Abstract
Sideritis scardica Griseb., Lamiaceae (ironwort, mountain tea), an endemic plant of the Balkan Peninsula, has been used in traditional medicine in the treatment of antimicrobial infections, gastrointestinal complaints, inflammation and rheumatic disorders. This study reports a comparison between conventional (hydrodistillation HD and solvent extraction SE) and alternative (supercritical carbon dioxide SC CO2) extraction methods regarding the qualitative and quantitative composition of the obtained extracts as analyzed by GC and GC-MS techniques and their anitimicrobial activity. Different types of extracts were tested, the essential oil EO obtained by HD, EO-CO2 and AO-CO2 obtained by SC CO2 at different preasures 10 and 30 MPa, at 40 °C, respectively, and the fractions A, B, C and D obtained by successive solvent extraction (SE) A: ethanol, B: diethyl ether, C: ethyl acetate and D: n-butanol). While EO was characterized by the presence of the high percentage of oxygenated monoterpenes and sesquiterpenes (30.01 and 25.54%, respectively), the rest of the investigated samples were the most abundant in fatty acids and their esters and diterpenes (from 16.72 to 71.07% for fatty acids and their esters, and from 23.30 to 72.76%, for diterpenes). Microbial susceptibility tests revealed the strong to moderate activity of all investigated extracts against the tested microorganisms (MIC from 40 to 2,560 μg/mL). Although differences in the chemical compositions determined by GC and GC-MS analysis were established, the displayed antimicrobial activity was similar for the all investigated extracts.
Keywords: Sideritis scardica Griseb., Lamiaceae (mountain tea), essential oil, hydrodistillation, supercritical carbon dioxide extracts, solvent extraction, terpenoids, antimicrobial activity, GC and GC-MS analysis
1. Introduction
The results of numerous preliminary investigations of plants beloning to the genus Sideritis L. have revealed plant-derived compounds of particular pharmacological and nutritional interest. Sideritis L. (Lamiaceae) includes approximately 150 species of annual and perennial plants distributed mainly in the Medirerranean region. This genus is devided into two subgenera, Sideritis and Marrubiastrum, formed by the European and Macaronesian species, respectively. So far, different biological activities of Sideritis species have been reported: anti-inflammatory, anti-ulcer, analgesic, antimicrobial and antifungal [1,2,3,4,5], immunomodulating [6], macrophage NOS-2-expression inhibiting [7], and hypoglycemic [4]. Recently, aldose reductase inhibiting activity [8], antiproliferative, anticholinesterase and selective estrogen receptor modulator-like effects and cytotoxic properties have also been reported [9,10,11,12]. The previous studies of Sideritis species reported the presence of flavonoid aglycones and glycosides, phenolic acids, di- and triterpenoids, fatty acids, coumarins and iridoid glycosides [2,8,10,12,13,14,15,16]. The composition of various Sideritis species essential oils has also been studied exhaustively as well [1].
The genus Sideritis is represented in Serbia by only one species, S. montana L. [17], but because of its pro-oxidant properties this plant has not been used in traditional medicine [18]. S. scardica Griseb. (ironwort, mountain tea) is an endemic plant of the Balkan Peninsula belonging to the Empedoclea section. Aerial parts of “mountain tea” are traditionally known for their anti-inflammatory, anti-microbial, anti-bacterial, anti-rheumatic and gastroprotective properties. S. scardica is used as a loosening agent in bronchitis and bronchial asthma, against common cold and lung emphysema, as well.
With the current trend towards increasing use of traditional medicines, plant-derived agents have been attracting much interest as natural alternatives to synthetic compounds. Since the Middle Age, essential oils have been widely used in the pharmaceutical, sanitary, cosmetic, agricultural and food industries. In recent years, there has been the considerable interest in essential oils extracted from various medicinal plants with the goal of discovering their multifunctional properties in addition to their classical roles as food additives and/or fragrances. Known properties of essential oils include antibacterial, antifungal, antioxidant and anti-inflammatory activities. The most common method of essential oil isolation is hydrodistillation (HD). Although a very simple process, it suffers of many drawbacks: thermal degradation, hydrolysis and solubilization in water of some compounds that alter the flavor and fragrance profile of many essential oils extracted by this technique. Organic solvent extraction (SE) is often used for isolation of active components from plant material in order to preserve thermolabile and highly volatile compounds, but it requires the use of organic solvents. Recently clean techniques, such as supercritical fluid extraction (SFE) have been developed for extracting not only essential oils. but other active component from complex matrices. SFE is a separation technology that uses a supercritical fluid as the solvent, carbon dioxide (CO2) being the main supercritical solvent. Carbon dioxide (critical conditions = 30.9 °C and 73.8 bar) is cheap, environmentally friendly and generally recognized as safe. Supercritical CO2 (SC-CO2) is attractive because of its high diffusivity and its easily tunable solvent strength. Another advantage is that CO2 is gaseous at room temperature and ordinary pressure, which makes the recovery of analytes very simple and provides solvent-free analytes [19]. Besides, SFE using CO2 allows the extraction of termally labile or easily oxidized compounds. The main drawback of SC-CO2 is its low polarity, a problem that can be overcome by employing polar modifiers (co-solvents) to change the polarity of the supercritical fluid and to increase its solvating power towards the analyte of interest.
Considering the differences between the applied extraction methods, which might reflect primarily in enhanced bioavailability of the active principles and the consequent increase of its therapeutic properties, the aim of this study was to evaluate the chemical profile and antimicrobial properties of conventional and supercritical S. scardica Griseb., Lamiaceae extracts, EO: essential oil obtained by hydrodistillation, EO-CO2: low volatile fraction obtained by supercritical carbon dioxide extraction at 10 MPa and 40 °C, AO-CO2: the fraction obtained by supercritical fluid extraction at higher pressure, 40 MPa and 40 °C, and fractions obtained by successive solvent extraction: samples A - ethanol, B - diethyl ether, C - ethyl acetate and D - n-butanol extracts. The chemical composition of the investigated extracts was determined by GC and GC-MS analysis. In addition, the in vitro antimicrobial activity of the investigated extracts was assessed against Gram-positive and Gram-negative bacteria and yeast strains of medicinal relevance applying the broth microdillution method. As far as a literature survey ascertained, there have been no reports on the chemical composition or antimicrobial activity of SC-CO2 extracts isolated from S. scardica at 10 MPa and 30 MPa at 40 °C. Papaefstathiou et al. reported the successful SFE extraction of added value components from S. raeseri [20], however, we have not found in the open literature any report regarding the chemical composition and antimicrobial activity of SC-CO2 extracts isolated from S. scardica.
2. Results and Discussion
The content and chemical profile of the investigated supercritical and conventional S. scardica extracts (essential oil, EO obtained by HD, low volatile fraction, sample EO-CO2, obtained by supercritical fluid extraction at 10 MPa and 40 °C, the second fraction, sample AO-CO2, obtained by supercritical fluid extraction at 30 MPa and 40 °C, extracts A, B, C and D, obtained by successive SE were determined by GC and GC-MS analysis to establish the effect of the extraction method on the chemical composition (Table 1 and Figure 1) and on the antimicrobial activity of each extract (Table 2).
Table 1.
Chemical composition of the supercritical (EO-CO2, AO-CO2) and conventional (EO, A, B, C, and D) S. scardica investigated extracts.
