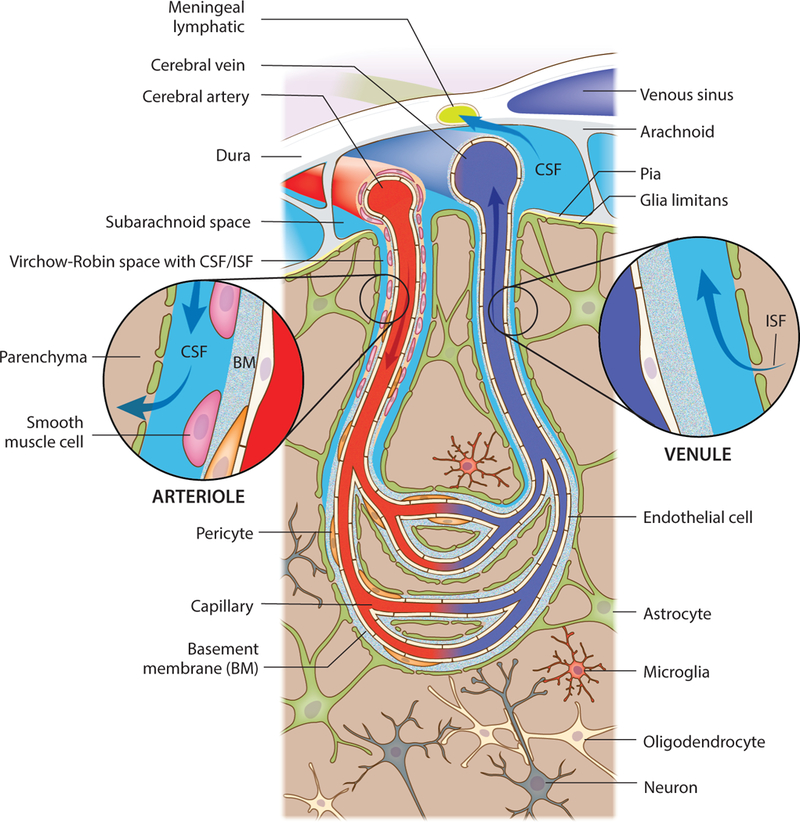
A schematic representation of the brain meninges constituted by dura, arachnoid and pia layers. Lymphatic vessels that are present in the meningeal dura drain components of the cerebrospinal fluid (CSF) that fills the subarachnoid space. Arising from the brain surface, cerebral arteries extend into pial and then subpial arteries. Higher caliber pial arteries extend into smaller caliber arterioles (both wrapped by smooth muscle cells) that dive into the brain parenchyma. Clearly defined paravascular spaces of about 50–100 nm, the Virchow-Robin spaces, are filled with CSF that flows into deeper brain regions, along the arterioles and capillaries, and diffuses through the glia limitans into the parenchyma. Efflux of interstitial fluid (ISF) happens through paravenous spaces back into the subarachnoid CSF.
Changes in CSF composition and renewal are involved in several key aspects of CNS physiology, including intracranial pressure equilibrium (through counterbalance of CSF formation and drainage (Botfield et al., 2017; Oshio et al., 2005)), removal of waste metabolites from brain-cell activity, such as amyloid beta (Aβ), which ends up in the CSF sink (Johanson et al., 2008; Palmert et al., 1989; Tarasoff-Conway et al., 2016; Xie et al., 2013), and neuroinflammation (Baruch et al., 2014; Marques et al., 2009). Changes in CSF composition also affect neural cell development and function (Ghersi-Egea et al., 2018; Saunders et al., 2013), for example by modulating the proliferation of neuronal progenitors during brain development (Lehtinen et al., 2011), or neurogenesis in the adult brain (Silva-Vargas et al., 2016). Decrease in CSF production and clearance is considered to be a serious aggravating factor in different models of neuropathology such as hydrocephalus and ischemia (Botfield et al., 2017; Ennis and Keep, 2006), and contributes to the decay of brain function in aging and in aging-associated neurodegenerative diseases such as AD (Johanson et al., 2008; May et al., 1990; Roh et al., 2012; Silverberg et al., 2001; Skillback et al., 2017; Tarasoff-Conway et al., 2016). In patients with late-onset AD, faulty clearance of Aβ from the ISF/CSF (rather than its increased production) is closely related to its accumulation in the brain (Mawuenyega et al., 2010; Tarasoff-Conway et al., 2016). The protein content of the CSF increases with aging (Kleine et al., 1993a; Kleine et al., 1993b), and was shown to be even higher in patients with aging-associated dementia (Skillback et al., 2017). Both in rodents and in humans the CSF turnover rate declines with age; the total CSF volume is twice as high in the elderly as in young adults, mostly because of agerelated atrophy of the brain parenchyma (Preston, 2001).
It is well accepted that alterations in CSF homeostasis, either acute or chronic, are closely associated with changes in brain function. Below, we discuss the importance of the paravascular glymphatic route, as well as the contribution of meningeal lymphatic drainage to CSF/ISF recirculation and to the maintenance of CNS fluid composition. Finally, we address the impact of aging-associated meningeal lymphatic dysfunction on the manifestation of behavioral deficits, as well as on the development of neurodegenerative disorders, with particular focus on AD.
Glymphatic route and neurophysiology
The CNS parenchyma presents a complex network of blood vessels, which provide oxygen and nutrients to neural cells (Zhao et al., 2015a), but is devoid of lymphatic vessels (Louveau et al., 2016). In contrast, all peripheral organs possess lymphatic vasculatures that are critical for the maintenance of tissue homeostasis (Alitalo, 2011). In the CNS parenchyma, drainage of cellular debris and waste products from cell metabolism, which in the periphery is usually attributed to the conventional lymphatic vasculature, is in part performed by a paravascular route through which interchange between the CSF and the ISF takes place (Iliff et al., 2013a; Iliff et al., 2012; Kress et al., 2014; Rennels et al., 1985; Xie et al., 2013). Classical studies performed in the 1970s showed that molecular tracers injected into the brain parenchyma circulate within the interstitium and end up in paravascular spaces (Cserr et al., 1977; Cserr and Ostrach, 1974). On the other hand, a study performed in the 1980s showed that molecules injected into the subarachnoid CSF could follow a paravascular route and enter the brain in under 10 minutes, providing one of the first indications of a continuous interchange between the CSF and the ISF (Rennels et al., 1985). It was not until recently, however, that more light could be shed on this route of communication between the subarachnoid CSF and the parenchymal ISF, by the use of fluorescent molecular tracers and in vivo two-photon laser scanning microscopy (Iliff et al., 2012).
To understand how the interchange between the CSF and ISF takes place, we first need to examine the cytoarchitecture of the brain vasculature (Figure 1). Cerebral arteries at the cortical surface extend into pial and then subpial arteries, all of which are wrapped by smooth muscle cells and are in direct contact with the subarachnoid CSF (Zhang et al., 1990; Zhao et al., 2015a). Higher-caliber pial arteries then branch into lower-caliber arterioles that penetrate the brain parenchyma (Zhang et al., 1990), forming clearly defined CSF-filled Virchow-Robin spaces (of about 50−100 nm) between the arterial basement membrane and the glia limitans composed by the astrocytic endfeet (Haj-Yasein et al., 2011). The Virchow-Robin (or paravascular) spaces exist along the arterioles and capillaries that dive into deeper brain regions (Haj-Yasein et al., 2011; Zhang et al., 1990; Zhao et al., 2015a). The arterial capillaries then converge into enlarged venules, which are still ensheathed by the glia limitans and also present a paravascular CSF/ISFfilled space (Zhao et al., 2015a). The venules converge into larger subcortical and cortical veins, which in turn converge into sinus networks (such as the meningeal superior sagittal sinus) that exit the CNS to drain into the jugular veins (Louveau et al., 2017; Zhao et al., 2015a). Pericytes, essential for maintenance of the BBB integrity and function (Armulik et al., 2010; Bell et al., 2010; Daneman et al., 2010), are found in close contact both with the basal lamina extracellular matrix (ECM) of arteries and capillaries and with the CSF/ISF that fills the paravascular space (Armulik et al., 2011; Zhao et al., 2015a). Importantly, the basal lamina ECM of brain capillaries and venules is composed mainly of laminin, fibronectin, type IV collagen and heparan sulfate proteoglycans (Baeten and Akassoglou, 2011), and therefore provides only minimal resistance to the paravascular fluid exchange and recirculation.
This paravascular route was proposed as a mechanism for small-molecule exchange between the CSF and the ISF (Iliff et al., 2013a; Iliff et al., 2012). Briefly, subarachnoid CSF flows along the Virchow-Robin spaces deep into the brain (paravascular influx), leaves the periarterial space to interchange with ISF within the parenchyma, and then exits along perivenous spaces (paravascular efflux) back into the subarachnoid space. Molecules of 100 kDa or less (such as horseradish peroxidase or ovalbumin, both ~45 kDa) encounter no major resistance to entering or leaving the brain parenchyma through the endfeet clefts of the glia limitans (Iliff et al., 2012; Rennels et al., 1985). Also in disagreement with this paravascular pathway theory (Iliff et al., 2013a; Iliff et al., 2012), it was initially hypothesized that interstitial molecules such as Aβ would leave the brain exclusively through the periarterial spaces, which in the case of Aβ peptides would lead to its aggregation and accumulation around the walls of brain arteries (Carare et al., 2008; Hawkes et al., 2011). However, it is still debatable whether blood vessel capillaries (which account for the vast majority of the blood vessel length in the brain (Sweeney et al., 2018)) have a perivascular space filled with CSF/ISF or if this space is at some point fully replaced by basal lamina ECM content. Additional studies (using ultrastructural imaging techniques) should be performed to fully address this aspect of brain vascular cytoarchitecture. Ultimately, an improved knowledge of the extent of perivascular spaces in the brain would allow us to better understand the phenomenon of macromolecule recirculation through the glymphatic pathway. Although some controversy remains about the mechanisms that modulate the paravascular CSF/ISF recirculation (Engelhardt et al., 2017; Smith et al., 2017), one of the features of this route is the crucial role of aquaporin 4 (AQP4), which is expressed in the astrocytic endfeet that ensheathe the brain’s blood vasculature (Iliff et al., 2012; Mestre et al., 2017). Furthermore, in addition to its contribution to paravascular fluid and macromolecule exchange, astrocytic AQP4 was shown to be important for the preservation of glia limitans integrity at the BBB (Haj-Yasein et al., 2011). Cerebral arterial pulsation was proposed as another essential mechanism for promoting the paravascular flow of CSF into the brain (Iliff et al., 2013b), although recent studies have claimed otherwise (Asgari et al., 2016; Smith et al., 2017). Interestingly, it was shown that paravascular solute influx/efflux mechanisms become more efficient under anesthesia or sleep-state (Xie et al., 2013), contributing to increased brain ISF metabolite (like lactate and Aβ) clearance (Iliff et al., 2012; Lundgaard et al., 2017; Roh et al., 2012).
Decreased brain-wide paravascular solute influx/efflux, with subsequently impaired clearance of ISF waste, has been associated with poor outcome in different models of brain disease and pathology. Decreased paravascular fluid influx/efflux was observed in rodent models of traumatic brain injury (a risk factor for early-onset dementia (Iliff et al., 2014)), of cortical spreading depression (an animal model of migraine aura (Schain et al., 2017)), and of multiple microinfarcts (closely associated with the development of vascular dementia (Venkat et al., 2017; Wang et al., 2017)). In the traumatic brain injury model, decreased paravascular efflux of tau protein results in augmented pathology (Iliff et al., 2014), which might have repercussions for frontotemporal dementia and AD, two diseases with raised levels of intraneuronal neurofibrillary tangles and extracellular tau in the brain interstitium (Shi et al., 2017; Yamada et al., 2011; Yanamandra et al., 2017). Importantly, aged mice demonstrate a marked decrease in glymphatic function (Da Mesquita et al., 2018; Kress et al., 2014), which might represent an aggravating factor for inherent or existing pathology and contribute to aging-related brain dysfunction and cognitive deficits. In AD, toxic Aβ peptides are present in the extracellular ISF (Cirrito et al., 2003; Roh et al., 2012) and can be excreted into the blood at the BBB by receptormediated transcytosis (by low-density lipoprotein receptor-related protein 1 (Deane et al., 2008; Deane et al., 2004; Shibata et al., 2000) in a process mediated by phosphatidylinositol binding clathrin assembly protein or PICALM (Zhao et al., 2015b)), be internalized and degraded by brain phagocytes (Keren-Shaul et al., 2017; Mildner et al., 2011; Wang et al., 2016), or be transported back into the CSF through the glymphatic route (Iliff et al., 2012) (Figure 2). Excretion of Aβ from the ISF into the CSF is dependent on the fitness of the paravascular route and is markedly decreased when the ISF efflux is dampened by AQP4 deficiency (Iliff et al., 2012). Consistently, brain amyloid pathology is significantly worsened in AD transgenic mice that are crossed with mice on an Aqp4-null background (Xu et al., 2015). Moreover, decreased paravascular CSF influx/ISF efflux, possibly due to early Aβ accumulation in both perivenous and periarterial spaces (Dorr et al., 2012; Michaud et al., 2013), precedes amyloid plaque deposition in the brain parenchyma of AD transgenic mice (Peng et al., 2016).
Figure 2. Mechanisms of amyloid beta clearance from the brain.
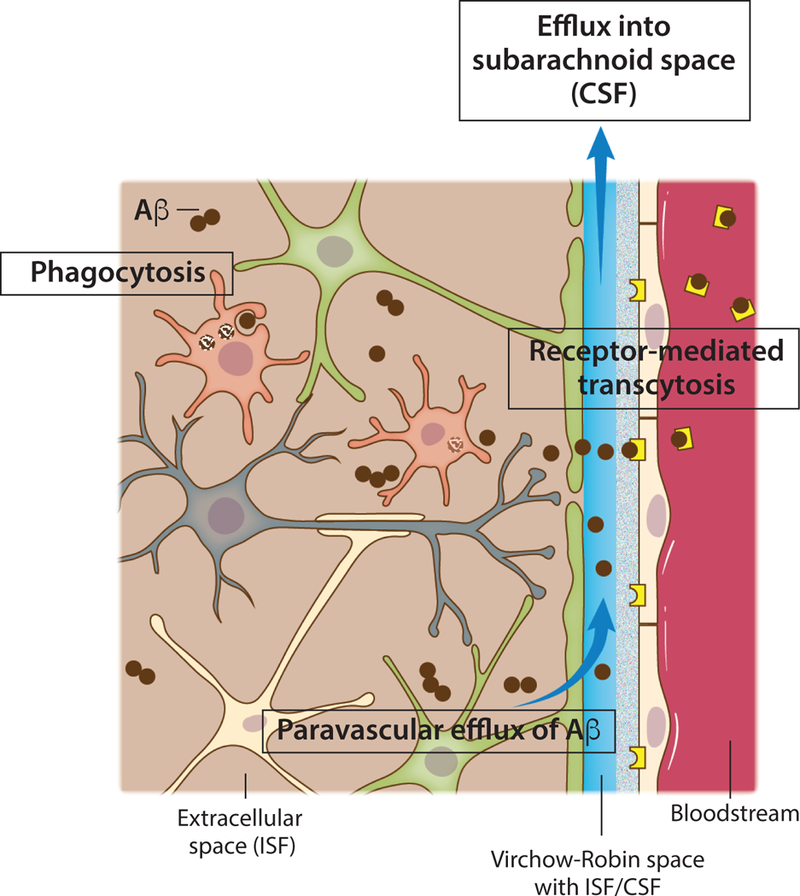
Toxic amyloid beta (Aβ) peptides are present in the extracellular brain ISF. One of the most important mechanisms of Aβ clearance from the brain extracellular environment is receptormediated transcytosis across the blood-brain barrier. Monomeric or aggregated Aβ can also be internalized and degraded by brain phagocytes, resident microglia or monocyte-derived macrophages that might be recruited and engraft the brain. Efflux of soluble Aβ from the brain ISF back into the subarachnoid CSF sink also takes place through paravascular spaces (glymphatic route).
Despite substantial research efforts, the cellular and molecular culprits for the age-related weakening of glymphatic function remain poorly understood. Furthermore, longitudinal and mechanistic experiments will be needed in order to address, in greater detail, the implications of decreased brain paravascular fluid circulation for specific aspects of brain physiology, both under healthy conditions and in models of aging and AD.
The meningeal lymphatics−glymphatics connection in aging
Based on current knowledge about the exchange of solutes between CNS compartments, it can reasonably be hypothesized that solutes present in the CSF may either travel via the paravascular route to reach the brain parenchyma and be drained by meningeal lymphatics into the periphery. However, until recently, it was unknown whether these two systems, the meningeal lymphatics and the paravascular flow (glymphatics), worked in tandem to regulate CSF/ISF homeostasis. Recent work from our lab demonstrated that the fitness of glymphatics (paravascular CSF influx and ISF efflux of macromolecules) is modulated by meningeal lymphatic function (Da Mesquita et al., 2018), suggesting a direct linkage between the two systems via brain fluids without any obvious anatomical connection (Figure 3). Using pharmacological, surgical, and genetic models (Harvey et al., 2005; Louveau et al., 2015b, 2018; Tammela et al., 2011), we showed that decreased drainage by meningeal lymphatic vessels results in impaired influx of CSF molecules into the brain (Da Mesquita et al., 2018). Notably, one month after pharmacological ablation of brain meningeal lymphatics in young-adult mice, learning and memory deficits were elicited without any detectable side effects on blood vasculature (Da Mesquita et al., 2018).
Figure 3. Aging diminishes meningeal lymphatic drainage and paravascular recirculation of CSF.