| No | Extraction mode | HD | SC CO2 | SE | |||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | K.I. a | EO | EO-CO2 | AO-CO2 | A | B | C | D | |
| 1 | α-thujene | 925.5 | 0.30 | - | - | - | - | - | - |
| 2 | thuja-2,4(10)-diene | 953.0 | - | 1.33 | - | - | - | - | - |
| 3 | sabinene | 967.3 | 0.05 | - | - | - | - | - | - |
| 4 | myrcene | 967.4 | 1.25 | 0.24 | - | - | - | - | - |
| 5 | n-Decane | 985.5 | 0.07 | - | - | - | - | - | - |
| 6 | β-phellandrene/limonene | 1021.7 | 0.05 | - | - | - | - | - | - |
| 7 | 1,8-cineole | 1023.2 | 0.08 | - | - | - | - | - | - |
| 8 | γ-terpinene | 1052.8 | 0.11 | - | - | - | - | - | - |
| 9 | isobutyl acetoacetate | 1084.9 | 0.31 | - | - | - | - | - | - |
| 10 | linalool | 1095.3 | 1.53 | - | - | - | - | - | - |
| 11 | β-thujone | 1097.8 | 0.45 | - | - | - | - | - | - |
| 12 | α-thujone | 1098.7 | 0.56 | - | - | - | - | - | - |
| 13 | trans-pinocarveol | 1130.8 | 0.34 | - | - | - | - | - | - |
| 14 | camphor | 1134.8 | 0.88 | - | - | - | - | - | - |
| 15 | trans-verbenol | 1138.8 | t | - | - | - | - | - | - |
| 16 | iso-menthone | 1145.8 | 1.75 | - | - | - | - | - | - |
| 17 | borneol | 1157.7 | 1.46 | - | - | - | - | - | - |
| 18 | menthol | 1165.6 | 4.90 | - | - | - | - | - | - |
| 19 | terpinen-4-ol | 1169.8 | 0.42 | - | - | - | - | - | - |
| 20 | α-terpineol | 1186.2 | 0.27 | - | - | - | - | - | - |
| 21 | myrtenal | 1190.8 | 0.42 | - | - | - | - | - | - |
| 22 | myrtenol | 1191.2 | 0.64 | - | - | - | - | - | - |
| 23 | trans-dihydrocarvone | 1199.5 | 0.07 | - | - | - | - | - | - |
| 24 | β-cyclocitral | 1214.0 | 0.09 | - | - | - | - | - | - |
| 25 | neoiso-dihydrocarveol | 1224.5 | 0.08 | - | - | - | - | - | - |
| 26 | thymol methyl ether | 1228.4 | 0.19 | - | - | - | - | - | - |
| 27 | pulegone | 1233.4 | 0.80 | - | - | - | - | - | - |
| 28 | carvacrol methyl ether | 1235.5 | 0.36 | - | - | - | - | - | - |
| 29 | d-carvone | 1242.1 | 0.37 | - | - | - | - | - | - |
| 30 | piperotone | 1250.7 | 0.27 | - | - | - | - | - | - |
| 31 | (Z)-chrysanthenyl acetate | 1257.8 | - | - | - | - | - | 1.63 | 4.58 |
| 32 | isobornyl acetate | 1277.5 | 1.10 | - | - | - | - | - | - |
| 33 | (E)-anethole | 1281.4 | 2.89 | - | - | - | - | - | - |
| 34 | menthyl acetate | 1286.4 | - | - | - | - | - | - | |
| 35 | thymol | 1291.1 | 1.97 | - | - | - | - | - | - |
| 36 | carvacrol | 1300.5 | 2.05 | - | - | 1.94 | 0.48 | 0.82 | 1.88 |
| 37 | (E)-dimetoxy citral | 1341.0 | - | - | - | 1.62 | - | - | - |
| 38 | 3’-metoxy-acetophenone | 1343.0 | 0.02 | - | - | - | - | - | - |
| 39 | α-cubebene | 1345.0 | 0.02 | - | 0.05 | - | - | - | - |
| 40 | α-terpenyl acetate | 1342.3 | 0.03 | - | - | - | - | - | - |
| 41 | decanoic acid | 1364.0 | - | - | - | - | - | 0.10 | 5.47 |
| 42 | α-copeane | 1365.6 | 0.11 | 0.34 | - | - | - | - | - |
| 43 | β-bourbonene | 1374.3 | 0.04 | - | - | - | - | - | - |
| 44 | trans-β-demascenone | 1376.9 | 0.21 | - | - | - | - | - | - |
| 45 | decyl acetate | 1407.0 | - | - | - | - | - | 2.66 | 12.54 |
| 46 | α-dihydroionone | 1389.0 | 0.27 | - | - | - | - | - | - |
| 47 | β-funebrene | 1395.2 | 0.30 | - | - | - | - | - | - |
| 48 | trans-β-caryophyllene | 1408.0 | 0.60 | 1.39 | - | - | - | - | - |
| 49 | 2,5-dimethyl-p-cymene | 1417.4 | 0.07 | - | - | - | - | - | - |
| 50 | trans-α-bergamotene | 1425.9 | 0.06 | - | - | - | - | - | - |
| 51 | α-humulene | 1442.4 | 0.10 | - | - | - | - | - | - |
| 52 | (E)-β-farnesene | 1449.2 | 0.21 | - | - | - | - | - | - |
| 53 | (2E)-dodecanal | 1464.0 | 0.10 | - | - | - | - | - | - |
| 54 | germacrene D | 1470.3 | 0.52 | - | - | - | - | - | - |
| 55 | (E)-β-ionone | 1478.0 | 1.15 | - | - | - | - | - | - |
| 56 | (E)-muurola-4(14),5-diene | 1482.2 | 0.03 | - | - | - | - | - | - |
| 57 | valencene | 1484.8 | 0.35 | - | - | - | - | - | - |
| 58 | α-muurolene | 1490.2 | 0.33 | - | - | - | - | - | - |
| 59 | β-bisabolene | 1499.3 | 0.19 | 0.41 | - | - | - | - | - |
| 60 | χ-cadinene | 1503.1 | 0.13 | 0.89 | - | - | - | - | - |
| 61 | 7-epi-α-selinene | 1520.0 | 0.66 | 0.94 | - | - | - | - | - |
| 62 | (E)-calamenene | 1521.0 | 0.36 | - | - | - | - | - | - |
| 63 | myristicin | 1522.0 | 5.23 | - | - | - | - | - | - |
| 64 | δ-cadinene | 1522.0 | 0.10 | - | - | - | - | - | - |
| 65 | ether-italicane | 1531.1 | t | - | - | - | - | - | - |
| 66 | α-calacorene | 1532.7 | 1.31 | - | - | - | - | - | - |
| 67 | β-calacorene | 1553.3 | 1.21 | - | - | - | - | - | - |
| 68 | (E)-nerolidol | 1557.1 | 0.06 | - | - | - | - | - | - |
| 69 | dodecanoic acid | 1565.0 | - | - | - | 17.05 | 6.96 | 25.56 | 35.78 |
| 70 | spathulenol | 1577.0 | 1.97 | - | - | - | - | - | - |
| 71 | caryophyllene oxide | 1582.0 | 4.84 | 2.44 | 0.07 | - | - | - | - |
| 72 | viridiflorol | 1592.0 | 1.23 | - | - | - | - | - | - |
| 73 | carotol | 1593.5 | t | - | - | - | - | - | - |
| 74 | ledol | 1594.3 | 0.99 | - | - | - | - | - | - |
| 75 | diepi-α-cedrenepoxide | 1607.0 | t | - | - | - | - | - | - |
| 76 | humulene epoxide II | 1608.0 | 0.56 | - | - | - | - | - | - |
| 77 | ledene | 1613.0 | 0.32 | - | - | - | - | - | - |
| 78 | (E)-isolongifolanene | 1618.8 | 0.56 | - | - | - | - | - | - |
| 79 | α-colocalene | 1622.0 | 0.14 | - | - | - | - | - | - |
| 80 | muurola-4,10(14)-dien-1-β-ol | 1630.0 | 0.61 | 0.21 | - | - | - | - | - |
| 81 | caryophylla-4(12),8(13)-dien-5-β-ol | 1639.0 | 0.51 | - | - | - | - | - | - |
| 82 | τ-muurolol | 1640.6 | 3.62 | - | - | - | - | - | - |
| 83 | α-muurolol | 1645.7 | 0.19 | - | - | - | - | - | - |
| 84 | α-selin-11-en-4-ol | 1658.1 | 1.65 | - | - | - | - | - | - |
| 85 | (E)-calamenen-10-ol | 1668.2 | 1.45 | - | - | - | - | - | - |
| 86 | valeranone | 1674.4 | 2.15 | 1.06 | - | - | - | - | - |
| 87 | cadelene | 1675.0 | 1.51 | - | - | - | - | - | - |
| 88 | α-germacra-4(15),5,10(14)-trien-1-ol | 1685.3 | 1.21 | - | - | - | - | - | - |
| 89 | α-bisabolol | 1685.7 | 0.32 | 0.27 | - | - | - | - | - |
| 90 | acorenone | 1692.0 | 0.26 | - | - | - | - | - | - |
| 91 | 2-(E)-tridecanol acetate | 1703.0 | 2.50 | 0.65 | 0.19 | - | - | - | - |
| 92 | (E)-coniferyl alcohol | 1735.6 | - | - | - | - | - | 11.81 | 18.69 |
| 93 | benzyl benzoate | 1761.8 | 0.02 | - | - | - | - | - | - |