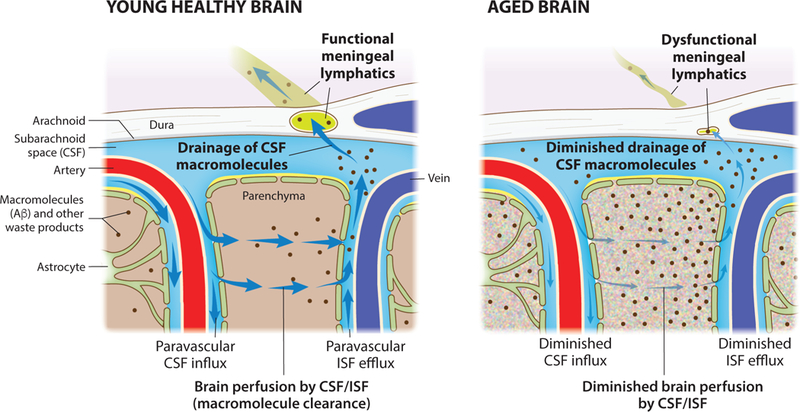
macromolecules Functional meningeal lymphatic vessels drain macromolecules (such as Aβ) from the CSF of the young and healthy brain. Influx of CSF through the paravascular (glymphatic) route leads to brain perfusion by CSF/ISF and paravascular efflux of macromolecules from the parenchymal ISF back into the CSF. Dysfunction of meningeal lymphatic vessels with aging contributes to impairment of influx/efflux mechanisms and to poor recirculation of CSF content.
Aging is known to negatively affect the function of lymphatic vessels in peripheral organs (Chevalier et al., 1996; Hos et al., 2008; Nagai et al., 2011). Likewise, in the CNS, aging was shown to be associated with impaired functioning of meningeal lymphatic vessels (Da Mesquita et al., 2018; Ma et al., 2017) (Figure 3). Sequencing of meningeal LECs from young-adult and aged mice revealed significant differences between the two groups in gene expression, pointing to impaired immune-related function, cytoarchitecture and morphology (along with differences in ECM) and response to growth factors in the older mice (Da Mesquita et al., 2018). Changes in meningeal LECs transcriptome in old mice were further supported by substantial alterations in the morphology and complexity of lymphatic vessels and their decreased capacity for drainage of CSF solutes into dCLNs (Da Mesquita et al., 2018). In a concomitant recent publication it was demonstrated that CSF solute outflow into the sCLNs is decreased in old mice (Ma et al., 2017). Notably, using different strategies for delivery of VEGF-C, a growth factor that can act on both peripheral and CNSdraining lymphatics (Antila et al., 2017; Karkkainen et al., 2004; Saaristo et al., 2002), into the meningeal milieu of old mice, we were able to enhance both lymphatic drainage into the dCLNs and brain paravascular CSF influx; moreover, old mice treated with VEGF-C showed improved performance in behavioral tests that assess learning and memory, namely the novel location recognition test and Morris water maze (Da Mesquita et al., 2018). The existence of a meningeal lymphatic-glymphatic connection with implications to brain function in aging raised other interesting questions that should be addressed in future studies. For example, despite the connection between the two systems, it is still unclear whether aging’s terrible toll acts first on meningeal lymphatic function, on glymphatics (or even on the components of the neurovascular unit that is indissociably linked to the glymphatic route), or if both systems become independently impaired. Alongside a more detailed longitudinal characterization of changes in meningeal lymphatic and glymphatic function with aging, it would be essential to explore possible connections with age-dependent alterations in CSF/ISF composition, molecular exchange mechanisms at the BBB (and with decreased barrier integrity with aging), and ultimately with the function of neural cells, particularly of microglia (Figure 2).
Meningeal lymphatics and Alzheimer’s disease
The major risk factor for late-onset AD is age (Brookmeyer et al., 2018; Erkkinen et al., 2018). Aging is accompanied by a progressive deterioration of brain vascular function, which in AD is further aggravated owing to cerebral amyloid angiopathy (Dorr et al., 2012; Iadecola, 2013; Montagne et al., 2015; Zhao et al., 2015a). Several of the genetic risk factors for late-onset AD, such as the apolipoprotein E4 (ApoE4) gene variant (Corder et al., 1993), or presence of single nucleotide polymorphisms in the genes that encode the proteins clusterin and PICALM (Harold et al., 2009), are associated with BBB dysfunction and impaired transvascular clearance of Aβ (Deane et al., 2008; Halliday et al., 2016; Zhao et al., 2015b; Zlokovic et al., 1996). Clearance mechanisms at the BBB are responsible for ~75% of Aβ excretion from the brain (Deane et al., 2004; Tarasoff-Conway et al., 2016). However, recognition of the glymphatic system as an alternative route of Aβ recirculation from brain ISF into the CSF sink (Iliff et al., 2013a; Iliff et al., 2012; Louveau et al., 2017) and detection of Aβ in the CLNs of AD transgenic mice (Pappolla et al., 2014) led to the hypothesis that impaired meningeal lymphatic drainage of CSF would affect Aβ clearance and hence exacerbate the brain’s amyloid burden in AD (Louveau et al., 2016). Besides describing the close relationship between meningeal lymphatic drainage and glymphatic function as well as the decline in meningeal lymphatic function with age (Da Mesquita et al., 2018), we have now also shown that ablation of meningeal lymphatic drainage in young-adult AD transgenic mice (both J20 and 5xFAD models) leads to more severe brain amyloid pathology. Surprisingly, we also found significant deposition of Aβ in the meninges of AD transgenic mice with ablated meningeal lymphatics, a feature not observed in their counterparts with intact meningeal lymphatics (Da Mesquita et al., 2018). Both the meningeal amyloid deposition and the recruitment of local macrophages around Aβ deposits that we observed in AD transgenic mice after lymphatic ablation were similar to what was observed in human dura from AD patients (Da Mesquita et al., 2018).
Taken together, our most recent observations suggest that the decreased meningeal lymphatic function in aging might exacerbate brain and meningeal amyloid pathology (Figure 3) and eventually precipitate the appearance of cognitive deficits in AD. However, there is still much to learn about the consequences of this age-related decrease in meningeal lymphatic drainage. Regulation of meningeal immunity and of inflammatory cytokine levels in the CSF by meningeal lymphatic function (Kipnis, 2016), together with their ability to modulate brain paravascular CSF influx/ISF efflux mechanisms, might represent a novel and unexplored mechanism to be manipulated for the benefit of the brain. This novel potential role for the meningeal lymphatics is discussed below.
Meningeal lymphatics−glymphatics and neuromodulation by cytokines
Neurons, by communicating with each other through electrical and chemical synapses, serve as the primary functional building units of the CNS (Eccles, 1982; Pereda, 2014), with glia playing vital support roles in neuronal function. Chemical transmission occurs when one or more types of neurotransmitters, in response to an action potential, are released from presynaptic buttons and bind with postsynaptic receptors (ionotropic or metabotropic), thereby inducing downstream changes in resting potential and/or activation of intracellular signaling transducing pathways that can influence gene expression (Greengard, 2001; Miesenbock et al., 1998; Pereda, 2014). Depending on their different postsynaptic downstream effects, neurotransmitters can be divided into two main categories, fast-acting and slow-acting (Hnasko and Edwards, 2012; Kaeser and Regehr, 2014). Foremost among the fast-acting neurotransmitters are glutamate and gammaaminobutyric acid (GABA). Upon their release at the synaptic cleft, they bind with postsynaptic ionotropic receptors to induce, within milliseconds, excitatory and inhibitory responses, respectively (Fonnum, 1984; Hnasko and Edwards, 2012; Klingauf et al., 1998). Examples of slow-acting neurotransmitters/neuromodulators are monoamines (such as dopamine, serotonin, norepinephrine and histamine), acetylcholine (ACh), purines (such as ATP) and gasotransmitters (such as nitric oxide and carbon monoxide) (Daubert and Condron, 2010; Graybiel, 1990; Picciotto et al., 2012). The term ‘slow-acting’ neuromodulator refers to their ability to modulate the neuronal response to fast-acting neurotransmitters upon being released separately or together with them (Caulfield and Birdsall, 1998; Hnasko and Edwards, 2012; Kebabian and Calne, 1979; Peroutka et al., 1981; Tritsch et al., 2016). After binding to metabotropic postsynaptic receptors, the slowacting neuromodulators do not directly induce neuronal membrane depolarization, but rather influence postsynaptic neuronal function by activating intracellular second-messenger signaling pathways (Beaulieu and Gainetdinov, 2011; Martinowich et al., 2007; Yogev and Shen, 2014).
In the peripheral nervous system, neurotransmitters and neuromodulators can modulate the immune response and inflammatory disease outcome at the level of peripheral organs, such as liver, lung or intestine (Ben-Shaanan et al., 2016; Borovikova et al., 2000; Cardoso et al., 2017; Klose et al., 2017; Tracey, 2009; Wallrapp et al., 2017). For example, vagal nerve stimulation (via release of (ACh)) attenuates systemic inflammation by inhibiting the production and release of tumor necrosis factor (TNF) by the liver, through a mechanism thought to be dependent on expression of the nicotinic ACh receptor alpha 7 subunit by macrophages (Borovikova et al., 2000; Wang et al., 2003). On the other hand, activation of dopaminergic neurons of the ventral tegmental area can boost innate and adaptive immunity (Ben-Shaanan et al., 2016). Notably, at mucosal surfaces the peripheral nervous system produces neuromedin U to promote antimicrobial responses by type-2 innate lymphoid cells, boosting the production of inflammatory and tissue repair cytokines (Cardoso et al., 2017; Klose et al., 2017; Wallrapp et al., 2017).
Besides relaying electrical and chemical signals and modulating inflammatory responses, neurons also express cytokine receptors, which mediate immune cell-cell communication and are essential players in both innate and adaptive immune responses (Gasteiger and Rudensky, 2014; Kipnis, 2016; Miyajima et al., 1992). Cytokines, together with neurotransmitters, play an important role in synaptic plasticity, a process that is strongly linked to learning and memory. Under physiological conditions, TNF released by astrocytes regulates homeostatic synaptic plasticity (Stellwagen and Malenka, 2006) and experience-dependent plasticity in the developing visual cortex (Kaneko et al., 2008). Increased cytokine production in response to exogenous pathological stimuli was also shown to affect neuronal synaptic plasticity. For example, increased levels of the double stranded RNA viral mimetic poly(I:C) led to the production of TNF by peripheral monocyte-derived immune cells that caused dendritic spine loss in the mouse primary motor cortex, impairments in learning-dependent dendritic spine formation and deficits in multiple learning tasks (Garre et al., 2017). Another pro-inflammatory cytokine, interleukin 1 beta (IL-1β), is also involved in memory formation and maintenance. Long term potentiation (LTP) in the hippocampus is accompanied by increased expression of Il1b (the gene encoding IL-1β) and incubation of brain slices with IL-1 receptor antagonist induces a reversible impairment of LTP maintenance (Schneider et al., 1998). Increased expression of Il1b in the hippocampus is also induced following fear conditioning training and blockade of IL-1β signaling, either by IL-1 receptor antagonist or lack of IL-1β receptor expression, dampens fear memory as well as spatial reference learning and memory (Avital et al., 2003; Goshen et al., 2007). These behavioral deficits were associated with enhanced paired-pulse inhibition in the hippocampus, in response to perforant path stimulation, and consequent impaired LTP in the dentate gyrus (Avital et al., 2003). Other cytokines such as IL-4, interferon gamma (IFN-γ) and type I IFNs, when present in the ISF and CSF, can bind to cytokine receptors on neurons, inducing changes in neuronal transmission and consequently affecting higher brain functions such as social and learning behaviors (Baruch et al., 2014; Derecki et al., 2010; Filiano et al., 2016; Kipnis, 2016, 2018). Performance of a cognitive task increases the numbers of IL-4producing T cells in the brain meninges (Derecki et al., 2010), and both learning and memory are impaired in T cell-depleted and Il4-null mice (Derecki et al., 2010; Kipnis et al., 2004). Those observations were further supported by the finding that IL-4 produced by T cells can be directly recognized by neurons (which express the IL-4 receptor) thereby inhibiting axonal degeneration and improving disease outcome in models of CNS injury or autoimmunity (Vogelaar et al., 2018; Walsh et al., 2015). Another T cell-derived cytokine, IFN-γ, is needed for synaptic transmission of GABAergic neurons in the prefrontal cortex, which support normal social interactions in mice (Filiano et al., 2016). Recent findings in the nematode Caenorhabditis elegans suggest that neuromodulation by cytokines might be conserved across species. In C. elegans, IL-17 acts directly on RMG interneurons, modulating their responsiveness to input from oxygen sensors (Chen et al., 2017). In mice, interestingly, fetal exposure to high levels of maternal IL-17 leads to development of behavioral deficits in the offspring (autism spectrum disorder-related behavioral impairments) and abnormal cortical development (Choi et al., 2016; Shin Yim et al., 2017), supporting a crucial role for IL-17 in brain development and homeostasis. In view of the wide but highly controlled distribution of cytokines within the CNS and their influence on brain development and function, in some cases through direct signaling on neurons, at least some cytokines could be considered as a new family of neuromodulators (Dantzer et al., 2008; Kipnis, 2018; Pereda, 2014).
Although recent studies suggest that cytokines can act as neuromodulators under both physiological and pathological conditions, it is nearly always difficult to clearly identify the specific cellular sources of cytokines and whether or how these cytokines recirculate within the brain. Here we consider three possible sources of neuromodulatory cytokines (Figure 4). The best characterized source is that of the brain parenchymal cells, particularly glia. For example, release of TNF by astrocytes is essential for the homeostatic plasticity of neurons during the critical period of their development (Lee et al., 1993; Stellwagen and Malenka, 2006). In most cases however, increased cytokine production in the brain occurs in response to noxious stimuli, such as the release of IL-33 by oligodendrocytes in response to injury (Gadani et al., 2015a; Gadani et al., 2015b), or the production of TNF, IL-6 and IL-1β by myeloid cells recruited into the brain after infection (Klein et al., 2017; Mildner et al., 2008) or by microglia, the brain resident phagocytes, in models of AD-related amyloidosis (Heneka et al., 2013; Meda et al., 1995).
Figure 4. Major sources of cytokines in the CNS.
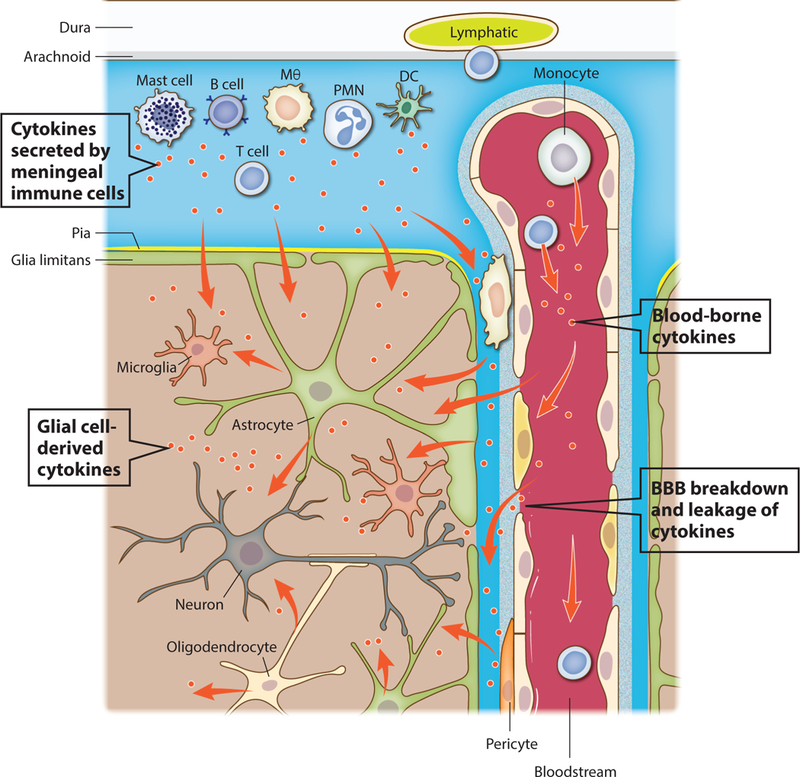
Cytokines can act as neuromodulators in both physiological and pathological conditions and their major sources are depicted. One of the major and best characterized sources of cytokines in the brain are parenchymal cells, particularly glial cells like microglia, astrocytes and oligodendrocytes. Cells found in the vicinity of the blood-brain barrier (BBB), namely perivascular macrophages and pericytes, also secrete cytokines that can reach the brain extracellular milieu. Myeloid and lymphoid immune cells present in the brain meninges produce and secrete cytokines into the CSF, which may then be transported into the brain parenchyma trough the glymphatic route. Finally, blood-borne cytokines might act directly on endothelial cells of the brain vasculature (like type I interferons upon infection), or reach the brain parenchyma in conditions where the BBB is disrupted and leaky.
An alternative source of cytokines in the CNS is represented by brain meningeal immune cells, both myeloid and lymphoid (Derecki et al., 2010; Filiano et al., 2016; Herz et al., 2017). Cytokines produced by meningeal immune cells and released into the CSF may diffuse into the brain via the glymphatic pathway and thus interact with receptor-bearing neurons and glial cells (Figure 4). It will be interesting to examine whether immune cells residing in different locations of the brain meninges produce different cytokines, thereby affecting particular neuronal subpopulations and generating different behavioral responses. It is also possible that the response of meningeal immune cells to different stimuli from the brain may result in their expression of different cytokines.
A third potential source of cytokines in the CNS is the blood. Blood-borne cytokines might act directly on brain endothelial cells (like type I IFNs upon infection (Blank et al., 2016)), or they might reach the brain parenchyma, especially under brain inflammatory conditions, when BBB permeability is dramatically increased. In this situation, not only macromolecules but also circulating immune cells can readily access paravascular spaces and cross the glia limitans to reach the brain interstitium (Banks et al., 1995; Becher et al., 2017; Blank et al., 2016; Dunn, 2006) (Figure 4).