| 94 | β-bisabolenal | 1768.9 | 0.22 | - | - | - | - | - | - |
| 95 | β-bisabolenol | 1786.1 | 1.40 | 0.53 | - | - | - | - | - |
| 96 | (2Z,6E)-farnesyl acetate | 1821.0 | - | 0.44 | - | - | - | - | - |
| 97 | cyclopentadecanolide | 1826.6 | - | - | - | 4.01 | 0.34 | 15.35 | - |
| 98 | (Z)-lanceol acetate | 1858.0 | - | 0.66 | - | - | - | - | - |
| 99 | hexadecanol | 1878.8 | - | - | - | - | - | 0.63 | 0.42 |
| 100 | (5E,9E)-farnesyl acetone | 1907.9 | 0.45 | - | 0.12 | - | - | - | - |
| 101 | methyl hexadecanoate | 1922.0 | - | - | 0.27 | - | - | - | - |
| 102 | ent-rosa-5,15-diene | 1933.9 | 0.15 | - | - | - | - | - | - |
| 103 | pimaradiene | 1948.8 | 0.05 | 1.52 | 2.84 | - | - | - | - |
| 104 | hexadecanoic acid | 1966.6 | 12.92 | 18.59 | 43.22 | 46.57 | 8.53 | 29.89 | 0.38 |
| 105 | ethyl hexadecanoate | 1992.0 | 0.20 | 11.82 | - | 4.13 | 1.23 | 7.64 | - |
| 106 | kaur-15-ene | 1997.0 | 1.88 | 0.55 | - | - | - | - | - |
| 107 | 13-epi-manool oxide | 2009.9 | 0.62 | - | - | - | - | - | - |
| 108 | manool | 2041.7 | 0.54 | - | - | - | - | - | - |
| 109 | 13-epi-manool | 2059.0 | 0.53 | - | - | - | - | - | - |
| 110 | octadecanol | 2077.0 | 0.21 | 6.20 | 1.84 | t | t | - | - |
| 111 | methyl linoleate | 2095.0 | 0.01 | 1.34 | 0.11 | - | - | - | - |
| 112 | methyl oleate | 2104.0 | - | - | 0.18 | t | - | - | - |
| 113 | linoleic acid | 2132.0 | 0.12 | 0.71 | 24.80 | - | t | - | - |
| 114 | oleic acid | 2141.0 | - | 0.80 | - | - | - | - | - |
| 115 | phytol acetate | 2170.6 | - | - | 0.46 | - | - | - | - |
| 116 | ugandensodial | 2190.0 | - | - | - | 1.36 | - | - | - |
| 117 | 7α-hydroxy manool | 2237.0 | 0.41 | 0.61 | - | - | - | - | - |
| 118 | 3β-sandaracopimardienol | 2269.0 | 0.20 | - | - | t | t | - | - |
| 119 | sandaracopimarinol | 2269.0 | 0.37 | 1.26 | 1.26 | - | - | - | - |
| 120 | tricosane | 2300.0 | 0.38 | 0.40 | - | - | t | - | - |
| 121 | isopimarol | 2310.4 | 0.22 | 1.49 | - | - | - | - | - |
| 122 | (E) -ferruginol acetate | 2357.0 | - | 11.73 | 11.22 | 8.25 | 65.47 | - | - |
| 123 | methyl strictate | 2387.0 | - | 1.70 | - | - | - | - | - |
| 124 | 9-octadecen-1-ol | 2396.4 | - | 1.05 | - | - | - | - | - |
| 125 | (Z) -ferruginol acetate | 2406.0 | - | - | - | 4.26 | 1.04 | - | - |
| 126 | pentacosane | 2486.0 | 0.41 | 1.23 | - | - | - | - | - |
| 127 | dihydroxysandaracopimar-8(14),15-diene b | 2506.0 | - | 14.89 | 11.49 | 10.79 | 6.25 | - | - |
| 128 | hexacosane | 2600 | - | t | - | - | - | - | - |
| 129 | heptacosane | 2700.0 | 1.28 | t | t | - | - | - | - |
| 130 | octacosane | 2800.0 | - | t | t | - | - | - | - |
| 131 | nonacosane | 2900.0 | - | t | - | - | - | - | - |
| 132 | triacontane | 3000.0 | 0.08 | - | - | - | - | - | - |
| 133 | dotriacontane | 3200.0 | 0.01 | - | - | - | - | - | - |
| Total | 89.12 | 87.69 | 98.12 | 99.98 | 90.30 | 96.09 | 79.74 | ||
| Monoterpene hydrocarbons | 1.83 | 1.57 | - | - | - | - | - | ||
| Oxygenated monoterpenes | 30.01 | - | - | 3.56 | 0.48 | 14.26 | 25.15 | ||
| Sesquiterpene hydrocarbons | 8.63 | 3.97 | 0.05 | - | - | - | - | ||
| Oxygenated sesquiterpenes | 25.54 | 5.61 | 0.19 | - | - | - | - | ||
| Diterpenes | 4.97 | 33.75 | 26.81 | 23.30 | 72.76 | - | - | ||
| Fatty acids&esters&aldehydes&alcohols | 15.96 | 41.16 | 71.07 | 67.75 | 16.72 | 66.48 | 54.59 | ||
| Hydrocarbones | 2.16 | 1.63 | t | - | t | - | - | ||
| Others | 0.02 | - | - | 5..37 | 0.34 | 15.35 | - | ||
a Kovats index; t = trace (percentage less than 0.01%); b MW 304, the peaks at m/z 121 and 133 supports a sandaracopimara-8(14),15-diene diterpene structure and the fragments 286[M − H2O]+ (100), 268 (30) indicate hydroxyl groups; Position of OH groups not determined.
Figure 1.
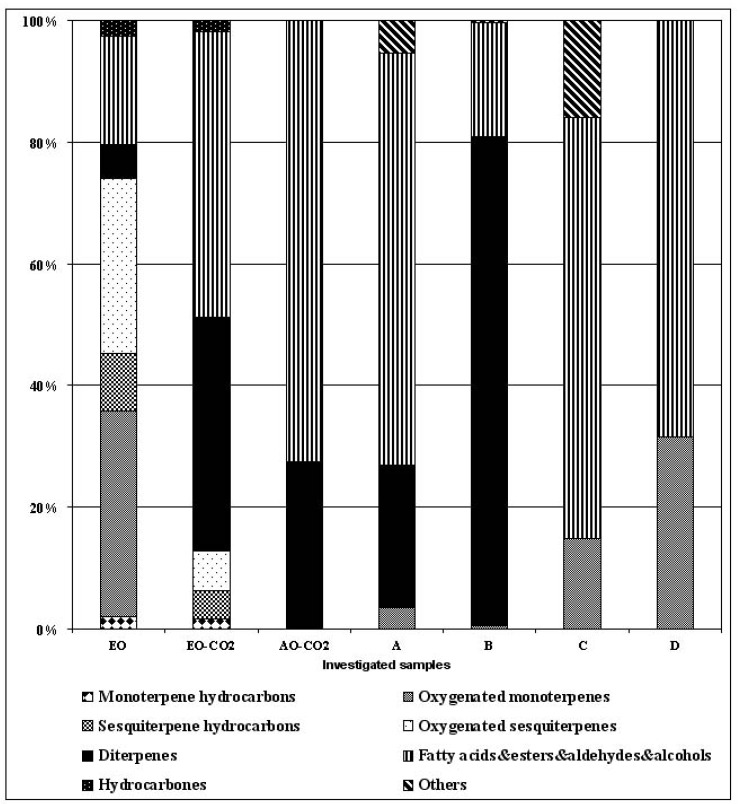
Comparative representation of particular groups of compounds (monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, oxygenated sesquiterpene, diterpenes, fatty acids&esters&aldehydes&alcohols, hydrocarbons and others) in the investigated extracts obtained by hydrodistillation - HD (EO), supercritical carbon dioxide extraction - SC CO2 (EO-CO2 and AO-CO2) and successive solvent extraction - SE (A, B, C, and D).
Table 2.
Results of testing the antibacterial activity on Gram-positive, Gram-negative bacteria and yeast of the supercritical (EO-CO2, AO-CO2) and conventional (EO, A, B, C, and D) S. scardica investigated extracts.
| Strain | MIC VALUES µg/mL | |||||||
|---|---|---|---|---|---|---|---|---|
| EO | EO-CO2 | AO-CO2 | A | B | C | D | Gentamicin | |
| Gram-positive bacteria | ||||||||
| Streptococcus pyogenes 1, tonsils swab | 1280 | 640 | 640 | 1280 | 640 | 1280 | 1280 | ≤4 |
| Streptococcus pyogenes 2, tonsils swab | 1280 | 640 | 640 | 1280 | 1280 | 1280 | 2560 | ≤4 |
| Streptococcus canis, tonsils swab dog | 2560 | 2560 | 2560 | 2560 | 2560 | 2560 | >2560 | ≤4 |
| Moraxella catarrhalis, tonsils swab | 1280 | 2560 | 2560 | 1280 | 1280 | 1280 | 2560 | ≤4 |
| Staphylococcus aureus, ATCC 25923 | >2560 | >2560 | >2560 | 1280 | 1280 | 1280 | 1280 | ≤4 |
| Staphylococcus aureus, CI, tonsils swab | >2560 | 2560 | 2560 | 1280 | 1280 | 640 | 1280 | ≤4 |
| MRSA ATCC 43300 | >2560 | 2560 | 2560 | 1280 | 1280 | 640 | 640 | ≤4 |
| Corynebacterium pseudotuberculosis, tonsils swab | 640 | 320 | 320 | 160 | 320 | 80 | 80 | ≤4 |
| Enterococcus faecalis, tonsils swab | 2560 | 2560 | 2560 | >2560 | >2560 | >2560 | 1280 | ≤4 |
| Gram-negative bacteria | ||||||||
| Escherichia coli, ATCC 25922 | 2560 | >2560 | >2560 | 2560 | >2560 | >2560 | 2560 | ≤4 |
| Escherichia coli, CI, skin swab | >2560 | >2560 | >2560 | 2560 | >2560 | >2560 | 2560 | ≤4 |
| Pseudomonas aeruginosa, tonsils swab | 2560 | 2560 | 2560 | 2560 | >2560 | >2560 | 2560 | ≤4 |
| Klebsiella pneumoniae, tonsils swab | >2560 | >2560 | >2560 | 2560 | >2560 | >2560 | 2560 | ≤4 |
| Pasteurella multocida tonsils swab, dog | 1280 | 1280 | 1280 | 640 | 640 | 320 | 320 | ≤4 |
| Haemophilus sp., nose swab | 640 | 640 | 640 | 320 | 320 | 40 | 80 | ≤4 |
| Yeast | ||||||||
| Candida albicans, tonsils swab | 2560 | 2560 | 2560 | 2560 | >2560 | >2560 | 2560 | - |
All swabs were taken from humans, except were indicated. CI - clinical isolates.