The release of cytokines by different cells, as well as their recirculation within the CNS, is thought to be regulated by circadian rhythm. Circadian control of BBB permeability, CSF production, and cellular cytokine release are all suggestive of a precise spatiotemporal mechanism that controls cytokine levels in the brain and also affects neuronal activity (Agorastos et al., 2014; Banks et al., 1985; Entzian et al., 1996; Keller et al., 2009; Nilsson et al., 1992).
Little is known about possible mechanisms that modulate cytokine recirculation and exchange between the ISF and the CSF, or about the kinetics of cytokine clearance from the brain, all of which might differ depending on the brain region and the time of day. As mentioned before, natural sleep or anesthesia are associated with a 60% increase in the interstitial space and result in a dramatic increase in exchange between CSF and ISF through glymphatics (Ding et al., 2016; Xie et al., 2013). In this context, it is important to gain a better understanding of the circadian changes in meningeal lymphatic drainage (and its relationship with the circadian-regulation of glymphatic function) and of the role of the meningeal lymphatics-glymphatics connection, particularly as a mechanism for controlling the paravascular influx of cytokines secreted by meningeal immune cells. Cytokine efflux from the parenchymal ISF might also be affected by aging or by situations in which meningeal lymphatic function is diminished or impaired (Da Mesquita et al., 2018; Kress et al., 2014), possibly resulting in delayed cytokine clearance (by impaired drainage of meningeal lymphatics) and prolonged action (protective or noxious) on neural cells (Dantzer et al., 2008; Deczkowska et al., 2016; Deleidi et al., 2015; Filiano et al., 2017). It is also possible that altered levels of cytokines in the brain parenchyma or its fluids might modulate the meningeal lymphatic-glymphatic connection and, hence, brain CSF/ISF exchange and CSF drainage. This might happen actively, through direct signaling on the cellular components within each system (for example on astrocytes along the glymphatic route or on endothelial cells of the meningeal lymphatics), or passively, through indirect regulation of the sleep-wake cycle, which can be profoundly affected by different proinflammatory cytokines (Krueger, 2008; Krueger et al., 1984; Scheiermann et al., 2018).
Concluding remarks and future directions
Meningeal lymphatic dysfunction, associated for example with aging, correlates closely with impaired paravascular CSF influx/ISF efflux of solutes in the brain through the glymphatic route, as well as with the manifestation of cognitive deficits and with more severe amyloid pathology in models of AD (Da Mesquita et al., 2018). However, knowledge about the effect of meningeal lymphatic dysfunction on the properties of the brain neurovascular unit is almost nonexistent. Prolonged or chronic impairment of meningeal lymphatics, through decreased drainage of CSF contents and resulting decrease in CSF/ISF macromolecule renewal through glymphatics (Da Mesquita et al., 2018; Louveau et al., 2017), might induce functional changes in astrocytes, pericytes, smooth muscle cells and even in brain endothelial cells, which altogether might trigger neurodegeneration (Sweeney et al., 2018; Tarasoff-Conway et al., 2016; Zhao et al., 2015a). Likewise, it is not known whether genetic risk factors of late-onset AD, such as ApoE4 (Corder et al., 1993; Deane et al., 2008; Shi et al., 2017), have any effect on meningeal lymphatic function.
We hypothesize that age-dependent impaired lymphatic drainage will lead to changes in the frequency of particular populations of meningeal immune cells, to alterations in the accessibility of immune-derived cytokines to the brain parenchyma (by indirectly affecting molecular recirculation through the glymphatic pathway) and will ultimately affect glial and neuronal activity (Derecki et al., 2010; Filiano et al., 2016; Louveau et al., 2016; Louveau et al., 2015a; Louveau et al., 2017; Louveau et al., 2015b). Recent data also indicates that aging-induced alterations in meningeal lymphatic drainage and immunity might contribute to the build-up of brain amyloid pathology and manifestation of cognitive decline in AD (Da Mesquita et al., 2018; Ma et al., 2017). Modulation of cytokine exchange between the CSF and the ISF through changes in meningeal lymphatics-glymphatics (with possible fluctuations along the sleep-wake cycle) might serve as another key mechanism for the fine-tuning of neuronal activity (and overall brain homeostasis), as well as for the outcome of brain disease. Conditions with strong neuroinflammatory components, such as CNS autoimmune diseases like multiple sclerosis (Becher et al., 2017; Louveau et al., 2017), CNS infection or injury (Gadani et al., 2015a; Perry et al., 2007), sickness behavior and inflammaging (Dantzer et al., 2008; Deleidi et al., 2015), might be influenced by the fitness of the meningeal lymphatic vasculature as well as by brain macromolecule and cellular recirculation through the meningeal lymphatic-glymphatic axis. All of these aspects should be addressed in future studies.
Owing to its effect on brain-wide macromolecule exchange and clearance mechanisms, which are of particular relevance for removal of toxic Aβ from the AD brain and for cytokine signaling within the CNS, we propose that progressive dysfunction of the meningeal lymphatic system should be considered as a risk factor for aging-related brain disorders. We also anticipate the development of better imaging techniques (Absinta et al., 2017; Eide et al., 2018; Ringstad et al., 2017), which will make it possible to employ meningeal lymphatic drainage and glymphatic influx/efflux measurements in humans as diagnostic and/or prognostic tools in patients with neuroinflammatory and neurodegenerative disorders.
Acknowledgements
We would like to thank Shirley Smith for editing the manuscript and Anita Impagliazzo for the artwork. We would also like to thank all the members of the Kipnis Laboratory and of the Center for Brain Immunology and Glia (BIG) for their valuable comments. This work was supported by grants from the National Institutes of Health/National Institute on Aging (AG034113 and AG057496) and from the Cure Alzheimer’s Fund.
Footnotes
Declaration of interests
J.K. is an Advisor to PureTech Health/Ariya.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Absinta M, Ha SK, Nair G, Sati P, Luciano NJ, Palisoc M, Louveau A, Zaghloul KA, Pittaluga S, Kipnis J, and Reich DS (2017). Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agorastos A, Hauger RL, Barkauskas DA, Moeller-Bertram T, Clopton PL, Haji U, Lohr JB, Geracioti TD Jr., Patel PM, Chrousos GP, and Baker DG (2014). Circadian rhythmicity, variability and correlation of interleukin-6 levels in plasma and cerebrospinal fluid of healthy men. Psychoneuroendocrinology 44, 71–82. [DOI] [PubMed] [Google Scholar]
- Alitalo K (2011). The lymphatic vasculature in disease. Nat Med 17, 1371–1380. [DOI] [PubMed] [Google Scholar]
- Alitalo K, Tammela T, and Petrova TV (2005). Lymphangiogenesis in development and human disease. Nature 438, 946–953. [DOI] [PubMed] [Google Scholar]
- Antila S, Karaman S, Nurmi H, Airavaara M, Voutilainen MH, Mathivet T, Chilov D, Li Z, Koppinen T, Park JH, et al. (2017). Development and plasticity of meningeal lymphatic vessels. J Exp Med 214, 3645–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genove G, and Betsholtz C (2011). Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21, 193–215. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. (2010). Pericytes regulate the blood-brain barrier. Nature 468, 557–561. [DOI] [PubMed] [Google Scholar]
- Asgari M, de Zelicourt D, and Kurtcuoglu V (2016). Glymphatic solute transport does not require bulk flow. Sci Rep 6, 38635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, and Alitalo K (2015). A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212, 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, and Yirmiya R (2003). Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus 13, 826–834. [DOI] [PubMed] [Google Scholar]
- Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, and Herculano-Houzel S (2009). Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 513, 532–541. [DOI] [PubMed] [Google Scholar]
- Baeten KM, and Akassoglou K (2011). Extracellular matrix and matrix receptors in bloodbrain barrier formation and stroke. Dev Neurobiol 71, 1018–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, and McDonald DM (2007). Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 204, 2349–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, and Broadwell RD (1995). Passage of cytokines across the bloodbrain barrier. Neuroimmunomodulation 2, 241–248. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, and Selznick JK (1985). Modulation of immunoactive levels of DSIP and blood-brain permeability by lighting and diurnal rhythm. J Neurosci Res 14, 347–355. [DOI] [PubMed] [Google Scholar]
- Baruch K, Deczkowska A, David E, Castellano JM, Miller O, Kertser A, Berkutzki T, Barnett-Itzhaki Z, Bezalel D, Wyss-Coray T, et al. (2014). Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science 346, 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, and Gainetdinov RR (2011). The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63, 182–217. [DOI] [PubMed] [Google Scholar]
- Becher B, Spath S, and Goverman J (2017). Cytokine networks in neuroinflammation. Nat Rev Immunol 17, 49–59. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, and Zlokovic BV (2010). Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shaanan TL, Azulay-Debby H, Dubovik T, Starosvetsky E, Korin B, Schiller M, Green NL, Admon Y, Hakim F, Shen-Orr SS, and Rolls A (2016). Activation of the reward system boosts innate and adaptive immunity. Nat Med 22, 940–944. [DOI] [PubMed] [Google Scholar]