Taking into account that the fraction A was obtained by SE using ethanol as non-selective solvent, we performed successive extraction of the A applying solvents with different polarity in order to enable the separation of the constituents regarding their polarity. Hence, we obtained extracts B (diethyl ether fraction), C (ethyl acetate fraction), and D (n-butanol fraction).
2.1. Chemical Composition of Investigated Samples
The constituents were analyzed by GC and GC-MS followed by calculation of Kovatz indices. In total, 133 compounds were identified (Table 1) in the investigated samples EO, EO-CO2, AO-CO2, A, B, C, and D accounting 89.12, 87.69, 98.12, 99.98, 90.30, 96.09 and 79.74% (respectively).
In the EO sample, oxygenated monoterpenes were the major constituents, but with significant amount of oxygenated sesquiterpenes and fatty acids with their esters, as well (30.01, 25.54 and 15.96%, respectively). Although monoterpene hydrocarbons were previously reported as the main constituents of the essential oil of several Sideritis species, including S. scardica [21], in EO monoterpene hydrocarbons compounds represented only 1.83%. According to the previously published data, in the Macedonian S. scardica essential oil, the most abundant compound was α-cadinol, whereas in the oil of Bulgarian origin the main components were diterpenic compounds and octadecanol (over 20%) [22]. In our sample, diterpenes constituted a significant percentage; with octadecanol representing only 0.21% in oil. The most abundant compounds were hexadecanoic acid, myristicin, menthol, caryophyllene oxide, and τ-muurolol (12.92, 5.23, 4.90, 4.84, and 3.62%, respectively).
Significant differences were established between the chemical profiles of the essential oils obtained by HD and SC CO2 applying the pressure of 10 MPa and temperature of 40 °C as extraction conditions (the samples EO and EO-CO2, respectively). Namely, fatty acids with their esters and diterpenes represented the main groups of the compounds in EO-CO2, with 41.16 and 33.75%, respectively (Table 1, Figure 1). The main components were hexadecanoic acid, dihydroxyl derivative of sandaracopimar-8(14),15-diene, (E)-ferruginol acetate and ethyl hexadecanoate (18.59, 14.89, 11.82 and 11.73%, respectively).
The second fraction AO-CO2 obtained by SC CO2 extraction, maintaining the same temperature, but increasing the pressure to 30 MPa, was mainly characterized by the presence of fatty acids and diterpenes, as well, (71.07 and 26.81%, respectively). The major compounds were hexadecanoic and linoleic acids (43.22 and 24.80%, respectively), and diterpenes—the dihydroxy derivative of sandaracopimar-8(14),15-diene and (E)-ferruginol acetate (11.49 and 11.22% respectively). Besides, in all other investigated extracts fatty acids with their esters remained the major components, with the exception of fraction B, the extracts obtained by SE with diethyl ether as non-polar solvent, which was abundant in diterpenes, representing more than 70% of the chemical composition analyzed by GC-MS. The samples AO-CO2 and A had relatively similar patterns of the constituents fatty acids and diterpenes were the most abundant in A, as well, representing 67.75 and 23.3%, respectively. Hexadecanoic and dodecanoic acid (46.57 and 17.05%, respectively) and the dihydroxy derivative of sandaracopimar-8(14),15-diene, (E)-ferruginol acetate and (Z)-ferruginol acetate (10.79, 8.25 and 4.26%, respectively) diterpenes were the major components.
As stated above, fraction B contained mainly diterpenes, which were not detected in the extracts C and D. The main components in B were (E)-ferruginol acetate and the dihydroxy derivative of sandaracopimar-8(14),15-diene, (65.47 and 6.25%, respectively). Fractions C and D were the fractions abundant in fatty acids and their esters (66.48 and 54.59%, respectively), with differences in the type of fatty acids and their esters present in greatest quantity—as determined, hexadecanoic and dodecanoic acids were the major components in C (29.89 and 25.56%, respectively), whereas dodecanoic acid and decyl acetate (35.78 and 12.54%, respectively) were the most abundant in D. Besides, both samples C and D, contained the oxygenated monoterpene (E)-coniferyl alcohol in a significant percentage (11.81 and 18.69%, respectively), while the sample C contained cyclopentadecanolide (15.35%), as well. The comparative representation of the identified compounds in all investigated samples, classified in the different chemical groups, was typified in Figure 1.
Many studies have been performed on the chemical composition of essential oil from Sideritis species using the GC–MS and GC techniques. In spite of fact that the Lamiaceae family is well-known because of its essential oil content, Sideritis species cannot be considered rich in essential oil. Nevertheless a correlation between the oil yield and the main group of constituents has been established the higher the essential oil yield, the higher the monoterpene hydrogencarbon content. The large number of studies on essential oils composition in Sideritis can explain the polymorphism among the populations and the existence of new species, chemical varieties and hybrids. Several Sideritis essential oils are characterized by high contents of monoterpene hydrocarbons with α-pinene, β-pinene, sabinene, myrcene or limonene as the main compounds [21,22]. The presence of important sesquiterpene hydrocarbons, particularly δ-cadinene and β-caryophyllene, has been usually confirmed. Other essential oils are rich in oxygenated sesquiterpenes, such as α-cadinol, bisabolol or muurol-5-en-4β-ol as the main compounds, and finally diterpene compounds have been found in Sideritis essential oils. The presence of diterpenes as volatile compounds has been described in other genus such as Cistus, Wollemia, Juniperus and Helichrysum, characterized by what occurs in Sideritis with the presence of a large number of these compounds in the aerial part extracts. Turkish endemic species S. bilgerana, S. ozturkii and S. cilicica were rich in the monoterpene hydrocarbons α- and β-pinene. S. cilicica has been shown to have relatively high content of β-phellandrene [23]. In the group of Sideritis species rich in sesquiterpenes the main constituents have been found to be β-caryophyllene, D germacrene and calamene (S. curvidens, S. montana). Oxygenated derivatives are not common as main constituents in Sideritis species. Oxygenated monoterpenes, alongside with thymol, are characteristic consituents in S. romana. Oxygenated sesquiterpenes predominate in essential oils of S. phlomoides and S. taurica. The main constituents of S. congesta and S. argyrea essential oil were α- and β-pinene, while limonene was the major one in S. perfoliata essential oil. S. condensata provided an essential oil with high proportions of β-caryophyllene and α-pinene [24]. S. perfoliata and S. dichotoma essential oils are rich in diterpenes [25]. Monoterpene hydrocarbones has also been reported as main constituent in Sideritis species growing in Greece, and in some Spanish species, as well. In the essential oil of Spanish endemic species S. ibanyezii, sabinene and α-pinene have been found as main compounds [26,27]. The same monoterpene hydrocarbons, as well fenchone and cineole were the main constituents in the essential oil of S. pusillafrom the Iberian Peninsula [28].
According to presented data, the chemical composition of the analyzed samples revealed the existence of different patterns in comparison to the chemical profile of S. scardica already investigated by other authors [21,22]. Namely, diterpenes and fatty acids and their derivatives represented significant groups of compounds in our samples in contrast to others abundant in monoterpene hydrocarbons or oxygenated sesquiterpenes. Besides, in this work the investigated S. scardica essential oil obtained by hydrodistillation, contained oxygenated monoterpenes in the highest percentage.