- Blank T, Detje CN, Spiess A, Hagemeyer N, Brendecke SM, Wolfart J, Staszewski O, Zoller T, Papageorgiou I, Schneider J, et al. (2016). Brain Endothelial- and EpithelialSpecific Interferon Receptor Chain 1 Drives Virus-Induced Sickness Behavior and Cognitive Impairment. Immunity 44, 901–912. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, and Tracey KJ (2000). Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462. [DOI] [PubMed] [Google Scholar]
- Botfield HF, Uldall MS, Westgate CSJ, Mitchell JL, Hagen SM, Gonzalez AM, Hodson DJ, Jensen RH, and Sinclair AJ (2017). A glucagon-like peptide-1 receptor agonist reduces intracranial pressure in a rat model of hydrocephalus. Sci Transl Med 9. [DOI] [PubMed] [Google Scholar]
- Bower NI, Koltowska K, Pichol-Thievend C, Virshup I, Paterson S, Lagendijk AK, Wang W, Lindsey BW, Bent SJ, Baek S, et al. (2017). Mural lymphatic endothelial cells regulate meningeal angiogenesis in the zebrafish. Nat Neurosci 20, 774–783. [DOI] [PubMed] [Google Scholar]
- Bradbury MW, and Westrop RJ (1983). Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J Physiol 339, 519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Abdalla N, Kawas CH, and Corrada MM (2018). Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement 14, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucchieri F, Farina F, Zummo G, and Cappello F (2015). Lymphatic vessels of the dura mater: a new discovery? J Anat 227, 702–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, and Weller RO (2008). Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol 34, 131–144. [DOI] [PubMed] [Google Scholar]
- Cardoso V, Chesne J, Ribeiro H, Garcia-Cassani B, Carvalho T, Bouchery T, Shah K, Barbosa-Morais NL, Harris N, and Veiga-Fernandes H (2017). Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549, 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield MP, and Birdsall NJ (1998). International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 50, 279–290. [PubMed] [Google Scholar]
- Chen C, Itakura E, Nelson GM, Sheng M, Laurent P, Fenk LA, Butcher RA, Hegde RS, and de Bono M (2017). IL-17 is a neuromodulator of Caenorhabditis elegans sensory responses. Nature 542, 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier S, Ferland G, and Tuchweber B (1996). Lymphatic absorption of retinol in young, mature, and old rats: influence of dietary restriction. FASEB J 10, 1085–1090. [DOI] [PubMed] [Google Scholar]
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, and Huh JR (2016). The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, May PC, O’Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, et al. (2003). In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci 23, 8844–8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, and Pericak-Vance MA (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923. [DOI] [PubMed] [Google Scholar]
- Cserr HF, Cooper DN, and Milhorat TH (1977). Flow of cerebral interstitial fluid as indicated by the removal of extracellular markers from rat caudate nucleus. Exp Eye Res 25 Suppl, 461–473. [DOI] [PubMed] [Google Scholar]
- Cserr HF, and Ostrach LH (1974). Bulk flow of interstitial fluid after intracranial injection of blue dextran 2000. Exp Neurol 45, 50–60. [DOI] [PubMed] [Google Scholar]
- Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, Contarino C, Onengut-Gumuscu S, Farber E, Raper D, et al. (2018). Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560, 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, and Barres BA (2010). Pericytes are required for bloodbrain barrier integrity during embryogenesis. Nature 468, 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, and Kelley KW (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubert EA, and Condron BG (2010). Serotonin: a regulator of neuronal morphology and circuitry. Trends Neurosci 33, 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, and Zlokovic BV (2008). apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest 118, 4002–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, et al. (2004). LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron 43, 333–344. [DOI] [PubMed] [Google Scholar]
- Deczkowska A, Baruch K, and Schwartz M (2016). Type I/II Interferon Balance in the Regulation of Brain Physiology and Pathology. Trends Immunol 37, 181–192. [DOI] [PubMed] [Google Scholar]
- Deleidi M, Jaggle M, and Rubino G (2015). Immune aging, dysmetabolism, and inflammation in neurological diseases. Front Neurosci 9, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, and Kipnis J (2010). Regulation of learning and memory by meningeal immunity: a key role for IL4. J Exp Med 207, 1067–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, O’Donnell J, Xu Q, Kang N, Goldman N, and Nedergaard M (2016). Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 352, 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr A, Sahota B, Chinta LV, Brown ME, Lai AY, Ma K, Hawkes CA, McLaurin J, and Stefanovic B (2012). Amyloid-beta-dependent compromise of microvascular structure and function in a model of Alzheimer’s disease. Brain 135, 3039–3050. [DOI] [PubMed] [Google Scholar]
- Dunn AJ (2006). Effects of cytokines and infections on brain neurochemistry. Clin Neurosci Res 6, 52–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC (1982). The synapse: from electrical to chemical transmission. Annu Rev Neurosci 5, 325–339. [DOI] [PubMed] [Google Scholar]
- Eide PK, Vatnehol SAS, Emblem KE, and Ringstad G (2018). Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Sci Rep 8, 7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B, Vajkoczy P, and Weller RO (2017). The movers and shapers in immune privilege of the CNS. Nat Immunol 18, 123–131. [DOI] [PubMed] [Google Scholar]
- Ennis SR, and Keep RF (2006). The effects of cerebral ischemia on the rat choroid plexus. J Cereb Blood Flow Metab 26, 675–683. [DOI] [PubMed] [Google Scholar]
- Entzian P, Linnemann K, Schlaak M, and Zabel P (1996). Obstructive sleep apnea syndrome and circadian rhythms of hormones and cytokines. Am J Respir Crit Care Med 153, 1080–1086. [DOI] [PubMed] [Google Scholar]
- Erkkinen MG, Kim MO, and Geschwind MD (2018). Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb Perspect Biol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubel R, Westendorf C, Bodenschatz E, and Eichele G (2016). Cilia-based flow network in the brain ventricles. Science 353, 176–178. [DOI] [PubMed] [Google Scholar]
- Filiano AJ, Gadani SP, and Kipnis J (2017). How and why do T cells and their derived cytokines affect the injured and healthy brain? Nat Rev Neurosci 18, 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano AJ, Xu Y, Tustison NJ, Marsh RL, Baker W, Smirnov I, Overall CC, Gadani SP, Turner SD, Weng Z, et al. (2016). Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature 535, 425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldi M, Gellert A, Kozma M, Poberai M, Zoltan OT, and Csanda E (1966). New contributions to the anatomical connections of the brain and the lymphatic system. Acta Anat (Basel) 64, 498–505. [DOI] [PubMed] [Google Scholar]
- Fonnum F (1984). Glutamate: a neurotransmitter in mammalian brain. J Neurochem 42, 1–11. [DOI] [PubMed] [Google Scholar]
- Gadani SP, Walsh JT, Lukens JR, and Kipnis J (2015a). Dealing with Danger in the CNS: The Response of the Immune System to Injury. Neuron 87, 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani SP, Walsh JT, Smirnov I, Zheng J, and Kipnis J (2015b). The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron 85, 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garre JM, Silva HM, Lafaille JJ, and Yang G (2017). CX3CR1(+) monocytes modulate learning and learning-dependent dendritic spine remodeling via TNF-alpha. Nat Med 23, 714722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasteiger G, and Rudensky AY (2014). Interactions between innate and adaptive lymphocytes. Nat Rev Immunol 14, 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghersi-Egea JF, Strazielle N, Catala M, Silva-Vargas V, Doetsch F, and Engelhardt B (2018). Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol 135, 337–361. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, LevyLahad E, and Yirmiya R (2007). A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology 32, 1106–1115. [DOI] [PubMed] [Google Scholar]
- Graybiel AM (1990). Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci 13, 244–254. [DOI] [PubMed] [Google Scholar]
- Greengard P (2001). The neurobiology of slow synaptic transmission. Science 294, 1024–1030. [DOI] [PubMed] [Google Scholar]
- Haj-Yasein NN, Vindedal GF, Eilert-Olsen M, Gundersen GA, Skare O, Laake P, Klungland A, Thoren AE, Burkhardt JM, Ottersen OP, and Nagelhus EA (2011). Glialconditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc Natl Acad Sci U S A 108, 17815–17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, and Zlokovic BV (2016). Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab 36, 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock MK, and Milhorat TH (1976). The cerebrospinal fluid: current concepts of its formation. Ann Clin Lab Sci 6, 22–26. [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, et al. (2009). Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet 41, 1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, and Oliver G (2005). Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet 37, 1072–1081. [DOI] [PubMed] [Google Scholar]
- Hawkes CA, Hartig W, Kacza J, Schliebs R, Weller RO, Nicoll JA, and Carare RO (2011). Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol 121, 431–443. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, et al. (2013). NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Filiano AJ, Smith A, Yogev N, and Kipnis J (2017). Myeloid Cells in the Central Nervous System. Immunity 46, 943–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, and Edwards RH (2012). Neurotransmitter corelease: mechanism and physiological role. Annu Rev Physiol 74, 225–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hos D, Bachmann B, Bock F, Onderka J, and Cursiefen C (2008). Age-related changes in murine limbal lymphatic vessels and corneal lymphangiogenesis. Exp Eye Res 87, 427–432. [DOI] [PubMed] [Google Scholar]
- Iadecola C (2013). The pathobiology of vascular dementia. Neuron 80, 844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, Singh I, Deane R, and Nedergaard M (2014). Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 34, 16180–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, and Benveniste H (2013a). Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 123, 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, et al. (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4, 147ra–111.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, and Nedergaard M (2013b). Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 33, 18190–18199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Duncan JA 3rd, Klinge PM, Brinker T, Stopa EG, and Silverberg GD (2008). Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M, Zakharov A, Papaiconomou C, Salmasi G, and Armstrong D (2004). Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res 1, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, and Alitalo K (1996). A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 15, 290–298. [PMC free article] [PubMed] [Google Scholar]
- Jung E, Gardner D, Choi D, Park E, Jin Seong Y, Yang S, Castorena-Gonzalez J, Louveau A, Zhou Z, Lee GK, et al. (2017). Development and Characterization of A Novel Prox1-EGFP Lymphatic and Schlemm’s Canal Reporter Rat. Sci Rep 7, 5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, and Regehr WG (2014). Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu Rev Physiol 76, 333–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Stellwagen D, Malenka RC, and Stryker MP (2008). Tumor necrosis factoralpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron 58, 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, et al. (2004). Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 5, 7480. [DOI] [PubMed] [Google Scholar]
- Kebabian JW, and Calne DB (1979). Multiple receptors for dopamine. Nature 277, 93–96. [DOI] [PubMed] [Google Scholar]
- Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, and Maier B (2009). A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A 106, 21407–21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, et al. (2017). A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 169, 1276–1290 e1217. [DOI] [PubMed] [Google Scholar]
- Kida S, Pantazis A, and Weller RO (1993). CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol 19, 480–488. [DOI] [PubMed] [Google Scholar]
- Kipnis J (2016). Multifaceted interactions between adaptive immunity and the central nervous system. Science 353, 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J (2018). Immune system: The “seventh sense”. J Exp Med 215, 397–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, Ziv Y, and Schwartz M (2004). T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A 101, 8180–8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RS, Garber C, and Howard N (2017). Infectious immunity in the central nervous system and brain function. Nat Immunol 18, 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine TO, Hackler R, Lutcke A, Dauch W, and Zofel P (1993a). Transport and production of cerebrospinal fluid (CSF) change in aging humans under normal and diseased conditions. Z Gerontol 26, 251–255. [PubMed] [Google Scholar]
- Kleine TO, Hackler R, and Zofel P (1993b). Age-related alterations of the blood-brainbarrier (bbb) permeability to protein molecules of different size. Z Gerontol 26, 256–259. [PubMed] [Google Scholar]
- Klingauf J, Kavalali ET, and Tsien RW (1998). Kinetics and regulation of fast endocytosis at hippocampal synapses. Nature 394, 581–585. [DOI] [PubMed] [Google Scholar]
- Klose CSN, Mahlakoiv T, Moeller JB, Rankin LC, Flamar AL, Kabata H, Monticelli LA, Moriyama S, Putzel GG, Rakhilin N, et al. (2017). The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549, 282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, et al. (2014). Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76, 845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM (2008). The role of cytokines in sleep regulation. Curr Pharm Des 14, 3408–3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Walter J, Dinarello CA, Wolff SM, and Chedid L (1984). Sleep-promoting effects of endogenous pyrogen (interleukin-1). Am J Physiol 246, R994–999. [DOI] [PubMed] [Google Scholar]
- Lecco V (1953). [Probable modification of the lymphatic fissures of the walls of the venous sinuses of the dura mater]. Arch Ital Otol Rinol Laringol 64, 287–296. [PubMed] [Google Scholar]
- Lee SC, Liu W, Dickson DW, Brosnan CF, and Berman JW (1993). Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol 150, 2659–2667. [PubMed] [Google Scholar]
- Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, Maynard T, Gonzalez D, Kim S, Ye P, et al. (2011). The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69, 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhou J, and Shi Y (1996). Scanning electron microscopy of human cerebral meningeal stomata. Ann Anat 178, 259–261. [DOI] [PubMed] [Google Scholar]
- Louveau A, Da Mesquita S, and Kipnis J (2016). Lymphatics in Neurological Disorders: A Neuro-Lympho-Vascular Component of Multiple Sclerosis and Alzheimer’s Disease? Neuron 91, 957–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Harris TH, and Kipnis J (2015a). Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol 36, 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, and Kipnis J (2017). Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest 127, 3210–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, et al. (2018). CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 21, 1380–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et al. (2015b). Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukic IK, Gluncic V, Ivkic G, Hubenstorf M, and Marusic A (2003). Virtual dissection: a lesson from the 18th century. Lancet 362, 2110–2113. [DOI] [PubMed] [Google Scholar]
- Lundgaard I, Lu ML, Yang E, Peng W, Mestre H, Hitomi E, Deane R, and Nedergaard M (2017). Glymphatic clearance controls state-dependent changes in brain lactate concentration. J Cereb Blood Flow Metab 37, 2112–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Ineichen BV, Detmar M, and Proulx ST (2017). Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun 8, 1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques F, Sousa JC, Coppola G, Falcao AM, Rodrigues AJ, Geschwind DH, Sousa N, Correia-Neves M, and Palha JA (2009). Kinetic profile of the transcriptome changes induced in the choroid plexus by peripheral inflammation. J Cereb Blood Flow Metab 29, 921932. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H, and Lu B (2007). New insights into BDNF function in depression and anxiety. Nat Neurosci 10, 1089–1093. [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, and Bateman RJ (2010). Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 330, 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May C, Kaye JA, Atack JR, Schapiro MB, Friedland RP, and Rapoport SI (1990). Cerebrospinal fluid production is reduced in healthy aging. Neurology 40, 500–503. [DOI] [PubMed] [Google Scholar]
- Meda L, Cassatella MA, Szendrei GI, Otvos L Jr., Baron P, Villalba M, Ferrari D, and Rossi F (1995). Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature 374, 647–650. [DOI] [PubMed] [Google Scholar]
- Mestre H, Kress BT, Zou W, Pu T, Murlidharan G, Castellanos Rivera RM, Simon MJ, Pike MM, Plog BA, Xavier ALR, et al. (2017). Aquaporin-4 dependent glymphatic solute transport in rodent brain. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud JP, Bellavance MA, Prefontaine P, and Rivest S (2013). Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Rep 5, 646–653. [DOI] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, and Rothman JE (1998). Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192–195. [DOI] [PubMed] [Google Scholar]
- Mildner A, Djukic M, Garbe D, Wellmer A, Kuziel WA, Mack M, Nau R, and Prinz M (2008). Ly-6G+CCR2- myeloid cells rather than Ly-6ChighCCR2+ monocytes are required for the control of bacterial infection in the central nervous system. J Immunol 181, 2713–2722. [DOI] [PubMed] [Google Scholar]
- Mildner A, Schlevogt B, Kierdorf K, Bottcher C, Erny D, Kummer MP, Quinn M, Bruck W, Bechmann I, Heneka MT, et al. (2011). Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer’s disease. J Neurosci 31, 1115911171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima A, Kitamura T, Harada N, Yokota T, and Arai K (1992). Cytokine receptors and signal transduction. Annu Rev Immunol 10, 295–331. [DOI] [PubMed] [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, et al. (2015). Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota B, and Herculano-Houzel S (2015). BRAIN STRUCTURE. Cortical folding scales universally with surface area and thickness, not number of neurons. Science 349, 74–77. [DOI] [PubMed] [Google Scholar]
- Nagai T, Bridenbaugh EA, and Gashev AA (2011). Aging-associated alterations in contractility of rat mesenteric lymphatic vessels. Microcirculation 18, 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson C, Stahlberg F, Thomsen C, Henriksen O, Herning M, and Owman C (1992). Circadian variation in human cerebrospinal fluid production measured by magnetic resonance imaging. Am J Physiol 262, R20–24. [DOI] [PubMed] [Google Scholar]
- Oshio K, Watanabe H, Song Y, Verkman AS, and Manley GT (2005). Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J 19, 76–78. [DOI] [PubMed] [Google Scholar]