2.2. Antimicrobial Activity
In this work, Gram-positive bacteria, Streptococcus pyogenes, Streptococcus canis, Moraxella catarrhalis, Staphylococcus aureus, methicillin resistant Staphylococcus aureus, Corynebacterium pseudotuberculosis, Enterococcus faecalis, Gram-negative bacteria Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Pasteurella multocida and Haemophilus sp., and yeast Candida albicans were the tested microorganisms. Pseudomonas aeruginosa is a huge medical and veterinary problem with its intrinsic resistance to many antibiotics and disinfectants, and its ability to develop resistance to every so called “antipseudomonal antibiotic”, including carbapenems and ureidopenicillins [29,30]. Candida albicans infections are usually chronic and hard to treat, especially in children because of strong nephrotoxic and hepatotoxic side effects of some antifungals, especially ketoconazole [31,32]. On the other hand, staphylococci and streptococci, statistically are the most frequent cause of skin infections in humans and animals, whether in hospitals or in the community [32]. Unlike streptococci, which are usually susceptible to penicillins, staphylococci are hard to treat due to their ability of developing resistance to antibiotics and disinfectants [32,33]. At the same time, staphylococci are often the causative agents of secondary skin infections, usually after bites of insects or allergies, so, in all this cases antistaphylococcal therapy is needed [31]. The rest of the investigated strains only occasionally occur as causative agents of infections, mostly in immunocompromised patients.
Overall, minimal inhibitory concentration values (MIC values from 40 to ≥2,560 μg/mL) of the investigated extracts, presented in Table 2, indicated a strong to a moderate antibacterial activity of the investigated S. scardica extracts against the tested microorganisms. Investigated Gram-positive bacteria were more susceptible in comparison to investigated Gram-negative bacteria, with the exception of Pasteurella multocida and Haemophilus sp. Investigated extracts showed slight differences in their antimicrobial activity, but the common feature for all of them was the strongest activity against Gram-negative bacteria Pasteurella multocida and Haemophilus sp. and Gram-positive bacterium Corynebacterium pseudotuberculosis (MIC values 40–640 μg/mL). The strongest antibacterial activity was determined against Haemophylus sp. for the extracts C and D, with obtained MIC values of 40 and 80 µg/mL, respectively. The same extracts exhibited strong antimicrobial activity against Corynebacterium pseudotuberculosis, with MIC values of 80 µg/mL. Essential oil obtained by HD exhibited the strongest activity against Corynebacterium pseudotuberculosis and Haemophylus sp. with MIC values of 640 μg/mL, while moderate activity was determined against Staphylococcus pyogenes, Moraxella catarrhalis and Pasteurella multocida. Regarding the SC CO2 extracts (EO-CO2 and AO-CO2), practically all tested strains showed the same susceptibility; with the best results being obtained against Corynebacterium pseudotuberculosis (MIC value was 320 µg/mL) for the both extracts. All extracts obtained by SE (A, B, C, and D) demonstrated moderately strong or strong antibacterial activity against all investigated Gram-positive bacteria, with MIC values from 80 to 2,560 µg/mL. Interestingly, extracts C and D exhibited moderate activity against MRSA with MIC values 640 µg/mL. Investigated Escherichia coli and Klebsiella pneumoniae strains proved to be the most resistant to the applied concentration of the all investigated extracts, with MIC ≥ 2,560 µg/mL. All investigated extracts inhibited the growth of Candida albicans and Pseudomonas aeruginosa strains at a concentration at 2,560 µg/mL, with the exception of B and C (MIC ≥ 2,560 µg/mL).
There are several reports on the antimicrobial activity of Sideritis essential oils. The antimicrobial activity of S. perfoliata and S. trojana essential oils was tested against Escherichia coli, methicillin-resistant Staphylococcus aureus, Enterobacter aerogenes, Salmonella typhimurium, Bacillus cereus, Staphylococcus epidermidis and Candida albicans. The antimicrobial assay results indicated that E. coli, methicillin-resistant S. aureus, E. aerogenes, B. cereus, and C. albicans were moderately inhibited by the oil of S. trojana, but the oil showed strong inhibitory effects against S. epidermidis. S. perfoliata oil, on the other hand, was less active against the test microorganisms except for C. albicans. The occurrence of a higher content of oxygenated derivatives of mono- and sesquiterpenes (20%) in the oil of S. trojana may be responsible for the better antimicrobial activity [23,34]. In addition, there are several reports about the antimicrobial activity of essential oil from Spanish Sideritis species, S. angustifolia, S. funkiana, S. javalambrensis, S. leucantha, S. mugronensis and S. tragoriganum inhibited Gram-positive bacteria, Staphylococcus aureus, Mycobacterium phlei and the fungi Candida albicans growth, whereas they did not show any activity against Gram-negative bacteria. Similar results were achieved in the investigation of essential oil of S. curvidens and S. lanata, which had no effect against any Gram-negative bacteria, but with a significant activity on Gram-positive bacteria [35,36].
On the contrary, essential oils from S. cilicica and S. bilgerana exerted a significant inhibitory effect against several Gram-negative (Salmonella typhimurium, Escherichia coli) and Gram-positive (Staphylococcus aureus, Bacillus cereus, Staphylococcus epidermidis) bacteria, with a MIC value from 0.125 to 0.5 mg/mL, as well as against Candida albicans (MIC 0.03 mg/mL). This antibacterial activity could be due to the presence of α-pinene and β-pinene as the main constituents of both species [37]. Also, S. italica essential oil was investigated because of its antimicrobial activity which has been shown to be the higher against Gram-negative than Gram-positive bacteria, especially against Pseudomonas aeruginosa responsible for severe opportunistic infections and very often resistant to conventional antibiotics [1]. Besides, the shown strong inhibiting activity against Helicobacter pylori justified the ethnopharmacological use of S. italica as an antiulcer agent.
Not only the essential oil, but various Sideritis extracts possess significant antibacterial activity. According to the performed study by Sagdic et al. [38], the methanolic extracts of S. ozturkii and S. caesarea, had considerable antimicrobial activity. Linearol, foliol, epicandicandiol and siderol which were found in the mentioned Sideritis species were investigated for antibacterial activities as well and epicandicandiol had the highest antimicrobial activity against E. coli. The acetone and methanol extracts of S. tmolea P. H. Davis were tested against standard bacterial strains. As the result of the activity studies, it is found that Sideritis species crude acetone and methanol extracts have not shown considerable antimicrobial or antituberculous activity [39], but the inhibition of clotrimmazole-resistant C. albicans by some Sideritis species from Turkey were reported [40]. As well, in vitro studies indicated that a series of ent-manoyl oxides from S. varoi and their synthetically obtained derivatives inhibited the growth of Leishmania donovani [41].
The observed antimicrobial activity of the investigated extracts in this work might be attributed to the presence different types of terpenoids. Besides the established antimicrobial potential of monoterpenes, diterpenes have attracted considerable attention recently. Namely, diterpenes (especially pimarane type) have been reported to display important biological activities, including antimicrobial activity [42]. Also, the antimicrobial activity might be the results of the various compounds present in the investigated extracts, but not identified by the applied analysis techniques.
There are no data on referential MIC values of plant extracts upon which categorization of investigated microorganisms could be done (susceptible or resistant) and antimicrobial potency of plant extracts is often estimated by comparing MIC values of plant extracts to MIC values of antibiotics. The scientific basis of such practice is unclear and microbiologically and pharmacologically this is the wrong principle because of different pharmacokinetics and metabolism of plant extract and antibiotics. In other words, higher MIC values of plant extracts do not necessarily mean weak antimicrobial potency [43,44]. For the purpose of the determination of MIC values of antibiotics or plant extracts, investigated substances first have to be diluted in order to find the lowest concentration in which they demonstrate antimicrobial activity [45,46,47]. Regarding this, in this research, extracts were previously diluted as described in Experimental. Dilution has been done by adding 25.6 µL (0.0256 mL) of investigated sample in 1 mL of DMSO (with density correction for every extract). The measured volume of investigated extracts was very small and from the microbiological point of a view, MIC values of 1,280 µg/mL and 2,560 µg/mL might be interpreted as no or weak antimicrobial activity [43,48]. Contrary to that, according to Aligiannis et al. [26], the antimicrobial activity of investigated essential oils of S. sipyle, S. clandestine and S. raeseri was characterized as strong or moderate, with MIC values ranging from 650 to 9,900 µg/mL, being significantly higher in comparison to those observed in this work.
2.3. The Extraction Yields
The yields of the performed extractions are presented in the Table 3. The results were as expected, according to the low selectivity of the polar solvent used for obtaining the sample A.
Table 3.
The yields, calculated as the amount of extract compared to the total mass of solid material at the beginning of the extraction process for the supercritical (EO-CO2, AO-CO2) and conventional (EO, A, B, C, and D) S. scardica investigated extracts.
| Investigated samples | Yield of extraction (%) |
|---|---|
| EO | 0.03 |
| EO-CO2 | 1.04 |
| AO-CO2 | 0.63 |
| A | 16.70 |
| B | 0.50 |
| C | 0.20 |
| D | 0.70 |
2.4. The Kinetics of SC-CO2 Extraction
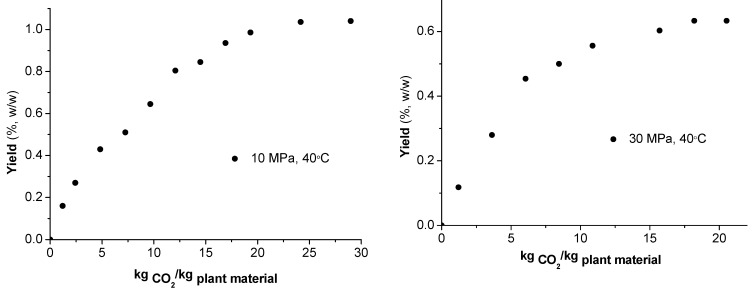
The kinetics of the extraction processes are depicted in Figure 2. The yield is shown as a function of the specific SC CO2 consumption. The results indicate that the total CO2 consumption was 1,200 g for the first, and 870 g for the second fraction extraction. The kinetics of both experiments was obviously similar. However, the first fraction consists mainly of low volatile components that represent essential oil, while the second fraction (defined as nonvolatile) consists mainly of components characterized with higher molecular weight.