- Palmert MR, Podlisny MB, Witker DS, Oltersdorf T, Younkin LH, Selkoe DJ, and Younkin SG (1989). The beta-amyloid protein precursor of Alzheimer disease has soluble derivatives found in human brain and cerebrospinal fluid. Proc Natl Acad Sci U S A 86, 63386342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappolla M, Sambamurti K, Vidal R, Pacheco-Quinto J, Poeggeler B, and Matsubara E (2014). Evidence for lymphatic Abeta clearance in Alzheimer’s transgenic mice. Neurobiol Dis 71, 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, Regan S, Kasper T, Peng S, Ding F, et al. (2016). Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis 93, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda AE (2014). Electrical synapses and their functional interactions with chemical synapses. Nat Rev Neurosci 15, 250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peroutka SJ, Lebovitz RM, and Snyder SH (1981). Two distinct central serotonin receptors with different physiological functions. Science 212, 827–829. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, and Holmes C (2007). Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol 7, 161–167. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Higley MJ, and Mineur YS (2012). Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76, 116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollay M, and Welch K (1962). The function and structure of canine arachnoid villi. J Surg Res 2, 307–311. [DOI] [PubMed] [Google Scholar]
- Preston JE (2001). Ageing choroid plexus-cerebrospinal fluid system. Microsc Res Tech 52, 31–37. [DOI] [PubMed] [Google Scholar]
- Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, and Grady PA (1985). Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res 326, 47–63. [DOI] [PubMed] [Google Scholar]
- Ridgway JP, Turnbull LW, and Smith MA (1987). Demonstration of pulsatile cerebrospinal-fluid flow using magnetic resonance phase imaging. Br J Radiol 60, 423–427. [DOI] [PubMed] [Google Scholar]
- Ringstad G, Vatnehol SAS, and Eide PK (2017). Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 140, 2691–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, and Holtzman DM (2012). Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med 4, 150ra–122.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaristo A, Veikkola T, Tammela T, Enholm B, Karkkainen MJ, Pajusola K, Bueler H, Yla-Herttuala S, and Alitalo K (2002). Lymphangiogenic gene therapy with minimal blood vascular side effects. J Exp Med 196, 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders NR, Daneman R, Dziegielewska KM, and Liddelow SA (2013). Transporters of the blood-brain and blood-CSF interfaces in development and in the adult. Mol Aspects Med 34, 742–752. [DOI] [PubMed] [Google Scholar]
- Schain AJ, Melo-Carrillo A, Strassman AM, and Burstein R (2017). Cortical Spreading Depression Closes Paravascular Space and Impairs Glymphatic Flow: Implications for Migraine Headache. J Neurosci 37, 2904–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann C, Gibbs J, Ince L, and Loudon A (2018). Clocking in to immunity. Nat Rev Immunol 18, 423–437. [DOI] [PubMed] [Google Scholar]
- Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, and Besedovsky HO (1998). A neuromodulatory role of interleukin-1beta in the hippocampus. Proc Natl Acad Sci U S A 95, 7778–7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber G, Aldred AR, Jaworowski A, Nilsson C, Achen MG, and Segal MB (1990). Thyroxine transport from blood to brain via transthyretin synthesis in choroid plexus. Am J Physiol 258, R338–345. [DOI] [PubMed] [Google Scholar]
- Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W, Tsai RM, Spina S, Grinberg LT, Rojas JC, et al. (2017). ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549, 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, and Zlokovic BV (2000). Clearance of Alzheimer’s amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 106, 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Yim Y, Park A, Berrios J, Lafourcade M, Pascual LM, Soares N, Yeon Kim J, Kim S, Kim H, Waisman A, et al. (2017). Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature 549, 482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Vargas V, Maldonado-Soto AR, Mizrak D, Codega P, and Doetsch F (2016). AgeDependent Niche Signals from the Choroid Plexus Regulate Adult Neural Stem Cells. Cell Stem Cell 19, 643–652. [DOI] [PubMed] [Google Scholar]
- Silverberg GD, Heit G, Huhn S, Jaffe RA, Chang SD, Bronte-Stewart H, Rubenstein E, Possin K, and Saul TA (2001). The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurology 57, 1763–1766. [DOI] [PubMed] [Google Scholar]
- Skillback T, Delsing L, Synnergren J, Mattsson N, Janelidze S, Nagga K, Kilander L, Hicks R, Wimo A, Winblad B, et al. (2017). CSF/serum albumin ratio in dementias: a crosssectional study on 1861 patients. Neurobiol Aging 59, 1–9. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Yao X, Dix JA, Jin BJ, and Verkman AS (2017). Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, and Malenka RC (2006). Synaptic scaling mediated by glial TNF-alpha. Nature 440, 1054–1059. [DOI] [PubMed] [Google Scholar]
- Sweeney MD, Sagare AP, and Zlokovic BV (2018). Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14, 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa K, Matsumae M, Sunohara S, Yatsushiro S, and Kuroda K (2017). Characterization of cardiac- and respiratory-driven cerebrospinal fluid motion based on asynchronous phase-contrast magnetic resonance imaging in volunteers. Fluids Barriers CNS 14, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammela T, Saaristo A, Holopainen T, Yla-Herttuala S, Andersson LC, Virolainen S, Immonen I, and Alitalo K (2011). Photodynamic ablation of lymphatic vessels and intralymphatic cancer cells prevents metastasis. Sci Transl Med 3, 69ra–11.. [DOI] [PubMed] [Google Scholar]
- Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, et al. (2016). Clearance systems in the brain-implications for Alzheimer diseaser. Nat Rev Neurol 12, 248. [DOI] [PubMed] [Google Scholar]
- Tracey KJ (2009). Reflex control of immunity. Nat Rev Immunol 9, 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Granger AJ, and Sabatini BL (2016). Mechanisms and functions of GABA corelease. Nat Rev Neurosci 17, 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkat P, Chopp M, Zacharek A, Cui C, Zhang L, Li Q, Lu M, Zhang T, Liu A, and Chen J (2017). White matter damage and glymphatic dysfunction in a model of vascular dementia in rats with no prior vascular pathologies. Neurobiol Aging 50, 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelaar CF, Mandal S, Lerch S, Birkner K, Birkenstock J, Buhler U, Schnatz A, Raine CS, Bittner S, Vogt J, et al. (2018). Fast direct neuronal signaling via the IL-4 receptor as therapeutic target in neuroinflammation. Sci Transl Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour RE, Nyman J, Dionne D, Hofree M, Cuoco MS, Rodman C, Farouq D, et al. (2017). The neuropeptide NMU amplifies ILC2driven allergic lung inflammation. Nature 549, 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JT, Hendrix S, Boato F, Smirnov I, Zheng J, Lukens JR, Gadani S, Hechler D, Golz G, Rosenberger K, et al. (2015). MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J Clin Invest 125, 699–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. (2003). Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421, 384–388. [DOI] [PubMed] [Google Scholar]
- Wang M, Ding F, Deng S, Guo X, Wang W, Iliff JJ, and Nedergaard M (2017). Focal Solute Trapping and Global Glymphatic Pathway Impairment in a Murine Model of Multiple Microinfarcts. J Neurosci 37, 2870–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ulland TK, Ulrich JD, Song W, Tzaferis JA, Hole JT, Yuan P, Mahan TE, Shi Y, Gilfillan S, et al. (2016). TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J Exp Med 213, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch K, and Pollay M (1961). Perfusion of particles through arachnoid villi of the monkey. Am J Physiol 201, 651–654. [DOI] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, et al. (2013). Sleep drives metabolite clearance from the adult brain. Science 342, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Xiao N, Chen Y, Huang H, Marshall C, Gao J, Cai Z, Wu T, Hu G, and Xiao M (2015). Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Abeta accumulation and memory deficits. Mol Neurodegener 10, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Cirrito JR, Stewart FR, Jiang H, Finn MB, Holmes BB, Binder LI, Mandelkow EM, Diamond MI, Lee VM, and Holtzman DM (2011). In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J Neurosci 31, 13110–13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanamandra K, Patel TK, Jiang H, Schindler S, Ulrich JD, Boxer AL, Miller BL, Kerwin DR, Gallardo G, Stewart F, et al. (2017). Anti-tau antibody administration increases plasma tau in transgenic mice and patients with tauopathy. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev S, and Shen K (2014). Cellular and molecular mechanisms of synaptic specificity. Annu Rev Cell Dev Biol 30, 417–437. [DOI] [PubMed] [Google Scholar]
- Zhang ET, Inman CB, and Weller RO (1990). Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat 170, 111–123. [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Nelson AR, Betsholtz C, and Zlokovic BV (2015a). Establishment and Dysfunction of the Blood-Brain Barrier. Cell 163, 1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Sagare AP, Ma Q, Halliday MR, Kong P, Kisler K, Winkler EA, Ramanathan A, Kanekiyo T, Bu G, et al. (2015b). Central role for PICALM in amyloid-beta blood-brain barrier transcytosis and clearance. Nat Neurosci 18, 978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV, Jovanovic S, Miao W, Samara S, Verma S, and Farrell CL (2000). Differential regulation of leptin transport by the choroid plexus and blood-brain barrier and high affinity transport systems for entry into hypothalamus and across the blood-cerebrospinal fluid barrier. Endocrinology 141, 1434–1441. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Martel CL, Matsubara E, McComb JG, Zheng G, McCluskey RT, Frangione B, and Ghiso J (1996). Glycoprotein 330/megalin: probable role in receptormediated transport of apolipoprotein J alone and in a complex with Alzheimer disease amyloid beta at the blood-brain and blood-cerebrospinal fluid barriers. Proc Natl Acad Sci U S A 93, 4229–4234. [DOI] [PMC free article] [PubMed] [Google Scholar]