Figure 2.
Experimental results for supercritical fluid extraction from S. scardica at 10 MPa and 40 °C (left), and 30 MPa and 40 °C (right).
3. Experimental
3.1. Plant Material
Wild growing species Sideritis scardica Griseb., Lamiaceae were collected on Shara Mountain (at the foothills of the Ljuboten, at ca. 1300 m) during the time of flowering. Plant material was air dried, packed in paper bags and kept in a dark and cool place until analysis. Plant material was verified and the voucher specimen of the plant (SS/08) was deposited at Herbarium of Botanical Garden, Jevremovac, Belgrade, Serbia. The plant material was milled in a blender for 60 s and immediately subjected to hydrodistillation (HD) or supercritical CO2 extraction (SC CO2), and solvent extraction, as well. The average particle size of milled herbs was 0.40 mm (used for all performed extractions).
3.2. Essential Oil Extraction by Hydrodistillation
Dried, milled herb (50 g) of S. scardica was distilled using 700 mL distilled water according to the standard Clevenger method (4 h) and a yellow viscous volatile essential oil with a balsamic odor was collected. The obtained essential oil (Sample EO) was kept in a sealed vial at 4 °C. The yield (w/w) of essential oil was 0.03% (on a dry weight basis).
3.3. Supercritical Fluid Extraction
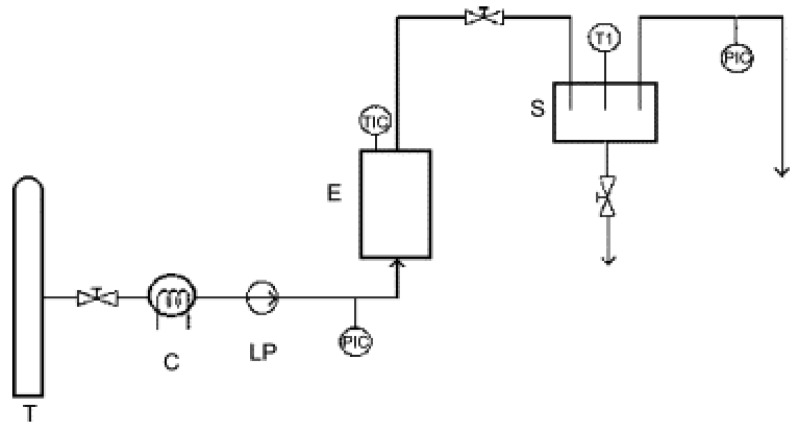
Extractions with supercritical carbon dioxide (SC CO2) were performed on a laboratory scale equipment, in an Autoclave Engineers SCE Screening System with a 150 cm3 extractor vessel previously described [49] and shown in Figure 3.
Figure 3.
Schematic presentation of the autoclave engineers screening system—T: CO2 storage tank; C: cryostat; LP: high pressure liquid pump; E: extractor vessel; S: separator vessel.
Plant material (41.4 g) was milled and sieved. The fraction with an average particle diameter of 0.4 mm (collected between sieves of 0.2 mm and 0.6 mm) was used for the experiments. Supercritical extractions with carbon dioxide were performed fractionally. The pressure and temperature conditions for the extraction of the first fraction were 10 MPa and 40 °C respectively, while the SC CO2 flow rate was 0.67 kg/h (obtained sample EO-CO2). After the plant material was exhausted, the pressure was raised to 30 MPa and the extraction of the second fraction followed. The SC CO2 flow rate was 0.32 kg/h (obtained sample AO-CO2). Commercial carbon dioxide (99% purity) supplied by Tehnogas (Messer-Tehnogas, Serbia) was used for SC CO2, and dichloromethane and alcohol (GC purity, Sigma–Aldrich, Germany) was used for dissolution of supercritical extracts prior to GC-FID-MS analyses.
3.4. Solvent Extraction (SE)
The shade-dried and powdered aerial parts of S. scardica (200 g) were coarsely extracted using 70% (v/v) ethanol. The crude ethanol extract (A) was re-dissolved in distilled water, shaken vigorously and successively extracted with 200 mL of diethyl ether, 200 mL ethyl acetate, and 200 mL saturated n-butanol in a separating funnel,. The obtained extracts were labelled as the diethyl ether extract, B (0.9 g) ethyl acetate extract, C (0.4 g). and n-butanol extract, D (1.5 g), respectively.
3.5. Gas Chromatography (GC-FID)
Gas chromatography analysis of the extracts was carried out on a HP-5890 Series II GC apparatus [Hewlett-Packard, Waldbronn (Germany)], equipped with a split–splitless injector and automatic liquid sampler, attached to a HP-5 column (25 m × 0.32 mm, 0.52 μm film thickness) and fitted with a flame ionization detector (FID). Carrier gas flow rate (H2) was 1 mL/min, split ratio 1:30, injector temperature was 250 °C, detector temperature 300 °C, while column temperature was linearly programmed from 40 to 260 °C (at rate of 4 °C /min), and then kept isothermally at 260 °C for 10 min. Solutions of samples in dichloromethane or alcohol were consecutively injected in amount of 1 μL. Area percent reports, obtained as result of standard processing of chromatograms, were used as base for the quantification analysis.
3.6. Gas Chromatography/Mass Spectrometry (GC-MS)
The same analytical conditions as those mentioned for GC-FID were employed for GC/MS analysis, along with a column HP-5MS (30 m × 0.25 mm, 0.25 μm film thickness), using a HP G 1800C Series II GCD system [Hewlett-Packard, Palo Alto, CA, USA]. Helium was used as carrier gas. The transfer line was heated at 260 °C. Mass spectra were acquired in EI mode (70 eV); in the 40–450 m/z range. An amount of 0.2 μL of sample solution in dichloromethane or alcohol was injected. The components of the oil were identified by comparison of their mass spectra to those from the Wiley 275 and NIST/NBS libraries, using different search engines. Identification of the compounds was achieved by comparing their retention indices and mass spectra with those found in the literature [50] and supplemented by the Automated Mass Spectral Deconvolution and Identification System software (AMDIS ver. 2.1), GC-MS Librairies [51]. The experimental values for retention indices were determined by the use of calibrated Automated Mass Spectral Deconvolution and Identification System Software (AMDIS ver. 2.1), GC-MS Libraries [51], compared to those from available literature (Adams 2007) [50] and used as additional tool to confirm the MS findings. The relative proportion of the essential oil constituents were expressed as percentages obtained by peak area normaliyation, all relative response factors being taking as one.
3.7. In Vitro Antimicrobial Activity
The investigation of the antibacterial activity of investigated samples EO, EO-CO2, AO-CO2, A, B, C, and D was performed on Gram-positive and Gram-negative bacterial species. From the group of Gram positive microorganisms, Streptococcus pyogenes, Streptococcus canis, Moraxella catarrhalis, Staphylococcus aureus, Corynebacterium pseudotuberculosis, and Enterococcus faecalis strains were chosen. From the group of Gram-negatives, Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli, Pasteurella multocida and Haemophilus strains were selected. Pathogenic yeasts was also included in the investigation and a Candida albicans strain was chosen for that purpose. The investigated strains were isolated from skin and tonsils swabs taken from diseased persons and animals with infection symptoms, except Staphylococcus aureus ATCC 25923 and the methicillin-resistant Staphylococcus aureus referential strains (MRSA ATCC 43300) which were purchased from Becton Dickinson, USA. The isolation was made from clinical material delivered to the Microbiology Department, Faculty of Veterinary Medicine, Belgrade University.
Conventional microbiological methods were applied for the purpose of isolation and identification and Columbia sheep blood agar (bioMerieux), MacConkey agar (bioMerieux), CNA agar with colistin and nalidixic acid (Becton Dickinson) and nutrient broth (BioLab) were used. For the isolation of Candida albicans, Sabouraud dextrose agar was used (BioLife). Identification of isolated strains was performed with BBL Crystal Gram-positive ID kit, BBL Crystal enteric/nonfermenter ID kit (Becton Dickinson), API 32 STAPH, API 20 NE and API 20 C AUX (bioMerieux).
For the investigation of antibacterial activity and the determination of MIC values of the investigated samples, broth microdillution method was applied in accordance with the CLSI prescriptions for antimicrobial susceptibility testing [45,46,47]. For that purpose, Cation adjusted Mueller Hinton II broth was used (CAMHB, Becton Dickinson) with the addition of 1.6% bromcresol purple (Merck) in final concentration at 0.2 mL/200 mL for Gram-positives and 1% phenol red (Merck) at 1 mL/200 mL for Gram-negatives. Bromcresol purple and phenol red were added to obtain bacterial growth visibility. Sabouraud dextrose broth (BioLife) was used for yeasts with no indicators added. For streptococci, foetal bovine serum (Sigma) was added in CAMHB at final concentration at 5%. Dimethyl sulfoxide, (DMSO, Merck) was used as solvent for investigated samples. Investigated concentrations of investigated samples were 2560, 1280, 640, 320, 160, 80, 40, 20, 10, 5, 2,5 and 1,25 expressed in μg/mL. The samples were dissolved in DMSO at 25.600 μg/mL, then 1:10 dilution with CAMHB was made. Titration until desired concentrations was performed in microplate wells as previously described [45,46,47]. The final bacterial inoculum density of 5 × 105 CFU/mL was achieved by adding 5 μL of 1–2 × 107 CFU/mL suspension of investigated strain in microplate wells with 100 μL of previously added CAMHB. Microplates were incubated 18–24 h on 37 °C. For MIC values the broth with lowest oil concentration, with no visible bacterial growth, was used.
4. Conclusions
The overall aim of this study was to contribute to the global search for bioactive natural products and convenient methods for their extraction. Hydrodistillation and solvent extraction are traditional techniques to recover compounds from aromatic plants. As an alternative method, supercritical carbon dioxide extraction was used and proved to be suitable for obtaining different plant extracts. The extraction method influenced the yield of the extraction and chemical composition performed by GC and GC-MS techniques of the investigated S. scardica extracts. The chemical profiles of the investigated extracts by GC-MS analysis revealed differences regarding the content of different compounds group - monoterpene and sesquiterpene hydrocarbons, oxygenated monoterpenes and sesquiterpenes, diterpenes, and fatty acids and their esters, as well. As observed differences in chemical compositions of investigated extracts were significant, the alternative SC CO2 extractions could not replace the conventional ones, regardless of the better yields of the extraction. Considering the literature, there were no data regarding the chemical composition of SC-CO2 extracts isolated from S. scardica at 10 MPa and 30 MPa at 40 °C, nor for the antimicrobial activity of S. scardica extracts or essential oil. The antimicrobial activity was detected at comparable levels for all investigated extracts, with obtained MIC values of 40–2,560 μg/mL. The lowest MIC values were detected for the extracts obtained by solvent extraction. The extracts obtained by supercritical carbon dioxide extraction exhibited more or the same activity against almost all investigated microorganisms in comparison to essential oil obtained by hydrodestilliation. In spite of the differences in the methods applied for the extraction, and the chemical composition of the investigated S. scardica extracts, as well, antimicrobial activity was not significantly influenced, revealing the possibility that the combination of diterpenes and fatty acids and their derivatives might be, at least, partly responsible for the shown activity, but the presence of other compound not identified by applied techniques should not be ignored.
Acknowledgments
The authors wish to send their gratitude to Serbian Ministry of Education and Science (Project No. III 45017).
Conflict of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the analyzed samples are available from the authors.
References and Notes
- 1.Basile A., Senatore F., Gargano R., Sorbo S., Del Pezzo M., Lavitola A., Ritieni A., Bruno M., Spatuzzi D., Rigano D., Vuotto M.L. Antibacterial and antioxidant activities in Sideritis italica (Miller) Greuter et Burdet essential oils. J. Ethnopharmacol. 2006;107:240–248. doi: 10.1016/j.jep.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Charami M., Lazari D., Karioti A., Skaltsa H., Hadjipavlou-Litina D., Souleles C. Antioxidant and Antiinflammatory Activities of Sideritis perfoliata subsp. perfoliata (Lamiaceae) Phytother. Res. 2008;22:450–454. doi: 10.1002/ptr.2333. [DOI] [PubMed] [Google Scholar]
- 3.Küpeli E., Şahin F.P., Çalış I., Yeşilada E., Ezer N. Phenolic compounds of Sideritis ozturkii and their in vivo anti-inflammatory and antinociceptive activities. J. Ethnopharmacol. 2007;118:356–360. doi: 10.1016/j.jep.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Aboutabl E.A., Nassar M.I., Elsakhawy F.M., Maklad Y.A., Osman A.F., El-Khrisy E.A.M. Phytochemical and pharmacological studies on Sideritis taurica Stephan ex Wild. J. Ethnopharmacol. 2002;82:177–184. doi: 10.1016/S0378-8741(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 5.Hernàndez-Pérez M., Rabanal R.M. Evaluation of the antinflammatory and analgesic activity of Sideritis canariensis var. pannosa in mice. J. Ethnopharmacol. 2002;81:43–47. doi: 10.1016/S0378-8741(02)00033-8. [DOI] [PubMed] [Google Scholar]
- 6.Navarro A., de las Heras B., Villar A. Anti-inflammatory and immunomodulating properties of a sterol fraction from Sideitis foetens Clem. Biol. Pharm. Bull. 2001;24:470–473. doi: 10.1248/bpb.24.470. [DOI] [PubMed] [Google Scholar]
- 7.De las Heras B., Navarro A., Díaz-Guerra M.J., Bermejo P., Castrillo A., Boscá L., Villar A. Inhibition of NOS-2 expression in macrophages through the inactivation of NF-kB by andalusol. Br. J. Pharmacol. 1999;28:605–612. doi: 10.1038/sj.bjp.0702844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Güvenç A., Okada Y., Küpeli Akkol E., Duman H., Okuyama T., Çalıs I. Investigations of anti-inflammatory, antinociceptive, antioxidant and aldose reductase inhibitory activities of phenolic compounds from Sideritis brevibracteata. Food Chem. 2010;118:686–692. doi: 10.1016/j.foodchem.2009.05.034. [DOI] [Google Scholar]
- 9.Demirtas I., Sahin A., Ayhan B., Tekin S., Telci I. Antiproliferative effects of the methanolic extracts of Sideritis libanotica Labill. subsp. Linearis. Rec. Nat. Prod. 2009;3:104–109. [Google Scholar]
- 10.Ertaş A., Öztürk M., Boga B., Topçu G. Antioxidant and anticholinesterase activity evaluation of ent-kaurane diterpenoids from Sideritis arguta. J. Nat. Prod. 2009;72:500–502. doi: 10.1021/np800671p. [DOI] [PubMed] [Google Scholar]
- 11.Kassi E., Papoutsi Z., Fokialakis N., Messari J., Mitakou S., Moutsatsou P. Greek plant extracts exhibit selective estrogen receptor modulator (SERM)-like properties. J. Agric. Food. Chem. 2004;52:6956–6961. doi: 10.1021/jf0400765. [DOI] [PubMed] [Google Scholar]
- 12.Tadic V.M., Jeremic I., Dobric S., Isakovic A., Markovic I., Trajkovic V., Bojovic D., Arsic I. Anti-inflammatory, gastroprotective and cytotoxic effects of Sideritis scardica extracts. Planta Med. 2012;78:1–13. doi: 10.1055/s-0031-1298172. [DOI] [PubMed] [Google Scholar]
- 13.Plioukas M., Termentzi A., Gabrieli C., Zervou M., Kefalas P., Kokkalou E. Novel acylflavones from Sideritis syriaca ssp. syriaca. Food Chem. 2010;123:1136–1141. doi: 10.1016/j.foodchem.2010.05.076. [DOI] [Google Scholar]
- 14.Petreska J., Stefova M., Ferreres F., Moreno D.A., Thomas-Barberan F.A., Stefkov G., Kulevanova S., Gil-Izquiredo A. Potential bioactive phenolics of Macedonian Sideritis species used for medicinal “Mountain tea”. Food Chem. 2011;125:13–20. doi: 10.1016/j.foodchem.2010.08.019. [DOI] [Google Scholar]
- 15.Alipieva K.I., Kostadinova E.P., Evstatieva L.N., Stefova M., Bankova V.S. An iridoid and a flavonoid from Sideritis lanata L. Fitoterapia. 2009;80:51–53. doi: 10.1016/j.fitote.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Kilic T. Isolation and biological activity of new and known diterpenoids from Sideritis stricta Boiss. & Heldr. Molecules. 2006;11:257–262. doi: 10.3390/11040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diklić N. Genus Sideritis L. In: Josifović M., editor. Flora of Serbia. Volume 4. Serbian academy of Sciecne and Art; Belgrade, Serbia: 1974. pp. 371–372. [Google Scholar]
- 18.Koleva I., Linssen J.P.H., van Beek T.A., Evstatieva L.N., Kortenska V., Handjieva N. Antioxidant activity screening of extracts from Sideritis species (Labiatae) grown in Bulgaria. J. Sci. Food Agric. 2003;83:809–819. doi: 10.1002/jsfa.1415. [DOI] [Google Scholar]
- 19.Reverchon E. Supercritical fluid extraction and fractionation of essential oils and related products. J. Supercrit. Fluid. 1997;10:1–37. doi: 10.1016/S0896-8446(97)00014-4. [DOI] [Google Scholar]
- 20.Papaefstathiou G., Polychronopoulos P., Aligiannis N., Skaltsounis A.L., Mitaku S. Comparative study of accelerated solvent extraction and supercritical fluid extraction of total phenolics and flavonoids from Sideritis raeseri subsp. attica and study of antioxidant activity. Planta Med. 2008;74:1122. [Google Scholar]
- 21.Krimer N., Tabanaca N., Özek T., Tümen G., Başer K.H.C. Essential oil of annual Sideritis species growing in Turkey. Pharm. Biol. 2000;38:106–111. doi: 10.1076/1388-0209(200004)3821-1FT106. [DOI] [PubMed] [Google Scholar]
- 22.Kostadinova E., Nikolova D., Alipieva K., Stefova M., Stefkov G., Evstatieva L., Matevski V., Bankova V. Chemical constituents of the essential oils of Sideritis scardica Griseb. and Sideritis raeseri Boiss and Heldr. from Bulgaria and Macedolnia. Nat. Prod. Res. 2007;21:819–823. doi: 10.1080/14786410701394142. [DOI] [PubMed] [Google Scholar]
- 23.Kirimer N., Baser K.H.C., Demirci B., Duman H. Essential oils of Sideritis species of Turkey belonging to the section Empedoclia. Chem. Nat. Compd. 2004;40:19–23. doi: 10.1023/B:CONC.0000025458.00475.cf. [DOI] [Google Scholar]
- 24.Ezer N., Vila R., Caiqigueraland S., Adzet T. Essential oil composition of four Turkish species of Sideritis. Phytochemistry. 1996;41:203–205. doi: 10.1016/0031-9422(95)00601-X. [DOI] [Google Scholar]
- 25.Baser K.H.C. Aromatic biodiversity among the flowering plant taxa of Turkey. Pure App. Chem. 2002;74:527–545. doi: 10.1351/pac200274040527. [DOI] [Google Scholar]
- 26.Aligiannis N., Kalpoutzakis I., Chinou B., Mitakou S. Composition and antimicrobial activity of the essential oils of five taxa of Sideritis from Greece. J. Agric. Food Chem. 2001;49:811–815. doi: 10.1021/jf001018w. [DOI] [PubMed] [Google Scholar]
- 27.Palá-Paúl J., Pérez-Alonso M.J., Velasco-Negueruela A., Ballesteros M.T., Sanz J. Essential oil composition of Sideritis hirsuta L. from Guadalajara Province, Spain. Flavour. Frag. J. 2006;21:410–415. doi: 10.1002/ffj.1727. [DOI] [Google Scholar]
- 28.Rodriguez-Garcia I., Munoz-Dorado M., Gomez-Mercado F., Garcia-Maroto F., Guil-Guerrero J.L. Essential oil composition of Sideritis pusilla (Lange) Pau ssp. J. Essent. Oil Res. 2004;16:535–538. doi: 10.1080/10412905.2004.9698791. [DOI] [Google Scholar]
- 29.Aloush V., Navon-Venezia S., Seigman-Igra Y., Cabili S., Carmeli Y. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob. Agents Ch. 2006;1:43–48. doi: 10.1128/AAC.50.1.43-48.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livermore D. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: Our worst nightmare. Clin. Infect. Dis. 2002;34:634–640. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- 31.Quinn P., Markey B. Concise Review of Veterinary Microbiology. 1st ed. Blackwell Publishing; Oxford, UK: 2003. pp. 8–10. [Google Scholar]
- 32.Winn W., Allen S., Janda W., Koneman E., Procop G., Schreckenberger P., Woods G. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. 6th ed. Lippincott Williams & Wilkins; Baltimore, MD, USA: 2006. pp. 945–1021. [Google Scholar]
- 33.Brooks G., Butel J., Morse S. Jawetz, Melnick & Adelberg’s Medical Microbiology. 21st ed. Appleton & Lange; Stamford, CT, USA: 1998. pp. 145–177. [Google Scholar]
- 34.Kirimer N., Demirci B., Iscan G., Baser K.H.C., Duman H. Composition of the essential oils of two Sideritis species from Turkey and antimicrobial activity. Chem. Nat. Comp. 2008;44:121–123. doi: 10.1007/s10600-008-0037-5. [DOI] [Google Scholar]
- 35.Ŭgur A., Varol O., Ceylan O. Antibacterial activity of Sideritis curvidens and Sideritis lanata from Turkey. Pharm. Biol. 2005;43:47–52. doi: 10.1080/13880200590903354. [DOI] [Google Scholar]
- 36.Villar A., Recio M.C., Ríos J.L., Zafra-Polo M.C. Antimicrobial activity of essential oils from Sideritis species. Pharmazie. 1986;41:298–299. [PubMed] [Google Scholar]
- 37.Iscan G., Kirimer N., Kurkcuoglu M., Baser K.H.C. Composition and antimicrobial activity of the essential oils of two endemic species from Turkey: Sideritis cilicica and Sideritis bilgerana. Chem. Nat. Comp. 2005;41:679–682. doi: 10.1007/s10600-006-0010-0. [DOI] [Google Scholar]
- 38.Sagdic O., Aksoy A., Ozkan G., Ekici L., Albayrak S. Biological activities of the extracts of two endemic Sideritis species in Turkey. Innov. Food Sci. Emerg. Technol. 2008;9:80–84. doi: 10.1016/j.ifset.2007.06.001. [DOI] [Google Scholar]
- 39.Çarıkç S., Çöl Ç., Kılıç T., Azizoglu A. Diterpenoids from Sideritis tmolea P. H. Davis. Rec. Nat. Prod. 2007;4:44–50. [Google Scholar]
- 40.Dulger B., Gonuz A., Aysel V. Inhibition of clotrimmazole-resistant Candida albicans by some Sideritis species from Turkey. Fitoterapia. 2006;77:404–405. doi: 10.1016/j.fitote.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 41.García-Granados A., Liñán E., Martínez A., Rivas F., Mesa-Valle C.M., Castilla-Calvente J.J., Osuna A. In vitro action of ent-manoyl oxides against Leishmania donovani. J. Nat. Prod. 1997;60:13–16. doi: 10.1021/np9603636. [DOI] [Google Scholar]
- 42.Ambrósio S.R., Arakawa N.S., Esperandim V.R., de Albuquerque S., Da Costa F.B. Trzpanocidal activity of pimarane diterpenes from Viguiera arenaria (Asteraceae) Phytother. Res. 2008;22:1413–1415. doi: 10.1002/ptr.2512. [DOI] [PubMed] [Google Scholar]
- 43.Weckesser S., Engel K., Simon-Haarhaus B., Wittmer A., Pelz K., Schempp C.M. Screening of plant extracts for antimicrobial activity against bacteria and yeasts with dermatological relevance. Phytomedicine. 2007;14:508–516. doi: 10.1016/j.phymed.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 44.Saddique Z., Naeem I., Maimoona A. A review of the antibacterial activity of Hypericum perforatum L. J. Ethnopharmacol. 2010;31:511–521. doi: 10.1016/j.jep.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 45.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2010. CLSI document M100-S20. [Google Scholar]
- 46.Isenberg H.D. Antimicrobial Susceptibility Testing. In: Isenberg H.D., editor. Clinical Microbiology Procedures Handbook. 1st ed. Volume 2. ASM Press; Washington, DC, USA: 2004. pp. 5.1.1.–5.18.2.1. [Google Scholar]
- 47.Schwalbe R., Steele-Moore L., Goodwin A. Antimicrobial Susceptibility Testing Protocols. 1st ed. CRC Press; Boca Raton, FL, USA: 2007. pp. 150–182. [Google Scholar]
- 48.Mišić D., Ašanin R., Ivanović J., Žižović I. Investigation of antibacterial activity of supercriticsl extracts of plants, as well as of extracts obtained by other technological processes on some bacteria isolated from animals. Acta Vet. 2009;59:557–568. [Google Scholar]
- 49.Zizovic I., Stamenic M., Orlovic A., Skala D. Supercritical carbon dioxide extraction of essential oils from plants with secretory ducts: Mathematical modeling on the micro-scale. J. Supercrit. Fluids. 2007;39:338–346. doi: 10.1016/j.supflu.2006.03.009. [DOI] [Google Scholar]
- 50.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 51.Automated Mass Spectral Deconvolution and Identification System software (AMDIS ver. 2.1National Institute of Standards and Technology (NIST), Standard Reference Data Program, Gaithersburg, MD, USA, 2005.